Abstract
Background: Observational studies and crossover feeding studies suggest that moderate alcohol use may benefit cardiovascular risk, but we know of no long-term randomized trials that have tested this hypothesis.
Objective: We evaluated the feasibility of an efficacy study of daily ethanol use in a 6-mo randomized pilot study in adults at higher cardiovascular risk.
Methods: In a double-blind, randomized, controlled parallel-design trial, we screened 67 adults aged ≥55 y and randomly assigned 45 participants to consume 150 mL of an artificially sweetened beverage with or without 10% grain alcohol daily for 6 mo. Participants were asked to consume no other alcohol and returned monthly to receive the beverage and undergo measurement of HDL cholesterol, liver function tests, and complete blood counts.
Results: Of the 45 randomly assigned participants, 39 completed the trial; the primary reason cited for attrition was inconvenience. None of the participants reported problem drinking or developed any serious adverse events or abnormal biochemical findings. However, we observed no differences in concentrations of HDL cholesterol, HDL lipoprotein subclasses, aspartate aminotransferase, alanine aminotransferase, γ-glutamyltransferase, mean corpuscular volume, or adiponectin between the alcohol and control arms, suggesting that adherence was poor. Every participant accurately identified their assigned beverage, most with great certainty.
Conclusions: In this parallel-design pilot study of daily alcohol use, we observed none of the expected changes in markers of alcohol intake, which suggests poor adherence to this pure alcohol intervention. Our results suggest that long-term trials of alcohol consumption, if they are conducted in light drinkers similar to these, must use pragmatic designs for maximal feasibility. This study was registered at clinicaltrials.gov as NCT01377727.
Keywords: randomized controlled trial, protocol compliance, alcohol consumption, pilot study, biomarkers
Introduction
Substantial epidemiologic evidence suggests that alcohol use within recommended limits of intake is associated with a lower risk of cardiovascular disease and diabetes (1, 2). In addition to observational studies, crossover feeding studies, typically of a few weeks' duration, have identified plausible pathways linking alcohol consumption to lower risk, including higher concentrations of HDL cholesterol and adiponectin and lower concentrations of fibrinogen (3).
However, this body of evidence is not without important limitations. Observational studies of drinking are prone to confounding due to ill health, socioeconomic status, and social integration, which may be difficult to control with standard epidemiologic techniques (4). Studies that used genetic polymorphisms in alcohol-metabolism pathways as instruments or proxies for alcohol intake have reached inconsistent conclusions (5, 6), as have animal studies (7, 8). Feeding studies, although free of confounding, have commonly been a few weeks in duration and thus only able to address short-term changes in biomarkers, not coronary risk per se.
Given these limitations, a strong interest in long-term randomized trials of alcohol has developed (9), although their feasibility remains uncertain. The USDA has conducted feeding studies of several months' duration (10, 11), but these provided complete diets to participants and hence are of limited generalizability to the type of large, free-living population necessary for a randomized trial. Parallel-design trials in Italy and Israel administered wine to diabetic participants over 12–24 mo, with clear benefits on glucose metabolism, but control beverages were either not provided or did not contain comparable polyphenols, making it impossible to determine if the observed effects were due to ethanol or the antioxidants in wine (12, 13). To address the feasibility of a long-term efficacy study of daily ethanol intake, we conducted the Alcohol and Atherosclerosis Pilot Study, a 6-mo pilot study in community-dwelling adults at higher cardiovascular risk, in which participants received a control beverage with or without the addition of pure ethanol.
Methods
Participants
We recruited free-living adults aged ≥55 y in the Boston, Massachusetts, area from December 2008 to March 2011 to participate in this parallel-design, double-blind randomized controlled trial. Inclusion criteria included the presence of either diabetes or 2 other cardiovascular risk factors, including hypertension, smoking, family history of premature heart disease, BMI (in kg/m2) ≥30, or waist circumference ≥40 inches for men and ≥35 inches for women (Table 1).
TABLE 1.
Baseline characteristics of participants by intervention1
| Placebo (n = 20) | Alcohol (n = 19) | P | |
|---|---|---|---|
| Demographic characteristics | |||
| Age, y | 64 ± 7 | 65 ± 6 | 0.33 |
| Female, n (%) | 7 (35) | 7 (37) | >0.99 |
| Race, n (%) | |||
| White | 15 (75) | 16 (84) | >0.99 |
| Black | 2 (10) | 3 (16) | |
| Asian | 2 (10) | 1 (5) | |
| Other | 1 (5) | 2 (10) | |
| Marital status, n (%) | 0.12 | ||
| Married | 12 (60) | 5 (26) | |
| Widowed | 1 (5) | 1 (5) | |
| Never married | 3 (15) | 5 (26) | |
| Divorced/separated | 4 (20) | 8 (42) | |
| Employment, n (%) | 0.54 | ||
| Employed | 12 (60) | 7 (37) | |
| Not employed | 0 | 1 (5) | |
| Retired | 8 (40) | 10 (53) | |
| Disabled | 0 | 1 (5) | |
| Education, n (%) | 0.42 | ||
| High school or less | 1 (5) | 2 (11) | |
| College | 12 (60) | 7 (37) | |
| Graduate school | 7 (35) | 10 (53) | |
| Smoking status, n (%) | 0.02 | ||
| Former | 6 (30) | 8 (44)2 | |
| Current | 0 | 5 (26) | |
| Alcohol use, drinks/mo | 8.3 ± 9.8 | 5.6 ± 8.2 | 0.20 |
| BMI, kg/m2 | 28.5 ± 5.0 | 30.0 ± 5.8 | 0.75 |
| Blood pressure, mm Hg | 126 ± 16/75 ± 10 | 135 ± 21/75 ± 13 | 0.11/0.53 |
| Hypertension, n (%) | 12 (60) | 13 (68) | 0.74 |
| Diabetes, n (%) | 5 (25) | 1 (5) | 0.18 |
| Previous myocardial infarction, n (%) | 1 (5) | 0 | >0.99 |
| Previous stroke, n (%) | 1 (5) | 0 | >0.99 |
| Statin use, n (%) | 10 (50) | 6 (32) | 0.33 |
| Laboratories | |||
| White blood cells, K/µL | 6.2 ± 1.6 | 6.7 ± 1.3 | 0.75 |
| Hematocrit, % | 40.8 ± 3.0 | 41.9 ± 4.7 | 0.22 |
| Platelets, K/µL | 210 ± 54 | 236 ± 43 | 0.03 |
| HDL cholesterol, mg/dL | 52 ± 123 | 49 ± 13 | 0.19 |
| Glycated hemoglobin, % | 6.2 ± 0.6 | 5.9 ± 0.5 | 0.06 |
| AST, IU/L | 24 ± 8 | 23 ± 5 | 0.75 |
| ALT, IU/L | 23 ± 8 | 24 ± 10 | 0.75 |
| GGT, IU/L | 24 ± 10 | 27 ± 19 | 0.42 |
| Glucose, mg/dL | 103 ± 293 | 95 ± 172 | 0.33 |
| Questionnaires | |||
| Alcohol Use Disorders Identification Test, score | 2 ± 1 | 2 ± 1 | 0.26 |
| Center for Epidemiologic Studies-Depression Scale, score | 2 ± 4 | 3 ± 3 | 0.03 |
| Pittsburgh Sleep Quality Index, score | 8 ± 5 | 10 ± 7 | 0.75 |
| Yale Physical Activity Survey (total activity time), h/wk | 30 ± 22 | 27 ± 16 | >0.99 |
Values are means ± SDs unless otherwise indicated. P values were derived with Fisher's exact tests for categorical variables and with median tests for continuous variables. ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ-glutamyltransferase; K, thousands.
n = 18.
n = 19.
Exclusion criteria included the following: a history of myocardial infarction or revascularization procedure (coronary, carotid, or peripheral) within the previous 6 mo; current atrial fibrillation; any illness expected to cause death or disability within 6 mo; blood pressure ≥180/110 mm Hg; alcohol intolerance or allergy (including flushing); allergies to aspartame, acesulfame, or artificial food coloring; current depressive symptoms (based on a Center for Epidemiologic Studies–Depression Scale score of ≥16); history of any chronic liver disease or breast or any gastrointestinal cancer; end-stage kidney disease; use of metronidazole, warfarin, or any sedative or hypnotics >4 d/wk; severe psychiatric illness; current intake of ≥7 drinks/wk; previous history of alcohol abuse; intake of >4 drinks in 1 d in the past 6 mo; no alcohol consumption in the past month; inability to speak English; and lack of a working telephone. At a screening examination, we also excluded individuals with abnormalities in aspartate aminotransferase (AST), alanine aminotransferase (ALT), or γ-glutamyltransferase (GGT); a platelet count <120,000 cells/µL; a hematocrit <37% for men and <33% for women; or a glycated hemoglobin concentration >10%. To test the feasibility of repeated MRI as a measure of subclinical vascular disease, we also excluded individuals with claustrophobia, pacemakers, and intra-auricular or intracranial clips.
The Committee on Clinical Investigations at Beth Israel Deaconess Medical Center (BIDMC) in Boston, Massachusetts, approved our protocol; and all participants provided written informed consent. The clinicaltrials.gov identifier for the study is NCT01377727. The study was funded by the National Institute on Alcohol Abuse and Alcoholism, which had no role in the conduct or presentation of the study.
Study procedures
Potential participants who responded to study advertisements were first assessed for eligibility by telephone and screened for eligibility at the BIDMC Harvard Catalyst Clinical Research Center. Eligible participants entered a 2-wk run-in period during which they were asked to refrain from alcohol use. Participants subsequently returned for a baseline visit and were randomly assigned through permuted blocks to consume either placebo or 150 mL of 10% ethyl alcohol (i.e., 15 g ethanol)/d for 6 mo. Subsequent study visits occurred at 2 wk, 1 mo, and monthly thereafter until 6 mo. Participants and study staff were blinded to treatment assignment.
We provided participants with 5 L of the study beverage prepared by research pharmacists on a monthly basis. The beverage consisted of Crystal Light Lemonade or Raspberry Lemonade (Kraft Foods), with or without supplementation with 95% ethanol (Letco Medical and Spectrum Chemical) to achieve a final concentration of 10%. We also provided participants with a 200-mL standardized dosing glass and instructed them to consume 150 mL of the study beverage daily. We asked that they drink only after completing tasks requiring substantial dexterity and that they refrain from alcohol use outside of the study.
The baseline and final study visits occurred after an 8-h fast; other visits occurred nonfasting. At all study visits, we assessed participant vital signs, administered standard alcohol questionnaires [Alcohol Use Disorders Identification Test, CAGE, TWEAK (14)], and performed phlebotomy. At the baseline and 3- and 6-mo visits, we administered the Yale Physical Activity Survey, the Pittsburgh Sleep Quality Index, and the Center for Epidemiologic Studies–Depression Scale (15–17). At the baseline and final visits, we performed 12-lead electrocardiography and an electrocardiogram-gated 1.5 or 3T T2-weighted spin-echo MRI of the abdomen with contrast and measured NMR spectroscopy–based lipoprotein subclasses, total adiponectin, and glycated hemoglobin.
At the final study visit, additional questions included the self-reported average number of missed doses of the study beverage per week, the total number of alcoholic beverages consumed outside of the study beverage, the participant's level of certainty about which beverage they received (i.e., masking), and their willingness to participate in a similar study lasting 2–3 y.
Laboratory methods
At every visit, samples were immediately spun and placed into aliquots after routine phlebotomy; samples for immediate assays were centrifuged at 1300 × g for 10 min at room temperature, and those for storage at 2000 × g for 15 min at 4°C. Aliquots for all but 2 measurements were assayed immediately by the BIDMC clinical laboratory at these visits. At the baseline and 3- and 6-mo visits, additional aliquots were stored briefly at −20°C and frozen at −80°C the same day; these were subsequently assayed for adiponectin and lipoprotein subclasses after the trial, as described below.
At each visit, we measured a series of markers intended either for safety monitoring (complete blood count, ALT, and glucose) or for adherence. For adherence, our primary measure was HDL cholesterol, which has been used to validate self-reported alcohol consumption at the population level for decades (18) and which increases in a dose-dependent manner with greater alcohol consumption in meta-analyses of directly administered feeding studies (3, 19). Although more reliably considered markers of heavy alcohol consumption, we also measured GGT, AST, and mean corpuscular volume (MCV) as secondary markers of adherence that perform as well as many more novel measures (20). As tertiary markers, we measured adiponectin, which is also directly increased by alcohol consumption in multiple feeding studies (3), and lipoprotein subclasses, which have been associated with alcohol consumption in epidemiologic studies (21).
HDL cholesterol was measured by enzymatic assays on a Hitachi analyzer (Roche Diagnostics), with intra-assay CVs of 0.60–0.95%. Complete blood counts were measured by using a Sysmex X-series analyzer (Sysmex Corporation). AST, ALT, GGT, and glucose were also measured on standard Roche autoanalyzers.
Adiponectin was measured at the baseline and 3- and 6-mo visits with an ELISA (Millipore Corporation). Intra-assay CVs were 3.2–7.0%. Lipoprotein subclasses were assessed with magnetic resonance spectroscopy (LipoScience, Inc.) at baseline and at 6 mo (22). In brief, lipoprotein subparticles of different sizes emit distinct lipid methyl group signals, and the measured amplitudes of these signals are directly proportional to concentrations of lipoprotein subparticles, which are grouped into very-low-density, low-density, and high-density lipoproteins. Mean lipoprotein subparticle sizes were then calculated from the diameter of each lipoprotein subparticle and its relative concentration.
Statistical analysis
We describe demographic and clinical characteristics of participants according to intervention arm in contingency tables and test differences with Fisher's exact tests for categorical variables and with median tests for continuous variables. We a priori identified 4 commonly measured biomarkers of adherence that have been related to alcohol consumption at varying levels: HDL cholesterol, GGT, AST, and MCV (19, 23, 24). For analyses of these biomarkers, along with ALT and BMI as markers of potential adverse effects, we show means and SEs at the 8 study visits according to intervention arm. We also examined adiponectin (25) and HDL particle concentrations as exploratory markers of adherence. We tested for differences between the study arms by using generalized estimating equations and an exchangeable correlation structure; all of the models included terms for intervention arm, time, and the time-by-intervention interaction. We tested time both as a categorical and continuous variable and examined models with and without additional adjustment for variables that differed at baseline between intervention and control arms (at P < 0.10). We log-transformed liver function tests and adiponectin in these models to maximize normality.
We collected data by using the Research Electronic Data Capture platform (26) and conducted analysis with the use of SAS version 9.3 (SAS Institute, Inc.). All of the analyses were performed on an intention-to-treat basis.
Results
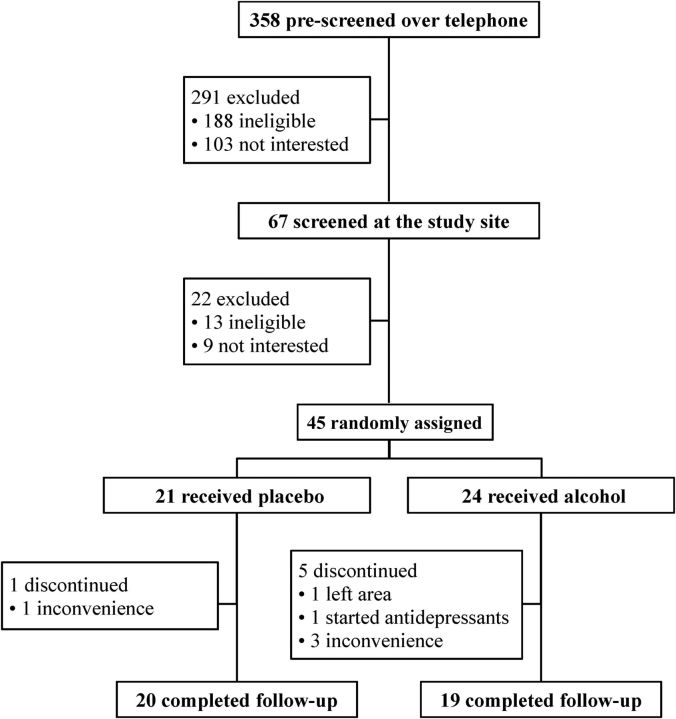
A total of 67 participants underwent a screening visit after initial telephone prescreening of 358 individuals (Figure 1). Of 291 participants excluded initially, most were ineligible because recruitment advertisements did not specify entry criteria, whereas ∼100 declined due to lack of interest. At the screening visit, an additional 22 participants were excluded with similar numbers due to lack of eligibility and lack of interest, time, or both.
FIGURE 1.
Flow of participants through the trial.
After screening, 45 participants were randomly assigned to the control or the intervention group, and 39 completed the study. Reasons for attrition were largely related to the burden and inconvenience of clinic visits (n = 4) and not to beverage side effects, with no serious adverse events. One participant assigned to alcohol discontinued due to life stressors unrelated to the study, resulting in depression, and another left the area. Minor adverse events included a case of presumed influenza in a control participant and gastrointestinal upset secondary to reflux in a participant in the intervention arm.
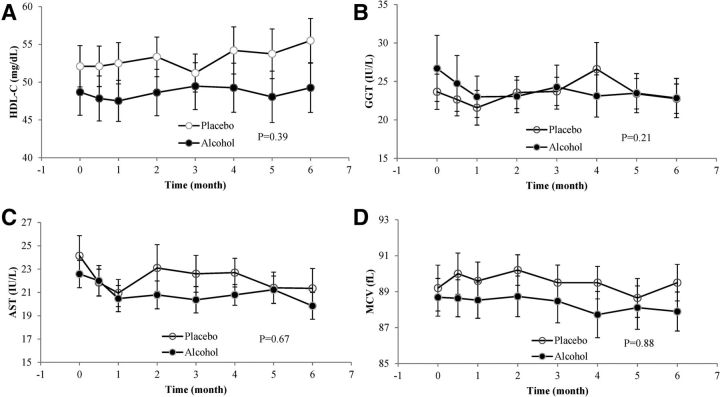
We monitored concentrations of HDL cholesterol, AST, GGT, and MCV at each study visit as plausible markers of alcohol intake. Results of these measurements are shown in Figure 2; in no case did we observe a clear difference in the trend over time between the alcohol and intervention arms, nor did any participant develop marked (>3 times the upper limit of normal) or persistent abnormalities in ALT, AST, or GGT. For HDL cholesterol, our primary marker of adherence, assignment to alcohol reduced the change over time by −0.24 ± 0.29 mg · dL−1 · mo−1 (95% CI: −0.80, 0.31 mg · dL−1 · mo−1; P = 0.39). Although several of the markers exhibited overall time trends, we identified no statistical differences between the time trends in the control and intervention arms for any of the biomarkers. We also observed no differences between the 2 arms over time in adiponectin concentrations (P = 0.99) or in concentrations of total, large, medium, or small HDL particles (data not shown).
FIGURE 2.
Biomarker concentrations of HDL-C (A), GGT (B), AST (C), and MCV (D) among participants assigned to alcohol or the control beverage at all measured time points. Values are means ± SEs. P values represent the difference in linear time trends between groups from mixed models, adjusted for baseline differences in smoking, platelet count, glycated hemoglobin, and depressive symptoms. AST, aspartate aminotransferase; GGT, γ-glutamyltransferase; HDL-C, HDL cholesterol; MCV, mean corpuscular volume.
For safety purposes, we also examined ALT concentrations and BMI at each study visit. Although ALT concentrations did not differ (P = 0.67), we observed a small difference between the 2 arms in BMI over time, with an increase from 28.5 to 28.9 in the control arm (P = 0.02) that was not present among those assigned to alcohol (P = 0.01 for time × alcohol interaction; Supplemental Figure 1).
When participants were surveyed at a final debriefing, those in the intervention arm endorsed missing a mean ± SE of only 0.4 ± 0.1 drinks/wk, although control participants reported missing only 0.1 ± 0.1 drinks/wk (P = 0.04). The 2 groups reported consuming alcohol outside of the study beverage a mean of 1.9 times anytime during the 6-mo study period, which did not vary between arms (P = 0.56). Every participant correctly identified their assigned study beverage. On a 5-point scale ranging from “very uncertain” to “very certain” about this identification, 32 of the 39 completers described themselves as very certain about the beverage.
When asked about their willingness to enter a multiyear study, 24 of 39 completers reported a willingness to do so, although this differed significantly by arm (P = 0.008); 16 of 19 participants in the alcohol arm but only 8 of 20 in the control arm would participate in a longer trial. Of the 12 individuals in the control arm unwilling to participate further, open-ended answers among 10 respondents suggested that the impact of complete alcohol abstention on lifestyle and the time commitment were the major factors that limited their future participation. All 39 participants completed technically satisfactory MRI examinations (i.e., scans that were not interrupted and that imaged the aorta without interfering artifact) at baseline; 2 participants declined follow-up examinations.
Discussion
In this 6-mo pilot randomized trial of daily consumption of a control beverage with or without added ethanol, no participant began problem drinking, reported serious adverse events, or developed abnormal biochemical findings. However, biomarker findings suggested that adherence to the alcohol intervention was poor. Every participant accurately identified his or her assigned beverage, the large majority with great certainty.
The results of this trial pose a number of important lessons and challenges for the study of moderate drinking and its role in chronic disease. First, adherence to daily drinking appeared to be poor by all biomarker criteria. We believe that it is likely that this relates to the specific beverage we used, which was designed as a vehicle for pure ethanol but not for palatability. Although this type of beverage has been used successfully in shorter, highly controlled settings like those at the USDA (10, 27), it seems doubtful that such a beverage could be used with any assurance of adherence in long-term trials. As a result, outcome trials will need to rely on more pragmatic designs that use commercial beverages, which have the particular advantage of being developed and marketed for taste and have been used in trials of several months' duration or longer in both Israel and Italy (12, 13, 28). Unfortunately, this implies that future long-term trials may be unable to test pure ethanol as an exposure, because dealcoholized beverages will inevitably differ to at least a modest extent in their nonalcoholic content from their alcoholic counterparts.
Second, our trial provided useful insight on both recruitment and attrition. Because our advertising did not specify our full entry criteria, most respondents were simply ineligible. This is likely to be true in any long-term trial of alcohol use, in which both lifelong abstainers and problem drinkers would necessarily be excluded, limiting the eligible pool to the minority of light drinkers. Furthermore, less than half of apparently eligible respondents by telephone were sufficiently enthused to attend a screening visit. Although this partly relates to the specific obstacles of this trial, including monthly visits with phlebotomy, it further highlights the challenge of identifying those light-drinking individuals who are relatively agnostic between the choices of complete abstention and daily drinking. Our results suggest that such individuals can be found, but they represent the minority of light drinkers and may find complete alcohol abstention particularly difficult to reconcile with their lifestyles. At the same time, we had relatively modest attrition among those participants who were randomly assigned, which in most cases was attributed mainly to the repeated nature of study visits and calls. Although beverage adherence appeared to be poor, this persistence among participants suggests that a pragmatic trial could well expect to run to completion if a sufficiently attractive intervention can be developed and if sufficient allowance can be made for occasional drinking at designated social events among individuals assigned to abstention.
Third, our results show both the strengths and limitations of markers of alcohol consumption. Participants in the alcohol arm were significantly more likely to report missed beverages than were those in the control arm, which is consistent with the lack of palatability noted above. However, the reported adherence in the intervention arm at the conclusion of the study was much greater than would be suggested by the biochemical markers we measured. Although these markers are, at the individual level, imperfect tests of moderate alcohol intake, they have proven to be highly successful at documenting adherence at the group level in previous trials. For example, a meta-analysis of feeding studies estimated that 15 g of alcohol consumption should increase HDL cholesterol by ∼2 mg/dL (19). In our trial, the change in HDL cholesterol over time was numerically smaller in the alcohol arm than in the placebo arm, and the upper bound of the CI for the difference in time trends between arms was statistically compatible with, at most, an increase of 1.86 mg/dL (i.e., 6 × 0.31 mg/dL). Taken together, these findings support concern for social desirability bias in reporting and suggest that great caution will be needed in ensuring a highly palatable and easily consumed beverage in any long-term trial. Although some progress has been made in finding highly specific biomarkers for even light drinking (29–31), it remains uncertain whether any biomarker will prove sufficiently sensitive, accurate, reproducible, and easily measured to be used in real time during a clinical trial to monitor participant compliance. In this regard, electronic momentary assessment may offer a useful and potentially cost-effective alternative in future studies (32).
Last, all participants correctly identified their assigned beverage, despite the fact that the beverages were identical beyond their alcohol content and all study staff were blinded to treatment assignment. Although blinding of alcoholic beverages is possible in highly controlled, brief settings, such as those used to test expectancy in understanding the behavioral effects of alcohol intake (33), our results suggest that it is implausible to expect blinding to be maintained in free-living settings with repeated exposure to the assigned beverage. As such, our trial not only suggests that tests of pure ethanol are unlikely to succeed but also that tests of commercial alcoholic beverages are unlikely to be successfully blinded to participants.
Several caveats warrant discussion. Our results, although disappointing and instructive, reflect one center's experience and may not generalize well, although the BIDMC Clinical Research Center performs trials of this type regularly (34, 35) and we know of no other US centers that have pilot-tested alcohol consumption for such a long duration. As noted above, we interpret our findings as showing a lack of adherence to the alcohol arm, and although self-reported adherence did differ significantly between arms, the absolute level of self-reported adherence was high. As such, it is possible that participants were indeed compliant but that long-term ethanol intake does not influence biomarkers in the same manner as does short-term intake (28). However, trials of even longer duration have observed expected effects on HDL cholesterol (12, 13), and we observed no effects on HDL cholesterol even at early time points. We only tested a single artificially sweetened beverage, and adherence may have been better with another control beverage with added ethanol, although none are likely to achieve better acceptance than the use of commercially available alcoholic beverages in a pragmatic trial.
In summary, in this 6-mo randomized pilot study, we identified no problems with alcohol misuse or serious adverse events in a well-screened population, but biochemical analyses suggest that adherence to the alcohol intervention was poor, and all participants correctly identified their beverage. Our experience highlights important challenges to the study of ethanol and argues for pragmatic designs that have garnered increasing interest within the biomedical community in recent years.
Acknowledgments
We acknowledge the invaluable support of Audrey Nathanson of the BIDMC Clinical Research Center and the dedication and insight of David Feillin, Meredith Regan, Richard Saitz, Howard Shaffer, and Adam Silk, the members of our data safety and monitoring board. The authors' responsibilities were as follows—KJM and MAM: designed the research; KJM and BN: conducted the research; CSM and WJM: provided essential materials; LM: analyzed the data; KJM: wrote the manuscript; KJM: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BIDMC
Beth Israel Deaconess Medical Center
- GGT
γ-glutamyltransferase
- MCV
mean corpuscular volume
Footnotes
Supported by the National Institute on Alcohol Abuse and Alcoholism (R21AA016110 and U10AA025286), the National Center for Research Resources, and the National Center for Advancing Translational Sciences. The Beth Israel Deaconess Medical Center Clinical Research Center is supported by the Harvard Catalyst/The Harvard Clinical and Translational Science Center (8UL1TR000170-05 from the National Center for Research Resources and the National Center for Advancing Translational Sciences and financial contributions from Harvard University and its affiliated academic health care centers).
Supplemental Figure 1 is available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at http://cdn.nutrition.org.
References
- 1. Koppes LL, Dekker JM, Hendriks HF, Bouter LM, Heine RJ. Moderate alcohol consumption lowers the risk of type 2 diabetes: a meta-analysis of prospective observational studies. Diabetes Care 2005;28:719–25. [DOI] [PubMed] [Google Scholar]
- 2. Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ 2011;342:d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brien SE, Ronksley PE, Turner BJ, Mukamal KJ, Ghali WA. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta-analysis of interventional studies. BMJ 2011;342:d636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jackson R, Broad J, Connor J, Wells S. Alcohol and ischaemic heart disease: probably no free lunch. Lancet 2005;366:1911–2. [DOI] [PubMed] [Google Scholar]
- 5. Wang Q, Zhou S, Wang L, Lei M, Wang Y, Miao C, Jin Y. ALDH2 rs671 polymorphism and coronary heart disease risk among Asian populations: a meta-analysis and meta-regression. DNA Cell Biol 2013;32:393–9. [DOI] [PubMed] [Google Scholar]
- 6. Au Yeung SL, Jiang C, Cheng KK, Cowling BJ, Liu B, Zhang W, Lam TH, Leung GM, Schooling CM. Moderate alcohol use and cardiovascular disease from Mendelian randomization. PLoS One 2013;8:e68054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vinson JA, Teufel K, Wu N. Red wine, dealcoholized red wine, and especially grape juice, inhibit atherosclerosis in a hamster model. Atherosclerosis 2001;156:67–72. [DOI] [PubMed] [Google Scholar]
- 8. Escolà-Gil JC, Calpe-Berdiel L, Ribas V, Blanco-Vaca F. Moderate beer consumption does not change early or mature atherosclerosis in mice. Nutr J 2004;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Freiberg MS, Samet JH. Alcohol and coronary heart disease: the answer awaits a randomized controlled trial. Circulation 2005;112:1379–81. [DOI] [PubMed] [Google Scholar]
- 10. Reichman ME, Judd JT, Longcope C, Schatzkin A, Clevidence BA, Nair PP, Campbell WS, Taylor PR. Effects of alcohol consumption on plasma and urinary hormone concentrations in premenopausal women. J Natl Cancer Inst 1993;85:722–7. [DOI] [PubMed] [Google Scholar]
- 11. Baer DJ, Judd JT, Clevidence BA, Muesing RA, Campbell WS, Brown ED, Taylor PR. Moderate alcohol consumption lowers risk factors for cardiovascular disease in postmenopausal women fed a controlled diet. Am J Clin Nutr 2002;75:593–9. [DOI] [PubMed] [Google Scholar]
- 12. Gepner Y, Golan R, Harman-Boehm I, Henkin Y, Schwarzfuchs D, Shelef I, Durst R, Kovsan J, Bolotin A, Leitersdorf E, et al. Effects of initiating moderate alcohol intake on cardiometabolic risk in adults with type 2 diabetes: a 2-year randomized, controlled trial. Ann Intern Med 2015;163:569–79. [DOI] [PubMed] [Google Scholar]
- 13. Marfella R, Cacciapuoti F, Siniscalchi M, Sasso FC, Marchese F, Cinone F, Musacchio E, Marfella MA, Ruggiero L, Chiorazzo G, et al. Effect of moderate red wine intake on cardiac prognosis after recent acute myocardial infarction of subjects with type 2 diabetes mellitus. Diabet Med 2006;23:974–81. [DOI] [PubMed] [Google Scholar]
- 14. Bradley KA, Boyd-Wickizer J, Powell SH, Burman ML. Alcohol screening questionnaires in women: a critical review. JAMA 1998;280:166–71. [DOI] [PubMed] [Google Scholar]
- 15. Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 16. Dipietro L, Caspersen CJ, Ostfeld AM, Nadel ER. A survey for assessing physical activity among older adults. Med Sci Sports Exerc 1993;25:628–42. [PubMed] [Google Scholar]
- 17. Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. [Google Scholar]
- 18. Giovannucci E, Colditz G, Stampfer MJ, Rimm EB, Litin L, Sampson L, Willett WC. The assessment of alcohol consumption by a simple self-administered questionnaire. Am J Epidemiol 1991;133:810–7. [DOI] [PubMed] [Google Scholar]
- 19. Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. BMJ 1999;319:1523–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sharpe PC, McBride R, Archbold GP. Biochemical markers of alcohol abuse. QJM 1996;89:137–44. [DOI] [PubMed] [Google Scholar]
- 21. Mukamal KJ, Mackey RH, Kuller LH, Tracy RP, Kronmal RA, Mittleman MA, Siscovick DS. Alcohol consumption and lipoprotein subclasses in older adults. J Clin Endocrinol Metab 2007;92:2559–66. [DOI] [PubMed] [Google Scholar]
- 22. Otvos JD, Jeyarajah EJ, Bennett DW, Krauss RM. Development of a proton nuclear magnetic resonance spectroscopic method for determining plasma lipoprotein concentrations and subspecies distributions from a single, rapid measurement. Clin Chem 1992;38:1632–8. [PubMed] [Google Scholar]
- 23. Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol 1995;56:423–32. [DOI] [PubMed] [Google Scholar]
- 24. Salaspuro M. Carbohydrate-deficient transferrin as compared to other markers of alcoholism: a systematic review. Alcohol 1999;19:261–71. [DOI] [PubMed] [Google Scholar]
- 25. Chiva-Blanch G, Magraner E, Condines X, Valderas-Martinez P, Roth I, Arranz S, Casas R, Navarro M, Hervas A, Sisó A, et al. Effects of alcohol and polyphenols from beer on atherosclerotic biomarkers in high cardiovascular risk men: a randomized feeding trial. Nutr Metab Cardiovasc Dis 2015;25:36–45. [DOI] [PubMed] [Google Scholar]
- 26. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Davies MJ, Baer DJ, Judd JT, Brown ED, Campbell WS, Taylor PR. Effects of moderate alcohol intake on fasting insulin and glucose concentrations and insulin sensitivity in postmenopausal women: a randomized controlled trial. JAMA 2002;287:2559–62. [DOI] [PubMed] [Google Scholar]
- 28. Shai I, Wainstein J, Harman-Boehm I, Raz I, Fraser D, Rudich A, Stampfer MJ. Glycemic effects of moderate alcohol intake among patients with type 2 diabetes: a multi-center, randomized clinical intervention trial. Diabetes Care 2007;30:3011–6. [DOI] [PubMed] [Google Scholar]
- 29. Zamora-Ros R, Lamuela-Raventos RM, Estruch R, Andres-Lacueva C. Resveratrol, a new biomarker of moderate wine intake? Br J Nutr 2009;101:148. [DOI] [PubMed] [Google Scholar]
- 30. Regueiro J, Vallverdu-Queralt A, Simal-Gandara J, Estruch R, Lamuela-Raventos RM. Urinary tartaric acid as a potential biomarker for the dietary assessment of moderate wine consumption: a randomised controlled trial. Br J Nutr 2014;111:1680–5. [DOI] [PubMed] [Google Scholar]
- 31. Jatlow PI, Agro A, Wu R, Nadim H, Toll BA, Ralevski E, Nogueira C, Shi J, Dziura JD, Petrakis IL, et al. Ethyl glucuronide and ethyl sulfate assays in clinical trials, interpretation, and limitations: results of a dose ranging alcohol challenge study and 2 clinical trials. Alcohol Clin Exp Res 2014;38:2056–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Beckjord E, Shiffman S. Background for real-time monitoring and intervention related to alcohol use. Alcohol Res 2014;36:9–18. [PMC free article] [PubMed] [Google Scholar]
- 33. Oei TP, Baldwin AR. Expectancy theory: a two-process model of alcohol use and abuse. J Stud Alcohol 1994;55:525–34. [DOI] [PubMed] [Google Scholar]
- 34. Naqvi AZ, Hasturk H, Mu L, Phillips RS, Davis RB, Halem S, Campos H, Goodson JM, Van Dyke TE, Mukamal KJ. Docosahexaenoic acid and periodontitis in adults: a randomized controlled trial. J Dent Res 2014;93:767–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wedick NM, Brennan AM, Sun Q, Hu FB, Mantzoros CS, van Dam RM. Effects of caffeinated and decaffeinated coffee on biological risk factors for type 2 diabetes: a randomized controlled trial. Nutr J 2011;10:93. [DOI] [PMC free article] [PubMed] [Google Scholar]