Abstract
Background: Ketones are the brain's main alternative fuel to glucose. Dietary medium-chain triglyceride (MCT) supplements increase plasma ketones, but their ketogenic efficacy relative to coconut oil (CO) is not clear.
Objective: The aim was to compare the acute ketogenic effects of the following test oils in healthy adults: coconut oil [CO; 3% tricaprylin (C8), 5% tricaprin (C10)], classical MCT oil (C8-C10; 55% C8, 35% C10), C8 (>95% C8), C10 (>95% C10), or CO mixed 50:50 with C8-C10 or C8.
Methods: In a crossover design, 9 participants with mean ± SD ages 34 ± 12 y received two 20-mL doses of the test oils prepared as an emulsion in 250 mL lactose-free skim milk. During the control (CTL) test, participants received only the milk vehicle. The first test dose was taken with breakfast and the second was taken at noon but without lunch. Blood was sampled every 30 min over 8 h for plasma acetoacetate and β-hydroxybutyrate (β-HB) analysis.
Results: C8 was the most ketogenic test oil with a day-long mean ± SEM of +295 ± 155 µmol/L above the CTL. C8 alone induced the highest plasma ketones expressed as the areas under the curve (AUCs) for 0–4 and 4–8 h (780 ± 426 µmol ⋅ h/L and 1876 ± 772 µmol ⋅ h/L, respectively); these values were 813% and 870% higher than CTL values (P < 0.01). CO plasma ketones peaked at +200 µmol/L, or 25% of the C8 ketone peak. The acetoacetate-to-β-HB ratio increased 56% more after CO than after C8 after both doses.
Conclusions: In healthy adults, C8 alone had the highest net ketogenic effect over 8 h, but induced only half the increase in the acetoacetate-to-β-HB ratio compared with CO. Optimizing the type of MCT may help in developing ketogenic supplements designed to counteract deteriorating brain glucose uptake associated with aging. This trial was registered at clinicaltrials.gov as NCT 02679222.
Keywords: coconut oil, medium-chain triglycerides, ketones, tricaprylin, tricaprin, beta-hydroxybutyrate, acetoacetate
Introduction
The ketones acetoacetate and β-hydroxybutyrate (β-HB) are the brain's principal alternative fuel to glucose under conditions when carbohydrate intake is significantly reduced, or during fasting or strenuous aerobic exercise (1, 2). Ketones replace glucose and supply ≤80% of the brain's energy requirements during medically supervised starvation of 40–60 d (3, 4). The very-high-fat ketogenic diet has been used for many years to treat refractory childhood epilepsy and increases plasma ketones by virtue of extreme carbohydrate restriction and hypoinsulinemia (5).
An alternative means of moderately increasing ketones without radically altering food intake or restricting carbohydrate is by consuming medium-chain TGs (MCTs; FAs of 8–12 carbons atoms) (2, 6–9). MCTs induce mild to moderate ketonemia when added to a regular meal because they are quickly absorbed via the portal vein and rapidly β-oxidized to acetyl-CoA in the liver (2, 10). In contrast, common long-chain dietary FAs are absorbed as chylomicrons via the lymphatic system and distributed throughout the circulation before being metabolized. Unlike long-chain FAs, medium-chain FAs do not need to be activated by carnitine in order to access the inner mitochondrial membrane for β-oxidation (7).
MCTs are typically purified from coconut oil (CO). To our knowledge, the ketogenic effect of different MCTs in humans has not been directly compared among themselves or with CO. Therefore, we aimed to compare the acute ketogenic effect of CO with that of tricaprylin alone (C8), tricaprin alone (C10), a typical MCT mixture (C8-C10), or CO mixed 50:50 with C8-C10 or C8 alone. The 8-h metabolic study day protocol was designed to compare the ketogenic effect of the dose of test oil taken with a meal (breakfast) with the same dose taken at midday but without an accompanying meal.
Methods
Participants
Ethical approval for this study was obtained from the Research Ethics Committee of the Integrated University Health and Social Services of Eastern Townships–Sherbrooke University Hospital Center, which oversees all human research conducted at the Research Center on Aging (Sherbrooke, Quebec, Canada). All of the participants provided written informed consent before beginning the study and were recruited from August to December 2015. They underwent a screening visit, including an analysis of a blood sample collected after a 12-h overnight fast. Exclusion criteria included the following: smoking, diabetes or glucose intolerance (fasting glucose >6.1 mmol/L and glycosylated hemoglobin >6.0%), strenuous aerobic exercise >3 times/wk, CO allergy, untreated hypertension, dyslipidemia, and abnormal renal, liver, heart, or thyroid function. This project is registered at clinicaltrials.gov (NCT02679222).
Test oils
The composition of the CO (President's Choice, Loblaws) is shown in Table 1. The MCT oil was 55% C8, 35% C10 (Captex 355; Abitec). The C8 oil was 95% pure tricaprylin (Captex 8000; Abitec). The C10 oil was 95% pure tricaprin (Captex 1000; Abitec). A 20-mL dose of each test oil was mixed with 250 mL lactose-free skim milk (Natrel, Agropur) by using a blender (Magic Bullet, Homeland Houseware, LLC). CO and C10 are solid at room temperature, so they were melted in a water bath at 60°C before blending into the milk base.
TABLE 1.
Test oils given on the metabolic study days1
| Composition, % | Quantity/dose, mL | ||||
|---|---|---|---|---|---|
| CO | C8 | C10 | C8 | C10 | |
| CTL | 0 | 0 | 0 | 0 | 0 |
| CO | 100 | 0 | 0 | 0.6 | 1 |
| C8-C10 | 0 | 60 | 40 | 12 | 8 |
| CO + C8-C10 | 50 | 30 | 20 | 6.3 | 4.5 |
| CO + C8 | 50 | 50 | 0 | 10.3 | 0.5 |
| C8 | 0 | 100 | 0 | 20 | 0 |
| C10 | 0 | 0 | 100 | 0 | 20 |
CO FA composition (%): C8:0 (3); C10:0 (5); C12:0 (45); C14:0 (18); C16:0 (15); C18:0 (7); C18:1 (7). CO, coconut oil; CTL, control; C8, tricaprylin; C10, tricaprin.
Experimental design
The protocol involved 7 separate but identical metabolic study days for each participant, hence a repeated or longitudinal measurements design during which the test substances were evaluated in random order: vehicle [control (CTL); 250 mL lactose-free skim milk] or 20 mL of the test oils mixed with 250 mL lactose-free skim milk [CO, C10, C8, C8-C10, CO+C8-C10 (50:50), CO+C8 (50:50)] taken twice, once at breakfast and once at midday. Participants were single-blinded and crossed over from one treatment to the next during the trial course. The test sequence was determined a priori by the investigator, and the participants were randomly assigned to sequences. On each metabolic study day, the participants arrived at 0730 after a 12-h overnight fast and a minimum of 24 h without alcohol intake. A forearm venous catheter was installed, and a baseline blood sample (time 0) collected. Participants then received a standard breakfast during which they consumed the test beverage. The breakfast consisted of 2 pieces of toast with raspberry jam, a piece of cheese, and 2 scrambled eggs. A second dose of the test beverage was given alone for lunch (i.e., with no other food) (Table 1). Water was available ad libitum throughout the study day. Blood samples were taken at baseline (predose) and every 30 min during 8 h, with the first postdose sample being taken 30 min after the test beverage was consumed. Blood samples were centrifuged at 2846 × g for 10 min at 4°C, and plasma stored at −80°C until analyzed.
Plasma metabolite analyses
Plasma β-HB and acetoacetate were measured by an automated colorimetric assay as previously described (9). Briefly, for acetoacetate, 25 μL plasma was mixed with 330 μL fresh reagent [Tris buffer, pH 7.0, 100 mmol/L; 20 mmol sodium oxamate/L; 0.15 mmol NAD(H)/L, and 1 U β-HB dehydrogenase (β-HBDH)/mL]. For β-HB, the reagent was Tris buffer (pH 9.0; 20 mM sodium oxamate, 1 mmol NAD/L; and 1 U β-HBDH/mL). Tris, oxamic acid, DL-β-HB sodium salt, Li-acetoacetate standard, and NAD were purchased from Sigma; NAD(H) was purchased from Roche; and β-HBDH was purchased from Toyobo. The change in absorbance at 340 nm between 15 and 120 s after the addition of the reagent was measured by using an automated clinical chemistry analyzer (Dimension Xpand Plus; Siemens). The assay was calibrated with freshly diluted standards from frozen aliquots of a 10-mmol/L standard of Li-acetoacetate or DL-β-HB sodium salt, which are stable at −20°C for 2 and 6 mo, respectively. Calibrations and quality controls were performed for each assay to ensure the precision of the kits (CV between tests: 5% ± 1%). Plasma glucose, lactate, TGs, cholesterol (Siemens Medical Solutions USA, Inc.), and FFAs (Randox Laboratories Ltd.) were analyzed by using commercial kits. Glycated hemoglobin was measured by HPLC-723G7, a fully automated HPLC instrument-reagent system (Tosoh Bioscience).
Statistical analysis
All of the results are given as means ± SEMs. The sample size calculation was based on a previous study in which 8 participants were sufficient to measure a significant difference (β = 0.80) in plasma ketones after consuming 30 g MCT oil (9). Our sample size was n = 9 for the present study in case of a dropout during 1 of the 7 tests. All of the statistical analyses were carried out by using SPSS 23.0 software (SPSS, Inc.). Plasma ketone data are all reported in relation to time 0 (baseline). When plasma ketones are given in the plural, this refers to the total of acetoacetate and β-HB combined. The second test dose was given 4 h after the first, so the half-day periods are reported as 0–4 and 4–8 h. For total ketones, the AUCs from 0 to 4 and 4 to 8 h were calculated according to the trapezoid method (11). Because the data were not normally distributed (n < 30), results of the 7 tests were compared by using Friedman's test, and the effect of the treatments as well as changes over time were determined in each group by using Wilcoxon's Signed Rank test. False discovery rate procedures for multiple comparisons were used to control for incorrect rejection of the null hypothesis (12). Spearman correlations were used to measure the statistical dependence between 2 variables. Differences were considered significant at P ≤ 0.05. Graphs were prepared with the use of Prism version 6.0 (GraphPad Software, Inc.).
Results
Seven men and 2 women (total n = 9) completed all of the tests except for C10 (n = 8; Table 2). The participants' baseline biochemical variables corresponded to normal reference values from the Sherbrooke University Hospital Center (9). No significant gastrointestinal side effects were reported. There were no differences in plasma glucose, lactate, TG, or FFA responses among the 7 metabolic days (data not shown).
TABLE 2.
Baseline demographic and biochemical variables of the participants1
| Characteristics | Values |
| Age, y | 34 ± 12 |
| Weight, kg | 72 ± 10 |
| Height, cm | 175 ± 6 |
| BMI, kg/m2 | 24 ± 3 |
| Plasma metabolites | |
| Glucose, mmol/L | 4.3 ± 0.2 |
| Ketones,2 μmol/L | 90 ± 61 |
| Glycated hemoglobin, % | 5.2 ± 0.4 |
| Total cholesterol, mmol/L | 4.3 ± 0.9 |
| TGs, mmol/L | 0.7 ± 0.3 |
Values are means ± SDs, n = 9.
Acetoacetate + β-hydroxybutyrate.
Plasma ketones
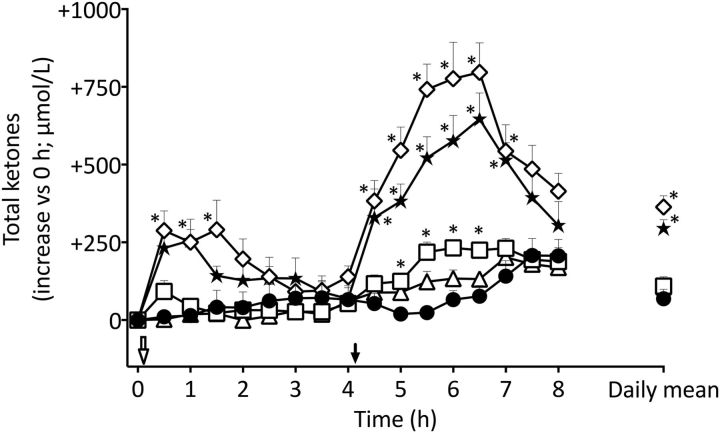
Compared with the CTL, there was no difference in plasma ketones during the metabolic day on which CO was evaluated (P = 0.11; Figure 1). C10 alone significantly elevated plasma ketones by up to 2-fold between 5 and 6.5 h compared with the CTL (P < 0.01). Compared with the CTL, C8 alone significantly increased plasma ketones by +288 ± 190 μmol/L above baseline at 0.5–1.5 h (P < 0.01), after which ketones returned to CTL values by 4 h. The second dose of C8 at midday again significantly elevated plasma ketones compared with the CTL, reaching a maximal peak concentration from 4.5 to 6.5 h of +797 ± 285 μmol/L above baseline (P < 0.01). Compared with the CTL, C8-C10 also induced a significant increase in ketones at 1 h (+250 ± 106 μmol/L above baseline; P < 0.01). In the second half of the metabolic day, C8-C10 significantly increased plasma ketones from 4.5 to 7 h, with a maximum of +646 ± 256 μmol/L above baseline (P < 0.05). Both CO+C8-C10 and CO+C8 produced intermediary effects on plasma total ketones that were significantly higher than the CTL at 0.5 and 1 h (+126 ± 55 and +129 ± 113 μmol/L above baseline; P < 0.01) and significantly higher again at 5.5 and 6 h (+341 ± 169 and +483 ± 206 μmol/L, respectively, above baseline) in comparison with the CTL (P < 0.01; data not shown). Mean ketones averaged over the whole metabolic study day were 5.3 times and 4.3 times higher with C8 alone and C8-C10 alone than with the CTL (P < 0.05).
FIGURE 1.
Plasma concentration and summed daily means (far right) during the metabolic study days for total ketones (β-HB and AcAc) obtained without an added test oil (CTL; ●) or after taking two 20-mL doses of CO alone (▵), C10 alone (□), medium-chain TGs (C8-C10; ★), or C8 alone (⋄). The open arrow indicates when the breakfast plus test oil was consumed; the solid arrow indicates when the test oil alone was consumed without an accompanying meal at midday. Data for metabolic study days on which CO+C8-C10 and CO+C8 were tested are not shown here for clarity, but their AUC data are shown in Figure 2. Values are means ± SEMs; n = 9/point. *Different from CTL, P < 0.05. AcAc, acetoacetate; CO, coconut oil; CTL, control; C8, tricaprylin; C10, tricaprin; β-HB, β-hydroxybutyrate.
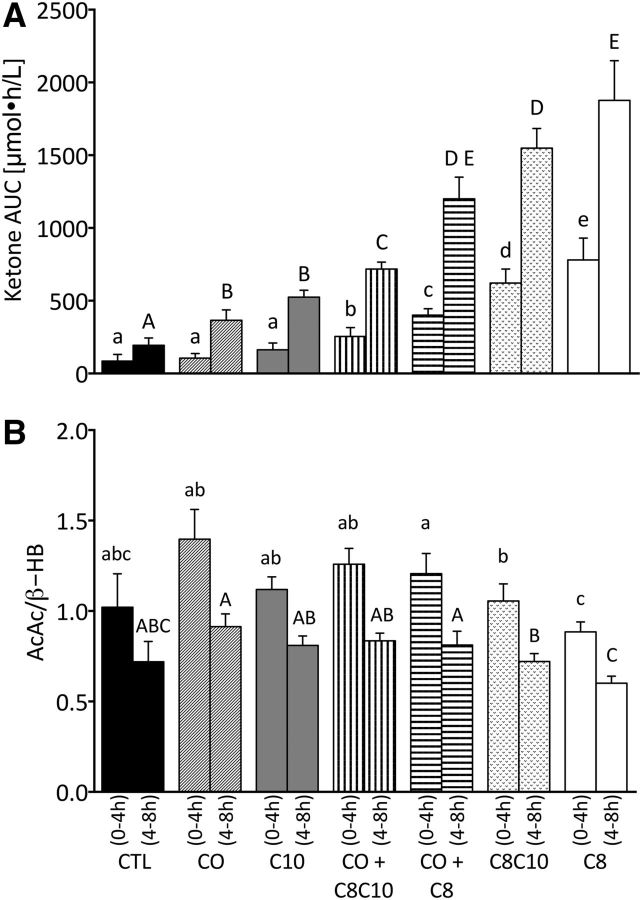
There was no difference in the plasma ketone AUC over the first 4-h period between the CTL, CO, and C10 (P < 0.12; Figure 2A). However, the AUC from 4 to 8 h of CO and C10 was significantly higher than the CTL (+88% and +171%, respectively; P < 0.05). C8 alone induced the highest plasma ketone AUCs from 0–4 h (780 ± 348 μmol ⋅ h/L) and from 4–8 h (1876 ± 564 μmol ⋅ h/L), values that were 26% and 21% more than C8-C10 alone and 813% and 870% more than the CTL, respectively (P < 0.01). The 2 half-day AUCs (0–4 and 4–8 h) were significantly different from each other during all tests (P < 0.05).
FIGURE 2.
Plasma concentration and summed means of 0- to 4-h and 4- to 8-h AUCs for plasma total ketones (i.e., AcAc and β-HB combined) (A) and for the mean AcAc-to-β-HB ratio (B). Bars represent no test oil consumed (CTL) or values after taking 2 doses of CO alone, C10 alone, medium-chain TGs (C8-C10), C8 alone, CO+C8-C10 (50:50), or CO+C8 (50:50). Values are means ± SEMs; n = 9. The AUC for 0–4 h was significantly different from the AUC for 4–8 h under all conditions. Labeled means without a common letter differ (a < b < c < d < e and A < B < C < D < E), P < 0.05. AcAc, acetoacetate; CO, coconut oil; CTL, control; C8, tricaprylin; C10, tricaprin; β-HB, β-hydroxybutyrate.
Plasma acetoacetate-to-β-HB ratio
CO significantly increased the plasma acetoacetate-to-β-HB ratio compared with C8 from 0 to 4 h and from 4 to 8 h (P < 0.05; Figure 2B). Acetoacetate:β-HB was also higher after C10 than after C8 during both time periods (P < 0.05). The acetoacetate-to-β-HB ratios for 0–4 and 4–8 h with the CTL treatment were not significantly different from those of any of the test oils (1.02 ± 0.50 for 0–4 h and 0.72 ± 0.27 for 4–8 h; P > 0.05). Plasma acetoacetate-to-β-HB ratios were significantly higher with CO+C8-C10 and with CO+C8 than with C8 alone (P < 0.01; Figure 2B). The ratio of acetoacetate to β-HB was significantly higher during 0–4 h than during 4–8 h, regardless of the test substance (P < 0.05).
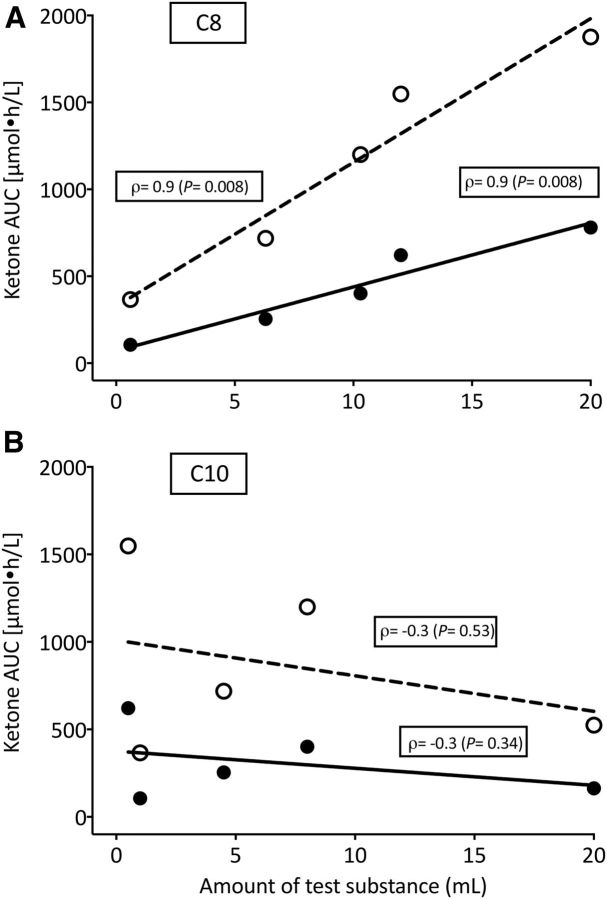
Plasma ketones compared with dose of test oil
The AUCs for 0–4 and 4–8 h for the increase in plasma total ketones after C8 were significantly positively correlated to the total dose of C8 given (ρ = 0.9, P = 0.008, and ρ = 0.9, P = 0.008, respectively; Figure 3A). Plasma total ketone AUCs after C10 were not significantly correlated with the dose of C10 (P ≥ 0.34; Figure 3B).
FIGURE 3.
Direct, linear relation between the 0–4 h (●) or 4–8 h (○) AUCs for plasma total ketones in relation to the dose of C8 (A) or C10 (B) consumed. Values are means ± SEMs; n = 9/point (P < 0.05). C8, tricaprylin; C10, tricaprin.
Discussion
Our main observation was that C8 was the most ketogenic of the MCTs tested (Figure 2A). When C8 was mixed with CO in a 50:50 ratio, the combination dampened the net ketogenic effect by 75% ± 27% compared with that of C8 alone. Hence the 5–10% medium-chain FA content of CO was only sufficient to modestly stimulate ketone production and only without a meal (4–8 h in our study day). In contrast, when given alone, C8 induced a 3.4-fold higher total plasma ketone daily mean response than CO alone (Figure 1). During the CTL test, total ketones increased at 7–8 h due to the low carbohydrate content in the CTL beverage (vehicle for all of the tests); this increase during the CTL test was not different from that observed during the CO and C10 tests.
The significant positive correlation of medium-chain FA intake and plasma ketone response was only observed for C8 (Figure 3A) but not for C10 (Figure 3B), suggesting that C8 drives the ketogenic effect of MCTs containing a mixture of C8 and C10. C8 and C10 differ in chain length by only 2 methylene groups, but C8 was clearly more ketogenic under our conditions. A family of medium-chain fatty acyl-CoA dehydrogenases catalyzes the first step of β-oxidation of C6–C12 FAs with a higher specific activity for C8 (13). As seen in astrocytes in culture (14), C8 also appears to be preferentially β-oxidized over C10 in humans, resulting in more rapid formation of acetyl-CoA, the substrate for ketogenesis. Although C10 is not very ketogenic, it may have an indirect effect on brain fuel availability because it promotes glycolysis and stimulates lactate release in isolated cultured astrocytes (14).
Nutritional state has an important effect on ketogenesis, with fasting stimulating ketone production more than the postprandial state for any given load of C8 (15). FA synthesis is generally decreased under fasting or very low food intake conditions in which acetyl-CoA generation is unchanged or increased, which results in a larger acetyl-CoA pool and increased ketogenesis. This could explain the higher plasma ketone response during our 4- to 8-h study period compared with the 0- to 4-h period.
Despite its relatively poor ketogenic effect, CO induced a significantly higher acetoacetate-to-β-HB ratio than did C8 or C8-C10 (Figure 2B). During ketogenesis, β-HB and acetoacetate are exported from the liver into the bloodstream for use by extrahepatic tissues such as the brain (16). It is acetoacetate that is metabolized to carbon dioxide, so β-HB needs to undergo conversion to acetoacetate via β-HBDH before it can affect ATP production (17). A higher acetoacetate-to-β-HB ratio could therefore potentially favor more direct energy availability from ketones. However, it is not clear that the acetoacetate-to-β-HB ratio observed in plasma represents the same ratio in mitochondria, so the implications of a higher or lower plasma acetoacetate-to-β-HB ratio need further investigation.
Brain glucose uptake is lower in Alzheimer disease (AD) (2, 18). This problem develops gradually before cognitive symptoms are present, continues as symptoms progress, and becomes lower than the brain glucose hypometabolism occurring in normal aging (19, 20). In contrast to glucose, brain ketone uptake in AD is similar to that in cognitively healthy, age-matched controls (2, 18). For ketones to be a useful energy source in glucose-deprived parts of the AD brain, the estimated mean daily plasma ketone concentration needs to be >200 μmol/L (21). With a total 1-d dose of 40 mL C8, plasma ketones peaked at 900 μmol/L and the day-long mean was 363 ± 93 μmol/L, whereas with the same amount of CO, they peaked at 300 and 107 ± 57 μmol/L, respectively. Our 2-dose test protocol (breakfast and midday) generated 2 peaks of plasma total ketones throughout 8 h, with the second dose inducing 3.5 and 2.4 times higher ketones with C8 than with CO, respectively. The first dose taken with a meal would be a more typical pattern but resulted in less ketosis that without a meal. One limitation of this study design is that the metabolic study period was only 8 h. A longer-term study lasting several weeks to months would be useful to assess the impact of regular MCT supplementation on ketone metabolism.
In summary, C8 was the most ketogenic MCT tested in this acute 8-h study and its ketogenic effect was significantly higher in the absence of an accompanying meal. Despite a low net ketogenic effect, CO may still be of interest because of its effect on plasma acetoacetate-to-β-HB ratio. With the help of positron emission tomographic imaging and the ketone tracer 11C-acetoacetate (2, 18, 20), it is now possible to investigate the impact on tissue ketone uptake of various ketogenic interventions.
Acknowledgments
We thank our research nurses, Christine Brodeur-Dubreuil and Georgette Proulx, for their assistance in participant screening, blood sampling, and care of the participants. The authors' responsibilities were as follows—MF, C-AC, and SCC: designed the study; CV, VS-P, and TP: conducted the study; CV, VS-P, and SCC: analyzed and interpreted the data; and all authors: read and approved the final manuscript.
Abbreviations
- AD
Alzheimer disease
- CO
coconut oil
- CTL
control
- C8
tricaprylin
- C10
tricaprin
- MCT
medium-chain triglyceride
- β-HB
β-hydroxybutyrate
- β-HBDH
β-hydroxybutyrate dehydrogenase
References
- 1. Cahill GF.. Fuel metabolism in starvation. Annu Rev Nutr 2006;26:1–22. [DOI] [PubMed] [Google Scholar]
- 2. Cunnane SC, Courchesne-Loyer A, St-Pierre V, Vandenberghe C, Pierotti T, Fortier M, Croteau E, Castellano CA.. Can ketones compensate for deteriorating brain glucose uptake during aging? Implications for the risk and treatment of Alzheimer's disease. Ann N Y Acad Sci 2016;1367:12–20. [DOI] [PubMed] [Google Scholar]
- 3. Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill GF.. Brain metabolism during fasting. J Clin Invest 1967;46:1589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Drenick EJ, Alvarez LC, Tamasi GC, Brickman AS.. Resistance to symptomatic insulin reactions after fasting. J Clin Invest 1972;51:2757–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cross H, Ferrie C, Lascelles K, Livingston J, Mewasingh L.. Old versus new antiepileptic drugs: the SANAD study. Lancet 2007;370:314; author reply: 315–6. [DOI] [PubMed] [Google Scholar]
- 6. Seaton TB, Welle SL, Warenko MK, Campbell RG.. Thermic effect of medium-chain and long-chain triglycerides in man. Am J Clin Nutr 1986;44:630–4. [DOI] [PubMed] [Google Scholar]
- 7. Martens B, Pfeuffer M, Schrezenmeir J.. Medium-chain triglycerides. Int Dairy J 2006;16:1374–82. [Google Scholar]
- 8. Henderson ST.. Ketone bodies as a therapeutic for Alzheimer's disease. Neurotherapeutics 2008;5:470–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Courchesne-Loyer A, Fortier M, Tremblay-Mercier J, Chouinard-Watkins R, Roy M, Nugent S, Castellano CA, Cunnane SC.. Stimulation of mild, sustained ketonemia by medium-chain triacylglycerols in healthy humans: estimated potential contribution to brain energy metabolism. Nutrition 2013;29:635–40. [DOI] [PubMed] [Google Scholar]
- 10. Schönfeld P, Wojtczak L.. Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. J Lipid Res 2016;57:943–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gagnon RC, Peterson JJ.. Estimation of confidence intervals for area under the curve from destructively obtained pharmacokinetic data. J Pharmacokinet Biopharm 1998;26:87–102. [DOI] [PubMed] [Google Scholar]
- 12. Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I.. Controlling the false discovery rate in behavior genetics research. Behav Brain Res 2001;125:279–84. [DOI] [PubMed] [Google Scholar]
- 13. Ikeda Y, Okamura-Ikeda K, Tanaka K.. Purification and characterization of short-chain, medium-chain, and long-chain acyl-CoA dehydrogenases from rat liver mitochondria: isolation of the holo- and apoenzymes and conversion of the apoenzyme to the holoenzyme. J Biol Chem 1985;260:1311–25. [PubMed] [Google Scholar]
- 14. Thevenet J, De Marchi U, Domingo JS, Christinat N, Bultot L, Lefebvre G, Sakamoto K, Descombes P, Masoodi M, Wiederkehr A.. Medium-chain fatty acids inhibit mitochondrial metabolism in astrocytes promoting astrocyte-neuron lactate and ketone body shuttle systems. FASEB J 2016;30:1913–26. [DOI] [PubMed] [Google Scholar]
- 15. McGarry JD, Foster DW.. The regulation of ketogenesis from octanoic acid: the role of the tricarboxylic acid cycle and fatty acid synthesis. J Biol Chem 1971;246:1149–59. [PubMed] [Google Scholar]
- 16. Rojas-Morales P, Tapia E, Pedraza-Chaverri J.. β-Hydroxybutyrate: a signaling metabolite in starvation response? Cell Signal 2016;28:917–23. [DOI] [PubMed] [Google Scholar]
- 17. Cotter DG, Schugar RC, Crawford PA.. Ketone body metabolism and cardiovascular disease. Am J Physiol Heart Circ Physiol 2013;304:H1060–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Castellano CA, Nugent S, Paquet N, Tremblay S, Bocti C, Lacombe G, Imbeault H, Turcotte É, Fulop T, Cunnane SC.. Lower brain 18F-fluorodeoxyglucose uptake but normal 11C-acetoacetate metabolism in mild Alzheimer's disease dementia. J Alzheimers Dis 2015;43:1343–53. [DOI] [PubMed] [Google Scholar]
- 19. Costantini LC, Barr LJ, Vogel JL, Henderson ST.. Hypometabolism as a therapeutic target in Alzheimer's disease. BMC Neurosci 2008;9(Suppl 2):S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nugent S, Tremblay S, Chen KW, Ayutyanont N, Roontiva A, Castellano CA, Fortier M, Roy M, Courchesne-Loyer A, Bocti C, et al.. Brain glucose and acetoacetate metabolism: a comparison of young and older adults. Neurobiol Aging 2014;35:1386–95. [DOI] [PubMed] [Google Scholar]
- 21. VanItallie TB, Nufert TH.. Ketones: metabolism's ugly duckling. Nutr Rev 2003;61:327–41. [DOI] [PubMed] [Google Scholar]