Abstract
Background: Historically, Holder pasteurization has been used to pasteurize donor human milk available in a hospital setting. There is extensive research that provides an overview of the impact of Holder pasteurization on bioactive components of human milk. A shelf-stable (SS) human milk product, created using retort processing, recently became available; however, to our knowledge, little has been published about the effect of retort processing on human milk.
Objective: We aimed to assess the ability of retort processing to eliminate bacteria and to quantify the difference in lysozyme and secretory immunoglobulin A (sIgA) activity between Holder pasteurized (HP) and SS human milk.
Methods: Milk samples from 60 mothers were pooled. From this pool, 36 samples were taken: 12 samples were kept raw, 12 samples were HP, and 12 samples were retort processed to create an SS product. All samples were analyzed for total aerobic bacteria, coliform bacteria, Bacillus cereus, sIgA activity, and lysozyme activity. Raw samples served as the control.
Results: One raw sample and 3 HP samples contained B. cereus at the time of culture. There were no detectable bacteria in SS samples at the time of culture. Raw samples had significantly greater lysozyme and sIgA activity than HP and SS samples (P < 0.0001). HP samples retained significantly more lysozyme and sIgA activity (54% and 87%, respectively) than SS samples (0% and 11%, respectively).
Conclusions: Human milk processed using Holder pasteurization should continue to be screened for the presence of B. cereus. Clinicians should be aware of the differences in the retention of lysozyme and sIgA activity in HP and SS products when making feeding decisions for medically fragile or immunocompromised infants to ensure that patients are receiving the maximum immune protection.
Keywords: heat processing, infant nutrition, donor human milk, commercial sterilization, shelf-stable human milk
Introduction
The nutritional requirements of premature infants can be difficult to meet and their mother's own milk (MOM) is the preferred food source after premature delivery, owing to the increased nutritional value of human milk (1, 2). Human milk contains bioactive components that help to protect the medically fragile infant from the development of complications such as sepsis, retinopathy of prematurity, and necrotizing enterocolitis (3–5). The protective benefits of human milk are maximized when an exclusively human milk diet is maintained, decreasing retinopathy rates by >20% and necrotizing enterocolitis rates by 12–14% (4–6). When babies are born prematurely, the mother is at an increased risk of delayed onset of lactogenesis II or low milk volume (7–9). Donor human milk (DHM) can be used to maintain an exclusively human milk diet until the mother's milk supply is established (10, 11).
Historically, the Human Milk Banking Association of North America (HMBANA) has been the provider of DHM in medical settings. To protect infants from potentially pathogenic bacteria, all donated milk is pasteurized and screened for bacteria. Holder pasteurization (62.5°C for 30 min) is the standard pasteurization method for HMBANA milk banks and has been shown to eliminate all pathogenic bacteria except Bacillus cereus (12). In addition, Holder pasteurization retains many bioactive compounds in human milk, including 40–75% of lysozyme function and 50–100% of secretory IgA (sIgA) function (12, 13).
Recently, a shelf-stable (SS) DHM product developed with retort processing became available for use in a neonatal intensive care unit (NICU) setting in the United States. Retort processing differs from Holder and high-temperature, short-time pasteurization, which are currently the most widely used and researched thermal pasteurization methods, in the temperatures utilized, time of heat exposure, pressure utilized, and shelf life of the product (14).
SS DHM may be an option for smaller facilities lacking storage and refrigeration space. However, to our knowledge, there is currently only one published study that investigates the effect of retort processing on bioactive components of human milk (15). Results from the study by Meredith-Dennis et al. (15) indicate destruction of bioactive components in retort processed milk compared with Holder pasteurized (HP) milk. Unfortunately, only 3 samples were analyzed per treatment group and all samples originated from different donor pools. Because concentrations of bioactive components in human milk can vary widely between mothers (16), it is difficult to draw definitive conclusions from the results of the study by Meredith-Dennis et al. (15).
Characterizing the bioactivity in different forms of DHM will allow informed choices regarding nutritional interventions for premature infants. Lysozyme and sIgA were chosen for analysis because of their roles in immune protection in the gastrointestinal tract. Lysozyme degrades the outer cell wall of gram-positive bacteria (17) and contributes to destruction of gram-negative bacteria in vitro (18). sIgA is synthesized by the mother's immune system in response to environmental cues and binds microbes in the infant's gastrointestinal tract to prevent their passage into other tissues (19).
Fragile infants who receive MOM or DHM with active sIgA and lysozyme have increased protection against pathogens within their environment (1, 20). Holder pasteurization and retort processing may yield DHM with different bioactive profiles. Research has shown that as the heat of the treatment increases, the destruction of bioactive components in human milk also increases (21). This study assesses the ability of retort processing to eliminate bacteria and quantifies the difference in lysozyme and sIgA activity between HP and SS human milk.
Methods
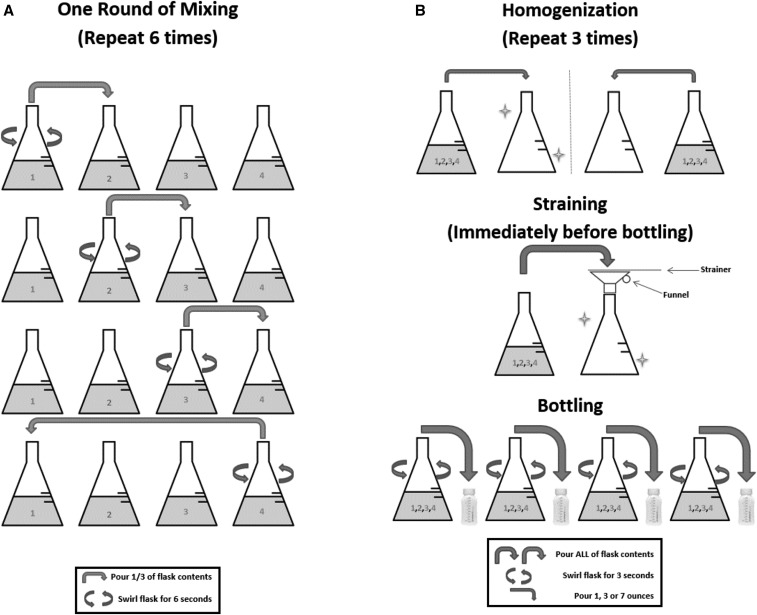
The North Carolina State University Institutional Review Board granted ethical approval for this study. Raw human milk was obtained from 60 approved donors through WakeMed Mothers' Milk Bank (Cary, NC). When women donate to WakeMed Mothers' Milk Bank, they consent that their milk may be used for research studies if it is unable to be used for medical purposes. One sample from each mother was obtained and used to create a pool of raw human milk totaling 260 ounces (7.69 liters). The samples were pooled in the WakeMed Mothers' Milk Bank by a trained technician using a standard pooling protocol. Each individual sample was thawed in the refrigerator prior to pooling. Proper personal protective equipment was worn during handling of the milk per HMBANA guidelines. When samples were adequately thawed, they were moved under a sanitized laminar flow hood to prevent contamination during the pooling process. To minimize fat separation and ensure maximum transfer of the contents, samples were removed from the original milk storage containers and transferred into 4000-mL beakers before they were completely thawed. During thawing, temperatures were maintained ≤4°C to discourage additional bacterial growth. When all individual samples were completely thawed, they were combined into multiple wide-mouth Erlenmeyer flasks and mixed using a pour-down method 6 times (Figure 1A). Once mixed, samples were swirled gently for homogenization and were strained prior to bottling (Figure 1B).
FIGURE 1.
(A) WakeMed Mothers' Milk Bank standard mixing protocol for combination of 4 flasks of human milk. (B) WakeMed Mothers' Milk Bank standard homogenization, straining, and bottling protocol for human milk.
Twenty-four 3-ounce (88.7 mL) samples were taken from the pooled milk and stored in Orthofix AXifeed 100-mL bottles (product no. 022001010; Nolato Jaycare Limited). Twelve samples received no further treatment (referred to hereafter as raw human milk samples) and 12 samples were processed using Holder pasteurization (62.5°C for 30 min) in an ACE Intermed Special Feed Pasteurizer (model HMP2070-40HCUL) at the WakeMed Mothers' Milk Bank.
All milk was kept refrigerated whenever it was not being pooled or processed. The remaining pooled milk was transferred into 2000-mL Erlenmeyer flasks and put on ice for transport to North Carolina State University (<30 min) for bottling and retort processing to create a SS product. Upon arrival, the remaining pooled milk was swirled gently for homogenization (Figure 1B) and was then poured into twelve 10-ounce (295.7 mL) aluminum cans, leaving 3–6 mm of air space at the top. Cans were sealed using a can sealer (Dixie Canner Company) and were retort processed to create a SS product (121°C, 20 psi for 5 min) using a full water immersion retort processer (PR-I900; Stock America Inc.). After all processing was completed, all samples were aliquoted and stored at −80°C until analysis. Note that all milk only underwent 1 freeze-thaw cycle to mimic the freeze-thaw cycle that occurs in a HMBANA milk bank. Each sample was analyzed for bacterial content (per standard protocols at the WakeMed Pathology Laboratory), sIgA activity, and lysozyme activity, as described below.
Bacterial screening
Bacterial analysis was completed at the WakeMed Pathology Laboratory in accordance with the HMBANA guidelines for quantitative bacterial analysis for mothers' milk. HP and SS samples underwent a full postprocessing culture to identify any present bacteria. Raw human milk samples were screened for the presence of B. cereus, Escherichia coli, general appearance of Enterococcus, gram-negative rods, yeast, and Staphylococcus aureus. Because of the large variety of bacteria potentially present in raw human milk samples (22), we chose to only screen for bacteria that may be of concern in a DHM setting, specifically with use in the NICU.
sIgA activity
sIgA activity in our samples was measured using a modified indirect ELISA. Briefly, flat-bottom, high-binding, 96-well plates were incubated for 12–18 h with an E. coli antigen. After completion of the incubation period, plates were washed 3 times with PBS plus 0.05% Tween 20 (PBST). Human milk samples and human IgA from colostrum standards (I-2636; Sigma-Aldrich) were then plated in triplicate and incubated for 3 h at room temperature. Plates were washed with PBST after the incubation period and were then loaded with HRP anti-human IgA (A-0295; Sigma-Aldrich) and incubated for 1 h at room temperature. Afterward, plates were washed a final time with PBST. The substrate solution (20 mL of 0.05 M citrate buffer, 0.1 mL of 3% hydrogen peroxide, and 0.5 mL of 40 mM 2,2′-azinodi-3-ethylbenzothiazoline-6-sulfonic acid) was then added and immediately read on a microplate reader (Multiskan EX; Thermo Electron Corp.) at 405 nm at time 0 and every 2 min for 20 min. To determine sIgA activity, the changes in absorption over time were graphed and a regression line was computed for each of the samples and the standards. The samples were then compared with the IgA standards to determine activity. CVs for triplicates were between 1% and 6%.
Lysozyme activity
Lysozyme activity was measured using the change in turbidity of a microbial suspension of Micrococcus lysodeikticus, a method developed and adapted for use in a 96-well plate (23, 24). Briefly, 25 µL of each human milk sample was plated in triplicate in a 96-well plate (1:20 diluted raw human milk samples, 1:10 diluted HP samples, and undiluted SS samples), and 200 µL of the Micrococcus lysodeikticus suspension (M3770; Sigma-Aldrich) (reading ∼1.00 at 450 nm on a 2600 Gilford spectrophotometer) was added to each well using a multichannel pipette. The 96-well plate was read on a microplate reader (Multiskan EX; Thermo Electron Corp.) at 450 nm every 30 s for 6 min. R2 values were calculated to ensure appropriate function of the assay and the CV was used to determine reliability. CVs for triplicates were between 5% and 7%. Lysozyme activity was then calculated using the following equation:
 |
Statistical analysis
Human milk samples from each treatment group were analyzed in triplicate for analysis of lysozyme and sIgA activity. A statistical comparison of lysozyme activity and sIgA activity between raw milk, HP milk, and SS milk was done with one-factor ANOVA. Differences between means ± SDs were tested for significance (α = 0.05) with Tukey's honest significant difference test.
Results
Bacteria
Raw milk samples were screened for the presence of B. cereus, E. coli, general appearance of Enterococcus, gram-negative rods, yeast, S. aureus, and Pseudomonas species. All raw milk samples contained Enterococcus species, gram-negative rods, yeast, and Pseudomonas species. One raw milk sample contained B. cereus. These results were typical of raw human milk and served as a control for HP samples and SS samples (Table 1).
TABLE 1.
Results of bacterial analysis in raw, Holder pasteurized, and shelf-stable human milk1
| Samples containing bacteria | |||||||
|---|---|---|---|---|---|---|---|
| Sample | Pseudomonas species | Gram-negative rods | Enterococcus species | Yeast | Bacilluscereus | Escherichiacoli | Staphylococcus aureus |
| Raw (unpasteurized) | 12 (100) | 12 (100) | 12 (100) | 12 (100) | 1 (8.3) | 0 (0) | 0 (0) |
| Holder pasteurized (62.5°C for 30 min) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (25) | 0 (0) | 0 (0) |
| Shelf-stable, retort processed (121°C, 20 psi for 5 min) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Values are n (%).
HP samples and SS samples went through a complete postprocessing screen to characterize any bacteria present. Three samples of HP milk had growth of B. cereus. No other growth was observed in HP milk. SS samples had no bacterial growth (Table 1).
sIgA activity
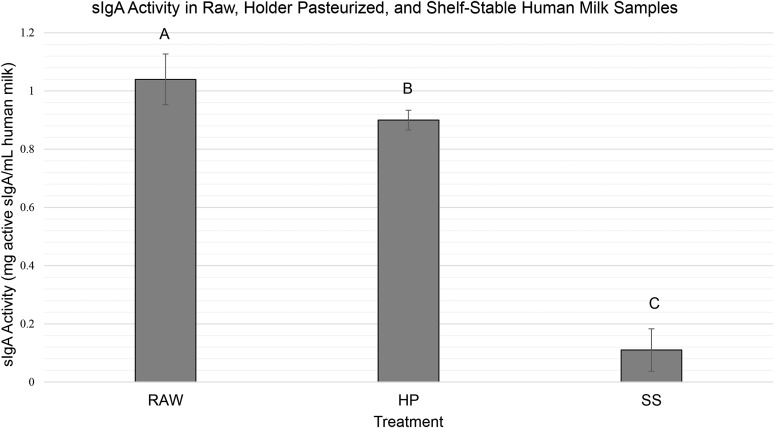
sIgA was measured in all samples, using raw human milk samples as the control. The analysis showed a mean of 1.04 ± 0.09 mg active sIgA/mL in raw human milk samples and was significantly more than HP and SS human milk from the same pool (0.90 ± 0.03 and 0.11 ± 0.07 mg active sIgA/mL, respectively, P < 0.0001; Figure 2).
FIGURE 2.
sIgA activity in raw, HP, and SS human milk. Values are means ± SDs. Bars with different letters are significantly different (P < 0.001). HP, Holder pasteurized; RAW, raw human milk; sIgA, secretory immunoglobulin A; SS, shelf stable.
Lysozyme activity
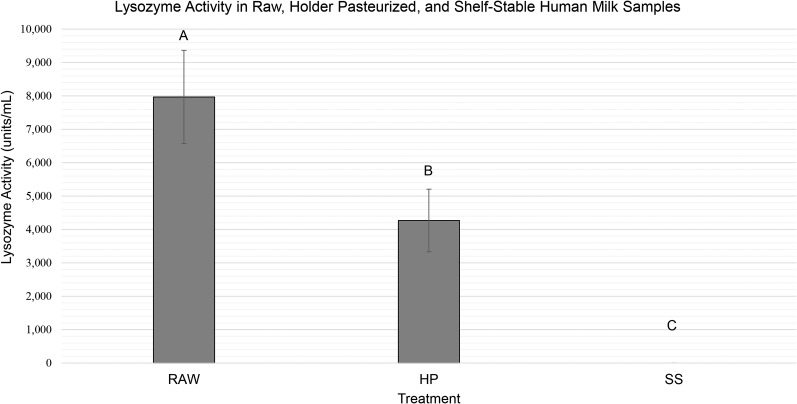
Lysozyme activity was measured in all samples, using raw human milk samples as the control. Raw human milk samples had a mean lysozyme activity of 7969 ± 1394 U/mL, which was significantly greater (P < 0.001) than both HP lysozyme activity (4269 ± 963 U/mL) and SS lysozyme activity (no activity detectible; Figure 3).
FIGURE 3.
Lysozyme activity in raw, HP, and SS human milk. Values are means ± SDs. Bars with different letters are significantly different (P < 0.001). HP, Holder pasteurized; RAW, raw human milk; SS, shelf stable.
Discussion
In the NICU setting, it is imperative to scrutinize nutritional interventions to avoid expensive, life-threatening complications (6, 25). With emerging options for human milk–based feeding, evidence on the full spectrum of feeding choices is needed in order to inform best practice. MOM is the recommended feeding choice for premature infants (1, 2). Whenever possible, breastfeeding should be supported and encouraged if the mother's goal is to breastfeed. When the mother faces obstacles in establishing an adequate milk supply for the infant(s), DHM provides a mechanism for the medically fragile infant to maintain exclusively human milk feedings. The effects of various human milk processing methods on nutrient and bioactive retention may impact health outcomes and are an important area of future research.
Compared with raw human milk, our results show that human milk processed via Holder pasteurization retains more sIgA activity and lysozyme activity (87% and 54%, respectively) than SS human milk (11% and 0%, respectively). Compared with raw human milk, the reduction of activity observed in HP human milk is consistent with ranges reported in the literature (13, 14). Because our study looked specifically at biological activity rather than the concentration of lysozyme and sIgA, there was no published literature to use as a reference for expected values or ranges. Meredith-Dennis et al. (15) found lower concentrations of IgA and no difference in lysozyme concentrations when comparing Holder and retort processed milk. These results may or may not be in agreement with our findings, because the measured protein concentration can remain the same even when there is a loss of biological activity owing to partial denaturation. In addition, it was a cross-sectional study with different donor pools represented in each treatment group (15); therefore, differences in milk composition cannot be specifically attributed to processing effects.
In our study, Holder pasteurization eliminated all bacteria except B. cereus. It is understood that Holder pasteurization does not kill B. cereus and causes B. cereus spores to sporulate during heating (12, 26). HMBANA milk banks have chosen to continue using this method and to screen for and discard any batches that are positive for B. cereus postprocessing to preserve the value of the milk. Retort processing used to create the SS product eliminated all bacteria. Our study provides evidence that retort processing is effective at eliminating all bacteria from human milk, whereas HP DHM must continue to be screened for B. cereus pre- and postprocessing to ensure its safety for consumption by medically fragile infants. The results for SS samples confirm that retort processed DHM is a sterile product (27).
The small sample size is a limitation of our study. However, clear patterns emerged regarding bioactivity retention during Holder pasteurization and retort processing. In addition, this study only looked at 2 of many possible heat-sensitive bioactive components and our processing and packaging methods may not reflect the exact methods used by the manufacturer of the SS human milk product. Additional research on other components in human milk is needed to provide a more complete understanding of the impact of retort processing.
Considering the observed differences in bioactivity of lysozyme and sIgA, a more complete analysis should be performed to determine the impact of retort processing on all heat-sensitive components of human milk, including additional nutrients and bioactive components. Furthermore, to our knowledge, there is currently no peer-reviewed literature on health outcomes of medically fragile infants fed retort processed human milk. Results from this study are important for clinicians to consider when choosing a feeding method for any medically fragile or immunocompromised infant.
Acknowledgments
We thank Michael Bumgardner and KP Sandeep at North Carolina State University for assisting with the retort processing. The authors' responsibilities were as follows—HKL, MTP, and ADF: conceived and designed the experiments; MW-G: collected the samples; HKL and MW-G: performed the experiment; HKL and MTP: analyzed the samples; HKL and ADF: analyzed the data and wrote the manuscript; and all authors: assisted in the revision of the manuscript and read and approved the final manuscript.
Abbreviations
- DHM
donor human milk
- HMBANA
Human Milk Banking Association of North America
- HP
Holder pasteurized
- MOM
mother's own milk
- NICU
neonatal intensive care unit
- PBST
PBS plus 0.05% Tween 20
- sIgA
secretory immunoglobulin A
- SS
shelf stable
Footnotes
Supported by North Carolina State University.
References
- 1. Underwood MA.. Human milk for the premature infant. Pediatr Clin North Am 2013;60:189–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schanler RJ, Shulman RJ, Lau C.. Feeding strategies for premature infants: beneficial outcomes of feeding fortified human milk versus preterm formula. Pediatrics 1999;103:1150–7. [DOI] [PubMed] [Google Scholar]
- 3. Sisk PM, Lovelady CA, Dillard RD, Gruber KJ, O'Shea TM.. Early human milk feeding is associated with a lower risk of necrotizing enterocolitis in very low birth weight infants. J Perinatol 2007;27:428–33. [DOI] [PubMed] [Google Scholar]
- 4. Hylander MA, Strobino DM, Pezzullo JC, Dhanireddy R.. Association of human milk feedings with a reduction in retinopathy of prematurity among very low birthweight infants. J Perinatol 2001;21:356–62. [DOI] [PubMed] [Google Scholar]
- 5. Hanson LA, Korotkova M.. The role of breastfeeding in prevention of neonatal infection. Semin Neonatol 2002;7:275–81. [DOI] [PubMed] [Google Scholar]
- 6. Sullivan S, Schanler RJ, Kim JH, Patel AL, Trawöger R, Kiechl-Kohlendorfer U, Chan GM, Blanco CL, Abrams S, Cotten CM, et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr 2010;156:562–7.e1. [DOI] [PubMed] [Google Scholar]
- 7. Dewey KG, Nommsen-Rivers LA, Heinig MJ, Cohen RJ.. Risk factors for suboptimal infant breastfeeding behavior, delayed onset of lactation, and excess neonatal weight loss. Pediatics 2003;112:607–19. [DOI] [PubMed] [Google Scholar]
- 8. Henderson JJ, Hartmann PE, Newnham JP, Simmer K.. Effect of preterm birth and antenatal corticosteroid treatment on lactogenesis II in women. Pediatrics 2008;121:e92–100. [DOI] [PubMed] [Google Scholar]
- 9. Parker LA, Sullivan S, Krueger C, et al. Effect of early breast milk expression on milk volume and timing of lactogenesis stage II among mothers of very low birth weight infants: a pilot study. J Perinatol 2012;32:205–9. [DOI] [PubMed] [Google Scholar]
- 10. Corpelejin WE, de Waard M, Christmann V, van Goudoever JB, Jansen-van der Weide MC, Kooi EM, Koper JF, Kouwenhoven SM, Lafeber HN, Mank E, et al. Effect of donor milk on severe infections and mortality in very low-birth-weight infants: the Early Nutrition Study Randomized Clinical Trial. JAMA Pediatr 2016;170:654–61. [DOI] [PubMed] [Google Scholar]
- 11. Kantorowska A, Wei JC, Cohen RS, Lawrence RA, Gould JB, Lee HC.. Impact of donor milk availability on breast milk use and necrotizing enterocolitis rates. Pediatrics 2016;137:e20153123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Landers S, Updegrove K.. Bacteriological screening of donor human milk before and after Holder pasteurization. Breastfeed Med 2010;5:117–21. [DOI] [PubMed] [Google Scholar]
- 13. Ewaschuk JB, Unger S, Harvey S, O'Connor DL, Field CJ.. Effect of pasteurization on immune components of milk: implications for feeding preterm infants. Appl Physiol Nutr Metab 2011;36:175–82. [DOI] [PubMed] [Google Scholar]
- 14. Peila C, Moro GE, Bertino E, Cavallarin L, Giribaldi M, Gviuliani F, Cresi F, Coscia A.. The effect of holder pasteurization on nutrients and biologically-active components in donor human milk: a review. Nutrients 2016;8:477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meredith-Dennis L, Xu G, Goonatilleke E, Lebrilla CB, Underwood MA, Smilowitz JT.. Composition and variation of macronutrients, immune proteins, and human milk oligosaccharides in human milk from nonprofit and commercial milk banks. J Hum Lact 2017:890334417710635. [DOI] [PubMed] [Google Scholar]
- 16. Perrin MT, Fogleman AD, Newburg DS, Allen JC.. A longitudinal study of human milk composition in the second year postpartum: implications for human milk banking. Matern Child Nutr 2017;13:e12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chipman DM, Sharon N.. Mechanism of lysozyme action. Science 1969;165:454–65. [DOI] [PubMed] [Google Scholar]
- 18. Ellison RTJ, Giehl TJ.. Killing of gram-negative bacteria by lactoferrin and lysozyme. J Clin Invest 1991;88:1080–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tully DB, Jones F, Tully MR.. Donor milk: what's in it and what's not. J Hum Lact 2001;17:152–5. [DOI] [PubMed] [Google Scholar]
- 20. Lönnerdal B.. Nutritional and physiologic significance of human milk proteins. Am J Clin Nutr 2003;77:1537S–43S. [DOI] [PubMed] [Google Scholar]
- 21. Ford JE, Law BA, Marshall VM, Reiter B.. Influence of heat treatment of human milk on some of its protective constituents. J Pediatr 1977;90:29–35. [DOI] [PubMed] [Google Scholar]
- 22. Cabrera-Rubio R, Collado MC, Laitinen K, Salminen S, Isolauri E, Mira A.. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am J Clin Nutr 2012;96:544–51. [DOI] [PubMed] [Google Scholar]
- 23. Shugar D.. The measurement of lysozyme activity and the ultra-violet inactivation of lysozyme. Biochim Biophys Acta 1952;8:302–9. [DOI] [PubMed] [Google Scholar]
- 24. Lee YC, Yank D.. Determination of lysozyme activities in a microplate format. Anal Biochem 2002;310:223–4. [DOI] [PubMed] [Google Scholar]
- 25. Colaizy TT, Bartick MC, Jegier BJ, Green BD, Reinhold AG, Schaefer AJ, Bogen DL, Schwarz EB, Stuebe AM; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Impact of optimized breastfeeding on the costs of necrotizing enterocolitis in extremely low birthweight infants. J Pediatr 2016;175:100–5.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Holsinger VH, Rajkowski KT, Stabel JR.. Milk pasteurisation and safety: a brief history and update. Rev Sci Tech 1997;16:441–51. [DOI] [PubMed] [Google Scholar]
- 27. Medo ET.. Is the donor milk used in your NICU commercially sterile? Neonatal Intensive Care 2015;28:34–7. [Google Scholar]