Abstract
Extracellular exosomes are formed inside the cytoplasm of cells in compartments known as multivesicular bodies. Thus, exosomes contain cytoplasmic content. Multivesicular bodies fuse with the plasma membrane and release exosomes into the extracellular environment. Comprehensive research suggests that exosomes act as both inflammatory intermediaries and critical inducers of oxidative stress to drive progression of Alzheimer’s disease. An important role of exosomes in Alzheimer’s disease includes the formation of neurofibrillary tangles and beta-amyloid production, clearance, and accumulation. In addition, exosomes are involved in neuroinflammation and oxidative stress, which both act as triggers for beta-amyloid pathogenesis and tau hyperphosphorylation. Further, it has been shown that exosomes are strongly associated with beta-amyloid clearance. Thus, effective measures for regulating exosome metabolism may be novel drug targets for Alzheimer’s disease.
Keywords: nerve regeneration, microvesicle, beta-amyloid, tau, neuroinflammation, oxidative stress, therapeutic target, neurodegeneration, dementia, neural regeneration
Introduction
Alzheimer’s disease (AD) is the most common cause of dementia, and an age-related neurodegenerative disease characterized by progressive memory loss and declining cognitive function. Multiple pathogenic hypotheses have been proposed including amyloid, extracellular beta-amyloid (Aβ) peptide deposition (Lloret et al., 2011; Busche et al., 2016), intracellular accumulation of hyperphosphorylated tau protein (formation of neurofibrillary tangles) (Lloret et al., 2011; Busche et al., 2016; Panza et al., 2016), cholinergic dysfunction (Picon et al., 2010; Cacabelos et al., 2014), neuroinflammation, and oxidative stress (Latta et al., 2015; Liu et al., 2015). Although several medications are available to treat AD, none of them stop or reverse the disease (de la Torre, 2010). Increased knowledge on available treatments and the existing pathogenesis of AD will be beneficial for reaching the best decisions on medications and preventing progression of AD.
Apart from macromolecular complexes and small molecules, a large number of microvesicles are secreted from cells into the extracellular space (Vlassov et al., 2012; Beach et al., 2014). Microvesicles (also known as circulating microvesicles or microparticles) are fragments of plasma membrane between 100 nm and 1,000 nm in diameter. Exosomes are microvesicles secreted by all cells. Exosomes first fuse with multivesicular bodies (MVBs) and the cell surface, and are subsequently released from MVBs into the extracellular space (Laulagnier et al., 2004; Vlassov et al., 2012). MVBs are referred to as late endosomes and contain internal vesicles. The most important role of exosomes is to act as intercellular communication messengers by delivering macromolecules between cells (Thery et al., 2002; Beach et al., 2014). Substantial evidence suggests that exosomes serve as active mediators of neurodegenerative disorders, and transport disease particles such as α-synuclein (Chang et al., 2013; Kong et al., 2014; Tsunemi et al., 2014; Grey et al., 2015), Aβ, and prions from their cells of origin to other cells (Kalani et al., 2014; Yuyama et al., 2014; Fiandaca et al., 2015; Yuyama et al., 2015). This review discusses the role of exosomes in the pathogenesis of AD, and addresses the association between exosomes and relevant AD pathologies (Aβ, neurofibrillary tangles, oxidative stress, and inflammation). Moreover, this review provides a proposed general role of exosomes in AD pathogenesis, and discusses novel therapeutic interventions of exosomes for AD.
Exosomes
Exosomes are microvesicles of 30–100 nm in diameter, and small lipid vesicles secreted by all cell types (Kastelowitz and Yin, 2014; Sluijter et al., 2014). Internal vesicles formed by the inward budding of cellular compartments are known as MVBs (Vlassov et al., 2012; Al-Nedawi, 2014). When MVBs fuse with the plasma membrane, exosomes are released from these internal vesicles. Exosomes exist in blood, saliva, urine, breast milk, and other body fluids (Vlassov et al., 2012; Qin and Xu, 2014). Exosomes not only maintain normal cellular functions and cellular viability via housekeeping roles, but also safeguard various functions of multicellular organisms (Ohno, 2006).
Exosomes contain various substances, including small RNAs, lipids, and a variety of proteins (Vitek et al., 1994; Beach et al., 2014). MicroRNAs (miRNAs) target messenger RNAs for degradation and prevent translation. It has been shown that exosome-released miRNAs regulate the inflammatory response to external environmental changes. For example, exosome-delivered miR-155 increases expression of inflammatory genes, while miR-146a decreases their expression (Gao et al., 2016). The most intriguing role of exosomes are as conveyors of proteins and lipids, which affect downstream signaling events in recipient cells and influence various aspects of cell behavior and physiology, including nerve regeneration, and synaptic function and behavior (Arscott et al., 2013; Chen et al., 2014). Exosomes may serve as vesicular carriers for intercellular communication in neurodegenerative disorders (Schneider and Simons, 2013). Further, they play an ominous role in propagation of toxic Aβ pathology (enhancing Aβ generation and deposition, and inhibiting Aβ clearance), abnormal tau phosphorylation, and triggering neuroinflammation and oxidative stress by exchange of information between neurons and glia in neurodegenerative AD conditions (Saman et al., 2014; Yuyama et al., 2014, 2015; Fiandaca et al., 2015; Goetzl et al., 2016; Shi et al., 2016).
Exosomes and Neurodegeneration
Neurodegeneration encompasses a series of diseases due to loss of structure and function of nerve cells in the brain. Most attention has been focused on Parkinson’s disease (PD), Huntington’s disease, and AD (Tonekaboni and Mollamohammadi, 2014; Mouton-Liger et al., 2015). Indeed, a large proportion of less popular diseases have been ignored (Kerner, 2014), such as multiple sclerosis (Orack et al., 2015), epilepsy (Aboud et al., 2013; Pottoo et al., 2014), and stroke (Seifert et al., 2014; Walberer et al., 2014). Increasing evidence indicates that exosomes are involved in neurodegenerative disorders as potential carriers of misfolded proteins (Russo et al., 2012; Candelario and Steindler, 2014; Kalani et al., 2014).
Two of the most common neurodegenerative diseases are AD and PD. The main cause of PD is death of dopaminergic cells in the substantia nigra. Emerging studies have shown that progression of neurodegeneration in PD may involve release of toxic forms of α-synuclein, which are taken up by neighboring neurons and trigger dysfunction (Russo et al., 2012). Several studies have noted that exosomes are involved in PD pathogenesis such as acceleration of α-synuclein aggregation (Tsunemi et al., 2014; Grey et al., 2015). Molecular biology data support the proposition that lysosomal dysfunction leads to increased α-synuclein release in exosomes, and a concomitant increase in α-synuclein transmission to recipient cells (Alvarez-Erviti et al., 2011). Additionally, an in vitro study suggested involvement of ATP13A2/(PARK9) in exosome biogenesis and α-synuclein secretion (Tsunemi et al., 2014). While PD-linked human ATP13A2/(PARK9) promotes α-synuclein externalization via exosomes (Kong et al., 2014). Furthermore, exosomes secreted from activated microglia are important mediators of α-synuclein-induced neurodegeneration (Chang et al., 2013).
Exosomes and Amyloid Pathology
Senile plaques produced by accumulation of Aβ are a classical hallmark of AD. Aβ originates from sequential cleavage of amyloid precursor protein (APP) (Tam et al., 2014; Agostinho et al., 2015). Cleavage by β-secretase within the luminal/extracellular domain leads to generation of β-carboxyl-terminal fragments (CTFs) (Cai et al., 2012; Ortega et al., 2013). Following β-secretase cleavage, γ-secretase processes APP at the carboxyl-terminus to produce Aβ. CTFs of APP can accumulate in MVBs and be released from the cell in exosomes (Sharples et al., 2008). Exosomes also contain CTFs and β- and γ-secretases (Sharples et al., 2008), indicating a wider role in APP metabolism.
Formation and clearance of Aβ are associated with endosomal compartments as Aβ and CTFs are secreted from exosomes (Rajendran et al., 2006). Cleavage of APP by β-secretase occurs in early endosomes (Rajendran et al., 2006). Exosome-associated Aβ levels increased more significantly in the cerebrospinal fluid of younger cynomolgus monkeys and APP transgenic mice compared with older animals (Yuyama et al., 2015). Additional evidence has confirmed that exosomes promote Aβ aggregation and accelerate amyloid plaque formation (Dinkins et al., 2014). Meanwhile, in vivo exosome reduction contributes to lower amyloid plaque load in the 5xFAD mouse model, a mouse line that expresses five mutations of familial AD (Dinkins et al., 2014).
Recent evidence revealed that infusion of neuronal exosomes into the brain of APP transgenic mice decreased Aβ generation and deposition, which was not observed with glial exosomes (Yuyama et al., 2015). This finding highlights the role of neuronal exosomes in Aβ clearance (Yuyama et al., 2015), and suggests that diminished secretion of neuronal exosomes may relate to Aβ accumulation, and ultimately, development of AD pathology (Figure 1). Indeed, it appears that improving Aβ clearance by exosome administration may provide a novel therapeutic intervention for AD (Yuyama et al., 2014).
Figure 1.
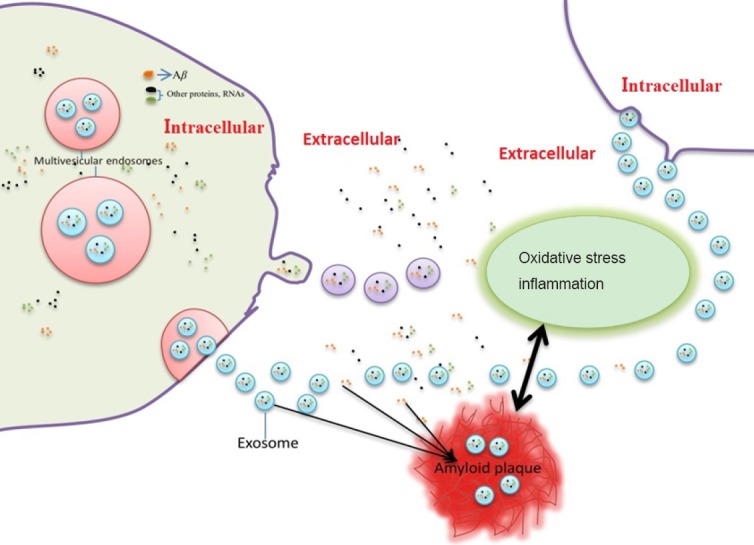
Schematic diagram of the emerging role of exosomes in beta-amyloid peptide pathology.
Exosomes are formed inside multivesicular bodies in cells. Exosomes are released into the extracellular environment when multivesicular bodies fuse with the plasma membrane. Beta-amyloid (Aβ) can be secreted from cells by association with exosomes. Aβ secreted from exosomes in the extracellular space contributes to Aβ plaque formation, which in turn triggers neuroinflammation and oxidative stress.
Exosomes and Neurofibrillary Tangles
Neurofibrillary tangles are aggregates of hyperphosphorylated tau protein (Gendreau and Hall, 2013). Definitive diagnosis of AD requires postmortem identification of amyloid plaques and neurofibrillary tangles. Several studies suggest that tau can be secreted from neurons via exosomes, and exosome-related tau may be an important contributor to spreading neurofibrillary lesions (Vingtdeux et al., 2012; Saman et al., 2014).
Exosomes as a novel way of interneuronal communication, participate in spreading pathological proteins (such as APP fragments, phosphorylated tau, or α-synuclein) across the nervous system (Chivet et al., 2012, 2013). There is significant correlation between multiple genes of AD and proteins recruited to exosomes by tau overexpression (Saman et al., 2014). A clinical study showed that exosome levels of total tau (pT181-tau and pS396-tau) were significantly higher in AD patients than case-controls, both 1–10 years before and at AD diagnosis, suggesting that pS396-tau and pT181-tau levels in extracts of neutrally-derived blood exosomes predict AD development before clinical onset (Fiandaca et al., 2015). In addition, exosome-associated tau phosphorylated at Thr-181 (AT270) is present in human cerebrospinal fluid samples, suggesting that phosphorylated tau induced by exosome secretion may contribute to abnormal tau processing (Saman et al., 2012).
Exosomes as Mediators of Neuroinflammation Associated with AD
Inflammation represents a response induced by injury or destruction of tissues, which enables removal, dilution, or isolation of both injurious substances and injured tissue. Neuroinflammation is inflammation of nervous tissue, and is a pathological and physiological process in response to a variety of events (Cai et al., 2013a, 2014), including microbial infections (Cox et al., 2013), chemical substances (de Rivero Vaccari et al., 2016), tissue necrosis from ischemia and anoxia (Maddahi and Edvinsson, 2010), traumatic brain injury (Lozano et al., 2015; de Rivero Vaccari et al., 2016), toxic metabolites (Butterworth, 2011; McMillin et al., 2014), and autoimmunity (Liu et al., 2014; Morales et al., 2014). It is well known that inflammation can be classified as either acute or chronic. As a common inflammatory process, acute neuroinflammation occurs immediately following injury to the central nervous system. It is characterized by the release of inflammatory molecules, glial cell activation, endothelial cell activation, platelet deposition, and tissue edema. Meanwhile, chronic neuroinflammation is of longer duration, with maintained glial cell activation and recruitment of other immune cells in the brain (Millington et al., 2014; Phillips et al., 2014). Neuroinflammation is regarded as chronic inflammation of the central nervous system. Mounting evidence shows that AD is associated with chronic inflammatory responses, with sustained presence of inflammatory cytokines from activated microglia and astrocytes, free radicals, and oxidative stress (Kaur et al., 2015; Latta et al., 2015; Zhang and Jiang, 2015).
Exosomes are emerging as important inflammatory mediators because of their role as cargo of inflammatory molecules, and thereby induce neuroinflammation by exchange of information between neurons and glia (Gupta and Pulliam, 2014; Kore and Abraham, 2014; Rajendran et al., 2014; Fernandez-Messina et al., 2015; de Rivero Vaccari et al., 2016). Aβ is effectively packaged into exosomes and spread from one cell to another, initiating an inflammatory cascade (Gupta and Pulliam, 2014). In addition to releasing inflammatory factors, exosomes secreted by dead brain cells can influence bystander cells by the transfer of inflammatory mediators in response to pathogenic stimuli (Prado et al., 2010; Sun et al., 2010; Gupta and Pulliam, 2014). Extracellular exosomes release Aβ and accelerate amyloid plaque formation, which are important causes of neuroinflammation (Engel, 2014). Considering their ability to mediate intercellular communication between cells (Record, 2014; Salido-Guadarrama et al., 2014; Zhang and Grizzle, 2014), exosomes represent one of the key players in transporting neurotoxic inflammatory agents and spreading progression of inflammation in brain cells. Oversecretion of exosomes is harmful and can strengthen progression of inflammation in the extracellular microenvironment. Nonetheless, despite abundant evidence demonstrating a role for exosomes in regulating the inflammatory response, the exact mechanisms remain unclear. Therefore, improved understanding of the role of exosomes in inflammation at different stages of AD will benefit prevention and treatment of AD.
Oxidative Stress: A Direct Mediator of Exosome Release in AD?
Extensive research has shown that oxidative stress is strongly linked to AD pathogenesis (Cai et al., 2011, 2013b; Ferreira et al., 2015). An important feature of AD is an active and self-perpetuating cycle of chronic neuroinflammation and oxidative stress that may contribute to irreversible neuronal dysfunction and cell death (Cai, 2014). Oxidative stress is proposed to contribute to Aβ generation and formation of neurofibrillary tangles (Santos et al., 2014; Kanamaru et al., 2015; Kamat et al., 2016). Many results show that neuroinflammation-induced oxidative stress increases Aβ generation by enhancing β- and γ-secretase activity (Cai et al., 2011; Bonda et al., 2014; Chang et al., 2014). In addition, intracellular Aβ accumulation promotes significant oxidative and inflammatory mechanisms that generate a vicious cycle of Aβ generation and oxidation, each accelerating the other (Luque-Contreras et al., 2014; Persson et al., 2014).
Many studies have noted that exosome release from MVBs are induced and accelerated by oxidative stress (Soderberg et al., 2007; Eldh et al., 2010; Zhou et al., 2013; Tsanova et al., 2014). Previous studies have indicated that exosome release from MVBs is associated with the pathogenesis of many diseases involved in oxidative stress (Tsanova et al., 2014), such as multiple sclerosis and dysmyelinating syndromes (Pusic et al., 2014), cancer (Goldkorn et al., 2013; Meseure et al., 2014), cerebral ischemia disease (DeGracia et al., 2008; Fröhlich et al., 2014), as well as cardiovascular disease (Fleury et al., 2014; Yamaguchi et al., 2015). However, many questions have not been answered: what is the exact role of exosome release mediated by oxidative damage in AD pathogenesis? Is release of exosomes from MVBs a cause or consequence of oxidative stress in AD? What is the relationship between oxidative-mediated release of exosomes and AD pathology?
Exosomes: A Novel Therapeutic Strategy for AD?
AD is a progressive brain disorder and the most common form of dementia. To date, there is still no cure for AD that can reverse or halt its progress, although there are medications that can help improve symptoms in some cases. Exosomes are extracellular vesicles that transport different molecules between cells. They are formed and stored inside MVBs until they are released to the extracellular environment. It is apparent that the brain microenvironment correlates with neurodegeneration, and brain intercellular communication induced by exosomes is necessary for this to occur. In the past decade, exosomes have been shown to be efficient carriers of genetic information, which can be transferred between cells to regulate gene expression and function of recipient cells (Fernandez-Messina et al., 2015). Hence, they may be an important means of regulating the neurodegenerative process underlying AD, and improve the brain microenvironment by affecting the intercellular communication induced by exosomes.
Exosomes can cross the blood-brain barrier and therefore be used as delivery vehicles of drugs and genetic elements for treatment of neurological disorders. Several studies have suggested that exosomes derived from multipluripotent mesenchymal stromal cells play a neuroprotective role by promoting functional recovery (Xin et al., 2014), neurovascular plasticity (Xin et al., 2013a, b; Zhang et al., 2015), and repairing injured tissue in traumatic brain injury and neurodegenerative disorders. Thus, it may be possible to use mesenchymal stromal cell exosomes in therapies for AD (Katsuda et al., 2015). Furthermore, intracerebrally administered exosomes can act as potent Aβ scavengers by binding to Aβ through enriched glycans on glycosphingolipids on the exosome surface, suggesting a role for exosomes in Aβ clearance in the central nervous system (Yuyama et al., 2014). Improving Aβ clearance by exosome administration provides a novel therapeutic intervention for AD.
Ambiguous knowledge of the underlying mechanisms responsible for causing AD and its progression is the major impediment to therapeutic advances. The potential role of exosomes in neurological disorders and knowledge of their biology show promising leads that are close to clinical translation. Regulating the status and state of exosomes may be a ‘Trojan-horse’ approach to deliver drugs into the brain and treat neurodegenerative and other disorders.
Footnotes
Conflicts of interest: None declared.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Sage Arbor, Marian University College of Osteopathic Medicine, USA.
Funding: This study was financially supported by the Health and Family Planning Scientific Research Project of Hubei Province of China, No. WJ2015MB219.
(Copyedited by James R, Frenchman B, Yu J, Li CH, Qiu Y, Song LP, Zhao M)
References
- 1.Aboud O, Mrak RE, Boop FA, Griffin WS. Epilepsy: neuroinflammation, neurodegeneration, and APOE genotype. Acta Neuropathol Commun. 2013;1:41. doi: 10.1186/2051-5960-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agostinho P, Pliassova A, Oliveira CR, Cunha RA. Localization and trafficking of amyloid-beta protein precursor and secretases: impact on Alzheimer’s disease. J Alzheimers Dis. 2015;45:329–347. doi: 10.3233/JAD-142730. [DOI] [PubMed] [Google Scholar]
- 3.Al-Nedawi K. The yin-yang of microvesicles (exosomes) in cancer biology. Front Oncol. 2014;4:172. doi: 10.3389/fonc.2014.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez-Erviti L, Seow Y, Schapira AH, Gardiner C, Sargent IL, Wood MJ, Cooper JM. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol Dis. 2011;42:360–367. doi: 10.1016/j.nbd.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arscott WT, Tandle AT, Zhao S, Shabason JE, Gordon IK, Schlaff CD, Zhang G, Tofilon PJ, Camphausen KA. Ionizing radiation and glioblastoma exosomes: implications in tumor biology and cell migration. Transl Oncol. 2013;6:638–648. doi: 10.1593/tlo.13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beach A, Zhang HG, Ratajczak MZ, Kakar SS. Exosomes: an overview of biogenesis, composition and role in ovarian cancer. J Ovarian Res. 2014;7:14. doi: 10.1186/1757-2215-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonda DJ, Wang X, Lee HG, Smith MA, Perry G, Zhu X. Neuronal failure in Alzheimer’s disease: a view through the oxidative stress looking-glass. Neurosci Bull. 2014;30:243–252. doi: 10.1007/s12264-013-1424-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busche MA, Staufenbiel M, Willem M, Haass C, Förstl H. Mechanisms of Alzheimer’s disease : Neuronal hyperactivity and hypoactivity as new therapeutic targets. Nervenarzt. 2016;87:1163–1174. doi: 10.1007/s00115-015-0041-5. [DOI] [PubMed] [Google Scholar]
- 9.Butterworth RF. Hepatic encephalopathy: a central neuroinflammatory disorder. Hepatology. 2011;53:1372–1376. doi: 10.1002/hep.24228. [DOI] [PubMed] [Google Scholar]
- 10.Cacabelos R, Cacabelos P, Torrellas C, Tellado I, Carril JC. Pharmacogenomics of Alzheimer’s disease: novel therapeutic strategies for drug development. Methods Mol Biol. 2014;1175:323–556. doi: 10.1007/978-1-4939-0956-8_13. [DOI] [PubMed] [Google Scholar]
- 11.Cai Z. Monoamine oxidase inhibitors: promising therapeutic agents for Alzheimer’s disease (Review) Mol Med Rep. 2014;9:1533–1541. doi: 10.3892/mmr.2014.2040. [DOI] [PubMed] [Google Scholar]
- 12.Cai Z, Zhao B, Ratka A. Oxidative stress and beta-amyloid protein in Alzheimer’s disease. Neuromolecular Med. 2011;13:223–250. doi: 10.1007/s12017-011-8155-9. [DOI] [PubMed] [Google Scholar]
- 13.Cai Z, Yan Y, Wang Y. Minocycline alleviates beta-amyloid protein and tau pathology via restraining neuroinflammation induced by diabetic metabolic disorder. Clin Interv Aging. 2013a;8:1089–1095. doi: 10.2147/CIA.S46536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai Z, Yan LJ, Ratka A. Telomere shortening and Alzheimer’s disease. Neuromolecular Med. 2013b;15:25–48. doi: 10.1007/s12017-012-8207-9. [DOI] [PubMed] [Google Scholar]
- 15.Cai Z, Hussain MD, Yan LJ. Microglia neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int J Neurosci. 2014;124:307–321. doi: 10.3109/00207454.2013.833510. [DOI] [PubMed] [Google Scholar]
- 16.Cai Z, Zhao B, Li K, Zhang L, Li C, Quazi SH, Tan Y. Mammalian target of rapamycin: a valid therapeutic target through the autophagy pathway for Alzheimer's disease? J Neurosci Res. 2012;90:1105–1118. doi: 10.1002/jnr.23011. [DOI] [PubMed] [Google Scholar]
- 17.Candelario KM, Steindler DA. The role of extracellular vesicles in the progression of neurodegenerative disease and cancer. Trends Mol Med. 2014;20:368–374. doi: 10.1016/j.molmed.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang C, Lang H, Geng N, Wang J, Li N, Wang X. Exosomes of BV-2 cells induced by alpha-synuclein: important mediator of neurodegeneration in PD. Neurosci Lett. 2013;548:190–195. doi: 10.1016/j.neulet.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Chang YT, Chang WN, Tsai NW, Huang CC, Kung CT, Su YJ, Lin WC, Cheng BC, Su CM, Chiang YF, Lu CH. The roles of biomarkers of oxidative stress and antioxidant in Alzheimer’s disease: a systematic review. Biomed Res Int. 2014;2014:182303. doi: 10.1155/2014/182303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen L, Charrier A, Zhou Y, Chen R, Yu B, Agarwal K, Tsukamoto H, Lee LJ, Paulaitis ME, Brigstock DR. Epigenetic regulation of connective tissue growth factor by MicroRNA-214 delivery in exosomes from mouse or human hepatic stellate cells. Hepatology. 2014;59:1118–1129. doi: 10.1002/hep.26768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chivet M, Hemming F, Pernet-Gallay K, Fraboulet S, Sadoul R. Emerging role of neuronal exosomes in the central nervous system. Front Physiol. 2012;3:145. doi: 10.3389/fphys.2012.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chivet M, Javalet C, Hemming F, Pernet-Gallay K, Laulagnier K, Fraboulet S, Sadoul R. Exosomes as a novel way of interneuronal communication. Biochem Soc Trans. 2013;41:241–244. doi: 10.1042/BST20120266. [DOI] [PubMed] [Google Scholar]
- 23.Cox GM, Kithcart AP, Pitt D, Guan Z, Alexander J, Williams JL, Shawler T, Dagia NM, Popovich PG, Satoskar AR, Whitacre CC. Macrophage migration inhibitory factor potentiates autoimmune-mediated neuroinflammation. J Immunol. 2013;191:1043–1054. doi: 10.4049/jimmunol.1200485. [DOI] [PubMed] [Google Scholar]
- 24.de la Torre JC. Alzheimer’s disease is incurable but preventable. J Alzheimers Dis. 2010;20:861–870. doi: 10.3233/JAD-2010-091579. [DOI] [PubMed] [Google Scholar]
- 25.de Rivero Vaccari JP, Brand F, 3rd, Adamczak S, Lee SW, Perez-Barcena J, Wang MY, Bullock MR, Dietrich WD, Keane RW. Exosome-mediated inflammasome signaling after central nervous system injury. J Neurochem. 2016;136(Suppl 1):39–48. doi: 10.1111/jnc.13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeGracia DJ, Jamison JT, Szymanski JJ, Lewis MK. Translation arrest and ribonomics in post-ischemic brain: layers and layers of players. J Neurochem. 2008;106:2288–2301. doi: 10.1111/j.1471-4159.2008.05561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinkins MB, Dasgupta S, Wang G, Zhu G, Bieberich E. Exosome reduction in vivo is associated with lower amyloid plaque load in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol Aging. 2014;35:1792–1800. doi: 10.1016/j.neurobiolaging.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eldh M, Ekström K, Valadi H, Sjöstrand M, Olsson B, Jernås M, Lötvall J. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS One. 2010;5:e15353. doi: 10.1371/journal.pone.0015353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engel PA. Does metabolic failure at the synapse cause Alzheimer’s disease. Med Hypotheses. 2014;83:802-808. doi: 10.1016/j.mehy.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez-Messina L, Gutierrez-Vazquez C, Rivas-Garcia E, Sanchez-Madrid F, de la Fuente H. Immunomodulatory role of microRNAs transferred by extracellular vesicles. Biol Cell. 2015;107:61–77. doi: 10.1111/boc.201400081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferreira ME, de Vasconcelos AS, da Costa Vilhena T, da Silva TL, da Silva Barbosa A, Gomes AR, Dolabela MF, Percario S. Oxidative stress in Alzheimer’s disease: should we keep trying antioxidant therapies. Cell Mol Neurobiol. 2015;35:595-614. doi: 10.1007/s10571-015-0157-y. [DOI] [PubMed] [Google Scholar]
- 32.Fiandaca MS, Kapogiannis D, Mapstone M, Boxer A, Eitan E, Schwartz JB, Abner EL, Petersen RC, Federoff HJ, Miller BL, Goetzl EJ. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimers Dement. 2015;11:600–607. doi: 10.1016/j.jalz.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fleury A, Martinez MC, Le Lay S. Extracellular vesicles as therapeutic tools in cardiovascular diseases. Front Immunol. 2014;5:370. doi: 10.3389/fimmu.2014.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fröhlich D, Kuo WP, Frühbeis C, Sun JJ, Zehendner CM, Luhmann HJ, Pinto S, Toedling J, Trotter J, Krämer-Albers EM. Multifaceted effects of oligodendroglial exosomes on neurons: impact on neuronal firing rate, signal transduction and gene regulation. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130510. doi: 10.1098/rstb.2013.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao W, Liu H, Yuan J, Wu C, Huang D, Ma Y, Zhu J, Ma L, Guo J, Shi H, Zou Y, Ge J. Exosomes derived from mature dendritic cells increase endothelial inflammation and atherosclerosis via membrane TNF-alpha mediated NF-kappaB pathway. J Cell Mol Med. 2016;20:2318–2327. doi: 10.1111/jcmm.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gendreau KL, Hall GF. Tangles toxicity, and Tau secretion in AD - new approaches to a vexing problem. Front Neurol. 2013;4:160. doi: 10.3389/fneur.2013.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goetzl EJ, Mustapic M, Kapogiannis D, Eitan E, Lobach IV, Goetzl L, Schwartz JB, Miller BL. Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer’s disease. FASEB J. 2016;30:3853–3859. doi: 10.1096/fj.201600756R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldkorn T, Chung S, Filosto S. Lung cancer and lung injury: the dual role of ceramide. Handb Exp Pharmacol. 2013:93–113. doi: 10.1007/978-3-7091-1511-4_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grey M, Dunning CJ, Gaspar R, Grey C, Brundin P, Sparr E, Linse S. Acceleration of alpha-synuclein aggregation by exosomes. J Biol Chem. 2015;290:2969–2982. doi: 10.1074/jbc.M114.585703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta A, Pulliam L. Exosomes as mediators of neuroinflammation. J Neuroinflammation. 2014;11:68. doi: 10.1186/1742-2094-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalani A, Tyagi A, Tyagi N. Exosomes: mediators of neurodegeneration, neuroprotection and therapeutics. Mol Neurobiol. 2014;49:590–600. doi: 10.1007/s12035-013-8544-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamat PK, Kalani A, Rai S, Swarnkar S, Tota S, Nath C, Tyagi N. Mechanism of oxidative stress and synapse dysfunction in the pathogenesis of Alzheimer’s disease: understanding the therapeutics strategies. Mol Neurobiol. 2016;53:648–661. doi: 10.1007/s12035-014-9053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanamaru T, Kamimura N, Yokota T, Iuchi K, Nishimaki K, Takami S, Akashiba H, Shitaka Y, Katsura K, Kimura K, Ohta S. Oxidative stress accelerates amyloid deposition and memory impairment in a double-transgenic mouse model of Alzheimer’s disease. Neurosci Lett. 2015;587:126–131. doi: 10.1016/j.neulet.2014.12.033. [DOI] [PubMed] [Google Scholar]
- 44.Kastelowitz N, Yin H. Exosomes and microvesicles: identification and targeting by particle size and lipid chemical probes. ChemBioChem. 2014;15:923–928. doi: 10.1002/cbic.201400043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katsuda T, Oki K, Ochiya T. Potential application of extracellular vesicles of human adipose tissue-derived mesenchymal stem cells in Alzheimer’s disease therapeutics. Methods Mol Biol. 2015;1212:171–181. doi: 10.1007/7651_2014_98. [DOI] [PubMed] [Google Scholar]
- 46.Kaur U, Banerjee P, Bir A, Sinha M, Biswas A, Chakrabarti S. Reactive oxygen species, redox signaling and neuroinflammation in Alzheimer’s disease: the NF-kappaB connection. Curr Top Med Chem. 2015;15:446–457. doi: 10.2174/1568026615666150114160543. [DOI] [PubMed] [Google Scholar]
- 47.Kerner B. Psychiatric genetics, neurogenetics, and neurodegeneration. Front Genet. 2014;5:467. doi: 10.3389/fgene.2014.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kong SM, Chan BK, Park JS, Hill KJ, Aitken JB, Cottle L, Farghaian H, Cole AR, Lay PA, Sue CM, Cooper AA. Parkinson’s disease-linked human PARK9/ATP13A2 maintains zinc homeostasis and promotes alpha-Synuclein externalization via exosomes. Hum Mol Genet. 2014;23:2816–2833. doi: 10.1093/hmg/ddu099. [DOI] [PubMed] [Google Scholar]
- 49.Kore RA, Abraham EC. Inflammatory cytokines, interleukin-1 beta and tumor necrosis factor-alpha, upregulated in glioblastoma multiforme, raise the levels of CRYAB in exosomes secreted by U373 glioma cells. Biochem Biophys Res Commun. 2014;453:326–331. doi: 10.1016/j.bbrc.2014.09.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Latta CH, Brothers HM, Wilcock DM. Neuroinflammation in Alzheimer’s disease; A source of heterogeneity and target for personalized therapy. Neuroscience. 2015;302:103–111. doi: 10.1016/j.neuroscience.2014.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laulagnier K, Motta C, Hamdi S, Roy S, Fauvelle F, Pageaux JF, Kobayashi T, Salles JP, Perret B, Bonnerot C, Record M. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem J. 2004;380:161–171. doi: 10.1042/BJ20031594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu C, Cui G, Zhu M, Kang X, Guo H. Neuroinflammation in Alzheimer’s disease: chemokines produced by astrocytes and chemokine receptors. Int J Clin Exp Pathol. 2014;7:8342–8355. [PMC free article] [PubMed] [Google Scholar]
- 53.Liu Z, Li T, Li P, Wei N, Zhao Z, Liang H, Ji X, Chen W, Xue M, Wei J. The ambiguous relationship of oxidative stress, tau hyperphosphorylation, and autophagy dysfunction in Alzheimer’s disease. Oxid Med Cell Longev. 2015;2015:352723. doi: 10.1155/2015/352723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lloret A, Badia MC, Giraldo E, Ermak G, Alonso MD, Pallardo FV, Davies KJ, Vina J. Amyloid-beta toxicity and tau hyperphosphorylation are linked via RCAN1 in Alzheimer’s disease. J Alzheimers dis. 2011;27:701–709. doi: 10.3233/JAD-2011-110890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lozano D, Gonzales-Portillo GS, Acosta S, de la Pena I, Tajiri N, Kaneko Y, Borlongan CV. Neuroinflammatory responses to traumatic brain injury: etiology, clinical consequences, and therapeutic opportunities. Neuropsychiatr Dis Treat. 2015;11:97–106. doi: 10.2147/NDT.S65815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luque-Contreras D, Carvajal K, Toral-Rios D, Franco-Bocanegra D, Campos-Peña V. Oxidative stress and metabolic syndrome: cause or consequence of Alzheimer’s disease. Oxid Med Cell Longev. 2014;2014:497802. doi: 10.1155/2014/497802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maddahi A, Edvinsson L. Cerebral ischemia induces microvascular pro-inflammatory cytokine expression via the MEK/ERK pathway. J Neuroinflammation. 2010;7:14. doi: 10.1186/1742-2094-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McMillin M, Frampton G, Thompson M, Galindo C, Standeford H, Whittington E, Alpini G, DeMorrow S. Neuronal CCL2 is upregulated during hepatic encephalopathy and contributes to microglia activation and neurological decline. J Neuroinflammation. 2014;11:121. doi: 10.1186/1742-2094-11-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meseure D, Drak Alsibai K, Nicolas A. Pivotal role of pervasive neoplastic and stromal cells reprogramming in circulating tumor cells dissemination and metastatic colonization. Cancer Microenviron. 2014;7:95–115. doi: 10.1007/s12307-014-0158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Millington C, Sonego S, Karunaweera N, Rangel A, Aldrich-Wright JR, Campbell IL, Gyengesi E, Münch G. Chronic neuroinflammation in Alzheimer’s disease: new perspectives on animal models and promising candidate drugs. Biomed Res Int. 2014;2014:309129. doi: 10.1155/2014/309129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morales I, Guzmán-Martínez L, Cerda-Troncoso C, Farias GA, Maccioni RB. Neuroinflammation in the pathogenesis of Alzheimer’s disease. A rational framework for the search of novel therapeutic approaches. Front Cell Neurosci. 2014;8:112. doi: 10.3389/fncel.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mouton-Liger F, Rebillat AS, Gourmaud S, Paquet C, Leguen A, Dumurgier J, Bernadelli P, Taupin V, Pradier L, Rooney T, Hugon J. PKR downregulation prevents neurodegeneration and beta-amyloid production in a thiamine-deficient model. Cell Death Dis. 2015;6:e1594. doi: 10.1038/cddis.2014.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ohno H. Overview: membrane traffic in multicellular systems: more than just a housekeeper. J Biochem. 2006;139:941–942. doi: 10.1093/jb/mvj119. [DOI] [PubMed] [Google Scholar]
- 64.Orack JC, Deleidi M, Pitt D, Mahajan K, Nicholas JA, Boster AL, Racke MK, Comabella M, Watanabe F, Imitola J. Concise review: modeling multiple sclerosis with stem cell biological platforms: toward functional validation of cellular and molecular phenotypes in inflammation-induced neurodegeneration. Stem Cells Transl Med. 2015;4:252–260. doi: 10.5966/sctm.2014-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ortega F, Stott J, Visser SA, Bendtsen C. Interplay between alpha-, beta-, and gamma-secretases determines biphasic amyloid-beta protein level in the presence of a gamma-secretase inhibitor. J Biol Chem. 2013;288:785–792. doi: 10.1074/jbc.M112.419135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Panza F, Solfrizzi V, Seripa D, Imbimbo BP, Lozupone M, Santamato A, Zecca C, Barulli MR, Bellomo A, Pilotto A, Daniele A, Greco A, Logroscino G. Tau-centric targets and drugs in clinical development for the treatment of Alzheimer’s disease. Biomed Res Int. 2016;2016:3245935. doi: 10.1155/2016/3245935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Persson T, Popescu BO, Cedazo-Minguez A. Oxidative stress in Alzheimer’s disease: why did antioxidant therapy fail. Oxid Med Cell Longev. 2014;2014:427318. doi: 10.1155/2014/427318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Phillips EC, Croft CL, Kurbatskaya K, O’Neill MJ, Hutton ML, Hanger DP, Garwood CJ, Noble W. Astrocytes and neuroinflammation in Alzheimer’s disease. Biochem Soc Trans. 2014;42:1321–1325. doi: 10.1042/BST20140155. [DOI] [PubMed] [Google Scholar]
- 69.Picon PD, Camozzato AL, Lapporte EA, Picon RV, Moser Filho H, Cerveira MO, Chaves ML. Increasing rational use of cholinesterase inhibitors for Alzheimer’s disease in Brazil: public health strategy combining guideline with peer-review of prescriptions. Int J Technol Assess Health Care. 2010;26:205–210. doi: 10.1017/S0266462310000097. [DOI] [PubMed] [Google Scholar]
- 70.Pottoo FH, Bhowmik M, Vohora D. Raloxifene protects against seizures and neurodegeneration in a mouse model mimicking epilepsy in postmenopausal woman. Eur J Pharm Sci. 2014;65:167–173. doi: 10.1016/j.ejps.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 71.Prado N, Cañamero M, Villalba M, Rodriguez R, Batanero E. Bystander suppression to unrelated allergen sensitization through intranasal administration of tolerogenic exosomes in mouse. Mol Immunol. 2010;47:2148–2151. doi: 10.1016/j.molimm.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 72.Pusic AD, Pusic KM, Clayton BL, Kraig RP. IFNgamma-stimulated dendritic cell exosomes as a potential therapeutic for remyelination. J Neuroimmunol. 2014;266:12–23. doi: 10.1016/j.jneuroim.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qin J, Xu Q. Functions and application of exosomes. Acta Pol Pharm. 2014;71:537–543. [PubMed] [Google Scholar]
- 74.Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, Simons K. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A. 2006;103:11172–11177. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rajendran L, Bali J, Barr MM, Court FA, Kramer-Albers EM, Picou F, Raposo G, van der Vos KE, van Niel G, Wang J, Breakefield XO. Emerging roles of extracellular vesicles in the nervous system. J Neurosci. 2014;34:15482–15489. doi: 10.1523/JNEUROSCI.3258-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Record M. Intercellular communication by exosomes in placenta: a possible role in cell fusion. Placenta. 2014;35:297-302. doi: 10.1016/j.placenta.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 77.Russo I, Bubacco L, Greggio E. Exosomes-associated neurodegeneration and progression of Parkinson’s disease. Am J Neurodegener Dis. 2012;1:217–225. [PMC free article] [PubMed] [Google Scholar]
- 78.Salido-Guadarrama I, Romero-Cordoba S, Peralta-Zaragoza O, Hidalgo-Miranda A, Rodríguez-Dorantes M. MicroRNAs transported by exosomes in body fluids as mediators of intercellular communication in cancer. Onco Targets Ther. 2014;7:1327–1338. doi: 10.2147/OTT.S61562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saman S, Lee NC, Inoyo I, Jin J, Li Z, Doyle T, McKee AC, Hall GF. Proteins recruited to exosomes by tau overexpression implicate novel cellular mechanisms linking tau secretion with Alzheimer’s disease. J Alzheimers Dis. 2014;40(Suppl 1):S47–70. doi: 10.3233/JAD-132135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saman S, Kim W, Raya M, Visnick Y, Miro S, Saman S, Jackson B, McKee AC, Alvarez VE, Lee NC, Hall GF. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem. 2012;287:3842–3849. doi: 10.1074/jbc.M111.277061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Santos JR, Gois AM, Mendonca DM, Freire MA. Nutritional status, oxidative stress and dementia: the role of selenium in Alzheimer’s disease. Front Aging Neurosci. 2014;6:206. doi: 10.3389/fnagi.2014.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schneider A, Simons M. Exosomes: vesicular carriers for intercellular communication in neurodegenerative disorders. Cell Tissue Res. 2013;352:33–47. doi: 10.1007/s00441-012-1428-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seifert HA, Collier LA, Chapman CB, Benkovic SA, Willing AE, Pennypacker KR. Pro-inflammatory interferon gamma signaling is directly associated with stroke induced neurodegeneration. J Neuroimmune Pharmacol. 2014;9:679–689. doi: 10.1007/s11481-014-9560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sharples RA, Vella LJ, Nisbet RM, Naylor R, Perez K, Barnham KJ, Masters CL, Hill AF. Inhibition of gamma-secretase causes increased secretion of amyloid precursor protein C-terminal fragments in association with exosomes. FASEB J. 2008;22:1469–1478. doi: 10.1096/fj.07-9357com. [DOI] [PubMed] [Google Scholar]
- 85.Shi M, Kovac A, Korff A, Cook TJ, Ginghina C, Bullock KM, Yang L, Stewart T, Zheng D, Aro P, Atik A, Kerr KF, Zabetian CP, Peskind ER, Hu SC, Quinn JF, Galasko DR, Montine TJ, Banks WA, Zhang J. CNS tau efflux via exosomes is likely increased in Parkinson’s disease but not in Alzheimer’s disease. Alzheimers Dement. 2016;12:1125–1131. doi: 10.1016/j.jalz.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sluijter JP, Verhage V, Deddens JC, van den Akker F, Doevendans PA. Microvesicles and exosomes for intracardiac communication. Cardiovasc Res. 2014;102:302–311. doi: 10.1093/cvr/cvu022. [DOI] [PubMed] [Google Scholar]
- 87.Soderberg A, Barral AM, Söderström M, Sander B, Rosén A. Redox-signaling transmitted in trans to neighboring cells by melanoma-derived TNF-containing exosomes. Free Radic Biol Med. 2007;43:90–99. doi: 10.1016/j.freeradbiomed.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 88.Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang HG. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18:1606–1614. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tam JH, Seah C, Pasternak SH. The Amyloid Precursor Protein is rapidly transported from the Golgi apparatus to the lysosome and where it is processed into beta-amyloid. Mol Brain. 2014;7:54. doi: 10.1186/s13041-014-0054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 91.Tonekaboni SH, Mollamohammadi M. Neurodegeneration with brain iron accumulation: an overview. Iran J Child Neurol. 2014;8:1–8. [PMC free article] [PubMed] [Google Scholar]
- 92.Tsanova B, Spatrick P, Jacobson A, van Hoof A. The RNA exosome affects iron response and sensitivity to oxidative stress. RNA. 2014;20:1057–1067. doi: 10.1261/rna.043257.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tsunemi T, Hamada K, Krainc D. ATP13A2/PARK9 regulates secretion of exosomes and alpha-synuclein. J Neurosci. 2014;34:15281–15287. doi: 10.1523/JNEUROSCI.1629-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vingtdeux V, Sergeant N, Buee L. Potential contribution of exosomes to the prion-like propagation of lesions in Alzheimer’s disease. Front Physiol. 2012;3:229. doi: 10.3389/fphys.2012.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vitek MP, Bhattacharya K, Glendening JM, Stopa E, Vlassara H, Bucala R, Manogue K, Cerami A. Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proc Natl Acad Sci U S A. 1994;91:4766–4770. doi: 10.1073/pnas.91.11.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820:940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 97.Walberer M, Jantzen SU, Backes H, Rueger MA, Keuters MH, Neumaier B, Hoehn M, Fink GR, Graf R, Schroeter M. In-vivo detection of inflammation and neurodegeneration in the chronic phase after permanent embolic stroke in rats. Brain Res. 2014;1581:80–88. doi: 10.1016/j.brainres.2014.05.030. [DOI] [PubMed] [Google Scholar]
- 98.Xin H, Li Y, Chopp M. Exosomes/miRNAs as mediating cell-based therapy of stroke. Front Cell Neurosci. 2014;8:377. doi: 10.3389/fncel.2014.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013a;33:1711–1715. doi: 10.1038/jcbfm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, Zhang ZG, Chopp M. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013b;31:2737–2746. doi: 10.1002/stem.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yamaguchi T, Izumi Y, Nakamura Y, Yamazaki T, Shiota M, Sano S, Tanaka M, Osada-Oka M, Shimada K, Miura K, Yoshiyama M, Iwao H. Repeated remote ischemic conditioning attenuates left ventricular remodeling via exosome-mediated intercellular communication on chronic heart failure after myocardial infarction. Int J Cardiol. 2015;178:239–246. doi: 10.1016/j.ijcard.2014.10.144. [DOI] [PubMed] [Google Scholar]
- 102.Yuyama K, Sun H, Sakai S, Mitsutake S, Okada M, Tahara H, Furukawa J, Fujitani N, Shinohara Y, Igarashi Y. Decreased amyloid-beta pathologies by intracerebral loading of glycosphingolipid-enriched exosomes in Alzheimer model mice. J Biol Chem. 2014;289:24488–24498. doi: 10.1074/jbc.M114.577213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yuyama K, Sun H, Usuki S, Sakai S, Hanamatsu H, Mioka T, Kimura N, Okada M, Tahara H, Furukawa J, Fujitani N, Shinohara Y, Igarashi Y. A potential function for neuronal exosomes: sequestering intracerebral amyloid-beta peptide. FEBS Lett. 2015;589:84–88. doi: 10.1016/j.febslet.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 104.Zhang F, Jiang L. Neuroinflammation in Alzheimer’s disease. Neuropsychiatr Dis Treat. 2015;11:243–256. doi: 10.2147/NDT.S75546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang HG, Grizzle WE. Exosomes: a novel pathway of local and distant intercellular communication that facilitates the growth and metastasis of neoplastic lesions. Am J Pathol. 2014;184:28–41. doi: 10.1016/j.ajpath.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang Y, Chopp M, Meng Y, Katakowski M, Xin H, Mahmood A, Xiong Y. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 2015;122:856–867. doi: 10.3171/2014.11.JNS14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y, Zhang B, Wang M, Mao F, Yan Y, Gao S, Gu H, Zhu W, Qian H. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther. 2013;4:34. doi: 10.1186/scrt194. [DOI] [PMC free article] [PubMed] [Google Scholar]


