Abstract
Iron deficiency remains a global health issue, and antinutritional factors, such as tannins, are often cited as contributors to the high prevalence of deficiency. Despite this, tannin-rich diets may have potential beneficial cardiovascular and cancer-fighting properties because of the antioxidant activity of tannins. Furthermore, epidemiologic studies and long-term trials involving participants who consumed diets rich in antinutritional factors, particularly tannins, conflict with single-meal bioavailability studies. The purpose of this narrative review is to determine the effect of tannins on iron bioavailability and status and establish whether adaptation to tannins reduces the antinutritional effects of tannins over time. We also aimed to compare tannins used in iron studies. Common themes related to iron bioavailability and iron status with tannin consumption were collected and collated for summary and synthesis based on models and subjects used. Overall, there was dissonance between iron bioavailability and status in studies. Single-meal studies with hydrolyzable and oligomeric catechin and epicatechin tannins (tea and tannic acid) generally support reductions in bioavailability related to tannin consumption but not consumption of condensed tannin, which are more commonly found in food. Long-term animal model, epidemiologic, and multimeal studies generally do not support changes in iron status related to tannin intake. Studies suggest that long-term tannin consumption may impact iron status in a different manner than single-meal studies or bioavailability iron models predict. Furthermore, iron bioavailability studies that use condensed tannins, which are more commonly consumed, may better predict mealtime iron bioavailability. More research is needed to develop representative antinutritional iron studies and investigate mechanisms underlying the adaptation to tannins and other antinutritional factors that occur over time.
Keywords: iron bioavailability, antinutritional factors, tannins, proanthocyanidins, iron-deficiency anemia
Introduction
Iron deficiency is common worldwide, and nearly 1 billion people suffer from iron-deficiency anemia (IDA) (1). Adequate iron stores are required for normal growth and development, and IDA has been associated with loss of productivity; reduced cognitive functioning (2); increased prematurity; and perinatal, childhood, and maternal mortality (3). Whereas the WHO approximates that IDA contributes to 3% of all disability life-years lost, more recent estimates suggest that 2013 economic losses related to IDA in Indian children aged 6–59 mo alone were nearly $24 billion (4). Populations with a higher prevalence of IDA include women, children, people consuming a vegetarian or meatless diet, and those consuming insufficient amounts of iron in developing countries (1), and an estimated 30–40% of women and children <5 y of age develop IDA without iron fortification (1). Despite prevalence rates and multiple initiatives aimed at improvement of IDA, an estimated 29% of nonpregnant women were anemic in 2011, a reduction of only 4% from 1995 (5).
The absorption, incorporation, and use of iron in the body is a strictly regulated process in which the homeostatic regulation of iron is primarily mediated through absorption and recycling (6). Nearly 90% of iron stores are retained through senescent RBC recycling; nutritional intake accounts for the remaining 10% (7). Although a multitude of genetic- and disease-related factors influence the pathophysiology and prevalence of IDA (6), interest in its treatment has been largely focused on readily modifiable factors, such as nutritional enhancement and iron absorption inhibitors. Inhibitors found in diets rich in legumes and grains (termed “antinutritional factors”) are particularly criticized as contributors to the high prevalence of deficiency in developing and low-income countries (8, 9), despite the cited health benefits of diets rich in these staples (10, 11). Antinutritional factors such as tannins and phytates in cereals have been found to negatively affect the bioavailability of minerals such as iron when consumed in large quantities (12, 13). It is accepted that tannins reduce iron availability before absorption through the formation of insoluble antinutritional-mineral complexes (14), and reported exacerbation of IDA by foods high in phytates or tannins is common (15–17). Single-meal studies have confirmed iron bioavailability inhibition with phytate (12, 18, 19) and tannin (20–22) consumption.
The term “tannin” denotes a broad class of compounds that can be further classified into hydrolysable tannins or the more commonly consumed condensed tannins (also known as proanthocyanidins) (23) (Figure 1). The ability of tannins to precipitate proteins has been linked to the sensation of astringency (24), and plant tannin content is linked to insect, animal, and mold resistance (25). It may be these defense mechanisms that lead to the antioxidant (26), cancer-fighting (27), and cardiovascular benefits (28) derived from the antioxidant properties of tannin-rich foods, such as wine and tea. Considering both the potential detrimental and beneficial properties of tannins, a dichotomy exists between limiting the tannin consumption of those at risk of IDA and the potential health benefits derived from tannin-rich diets.
Figure 1.
Condensed tannin (A). Tannic acid (B). Panel A is reproduced from https://commons.wikimedia.org/wiki/File:Tannic_acid.svg. Panel B is reproduced from https://commons.wikimedia.org/wiki/File:Procyanidin_C1.svg. Both images are in the public domain.
Because of the iron absorption inhibition of tannins, prudent food system and agricultural efforts have been made to reduce the tannin content of grains, legumes, and foods in an effort to enhance the iron status of those consuming them (29). Despite this, there is a wealth of information that refutes conventional ideology that tannins contribute to chronic changes in iron deficiency. In studies that have supported a reduction in bioavailability through tannin consumption, individual iron absorption has been highly variable (30, 31), and the majority of individuals who consume diets with high concentrations of tannins, as well as antinutritional factors in general, have reported normal iron status (32, 33). Furthermore, the removal of antinutritional factors has not been shown to improve iron bioavailability (34), nor have diets rich in tannin content (35, 36).
Evidence suggests that individuals can adapt to antinutritional factors over time. Repeated consumption of antinutritional factors has been shown to blunt reductions in iron bioavailability in animal (37–39) and clinical (40, 41) models alike. Interestingly, tannins administered per rectum (42) or topically (43) have been linked to hepatotoxicity, whereas oral consumption of condensed tannins has not, suggesting that inherent defense mechanisms may exist that respond to tannin consumption over time. Although adaptation to antinutritional factors may be plausible, it is not currently well understood whether 1) tannins at commonly consumed amounts are linked to changes in iron status, 2) short-term tannin-mediated reductions in iron bioavailability continue over time, 3) tannins alone (rather than consumed with phytates) reduce iron status, and 4) single-meal study inhibition concentrations result in meaningful reductions in iron status. The focus of this review is to explore the effects of tannins on iron bioavailability and status.
Methods
Interventional and epidemiologic studies that examined the relation between tannin consumption and iron bioavailability or iron status were identified through PubMed, Web of Science, and Google Scholar databases with the use of the search terms “iron availability and/or iron bioavailability” and “tannin and/or polyphenol and/or antinutritional factors,” “iron” [Mesh] and “bioavailability” [Mesh] and “tannin” [Mesh], “polyphenol,” and iron bioavailability. Terms also included were “sorghum and/or tea” because of the common citation of these tannin-containing factors on iron bioavailability. In addition, the method of snowball article collection (citations from relevant journal articles) was also used.
In vivo articles were included that gave some indication of bioavailability and iron status at the end of the study. Studies excluded were ecological, rather than human nutrition application, in vitro, or focused on special populations (genetically defined illness), or did not attempt to quantify the effects of tannins alone when consumed with other antinutritional factors.
Results
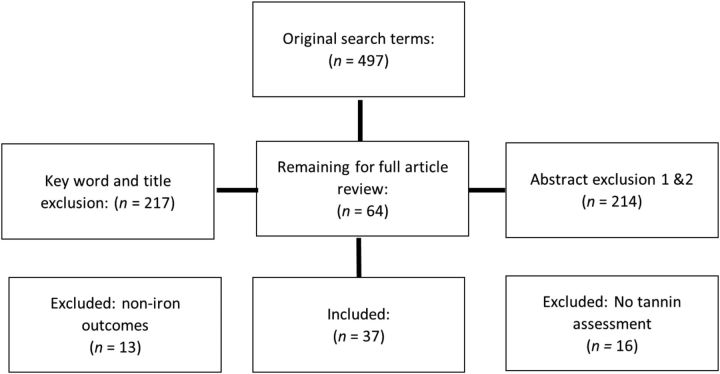
The original search terms generated 497 articles, which were narrowed first by inclusion of ≥2 search terms (n = 217). Exclusions for in vitro studies (n = 133), review articles (n = 38), and specialized populations (n = 9) were conducted during abstract reviews. A second abstract review excluded studies for manual combing of duplicates (n = 34). A full article review of the 64 remaining studies was conducted, and further exclusion for noniron outcomes (n = 13), as well as lack of direct assessment of the effects of tannins on iron status (n = 16), were applied. In total, 37 studies were reviewed (Figure 2).
Figure 2.
Inclusion and exclusion criteria for review. Articles were excluded on the basis of key terms, in vitro analysis, lack of tannin assessment in dietary analysis, and analysis of noniron outcomes.
Animal studies
Animal studies that used tea to measure the inhibition of iron status or bioavailability often exceeded or met amounts that could be expected in common consumption. For example, 1 cup of tea may contain ∼25–80 mg tannins per 150 mL (21, 22), and 3 cups of tea/d would mean consumption of anywhere from 75 to 240 mg tannins/d. Most studies exceeded this, especially when accounting for allometric dosing in animals. Many studies that have isolated the influence of tannin consumption over time without confounding antinutritional factors have been in animals. Without confounding factors, comparison of tissue-level iron depletion or repletion, and direct comparison between bioavailability and iron status with tannin consumption was possible.
Studies that support reduced iron bioavailability and/or iron status in animals consuming tannins over time
Studies that support reductions in iron bioavailability and/or iron status in animal models have typically used tannic acid or tea as study interventions (Table 1). The consumption of 100 g green tea polyphenols/L compared with water consumption in rats over 8 wk (46) resulted in a significant reduction in hepatic iron and hemoglobin (25% and 10%, respectively), although food intake was also significantly reduced. In a 28-d pig study, consumption of 125, 250, 500, or 1000 mg tannic acid/kg in feed resulted in a significant and linear depletion of serum iron concentrations, as well as hemoglobin. In this study, there was a significant decline in erythrocyte counts, hemoglobin, and hematocrit seen in the control group that was similar to the groups consuming 125, 250, and 500 mg/kg diet, and mean corpuscular volume was unaffected by tannic acid consumption (45). Interestingly, there were significant reductions in gain-to-feed ratios seen on days 0–14 that were normalized during days 15–28, suggesting adaptation to the diets over time. Serum and hepatic iron concentrations were significantly reduced in rats that consumed diets containing 5%, 10%, 15%, or 20% tannic acid/kg for 3 wk (44), but there were no significant differences in other tissue iron concentrations, body weight gain, or food intake with increasing tannin doses. Despite reductions in iron stores, rats that consumed tannic acid in weeks 2 and 3 had nonsignificant improvements in hematocrit concentrations, possibly suggesting adaptation or demand-facilitated increases in uptake to blunt iron losses. Another depletion-repletion rat study that used a bean ragout meal with green or black tea compared with water for 14 d found that iron bioavailability and change in hemoglobin decreased significantly with tea intake when iron and food intake were similar, but hepatic iron and total hemoglobin concentrations were not significantly different at study end (47). In a study that examined the effects of a habituated compared with a black tea–naïve diet on iron bioavailability in rats (n = 6), iron bioavailability, along with final body weight and food consumption, of rats consuming a powdered black tea diet was significantly reduced compared with control, although hepatic iron stores were normal (48). In the group consuming tea long term, food intake and iron bioavailability from baseline to end line significantly improved over time, suggesting adaptation.
Table 1.
Studies reporting reductions in iron bioavailability in animal models
| Reference | Subjects, n | Model | Intervention | Tannin type | Intervention length | Outcome |
|---|---|---|---|---|---|---|
| 44 | 6 | Rat | 5, 10, 15, and 20 g tannin/kg diet vs. control | Tannic acid | 3 wk | Significant linear reduction in hemoglobin (≤27%) and hepatic (≤61%) iron concentrations with tannic acid consumption. |
| 45 | 9 | Pig | 125, 250, 500, and 1000 mg tannin/kg diet vs. control | Tannic acid | 4 wk | Significant linear reduction in hemoglobin (≤21%; P = 0.028) and serum (29%, P = 0.12) iron concentrations with tannic acid consumption. |
| 46 | 7 | Rat | 100 g tea consumption vs. 100 g tea (beverage) with various concentrations of aluminum and control | Green tea | 8 wk | Significant reduction in hemoglobin (11.0 vs. 10.0 g/L; 9% reduction) and hepatic (750 vs. 250 µg/liver; 71% reduction) iron with tea consumption vs. control. |
| 47 | 8 | Rat | Green or black tea decoction with bean ragout meal vs. meal alone | Green or black tea | 14 d | Significant depletion of hemoglobin (−1.1 and −0.95 g/L with black and green tea, respectively) and iron bioavailability (19.6% and 14.9% with black and green tea, respectively) vs. control during the study. Normal hepatic iron in tea groups vs. control (65, 89.4, and 66.3 µg/g in control, black tea, and green tea groups). |
| 48 | 6 | Rat | Black tea powdered diet as 25 g/kg vs. control | Black tea | 12 d | Significant reduction (26%) in iron absorption vs. control, although there was a significant increase over time (24% vs. 42% at baseline and end line in tea consumers). No reduction in hepatic iron concentrations. |
Studies that support no differences in iron bioavailability or status with tannin consumption over time
Many animal studies have not reported differences in iron status after the consumption of tannin-rich diets by these animals (Table 2). In iron-replete and -depleted rats that consumed a diet containing 20 mg condensed tannin/kg body weight plus phytoferritin for 4 wk in a hemoglobin depletion-repletion model, a significant reduction in hemoglobin, weight gain, and serum iron in the rats that consumed condensed tannins plus phytoferritin compared with the rats that consumed phytoferritin alone was observed (50). Despite reductions in overall iron absorption, ferritin was not reduced, rats were not iron deficient, and they achieved iron repletion that was similar to control while consuming condensed tannins with adequate iron intake. Food intake was not measured in this study. It is important to note that anemic rats that consumed condensed tannins died by study end, pointing to toxicity at the daily dose of 20 mg/kg. In both 16-d and 30-d rat studies that compared 0.35%, 1.17%, or 3.50% black tea consumption or a green tea oral and powdered diet (daily dose of 20 mg/kg) with control diets, there were no differences in tissue iron concentrations or hemoglobin at study end (38, 49). In the study that used a green tea challenge, iron absorption was similarly unchanged among tea consumers compared with control without changes in body weight or feed intake (38). A study in weanling rats looked at differences in iron availability with condensed tannin in habituated compared with naïve rats. There were no significant differences between habituated and naïve rats' iron status at study end (51). A 4-wk hemoglobin depletion-repletion study in piglets that consumed meals with significantly different tannin amounts from red or white bean feed found no differences in hemoglobin, hemoglobin repletion-efficiency (hemoglobin replaced per iron intake), or weight gain at endpoint (36). Interestingly, this study did find initial downward trends in hemoglobin repletion efficiency on day 7 (49.9% compared with 55.6% in red compared with white bean consumers, respectively) that were compensated for by endpoint, potentially indicating adaption to tannin consumption over the study period. In pigs that consumed polyphenol-rich diets containing grape meal or hops compared with a control diet for 4 wk, there were no significant differences in plasma iron, total iron binding capacity, transferrin saturation, tissue iron, or fecal iron compared with control (52).
Table 2.
Studies reporting no or inconsistent reductions in iron bioavailability or iron status in animal models1
| Reference | Subjects, n | Model | Intervention | Tannin type | Intervention length | Outcome |
|---|---|---|---|---|---|---|
| 49 | 6 | Rat | Control vs. various types of tea in food | Black tea | 16 d | No significant differences in iron absorption or hepatic iron vs. control. |
| 38 | 6 | Rat | Green tea diet or gavage vs. control | Green tea | 30 d | No significant differences in iron absorption (3.7% vs. 5.6% over time and 43% vs. 63% in control vs. tea, respectively; P = 0.292) or hepatic iron (60.9 vs. 54.2 µg Fe/g liver, control vs. tea, respectively; P = 0.521) vs. control. |
| 50 | 10 | Rat | Meal with phytoferritin vs. condensed tannins (PA) and phytoferritin | Condensed tannins | 4 wk | Significant reduction in hemoglobin (11.9 vs. 10.0 g/L, respectively) and serum iron (10.33 vs. 21.43 µmol/L, respectively) for control vs. proanthocyanidins. Iron repletion and ferritin (23.4 vs. 20.98 ng/mL, respectively) not significantly different from no-proanthocyanidin group. |
| 51 | 7 | Rat | Meals consisting of casein, soy, chickpea, or red kidney bean flour | Condensed tannins | 1 wk | No significant differences in iron retained, total hemoglobin in rats consuming meals containing various amounts of polyphenols vs. control; no differences in iron retention between high- and low-tannin kidney bean meals. |
| 52 | 16 | Pig | Grape meal– and hops-based diets vs. control | Condensed tannins | 4 wk | No significant differences in iron, TIBC, transferrin, hepatic iron, and fecal iron between groups. |
| 36 | 8 | Pig | Red- vs. white-bean meals | Condensed tannins | 4 wk | No significant difference in hemoglobin/hemoglobin repletion efficiency in white and red beans (26% vs. 30%, respectively). |
PA, proanthocyanidins; TIBC, total iron binding capacity.
Human studies
Although many studies have linked tannin consumption to iron bioavailability, there is a paucity of human studies directly examining the relation between tannin intake and iron status. Studies are presented by their methodology to accurately portray the information available from the design. Single-meal studies that used tea often used a standard dose of 150–300 mL tea, whereas the majority of studies that used condensed polyphenols exceeded the 75–240 mg polyphenol/d that could be expected with tea consumption 3 times/d (21, 22).
Epidemiologic studies
The majority of epidemiologic studies that included isolated tannin-iron interactions focused on tea consumption and did not find an influence of tea consumption on iron status (Table 3). One study collected two 24-h recalls from 173 premenopausal parous Indian women, and found that in multiple regression analysis, tannin intake was not a significant regression factor correlated with anemia (53). Similarly, tea consumption in 2573 French men (n = 954) and women (n = 1639) had no influence on iron status (55). Another cross-sectional study with 157 Indian participants did not find differences in anemia prevalence between men and women who consumed diets that contained high and low tannin amounts (54). Notably, condensed tannin and polyphenol consumption varied widely in these studies (from 36 mg tannin/d to >5000 mL tea/wk); however, none of them found an impact from tannin consumption amounts on iron status.
Table 3.
Epidemiologic study outcomes related to iron status1
| Reference | Subjects, n | Intervention | Analysis | Tannin type | Conflation of phytates | Iron status affected? |
|---|---|---|---|---|---|---|
| 53 | 173 | Two 24-h recalls | Multiple regression | Dietary tannin including tea and other polyphenols | Yes | No IDA correlated with tannin intake. No significant effect in regression model. |
| 54 | 143 | 24-h diet recall | Multiple regression | Dietary tannin including tea and other polyphenols | Yes | No significant IDA correlation with tannin intake. |
| 55 | 1639 | Three 24-h diet recalls, venous blood draw | Multiple regression | Black, green, and herbal tea | Yes | IDA or marginal iron status not correlated with tannin intake (ferritin 48, 50, and 49 vs. 50, 47, and 46 µg/L in control group and regular black, green, and herbal tea drinkers, respectively; P = 0.71, 0.34, and 0.36 in premenopausal women with green, black, and herbal tea, respectively). |
IDA, iron-deficiency anemia.
Single-meal studies
Since the 1970s, researchers have found statistically significant reductions in iron absorption measurements with tannin consumption in single meal studies. The majority of these studies have been in iron-replete individuals, both male and female, who consumed a meal with tannin compared with a meal alone. Almost all single-meal bioavailability studies use radioactive iron, most use hemoglobin incorporation, and fewer use direct measurement of iron absorption through AUC serum iron concentrations to understand iron uptake. In addition, most studies have used iron absorption ratios to compare tannin-containing meals with control.
In premenopausal anemic and nonanemic Indian women (n = 10) who consumed either 200 mL black tea or warm tap water with a control meal on 2 consecutive days reduced iron absorption by 21%, although this inhibition was reduced when tea was consumed with milk (20). Since then, these findings have been supported in a variety of foods, but generally, reductions in iron bioavailability with tannin consumption are linked to consuming black tea (Table 4) (22, 56–63). These studies all reported notable iron absorption variability between participants. Furthermore, iron absorption between studies varied, from as little as 1% (64) to as much as 50% (31), pointing to the wide variability in maintenance of iron homeostasis through iron absorption.
Table 4.
Single-meal bioavailability studies showing reductions in iron bioavailability with tannin consumption1
| Reference | Subjects, n | Iron status | Population | Intervention | Tannin type | Outcome |
|---|---|---|---|---|---|---|
| 20 | 10 | Replete | Women | Control meal with water vs. meal with tea | Black tea | Significant reduction in iron bioavailability by 20%. |
| 56 | 10 | Replete and depleted | Men and women | Oregano, spinach, coffee, tea, or tannic acid vs. control | Black tea, tannic acid, polyphenols/condensed tannin | Tannic acid significantly reduced iron bioavailability; oregano, tea, and coffee percentage inhibited bioavailability by >60%, which was less than their respective equivalent tannic acid doses. Spinach reduced bioavailability by 30% despite tannic acid equivalents similar to its tannic acid, tea, coffee, and oregano counterparts. |
| 57 | 6 (C), 13 (I) | Replete | Men and women | High- vs. low-availability meal in vegetarians vs. nonvegetarians | Polyphenols/condensed tannin | Significant impairment of iron absorption from low-bioavailability meals in vegetarian and nonvegetarian consumers. Similar iron bioavailability between vegetarians (1.4% vs. 14.9% in bran vs. whole- wheat rolls, respectively) and nonvegetarians (22.3% vs. 2.2%) despite higher average phytate intake in vegetarian group. |
| 22 | 10 | Replete and depleted | Women | Meal with black tea or ascorbic acid, or control meal | Black tea | Significant reduction in iron bioavailability with tea consumption (18.2% vs. 7.1% in control vs. 150 mL tea drinkers, respectively, and 19.7% vs. 5.6% in control vs. 300 mL tea drinkers, respectively), not dependent on polyphenol burden (1 vs. 2 cups tea). |
| 58 | 8 | Replete and depleted | Men and women | 10 different beverages | Black tea, herbal tea, cocoa, or coffee | Significant reduction in iron bioavailability with tannin consumption (tea); range in reductions for tea vs. water: 3–27%, dependent on whether food consumed. |
| 59 | 13 | Replete and depleted | Men and women | Control breakfast vs. coffee or tea | Black tea, polyphenols | Significant reduction in bioavailability with tea or coffee consumption vs. control (60–90% reduction vs. control; average 10% less iron absorbed). |
| 60 | 22 | Replete | Women | Control meal vs. meal with tea | Black tea | Nonsignificant differences in iron absorption with tea consumption (reduction 1.7%), although effects of tea noted with ascorbic acid consumption (20% reduction vs. ascorbic acid alone). |
| 61 | 8 | Replete | Women | Broccoli with tannic acid (500 mg) or broccoli alone | Tannic acid | Significant reduction (10% vs. 0.3%) in iron absorption for broccoli meal vs. broccoli + tannic acid meal. |
| 31 | 14 (C), 15 (I) | Replete | Women | Control meal with 5 mg FeSO4 vs. tannic acid, phytic acid, or pectin | Tannic acid | 16–25% significant reduction in iron absorption with tannic acid vs. no tannin consumption. |
| 62 | 10 (C), 16 (I) | Replete | Women | Bread baked with tannic acid (12–884 mg) vs. control | Tannic acid | Significant reduction in iron absorption ratio and serum iron with consumption of tannic acid in bread (average reduction of iron absorbed, 3–10%). |
| 16 | 10 (C), 11 (I) | Replete | Men | Meal with varying amounts of tannin-rich condiment [yod kratin (a vegetable); 0–584 tannic acid equivalents] to control | Polyphenols/condensed tannins | 10% significant reduction in iron bioavailability with highest tannin content meal vs. control. |
C, control; I, intervention.
Contrary to the previous studies mentioned, other single-meal studies in men and women have suggested that tannin consumption alone, particularly condensed tannins, may not contribute to reductions in iron bioavailability (Table 5). One study in anemic and nonanemic Indian men found that although there was a 5.2% reduction in iron absorption after consumption of a phytate- and tannin-rich sorghum meal (20 compared with 136 mg/100 g and 160 compared with 273 mg/100 g tannin and phytates in low- and high-tannin meals, respectively), there was no significant difference in iron absorption when meals were normalized for phytic acid (n = 12; 4.0% compared with 3.1% iron absorption in low- and high-tannin meals) (35). A comparison of red and white cowpea meals resulted in no differences in iron bioavailability with a 2-fold increase in tannin amounts in premenopausal Dutch women (66). Another study found that, despite similar tannic acid equivalents, spinach consumption resulted in a 2-fold increase in iron bioavailability compared with the consumption of black tea (n = 9 and 5, respectively) in 19- to 51-y-old Swedish men and women (56). Other studies have reported minimal (16, 60, 64) or no impact on iron bioavailability (65), or enhanced uptake of iron absorption (67) with tannin consumption.
Table 5.
Single-meal bioavailability studies showing no or minimal reductions in iron bioavailability with tannin consumption1
| Reference | Subjects, n | Iron status | Population | Intervention | Tannin type | Outcome |
|---|---|---|---|---|---|---|
| 64 | 14 | Replete | Women | Meal with green tea, meal with rosemary extract, and control meal | Green tea or polyphenols | No significant differences in iron absorption: 12.1% vs. 8.9% (control vs. green tea, respectively) and 7.5% vs. 6.4% (control vs. rosemary oil, respectively). |
| 65 | 8 | Replete and depleted | Men and women | Meal with orange juice or tea vs. control (water) | Black tea | No significant differences in iron bioavailability between tea and control or ascorbic acid and control. |
| 35 | 7 (D), 12 (R) | Replete and depleted | Men | Vegetable and low- or high-tannin sorghum roti | Polyphenols/condensed tannins | Significant 5.2% reduction in absorption of iron in anemic men, normalized and nonsignificant when adjusted for phytates (0.83%). No significant difference in iron-replete men (5.05% vs. 3.81% in low- and high-tannin sorghum, respectively). |
| 63 | 16 (C), 18 (I) | Replete | Women | Meal with white vs. polyphenol-rich sorghum | Condensed tannins | 5.2% and 5.8% significant reduction (P < 0.001) in iron bioavailability from high- or medium-tannin sorghum vs. low-tannin sorghum. No differences noted with polyphenol oxidase addition to meal. |
| 66 | 16 | Replete | Women | Meal containing red or white cowpea | Condensed tannins | No significant differences in iron bioavailability between red and white cowpea (both groups 11%, P = 0.69). |
| 67 | 16 | Replete | Men and women | Maize meal vs. algae-containing maize meal | Polyphenols/condensed tannins | Dose-dependent significant enhancement of iron bioavailability with algae polyphenols (6.8–17.8% more iron absorbed with algae vs. maize meal). |
| 68 | 20 | Replete and depleted | Women | 2 × 2 factorial structure with low-phytate, low-polyphenol, high-phytate, or high-polyphenol bean meals | Polyphenols/condensed tannins | Polyphenol-rich, low-phytate beans nonsignificantly increased iron bioavailability (6.14%; 95% CI: 2.57%, 14.65% vs. 3.99%; 95% CI: 1.83%, 8.71%) vs. low-polyphenol, low-phytate beans, respectively. High-phytate beans significantly reduced iron bioavailability (6.14%; 95% CI: 2.57%, 14.65% vs. 3.84%, 1.76%, 8.38%). |
C, control; D, depleted; I, intervention; R, replete.
Phytate content may conflate findings in these studies. Women who consumed low-polyphenol beans (29 compared with 180 mg/100 g in low- compared with high-polyphenol beans) did not significantly increase their iron absorption, whereas high-phytate beans significantly reduced iron bioavailability (n = 20) (68). Similarly, adding polyphenol oxidase to high tannin sorghum meals did not improve iron bioavailability in iron-replete women who consumed them, suggesting that tannins were not the cause of reduced iron bioavailability (n = 16 and 18, respectively) (63).
Long-term and multimeal clinical studies
To our knowledge, few multimeal antinutritional-factor iron bioavailability studies have been conducted (Table 6). In 19- to 32-y-old German vegetarian (n = 6 and 8 in black and green tea groups, respectively) and omnivorous (n = 10, both groups) men and women who consumed green or black tea for 4 wk, ferritin was significantly decreased in omnivorous women who consumed black tea, as well as in a subgroup analysis of anemic women who consumed black or green tea. There were no changes in ferritin in men and nonanemic vegetarian women who consumed tea, nor did total iron binding capacity, hemoglobin, serum iron, or hematocrit change in groups from baseline to end line (69). In a study that compared the iron status of Indian men and women aged 20–25 y (n = 46), polyphenol-rich diets that contained leafy green vegetable supplements did not significantly affect individuals' hemoglobin concentrations after 3 wk of supplementation compared with those consuming a control meal with no leafy green vegetables (70).
Table 6.
Long-term studies investigating the impact of tannin consumption on iron bioavailability1
| Reference | Subjects, n | Iron status | Intervention | Tannin type | Intervention length | Outcome |
|---|---|---|---|---|---|---|
| 69 | 9 (D), 25 (R) | Depleted and replete | Green or black tea supplementation in either vegetarian or omnivorous participants | Green or black tea | 4 wk | Significant decrease in ferritin in anemic and omnivorous women consuming black tea without change in TIBC, Hb, or serum iron. |
| 70 | 11 (C), 12 (I) | Replete | Leafy green vegetable supplementation vs. standard meal | Polyphenols/condensed tannins | 3 wk | No significant inhibition of iron bioavailability after supplementation of meal with tannin vs. control; significant increase in Hb by 11% vs. baseline with leafy vegetable intake. |
| 40 | 14 (C), 17 (I) | Replete | High- or low-bioavailability diet | Polyphenols/condensed tannins | 12 wk | Trends toward increases in bioavailability of low-bioavailability diet over time with reduction in bioavailability of high-bioavailability diet. No significant differences in nonheme iron absorption between groups at study end. |
| 71 | 9 | Replete | High- or low-bioavailability diet | Polyphenols/condensed tannins | 12 wk | Significant 8.8% greater iron absorption efficiency in high-bioavailability group (P < 0.0001) vs. low-bioavailability group. |
| 41 | 16 | Depleted | High- or low-phytate diet with high-phytate diet challenge at baseline and endpoint | Polyphenols/condensed tannins | 8 wk | Significantly increased uptake of iron in high-phytate diet (29.3% increase) despite no changes in iron markers (ferritin, TIBC, and hepcidin) vs. low-phytate group. Decrease in absorption of iron to high phytate meal with low phytate–consuming group. |
C, control; D, depleted; Hb, hemoglobin; I, intervention; R, replete; TIBC, total iron binding capacity.
Confounding antinutritional diet factors make intervention increases in tannin consumption alone difficult. Two studies followed 31 healthy men aged 32–56 y (40) and, later, 36 premenopausal women (71) consuming a high- or low-bioavailability diet for >12 wk. High-bioavailability diets consisted of meat and poultry, refined cereal and grain products, no coffee or tea, and foods with >75 mg ascorbic acid in each meal, whereas low-bioavailability diets consisted of whole grains, with limited meat, tea, and 60 mg ascorbic acid equivalent/d. The iron and calcium content of the diets were similar, and researchers conducted two 24-h iron absorption studies that compared iron bioavailability between the 2 groups before and after the study period was complete. Although the tannin burden of the diets was not measured, it was noted that the absorption efficiency of the low-bioavailability diets significantly increased over time, whereas the absorption efficiency of the high-bioavailability diets decreased (40, 71). This was supported by a 2015 study, where marginally iron-deficient women who consumed a high-phytate (and -tannin, although this was not measured) diet for 8 wk (n = 16) significantly increased iron absorption from a high-phytate test meal, whereas iron absorption in consumers of a low-phytate diet was nonsignificantly decreased. In this study, the iron status of consumers was not changed by high- or low-phytate diets (41).
Discussion
The effect of tannins on iron bioavailability
The studies reviewed that noted reductions in iron bioavailability with tannin consumption commonly used hydrolyzable tannins (tannic acid) or epicatechin and catechin monomers, dimers, and oligomers found in tea. Animal or single-meal studies that resulted in significant reductions in iron bioavailability or iron status almost exclusively used tannic acid (31, 45, 44, 61, 62), a mixture of hydrolyzable gallo-tannins that are virtually absent from our diet (23), or tea (20, 22, 46, 47, 49, 56, 58, 60), which contains thearubigins and a low density of condensed tannins found in most foods (72). Of the studies reviewed, only 4 that used condensed tannins supported reductions in iron bioavailability during single meals (of 16 total). No studies reviewed that used multimeal animal or clinical models or epidemiologic analysis found reductions in iron status or bioavailability with condensed tannin consumption, which may point to a limitation of single-meal studies in assessing iron bioavailability and status for long-term outcomes. This could highlight the importance of using condensed rather than hydrolyzable tannins or tea to assess the bioavailability of iron in tannin-containing meals. It is also interesting to note that some significant findings have used polyphenol beverages, rather than food, which may increase the tannin-iron interaction in the food matrix (24). Plants such as sorghum contain proteins rich in proline, similar to salivary proteins that may protect consumers from the antinutritional properties of tannins (24). It may be that the consumption of such plants may result in an inherent mediation of tannin-nutrient binding with whole plant or food consumption, not found with tannin extracts or beverages. Phytic acid may be another factor that affects outcomes describing tannin-induced reductions in iron availability, considering the negation of antinutritional effects in the setting of normalized phytate concentrations found in 2 studies reviewed (35, 68).
It may be important to also consider outcome measures from single-meal studies. Although several single-meal studies reviewed noted reductions in iron bioavailability, these findings were often nonsignificant until data were adjusted into iron absorption ratios. Often, total iron absorption differences were <10% between tannin consumers and nonconsumers, which may not affect iron status in the long term. This is an important normalizing factor, given the wide variability of iron absorption, but it may point to significant outcomes that make little meaningful impact on iron status when tannin-rich diets are consumed over time. It is similarly important to take into consideration population, iron status, and study design when determining whether this research will result in meaningful outcomes in the context of global nutrition. Studies that consider iron bioavailability from iron-replete and -depleted populations, in which intervention order is fully randomized, may carry more significant weight in this context.
Effect of tannins on iron status
Although tannin consumption impairs iron bioavailability, the majority of epidemiologic and long-term human studies reviewed did not support reductions in iron status with the consumption of tannin-rich diets over time. In epidemiologic studies, tannin compounds that inhibit iron bioavailability in single-meal studies were not correlated with iron status changes (55) or iron deficiency (53, 54). Furthermore, studies lasting ≥4 wk that focused on tannin-rich foods did not find alterations in iron status (69, 70). Some animal (38, 48) and human (40, 41) studies that were reviewed point instead to the idea of long-term adaptation to antinutritional factors, including tannins. It is important to note that these findings are obscured by several factors, including lack of control of concurrent antinutritional or iron-enhancing factors in diets, as well as assessment of iron deficiency, rather than adequate iron stores. More importantly, to the best of our knowledge, there are few studies that have looked into long-term antinutritional effects on either iron bioavailability or iron status, and no studies that we found isolated the effects of condensed tannin consumption over time.
Conclusions
The focus of this review was to determine the effects of tannins on iron bioavailability and status, and investigate whether possible adaptation to tannins could reduce the antinutritional effects of tannin consumption over time. Evidence from animal and single-meal studies suggests that tannic acid and tea consumption more consistently impair iron bioavailability than does the consumption of condensed tannins, although the connection between these studies' findings and individual iron status is not established. Certain tannins may inhibit iron bioavailability, but a lack of long-term studies and confounding factors in most studies reviewed that assess the effects of tannins limit generalizability. In addition, epidemiologic studies and long-term trials reviewed suggest that the iron status of individuals is often not affected by tannin consumption, although there is a dearth of this type of research conducted compared with single-meal studies. Future studies focusing on the long-term effects of condensed tannins (proanthocyanidins) on iron status are needed to determine their impact on iron bioavailability and status, and whether adaptation is the missing piece to explain the inconsistency between single-meal and longer-term studies. In addition, further studies are needed to characterize the adaptation mechanism.
Acknowledgments
The authors' responsibilities were as follows—NMD and MDH: conceived of the research; NMD: conducted research for and wrote the manuscript; BLL: edited the manuscript; and all authors: reviewed, read, and approved the final manuscript.
References
- 1. Camaschella C.. Iron-deficiency anemia. N Engl J Med 2015;372:1832–43. [DOI] [PubMed] [Google Scholar]
- 2. Murray-Kolb LE.. Iron and brain functions. Curr Opin Clin Nutr Metab Care 2013;16:703–7. [DOI] [PubMed] [Google Scholar]
- 3. Di Renzo GC, Spano F, Giardina I, Brillo E, Clerici G, Roura LC.. Iron deficiency anemia in pregnancy. Womens Health (Lond) 2015;11:891–900. [DOI] [PubMed] [Google Scholar]
- 4. Plessow R, Arora NK, Brunner B, Tzogiou C, Eichler K, Brügger U, Wieser S.. Social costs of iron deficiency anemia in 6–59-month-old children in India. PLoS One 2015;10:e0136581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stevens GA, Finucane MM, De-Regil L, Paciorek CJ, Flaxman SR, Branca F, Peña-Rosas JP, Bhutta ZA, Ezzati M, Nutrition Impact Model Study Group (Anaemia) . Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non- pregnant women for 1995– 2011: a systematic analysis of population- representative data. Lancet Glob Health 2013;1:e16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gozzelino R, Arosio P.. Iron homeostasis in health and disease. Int J Mol Sci 2016;17:2–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang DL, Ghosh MC, Rouault TA.. The physiological functions of iron regulatory proteins in iron homeostasis - an update. Front Pharmacol 2014;5:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Petry N, Egli I, Zeder C, Walczyk T, Hurrell R.. Polyphenols and phytic acid contribute to the low iron bioavailability from common beans in young women. J Nutr 2010;140:1977–82. [DOI] [PubMed] [Google Scholar]
- 9. Tatala S, Svanberg U, Mduma B.. Low dietary iron availability is a major cause of anemia: a nutrition survey in the Lindi District of Tanzania. Am J Clin Nutr 1998;68:171–8. [DOI] [PubMed] [Google Scholar]
- 10. De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P.. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA 2010;107:14691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kastorini CM, Milionis HJ, Esposito K, Giugliano D, Goudevenos JA, Panagiotakos DB.. The effect of Mediterranean diet on metabolic syndrome and its components: a meta-analysis of 50 studies and 534,906 individuals. J Am Coll Cardiol 2011;57:1299–313. [DOI] [PubMed] [Google Scholar]
- 12. Hurrell RF, Juillerat MA, Reddy MB, Lynch SR, Dassenko SA, Cook JD.. Soy protein, phytate, and iron absorption in humans. Am J Clin Nutr 1992;56:573–8. [DOI] [PubMed] [Google Scholar]
- 13. Clemens S.. Zn and Fe biofortification: the right chemical environment for human bioavailability. Plant Sci 2014;225:52–7. [DOI] [PubMed] [Google Scholar]
- 14. Bohn L, Meyer A, Rasmussen S.. Phytate: impact on environment and human nutrition. A challenge for molecular breeding. J Zhejiang Univ Sci B 2008;9:165–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gillooly M, Bothwell TH, Torrance JD, MacPhail AP, Derman DP, Bezwoda WR, Mills W, Charlton RW, Mayet F.. The effects of organic acids, phytates and polyphenols on the absorption of iron from vegetables. Br J Nutr 1983;49:331–42. [DOI] [PubMed] [Google Scholar]
- 16. Tuntawiroon M, Sritongkul N, Brune M, Rossander-Hulten L, Pleehachinda R, Suwanik R, Hallberg L.. Dose- dependent inhibitory effect of phenolic compounds in foods on nonheme-iron absorption in men. Am J Clin Nutr 1991;53:554–7. [DOI] [PubMed] [Google Scholar]
- 17. Gorczyca D, Prescha A, Szeremeta K, Jankowski A.. Iron status and dietary iron intake of vegetarian children from Poland. Ann Nutr Metab 2013;62:291–7. [DOI] [PubMed] [Google Scholar]
- 18. Davidsson L, Galan P, Kastenmayer P, Cherouvrier F, Juillerat MA, Hercberg S, Hurrell RF.. Iron bioavailability studied in infants: the influence of phytic acid and ascorbic acid in infant formulas based on soy isolate. Pediatr Res 1994;36:816–22. [DOI] [PubMed] [Google Scholar]
- 19. Cook JD, Dassenko SA, Lynch SR.. Assessment of the role of nonheme-iron availability in iron balance. Am J Clin Nutr 1991;54:717–22. [DOI] [PubMed] [Google Scholar]
- 20. Disler PB, Lynch SR, Charlton RW, Torrance JD, Bothwell TH, Walker RB, Mayet F.. The effect of tea on iron absorption. Gut 1975;16:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zijp IM, Korver O, Tijburg LBM.. Effect of tea and other dietary factors on iron absorption. Crit Rev Food Sci Nutr 2000;40:371–98. [DOI] [PubMed] [Google Scholar]
- 22. Thankachan P, Walczyk T, Muthayya S, Kurpad AV, Hurrell RF.. Iron absorption in young Indian women: the interaction of iron status with the influence of tea and ascorbic acid. Am J Clin Nutr 2008;87:881–6. [DOI] [PubMed] [Google Scholar]
- 23. Santos‐buelga C, Scalbert A.. Proanthocyanidins and tannin‐like compounds—nature, occurrence, dietary intake and effects on nutrition and health. J Sci Food Agric 2000;80:1094–117. [Google Scholar]
- 24. Bennick A.. Interaction of plant polyphenols with salivary proteins. Crit Rev Oral Biol Med 2002;13:184–96. [DOI] [PubMed] [Google Scholar]
- 25. Esele J.. The genetics of grain mold resistance in sorghum (Sorghum bicolor (L.) Moench). Texas A & M University; 1991. p. 152. [Google Scholar]
- 26. Teissedre PL, Frankel EN, Waterhouse AL, Peleg H, German JB.. Inhibition of in vitro human LDL oxidation by phenolic antioxidants from grapes and wines. J Sci Food Agric 1996;70:55–61. [Google Scholar]
- 27. Katiyar SK, Mukhtar H.. Tea antioxidants in cancer chemoprevention. J Cell Biochem Suppl 1997;27:59–67. [PubMed] [Google Scholar]
- 28. Tijburg LB, Mattern T, Folts JD, Weisgerber UM, Katan MB.. Tea flavonoids and cardiovascular diseases: a review. Crit Rev Food Sci Nutr 1997;37:771–85. [DOI] [PubMed] [Google Scholar]
- 29. Webb P, Rogers BL, Rosenberg I, Schlossman N, Wanke C, Bagriansky J, Sadler K, Johnson Q, Tilahun J, Reese Masterson A.. Improving the nutritional quality of US food aid: recommendations for changes to products and programs. Boston: Tufts University; 2011. [Google Scholar]
- 30. Hunt JR, Roughead ZK.. Nonheme-iron absorption, fecal ferritin excretion, and blood indexes of iron status in women consuming controlled lactoovovegetarian diets for 8 wk. Am J Clin Nutr 1999;69:944–52. [DOI] [PubMed] [Google Scholar]
- 31. Jaramillo Á, Briones L, Andrews M, Arredondo M, Olivares M, Brito A, Pizarro F.. Effect of phytic acid, tannic acid and pectin on fasting iron bioavailability both in the presence and absence of calcium. J Trace Elem Med Biol 2015;30:112–7. [DOI] [PubMed] [Google Scholar]
- 32. Gibson RS, Heath AL, Szymlek-Gay EA.. Is iron and zinc nutrition a concern for vegetarian infants and young children in industrialized countries? Am J Clin Nutr 2014;100:459S–68S. [DOI] [PubMed] [Google Scholar]
- 33. Savva SC, Kafatos A.. Is red meat required for the prevention of iron deficiency among children and adolescents? Curr Pediatr Rev 2014;10:177–83. [PubMed] [Google Scholar]
- 34. Davidsson L, Galan P, Cherouvrier F, Kastenmayer P, Juillerat MA, Hercberg S, Hurrell RF.. Bioavailability in infants of iron from infant cereals: effect of dephytinization. Am J Clin Nutr 1997;65:916–20. [DOI] [PubMed] [Google Scholar]
- 35. Radhakrishnan MR, Sivaprasad J.. Tannin content of sorghum varieties and their role in iron bioavailability. J Agric Food Chem 1980;28:55–7. [DOI] [PubMed] [Google Scholar]
- 36. Tan SY, Yeung CK, Tako E, Glahn RP, Welch RM, Lei X, Miller DD.. Iron bioavailability to piglets from red and white common beans (Phaseolus vulgaris). J Agric Food Chem 2008;56:5008–14. [DOI] [PubMed] [Google Scholar]
- 37. Wauben IP, Atkinson SA.. Calcium does not inhibit iron absorption or alter iron status in infant piglets adapted to a high calcium diet. J Nutr 1999;129:707–11. [DOI] [PubMed] [Google Scholar]
- 38. Beverly AB, Zhu L, Fish TL, Thannhauser T, Rutzke MA, Miller DD.. Green tea ingestion by rats does not affect iron absorption but does alter the composition of the saliva proteome. J Food Sci 2012;77:H96–104. [DOI] [PubMed] [Google Scholar]
- 39. Lopez HW, Coudray C, Bellanger J, Younes H, Demigné C, Rémésy C.. Intestinal fermentation lessens the inhibitory effects of phytic acid on mineral utilization in rats. J Nutr 1998;128:1192–8. [DOI] [PubMed] [Google Scholar]
- 40. Hunt JR, Roughead ZK.. Adaptation of iron absorption in men consuming diets with high or low iron bioavailability. Am J Clin Nutr 2000;71:94–102. [DOI] [PubMed] [Google Scholar]
- 41. Armah SM, Boy E, Chen D, Candal P, Reddy MB.. Regular consumption of a high-phytate diet reduces the inhibitory effect of phytate on nonheme-iron absorption in women with suboptimal iron stores. J Nutr 2015;145:1735–9. [DOI] [PubMed] [Google Scholar]
- 42. Lucke HH, Hodge KE, Patt NL.. Fatal liver damage after barium enemas containing tannic acid. Can Med Assoc J 1963;89:1111–4. [PMC free article] [PubMed] [Google Scholar]
- 43. Erb IH, Morgan EM, Farmer AW.. The pathology of burns: the pathologic picture as revealed at autopsy in a series of 61 fatal cases treated at the hospital for sick children, Toronto, Canada. Ann Surg 1943;117:234–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Afsana K, Shiga K, Ishizuka S, Hara H.. Reducing effect of ingesting tannic acid on the absorption of iron, but not of zinc, copper and manganese by rats. Biosci Biotechnol Biochem 2004;68:584–92. [DOI] [PubMed] [Google Scholar]
- 45. Lee SH, Shinde PL, Choi JY, Kwon IK, Lee JK, Pak SI, Cho WT, Chae BJ.. Effects of tannic acid supplementation on growth performance, blood hematology, iron status and faecal microflora in weanling pigs. Livest Sci 2010;131:281–6. [Google Scholar]
- 46. Marouani N, Chahed A, Hédhili A, Hamdaoui MH.. Both aluminum and polyphenols in green tea decoction (Camellia sinensis) affect iron status and hematological parameters in rats. Eur J Nutr 2007;46:453–9. [DOI] [PubMed] [Google Scholar]
- 47. Hamdaoui MH, Chabchoub S, Hédhili A.. Iron bioavailability and weight gains to iron-deficient rats fed a commonly consumed Tunisian meal ‘bean seeds ragout’ with or without beef and with green or black tea decoction. J Trace Elem Med Biol 2003;17:159–64. [DOI] [PubMed] [Google Scholar]
- 48. Kim HS, Miller DD.. Proline- rich proteins moderate the inhibitory effect of tea on iron absorption in rats. J Nutr 2005;135:532–7. [DOI] [PubMed] [Google Scholar]
- 49. Greger JL, Lyle BJ.. Iron, copper and zinc metabolism of rats fed various levels and types of tea. J Nutr 1988;118:52–60. [DOI] [PubMed] [Google Scholar]
- 50. Yun S, Zhang T, Li M, Chen B, Zhao G.. Proanthocyanidins inhibit iron absorption from soybean (Glycine max) seed ferritin in rats with iron deficiency anemia. Plant Foods Hum Nutr 2011;66:212–7. [DOI] [PubMed] [Google Scholar]
- 51. Garcia-Lopez JS, Erdman JW Jr, Sherman AR.. Iron retention by rats from casein- legume test meals: effect of tannin level and previous diet. J Nutr 1990;120:760–6. [DOI] [PubMed] [Google Scholar]
- 52. Fiesel A, Ehrmann M, Geßner DK, Most E, Eder K.. Effects of polyphenol- rich plant products from grape or hop as feed supplements on iron, zinc and copper status in piglets. Arch Anim Nutr 2015;69:276–84. [DOI] [PubMed] [Google Scholar]
- 53. Tupe R, Chiplonkar SA, Kapadia-Kundu N.. Influence of dietary and socio-demographic factors on the iron status of married adolescent girls from Indian urban slums. Int J Food Sci Nutr 2009;60:51–9. [DOI] [PubMed] [Google Scholar]
- 54. Mennen L, Hirvonen T, Arnault N, Bertrais S, Galan P, Hercberg S.. Consumption of black, green and herbal tea and iron status in French adults. Eur J Clin Nutr 2007;61:1174–9. [DOI] [PubMed] [Google Scholar]
- 55. Chiplonkar SA, Agte VV.. Statistical model for predicting non-heme iron bioavailability from vegetarian meals. Int J Food Sci Nutr 2006;57:434–50. [DOI] [PubMed] [Google Scholar]
- 56. Brune M, Rossander L, Hallberg L.. Iron absorption and phenolic compounds: importance of different phenolic structures. Eur J Clin Nutr 1989;43:547–57. [PubMed] [Google Scholar]
- 57. Brune M, Rossander L, Hallberg L.. Iron absorption: no intestinal adaptation to a high-phytate diet. Am J Clin Nutr 1989;49:542–5. [DOI] [PubMed] [Google Scholar]
- 58. Hurrell RF, Reddy M, Cook JD.. Inhibition of non-haem iron absorption in man by polyphenolic- containing beverages. Br J Nutr 1999;81:289–95. [PubMed] [Google Scholar]
- 59. Layrisse M, Garcia-Casal M, Solano L, Barón MA, Arguello F, Llovera D, Ramirez J, Leets I, Tropper E.. Iron bioavailability in humans from breakfasts enriched with iron bis-glycine chelate, phytates and polyphenols. J Nutr 2000;130:2195–9. [DOI] [PubMed] [Google Scholar]
- 60. Derman D, Sayers M, Lynch SR, Charlton RW, Bothwell TH, Mayet F.. Iron absorption from a cereal-based meal containing cane sugar fortified with ascorbic acid. Br J Nutr 1977;38:261–9. [DOI] [PubMed] [Google Scholar]
- 61. Gillooly M, Bothwell TH, Charlton RW, Torrance JD, Bezwoda WR, MacPhail AP, Derman DP, Novelli L, Morrall P, Mayet F.. Factors affecting the absorption of iron from cereals. Br J Nutr 1984;51:37–46. [DOI] [PubMed] [Google Scholar]
- 62. Siegenberg D, Baynes RD, Bothwell TH, Macfarlane BJ, Lamparelli RD, Car NG, MacPhail P, Schmidt U, Tal A, Mayet F.. Ascorbic acid prevents the dose- dependent inhibitory effects of polyphenols and phytates on nonheme-iron absorption. Am J Clin Nutr 1991;53:537–41. [DOI] [PubMed] [Google Scholar]
- 63. Cercamondi CI, Egli IM, Zeder C, Hurrell RF.. Sodium iron EDTA and ascorbic acid, but not polyphenol oxidase treatment, counteract the strong inhibitory effect of polyphenols from brown sorghum on the absorption of fortification iron in young women. Br J Nutr 2014;111:481–9. [DOI] [PubMed] [Google Scholar]
- 64. Samman S, Sandström B, Toft MB, Bukhave K, Jensen M, Sørensen SS, Hansen M.. Green tea or rosemary extract added to foods reduces nonheme-iron absorption. Am J Clin Nutr 2001;73:607–12. [DOI] [PubMed] [Google Scholar]
- 65. Lundqvist H, Sjöberg F.. Food interaction of oral uptake of iron / a clinical trial using 59Fe. Arzneimittelforschung 2007;57:401–16. [DOI] [PubMed] [Google Scholar]
- 66. Abizari AR, Moretti D, Schuth S, Zimmermann MB, Armar-Klemesu M, Brouwer ID.. Phytic acid-to-iron molar ratio rather than polyphenol concentration determines iron bioavailability in whole- cowpea meal among young women. J Nutr 2012;142:1950–5. [DOI] [PubMed] [Google Scholar]
- 67. García-Casal MN, Ramírez J, Leets I, Pereira AC, Quiroga MF.. Antioxidant capacity, polyphenol content and iron bioavailability from algae (Ulva sp., Sargassum sp. and Porphyra sp.) in human subjects. Br J Nutr 2009;101:79–85. [DOI] [PubMed] [Google Scholar]
- 68. Petry N, Egli I, Campion B, Nielsen E, Hurrell R.. Genetic reduction of phytate in common bean (Phaseolus vulgaris L.) seeds increases iron absorption in young women. J Nutr 2013;143:1219–24. [DOI] [PubMed] [Google Scholar]
- 69. Schlesier K, Kühn B, Kiehntopf M, Winnefeld K, Roskos M, Bitsch R, Böhm V.. Comparative evaluation of green and black tea consumption on the iron status of omnivorous and vegetarian people. Food Res Int 2012;46:522–7. [Google Scholar]
- 70. Agte V, Jahagirdar M, Chiplonkar S.. GLV supplements increased plasma β-carotene, vitamin C, zinc and hemoglobin in young healthy adults. Eur J Nutr 2006;45:29–36. [DOI] [PubMed] [Google Scholar]
- 71. Hunt JR.. High-, but not low- bioavailability diets enable substantial control of women's iron absorption in relation to body iron stores, with minimal adaptation within several weeks. Am J Clin Nutr 2003;78:1168–77. [DOI] [PubMed] [Google Scholar]
- 72. Atoui A, Mansouri A, Boskou G, Kefalas P.. Tea and herbal infusions: their antioxidant activity and phenolic profile. Food Chem 2005;89:27–36. [Google Scholar]