Abstract
Background: In Canada, pregnant women are typically referred to Canada's Food Guide (CFG), a set of national dietary recommendations designed to promote adequate nutrient intake. Pregnant women are also advised to gain weight within the Institute of Medicine guidelines, which differ by prepregnancy body mass index (BMI). However, CFG recommendations do not account for prepregnancy BMI and provide no guidance on “less healthy” (LH) foods.
Objective: The aim of this study was to score women's diets according to adherence to CFG recommendations and consumption of LH foods and to examine differences between these diet scores by prepregnancy BMI.
Methods: Participants enrolled in the APrON (Alberta Pregnancy Outcomes and Nutrition) prospective cohort study completed a 24-h recall in their second trimester (n = 1630). A score was created on the basis of each daily dietary CFG recommendation met, ranging from 0 to 9. The distribution of consumption (grams per day) of 8 LH food groups was given a score of 0 (none) or 1, 2, or 3 (representing the lowest, middle, or highest tertiles, respectively) and summed giving a total LH score of 0–24.
Results: There were few differences in CFG recommendations met by prepregnancy BMI status, although fewer women who were overweight or obese prepregnancy met the specific recommendation to consume 7–8 servings of fruit or vegetables/d than did those who were under- or normal weight (47% and 41% compared with 50% and 54%, respectively). Although differences were small, women who were obese prepregnancy had lower CFG scores (β = −0.28; 95% CI:−0.53, −0.02) and higher LH scores (β = 0.45; 95% CI: 0.04, 0.86) than did those who were normal weight.
Conclusion: The study results suggest that more attention may need to be paid to individualized counseling on dietary recommendations that take account of prepregnancy BMI.
Keywords: Canada's Food Guide, pregnancy, prospective cohort, nutritional epidemiology, dietary guidelines, food policy, public health
Introduction
Optimal nutrition in pregnancy is vital for both limiting the risk of pregnancy complications for the mother and supporting the growth and development of the fetus (1, 2). To address this issue, many countries have developed specific national guidelines for diet during pregnancy (3–5). In Canada, pregnant women are typically referred to Canada's Food Guide (CFG), which makes recommendations about the number of daily servings of food groups, with the overall goal of promoting the consumption of adequate amounts of nutrients and calories needed to support a healthy pregnancy (4). Specifically, CFG recommends that pregnant women in the second trimester onward consume an extra 2–3 servings/d from any of the following food groups: fruit and vegetables, grains, meats and alternatives, or dairy and alternatives, in addition to the dietary recommendations for nonpregnant women. CFG offers little directed guidance on the consumption of less-healthy (LH) foods, defined in CFG as “foods and beverages high in calories, fat, sugar or salt” other than stating that these foods should be “limited” (4).
In addition to providing adequate micronutrients, Health Canada also recommends following CFG in order to achieve healthy gestational weight gain (GWG) (6). GWG outside of recommended target ranges is associated with adverse outcomes for both the mother and infant during pregnancy and the postpartum periods (7–11). Recent studies in North America and Europe have shown that excessive GWG is more common than inadequate weight gain (7, 12). As a strategy to improve pregnancy outcomes for the mother and offspring, Health Canada adopted and disseminated the 2009 US Institute of Medicine (IOM) recommendations for GWG in 2010 (6). These evidence-based recommendations provide optimal ranges of total and weekly rates of GWG according to a woman's prepregnancy BMI category. Total GWG recommendations are as follows: 12–18 kg for underweight, 11.5–16 kg for normal weight, 7–11.5 kg for overweight, and 5–9 kg for obese women.
Promoting adherence to dietary guidelines in pregnant women with no known complications is challenging at a clinical level, because clinicians must help women balance their need for additional calories and specific nutrients with overall pregnancy weight gain. In addition, although the IOM GWG guidelines (6) differ according to prepregnancy BMI, in Canada the CFG recommendations are universal, with a tacit assumption that all women need to increase their food intake to meet the metabolic demands of pregnancy and fetal development from the second trimester onward. If women are expected to gain different amounts of weight during pregnancy, it would be necessary for their food intake to also differ according to their prepregnancy BMI status (13).
To the best of our knowledge, there are no published studies that have explored women's adherence to CFG recommendations during pregnancy, and whether adherence differs by prepregnancy BMI status. To address this gap, we examined data from a prospective cohort of pregnant women in Alberta, Canada (14), to 1) create a simple score to examine their diets according to adherence to CFG recommendations during the second trimester of pregnancy and to create a second score to account for consumption of LH foods, 2) examine factors associated with both CFG adherence and the consumption of LH foods, and 3) examine these diet scores according to prepregnancy BMI status.
Methods
Study design and population
The Alberta Pregnancy Outcomes and Nutrition (APrON) study is a prospective cohort study that followed women during pregnancy and postpartum and their infants, with an overarching goal of assessing the impacts of nutrition on pregnancy-related outcomes. Participants were recruited through advertisements in the media and in physicians' offices in Calgary and Edmonton, Alberta, between May 2009 and November 2012. Women aged ≥16 y and at <27 wk of gestation were eligible for inclusion. Women were excluded if they were unable to answer questions in English or if they planned to move out of the region during the timeline of the study. Women who provided written informed consent were invited to attend a study center once in each trimester after enrollment and once postpartum at ∼3 mo after delivery. More detailed descriptions of participant recruitment and study protocols have been published elsewhere (14). Research ethics board approval for the APrON study was obtained from the Health Research Ethics Boards at the University of Alberta and the University of Calgary.
Procedure
Upon enrollment, participants completed questionnaires including information on prepregnancy weight, age, parity, marital status, ethnicity, family income, and education. Questions on demographic characteristics were adapted from the 2004 Canadian Community Health Survey (15). At each study visit, trained staff measured participants' weight to the nearest 0.01 kg (Healthometer Professional 752KL) and height to the nearest 0.001 m (Charder HM200P Portstad Portable Stadiometer). Prepregnancy BMI was calculated by using self-reported prepregnancy weight and measured height. BMI (in kg/m2) values were classified as underweight (<18.5), normal weight (18.5 to <25), overweight (25 to <30), or obese (≥30). The self-reported highest weight during pregnancy was recorded at the postpartum visit to calculate total GWG.
Dietary assessment
For the purpose of this study, only dietary data collected in the second trimester were used, because the CFG pregnancy-specific recommendations are relevant from the second trimester onward. Each woman completed one 24-h recall in her second trimester. During the recall, women were asked to report every food and beverage that they had consumed during the previous day, including details concerning cooking methods, location and time of eating, and food brand names. To prompt accurate recall, the multi-pass method was used. Portion sizes were estimated with the aid of pictures. Information from the recalls was converted to intakes of nutrients and foods as grams per day.
Calculating CFG scores
Intakes of the foods included in each of the recommendations were summed and categorized according to CFG recommendations as follows: total fruit and vegetables, green vegetables, orange vegetables, total grains, whole grains, total dairy, reduced-fat milk, and meat and alternatives. A score of 1 was given for each of the following daily recommendations that were met: 7–8 servings of fruit and vegetables, making ≥1 serving of fruit and vegetables a green vegetable and ≥1 serving of fruit and vegetables an orange vegetable, 6–7 servings of grain products, making ≥3–4 servings a whole-grain product, 2 servings of milk or alternatives, choosing reduced-fat milk, and 2 servings of meat and alternatives. Details of the scoring system are presented in Table 1. For women aged <19 y (n = 21), a score of 1 was given for the consumption of 3–4 servings of milk and alternatives, which is the recommendation for the age group of 16–18 y. An additional score of 1 was given when a woman of any age reported the consumption of an extra 2–3 servings of any food group, as per pregnancy-specific recommendations. Scores were summed to produce a total score between 0 and 9 for each woman, where 9 represented meeting all of the daily food-group recommendations for a pregnant woman from her second trimester onward. A binary approach to creating the score was adopted due to its ease of interpretation and transferability to other studies of adherence to national dietary guidelines. There were 4 dietary CFG recommendations relating to frequency of food consumption that could not be scored because we used a single 24-h recall as our dietary assessment tool, and it was not possible to quantify statements such as “often.” These included the following: “choose whole fruits and vegetables more often than juice,” “have 30–45 mL vegetable oil,” “choose meat alternatives such as beans and tofu often,” and “have 2 portions of fish per week.”
TABLE 1.
Method for calculating the CFG adherence score1
| Score | ||
|---|---|---|
| CFG recommendation | Not meeting recommendation | Meeting recommendation |
| 7–8 servings fruit and vegetables/d | 0 | 1 |
| ≥1 vegetable is an orange vegetable | 0 | 1 |
| ≥1 vegetable is a dark-green vegetable | 0 | 1 |
| 6–7 servings grains/d | 0 | 1 |
| ≥3–3.5 servings of grains are whole grains | 0 | 1 |
| 2 servings milk and alternatives/d | 0 | 1 |
| Choosing reduced-fat milk | 0 | 1 |
| 2 servings meat and alternatives/d | 0 | 1 |
| Include 2–3 extra servings from any food group | 0 | 1 |
The total for each recommendation was summed to give an overall score that directly relates to the number of CFG recommendations being met. A score of 0 represents none of the recommendations being met, a score of 1 represents meeting one of the recommendations, and a score of 9 represents meeting all 9 of the quantifiable recommendations. CFG, Canada's Food Guide.
Calculating the LH food score
CFG recommends “avoiding less-healthy food,” but given that no specific recommendations for serving sizes of LH foods exist in CFG, our procedure for scoring their consumption differed from that created for the CFG score. To classify LH foods, we grouped foods from the 24-h recall into the following groups: high-energy soft drinks, chips, fries, puddings and desserts, candy and chocolate, cakes and cookies, pancakes and waffles, and hot chocolate. These food groups were chosen because they have previously been identified as energy-dense, nutrient-poor foods (16). Distributions of intakes (grams per day ) for each of the 8 food groups were divided into tertiles. For each of the 8 LH food groups, a score of 0 was given for no consumption, a score of 1 was given if women were in the lowest third for consumption, a score of 2 if in the middle third for consumption, and a score of 3 if in the highest third for consumption. Scores from the 8 LH food groups were summed to give an overall LH diet score of between 0 and 24, where a score of 0 represented the intake reported by a woman who did not consume any foods from the 8 LH food groups and a score of 24 represented the intake of a woman who was in the highest tertile for the consumption of all 8 of the LH food groups.
GWG
Total GWG was calculated by subtracting prepregnancy body weight from the highest body weight during pregnancy (both measures were self-reported). In cases in which participants did not report their highest body weight, we calculated total GWG by using the highest measured weight in the third trimester (n = 248; 12%).
Statistical analysis
For adherence to IOM GWG guidelines, within each prepregnancy BMI group, women were categorized as “below” if they gained less than the lower limit of the recommended amount of total weight, “met” if they gained within the recommended weight range, or “above” if they exceeded the upper limit of the recommended amount of weight gain. Mean ± SD CFG and LH scores were evaluated by prepregnancy BMI status. Proportions of women who consumed the recommended number of servings of each food group included in CFG stratified by prepregnancy BMI were calculated. Proportions of women meeting any of the 9 dietary recommendations, stratified by prepregnancy BMI, were calculated. Linear regression models were used to assess characteristics associated with both CFG and LH scores. Models were mutually adjusted for the other exposures of interest, and P < 0.05 was considered significant. All of the analyses were carried out by using Stata version 14.0 (StataCorp) (17).
Results
A total of 2189 women were recruited to the APrON study. For the purposes of this study, we included 1630 women with term, singleton pregnancies who had complete dietary, demographic, and prepregnancy weight data. Baseline characteristics stratified by prepregnancy BMI are presented in Table 2. The majority of women had attained a university-level education (71%), were married or cohabiting (96%), and had a yearly household income of >$100,000 (58%). Just over half of the women were pregnant with their first child (56%) and very few smoked during pregnancy (1%). In total, 26%, 40%, 71%, and 66% of women who were underweight, normal weight, overweight, and obese prepregnancy exceeded GWG guidelines, respectively.
TABLE 2.
Characteristics of participants with complete diet, weight, and demographic data by prepregnancy BMI1
| Characteristics | All (n = 1630) | Underweight (n = 56) | Normal weight (n = 1056) | Overweight (n = 343) | Obese (n = 175) |
|---|---|---|---|---|---|
| Maternal age at enrollment, y | 31.3 ± 4.3 | 28.8 ± 4.2 | 31.3 ± 4.3 | 31.8 ± 4.4 | 31.2 ± 4.3 |
| Maternal education, n (%) | |||||
| Less than university | 477 (29) | 20 (36) | 264 (25) | 117 (34) | 76 (43) |
| University or higher | 1153 (71) | 36 (64) | 792 (75) | 226 (66) | 99 (57) |
| Marital status, n (%) | |||||
| Married/cohabiting | 1565 (96) | 51 (91) | 1015 (96) | 333 (97) | 166 (95) |
| Single/divorced/separated | 64 (4) | 5 (9) | 40 (4) | 10 (3) | 9 (5) |
| Annual household income,2n (%) | |||||
| <$39,999 | 117 (7) | 9 (16) | 73 (7) | 21 (6) | 14 (8) |
| $40,000–$69,999 | 212 (13) | 9 (16) | 124 (12) | 49 (14) | 30 (17) |
| $70,000–$99,999 | 354 (22) | 10 (18) | 222 (21) | 75 (22) | 47 (27) |
| >$100,000 | 930 (58) | 26 (46) | 624 (59) | 196 (57) | 84 (48) |
| Ethnicity, n (%) | |||||
| White | 1334 (82) | 39 (70) | 848 (80) | 296 (86) | 151 (86) |
| Nonwhite | 293 (18) | 17 (30) | 207 (20) | 45 (14) | 25 (14) |
| Smoking in pregnancy, n (%) | |||||
| Yes | 22 (1) | 2 (4) | 11 (1) | 3 (1) | 6 (3) |
| No | 1608 (99) | 54 (96) | 1044 (99) | 339 (99) | 169 (97) |
| Parity, n (%) | |||||
| Nulliparous | 909 (56) | 31 (55) | 614 (58) | 171 (50) | 93 (53) |
| Multiparous | 721 (44) | 25 (45) | 442 (42) | 172 (50) | 82 (47) |
| GWG guidelines, n (%) | |||||
| Below guidelines | 310 (19) | 14 (25) | 247 (23) | 23 (7) | 26 (15) |
| Met guidelines | 529 (32) | 26 (46) | 392 (37) | 78 (23) | 33 (19) |
| Exceeded guidelines | 791 (49) | 16 (29) | 417 (40) | 242 (70) | 116 (66) |
| CFG score | 3.2 ± 1.6 | 3.1 ± 1.7 | 3.3 ± 1.6 | 3.1 ± 1.5 | 2.9 ± 1.6 |
| LH score | 3.4 ± 2.6 | 3.2 ± 2.6 | 3.3 ± 2.5 | 3.4 ± 2.7 | 3.7 ± 2.5 |
Values are means ± SDs unless otherwise indicated. CFG, Canada's Food Guide; GWG, gestational weight gain; LH, less healthy.
Currency is in Canadian dollars.
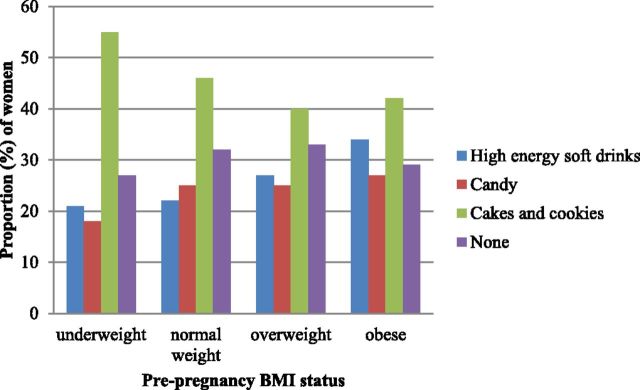
In this group of pregnant women, CFG scores ranged from 0 to 8, with a mean ± SD score of 3.2 ± 1.6, indicating that few women came close to meeting CFG daily dietary recommendations. Women with a normal prepregnancy BMI had marginally higher scores than did women in other BMI groups, with those who were obese having the lowest scores (3.3 compared with 3.1, 3.1, and 2.9 for normal weight compared with underweight, overweight, and obese, respectively). In addition, the range of LH scores was 0–19, indicating that no woman was in the top third for consumption for all 8 of the LH food groups. Mean ± SD LH score in the whole cohort was 3.4 ± 2.5, and mean LH scores by prepregnancy BMI were 3.2, 3.3, 3.4, and 3.7 for underweight, normal-weight, overweight, and obese women, respectively. The most commonly consumed LH food group was “cakes and cookies,” which were reported to have been consumed by 45% of women, followed by the food groups “high-energy soft drinks” and “candy,” with 25% of the cohort reporting the consumption of foods from each of these groups. Figure 1 shows the reported consumption of the most commonly consumed food groups included in the LH score by prepregnancy BMI. More than one-third (34%) of women who were obese prepregnancy reported the consumption of high-energy soft drinks compared with 27%, 22%, and 21% of women who were overweight, normal weight, and underweight, respectively. In addition, the greatest proportion of women in each BMI group reported the consumption of foods from the “cakes and cookies” group: 55%, 46%, 40%, and 42% of women who were underweight, normal weight, overweight, and obese, respectively.
FIGURE 1.
Proportion of women who consume the 3 most commonly consumed food groups and women who consume none of the food groups, included in the less-healthy score, by prepregnancy BMI status.
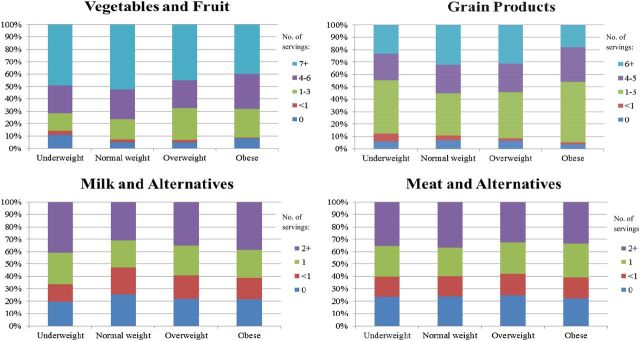
Patterns of the proportions of women who consumed numbers of servings of each food group included in CFG were largely similar across prepregnancy BMI groups (Figure 2). However, 13% and 9% of women in the underweight and obese groups, respectively, reported not consuming any fruit or vegetables compared with 5% of both normal-weight and overweight women. The proportions of women who met each specific CFG recommendation, stratified by prepregnancy BMI, are shown in Table 3. Just over half of the women (56%) met the recommendation to choose reduced-fat milk, whereas <1% of women reported meeting the pregnancy-specific recommendation of consuming an extra 2–3 servings from any food group/d. Further analyses showed that <1% of women consumed 1 extra serving from any food group, whereas none reported the consumption of 4 extra servings, indicating that women were not close to meeting the pregnancy-specific recommendation (data not shown). Compared with women in the normal-weight prepregnancy group, fewer women who were overweight or obese met the recommendations for fruit and vegetable consumption (54% compared with 47% and 41% for overweight and obese women, respectively). In addition, fewer women who were obese met the recommendation for the consumption of 6–7 portions of grains/d than did those who were normal weight (18% compared with 33% for obese and normal-weight women, respectively).
FIGURE 2.
Proportion of women who consume the numbers of servings per day by each food group in Canada's Food Guide, stratified by prepregnancy BMI. Canada's Food Guide recommendations for daily servings of these food groups are as follows: fruit and vegetables, 7–8 servings; grains, 6–7 servings; dairy and alternatives, 2 servings; and meat and alternatives, 2 servings.
TABLE 3.
Proportion of women meeting each of the 9 daily dietary recommendations outlined in CFG, stratified by prepregnancy BMI1
| CFG recommendation | All, n (%) | Underweight, n (%) | Normal weight, n (%) | Overweight, n (%) | Obese, n (%) |
|---|---|---|---|---|---|
| 7–8 servings fruit and vegetables/d | 827 (51) | 28 (50) | 566 (54) | 161 (47) | 72 (41) |
| ≥1 vegetable is an orange vegetable | 544 (33) | 19 (34) | 366 (35) | 107 (31) | 52 (30) |
| ≥1 vegetable is a dark-green vegetable | 625 (38) | 16 (29) | 421 (40) | 125 (36) | 63 (36) |
| 6–7 servings grains/d | 509 (31) | 14 (25) | 353 (33) | 110 (32) | 32 (18) |
| ≥3–3.5 servings of grains are whole grains | 483 (30) | 18 (32) | 317 (30) | 96 (28) | 52 (30) |
| 2 servings milk and alternatives/d2 | 673 (41) | 28 (50) | 419 (40) | 146 (43) | 80 (46) |
| Choosing reduced-fat milk | 917 (56) | 31 (55) | 598 (57) | 193 (56) | 95 (54) |
| 2 servings meat and alternatives/d | 698 (43) | 24 (43) | 467 (44) | 136 (40) | 71 (41) |
| Include 2–3 extra servings from any food group | 8 (0.5) | 0 | 6 (0.6) | 0 | 2 (1) |
CFG, Canada's Food Guide.
For women aged <19 y the recommendation is to consume 3–4 servings of milk and alternatives/d and was accounted for in this analysis.
Multivariate regression analyses showed significant associations between several participant characteristics with CFG score and LH score (Table 4). The 2 diet scores were inversely associated with each other, such that each additional 1-point increase in CFG score was associated with a −0.04-point decrease in LH diet score (95% CI: −0.07, −0.01 points). In addition, women with higher levels of education and higher incomes were more likely to have higher CFG scores. Notably, those who were obese were more likely to have lower CFG scores than those who were normal-weight prepregnancy. Conversely, women with higher incomes and those who were younger were more likely to have higher LH diet scores. Furthermore, women who were obese prepregnancy were more likely to have higher LH scores than those who were normal weight (β = 0.45; 95% CI: 0.04, 0.86). Models were adjusted for the presence of diabetes (n = 52), and no associations between diabetes and either of the diet scores were observed.
TABLE 4.
Characteristics associated with CFG compliance score and LH diet scores1
| Outcome | ||
|---|---|---|
| Characteristic | Model 1: CFG compliance score | Model 2: LH score |
| CFG compliance score | — | −0.10 (−0.18, −0.03)* |
| LH score | −0.04 (−0.07, −0.01)* | — |
| Maternal age | 0.01 (−0.01, 0.03) | −0.04 (−0.07, −0.01)* |
| Maternal education2 | 0.18 (0.09, 0.27)* | −0.03 (−0.18, 0.11)* |
| Household income3 | 0.10 (0.02, 0.17)* | 0.20 (0.07, 0.33) |
| Prepregnancy BMI4 | ||
| Underweight | 0.04 (−0.40, 0.48) | −0.11 (−0.81, 0.60) |
| Overweight | −0.18 (−0.37, 0.02) | 0.10 (−0.21, 0.42) |
| Obese | −0.28 (−0.53, −0.02)* | 0.45 (0.04, 0.86)* |
| Smoking in pregnancy | −0.16 (−0.84, 0.53) | 0.50 (−0.61, 1.6) |
| Parity5 | −0.01 (−0.12, 0.11) | 0.06 (−0.12, 0.24) |
Values are β coefficients (95% CIs). The table shows 2 separate multiple linear regression models with CFG score and LH score as the dependent variables. Models were adjusted for all of the listed variables and for the presence of diabetes. Total R2 values for models 1 and 2 were 0.03 and 0.02, respectively. *P < 0.05. CFG, Canada's Food Guide; LH, less healthy.
Maternal education: 1 = less than high school, 2 = high school or higher, 3 = less than university, 4 = university or higher.
Household income (annual, in Canadian dollars): 1 = <$39,999, 2 = $40,000–$69,999, 3 = $70,000–$99,999, 4 = >$100,000.
Normal weight was the reference category.
Parity: 0 = nulliparous, 1 = 1 child, 2 = 2 children, 3 = 3 children, 4 = 4 children.
Discussion
Summary of the findings
In this cohort of well-educated, high-income pregnant women in Alberta, we found that self-reported adherence to CFG recommendations during the second trimester of pregnancy was low. Women with better adherence to CFG, as represented by higher CFG scores, tended to report a lower consumption of LH foods. Women who had higher levels of education and income tended to have higher CFG scores, and higher LH diet scores were associated with being younger and having a higher income. The impact of prepregnancy BMI status on specific CFG recommendations was shown by a trend observed in mean CFG and LH scores, in that women who were obese prepregnancy had lower CFG scores and higher LH scores than did those who were normal weight.
Interpretation
Despite pregnancy tending to be a time of heightened motivation in terms of following health recommendations (18), many women in this cohort were not following the national daily dietary recommendations provided in CFG. A lack of adherence to the nutrition guidelines in the nonpregnant general Canadian population has been shown in other studies. A review of diet quality of >33,000 nonpregnant Canadians aged >2 y (19), in which the US Healthy Eating Index was adapted to score diets according to CFG, reported that the majority (82.9%) of Canadians had a diet that “required improvement.” Similarly, in an older study in 1543 adults and adolescents in which diet was assessed by using 24-h recall and intakes compared with the 1992 CFG, adherence to recommendations was relatively low (20). This older study also reported that in nonpregnant women, of those aged 18–49 y, 6%, 4%, 29%, and 20% reported the consumption of <1 portion of fruit and vegetables, grains, dairy, and meat and alternatives, respectively. This is comparable to pregnant women in our study in which 8%, 7%, 30%, and 29% reported the consumption of <1 portion of fruit and vegetables, grain, dairy, and meat and alternatives, respectively. This is of concern because it is an indication that, despite the reformulation of CFG guidelines in 2007, dietary patterns reported by Canadian women still need attention. Drawing comparisons between women in our study and nonpregnant Canadian women is appropriate, because all but one of the recommendations included in the formulation of our score are targeted to women aged 19–50 y in general, with only one food-based recommendation being made especially for pregnant women. It was of interest that <1% of women reported the consumption of the pregnancy-specific recommendation of 2–3 extra servings from any food group/d and that further analyses showed that this was due to women consuming fewer food servings rather than too many. Studies have indicated that awareness and knowledge of national dietary guidelines vary in the population. In a survey of 1210 nonpregnant adults in Canada, 86.5% of persons reported to have a general awareness of CFG and 82.5% reported to have specific knowledge of the recommendations (21). However, the extent of the participants' specific knowledge was not explored.
There is a paucity of studies that assess adherence to national dietary guidelines in pregnancy. However, in Australia, a cross-sectional study in 388 pregnant women reported that adherence to their national dietary guidelines was generally low, which is consistent with the findings of our study (22). In that study, women completed an FFQ along with questions testing participant knowledge of the Australian Dietary Guidelines for Healthy Eating. The authors found that only ∼34% were aware of the dietary guidelines and that the majority of women were not meeting the recommendations for numbers of servings of fruit and vegetables, dairy, and breads and cereals, whereas 52% exceeded the guidelines for meat consumption. These findings were despite the fact that women also reported to be motivated and confident that they had healthy diets during pregnancy. Given the time and resources used to develop national dietary guidelines, it is of concern that apparent adherence is so low. Currently, the promotion of CFG is included in Health Canada's information for health care providers “Prenatal Nutrition Guidelines for Health Professionals: Gestational Weight Gain” (23), which recommends that practitioners direct women to follow CFG as part of their prenatal care.
We observed an inverse association between reported CFG and LH score, which suggests that those who met more CFG recommendations were not simply those who were generally eating more of all foods. Other studies of dietary patterns have consistently shown similar associations (24, 25). Two large cohort studies in women in the United Kingdom showed that, in both prepregnancy and during pregnancy, those who consumed a diet rich in fruit, vegetables, and whole grains were also less likely to consume energy-dense, nutrient-poor foods such as chips, fries, and refined grains, and vice versa. We also observed that women with higher levels of education tended to report the consumption of diets more adherent to CFG recommendations. This is consistent with the findings from other studies, which showed that higher levels of education are associated with greater awareness (7) and adherence to dietary guidelines (22).
Although we observed a significant association indicating that women who were obese prepregnancy had higher LH scores and lower CFG scores than women with normal prepregnancy BMI, the effect sizes were minimal. However, this indication that diet quality during pregnancy is lower in those who are obese prepregnancy is consistent with findings from large cohort studies, including the Framingham and NHANES studies (26, 27). It was of interest that mean CFG scores were the same in women in the underweight and overweight categories. One possible explanation is that the underweight women were not consuming enough servings to meet the CFG recommendations because, in general, they ate less, whereas the overweight women were not consuming enough servings of the foods to meet CFG recommendations but were getting more of their calories from LH foods, indicated by a difference in mean LH score.
In our study, more women who were overweight or obese exceeded GWG guidelines. In a previous study in the same cohort we also observed that the trajectories of GWG were very similar across prepregnancy BMI groups (12). In a health care system in which GWG guidelines are based on prepregnancy BMI, it may be necessary to tailor dietary guidelines as well. This conclusion is shared by the Academy of Nutrition and Dietetics (formerly the American Dietetic Association), which reported that prepregnancy BMI and rates of GWG should be taken into account when providing dietary guidance to pregnant women (13). Although more research is required to explore this hypothesis, the idea that CFG may need to be revisited is consistent with previous suggestions that the lack of guidance concerning LH foods and the high energy intake that would be experienced if the CFG recommendations were followed would promote tacit overconsumption of calories in an “obesogenic environment” (28–30).
Strengths and limitations
This study was not without limitations. The use of a single 24-h recall for dietary assessment presents challenges. Because 24-h recalls tend to underestimate habitual intakes, it is possible that more of the CFG recommendations were being met over the longer term than was captured in our scoring method. However, studies that aimed to assess the relative validity of dietary assessment tools have suggested that those with higher levels of education may report diet more accurately (31); and in our population, 77% of women had a university-level education. In addition, underreporting is often associated with social-desirability bias, with the LH food groups being underreported (32). Therefore, it is possible that any underestimation in our study would be more likely to have occurred in the LH score rather than in the CFG score. However, our analyses were carried out at a food-group level, which is likely to vary less than the reporting of individual foods. Studies of dietary patterns during pregnancy with the use of repeated dietary assessment methods have shown that women are unlikely to vary in their food choices throughout pregnancy (33–35). In addition, CFG recommendations are for daily intake; therefore, we expect that our dietary assessment was useful for ranking women according to their adherence to CFG recommendations. An additional challenge was presented by creating a scoring approach that not only accounted for adherence to CFG recommendations but also attempted to account for the consumption of LH foods. Although Health Canada has developed a tool for assessing diets reported in surveillance activities (36), a recent study concluded that it was not suitable for use in analyses that aimed to use adherence to CFG as an exposure related to a health outcome (37). Finally, reliance on self-reported prepregnancy weight may have caused some women to be misclassified in their prepregnancy BMI categories. However, in an earlier publication describing patterns of GWG in this same cohort, we showed that BMI calculated by using self-reported prepregnancy weight was a reasonable estimate (12).
A key strength of our study is that our score is simple to use and provides an assessment that reflects overall adherence, which could be used in analyses of predictors and outcomes associated with this diet pattern. Because CFG does not include quantifiable recommendations on the consumption of LH foods, a separate score was necessary to account for the consumption of these foods as well. Other strengths of our study are that, in a large, contemporary cohort, this is the first study to our knowledge that assesses adherence to CFG recommendations in pregnancy. Overall, this simple scoring system allows for easy interpretation and is likely to be transferable to other studies that assess adherence to national dietary guidelines.
Conclusions
We developed a simple score to reflect adherence to Canadian dietary guidelines and the consumption of LH foods during pregnancy. Our observations suggest that adherence to national dietary recommendations in pregnancy is low and is an important area for further research to tease out the extent to which low adherence is due to lack of awareness, or if other factors are in play. Thus, future research to better understand the barriers to and facilitators of good-quality diets during pregnancy needs to take a mixed-methods approach. Observational data need to be complemented by robust qualitative research to unravel the complexity of the influences on diet in pregnancy. In addition, future research must involve both practitioners and women in order to advance practices that better support women to have healthy diets and gain appropriate amounts of weight during pregnancy.
Acknowledgments
We thank Mette Madsen, Sarah Loehr, Dayna-Lynn Dymianiw, Anne Gilbert, Ala Qabaja, Andrea Deane, Lubna Anis, and Yan Yuan for their assistance with data collection, preparation, and database development. The authors' responsibilities were as follows—MJ: designed the study with the guidance of PJR and RCB, conducted the analyses, and wrote the manuscript with assistance from PJR and RCB; RCB: is a principal investigator of the ENRICH study and a coinvestigator of the APrON cohort; RCB and PJR: supervised MJ; KN: provided a clinical perspective and assisted in the presentation of the results and writing of the manuscript; MJ, RCB, KN, and PJR: reviewed drafts of the manuscript; and all authors: read and approved the final manuscript.
Abbreviations
- APrON
Alberta Pregnancy Outcomes and Nutrition
- CFG
Canada's Food Guide
- GWG
gestational weight gain
- IOM
Institute of Medicine
- LH
less healthy
Contributor Information
APrON and ENRICH Study Teams:
Megan Jarman, Rhonda C Bell, Kara Nerenberg, and Paula J Robson
References
- 1. Koblinsky MA. Beyond maternal mortality—magnitude, interrelationship, and consequences of women's health, pregnancy-related complications and nutritional status on pregnancy outcomes. Int J Gynaecol Obstet 1995;48:S21–32. [DOI] [PubMed] [Google Scholar]
- 2. Bateson P, Barker D, Clutton-Brock T, Deb D, D'Udine B, Foley RA, Gluckman P, Godfrey K, Kirkwood T, Lahr MM, et al. Developmental plasticity and human health. Nature 2004;430:419–21. [DOI] [PubMed] [Google Scholar]
- 3. Australian Government National Health and Medical Research Council [Internet]. Australian Dietary Guidelines for Healthy Eating. Healthy Eating During Your Pregnancy. c2013 [cited 2016 Oct 15]. Available from: https://www.nhmrc.gov.au/guidelines-publications/n55.
- 4. Health Canada. Eating well with Canada's Food Guide [Internet]. 2007. [cited 2016 Apr 7]. Available from: www.hc-sc.gc.ca/fn-an/food-guide-aliment/index_e.html.
- 5. Brown KA, Timotijevic L, Barnett J, Shepherd R, Lahteenmaki L, Raats MM.. A review of consumer awareness, understanding and use of food-based dietary guidelines. Br J Nutr 2011;106:15–26. [DOI] [PubMed] [Google Scholar]
- 6. Rasmussen KM, Yaktine AL. editors. Weight gain during pregnancy: reexamining the guidelines. Washington (DC): National Academies Press; 2009. [PubMed] [Google Scholar]
- 7. Davis RR, Hofferth SL, Shenassa ED.. Gestational weight gain and risk of infant death in the United States. Am J Public Health 2014;104:S90–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stotland NE, Cheng YW, Hopkins LM, Caughey AB.. Gestational weight gain and adverse neonatal outcome among term infants. Obstet Gynecol 2006;108:635–43. [DOI] [PubMed] [Google Scholar]
- 9. Nehring I, Schmoll S, Beyerlein A, Hauner H, Von KR.. Gestational weight gain and long-term postpartum weight retention: a meta-analysis. Am J Clin Nutr 2011;94:1225–31. [DOI] [PubMed] [Google Scholar]
- 10. Johnson J, Clifton RG, Roberts JM, Myatt L, Hauth JC, Spong CY, Varner MW, Wapner RJ, Thorp JM Jr., Mercer BM, et al. Pregnancy outcomes with weight gain above or below the 2009 Institute of Medicine guidelines. Obstet Gynecol 2013;121:969–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nohr EA, Vaeth M, Baker JL, Sorensen T, Olsen J, Rasmussen KM.. Combined associations of pre-pregnancy body mass index and gestational weight gain with the outcome of pregnancy. Am J Clin Nutr 2008;87:1750–9. [DOI] [PubMed] [Google Scholar]
- 12. Jarman M, Yuan Y, Pakseresht M, Shi Q, Robson PJ, Bell RC.. Gestational weight gain is often excessive and early in pregnancy. CMAJ Open 2016;4:338–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaiser L, Allen LH.. Position of the American Dietetic Association: nutrition and lifestyle for a healthy pregnancy outcome. J Am Diet Assoc 2008;108:553–61. [DOI] [PubMed] [Google Scholar]
- 14. Kaplan BJ, Giesbrecht GF, Leung BM, Field CJ, Dewey D, Bell RC.. The Alberta Pregnancy Outcomes and Nutrition (APrON) cohort study: rationale and methods. Matern Child Nutr 2014;10:44–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Health Canada. Canadian Community Health Survey cycle 2.2 (2004): a guide to accessing and interpreting the data [Internet]. 2006. [cited 2017 May 1]. Available from: http://www.hc-sc.gc.ca/fn-an/surveill/nutrition/commun/cchs_guide_escc-eng.php#a1.2.17.
- 16. Maillot M, Darmon N, Darmon M, Lafay L, Drewnowski A.. Nutrient-dense food groups have high energy costs: an econometric approach to nutrient profiling. J Nutr 2007;137:1815–20. [DOI] [PubMed] [Google Scholar]
- 17. StataCorp. Stata statistical software: release 14. College Station (TX): StataCorp LP; 2015. [Google Scholar]
- 18. Hall M, Macintyre S, Porter M.. Antenatal care assessed: a case study of innovation in Aberdeen. Aberdeen (Scotland): Aberdeen University Press; 1985. [Google Scholar]
- 19. Garriguet D. Diet quality in Canada. Health Rep 2009;20:41–52. [PubMed] [Google Scholar]
- 20. Starkey LJ, Johnson-Down L, Gray-Donald K.. Food habits of Canadians: comparison of intakes in adults and adolescents to Canada's Food Guide to healthy eating. Can J Diet Pract Res 2001;62:61–9. [PubMed] [Google Scholar]
- 21. Mathe N, Van der Meer L, Agborsangaya CB, Murray T, Storey K, Johnson JA, Loitz CC, Johnson ST.. Prompted awareness and use of eating well with Canada's Food Guide: a population-based study. J Hum Nutr Diet 2015;28:64–71. [DOI] [PubMed] [Google Scholar]
- 22. Bookari K, Yeatman H, Williamson M.. Falling short of dietary guidelines—what do Australian pregnant women really know? A cross sectional study. Women Birth 2017;30:9–17. [DOI] [PubMed] [Google Scholar]
- 23. Health Canada. Prenatal nutrition guidelines for health professionals: gestational weight gain [Internet]. 2010. [cited 2016 May 1]. Available from: www.hc-sc.gc.ca/fn-an/nutrition/prenatal/ewba-mbsa-eng.php#a2.
- 24. Northstone K, Emmett P, Rogers I.. Dietary patterns in pregnancy and associations with socio-demographic and lifestyle factors. Eur J Clin Nutr 2008;62:471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Crozier SR, Robinson SM, Borland SE, Inskip HM.. Dietary patterns in the Southampton Women's Survey. Eur J Clin Nutr 2006;60:1391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wolongevicz DM, Zhu L, Pencina MJ, Kiokoti RW, Newby PK, D'Agostino RB, Millen BE.. Diet quality and obesity in women: the Framingham nutrition study. Br J Nutr 2010;103:1223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shin D, Lee KW, Song WO.. Pre-pregnancy weight status is associated with diet quality and nutritional biomarkers during pregnancy. Nutrients 2016;8:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kondro W. Proposed Canada Food Guide called ‘obesogenic’. CMAJ 2006;174:605–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Standing Senate Committee on Social Affairs. Obesity in Canada: a whole of society approach. Ottowa (Canada): The Science and Technology Senate; 2016. [Google Scholar]
- 30. Jessri M, L'Abbe MR.. The time for an updated Canadian food guide has arrived. Appl Physiol Nutr Metab 2015;40:854–7. [DOI] [PubMed] [Google Scholar]
- 31. Klesges RC, Eck LH, Ray JW.. Who underreports dietary intake in a dietary recall? Evidence from the Second National Health and Nutrition Examination Survey. J Consult Clin Psychol 1995;63:438–44. [DOI] [PubMed] [Google Scholar]
- 32. Johansson G, Wikman A, Ahren AM, Hallmans G, Johansson I.. Underreporting of energy intake in repeated 24-hour recalls related to gender, age, weight status, day of interview, educational level, reported food intake, smoking habits and area of living. Public Health Nutr 2001;4:919–27. [DOI] [PubMed] [Google Scholar]
- 33. McGowan CA, McAuliffe FM.. Maternal dietary patterns and associated nutrient intakes during each trimester of pregnancy. Public Health Nutr 2013;16:97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Crozier SR, Robinson SM, Godfrey KM, Cooper C, Inskip HM.. Women's dietary patterns change little from before to during pregnancy. J Nutr 2009;139:1956–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sotres-Alvarez D, Herring AH, Siega-Riz AM.. Latent transition models to study women's changing of dietary patterns from pregnancy to 1 year postpartum. Am J Epidemiol 2013;177:852–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Health Canada. The development and use of a surveillance tool: the classification of foods in the Canadian nutrient file according to eating well with Canada’s Food Guide. Ottawa (Canada): Government of Canada; 2014. [Google Scholar]
- 37. Jessri M, Nishi SK, L'Abbe MR.. Assessing the nutritional quality of diets of Canadian adults using the 2014 Health Canada surveillance tool tier system. Nutrients 2015;7:10447–68. [DOI] [PMC free article] [PubMed] [Google Scholar]