Abstract
Background: High intakes of total and animal protein are associated with the risk of type 2 diabetes (T2D). The influence of protein type on insulin resistance, a key precursor of T2D, has not been extensively studied.
Objective: The aim of this study was to determine the associations between dietary total, animal, and plant protein intakes as well as the animal-to-plant protein (AP) intake ratio with insulin resistance in middle-aged and older adults.
Methods: This was a cross-sectional analysis in 548 participants (mean ± SD age: 66.2 ± 13.7 y) from the calibration substudy of the AHS-2 (Adventist Health Study 2) cohort. Participants consumed diets with a low AP intake ratio. Dietary intakes of total and particular types of protein were calculated from six 24-h dietary recalls. Participants completed a self-administered questionnaire on demographic, lifestyle, health, diet intake, and physical activity characteristics. Anthropometric variables including weight, height, and waist circumference were measured. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated by using fasting serum glucose and insulin. Multiple linear regression models were used to test the relations between total and specific protein intakes with insulin resistance.
Results: The ranges of dietary intakes of animal and plant protein and the AP intake ratio were 0.4–87.4 and 14.0–79.2 g/d and 0.02–4.43, respectively. Dietary intakes per 10-g/d increments of total protein (β: 0.11; 95% CI: 0.02, 0.21) and animal protein (β: 0.11; 95% CI: 0.01, 0.20) and the AP intake ratio (β: 1.82; 95% CI: 0.80, 2.84) were positively related to HOMA-IR. Plant protein was not significantly related to insulin resistance.
Conclusion: Total and animal protein intakes and the AP intake ratio were positively associated with HOMA-IR in adults with relatively a low intake of animal protein and a high consumption of plant protein.
Keywords: animal protein, animal-to-plant protein intake ratio, insulin resistance, HOMA-IR, AHS-2
Introduction
Insulin resistance is a major predictor of type 2 diabetes (T2D) and an independent risk factor for cardiovascular disease (1–3). The early detection and treatment of insulin resistance can prevent T2D and alleviate its high economic and public health burden (4). Findings from the first Adventist Health Study group of white Californians suggested that a vegetarian diet reduces the risk of developing diabetes (5). In the current Adventist Health Study 2 (AHS-2) cohort, the prevalence and incidence of diabetes were the lowest among vegans compared with other vegetarians and nonvegetarians (6, 7). Recent reports in established longitudinal cohorts found a clear association between total and animal protein intakes and an increased risk of T2D (8–10), whereas plant protein intake had a protective (10) or no (8, 9) effect. In these cohorts, animal protein contributed the majority of the total protein, and although total and animal protein intakes varied among participants, the proportion of energy from plant protein diverged little (8, 9). In cross-sectional studies in adults without diabetes, a high meat intake was associated with insulin resistance evaluated by using the HOMA-IR (11, 12).
Although previous studies showed an association between dietary protein and diabetes risk or meat intake and insulin resistance, none to our knowledge have examined the possible influence of the type of protein on biomarker-validated insulin sensitivity. Due to the emphasis placed on plant-based diets among Seventh-day Adventists, the eating pattern of the AHS-2 cohort differs from other groups in the amount of plant protein and in the proportion of plant to animal protein consumed (13). For example, the average percentages of protein intake derived from animal and plant protein sources of AHS-2 participants are ∼34% and 65%, respectively, whereas those of the US population are 62% and 30%, respectively (14). Another feature of the cohort is the consistency of eating habits, with usual intakes representing long-term patterns (15). Whether dietary animal or plant protein or their relative amounts affect insulin sensitivity as such is largely unknown. The purpose of this study is to examine the relation between total protein intake and the type of dietary protein on insulin resistance in healthy older to elderly individuals who consume a wide range of plant and animal protein and a predominantly plant-based diet.
Methods
Study design and participants
This was a cross-sectional analysis of data from a randomly selected subsample of the AHS-2 participants, called the calibration substudy, on whom detailed dietary and biochemical data were obtained. The AHS-2 is a prospective population-based study in 96,592 Seventh-day Adventists from the United States and Canada whose main goal is to examine the associations of lifestyle factors with risk of cancers. Details of the cohort and study protocol have been described elsewhere (16). Briefly, participants completed a comprehensive self-administered questionnaire, providing information related to lifestyle and health, dietary patterns, and physical activity (17). The calibration substudy participants (n = 1011) were randomly selected from the parent cohort by church and then within church by sex and age (18). Participants were excluded if data on dietary intake, demographic variables, insulin resistance, physical activity, and waist circumference were incomplete and if energy intake was <500 or >4500 kcal. Lifestyle practices such as current smoking and alcohol consumption are confounders traditionally controlled for in a statistical analysis of diet-disease relations. In this report we intended to test the effect of protein intake on insulin resistance without the influence of these confounders; therefore, we also excluded participants who consumed alcohol and were current smokers. After all exclusions, the final analytic cohort consisted of 548 participants.
The Institutional Review Board of Loma Linda University approved the study protocol. All of the participants were informed of the study protocol and provided written consent at the time of enrollment.
Dietary assessment
Trained dietitians obtained unannounced telephone 24-h dietary recalls (24HDRs) to collect dietary intakes of foods, beverages, and supplements. The Nutrition Data System for Research (NDS-R) version 4.06 or 5.0 was used for data acquisition and entry, and nutrient composition was based on the NDS-R 2008 database (Nutrition Coordinating Center). Each participant provided 2 sets of 3 recalls for a total of six 24HDRs. Each set was taken 6 mo apart and consisted of intakes for 1 Saturday, 1 Sunday, and 1 weekday. Within each set of recalls, each recall was weighted appropriately so as to produce a weekly intake (Saturday intake + Sunday intake + 5 × weekday intakes), and then divided by 7 to estimate an average daily intake of a nutrient. Each individual thus provided 2 repeated measures of intake data. The NDS-R 2008 database includes data for protein, animal protein, and plant protein; thus, the daily intake of each protein was the sum from all food sources as well as from recipes of mixed dishes for each recall day. Intakes of total, animal, and plant protein were energy-adjusted by using the residual method (19). The animal-to-plant protein (AP) intake ratio was the ratio of the energy-adjusted values of these nutrients.
Anthropometric and activity measures
Participants' weight was measured with light clothing and without shoes by using a Tanita BF-350 scale (Tanita UK Ltd.) and was rounded up to the nearest 0.1 kg. Height was measured without shoes by using a Seca 214 portable Height Rod (Seca Corporation) and was rounded up to the nearest 0.25 cm. Waist circumference was measured 3 times with an anthropometric tape 2.5 cm above the navel. The average of 3 values was used for the analyses. Physical activity (hours per day) was estimated by questionnaire (17) and calculated as the sum of mild, moderate, vigorous, and extremely vigorous activity.
Biochemical measures
Fasting blood glucose was analyzed by using the Cholestech LDX System (Cholestech) (20). Fasting insulin was measured by using the Elecsys Insulin Assay (Roche Diagnostics). Insulin resistance was estimated by HOMA-IR, which was calculated with the following formula: fasting plasma glucose (milligrams per deciliter) × fasting serum insulin (microunits per deciliter) divided by 405 (21).
Statistical analysis
A comparison of HOMA-IR according to participant characteristics was calculated by an independent-samples t test or ANOVA. Dietary intakes of total, animal, and plant protein and the AP intake ratio were calculated as means ± SDs and 5th, 50th, and 95th percentiles. To determine the association between dietary protein and HOMA-IR, we used 2 approaches: regression calibration and multiple linear regression (MLR) models with HOMA-IR as the dependent variable and total protein, animal protein, and plant protein intakes or the AP intake ratio as independent variables. Relevant assumptions for linear regression models were checked and log-transformed variables were used when appropriate. All of the regression models were adjusted for age, ethnicity, physical activity, waist circumference, energy intake, dietary PUFA–to-SFA ratio, dietary glycemic load, and type of dietary protein. In the MLR approach, the 2 repeated measures of each protein variable (total, animal, and plant intakes and the AP intake ratio) were averaged. In regression calibration we corrected for within-subject variation and measurement error bias in the 2 sets of repeated 24HDRs according to Spiegelman et al. (22) and plotted the back-transformed predictors from these models. Based on the fact that waist circumference is a strong predictor of insulin resistance (23), we stratified our analysis by waist circumference to examine its role as an effect modifier. Cutoffs for waist circumference in men and women were 102 and 88 cm, respectively (24).
A descriptive analysis was performed by using the Statistical Package for the Social Sciences (version 22.0; SPSS). Data analysis and statistical software (STATA, version 13; StataCorp) was used for all regression analyses and to generate plots from the regression calibration models. P values <0.05 were considered significant.
Results
A total of 548 participants were considered for this study. Women comprised 66% of the study participants. The mean age of participants was 66.2 y. Men, whites, and those with a higher BMI and waist circumference had higher insulin resistance (Table 1). Mean ± SD daily intakes of total, animal, and plant protein and the AP intake ratio were 56.7 ± 11.5, 21.2 ± 16.0, and 35.5 ± 11.1 g/d and 0.83 ± 0.09, respectively (Table 2).
TABLE 1.
HOMA-IR values according to participant characteristics
| n | HOMA-IR1 | P 2 | |
|---|---|---|---|
| Sex | 0.018 | ||
| Male | 241 | 1.66 ± 2.11 | |
| Female | 466 | 1.44 ± 2.07 | |
| Age, y | 0.047 | ||
| <59 | 349 | 1.59 ± 2.13 | |
| ≥60 | 345 | 1.43 ± 2.03 | |
| Race | 0.014 | ||
| White | 309 | 1.64 ± 2.04 | |
| Nonwhite | 398 | 1.43 ± 2.13 | |
| Education3 | 0.441 | ||
| High school or less | 191 | 1.61 ± 2.09 | |
| Some college | 226 | 1.52 ± 2.18 | |
| College graduate or higher | 281 | 1.47 ± 2.05 | |
| BMI, kg/m2 | <0.0001 | ||
| <25 | 253 | 0.941 ± 1.81 | |
| ≥25 | 437 | 1.99 ± 1.97 | |
| Waist circumference, cm | <0.0001 | ||
| ≤102 in men or ≤88 in women | 408 | 1.09 ± 1.82 | |
| >102 in men or >88 in women | 297 | 2.38 ± 1.93 | |
| Physical activity, h/d | 0.776 | ||
| <3 | 395 | 1.58 ± 2.07 | |
| ≥3 | 227 | 1.55 ± 2.13 |
Values are means ± SDs.
P values were obtained by independent t test unless otherwise indicated.
P value was obtained by 1-factor ANOVA.
TABLE 2.
Means ± SDs and percentiles of daily dietary protein intake in the AHS-2 calibration substudy1
| Percentile of in take, g | Difference between 95th and 5th percentiles, g | ||||
|---|---|---|---|---|---|
| Dietary protein intake,2 g | 5th | 50th | 95th | ||
| Total protein | 56.68 ± 11.49 | 41.59 | 52.65 | 69.38 | 35.76 |
| Animal protein | 21.17 ± 16.03 | 3.39 | 17.76 | 44.24 | 49.78 |
| Plant protein | 35.51 ± 11.14 | 21.69 | 34.68 | 50.67 | 35.04 |
| AP intake ratio | 0.83 ± 0.94 | 0.056 | 0.52 | 2.01 | 2.49 |
n = 548. AHS-2, Adventist Health Study 2; AP, animal-to-plant protein.
Values are means ± SDs.
Percentiles of dietary protein intake are presented in Table 2. Differences between the 5th and 95th percentiles of total, animal, and plant protein intakes and the AP intake ratio were 35.8 g/d, 49.8 g/d, 35.0 g/d, and 2.5, respectively. Table 3 shows the association between dietary protein intake and insulin resistance in all participants and stratified by waist circumference. In the population as a whole, total and animal protein intakes and the AP intake ratio showed a positive association with HOMA-IR, and these results were consistent with the use of regression calibration and the MLR models. On stratification by waist circumference, a significant strong positive association between AP intake ratio and HOMA-IR was evident in those with a normal waist circumference, but the association was attenuated and became nonsignificant in abdominally obese participants with the use of regression calibration models.
TABLE 3.
β-Coefficients (95% CIs) of the associations between dietary protein intake in 10-g/d increments and insulin resistance in the AHS-2 calibration substudy total population and stratified by waist circumference1
| Regression calibration | Multiple linear regression | |
|---|---|---|
| Total (n = 548) | ||
| Total protein | 0.11 (0.02, 0. 21) | 0.07 (0.01, 0.12) |
| Animal protein | 0.11 (0.01, 0.20) | 0.08 (0.03, 0.14) |
| Plant protein | 0.00 (−0.19, 0.19) | 0.00 (−0.07, 0.08) |
| AP intake ratio | 1.82 (0.80, 2.84) | 1.30 (0.65, 1.95) |
| Normal waist circumference (n = 314) | ||
| Total protein | 0.10 (−0.03, 0.23) | 0.07 (−0.001, 0.14) |
| Animal protein | 0.09 (−0.03, 0.23) | 0.08 (0.01, 0.16) |
| Plant protein | −0.06 (−0.33, 0.19) | −0.01(−0.12, 0.09) |
| AP intake ratio | 2.52 (0.93, 4.11) | 1.70 (0.80, 2.60) |
| High waist circumference (n = 234) | ||
| Total protein | 0.13 (−0.01, 0.27) | 0.07 (−0.008, 0.15) |
| Animal protein | 0.12 (−0.02, 0.26) | 0.08 (0.00, 0.15) |
| Plant protein | 0.06 (−0.24, 0.37) | 0.01 (−0.10, 0.13) |
| AP intake ratio | 1.27 (−0.06, 2.62) | 0.90 (0.04, 1.85) |
Values for animal and plant protein were adjusted for age, sex, waist circumference, ethnicity, physical activity, dietary PUFA to SFA ratio, dietary glycemic load, type of dietary protein, and energy. AHS-2, Adventist Health Study 2; AP, animal-to-plant protein.
Plots from the regression calibration models show that HOMA-IR was positively associated with intakes of total and animal protein and was not associated with plant protein intake (Figure 1A). At the same amount of protein intake, insulin resistance was higher with animal protein than with total protein intake. Figure 1B shows that the association between HOMA-IR and the AP intake ratio was nonlinear, but that insulin resistance as shown by HOMA-IR showed a decrease with decreasing AP intake ratios.
FIGURE 1.
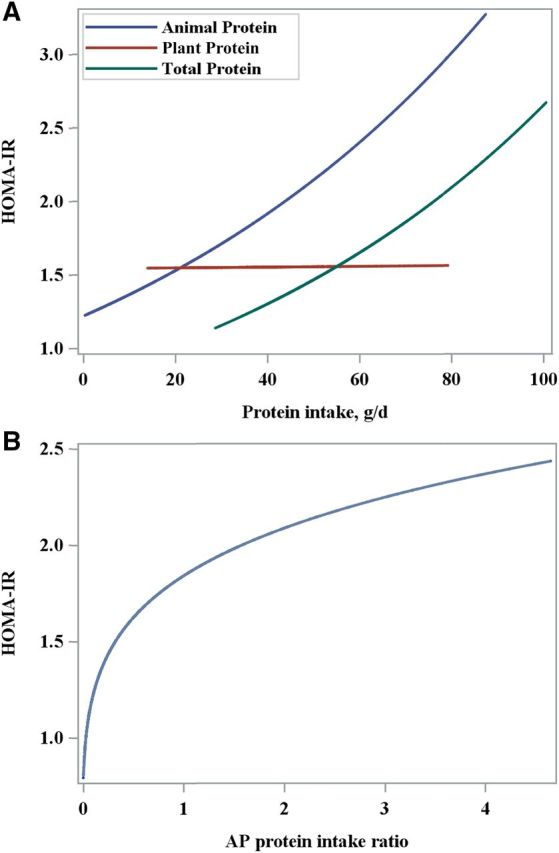
(A) Multiple linear regression of the association between HOMA-IR and dietary intakes of total (P = 0.018), animal (P = 0.022), and plant (P = 0.098) protein adjusted for age, ethnicity, physical activity, waist circumference, energy, ratio of PUFAs to SFAs, glycemic load, and type of dietary protein. (B) Multiple linear regression of the association between HOMA-IR and the AP protein intake ratio (P = 0.000) adjusted for age, ethnicity, physical activity, waist circumference, energy, ratio of PUFAs to SFAs, glycemic load, and type of dietary protein. AP, animal to plant.
Discussion
In this cohort of middle-aged and older adults, total and animal protein consumption and the AP intake ratio were associated with higher insulin resistance measured by using the HOMA-IR. Despite a wide range of plant protein intakes, no association between plant protein and insulin resistance was observed. Waist circumference modified the effect of the AP intake ratio, but not total, animal, or plant protein intakes, on insulin resistance.
Studies examining the association between dietary protein or protein-rich foods and measures of insulin sensitivity in nondiabetics are sparse, and none, to our knowledge, isolated the effect of animal and plant protein as such. In participants whose intake was monitored over a period of 6 mo, high dietary protein consumption (1.87 g/kg) compared with normal protein consumption (0.74 g/kg) was accompanied by increased glucose-stimulated insulin secretion and gluconeogenesis (25). Positive associations between meat intake (12), red meat intake (11), and a dietary pattern high in animal protein (26) with the HOMA-IR have been reported. In a recent cross-sectional analysis from the Nurses' Health Study (n = 3690), intakes of total, processed, and unprocessed red meat were linked to increased fasting insulin and glycated hemoglobin (27). In contrast, an inverse association was reported between soy-food consumption and HOMA-IR in a Japanese cohort (28). To the best of our knowledge, this is the first cross-sectional study to examine the association between intakes of total, animal, and plant protein and the AP intake ratio with insulin resistance assessed by using the HOMA-IR.
Our results showed that a higher total protein intake was associated with increased insulin resistance. Although high dietary protein in short-term clinical trials showed positive effects on glycemic control by augmenting insulin secretion and glucose clearance, studies on the long-term effects are equivocal and problematic (29, 30). In randomized dietary intervention studies, increases in one macronutrient resulted in the replacement of another. It is therefore not possible to isolate the nutrient responsible for the changes observed. This uncertainty was shown in a study in overweight adults with features of the metabolic syndrome, in whom a high-protein diet was compared with an isoenergic, low-protein, high-carbohydrate and high-fiber diet. The low-protein intervention resulted in a 25% increase in glucose sensitivity measured by using the euglycemic-hyperinsulinemic clamp technique—an effect that could be attributed either to a decrease in protein or an increase in carbohydrate and fiber (31).
Our observation that the proportion of AP in the diet, or the AP intake ratio, affects insulin sensitivity is somewhat supported by a number of clinical intervention trials. In women with abdominal obesity, partly replacing meat protein with soy resulted in greater insulin sensitivity assessed by the frequently sampled intravenous glucose-tolerance test (32). In a recent meta-analysis of randomized controlled trials, replacing some animal protein with plant protein foods resulted in modest improvements in glycemic control (33). Intervention studies that used vegan and vegetarian diets in diabetics reported improvements in glycemic control and increased insulin sensitivity (34, 35). Yet again, the observed beneficial effects could just as likely be attributed to components other than protein that plant foods provide.
The findings of the present study complement those of epidemiologic studies that reported associations between total protein (9, 36, 37), animal protein (8–10), and meat consumption (38, 39) and an increased risk of T2D. Recently, a combined analysis of 3 established prospective cohorts in the United States showed that a higher intake of animal protein was related to an increased risk of T2D and all-cause mortality (10). In the aforementioned cohorts, meat and/or processed meat were the main contributors of animal protein intake (8–10). However, reports regarding the effect of plant protein on T2D risk are inconsistent. Plant protein intake (10) and plant-based dietary patterns low in animal foods (40) were associated with a reduced risk of T2D or not found to have an effect (9).
Results from stratification by waist circumference suggest that waist circumference was an effect modifier in the association between protein intake and insulin resistance. Abdominal obesity is associated with insulin resistance in older adults (23). Our findings are in line with those in prospective cohorts that found an attenuation of the association between total and animal protein intakes and the risk of T2D when adjusting for adiposity (8, 10). In contrast, a recent case-cohort study reported a stronger association between total and animal protein intakes and the risk of T2D in obese women but not in obese men (9).
Although we did not explore the effect of animal or plant protein–containing food sources on insulin resistance, studies have shown that foods differ in their impact on glycemic control. Some evidence points to red meat and processed meat as the main contributors to an increased risk of T2D (38) and insulin resistance (11, 12). All animal protein sources may not have the same effect on glucose metabolism. Full-fat dairy products were associated with lower insulin resistance in a cross-sectional study (41), whereas in a randomized intervention, lean meat compared with dairy protein improved insulin sensitivity in insulin-resistant participants (42). A positive association between oily fish consumption and insulin sensitivity has been documented (43). If plant protein foods, such as whole grains (44), legumes (45), and nuts (46), with low glycemic load are the main sources of protein, a protective effect against T2D is shown (10). But plant foods with a high glycemic load, such as bread, pasta, and potatoes, do not show this protective effect (8, 9).
The rationale for focusing our research on animal and plant protein per se was to narrow the search for mechanisms underlying the association between dietary protein and glycemic control. Our finding that dietary animal protein is associated with insulin resistance may be due to its amino acid profile (47). A metabolomics study documented that high concentrations of plasma BCAAs and aromatic amino acids are related to the risk of T2D (48) and insulin resistance through stimulating insulin secretion and subsequent hyperinsulinemia (49). BCAAs, especially leucine, are found in higher proportions in animal food sources, and when absorbed may impair glucose uptake despite inducing a significant increase in insulin concentrations (47–50). Although it is unclear whether high circulating BCAA concentrations are a cause or a marker of the insulin-resistant state, recent research suggests that they are more likely a consequence of impaired insulin action and disturbed amino acid metabolism (51). It remains difficult to isolate the effect of specific types of protein on metabolism independent of the contribution of other nutrients in plant and animal food sources, the overall dietary pattern, and factors such as obesity and genetics (52).
The strengths of the present study lie in its relatively large sample size and in the quality of the dietary data obtained. Multiple 24HDRs are considered the gold standard against which other types of dietary assessments are often validated (53). Reported multiple 24HDRs are advantageous because within-subject variability can be removed by using regression calibration (22). There was a wide range of both animal protein and plant protein intakes in our population, which increased the likelihood of capturing a possible association with insulin resistance. Another strength of our study was the assessment of the relation between dietary protein intake and insulin resistance in nonsmokers and non–alcohol users, which enhances internal validity. Because we isolated total protein and the type of protein in the analyses, we were able to assess the net effect of plant or animal protein independent of their influence on one another. Our observations that animal protein per se is positively related to insulin resistance adds new information to the existing literature while strengthening and supporting what is already known about animal protein and its association with T2D. Beyond this, a unique contribution of this study is attributed to the study cohort of middle-aged and older adults who observe a range of eating patterns from plant-exclusive to typical omnivorous diets and many variations in between. Therefore, in essence, our study was able to show that, in the context of a relatively wide range of plant protein intake (from 14 to 79.2 g/d), total and animal protein intakes are associated positively with insulin resistance.
There are also limitations. The cross-sectional study design precludes any inference of causality. Because dietary and lifestyle practices of the AHS-2 participants may differ from those of the general population (13, 16), generalizing our results should probably be limited to groups with similar habits. More research is needed to investigate the effects of animal protein–rich food groups (red meats, poultry, fish, dairy, cheese, and eggs) on insulin resistance in populations with different sources and amounts of protein intake.
In conclusion, we were able to show that higher total and animal protein intakes are associated with higher HOMA-IR in a group of middle-aged and older adults. This observation is particularly unique given that this population, in addition to being nonsmokers and non–alcohol users, consumes a diverse range of dietary patterns that are relatively high in plant protein and vary from no animal protein to including animal protein. Although previously it has been shown that animal protein is associated with T2D, our study findings support this knowledge. These findings need to be explored further through randomized clinical trials. Overall, on the basis of our observations, it may be prudent to consider limiting animal protein intake and adopting an AP intake ratio of ∼1.0 or lower in order to decrease the risk of insulin resistance.
Acknowledgments
The authors' responsibilities were as follows—BA, SR, KJ-S, and EHH: designed the research; BA and DS: performed the statistical analysis; BA: drafted the article; BA, SR, KJ-S, JS, and EHH: revised the manuscript critically for important intellectual content; BA and EHH: had primary responsibility for final content; GEF: provided access to the AHS-2 data set; and all authors: read and approved the final manuscript.
Abbreviations
- AHS-2
Adventist Health Study 2
- AP
animal-to-plant protein (intake ratio)
- MLR
multiple linear regression
- NDS-R
Nutrition Data System for Research
- T2D
type 2 diabetes
- 24HDR
24-h dietary recall
References
- 1. Zavaroni I, Bonini L, Gasparini P.. Hyperinsulinemia in a normal population as a predictor of non-insulin-dependent diabetes mellitus, hypertension, and coronary heart disease: the Barilla factory revisited. Metabolism 1999;48:989–94. [DOI] [PubMed] [Google Scholar]
- 2. Abbasi F, Brown WB, Lamendola C, McLaughlin T, Reaven MG.. Relationship between obesity, insulin resistance, and coronary heart disease risk. J Am Coll Cardiol 2002;40:937–43. [DOI] [PubMed] [Google Scholar]
- 3. Imamura F, Mukamal KJ, Meigs JB, Luchsinger JA, Ix JH, Siscovick DS, Mozaffarian D.. Risk factors for type 2 diabetes mellitus preceded by β-cell dysfunction, insulin resistance, or both in older adults: the cardiovascular health study. Am J Epidemiol 2013;177:1418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fonseca VA.. Early identification and treatment of insulin resistance: impact on subsequent prediabetes and type 2 diabetes. Clin Cornerstone 2007;8:S7–18. [DOI] [PubMed] [Google Scholar]
- 5. Snowdon DA, Phillips RL.. Does a vegetarian diet reduce the occurrence of diabetes? Am J Public Health 1985;75:507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tonstad S, Butler T, Yan R, Fraser GE.. Type of vegetarian diet, body weight and prevalence of type 2 diabetes. Diabetes Care 2009;35:791–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tonstad S, Stewart K, Oda K, Batech M, Herring RP, Fraser GE.. Vegetarian diets and incidence of diabetes in the Adventist Health Study-2. Nutr Metab Cardiovasc Dis 2013;23:292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sluijs I, Beulens J, Van der D, Spijkerman A, Grobbee D, Van der Schouw Y.. Dietary intake of total, animal, and vegetable protein and risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-NL study. Diabetes Care 2010;33:43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Nielen M, Feskens EJ, Mensink M, Sluijs I, Molina E, Amiano P, Ardanaz E, Balkau B, Beulens JWJ, Boeing N, et al. Dietary protein intake and incidence of type 2 diabetes in Europe: the EPIC-InterAct case-cohort study. Diabetes Care 2014;37:1854–62. [DOI] [PubMed] [Google Scholar]
- 10. Malik VS, Li Y, Tobias DK, Pan A, Hu FB.. Dietary protein intake and risk of type 2 diabetes in US men and women. Am J Epidemiol 2016;183:715–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Panagiotakos DB, Tzima N, Pitsavos C, Chrysohoou C, Papakonstantinou E, Zampelas A, Stefanadis C.. The relationship between dietary habits blood glucose and insulin levels among people without cardiovascular disease and type 2 diabetes: the ATTICA study. Rev Diabet Stud 2005;2:208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tucker LA, LeCheminant JD, Bailey BW.. Meat intake and insulin resistance in women without type 2 diabetes. J Diabetes Res 2015;2015:174742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rizzo NS, Jaceldo-Siegl K, Sabate J, Fraser GE.. Nutrient profiles of vegetarian and non vegetarian dietary patterns. J Acad Nutr Diet 2013;113:1610–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pasiakos SM, Agarwal S, Lieberman HR, Fulgoni VL.. Sources and amounts of animal, dairy, and plant protein intake of US adults in 2007–2010. Nutrients 2015;7:7058–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Singh PN, Batech M, Faed P, Siegl KJ, Martins M, Fraser GE.. Reliability of meat, fish, dairy and egg intake over a 33-year interval in Adventist Health Study 2. Nutr Cancer 2014;66:1315–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Butler TL, Gary F, Beeson L, Synnøve FK, Patti HR, Jacqueline C, Sabaté J, Montgomery S, Haddad E, Preston-Martin S, et al. Cohort profile: the Adventist Health Study-2 (AHS-2). Int J Epidemiol 2008;37:260–5. [DOI] [PubMed] [Google Scholar]
- 17. Blair SN, Haskell WL, Ho P, Paffenbarger JR, Vranizan KM, Farquhar JW, Wood PD.. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol 1985;122:794–804. [DOI] [PubMed] [Google Scholar]
- 18. Jaceldo-Siegl K, Knutsen SF, Sabate J, Beeson WL, Chan J, Herring RP, Butler TL, Haddad E, Bennett H, Montgomery S, et al. Validation of nutrient intake using an FFQ and repeated 24 h recalls in black and white subjects of the Adventist Health Study-2 (AHS-2). Public Health Nutr 2010;13:812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Willett WC, Howe GR, Kushi LH.. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65(Suppl):1220S–28S. [DOI] [PubMed] [Google Scholar]
- 20. Bachorik PS, Rock R, Treciak E, Becker D, Sigmund W.. Cholesterol screening: comparative evaluation of on-site and laboratory-based measurements. Clin Chem 1990;36:255–60. [PubMed] [Google Scholar]
- 21. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC.. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- 22. Spiegelman D, McDermott A, Rosner B.. Regression calibration method for correcting measurement-error bias in nutritional epidemiology. Am J Clin Nutr 1997;65:1179S–86S. [DOI] [PubMed] [Google Scholar]
- 23. Racette SB, Evans EM, Weiss EP, Hagberg J, Holloszy JO.. Abdominal adiposity is a strong predictor of insulin resistance than fitness among 50–95 year olds. Diabetes Care 2006;29:673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang PL.. A comprehensive definition for metabolic syndrome. Dis Model Mech 2009;2:231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Linn T, Santosa B, Grönemeyer D, Aygen S, Scholz N, Busch M, Bretzel RG.. Effect of long-term dietary protein intake on glucose metabolism in humans. Diabetologia 2000;43:1257–65. [DOI] [PubMed] [Google Scholar]
- 26. Gadgil MD, Anderson CA, Kandula NR, Kanaya AM.. Dietary patterns are associated with metabolic risk factors in South Asians living in the United States. J Nutr 2015;145:1211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ley SH, Sun Q, Willett WC, Eliassen H, Wu K, Pan A, Grodstein F, Hu FB.. Associations between red meat intake and biomarkers of inflammation and glucose metabolism in women. Am J Clin Nutr 2014;99:352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakamoto M, Uemura H, Sakai T, Katsuura-Kamano S, Yamaguchi M, Hiyoshi M, Arisawa K.. Inverse association between soya food consumption and insulin resistance in Japanese adults. Public Health Nutr 2015;18:2031–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tremblay F, Lavigne C, Jacques H, Marette A.. Role of dietary proteins and amino acids in the pathogenesis of insulin resistance. Annu Rev Nutr 2007;27:293–310. [DOI] [PubMed] [Google Scholar]
- 30. Promintzer M, Krebs M.. Effects of dietary protein on glucose homeostasis. Curr Opin Clin Nutr Metab Care 2006;9:463–8. [DOI] [PubMed] [Google Scholar]
- 31. Weickert MO, Roden M, Isken F, Hoffmann D, Nowotny P, Osterhoff M, Blaut M, Alpert C, Gögebakan Ö, Bumke-Vogt C, et al. Effects of supplemented isoenergetic diets differing in cereal fiber and protein content on insulin sensitivity in overweight humans. Am J Clin Nutr 2011;94:459–71. [DOI] [PubMed] [Google Scholar]
- 32. van Nielen M, Feskens EJM, Rietman A, Siebelink E, Mensink M.. Partly replacing meat protein with soy protein alters insulin resistance and blood lipids in postmenopausal women with abdominal obesity. J Nutr 2014;144:1423–9. [DOI] [PubMed] [Google Scholar]
- 33. Viguiliouk E, Stewart SE, Jayalath VH, Ng AP, Mirrahimi A, de Souza RJ, Hanley AJ, Bazinet RP, Mejia SB, Leiter LA, et al. Effect of replacing animal protein with plant protein on glycemic control in diabetes: a systematic review and meta-analysis of randomized controlled trials. Nutrients 2015;7:9804–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barnard ND, Cohen J, Jenkins DJ, Turner-McGrievy G, Gloede L, Green A Ferdowsian H.. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-wk clinical trial. Am J Clin Nutr 2009;89:1588S–96S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kahleova H, Matoulek M, Malinska H, Oliyarnik O, Kazdova L, Neskudia T, Skoch A, Hajek M, Hill M, Kahle M, et al. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with type 2 diabetes. Diabet Med 2011;28:549–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tinker LF, Sarto GE, Howard BV, Huang Y, Neuhouser ML, Mossavar-Rahmani Y, Beasley JM, Margolis KL, Eaton CB, Phillips LS, et al. Biomarker-calibrated dietary energy and protein intake associations with diabetes risk among postmenopausal women from the Women's Health Initiative. Am J Clin Nutr 2011;94:1600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang ET, de Koning L, Kanaya AM.. Higher protein intake is associated with diabetes risk in South Asian Indians: the Metabolic Syndrome and Atherosclerosis in South Asians Living in America (MASALA) study. J Am Coll Nutr 2010;29:130–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bendinelli B, Palli D, Masala G, Sharp SJ, Schulze MB, Guevara M, van der AD, Sera F, Amiano P, Balkau B, et al. InterAct Consortium.. Association between dietary meat consumption and incident type 2 diabetes: the EPIC-InterAct Study. Diabetologia 2013;56:47–59. [DOI] [PubMed] [Google Scholar]
- 39. Song Y, Manson JE, Buring JE, Liu S.. A prospective study of red meat consumption and type 2 diabetes in middle-aged and elderly women. Diabetes Care 2004;27:2108–15. [DOI] [PubMed] [Google Scholar]
- 40. Satija A, Bhupathiraju SN, Rimm EB, Spiegelman D, Chiuve SE, Borgi L, Willett WC, Manson JE, Sun Q, Hu FB.. Plant-based dietary patterns and incidence of type 2 diabetes in US men and women: results from three prospective cohort studies. PLoS Med 2016;13:e1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Akter S, Kurotani K, Nanri A, Pham NM, Sato M, Hayabuchi H, Mizoue T.. Dairy consumption is associated with decreased insulin resistance among the Japanese. Nutr Res 2013;33:286–92. [DOI] [PubMed] [Google Scholar]
- 42. Turner KM, Keogh JB, Clifton PM.. Red meat, dairy and insulin sensitivity: a randomized crossover intervention study. Am J Clin Nutr 2015;101:1173–9. [DOI] [PubMed] [Google Scholar]
- 43. Navas-Carretero S, Pérez-Granados AM, Schoppen S, Vaquero MP.. An oily fish diet increases insulin sensitivity compared to a red meat diet in young iron deficient women. Br J Nutr 2009;102:546–53. [DOI] [PubMed] [Google Scholar]
- 44. Fung TT, Hu FB, Pereira MA, Liu S, Stampfer MJ, Colditz GA, Willett WC.. Whole-grain intake and the risk of type 2 diabetes: a prospective study in men. Am J Clin Nutr 2002;76:535–40. [DOI] [PubMed] [Google Scholar]
- 45. Villegas R, Gao YT, Yang G, Li HL, Elasy TA, Zheng W, Shu XO.. Legumes and soy food intake and the incidence of type 2 diabetes in the Shanghai Women's Health Study. Am J Clin Nutr 2008;87:162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pan A, Sun Q, Manson JE, Willett WC, Hu FB.. Walnut consumption is associated with lower risk of type 2 diabetes in women. J Nutr 2013;143:512–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Abumrad NN, Robinson RP, Gooch BR, Lacy WW.. The effect of leucine infusion on substrate flux across the human forearm. J Surg Res 1982;32:453–63. [DOI] [PubMed] [Google Scholar]
- 48. Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011;17:448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rietman A, Schwarz J, Tomé D, Kok FJ, Mensink M.. High dietary protein intake, reducing or eliciting insulin resistance? Eur J Clin Nutr 2014;68:973–9. [DOI] [PubMed] [Google Scholar]
- 50. Floegel A, Stefan N, Yu Z, Mühlenbruch K, Drogan D, Joost HG, Fritsche A, Häring HU, Hrabě de Angelis M, Peters A, et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomics approach. Diabetes 2013;62:639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mahendran Y, Jonsson A, Have CT, Allin KH, Witte DR, Jørgensen ME, Grarup N, Pedersen O, Kilpeläinen TO, Hansen T.. Genetic evidence of a causal effect of insulin resistance on branched-chain amino acid levels. Diabetologia 2017 Feb 10 (Epub ahead of print; DOI: 10.1007/s00125-017-4222-6). [DOI] [PubMed] [Google Scholar]
- 52. Yoon M-S.. The emerging role of branched-chain amino acids in insulin resistance and metabolism. Nutrients 2016;8:405–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Block G.. A review of validations of dietary assessment methods. Am J Epidemiol 1982;115:492–505. [DOI] [PubMed] [Google Scholar]


