Abstract
Objective: To provide a review on the use, percent positive agreement (PPA), percent negative agreement (PNA), and utilization of Syphilis Health Check for syphilis screening in community pharmacies (in coordination with public health departments) in an effort to increase overall syphilis screening in high-risk populations. Data Sources: PubMed was searched for the following keywords: syphilis, sexually transmitted diseases, diagnosis, public health, point-of-care tests. The search included all dates up to December 2016. Study Selection: Data from studies including the use of the Syphilis Health Check Rapid Immunochromatographic Test were included. Data Synthesis: There are many existing tests to aid in the diagnosis of syphilis. The Syphilis Health Check was compared with these assays using PPA and PNA, where it demonstrated a high level of accuracy in the detection of syphilis antibodies. Conclusion: The Syphilis Health Check Rapid Immunochromatographic Test is a Clinical Laboratory Improvement Amendments–waived assay that has been shown to be easy to use and produces results in minutes. As one of the most accessible health care providers, pharmacists have an opportunity to join the fight against syphilis, and in collaboration with public health departments, screen a vast number of high-risk patients and deliver follow-up care as needed.
Keywords: syphilis, sexually transmitted diseases, diagnosis, public health, point-of-care tests
Introduction
Sexually transmitted infections (STIs) are among the most prevalent and preventable infections in the United States. The Centers for Disease Control and Prevention (CDC) estimates there are more than 110 million cases of STIs nationwide, with 20 million new cases developing each year.1 These diseases account for about $16 billion annually.2 Common STIs include gonorrhea, hepatitis B virus (HBV), herpes simplex virus type 2 (HSV-2), human immunodeficiency virus (HIV), human papillomavirus, trichomoniasis, and syphilis.
After being in decline in the United States in the early 2000s, syphilis infections have been increasing. From 2011 to 2014, approximately 27% increase in the total number of new cases was observed, increasing from 46 060 to 63 450.3 Several factors are theorized to have attributed to the growing incidence of syphilis, including an increased prevalence in the gay, bisexual, and men who have sex with men (MSM) communities. The introduction of the Internet to the general public has also increased the ease of finding partners while traveling, and the breakthroughs in HIV therapy have made unprotected sex seem less harmful.4 The MSM population also has high rates of HIV coinfection, as both can be acquired following high-risk sexual behavior. One symptom of syphilis is the development of open sores, or chancers, in the genital area. These increase the risk of HIV transmission. Additionally, HIV-infected patients who contract syphilis may have an atypical serological response to the infection, which can complicate a diagnosis. Last, among those who become immunocompromised secondary to HIV, progression of syphilis to neurosyphilis can be accelerated.5 For these reasons, the CDC recommends anyone diagnosed with syphilis also be tested for HIV.3,5
Syphilis is a highly contagious infection. If left untreated, it can become a debilitating or fatal illness. Syphilis is spread through direct contact with infected mucus membranes, allowing the causative spirochete, Treponema pallidum (TP), access to a new host. After acquiring the disease, it progresses through various stages if left untreated. Primary syphilis develops following an initial incubation period of 10 to 90 days, characterized by a single or multiple chancres at the site of infection. These are usually firm, round, small, but painless. These sores will regress in a matter of weeks even without treatment, but if not treated, the infection will progress to the secondary stage. Secondary syphilis is typically associated with more systemic symptoms as the disease disseminates throughout the entire body. Classic symptoms include a rash or red spots on the palms and soles, but other common features include fever, swollen lymph nodes, fatigue, and increased mucocutaneous eruptions. Like primary syphilis, the symptoms of the secondary phase are also self-limiting, but if untreated can worsen. Latent syphilis occurs roughly 1 year after symptoms associated with the initial infection have dissipated. This stage is typically asymptomatic. Latent syphilis can be further classified as early latent (less than 1 year since exposure) and late latent (greater than 1 year since or unknown time of exposure) infection. During latent syphilis, the disease can reactivate and begin damaging the internal organs, including the cardiovascular and central nervous systems. The host may lose muscle movement coordination, eyesight, become paralyzed, or die as a result of the infection.5
Penicillin G remains the first-line treatment for all stages of syphilis, and it is highly effective. The formulation, dose, duration, and goals of therapy vary depending on the stage of illness. Adults with primary, secondary, and early-latent disease are treated with benzathine penicillin G 2.4 million units (MU) given intramuscularly (IM) once as a single dose. Late-latent and syphilis of unknown duration require multiple doses, given as benzathine penicillin G 2.4 MU IM once weekly for 3 weeks (7.2 MU total). In the event the patient is allergic to penicillin agents, oral doxycycline 100 mg given twice daily for 14 days is an accepted alternative. Those treated in the earlier stages (primary, secondary, early-latent) are more likely to become seronegative posttreatment, but those treated much later will likely remain seropositive, even after undergoing adequate treatment.5
Screening for Syphilis
Owing to strict growth requirements, Treponema pallidum cannot be cultured in vitro and is only viable in cell culture for a limited amount of time.3,6,7 Current screening and diagnosis methods for detecting syphilis involve both qualitative and quantitative measures. The types of tests used can be classified into 2 groups: those that detect antibodies (both nontreponemal and treponemal assays) and those seeking to identify T pallidum through polymerase chain reactions and dark-field microscopy. The reliability of tests is largely influenced by the stage of syphilis in the contracted patient (Table 1).5,6
Table 1.
Performance Characteristics Reported for Various Tests Used to Detect Syphilis.7
| Test Type | Sensitivitya | Specificityb |
|---|---|---|
| Dark-field microscopy | 85% to 92% | 85% to 100% |
| RPR | 78% to 100% | 85% |
| VDRL | 86% to 100% | 99% |
| TP-PA | 85% to 100% | 98% to 100% |
| FTA-Abs | 84% | 96% |
| EIA | 82% to 100% | 97% to 100% |
Abbreviations: RPR, rapid plasma reagin; VDRL, venereal disease research laboratory; TP-PA, treponemal pallidum particle agglutination assay; FTA-Abs, fluorescent treponemal antibody absorbed; EIA, enzyme immunoassay.
Sensitivities are lowest in primary infection, highest in secondary, and variable during the other stages.
Specificity is reduced in the presence of comorbid conditions (pregnancy, intravenous drug use, malaria, tuberculosis).
Diagnostic Assays
Historically, dark-field microscopy was a technique done to evaluate the patient’s chancre for the presence of T pallidum species. This is a process in which a sample from the chancre is placed under a microscope to detect spirochetes. Conducting this method is invasive, dependent on proper specimen collection, and useful only in the early stages of the infection.6
Treponemal antibody detection tests include both manual (fluorescent treponemal antibody absorbed [FTA-Abs] and T pallidum particulate agglutination [TP-PA]) as well as automated methods (enzyme immunoassays [EIA] and chemiluminescence immunoassays [CIA]). FTA-Abs and TP-PA are used less frequently with the coming of EIA technology. These qualitative tests are designed to identify anti-treponemal antibodies specific to T pallidum antigens, but cannot distinguish between past or present infection.6,8 All of these screening tests must be conducted in a certified clinical laboratory by trained personnel.
Nontreponemal screening methods include the venereal disease research laboratory (VDRL) test and the more commonly used rapid plasma reagin (RPR) test. These quantitative tests are designed to identify anti-cardiolipin antibodies that are released during syphilis infections. These markers are also elevated in other conditions, such as autoimmune diseases or following a recent vaccination. As a result, false positive test results are not uncommon. In addition, because these tests only detect active infections, false negative results may be noted in latent or tertiary illness.7
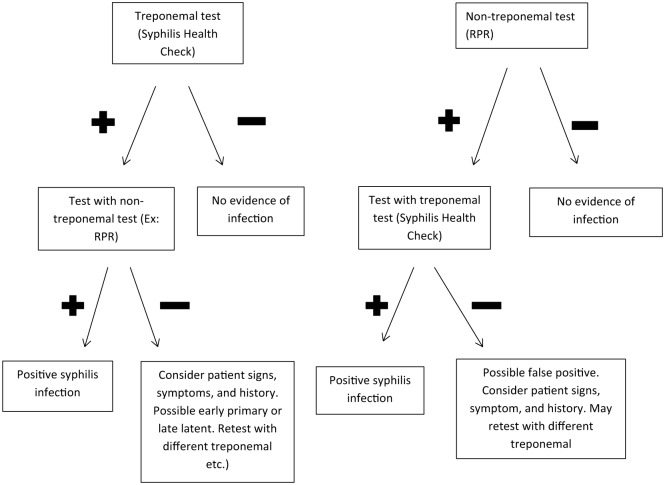
Due to the limitations of both the treponemal and non-treponemal tests, a syphilis diagnosis requires a combination of treponemal and non-treponemal results. Historically, a non-treponemal test such as RPR was performed first, as this was a cheaper and less complicated to perform. Conformation testing was then done with a treponemal test. In the past several years, several agencies have begun screening initially with a treponemal test and confirming it with a non-treponemal test. Currently, there is no recommendation from the CDC on the preferred order of screening (Figure 1).
Figure 1.
CLIA-Waived Screening Tests
In December 2014, Diagnostics Direct received a Clinical Laboratory Improvement Amendments (CLIA) waiver for their previously Food and Drug Administration–approved anti-TP rapid immunochromatographic test called the Syphilis Health Check. Similar to other immunoassays, the Syphilis Health Check uses a nitrocellulose strip loaded with T pallidum antigen designed to capture passing antibodies.10 In the past, treponemal screening tests have demonstrated high sensitivities and specificities, but their utility were restricted because of inaccessibility, high costs, and long turnaround times.6 The Syphilis Health Check was designed to limit barrier for use by being available in more patient-accessible areas, being simple to perform, reducing costs, and without sacrificing sensitivity and specificity parameters.11
The Syphilis Health Check Immunochromatographic Test
Methods for Data Collection and Analysis
PubMed was searched for the following keywords: syphilis, sexually transmitted diseases, diagnosis, public health, point-of-care tests. The search included all dates up to December 2016. Data from studies including the use of the Syphilis Health Check Rapid Immunochromatographic Test were included.
Intended Use
The Syphilis Health Check is a qualitative rapid membrane immunochromatographic assay for the detection of T pallidum antibodies in human whole blood, serum, or plasma. The assay can be used as an initial screening test or in conjunction with a non-treponemal laboratory test and clinical findings to aid in the diagnosis of syphilis. This test is not intended to be used for screening blood or plasma donors.11
Summary of the Test
The diagnosis of syphilis depends on clinical manifestations and antibody detection. Two types of antibody responses typically result following exposure to T pallidum: nonspecific (anti-cardiolipin) and specific (anti-treponemal). Nonspecific antibodies are usually present in patients with syphilis, but may also be present in other conditions (eg, antiphospholipid syndrome, livedoid vasculitis, vertebrobasilar insufficiency, Behçet’s syndrome, idiopathic spontaneous abortion, and systemic lupus erythematosus), thus hampering the specificity of non-treponemal tests that are designed to detect these antibodies. The Syphilis Health Check is a treponemal test intended to detect both IgG and IgM anti-treponemal antibodies and serve as a more specific diagnostic aid. In the test, recombinant treponemal antigens are immobilized as a single line on the assay strip. Antibodies present in the specimen will bind the immobilized antigens. This complex then reacts with colloidal gold conjugated protein A, ultimately yielding a visible line.6,11
Principals of the Test
The Syphilis Health Check Rapid Immunochromatographic Test was developed to detect human antibodies to a syphilis infection in serum, plasma, or whole blood. Samples are collected via finger stick method or venipuncture. Each kit comes with enough supplies to conduct 20 tests, including the test devices, disposable plastic pipettes, and a diluent composed of saline, detergent, and sodium azide 0.1%. Lancets, a timer, and the control solutions are not included.11
To perform a test, 50 µL of blood from either a finger stick or venipuncture is collected using the included plastic microtube. The tube is then held vertically over the collection area on the test cassette and 2 drops are dispensed (1 drop if serum or plasma). After the sample has been loaded onto the device, 4 drops of diluent are added to the collection area to facilitate sample flow across the test membrane. One or 2 additional drops of diluent may be required if no flow is observed. The testing cassette should be placed on a flat surface at room temperature (20°C to 26°C) and remain undisturbed for at least 10 minutes. Results can be read after 10 minutes but not longer than 15 minutes following addition of the diluent. As the sample flows through the cassette, anti-TP antibodies in the specimen bind to the gold conjugated protein A reagent in the test strip. Any reaction occurring in the test (T) or control (C) region will cause a pink-rose line to become visible. Tests are only valid if a solid line is seen in the C zone. The test should be read as reactive if a line is visible in both the C zone and any distinguishable band appears in the T zone. Line intensity of the T zone is not associated with the amount of anti-TP antibodies in the sample, so identification of any intensity should be considered a positive result.11
Quality Control
The manufacturer recommends that external positive and negative controls be run with each new lot, shipment, and operator. This should also be done monthly to ensure that storage conditions have not affected the test performance. Controls must be purchased separately. Each control should be processed like an unknown sample. If control results do not behave as expected, patients must not be tested, nor results reported.11
Limitations of the Test
Syphilis Health Check should be used in conjunction with clinical symptoms, medical history, and other laboratory findings to aid in diagnosis only. A positive result must be confirmed using a non-treponemal test and does not rule out the presence of other comorbid pathogens. Nonreactive results do not preclude the possibility of infection, as anti-TP antibody concentrations take up to several weeks to become detectable. Therefore, if patients are tested in the early stages of infection they may experience false negative results. Clinical performance has not been demonstrated among immunocompromised, immunosuppressed, or infant populations.11
Performance Characteristics
Sensitivity and specificity of any assay are traditionally compared to the accepted reference standard. Since there is no single gold standard method used to detect syphilis infections, performance is assessed by determining the percent positive agreement (PPA) and percent negative agreement (PNA) versus existing tests.6
One study prospectively tested specimens collected from 89 patients that presented to a clinic with symptoms consistent with syphilis (Table 2). Patients were tested with treponemal (FTA-Abs, TP-HA, Syphilis Health Check) and non-treponemal (RPR) methods. The results revealed 100% PPA with the Syphilis Health Check with comparator tests, but mixed PNA results. PNA ranged from 50% to 100%, meaning false positive tests may have been noted.10,11
Table 2.
Performance of the Syphilis Health Check Test.11
| Population/Specimens | Site | Comparator | PPA (95% CI) | PNA (95% CI) | |
|---|---|---|---|---|---|
| Study 1 | 89 symptomatic and suspected syphilis-positive patients at 4 STD clinics and 1 hospital clinic | STD clinic | RPR FTA-Abs |
100% (89.1-100) 100% (87.2-100) | 85.7% (42.1-99.6) 50% (21.1-78.9) |
| Hospital clinic | RPR | 100% (29.2-100) | 93.6% (82.5-98.7) | ||
| TP-HA | 100% (54.1-100) | 100% (92-100) | |||
| Study 2 | 694 patients at 3 study sites | Site 1 | RPR | 86.7% (59.5-98.3) | 96.1% (93.7-97.8) |
| TP-PA | 77.8% (57.7-91.4) | 97.9% (95.8-99.1) | |||
| Site 2 | RPR | 100% (15.8-100) | 97.7% (91.9-99.7) | ||
| TP-PA | 100% (39.8-100) | 100% (95.8-100) | |||
| Site 3 | RPR | 100% (54.1-100) | 98% (94.9-99.4) | ||
| EIA | 90% (55.5-99.7) | 99% (96.3-99.9) | |||
| Study 3 | 315 positive samples | Not specified | RPR | 93.4% (89.9-96) | 100% (100) |
| TP-PA | 99.6% (97.9-100) | 85.7% (63.7-97) | |||
| Study 4 | 97 suspected syphilis-positive samples | Laboratory | RPR | 100% (94.2-100) | 28.6% (14.6-46.3%) |
| TP-HA | 100% (95.8-100) | 100% (69.2-100) | |||
| MH-TP | 100% (95.8-100) | 100% (69.2-100) | |||
| Study 5 | 164 clinically diagnosed, syphilis-positive serum samples, both treated and untreated | Infectious disease clinic | RPR, TP-PA, and FTA-Abs | ||
| Untreated | |||||
| Primary | 100% (85.2-100) | ||||
| Secondary | 100% (86.3-100) | ||||
| Latent | 100% (84.6-100) | ||||
| Treated | |||||
| Primary | 100% (87.8-100) | ||||
| Secondary | 100% (86.8-100) | ||||
| Latent | 100% (81.5-100) | ||||
| Study 6 | 162 pregnant female samples with 93 syphilis-positive and 69 status unknown | Not specified | RPR TP-PA |
96.8% (91-99.3) 100% (96.2-100) | 100% (94.7-100) 100% (94.7-100) |
Abbreviations: RPR, rapid plasma reagin; VDRL, venereal disease research laboratory; TP-PA, Treponema pallidum particle agglutination assay; FTA-Abs, fluorescent treponemal antibody absorbed; EIA, enzyme immunoassay.
Another study compared results from the Syphilis Health Check to results with other tests. Specimens from 694 patients were gathered from 3 study sites (Table 2). Study site 1 had the most patients (400), with sites 2 and 3 having 89 and 205 patients, respectively. Six false negative results were reported with the Syphilis Health Check versus TP-PA. However, 2 of these were only positive with the TP-PA and negative in RPR and FTA-Abs. The PPA with TP-PA was 77.8% but would be higher if adjusted for these 2 data points, as only 3 total samples were positive for both treponemal tests but negative for Syphilis Health Check and RPR. Overall, the prospective studies yielded a PPA of 95.6% versus RPR and 98.5% versus other treponemal tests. PNA values were 90.5% and 97.3% for RPR and treponemal tests, respectively. Most important among these results is the high PPA between Syphilis Health Check and other treponemal tests, as these would be the tests the Syphilis Health Check would likely replace.10,11
In a retrospective analysis of 315 positive serum and plasma samples from blood centers, the Syphilis Health Check yielded a reactive response for 294 (93.3%) of specimens.10,11 Compared with RPR, the PPA between the 2 tests was 93.4%, and compared with TP-PA, the PPA was 99.6% (TP-PA was reactive in one additional instance when Syphilis Health Check was not). Again, it is important to note the high levels of agreement between the 2 treponemal tests (Syphilis Health Check and TP-PA), remembering that RPR is less specific for identifying a true syphilis infection.
In another trial, the PPAs and PNAs between Syphilis Health Check and other tests were determined among 164 patients at different stages of syphilis infection (primary, secondary, latent), and included both treated and untreated individuals.10,11 All tests (RPR, TP-PA, FTA-Abs, Syphilis Health Check) were in 100% agreement with one another for patients in all stages, regardless of treatment. It should be noted that during latent illness, RPR was nonreactive in both treatment groups, but the treponemal tests retained reactivity.
An additional study evaluated the performance of the Syphilis Health Check test in patients co-infected with HIV, HCV, or HBV who had tested syphilis-positive via RPR.10,11 A 100% agreement was noted among tests in this small population of subjects. Another study examined the performance of Syphilis Health Check in pregnant women.10,11 In this study, 69 samples of unknown syphilis status and 93 RPR-positive specimens from pregnant women were tested. Both the treponemal tests were in 100% agreement with one another, while the RPR detected 3 additional positive samples (PPA 96.8%).10,11
CLIA-Waiver Study
To demonstrate lack of complexity, the manufacturer evaluated the performance of the Syphilis Health Check in a variety of settings by intended users such as health care workers and trained laboratory technicians. The PPA of 98% and PNA of 97.2% achieved in this study demonstrate the ability of untrained operators to achieve the same results as clinical laboratory specialists were recording with other treponemal and non-treponemal assays.
Another study was performed to evaluate the ability of untrained users at 3 sites to correctly interpret weakly reactive samples. Known positive syphilis samples were diluted to just above or below the Syphilis Health Checks cutoff, and the result obtained by untrained users was compared with the expected results. All 3 sites had above a 95.8% agreement with expected result (minimum 24 samples testes). A P value of 1.00 was calculated using the Fisher-Freeman-Halton test for the difference between the result obtained by the untrained user and the result obtained by a laboratory professional, indicating there is no statistically significant difference between results of professionals and untrained operators.11
Acquisition Cost
Syphilis Health Check is available at a cost of $400.00 per kit, which contains 20 tests ($20.00 per test). Control solutions available separately for $49.00.
Role of the Syphilis Health Check Rapid Immunochromatographic Test in Pharmacy Practice
The landscape of health care in the United States brought about through the Affordable Care Act continues to change. Virtually overnight, thousands of individuals acquired insurance and began to seek entry into the health care network. The result of this was an immediate realization that our primary care system was not prepared to absorb this influx of new patients. As a result, pharmacists are being looked upon to improve access to care and play a greater role in the provision of primary care. Apart from the shortage of primary care physicians, accessibility and cost savings have also been identified as justifications for the development of more pharmacy-based care models.12 The development of pharmacy-based vaccination programs has highlighted the value of physicians and pharmacists developing collaborative practice agreements (CPAs) to improve patient care. For decades, as part of their clinical practice, pharmacists routinely evaluate patients and provide recommendations for care via over-the-counter products or referral to other providers. With advanced technologies, incorporation of CLIA-waived point-of-care tests into evidence-based CPAs could improve the pharmacist’s ability to manage broader ranges of ailments. Various physician-pharmacist collaborative disease management models have been validated for streptococcal pharyngitis and influenza.12,13 Furthermore, pharmacy-based screening for HIV and HCV with linkage to care have also been described.
Currently, the identification and management of an individual with syphilis is a 3-step process.14,15 First, an individual at risk for syphilis or pregnant woman is screened using a treponemal test, such as FTA-Abs, TP-PA, EIA, or Syphilis Health Check. Those who are positive based on this initial screen are then tested with a nontreponemal test, such as the RPR or VDRL. At this point treatment may be initiated. Unfortunately, not all individuals at risk for syphilis are identified and routinely screened. In a similar model to that described for pharmacy-based HIV and HCV, pharmacists could raise public awareness of the disease and offer screenings in the pharmacy. Those who test positive would then be referred to care to either their primary care provider or public health agency for confirmatory testing and initiation of therapy. In states that allow pharmacists and physicians to enter into CPAs, pharmacists may be allowed to order a confirmatory nontreponemal test in the event a patient tests positive with Syphilis Health Check. One barrier to the implementation of this model is reimbursement of the pharmacies. Currently, screening programs for HIV and HCV function largely as part of research programs or as collaborations with public health agencies. Another consideration prior to implementation would be the need to provide pharmacists with training in delivering test results to patients. This type of training is available as part of a national program offered by the National Association for Chain Drug Stores that is intended to provide pharmacist with the tools needed to effectively use CLIA-waived point-of-care testes in their practice.
It is widely believed that the prevalence of syphilis is vastly underestimated. Implementation of pharmacy-based syphilis screening programs could appreciably expand the numbers of patients screened. These data would help public health agencies acquire better data regarding the prevalence and locality of the disease.
Summary
The value of using CLIA-waived point-of-care tests by pharmacists in a variety of disease management models has been well-documented. With the receipt of a CLIA-waiver, the Syphilis Health Check test represents another tool available for inclusion in collaborative disease management programs. Since most patients that will be screened for syphilis do not have the disease, conducting screening tests and provision of information related to sexually transmitted infections by a pharmacist could not only improve access to care, but could also reduce congestion in the offices of primary care providers. The Syphilis Health Check Rapid Immunochromatographic Test represents an important tool to allow pharmacists to augment public health initiatives and improve screening for and detection of syphilis.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among U.S. women and men: Prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40:187-193. [DOI] [PubMed] [Google Scholar]
- 2. Owusu-Edusei K, Jr, Chesson HW, Gift TL, et al. The estimated direct medical cost of selected sexually transmitted infections in the United States, 2008. Sex Transm Dis. 2013;40:197-201. [DOI] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2013. Atlanta, GA: US Department of Health and Human Services; 2014. [Google Scholar]
- 4. Heffelfinger JD, Swint EB, Berman SM, Weinstock HS. Trends in primary and secondary syphilis among men who have sex with men in the United States. Am J Public Health. 2007;97:1076-1083. doi: 10.2105/AJPH.2005.070417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lukehart S. Syphilis. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Larry Jameson J, Loscalzo J, eds. Harrison’s Principles of Internal Medicine. 18th ed. New York, NY: McGraw-Hill Medical; 2011:1380-1388. [Google Scholar]
- 6. Ratnam S. The laboratory diagnosis of syphilis. Can J Infect Dis Med Microbiol. 2005;16:45-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. British Medical Journal Best Practice. Diagnostic tests: syphilis. http://bestpractice.bmj.com/best-practice/monograph/50/diagnosis/tests.html. Accessed January 21, 2017.
- 8. Sato N. Laboratory diagnosis of syphilis. http://cdn.intechopen.com/pdfs-wm/23788.pdf. Accessed January 21, 2017.
- 9. Means C, Waletzky J. Syphilis serological testing. http://portals.clevelandclinic.org/portals/66/pdf/technical-briefs/syphilis-serological-testing.pdf. Accessed January 21, 2017.
- 10. US Food and Drug Administration. 501(k) Substantial Equivalence Determination Decision Summary: Assay Only Template. http://www.accessdata.fda.gov/cdrh_docs/reviews/K113793.pdf. Accessed January 21, 2017.
- 11. Syphilis Health Check Rapid Immunochromatographic Test [Package insert]. Stone Harbor, NJ: Diagnostics Direct; 2011. [Google Scholar]
- 12. Klepser DG, Bisanz SE, Klepser ME. Cost-effectiveness of pharmacist provided treatment of adult pharyngitis. Am J Manag Care. 2012;18:e145-e154. [PubMed] [Google Scholar]
- 13. Gubbins PO, Klepser ME, Dering-Anderson AM, et al. Point-of-care testing for infectious disease: opportunities, barriers, and considerations in community pharmacy. J Am Pharm Assoc (2003). 2014;54:163-71. [DOI] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention. Recommendations for Public Health Surveillance of Syphilis in the United States. Atlanta, GA: US Department of Health and Human Services; March 2003. [Google Scholar]
- 15. Centers for Disease Control and Prevention. Sexually Transmitted Diseases Treatment Guidelines, 2015. Morbidity and Mortality Weekly Report Recommendations and Reports. 2015;64:1-138. [PubMed] [Google Scholar]