Abstract
Peripheral nerve injuries impose significant health and economic consequences, yet no surgical repair can deliver a complete recovery of sensory or motor function. Traditional methods of repair are less than ideal: direct coaptation can only be performed when tension-free repair is possible, and transplantation of nerve autograft can cause donor-site morbidity and neuroma formation. Cell-based therapy delivered via nerve conduits has thus been explored as an alternative method of nerve repair in recent years. Stem cells are promising sources of the regenerative core material in a nerve conduit because stem cells are multipotent in function, abundant in supply, and more accessible than the myelinating Schwann cells. Among different types of stem cells, undifferentiated adipose-derived stem cell (uASC), which can be processed from adipose tissue in less than two hours, is a promising yet underexplored cell type. Studies of uASC have emerged in the past decade and have shown that autologous uASCs are non-immunogenic, easy to access, abundant in supply, and efficacious at promoting nerve regeneration. Two theories have been proposed as the primary regenerative mechanisms of uASC: in situ trans-differentiation towards Schwann cells, and secretion of trophic and anti-inflammatory factors. Future studies need to fully elucidate the mechanisms, side effects, and efficacy of uASC-based nerve regeneration so that uASCs can be utilized in clinical settings.
Keywords: peripheral nerve injury, adipose-derived stem cells, Schwann cells, cell therapy, nerve conduits, axonal regeneration, stem cell differentiation, neurotrophic factors, anti-apoptosis, immunosuppression
Review Structure
The research of undifferentiated adipose-derived stem cells (uASCs) has emerged in the past decade, showing that these stem cells are efficacious at aiding peripheral nerve regeneration in a timely manner. uASCs have promising clinical utility because they can be accessed, processed, and ready-to-be deployed in a matter of hours. However, their efficacy and mechanisms in regenerating peripheral nerves are not fully understood. We therefore intended this review to serve a progress update of the field's current understanding of uASCs in peripheral nerve repair.
After illustrating the Seddon and Sunderland classification of peripheral nerve injury, we reviewed pathophysiology of peripheral nerve injury at different anatomical locations: injury site, distal stump, proximal stump, neuronal cell body, and end organ. What follows is a short introduction of stem cell-based therapy for peripheral nerve repair. Then, we summarized, both with a table and with narration, the findings from 39 original studies published in the past decade on the efficacy of uASCs. Lastly, we reviewed several possible mechanisms through which uASCs promote peripheral nerve repair.
By reviewing the recent studies, we concluded that uASCs were efficacious at aiding peripheral nerve repair through mechanisms that were still unclear. Among the different theories, the secretion of neurotrophic, neuroprotective, and anti-inflammatory factors appears to be the most likely mechanism through which uASCs exerted their impact. We suggest future research fully elucidate the mechanisms, side effects, and efficacy of uASC-based nerve regeneration.
Introduction
Nerve fibers in the peripheral nervous system, which relay sensory and motor information between the brain, the spinal cord and the rest of the body, regenerate more readily than nerve fibers in the central nervous system (Ide, 1996). However, surgical intervention is often if not always needed after severe peripheral nerve injuries secondary to motor vehicle accidents, penetrating traumas, gunshot wounds, and failing injuries (Kouyoumdjian, 2006; Campbell, 2008). Traditional methods of repair include direct coaptation of the proximal and distal stumps of a severed nerve and transplantation of nerve autografts using patient’s own cutaneous nerves (Lee and Wolfe, 2000). However, these techniques are less than ideal: direct coaptation is only beneficial when tension-free repair is achievable while nerve autografts run the risk of creating sensory deficits and even neuroma at the donor site (Grinsell et al., 2014). Therefore, nerve conduits, which are made up of biological, synthetic, or tissue-engineered materials, have been explored as an alternative (Carriel et al., 2014). Moreover, nerve conduits that have been pre-seeded with aligned structures, growth factors, or viable cells render better clinical outcome than empty conduits (Gu et al., 2011; Daly et al., 2012; Lin and Marra, 2012; Carriel et al., 2013). Autologous adipose-derived stem cell (ASC), which is a type of precursor cell obtained and processed from patient’s own adipose tissue, is a promising source of core material in nerve conduits because autologous ASCs are easily accessible, multipotent, and non-immunogenic (Mizuno, 2009; Klein et al., 2016). These ASCs can be differentiated towards a Schwann cell-like phenotype to promote regeneration (Jiang et al., 2008). However, such differentiation is time- and cost-intensive, yielding suboptimal outcomes when urgent nerve repair is called for. Here we review the efficacy and mechanism of autologous undifferentiated ASCs (uASCs) in promoting peripheral nerve regeneration.
Classification of Peripheral Nerve Injury
Seddon (Seddon, 1943) and Sunderland (1990) have developed the widely accepted classification schemes for peripheral nerve injuries (Rosen, 1981; Burnett and Zager, 2004). Neuropraxia (Seddon) or first-degree injury (Sunderland) is the mildest form of nerve injury, which disrupts the surrounding myelin and causes transient functional block but preserves nerve continuity. Axonotmesis (Seddon) or second-degree injury (Sunderland) crushes both the axon and the myelin but spares the surrounding mesenchymal structures including the endoneurium, perineurium, and epineurium. Axonotmesis has a good prognosis because axons can regenerate along the uninjured mesenchymal structures and reinnervate the target organ (Burnett and Zager, 2004). Neurotmesis (Seddon) is the complete severance of nervous continuity, cutting the nerve into a proximal and a distal stump. This is the most severe form of nerve injury and its prognosis is poor without surgical intervention.(Burnett and Zager, 2004) Sunderland further stratified neurotmesis into third, fourth, and fifth-degree injuries, based on the involvement of mesenchymal structures (Figure 1). Most research models are based on neurotmesis or fifth-degree injury, because complete laceration of a nerve is more reproducible than lesser degrees of injury (Derby et al., 1993; Fine et al., 2002; Burnett and Zager, 2004).
Figure 1.
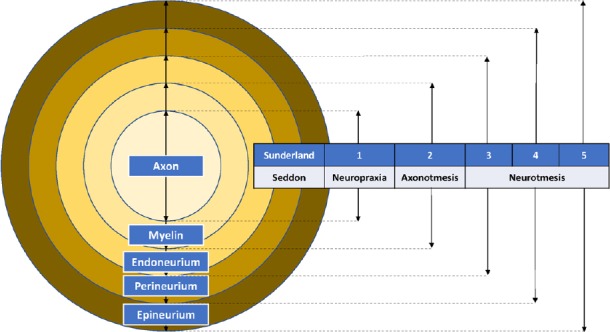
Seddon and Sunderland classification of peripheral nerve injury.
Pathophysiology of Peripheral Nerve Injury
Here we review the site-specific degenerative and regenerative processes following a neurotmesis or fifth-degree injury in the following order: injury site, distal stump, proximal stump, cell body, and end organ. Similar processes are reviewed by Terenghi (Terenghi, 1999) and Burnett and Zager (Burnett and Zager, 2004).
Injury site
After a complete severance of a nerve, the two cut ends will retract and capillary permeability will increase (Madura, 2012). The cellular environment at the injury site is edematous and messy, featuring capillaries, fibroblasts, collagen fibers, macrophages, and Schwann cells (Burnett and Zager, 2004). Burnett noted that degeneration has to occur before regeneration because such an environment is not conducive to healing (Burnett and Zager, 2004).
Distal stump
Within the first few days of injury, Wallerian degeneration takes place at the distal stump where axons degenerate and the myelin sheath detaches and degrades. Macrophages are recruited to phagocytose debris and to activate Schwann cells, which have two roles – assisting phagocytosis and, later on, guiding regenerating axons (Jessen and Mirsky, 2016). In regeneration, Schwann cells proliferate to form the bands of Büngner and secrete neurotrophic factors that travel retrogradely to guide regenerating axons (Frostick et al., 1998)
Proximal stump
After the injury, Wallerian degeneration also takes place in a retrograde fashion up to the first node of Ranvier at the proximal stump (Burnett and Zager, 2004). Within a few hours, however, neuronal sprouts are formed with terminal growth cones searching for neurotrophic factors secreted from the distal stump (Li et al., 2005). When the regenerating axons successfully reach the matrix of the distal stump, they will grow within the bands of Büngner formed by Schwann cells (Bunge, 1994).
Cell body
In a severe nerve injury, the cell body might be damaged even though the injury is distal in the axon. Histologically, the stressed cell body will undergo a characteristic process called chromatolysis, in which the cell body swells, its nucleus migrates to the periphery, and the Nissl bodies, the neuronal protein production sites, break up and disperse (Evans, 2001; Burnett and Zager, 2004). Approximately 40% of involved dorsal root ganglions undergo retrograde cell death after peripheral nerve injuries, primarily when there is deficient target-derived neurotrophic support (Schmidt and Leach, 2003; Hart et al., 2004; Hall, 2005).
End organ
The end organ involved in a peripheral nerve injury is often somatic muscle of the upper or the lower extremities. In a retrospective study of 456 peripheral nerve injuries, Kouyoumdjian (2006) reported that 73.5% injuries happened to the upper limbs while 21.5% to the lower limbs, leaving 5% injuries to the face. After prolonged denervation, muscles fibers will decrease in numbers, cross-sectional area, and force, only recovering partially after reinnervation (Gutmann and Young, 1944; Fu and Gordon, 1995; Rosen, 1981; Burnett and Zager, 2004).
Cell-Based Therapy for Peripheral Nerve Repair
As noted above, Schwann cells play important roles in peripheral nerve regeneration by clearing injury debris, secreting trophic factors, and guiding regenerating axons. Additionally, transplanted Schwann cells have been widely shown to enhance axonal regeneration after peripheral nerve injury (Guénard et al., 1992; Hadlock et al., 2000; Evans et al., 2002). However, Schwann cells are the less-than-ideal cell-therapy to repair peripheral nerves because they are difficult to harvest and time-consuming to expand in culture (Tohill and Terenghi, 2004). In search of a more suitable cell-therapy, researchers and clinicians have turned to stem cells, which have already been explored in many disease models, such as sickle cell anemia, Parkinson’s-like syndrome, and graft-versus-host disease (Daley and Scadden, 2008). Among different types of stem cells, autologous ASC is the most clinically promising option for the following reasons (Mizuno, 2009):
ASCs are processed from patients’ own adipose tissue, therefore they do not engender the ethical concerns often associated with embryonic stem cells (Lo and Parham, 2009);
Compared to the painful procurement of bone marrow-derived stem cells, ASCs can be harvested from adipose tissue obtained from the minimally invasive liposuction procedure under local anesthesia;
Adipose tissue has a higher stem cell yield than bone marrow does (Kern et al., 2006). One gram of adipose tissue can yield 3.5 × 105 to 1 × 106 stem cells, while one gram of bone marrow can only yield 500 to 5 × 104 stem cells (Tsuji, 2014).
ASCs are self-renewal and multipotent, capable of differentiating into mesodermal lineages such as bone, fat, cartilage, and muscle (Zuk, 2013). In peripheral nerve repair, Di Summa et al. (2010) demonstrated that fibrin conduits seeded with ASCs previously differentiated towards a Schwann cell-like phenotype induced greater axonal regeneration than empty conduits did. Although differentiated ASCs (dASCs) yield promising clinical outcome, the process of differentiation can take more than 2.5 weeks (Kingham et al., 2007; Di Summa et al., 2010) which would prolong denervation of the injured nerve and worsen functional recovery.
Efficacy of Autologous uASCs
In recent years, uASC has been explored as an accessible, abundant, multipotent, and efficient source of stem cells. The process of obtaining these stem cells is well documented in both animal experiments and clinical applications (Yoshimura et al., 2008; Sterodimas et al., 2010; Klein et al., 2016; Zhou et al., 2016) Adipose tissue is first harvested either by dissection (in animal models) or by liposuction (in humans) and is then enzymatically digested by collagenase in a buffered solution for 30–60 minutes at 37°C. The solution is filtered, and the infranatant is centrifuged to separate the stem cells from adipocytes and fluids. The cellular pellet is then rinsed and resuspended in a minimal essential medium with fetal bovine serum and antibiotics and is then passed through a mesh to remove debris. The hence obtained stromal vascular fraction, which consists of heterogeneous mesenchymal cells including progenitor cells (Bourin et al., 2013), can either be further expanded in culture for another seven days or can be directly mixed with a previously saved lipoaspirate fat graft and transplanted to the injury site. In urgent situations, the method that combines stromal vascular fraction with fat graft, or otherwise known as cell-assisted lipotransfer, takes no more than 90 minutes for cellular processing and 15 minutes for mixing (Yoshimura et al., 2008).
After searching PubMed (Additional file 1 (72.8KB, pdf) ) for studies from 2008 to 2018, we identified 39 original articles that directly examined the efficacy of uASCs in aiding peripheral nerve repair (Additional Table 1 (4.1MB, tif) ). We summarize the key findings here.
PubMed Search Strategy
In vitro and in vivo experiments that examined the efficacy of undifferentiated adipose-derived stem cells.
1. Utilization of uASCs delivered significantly better results than the control groups, such as empty conduits, at promoting peripheral nerve regeneration. Such improvement was shown in different experiment models, such as sciatic nerve defect (Bloancă et al., 2017), facial nerve defect (Abbas et al., 2016), and cavernous nerve injury (Fandel et al., 2012). The only exception to this pattern of positive effect of uASCs was shown by Tomita et al. (2013) who demonstrated that uASCs did not significantly promote neurite outgrowth compared to the control NG108-15 neuronal cells.
2. Compared to Schwann cells or ASCs differentiated towards the Schwann cell phenotype, uASCs have been found to achieve either similar (Orbay et al., 2012; Watanabe et al., 2014; Sowa et al., 2016) or worse clinical outcomes (Tomita et al., 2013; Kappos et al., 2015).
Hundepool et al. (2014) and Mohammadi et al. (2011) have separately shown that uASCs have similar regenerative efficacy as do bone marrow stromal cells.
3. There still is a debate on the primary regenerative mechanism of uASCs. The two competing hypotheses are in-situ trans-differentiation (Kingham et al., 2007; Orbay et al., 2012; Abbas et al., 2016) and secretion of trophic factors (Santiago et al., 2009; Erba et al., 2010; Carlson et al., 2011; Marconi et al., 2012; Suganuma et al., 2013; Hsieh et al., 2016).
4. Farinazzo et al. (2015), Mohammadi et al. (2016), and Qiu et al. (2012) separately suggest that stromal vascular fraction, which is the rapid acquisition of uASCs from adipose tissue, has therapeutic potential in treatment settings.
Mechanism of uASCs in Aiding Peripheral Nerve Regeneration
Proximal and distal stumps: axonal regeneration
Erba et al. (2010) showed that uASCs could stimulate axonal growth from the proximal stump and even greater Schwann cell proliferation from the distal stump of an injured peripheral nerve. Schwann cells and stem cells that have differentiated towards a Schwann cell phenotype have been shown to promote nerve regeneration (Guénard et al., 1992; Dezawa et al., 2001), but how do the naïve, undifferentiated stem cells freshly harvested from adipose tissue promote axon regrowth? Having found no significant regenerative benefits uASCs, Tomita et al. (2013) argues that these stem cells need to trans-differentiate in situ towards a downstream cell type, most likely Schwann cells, in order to promote axonal regeneration. Wei et al. (2010) showed that, after co-cultured with Schwann cells, ASCs could differentiate into Schwann-like cells, which suggests that Schwann cells at an injury site could induce trans-differentiation of ASCs. However, many studies argue that such trans-differentiation is unlikely. For example, Santiago et al. (2009), Carlson et al. (2011), Suganuma et al. (2013), and Hsieh et al. (2016) and their respective colleagues showed that markers of ASCs and their downstream lineages do not colocalize with markers of Schwann cells, usually S-100 protein, suggesting that those Schwann cells did not belong to the lineage of ASCs.
Besides trans-differentiation, the trophic effect mediated by secreted factors is the other contending explanation for the regenerative ability of uASCs. Salgado et al. (2010) provides an excellent summary of the various soluble factors produced from ASCs in various environments. Among them, glial-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), and insulin-like growth factor-I (IGF-I), nerve growth factor (NGF), and angiopoietin 1 (Ang-1) are the most relevant for nerve regeneration. For example, Shi et al. (2011) showed that intramuscular GDNF gene delivery improved myelination and functional recovery after constriction-induced nerve injury. Lopatina et al. (2011) showed ASCs could not stimulate nervous regrowth if BDNF neutralizing antibodies were introduced. Yamagishi et al. (2003) demonstrated that IGF-I prevented neuronal apoptosis by inhibiting the apoptotic p38-c-Jun pathway. Additionally, Anton et al. (1994) showed that antibodies to NGF strongly inhibited the otherwise robust migration of Schwann cells in denervated models. Besides directly secreting neurotrophic and neuroprotective factors, uASCs have also been shown to recruit and stimulate endogenous Schwann cells to aid regeneration (Hill et al., 2006; Erba et al., 2010; Marconi et al., 2012).
Neuron and end-organ: anti-apoptosis
Besides promoting axonal regrowth from the proximal and distal stumps, uASCs also have the potential to curb neuronal cell death retrogradely and muscular atrophy anterogradely (Reid et al., 2011; Fandel et al., 2012; Masgutov et al., 2016). Reid et al. (2011) showed that ASCs differentiated towards a Schwann cell phenotype significantly increased anti-apoptotic Bcl-2 mRNA expression and significantly decreased pro-apoptotic Bax and caspase-3 mRNA expressions compared to empty conduits. Furthermore, Reid et al. (2011) suggested that the anti-apoptotic property of dASCs were achieved by retrograde delivery of neurotrophic factors, which were shown to be increased in their study. While uASCs have not been shown to be anti-apoptotic, they do secrete neurotrophic factors similar to, although to a lesser extent than, those secreted by dASCs, such as BDNF, NGF, and GDNF (Tomita et al., 2013). In addition, Wei et al. (2009) demonstrated that uASCs secreted IGF-1 and BDNF, both of which are neuroprotective in brain hypoxic-ischemic injury. Therefore, it is likely that uASCs utilize a similar mechanism to prevent neuronal cell death in peripheral nerve injury.
uASCs also curb atrophy in denervated muscles as shown by Santiago et al. (2009). In a 6-mm sciatic nerve defect model, Santiago et al. (2009) measured the E/C ratio of experimental muscle mass to controlled muscle mass (uninjured leg) in four groups – no treatment (0.192 ± 0.024), nerve autograft (0.666 ± 0.070), conduit alone (0.487 ± 0.151), and conduit with uASCs (0.522 ± 0.108). The E/C ratio that is closer to 1 reflects better preservation of the muscle mass. Although the muscle preservative effect of the stem cells is not significant (P = 0.632), the group that received uASCs suffered less muscle atrophy than the group that received no treatment. Studies in different disease models also demonstrated the ability of ASCs to inhibit muscle atrophy. In a murine ischemic hindlimb model, Kang et al. (2010) showed that endothelial-differentiated ASCs promoted angiogenesis and myogenesis. In a burn injury model, Wu et al. (2015) demonstrated that uASCs significantly inhibited denervation atrophy of the gastrocnemius muscle and attenuated apoptotic death of burn injury-induced spinal cord ventral horn motor neurons. Taken together, it is evident that ASCs can curb denervation-induced muscular atrophy.
Regenerating environment: immunosuppression
The immunomodulatory effect of ASCs has been demonstrated in a wide range of disease models such as rheumatoid arthritis, graft-versus-host disease, and tissue repair (Yañez et al., 2006; Hong et al., 2010; Zhang et al., 2017). Similarly, in a sciatic nerve injury model, Marconi et al. (2012) noted that inflammatory infiltrates including both lymphocytes and macrophages have been reduced after uASCs were systemically delivered through intravenous administration. There are two main ways through which uASCs exert their immunomodulatory effects: boosting anti-inflammatory factors and reducing inflammatory ones. To enhance anti-inflammatory effect, ASCs 1) promote regulatory T cells, which trigger the alternative activation of macrophage towards the anti-inflammatory M2 phenotype (Gonzalez-Rey et al., 2010; Kawanishi et al., 2010; Guo et al., 2016; Bowles et al., 2017; Zhang et al., 2017), and 2) reduce the level of interleukin-10 (IL-10), which is a potent immunosuppressant in vitro (de Vries, 1995; Franchi et al., 2014; Lee et al., 2015; Zhang et al., 2017). Cui et al. (2007) also demonstrated that prostaglandin E2 (PGE2) might be the principal factor responsible for ASC-mediated immune suppression. When exposed to the pro-inflammatory environment of mixed lymphocyte reactions (MLRs), ASCs expressed significantly higher levels of PGE2, and subsequent PGE2-inhibitor counteracted the immunosuppression, thereby proving the pivotal role of PGE2 in immunomodulation. To downgrade inflammatory tone, ASCs 1) inhibit the proliferation and cytokine production of T cells in response to mitogens (Yañez et al., 2006; Gonzalez-Rey et al., 2010), and 2) decrease the production of inflammatory cytokines and growth factors, such as tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), and interleukin-6 (IL-6) (Premaratne et al., 2011; Franchi et al., 2014; Lee et al., 2015; Guo et al., 2016; Zhang et al., 2017). Melief et al. (2013) also notes that ASCs demonstrate better immunomodulatory potency than bone marrow-derived stem cells, which are considered the prototypical mesenchymal stem cells.
Limitations of uASCs in Peripheral Nerve Regeneration
There are at least three concerns when using uASCs for peripheral nerve regeneration.
First and foremost, uASCs can potentially differentiate into unwanted cell types of mesenchymal lineage or form teratomas. However, the risk of these processes is low. Differentiation of uASCs into specific mesenchymal lineages requires weeks of culturing using lineage-specific support medium (Banas et al., 2007). Santiago et al. (2009) has also shown that uASCs did not differentiate into Schwann cells after 12 weeks. The risk of teratoma formation is even lower, because uASCs only develop into cells of mesodermal lineages, while the composition of teratoma requires all three germ cell layers, namely ectoderm, mesoderm, and endoderm (Sun et al., 2009). Nonetheless, if uASCs were to be used clinically, future studies would still have to investigate the risk of spontaneous differentiation and teratoma formation.
Second, the regenerative potential of uASCs can be limited compared to that of Schwann cells or ASCs differentiated towards the Schwann cell phenotype (Tomita et al., 2013; Kappos et al., 2015). Although uASCs can be delivered in a time-efficient manner through stromal vascular fraction, the comparative efficacy of uASCs still needs to be studied.
Third, it is unclear whether the peripherally transplanted uASCs could affect far organs, such as the brain. Wei et al. (2009) and Marconi et al. (2013) have systemically injected uASCs to separately evaluate the effect of these stem cells on the brain and the peripheral nervous system. In both cases, the systemic delivery of uASCs has had favourable effect on their target organs. However, it is unclear whether the local transplantation of uASCs in conduits would have affected far organs.
Conclusion
Research in the past decade has demonstrated that uASCs are efficacious at promoting peripheral nerve repair, although the principal mechanism of repair is still under debate. Several possible mechanisms have been proposed, such as in-situ trans-differentiation towards Schwann cells, secretion of neurotrophic and neuroprotective factors, and immunosuppression. In-situ trans-differentiation seems the least likely explanation as several studies did not observe co-localization between Schwann cells and cells of ASC cell lineage. Secretion of soluble factors, whether anti-inflammatory, neurotrophic, or neuroprotective, seems to account for most of the regenerative ability of uASC. It is important to note that these stem cells are often transplanted at the injury site, therefore secreted factors must have been transported both retrogradely and anterogradely to deliver therapeutic benefits. However, future experiments should confirm the traveling course of these factors. From a clinical standpoint, the overwhelming advantage of autologous uASC lies in the fact that it can be harvested, processed, and ready-to-be-deployed in less than 2 hours through cell-assisted lipotransfer. In order to safely and effectively utilize uASCs in urgent peripheral nerve repair, future studies need to fully elucidate the mechanisms, side effects, and efficacy of uASC-based nerve regeneration.
Additional files:
Additional file 1 (72.8KB, pdf) : PubMed search strategy.
Additional Table 1 (4.1MB, tif) : In vitro and in vivo experiments that examined the efficacy of undifferentiated adipose-derived stem cells.
Acknowledgments
The authors would like to thank Mr. Jacob T. Borodovsky, Ph.D. student at The Dartmouth Institute for Health Policy and Clinical Practice, for his general advice on communication through scientific writing.
Footnotes
Conflicts of interest: JMR is a board-certified plastic surgeon who specializes in reconstructive surgery, peripheral nerve surgery, microsurgery, and many other areas of plastic surgery. He has published and presented extensively on peripheral nerve repair, regenerative medicine, and telemedicine and cybercare. JMR holds a United States patent on a microelectronic axon processor that restores nerve function after severance.
Financial support: This work was supported by the Summer Research Funding of Medical Student Research Fellowships at Dartmouth Geisel School of Medicine to RZ.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was supported by the Summer Research Funding of Medical Student Research Fellowships at Dartmouth Geisel School of Medicine to RZ.
References
- 1.Abbas OL, Borman H, Uysal ÇA, Gönen ZB, Aydin L, Helvacioğlu F, Ilhan Ş, Yazici AC. Adipose-derived stem cells enhance axonal regeneration through cross-facial nerve grafting in a rat model of facial paralysis. Plast Reconstr Surg. 2016;138:387–396. doi: 10.1097/PRS.0000000000002351. [DOI] [PubMed] [Google Scholar]
- 2.Anton ES, Weskamp G, Reichardt LF, Matthew WD. Nerve growth factor and its low-affinity receptor promote Schwann cell migration. Proc Natl Acad Sci U S A. 1994;91:2795–2799. doi: 10.1073/pnas.91.7.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Quinn G, Okochi H, Ochiya T. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology. 2007;46:219–228. doi: 10.1002/hep.21704. [DOI] [PubMed] [Google Scholar]
- 4.Bloancă V, Ceauşu AR, Jitariu AA, Barmayoun A, Moş R, Crăiniceanu Z, Bratu T. Adipose tissue graft improves early but not late stages of nerve regeneration. In Vivo. 2017;31:649–655. doi: 10.21873/invivo.11107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowles AC, Wise RM, Gerstein BY, Thomas RC, Ogelman R, Febbo I, Bunnell BA. Immunomodulatory effects of adipose stromal vascular fraction cells promote alternative activation macrophages to repair tissue damage. Stem Cells. 2017;35:2198–2207. doi: 10.1002/stem.2689. [DOI] [PubMed] [Google Scholar]
- 7.Bunge RP. The role of the Schwann cell in trophic support and regeneration. J Neurol. 1994;242:S19–S21. doi: 10.1007/BF00939235. [DOI] [PubMed] [Google Scholar]
- 8.Burnett MG, Zager EL. Pathophysiology of peripheral nerve injury: a brief review. Neurosurg Focus. 2004;16:1–7. doi: 10.3171/foc.2004.16.5.2. [DOI] [PubMed] [Google Scholar]
- 9.Campbell WW. Evaluation and management of peripheral nerve injury. Clin Neurophysiol. 2008;119:1951–1965. doi: 10.1016/j.clinph.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Carlson KB, Dhadilla PS, Feaster MM, Ramnarain A, Pavlides C, Chen ZL, Yu WM, Feltri ML, Strickland S. Mesenchymal stem cells facilitate axon sorting, myelination, and functional recovery in paralyzed mice deficient in Schwann cell-derived laminin. Glia. 2011;59:267–277. doi: 10.1002/glia.21099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carriel V, Alaminos M, Garzón I, Campos A, Cornelissen M. Tissue engineering of the peripheral nervous system. Expert Rev Neurother. 2014;14:301–318. doi: 10.1586/14737175.2014.887444. [DOI] [PubMed] [Google Scholar]
- 12.Carriel V, Garrido-Gómez J, Hernández-Cortés P, Garzón I, García-García S, Sáez-Moreno JA, del Carmen Sánchez-Quevedo M, Campos A, Alaminos M. Combination of fibrin-agarose hydrogels and adipose-derived mesenchymal stem cells for peripheral nerve regeneration. J Neural Eng. 2013;10:026022. doi: 10.1088/1741-2560/10/2/026022. [DOI] [PubMed] [Google Scholar]
- 13.Carriel V, Garzón I, Campos A, Cornelissen M, Alaminos M. Differential expression of GAP-43 and neurofilament during peripheral nerve regeneration through bio-artificial conduits: GAP-43 and neurofilament during nerve regeneration. J Tissue Eng Regen Med. 2017;11:553–563. doi: 10.1002/term.1949. [DOI] [PubMed] [Google Scholar]
- 14.Cherubino M, Pellegatta I, Crosio A, Valdatta L, Geuna S, Gornati R, Tos P. Use of human fat grafting in the prevention of perineural adherence: Experimental study in athymic mouse. PLoS One. 2017;12:e0176393. doi: 10.1371/journal.pone.0176393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui L, Yin S, Liu W, Li N, Zhang W, Cao Y. Expanded adipose-derived stem cells suppress mixed lymphocyte reaction by secretion of prostaglandin E2. Tissue Eng. 2007;13:1185–1195. doi: 10.1089/ten.2006.0315. [DOI] [PubMed] [Google Scholar]
- 16.Daley GQ, Scadden DT. Prospects for stem cell-based therapy. Cell. 2008;132:544–548. doi: 10.1016/j.cell.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Daly W, Yao L, Zeugolis D, Windebank A, Pandit A. A biomaterials approach to peripheral nerve regeneration: bridging the peripheral nerve gap and enhancing functional recovery. J R Soc Interface. 2012;9:202–221. doi: 10.1098/rsif.2011.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Vries JE. Immunosuppressive and anti-inflammatory properties of interleukin 10. Ann Med. 1995;27:537–541. doi: 10.3109/07853899509002465. [DOI] [PubMed] [Google Scholar]
- 19.Derby A, Engleman VW, Frierdich GE, Neises G, Rapp SR, Roufa DG. Nerve growth factor facilitates regeneration across nerve gaps: morphological and behavioral studies in rat sciatic nerve. Exp Neurol. 1993;119:176–191. doi: 10.1006/exnr.1993.1019. [DOI] [PubMed] [Google Scholar]
- 20.Dezawa M, Takahashi I, Esaki M, Takano M, Sawada H. Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiated bone-marrow stromal cells. Eur J Neurosci. 2001;14:1771–1776. doi: 10.1046/j.0953-816x.2001.01814.x. [DOI] [PubMed] [Google Scholar]
- 21.Di Summa PG, Kingham PJ, Raffoul W, Wiberg M, Terenghi G, Kalbermatten DF. Adipose-derived stem cells enhance peripheral nerve regeneration. J Plast Reconstr Aesthet Surg. 2010;63:1544–1552. doi: 10.1016/j.bjps.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Erba P, Mantovani C, Kalbermatten DF, Pierer G, Terenghi G, Kingham PJ. Regeneration potential and survival of transplanted undifferentiated adipose tissue-derived stem cells in peripheral nerve conduits. J Plast Reconstr Aesthet Surg. 2010;63:e811–e817. doi: 10.1016/j.bjps.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 23.Evans GR, Brandt K, Katz S, Chauvin P, Otto L, Bogle M, Wang B, Meszlenyi RK, Lu L, Mikos AG, Patrick CW. Bioactive poly(l-lactic acid) conduits seeded with Schwann cells for peripheral nerve regeneration. Biomaterials. 2002;23:841–848. doi: 10.1016/s0142-9612(01)00190-9. [DOI] [PubMed] [Google Scholar]
- 24.Evans GR. Peripheral nerve injury: a review and approach to tissue engineered constructs. Anat Rec. 2001;263:396–404. doi: 10.1002/ar.1120. [DOI] [PubMed] [Google Scholar]
- 25.Fandel TM, Albersen M, Lin G, Qiu X, Ning H, Banie L, Lue TF, Lin CS. Recruitment of intracavernously injected adipose-derived stem cells to the major pelvic ganglion improves erectile function in a rat model of cavernous nerve injury. Eur Urol. 2012;61:201–210. doi: 10.1016/j.eururo.2011.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farinazzo A, Turano E, Marconi S, Bistaffa E, Bazzoli E, Bonetti B. Murine adipose-derived mesenchymal stromal cell vesicles: in vitro clues for neuroprotective and neuroregenerative approaches. Cytotherapy. 2015;17:571–578. doi: 10.1016/j.jcyt.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Fine EG, Decosterd I, Papaloïzos M, Zurn AD, Aebischer P. GDNF and NGF released by synthetic guidance channels support sciatic nerve regeneration across a long gap. Eur J Neurosci. 2002;15:589–601. doi: 10.1046/j.1460-9568.2002.01892.x. [DOI] [PubMed] [Google Scholar]
- 28.Franchi S, Castelli M, Amodeo G, Niada S, Ferrari D, Vescovi A, Brini AT, Panerai AE, Sacerdote P. Adult stem cell as new advanced therapy for experimental neuropathic pain treatment. BioMed Res Int. 2014;2014:470983. doi: 10.1155/2014/470983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frostick SP, Yin Q, Kemp GJ. Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery. 1998;18:397–405. doi: 10.1002/(sici)1098-2752(1998)18:7<397::aid-micr2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 30.Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged denervation. J Neurosci Off J Soc Neurosci. 1995;15:3886–3895. doi: 10.1523/JNEUROSCI.15-05-03886.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghoreishian M, Rezaei M, Beni BH, Javanmard SH, Attar BM, Zalzali H. Facial nerve repair with Gore-Tex tube and adipose-derived stem cells: An animal study in dogs. J Oral Maxillofac Surg. 2013;71:577–587. doi: 10.1016/j.joms.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez-Rey E, Gonzalez MA, Varela N, O’Valle F, Hernandez-Cortes P, Rico L, Buscher D, Delgado M. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann Rheum Dis. 2010;69:241–248. doi: 10.1136/ard.2008.101881. [DOI] [PubMed] [Google Scholar]
- 33.Grinsell D, Keating CP, Grinsell D, Keating CP. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. BioMed Res Int. 2014;2014:1–13. doi: 10.1155/2014/698256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gu X, Ding F, Yang Y, Liu J. Construction of tissue engineered nerve grafts and their application in peripheral nerve regeneration. Prog Neurobiol. 2011;93:204–230. doi: 10.1016/j.pneurobio.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Guénard V, Kleitman N, Morrissey TK, Bunge RP, Aebischer P. Syngeneic Schwann cells derived from adult nerves seeded in semipermeable guidance channels enhance peripheral nerve regeneration. J Neurosci. 1992;12:3310–3320. doi: 10.1523/JNEUROSCI.12-09-03310.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo J, Nguyen A, Banyard DA, Fadavi D, Toranto JD, Wirth GA, Paydar KZ, Evans GRD, Widgerow AD. Stromal vascular fraction: A regenerative reality?. Part 2: Mechanisms of regenerative action. J Plast Reconstr Aesthet Surg. 2016;69:180–188. doi: 10.1016/j.bjps.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 37.Gutmann E, Young JZ. The re-innervation of muscle after various periods of atrophy. J Anat. 1944;78:15–43. [PMC free article] [PubMed] [Google Scholar]
- 38.Hadlock T, Sundback C, Hunter D, Cheney M, Vacanti JP. A polymer foam conduit seeded with schwann cells promotes guided peripheral nerve regeneration. Tissue Eng. 2000;6:119–127. doi: 10.1089/107632700320748. [DOI] [PubMed] [Google Scholar]
- 39.Hall S. The response to injury in the peripheral nervous system. J Bone Jt Surg. 2005;87:1309–1319. doi: 10.1302/0301-620X.87B10.16700. [DOI] [PubMed] [Google Scholar]
- 40.Hart AM, Terenghi G, Kellerth JO, Wiberg M. Sensory neuroprotection, mitochondrial preservation, and therapeutic potential of N-acetyl-cysteine after nerve injury. Neuroscience. 2004;125:91–101. doi: 10.1016/j.neuroscience.2003.12.040. [DOI] [PubMed] [Google Scholar]
- 41.Hernández-Cortés P, Toledo-Romero MA, Delgado M, Gonzalez-Rey E, Gómez Sánchez R, Prados-Olleta N, Aneiros-Fernández J, Crespo-Lora V, Aguilar M, Galindo-Moreno P, O’Valle F. Ghrelin and adipose-derived mesenchymal stromal cells improve nerve regeneration in a rat model of epsilon-caprolactone conduit reconstruction. Histol Histopathol. 2017;32:627–637. doi: 10.14670/HH-11-828. [DOI] [PubMed] [Google Scholar]
- 42.Hill CE, Moon LDF, Wood PM, Bunge MB. Labeled Schwann cell transplantation: Cell loss, host Schwann cell replacement, and strategies to enhance survival. Glia. 2006;53:338–343. doi: 10.1002/glia.20287. [DOI] [PubMed] [Google Scholar]
- 43.Hong SJ, Traktuev DO, March KL. Therapeutic potential of adipose-derived stem cells in vascular growth and tissue repair. Curr Opin Organ Transplant. 2010;15:86–91. doi: 10.1097/MOT.0b013e328334f074. [DOI] [PubMed] [Google Scholar]
- 44.Hsieh SC, Chang CJ, Cheng WT, Tseng TC, Hsu SH. Effect of an epineurial-like biohybrid nerve conduit on nerve regeneration. Cell Transplant. 2016;25:559–574. doi: 10.3727/096368915X688920. [DOI] [PubMed] [Google Scholar]
- 45.Hsueh YY, Chang YJ, Huang TC, Fan SC, Wang DH, Chen JJ, Wu CC, Lin SC. Functional recoveries of sciatic nerve regeneration by combining chitosan-coated conduit and neurosphere cells induced from adipose-derived stem cells. Biomaterials. 2014;35:2234–2244. doi: 10.1016/j.biomaterials.2013.11.081. [DOI] [PubMed] [Google Scholar]
- 46.Hundepool CA, Nijhuis THJ, Mohseny B, Selles RW, Hovius SER. The effect of stem cells in bridging peripheral nerve defects: a meta-analysis: A review. J Neurosurg. 2014;121:195–209. doi: 10.3171/2014.4.JNS131260. [DOI] [PubMed] [Google Scholar]
- 47.Ide C. Peripheral nerve regeneration. Neurosci Res. 1996;25:101–121. doi: 10.1016/0168-0102(96)01042-5. [DOI] [PubMed] [Google Scholar]
- 48.Jessen KR, Mirsky R. The repair Schwann cell and its function in regenerating nerves. J Physiol. 2016;594:3521–3531. doi: 10.1113/JP270874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang L, Zhu J, Liu X, Xiang P, Hu J, Yu WH. Differentiation of rat adipose tissue-derived stem cells into Schwann-like cells in vitro. Neuroreport. 2008;19:1015–1019. doi: 10.1097/WNR.0b013e3283040efc. [DOI] [PubMed] [Google Scholar]
- 50.Kalbermatten DF, Schaakxs D, Kingham PJ, Wiberg M. Neurotrophic activity of human adipose stem cells isolated from deep and superficial layers of abdominal fat. Cell Tissue Res. 2011;344:251–260. doi: 10.1007/s00441-011-1142-5. [DOI] [PubMed] [Google Scholar]
- 51.Kang Y, Park C, Kim D, Seong CM, Kwon K, Choi C. Unsorted human adipose tissue-derived stem cells promote angiogenesis and myogenesis in murine ischemic hindlimb model. Microvasc Res. 2010;80:310–316. doi: 10.1016/j.mvr.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 52.Kappos EA, Engels PE, Tremp M, Meyer zu Schwabedissen M, di Summa P, Fischmann A, von Felten S, Scherberich A, Schaefer DJ, Kalbermatten DF. Peripheral nerve repair: multimodal comparison of the long-term regenerative potential of adipose tissue-derived cells in a biodegradable conduit. Stem Cells Dev. 2015;24:2127–2141. doi: 10.1089/scd.2014.0424. [DOI] [PubMed] [Google Scholar]
- 53.Kawanishi N, Yano H, Yokogawa Y, Suzuki K. Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exerc Immunol Rev. 2010;16:105–118. [PubMed] [Google Scholar]
- 54.Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 55.Kingham PJ, Kalbermatten DF, Mahay D, Armstrong SJ, Wiberg M, Terenghi G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp Neurol. 2007;207:267–274. doi: 10.1016/j.expneurol.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 56.Klein SM, Vykoukal J, Li DP, Pan HL, Zeitler K, Alt E, Geis S, Felthaus O, Prantl L. Peripheral motor and sensory nerve conduction following transplantation of undifferentiated autologous adipose tissue-derived stem cells in a biodegradable U.S. Food and Drug Administration-approved nerve conduit. Plast Reconstr Surg. 2016;138:132–139. doi: 10.1097/PRS.0000000000002291. [DOI] [PubMed] [Google Scholar]
- 57.Kouyoumdjian JA. Peripheral nerve injuries: A retrospective survey of 456 cases. Muscle Nerve. 2006;34:785–788. doi: 10.1002/mus.20624. [DOI] [PubMed] [Google Scholar]
- 58.Lee HY, Lee HL, Yun Y, Kim JS, Ha Y, Yoon DH, Lee SH, Shin DA. Human adipose stem cells improve mechanical allodynia and enhance functional recovery in a rat model of neuropathic pain. Tissue Eng Part A. 2015;21:2044–2052. doi: 10.1089/ten.TEA.2014.0713. [DOI] [PubMed] [Google Scholar]
- 59.Lee SK, Wolfe SW. Peripheral nerve injury and repair. J Am Acad Orthop Surg. 2000;8:243–252. doi: 10.5435/00124635-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 60.Li Y, Jia Y-C, Cui K, Li N, Zheng Z-Y, Wang Y, Yuan X. Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature. 2005;434:894–898. doi: 10.1038/nature03477. [DOI] [PubMed] [Google Scholar]
- 61.Lin G, Albersen M, Harraz AM, Fandel TM, Garcia M, McGrath MH, Konety BR, Lue TF, Lin CS. Cavernous nerve repair with allogenic adipose matrix and autologous adipose-derived stem cells. Urology. 2011;77:1509.e1–8. doi: 10.1016/j.urology.2010.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin YC, Marra KG. Injectable systems and implantable conduits for peripheral nerve repair. Biomed Mater. 2012;7:024102. doi: 10.1088/1748-6041/7/2/024102. [DOI] [PubMed] [Google Scholar]
- 63.Lo B, Parham L. Ethical issues in stem cell research. Endocr Rev. 2009;30:204–213. doi: 10.1210/er.2008-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lopatina T, Kalinina N, Karagyaur M, Stambolsky D, Rubina K, Revischin A, Pavlova G, Parfyonova Y, Tkachuk V. Adipose-derived stem cells stimulate regeneration of peripheral nerves: BDNF secreted by these cells promotes nerve healing and axon growth de novo. PLoS ONE. 2011;6:e17899. doi: 10.1371/journal.pone.0017899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luo H, Zhang Y, Zhang Z, Jin Y. The protection of MSCs from apoptosis in nerve regeneration by TGFβ1 through reducing inflammation and promoting VEGF-dependent angiogenesis. Biomaterials. 2012;33:4277–4287. doi: 10.1016/j.biomaterials.2012.02.042. [DOI] [PubMed] [Google Scholar]
- 66.Madura T. Rayegani SM, editor. Pathophysiology of peripheral nerve injury. Basic Principles of Peripheral Nerve Disorders. 2012:1–16. InTech. [Google Scholar]
- 67.Marconi S, Bonaconsa M, Scambi I, Squintani GM, Rui W, Turano E, Ungaro D, D’Agostino S, Barbieri F, Angiari S, Farinazzo A, Constantin G, Del Carro U, Bonetti B, Mariotti R. Systemic treatment with adipose-derived mesenchymal stem cells ameliorates clinical and pathological features in the amyotrophic lateral sclerosis murine model. Neuroscience. 2013;248:333–343. doi: 10.1016/j.neuroscience.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 68.Marconi S, Castiglione G, Turano E, Bissolotti G, Angiari S, Farinazzo A, Constantin G, Bedogni G, Bedogni A, Bonetti B. Human adipose-derived mesenchymal stem cells systemically injected promote peripheral nerve regeneration in the mouse model of sciatic crush. Tissue Eng Part A. 2012;18:1264–1272. doi: 10.1089/ten.TEA.2011.0491. [DOI] [PubMed] [Google Scholar]
- 69.Masgutov RF, Masgutova GA, Zhuravleva MN, Salafutdinov II, Mukhametshina RT, Mukhamedshina YO, Lima LM, Reis HJ, Kiyasov AP, Palotás A, Rizvanov AA. Human adipose-derived stem cells stimulate neuroregeneration. Clin Exp Med. 2016;16:451–461. doi: 10.1007/s10238-015-0364-3. [DOI] [PubMed] [Google Scholar]
- 70.Melief SM, Zwaginga JJ, Fibbe WE, Helene R. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl Med. 2013;2:455–463. doi: 10.5966/sctm.2012-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mizuno H. Adipose-derived stem cells for tissue repair and regeneration: ten years of research and a literature review. J Nippon Med Sch. 2009;76:56–66. doi: 10.1272/jnms.76.56. [DOI] [PubMed] [Google Scholar]
- 72.Mohammadi R, Azizi S, Amini K. Effects of undifferentiated cultured omental adipose-derived stem cells on peripheral nerve regeneration. J Surg Res. 2013;180:e91–e97. doi: 10.1016/j.jss.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 73.Mohammadi R, Azizi S, Delirezh N, Hobbenaghi R, Amini K. Comparison of beneficial effects of undifferentiated cultured bone marrow stromal cells and omental adipose-derived nucleated cell fractions on sciatic nerve regeneration. Muscle Nerve. 2011;43:157–163. doi: 10.1002/mus.21895. [DOI] [PubMed] [Google Scholar]
- 74.Mohammadi R, Mehrtash M, Mehrtash M, Sajjadi SS. Nonexpanded adipose stromal vascular fraction local therapy on peripheral nerve regeneration using allografts. J Invest Surg. 2016;29:149–156. doi: 10.3109/08941939.2015.1093046. [DOI] [PubMed] [Google Scholar]
- 75.Orbay H, Uysal AC, Hyakusoku H, Mizuno H. Differentiated and undifferentiated adipose-derived stem cells improve function in rats with peripheral nerve gaps. J Plast Reconstr Aesthet Surg. 2012;65:657–664. doi: 10.1016/j.bjps.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 76.Perry VH, Brown MC, Gordon S. The macrophage response to central and peripheral nerve injury. J Exp Med. 1987;165:1218–1223. doi: 10.1084/jem.165.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Premaratne GU, Ma LP, Fujita M, Lin X, Bollano E, Fu M. Stromal vascular fraction transplantation as an alternative therapy for ischemic heart failure: anti-inflammatory role. J Cardiothorac Surg. 2011;6:43. doi: 10.1186/1749-8090-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qiu X, Fandel TM, Ferretti L, Albersen M, Zhang H, Lin G, Lin C-S, Schroeder T, Lue TF. Both immediate and delayed intracavernous injection of autologous adipose-derived stromal vascular fraction enhances recovery of erectile function in a rat model of cavernous nerve injury. Eur Urol. 2012;62:720–727. doi: 10.1016/j.eururo.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reid AJ, Sun M, Wiberg M, Downes S, Terenghi G, Kingham PJ. Nerve repair with adipose-derived stem cells protects dorsal root ganglia neurons from apoptosis. Neuroscience. 2011;199:515–522. doi: 10.1016/j.neuroscience.2011.09.064. [DOI] [PubMed] [Google Scholar]
- 80.Rosen J. Concepts of peripheral nerve repair. Ann Plast Surg. 1981;7:165–171. doi: 10.1097/00000637-198108000-00016. [DOI] [PubMed] [Google Scholar]
- 81.Salgado AJ, Reis RL, Sousa N, Gimble JM. Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther. 2010;5:103–110. doi: 10.2174/157488810791268564. [DOI] [PubMed] [Google Scholar]
- 82.Santiago LY, Clavijo-Alvarez J, Brayfield C, Rubin JP, Marra KG. Delivery of adipose-derived precursor cells for peripheral nerve repair. Cell Transpl. 2009;18:145–158. doi: 10.3727/096368909788341289. [DOI] [PubMed] [Google Scholar]
- 83.Schmidt CE, Leach JB. Neural tissue engineering: Strategies for repair and regeneration. Annu Rev Biomed Eng. 2003;5:293–347. doi: 10.1146/annurev.bioeng.5.011303.120731. [DOI] [PubMed] [Google Scholar]
- 84.Seddon H. Three types of nerve injury. Brain. 1943;6:237–288. [Google Scholar]
- 85.Shi JY, Liu GS, Liu LF, Kuo SM, Ton CH, Wen ZH, Tee R, Chen CH, Huang HT, Chen CL, Chao D, Tai MH. Glial cell line–derived neurotrophic factor gene transfer exerts protective effect on axons in sciatic nerve following constriction-induced peripheral nerve injury. Hum Gene Ther. 2011;22:721–731. doi: 10.1089/hum.2010.036. [DOI] [PubMed] [Google Scholar]
- 86.Son YJ, Thompson WJ. Schwann cell processes guide regeneration of peripheral axons. Neuron. 1995;14:125–132. doi: 10.1016/0896-6273(95)90246-5. [DOI] [PubMed] [Google Scholar]
- 87.Sowa Y, Imura T, Numajiri T, Nishino K, Fushiki S. Adipose-derived stem cells produce factors enhancing peripheral nerve regeneration: influence of age and anatomic site of origin. Stem Cells Dev. 2012;21:1852–1862. doi: 10.1089/scd.2011.0403. [DOI] [PubMed] [Google Scholar]
- 88.Sowa Y, Kishida T, Imura T, Numajiri T, Nishino K, Tabata Y, Mazda O. Adipose-derived stem cells promote peripheral nerve regeneration in vivo without differentiation into schwann-like lineage. Plast Reconstr Surg. 2016;137:318e–330e. doi: 10.1097/01.prs.0000475762.86580.36. [DOI] [PubMed] [Google Scholar]
- 89.Sterodimas A, de Faria J, Nicaretta B, Papadopoulos O, Papalambros E, Illouz YG. Cell-assisted lipotransfer. Aesthet Surg J. 2010;30:78–82. doi: 10.1177/1090820X10362730. [DOI] [PubMed] [Google Scholar]
- 90.Suganuma S, Tada K, Hayashi K. Uncultured adipose-derived regenerative cells promote peripheral nerve regeneration. J Orthop Sci. 2013;18:145–151. doi: 10.1007/s00776-012-0306-9. [DOI] [PubMed] [Google Scholar]
- 91.Sun F, Zhou K, Mi W, Qiu J. Repair of facial nerve defects with decellularized artery allografts containing autologous adipose-derived stem cells in a rat model. Neurosci Lett. 2011;499:104–108. doi: 10.1016/j.neulet.2011.05.043. [DOI] [PubMed] [Google Scholar]
- 92.Sun N, Panetta NJ, Gupta DM, Wilson KD, Lee A, Jia F, Hu S, Cherry AM, Robbins RC, Longaker MT, Wu JC. Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. Proc Natl Acad Sci. 2009;106:15720–15725. doi: 10.1073/pnas.0908450106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sunderland S. The Anatomy and Physiology of Nerve Injury. Muscle Nerve. 1990;13:771–784. doi: 10.1002/mus.880130903. [DOI] [PubMed] [Google Scholar]
- 94.Terenghi G. Peripheral nerve regeneration and neurotrophic factors. J Anat. 1999;194:1–14. doi: 10.1046/j.1469-7580.1999.19410001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tohill M, Terenghi G. Stem-cell plasticity and therapy for injuries of the peripheral nervous system. Biotechnol Appl Biochem. 2004;40:17–24. doi: 10.1042/BA20030173. [DOI] [PubMed] [Google Scholar]
- 96.Tomita K, Madura T, Sakai Y, Yano K, Terenghi G, Hosokawa K. Glial differentiation of human adipose-derived stem cells: Implications for cell-based transplantation therapy. Neuroscience. 2013;236:55–65. doi: 10.1016/j.neuroscience.2012.12.066. [DOI] [PubMed] [Google Scholar]
- 97.Tremp M, Meyer Zu Schwabedissen M, Kappos EA, Engels PE, Fischmann A, Scherberich A, Schaefer DJ, Kalbermatten DF. The regeneration potential after human and autologous stem cell transplantation in a rat sciatic nerve injury model can be monitored by MRI. Cell Transplant. 2015;24:203–211. doi: 10.3727/096368913X676934. [DOI] [PubMed] [Google Scholar]
- 98.Tsuji W. Adipose-derived stem cells: Implications in tissue regeneration. World J Stem Cells. 2014;6:312. doi: 10.4252/wjsc.v6.i3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Watanabe Y, Sasaki R, Matsumine H, Yamato M, Okano T. Undifferentiated and differentiated adipose-derived stem cells improve nerve regeneration in a rat model of facial nerve defect. J Tissue Eng Regen Med. 2014;11:362–374. doi: 10.1002/term.1919. [DOI] [PubMed] [Google Scholar]
- 100.Wei X, Du Z, Zhao L, Feng D, Wei G, He Y, Tan J, Lee WH, Hampel H, Dodel R, Johnstone BH, March KL, Farlow MR, Du Y. IFATS Collection: The conditioned media of adipose stromal cells protect against hypoxia-ischemia-induced brain damage in neonatal rats. Stem Cells. 2009;27:478–488. doi: 10.1634/stemcells.2008-0333. [DOI] [PubMed] [Google Scholar]
- 101.Wei Y, Gong K, Zheng Z, Liu L, Wang A, Zhang L, Ao Q, Gong Y, Zhang X. Schwann-like cell differentiation of rat adipose-derived stem cells by indirect co-culture with Schwann cells in vitro. Cell Prolif. 2010;43:606–616. doi: 10.1111/j.1365-2184.2010.00710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wei Y, Gong K, Zheng Z, Wang A, Ao Q, Gong Y, Zhang X. Chitosan/silk fibroin-based tissue-engineered graft seeded with adipose-derived stem cells enhances nerve regeneration in a rat model. J Mater Sci Mater Med. 2011;22:1947–1964. doi: 10.1007/s10856-011-4370-z. [DOI] [PubMed] [Google Scholar]
- 103.Wu SH, Huang SH, Lo YC, Chai CY, Lee SS, Chang KP, Lin SD, Lai CS, Yeh JL, Kwan AL. Autologous adipose-derived stem cells attenuate muscular atrophy and protect spinal cord ventral horn motor neurons in an animal model of burn injury. Cytotherapy. 2015;17:1066–1075. doi: 10.1016/j.jcyt.2015.03.687. [DOI] [PubMed] [Google Scholar]
- 104.Yamagishi S, Matsumoto T, Yokomaku D, Hatanaka H, Shimoke K, Yamada M, Ikeuchi T. Comparison of inhibitory effects of brain-derived neurotrophic factor and insulin-like growth factor on low potassium-induced apoptosis and activation of p38 MAPK and c-Jun in cultured cerebellar granule neurons. Mol Brain Res. 2003;119:184–191. doi: 10.1016/j.molbrainres.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 105.Yañez R, Lamana ML, García-Castro J, Colmenero I, Ramírez M, Bueren JA. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. 2006;24:2582–2591. doi: 10.1634/stemcells.2006-0228. [DOI] [PubMed] [Google Scholar]
- 106.Yang R, Fang F, Wang J, Guo H. Adipose-derived stem cells ameliorate erectile dysfunction after cavernous nerve cryoinjury. Andrology. 2015;3:694–701. doi: 10.1111/andr.12047. [DOI] [PubMed] [Google Scholar]
- 107.Ying C, Yang M, Zheng X, Hu W, Wang X. Effects of intracavernous injection of adipose-derived stem cells on cavernous nerve regeneration in a rat model. Cell Mol Neurobiol. 2013;33:233–240. doi: 10.1007/s10571-012-9890-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yoshimura K, Sato K, Aoi N, Kurita M, Inoue K, Suga H, Eto H, Kato H, Hirohi T, Harii K. Cell-assisted lipotransfer for facial lipoatrophy: Efficacy of clinical use of adipose-derived stem cells. Dermatol Surg. 2008;34:1178–1185. doi: 10.1111/j.1524-4725.2008.34256.x. [DOI] [PubMed] [Google Scholar]
- 109.You D, Jang MJ, Kim BH, Song G, Lee C, Suh N, Jeong IG, Ahn TY, Kim CS. Comparative study of autologous stromal vascular fraction and adipose-derived stem cells for erectile function recovery in a rat model of cavernous nerve injury: svf versus adscs in erectile function recovery. Stem Cells Transl Med. 2015;4:351–358. doi: 10.5966/sctm.2014-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.You D, Jang MJ, Lee J, Suh N, Jeong IG, Sohn DW, Kim SW, Ahn TY, Kim CS. Comparative analysis of periprostatic implantation and intracavernosal injection of human adipose tissue‐derived stem cells for erectile function recovery in a rat model of cavernous nerve injury. Prostate. 2013;73:278–286. doi: 10.1002/pros.22567. [DOI] [PubMed] [Google Scholar]
- 111.Zhang H, Yang R, Wang Z, Lin G, Lue TF, Lin C. Adipose tissue‐derived stem cells secrete cxcl5 cytokine with neurotrophic effects on cavernous nerve regeneration. J Sex Med. 2011;8:437–446. doi: 10.1111/j.1743-6109.2010.02128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang L, Wang XY, Zhou PJ, He Z, Yan HZ, Xu DD, Wang Y, Fu WY, Ruan BB, Wang S, Chen HX, Liu QY, Zhang YX, Liu Z, Wang YF. Use of immune modulation by human adipose-derived mesenchymal stem cells to treat experimental arthritis in mice. Am J Transl Res. 2017;9:2595–2607. [PMC free article] [PubMed] [Google Scholar]
- 113.Zhou Y, Wang J, Li H, Liang X, Bae J, Huang X, Li Q. Efficacy and safety of cell-assisted lipotransfer: a systemic review and meta-analysis. Plast Reconstr Surg. 2016;137:44e–57e. doi: 10.1097/PRS.0000000000001981. [DOI] [PubMed] [Google Scholar]
- 114.Zuk P. Adipose-derived stem cells in tissue regeneration: a review. ISRN Stem Cells. 2013;2013:e713959. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PubMed Search Strategy
In vitro and in vivo experiments that examined the efficacy of undifferentiated adipose-derived stem cells.


