Abstract
Background: Treatment of advanced BRAF-mutant melanoma has changed dramatically in the past 3 years thanks to the approval of new immunotherapy and targeted therapy agents. Objectives: The goal of our survey was to investigate when immunotherapy and targeted therapy are used in the management of advanced melanoma patients and whether differences exist between the types of setting. Methods: Oncologists from academic centers, community-based centers, and private clinics were invited to participate in an online survey. Survey questions addressed the proportion of BRAF-mutant patients per treatment line, proportion of patients on targeted therapy and immunotherapy available in the United States, and reasons for prescribing each drug class. Results: A total of 101 physicians completed the survey, of which 47 worked in a private clinic, 33 in an academic center, and 21 in a community-based center. Academic center participants tended to see more severe patients (P < .001) and had more patients in second-line treatment than participants from other setting types. In addition, academic center physicians had more patients in clinical trials (P < .001), and they prescribed the ipilimumab and nivolumab combination more frequently. In terms of sequencing, all participants used targeted therapy for severe or rapidly progressing patients and immunotherapy for those who were less severe or slowly progressing. Conclusions: The findings illustrate the differences in treatment approach per type of setting, with patients in academic centers more likely to receive recently approved products or to be enrolled in clinical trials than those in community-based settings.
Keywords: advanced melanoma, targeted therapy, immunotherapy, treatment sequencing
Introduction
With an estimated 87 110 new cases in 2017, melanoma is the fifth most diagnosed cancer in the United States.1 Its incidence has been steadily increasing over the past 30 years, and 9730 deaths are expected to occur in the United States in 2017.1 In recent years, the approval of immunotherapy and targeted therapies blocking BRAF and MEK changed the way advanced melanoma patients are treated, and improved survival.2
Ipilimumab, a monoclonal antibody against cytotoxic T lymphocyte antigen 4 (CTLA-4), has demonstrated benefits in overall survival (OS) and durable response, although it has been associated with a substantial risk of immune-related adverse events and drug-related death.3-6 Other immunotherapy agents that target the programmed cell death-1 receptor pathway (anti-PD1 antibodies), such as nivolumab and pembrolizumab, have also demonstrated to improve OS when compared with chemotherapy and ipilimumab.7,8 Both nivolumab and pembrolizumab were approved by the Food and Drug Administration (FDA) in 2014 for the treatment of metastatic melanoma after progression during ipilimumab or BRAF-inhibitor treatment. In 2016, both pembrolizumab and nivolumab were approved by the FDA as single agent in the first-line setting.8,9 Approval of pembrolizumab as a first-line single agent was based on data from the Keynote-006 clinical trial that demonstrated that patient with unresectable or metastatic melanoma experienced a superior overall survival compared with ipilimumab.8 Patients given pembrolizumab every 2 or 3 weeks had a 37% and 31% reduction in risk of death compared with ipilimumab, respectively (hazard ratio [HR] = 0.63, P < .001, and HR = 0.69, P < .004, respectively).8 As for nivolumab, the CheckMate-067 clinical trial showed that the combination nivolumab plus ipilimumab reduced the risk of progression by 58% compared with ipilimumab alone (HR = 0.42; P < .0001) in patients with advanced melanoma, and single-agent nivolumab reduced the risk of progression by 43% versus ipilimumab (HR = 0.57; P < .0001).9 Additionally, the combination of ipilimumab and nivolumab has shown clinical significance in the treatment of patients with metastatic melanoma10,11 and was approved in the United States in 2016 for the treatment of patients with unresectable or metastatic melanoma patients. In terms of safety, checkpoint immunotherapies have been associated with a high rate of immune-related toxicities including cutaneous toxicities, gastrointestinal toxicities, and fatigue, as well as endocrinopathies and hepatitis.3-11
Vemurafenib and dabrafenib were the first 2 type I BRAF inhibitors to enter clinical development, with both drugs showing more than 50% confirmed response rates as single agents.12-15 Vemurafenib and dabrafenib have also been shown to induce earlier antitumor activity than ipilimumab and anti-PD1 antibodies, and are therefore sometimes considered preferred options to treat patients with rapidly growing tumors.16 MEK inhibitors such as trametinib and cobimetinib are 2 targeted therapies that have been developed for the treatment of BRAF-mutant patients.17,18 Their use as single agents is limited by an unfavorable side effect profile,17,18 but combinations of BRAF and MEK inhibitors have been shown to reduce the single-agent toxicity of each agent and to result in improved clinical outcomes compared with monotherapy due to the delay in the onset of resistance observed with BRAF inhibitors alone.18-22
These new agents have changed the paradigm of treatment for patients with advanced melanoma.23 Numerous clinical trials have been designed to evaluate the efficacy of the various drug classes in various patient types, but the optimal sequence of immunotherapy and BRAF/MEK inhibitors remains unknown.24 In this context, a survey was designed to investigate current treatment of advanced BRAF-mutant melanoma patients. More specifically, this survey investigated the proportion of patients receiving immunotherapy versus those on targeted therapy in first line and second line and over, as well as the rationale for the treatment choice. Differences in treatment practices at the setting level were also investigated.
Method
Study Sample Design
Oncologists from the United States were invited to participate in an online survey aimed at better understanding the medical management and treatment regimens of unresectable stage III and IV BRAF-mutant melanoma patients.
A universe sample frame of oncologists was created by sourcing all practicing physicians who listed oncology as their primary medical specialty via the Centers for Medicare and Medicaid Services National Plan and Provider Enumeration System. This listing of 4003 oncologists served as the sample frame for this survey, and all were invited to the survey by email, postal mail, or both depending on the contact information that was available for each contact. The sample was self-selecting as the participants made the choice to access and complete the survey. Oncologists were eligible to participate if they personally currently managed at least 5 melanoma patients, of which at least one was unresectable stage III or stage IV patient. A total of 138 oncologists responded to the survey, with 101 meeting study eligibility criteria. A true response rate could not be calculated since it is unknown how many of the survey invitations were successfully received and reviewed by the 4003 medical oncologists who were part of the recruiting effort.
Participants were offered an industry-standard honorarium as compensation for their time in completing the survey. The survey was administered online and was fielded from March 9, 2017, to April 3, 2017.
Survey Design
A questionnaire was developed to collect anonymized information on patients with advanced melanoma. We developed and pretested this instrument through interviews and consultations with 4 melanoma-treating oncologists before launching the survey online. All 4 reviewers worked at academic centers. Three of them specialized in melanoma and treat a high volume of advanced melanoma patients, while the fourth one treats a wider variety of malignancies. The online questionnaire consisted largely of quantitative questions and covered the following topics: patients’ disease stages; proportion of BRAF-tested patients; outcome of BRAF tests; reasons for not testing; proportion of BRAF-mutant patients per line of therapy; management of advanced BRAF-mutant melanoma patients per line of therapy; and rationale for prescribing targeted therapy and immunotherapy (questionnaire available on request to corresponding author).
Statistical Analysis
All survey data were analyzed in aggregate and the individual identities of the survey respondents were blinded to the study authors. Data from each respondent were weighted by the total number of advanced melanoma patients that they follow to account for differences between large and small practices, and were reported as means and percentages. We used one-way analysis of variance (ANOVA) to compare the average proportion of patients per disease severity and per treatment options, where the percentages are treated as a continuous variable between 0 and 1 and ANOVA compares the means across settings after adjusting for cluster size. For ease of analysis, treatments provided in first line and second line or more were grouped per drug class, that is, immunotherapy (consisting of pembrolizumab, nivolumab, and ipilimumab in monotherapy and in combination), targeted therapy (consisting of the combination of dabrafenib and trametinib and the combination of cobimetinib and vemurafenib), and inclusion in clinical trial or other type of treatment. All analyses were performed with SPSS version 23.0 (IBM Corp, Armonk, NY) with statistical significance defined as P ≤ .05. Qualitative data were analyzed thematically and coded according to the main themes of the survey questions. Any response that addressed multiple themes was counted as multiple comments.
Ethics
By electing to complete the survey, respondents provided consent to use their anonymous responses to the survey questions. The study did not involve patients, and data on patient characteristics were provided only in the aggregate. As such, there was no institutional review board and/or licensing committee involved in approving the research and no need for informed consent from the participants per US regulations.25
Results
Out of the 101 medical oncologists who qualified and completed the survey, 47 were working in a private clinic, 33 in an academic center, and 21 in a community-based center.
Characteristics of Melanoma Patient Population
Respondents working in an academic center followed a higher number of melanoma patients than those based in either private clinic or community-based center (on average 89, 27, and 32 melanoma patients, respectively, P < .001; Table 1). Patients seen at private clinics and academic centers were more likely to be in stage III or stage IV disease than those followed in community-based centers (78%, 75%, and 53% respectively, P < .001; Table 1).
Table 1.
Split of Melanoma Patients by Setting and Disease Severitya.
| Academic Center | Community-Based Center | Private Clinic | P | |
|---|---|---|---|---|
| Mean number of patient | 88.6 | 31.6 | 27.1 | <.001 |
| Disease stages in percentage of patients | ||||
| Stage I | 11% | 26% | 11% | <.001 |
| Stage II | 14% | 21% | 11% | <.001 |
| Stage III | 25% | 18% | 21% | <.001 |
| Stage IV | 50% | 34% | 56% | <.001 |
Based on a total of 101 oncologists, of which 33 from academic centers, 21 community-based centers, and 47 private clinics.
BRAF Mutation Status
On average 88% of patients were tested for BRAF mutation, ranging from 86% in academic centers to 90% in private clinics and 93% in community-based centers. In community-based centers, the main reasons for not testing patients for BRAF mutation were the fact that stage III patients are not tested as it is not indicated for these patients (71% of mentions). In academic centers, the main barrier was the lack of tissue (47% of mentions), while in private practices 38% mentioned they do not test stage III patients, and 31% mentioned some cost and insurance constraints. Of the patients who were tested for BRAF mutations, 40% of patients in private clinics had a positive test, compared with 42% of patients in academic centers, and 46% of patients in community-based centers.
Pharmaceutical Management of Advanced BRAF-Mutant Melanoma Patients
Proportion of advanced BRAF-mutant melanoma patients per line of treatment per type of setting are shown in Online Supplement 1 (available in the online version of the journal). Community-based oncologists reported the highest proportion of patients in first line at 66% of patients, compared with 56% in private clinics and 48% in academic centers, while academic centers had the highest proportion of patients in second line or over with 52% of patients, compared with 44% in private clinics and 34% in community based centers.
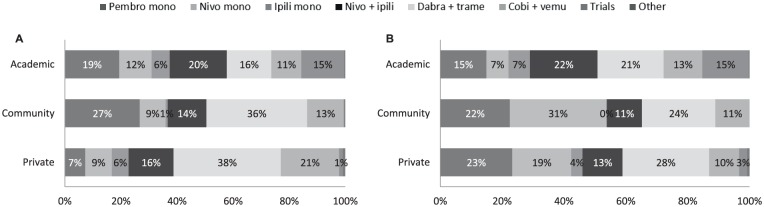
Figure 1 shows how advanced BRAF-mutant melanoma patients were treated per setting and per line of treatment. In first line, academic centers had the highest proportion of patients in clinical trials (15% vs none to 1% in other settings, P < .001), and higher prescription of the combination nivolumab plus ipilimumab (20% vs 16% in private centers and 14% in community-based centers). At the drug class level, academic centers prescribed immunotherapy treatments to 57% of their patients, versus 51% of patients at community-based centers and 38% at private clinics. Another difference was the statistically lower use of targeted therapy in academic centers (27% of patients) compared with private clinics (59%) and community-based centers (49%, P < .001).
Figure 1.
Treatment of advanced BRAF-mutant melanoma patients. Proportion of patients by type of setting. (a) Type of treatment received in first line; (b) Type of treatment received in second line or over.
Abbreviations: Pembro mono, pembrolizumab in monotherapy; nivo mono, nivolumab in monotherapy; Ipili mono, ipilimumab in monotherapy; Nivo + ipili, combination of nivolumab and ipilimumab; Dabra + trame, combination of dabrafenib and trametinib; Cobi + vemu, combination of cobimetinib and vemurafenib; Trials, included in a clinical trial.
Based on a total of 99 oncologists for the first-line treatment, of which 33 from academic centers, 20 community-based centers, and 46 private clinics; and 86 oncologists for the second line or over treatment, of which 29 from academic centers, 16 community-based centers, and 41 private clinics.
Statistical analysis for first-line treatment: P < .001 between settings for patients receiving an immunotherapy, a targeted therapy, included in a clinical trial. Immunotherapy consisting of pembrolizumab, nivolumab, and ipilimumab in monotherapy and in combination. Targeted therapy consisting of dabra + trame and Cobi + vemu.
Statistical analysis for second-line treatment or more: P < .001 between settings for patients receiving an immunotherapy or included in a clinical trial.
P = .210 between settings for patients receiving a targeted therapy.
In second line or over, the use of immunotherapy combination nivolumab plus ipilimumab increased slightly in academic centers from first line (22% of patients) while it decreased in community-based centers and private clinics. Another difference observed was the decrease in use of targeted therapy in community-based centers (from 49% in first line to 35% in second line or over) and private clinics (from 59% in first line to 38% in second line or over), while this use increased in academic centers (from 27% in first line to 34% in second line or over), although the difference was not statistically significant (P = .210). Importantly, academic centers still had 15% of patients included in clinical trials, versus 3% of patients in private clinics, and none in community-based centers (P < .001).
In terms of targeted therapy combination, all participants favored the dabrafenib plus trametinib combination over the vemurafenib plus cobimetinib combination, for all treatment lines, with use of dabrafenib plus trametinib ranging from 16% in academic centers to 38% in private clinics in first line, while cobimetinib plus vemurafenib only represented from 11% of patients in academic centers to 21% in private clinic. In second line or over, dabrafenib plus trametinib was prescribed from 21% of patients in academic center to 28% in private clinics, versus 13% in academic centers and 10% in private clinics for the cobimetinib plus vemurafenib combination
Rationale for Using Immunotherapies or Targeted Therapies
The rationale for prescribing an immunotherapy or a targeted therapy in first line depended on both patients’ clinical condition and drug class characteristics (Figure 2). Immunotherapies were mainly prescribed because they were perceived as having a longer duration of action and better survival efficacy (49%; Figure 2a). They were also prescribed to patients who had low volume disease, who were progressing slowly, or who had mild disease (35% of mentions; Figure 2a). Targeted therapies were used in first line in patients who had high volume disease, who were rapidly progressing, or were symptomatic (39% of mentions), and therefore needed a product that was quick acting (31% of mentions; Figure 2b). The quick acting effect of targeted therapy was most important for community-based oncologists, with 40% of mentions versus 31% of mentions by private oncologists and 27% of academic-based respondents.
Figure 2.
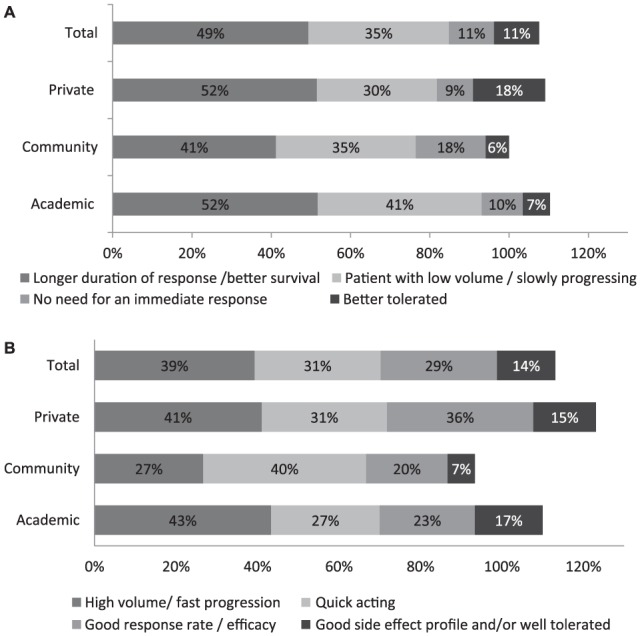
Rationale for choosing an immunotherapy or a targeted therapy in first line: (a) immunotherapy; (b) targeted therapy.
Oncologists reported their reasons for prescribing an immunotherapy (a) or a targeted therapy (b) to their advanced BRAF-mutant melanoma patients in first line. Results are shown as the percentage of oncologists reporting a given reason for prescribing an immunotherapy or a targeted therapy. The percentages of oncologists are shown within the bars. Totals can sum to over 100% as specialists were allowed to report more than one reason to prescribe an immunotherapy or a targeted therapy. (a) Based on a total of 79 oncologists, of which 29 from academic centers, 17 community-based centers, and 33 private clinics. (b) Based on a total of 84 oncologists, of which 30 from academic centers, 21 community-based centers, and 47 private clinics.
In second line or over, the approach consisted in prescribing the drug class that was not prescribed in first line (52% of mentions for immunotherapy and 63% for targeted therapy; Figure 3). Twenty-eighth percent of oncologists from academic centers also mentioned prescribing an immunotherapy in second line because of its longer duration of response.
Figure 3.
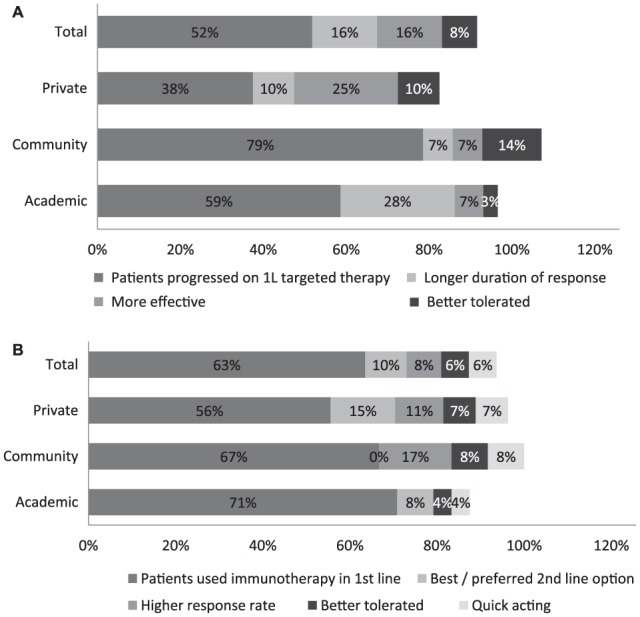
Rationale for choosing an immunotherapy or a targeted therapy in second line or over: (a) immunotherapy; (b) targeted therapy.
Oncologists reported their reasons for prescribing an immunotherapy (a) or a targeted therapy (b) to their advanced BRAF-mutant melanoma patients in second line or over. Results are shown as the percentage of oncologists reporting a given reason for prescribing an immunotherapy or a targeted therapy. The percentages of oncologists are shown within the bars. Totals can sum to over 100% as specialists were allowed to report more than one reason to prescribe an immunotherapy or a targeted therapy. (a) Based on a total of 83 oncologists, of which 29 from academic centers, 14 community-based centers, and 40 private clinics. (b) Based on a total of 63 oncologists, of which 24 from academic centers, 12 community-based centers, and 27 private clinics.
Discussion
The management of advanced melanoma has been rapidly changing in the past 3 years with the approval of targeted therapies and immunotherapies. We designed a survey to investigate how advanced BRAF-mutant melanoma patients are currently treated in the United States, and we investigated potential differences in treatment approaches depending on the type of setting.
Our survey identified a number of differences per type of setting when it comes to the management of advanced BRAF-mutant melanoma patients. First, the average number of patients treated per oncologist was higher in academic centers than in community-based centers and private clinics. Second, academic centers and private clinics tended to follow more severe patients than community-based centers. Third, academic centers had the highest proportion of patients in second line or over compared with private clinics and community-based centers.
Differences in terms of treatment decisions were also observed. In first line, academic centers favored immunotherapy treatments, while use of targeted therapy was higher in private clinics. In second line or over, private clinics switched from targeted therapy to immunotherapy, while academic centers kept a large proportion of their patients on immunotherapy. As for community-based centers, their use of targeted therapy decreased from 49% of patients in first line to 35% in second line or over. Last, academic centers had the highest proportion of patients included in clinical trials.
These differences illustrate the role that each setting has in the management of advanced melanoma patients in the United States. Community-based centers are involved at the onset of the disease, when the severity is still low, but refer patients to academic centers when the disease progresses. Academic centers have extended experience in the most recent treatments, and provide access to the newest agents through clinical trials. As for private clinics, although they had a very low proportion of patients in clinical trials, their use of recent treatments was similar to academic centers.
When it comes to the choice of targeted therapy molecules, the combination dabrafenib plus trametinib was always preferred over the combination of vemurafenib plus cobimetinib in all types of settings and for all lines of treatment. This is likely due to the fact that dabrafenib plus trametinib was approved first, and the perception that both combinations were equally efficacious with a more favorable side effect profile for dabrafenib plus trametinib, as was recently highlighted by Daud et al.26 Regarding immunotherapies, the use of the combination nivolumab plus ipilimumab was the highest in academic centers. Community-based centers tended to use immunotherapy as a single agent rather than in combination. For the combination nivolumab plus ipilimumab, the difference of use between academic centers and community-based centers could be explained by the fact that academic centers have better capability to treat high-grade autoimmune adverse effect are therefore more comfortable managing the toxic profile of immunotherapies. Other potential explanations include the lack of familiarity among community-based centers with the most recent drugs, and the fact that academic centers might have become familiar with this combination through clinical trials.
Regarding the sequencing of the various drug classes, in the context of immunotherapies having a longer acting duration and being associated with good survival, we investigated which patient characteristics were driving the choice between immunotherapy and targeted therapy. We found that the main decision driver for first-line prescription was the stage and speed of progression of the disease. Patients whose disease was not too severe or with low disease volume, or those whose disease was slowly progressing (including severe patients), were usually prescribed an immunotherapy. If a patient’s disease was severe or rapidly progressing, they received a targeted therapy regardless of the disease severity. This was based on the belief that targeted therapies act faster, and this speed of action counterbalances their severity profile. When patients progressed and required a second-line treatment, most oncologists indicated that they switched classes, prescribing a targeted therapy to those who received an immunotherapy and vice versa.
The impact of these treatment decisions on patients’ outcomes was not measured in our survey, and we can therefore not comment on which sequence or targeted therapy choice is the most beneficial. A recent article from Aya et al compared the outcomes of 2 different cohorts of patients treated with BRAF inhibitors first followed by immunotherapy or the reverse sequence.24 The authors concluded that no differences were observed in OS between the 2 cohorts, although the survey was based on a limited number of patients. Additionally, the ECOG 6134 trial (NCT 02224781) studies whether to start treatment with dabrafenib plus trametinib or with ipilimumab plus nivolumab. Results are expected in July 2019.
It must be noted that this survey has a number of limitations. Although this survey was distributed to a wide array of oncologists, this may not be a representative sample of melanoma-treating physicians as information about nonresponders was not collected. Therefore, caution should be used when generalizing results of this subset of oncologists to the entire advanced melanoma-treating physician population. Additionally, the number of respondents for each setting was low, especially for the community-based oncologists. Therefore, results for this type of setting need to be analyzed with caution, although we believe the data provide important directional information on how patients are treated. Last, the impact of treatment decisions on the patients’ outcomes was not measured as it was outside the scope of this survey, and would have required a more complex research methodology than the one used in this study.
Conclusions
Our survey identified differences in the management of advanced BRAF-mutant melanoma patients depending on the type of setting that cares for these patients. Adoption of new therapy as illustrated by the use of the combination nivolumab plus ipilimumab and targeted therapy was more advanced in academic centers and private clinics than in community-based centers. In terms of drug class sequencing, doctors had the same rationale for prescribing immunotherapy or targeted therapy. The impact of these treatment decisions on patients’ outcomes is not known and requires further research.
Supplementary Material
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Financial support for this research was provided by the Deerfield Institute, the internal research group at Deerfield Management Company, a health care investment firm dedicated to advancing health care through investment, information, and philanthropy.
ORCID iD: Mark Stuntz  https://orcid.org/0000-0001-9646-1781
https://orcid.org/0000-0001-9646-1781
Supplementary Material: Supplementary material is available for this article.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2. McArthur GA, Ribas A. Targeting oncogenic drivers and the immune system in melanoma. J Clin Oncol. 2013;31:499-506. doi: 10.1200/JCO.2012.45.5568. [DOI] [PubMed] [Google Scholar]
- 3. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517-2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 5. Prieto PA, Yang JC, Sherry RM, et al. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012;18:2039-2047. doi: 10.1158/1078-0432.CCR-11-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McDermott D, Lebbé C, Hodi FS, et al. Durable benefit and the potential for long-term survival with immunotherapy in advanced melanoma. Cancer Treat Rev. 2014;40:1056-1064. doi: 10.1016/j.ctrv.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 7. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 8. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521-2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 9. Wolchok J, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345-1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23-34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006-2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809-819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707-714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Falchook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 2012;379:1893-1901. doi: 10.1016/S0140-6736(12)60398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jarkowski A, 3rd, Norris L, Trinh VA. Controversies in the management of advanced melanoma: “gray” areas amid the “black and blue”. Ann Pharmacother. 2014;48:1456-1468. doi: 10.1177/1060028014544165. [DOI] [PubMed] [Google Scholar]
- 17. Falchook GS, Lewis KD, Infante JR, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:782-789. doi: 10.1016/S1470-2045(12)70269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Infante JR, Falchook GS, Lawrence DP, et al. Phase I/II study to assess safety, pharmacokinetics, and efficacy of the oral MEK 1/2 inhibitor GSK1120212 (GSK212) dosed in combination with the oral BRAF inhibitor GSK2118436 (GSK436). J Clin Oncol. 2011;29:CRA8503. [Google Scholar]
- 19. Tran KA, Cheng MY, Mitra A, et al. MEK inhibitors and their potential in the treatment of advanced melanoma: the advantages of combination therapy. Drug Des Devel Ther. 2015;10:43-52. doi: 10.2147/DDDT.S93545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Larkin J, Ascierto P, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867-1876. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 21. Sanlorenzo M, Choudhry A, Vujic I, et al. Comparative profile of cutaneous adverse events: BRAF/MEK inhibitor combination therapy versus BRAF monotherapy in melanoma. J Am Acad Dermatol. 2014;71:1102-1109.e1. doi: 10.1016/j.jaad.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Long G, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicenter, double-blind, phase 3 randomised controlled trial. Lancet. 2015;386:444-451. doi: 10.1016/S0140-6736(15)60898-4. [DOI] [PubMed] [Google Scholar]
- 23. Cooper ZA, Frederick DT, Ahmed Z, Wargo JA. Combining checkpoint inhibitors and BRAF-targeted agents against metastatic melanoma. Oncoimmunology. 2013;2:e24320. doi: 10.4161/onci.24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aya F, Fernandez-Martinez A, Gaba L, et al. Sequential treatment with immunotherapy and BRAF inhibitors in BRAF-mutant advanced melanoma. Clin Transl Oncol. 2017;19:119-124. doi: 10.1007/s12094-016-1514-0. [DOI] [PubMed] [Google Scholar]
- 25. US Department of Health and Human Services. Protection of human subjects 45 CFR §46.116 General requirements for informed consent. https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/index.html#46.116. Accessed November 23, 2017.
- 26. Daud A, Gill J, Kamra S, Chen L, Ahuja A. Indirect treatment comparison of dabrafenib plus trametinib versus vemurafenib plus cobimetinib in previously untreated metastatic melanoma patients. J Hematol Oncol. 2017;10:3. doi: 10.1186/s13045-016-0369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.