Keywords: nerve regeneration; endothelial progenitor cells; conditioned medium; spinal cord injury; inflammation; classical macrophages; angiogenesis; neuroprotection; alternatively activated macrophages; Basso, Beattie and Bresnahan score; neural regeneration
Abstract
Endothelial progenitor cells secrete a variety of growth factors that inhibit inflammation, promote angiogenesis and exert neuroprotective effects. Therefore, in this study, we investigated whether endothelial progenitor cell-conditioned medium might have therapeutic effectiveness for the treatment of spinal cord injury using both in vitro and in vivo experiments. After primary culture of bone marrow-derived macrophages, lipopolysaccharide stimulation was used to classically activate macrophages to their proinflammatory phenotype. These cells were then treated with endothelial progenitor cell-conditioned medium or control medium. Polymerase chain reaction was used to determine mRNA expression levels of related inflammatory factors. Afterwards, primary cultures of rat spinal cord neuronal cells were prepared and treated with H2O2 and either endothelial progenitor cell-conditioned medium or control medium. Hoechst 33258 and propidium iodide staining were used to calculate the proportion of neurons undergoing apoptosis. Aortic ring assay was performed to assess the effect of endothelial progenitor cell-conditioned medium on angiogenesis. Compared with control medium, endothelial progenitor cell-conditioned medium mitigated the macrophage inflammatory response at the spinal cord injury site, suppressed apoptosis, and promoted angiogenesis. Next, we used a rat model of spinal cord injury to examine the effects of the endothelial progenitor cell-conditioned medium in vivo. The rats were randomly administered intraperitoneal injection of PBS, control medium or endothelial progenitor cell-conditioned medium, once a day, for 6 consecutive weeks. Immunohistochemistry was used to observe neuronal morphology. Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling assay was performed to detect the proportion of apoptotic neurons in the gray matter. The Basso, Beattie and Bresnahan Locomotor Rating Scale was used to evaluate the recovery of motor function of the bilateral hind limbs after spinal cord injury. Compared with the other two groups, the number of axons was increased, cavities in the spinal cord were decreased, the proportion of apoptotic neurons in the gray matter was reduced, and the Basso, Beattie and Bresnahan score was higher in the endothelial progenitor cell-conditioned medium group. Taken together, the in vivo and in vitro results suggest that endothelial progenitor cell-conditioned medium suppresses inflammation, promotes angiogenesis, provides neuroprotection, and promotes functional recovery after spinal cord injury.
Introduction
Traumatic spinal cord injury (SCI) results in the destruction of nervous tissue and a partial or complete loss of neurological functions. However, effective treatments are still lacking. The pathology of SCI can be divided into two distinct phases: the initial physical events that result in axonal damage and the later secondary injury processes that involve persistent inflammation, apoptosis, glutamate excitotoxicity, lipid peroxidation and free radical production (Oyinbo, 2011; Hayta and Elden, 2017).
Therefore, SCI can be considered to consist of primary and secondary injury mechanisms (Norenberg et al., 2004). The primary injury occurs seconds to minutes after the initial insult, while the secondary injury proceeds over a time course of minutes to months. It is thought that the poor outcome following SCI is mainly a result of the secondary damage (Oyinbo, 2011). Among the various mechanisms of secondary damage, inflammation plays a particularly critical role (Anwar et al., 2016). The inflammatory cytokines and chemokines released from spinal cord cells lead to the sequential activation and migration of microglia towards the lesion and the recruitment of circulating monocytes to the site of injury (Taoka and Okajima, 2000; Donnelly and Popovich, 2008). There is a consensus that inflammation has both beneficial and detrimental effects. In the first week after SCI, the destructive effects outweigh the beneficial effects, perhaps because lesion-derived inflammatory cytokines induce migrating peripheral blood monocytes into classically activated pro-inflammatory macrophages that persist for 1 month. In comparison, alternatively activated macrophages, which are anti-inflammatory, persist for a very short period of time (Kigerl et al., 2009; Kong and Gao, 2017). The classically activated macrophages produce high levels of inflammatory factors, such as interleukin (IL)-1β, IL-6 and inducible nitric oxide synthase, resulting in a local microenvironment that is not conducive to the survival of resident neurons, oligodendrocytes or transplanted cells (Garcia et al., 2016).
Over the past decade, cell transplantation has shown limited effectiveness (Nando Tewariem et al., 2009; Cusimano et al., 2012; Quertainmont et al., 2012; Guest et al., 2013; Kamei et al., 2013; Kolar et al., 2014; Muheremu et al., 2016). A hostile microenvironment and adaptive immune responses in the injured spinal cord might contribute to the low effectiveness of cell transplantation. It is reported that only a small number of transplanted cells exert beneficial effects because more than 90% of cells are cleared during the first few days after transplantation (Zhang et al., 2001; Paul et al., 2009; Van der bogt et al., 2009; Torres-Espin et al., 2014). In addition, many studies suggest that the beneficial effects obtained after cell transplantation are more likely the results of paracrine mechanisms rather than effective integration and differentiation of the transplanted cells within the host tissue (Himes et al., 2006; Rooney et al., 2009). Accordingly, an increasing number of studies have focused on the use of conditioned media from stem cells or differentiated cells for treating SCI. The new methods have shown therapeutic potential by providing neuroprotection and by promoting axonal regeneration and alleviating inflammation (Montoya et al., 2009; Cantinieaux et al., 2013; Guo et al., 2016; Cheng et al., 2017; Gu et al., 2017).
Endothelial progenitor cells (EPCs), mainly obtained from bone marrow or peripheral blood, secrete a variety of growth factors, such as hepatocyte growth factor, IL-8, platelet-derived growth factor, stromal cell-derived factor 1, vascular endothelial growth factor and brain-derived neurotrophic factor (Di Santo et al., 2009; Di Stefano et al., 2009; Zhao et al., 2016). Fujioka et al. (2012) and Kamei et al. (2012, 2013) reported that transplantation of human blood-derived CD133+ cells improves functional recovery after SCI. Our previous study showed that EPCs promote neural stem cell proliferation and differentiation in vitro (Du et al., 2016). Moreover, human peripheral blood-derived EPC-conditioned medium (EPC-CM) has been shown to be as effective as cell transplantation in promoting tissue revascularization and functional recovery in a rat model of chronic hind limb ischemia (Di Santo et al., 2009). Nevertheless, it is unknown whether EPC-CM promotes functional recovery after contusive SCI. In the present study, we investigated whether EPC-CM inhibits inflammation, promotes angiogenesis and provides neuroprotection.
Materials and Methods
Animals
For primary culture of bone marrow EPCs, 24 4-week-old female Sprague-Dawley rats, weighing 80–100 g, were used. For primary culture of bone marrow-derived macrophages, 12 C57/BL6 mice, weighing 14–20 g, were used. For culture of spinal neurons, six 24-hour-old Sprague-Dawley pups were used. For in vivo experiments, 30 healthy adult female Sprague-Dawley rats, weighing 200–250 g, were used. All animals were specific-pathogen-free and provided by the Experimental Animal Center of Anhui Medical University of China (license No. SCXK (Wan) 2011-002). The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1985), and was in accordance with the Consensus Author Guidelines on Animal Ethics and Welfare produced by the International Association of Veterinary Editors. The study protocol was approved by the Animal Ethics Committee of Anhui Medical University of China (No. LLSC 20150344).
In vitro experiments
Primary culture of EPCs and EPC-CM collection
EPCs were cultured as described in a previous report with slight modification (Brunt et al., 2007). Bone marrow mononuclear cells were isolated from the bone marrow of 4-week-old female Sprague-Dawley rats by density gradient centrifugation, and these cells were incubated on culture dishes coated with fibronectin in endothelial cell growth medium (Lonza, MD, USA). To produce EPC-CM, EPCs were cultured for 48 hours under hypoxic conditions (1.5% O2, 5% CO2, 93.5% N2) in serum and growth factor-free endothelial cell basal medium (Lonza, MD, USA). The conditioned medium was collected and centrifuged at 4000 ×g for 30 minutes at 4°C using 3-kDa MW cut-off filter units (Millipore, Bedford, MA, USA), and then sterilized through a 0.4-µm filter. This concentrated medium (i.e., the EPC-CM) was stored at −80°C until use. Growth factor and serum-free endothelial cell basal medium served as control medium (Con-M).
Primary culture of mouse bone marrow-derived macrophages and assessment of the effect of EPC-CM on inflammation
Mouse bone marrow-derived macrophages were prepared as previously described (Ying et al., 2013). Briefly, bone marrow was harvested from C57/BL6 mouse femoral and tibial shafts. Cells were cultured for 7 days in Dulbecco’s modified Eagle’s medium/high glucose (DMEM/HG; Gibco, Grand Island, USA) supplemented with 10% fetal bovine serum (FBS), macrophage colony stimulating factor (10 ng/mL, PeproTech, NJ, USA) and 1% penicillin/streptomycin. On day 7, bone marrow-derived macrophages were divided into four groups. In the control group, cells were incubated in DMEM/HG containing 10% FBS. In the lipopolysaccharide group, cells were activated with 100 ng/mL lipopolysaccharide (Sigma-Aldrich, St. Louis, MO, USA) and incubated in DMEM/HG containing 10% FBS. In the Con-M and EPC-CM groups, cells were activated with 100 ng/mL lipopolysaccharide and incubated in DMEM/HG containing 10% FBS and either Con-M or EPC-CM (1:1) for 48 hours. Single-cell suspensions were prepared in phosphate-buffered saline (PBS) and blocked with anti-mouse CD16/32 for 10 minutes, and thereafter stained with rat anti-mouse F4/80, CD11b, CD86 or CD206 antibody (1 µg per 1 × 106 cells; BioLegend, CA, USA) for 20 minutes on ice. After three PBS washes, samples were analyzed with the BD FACSverse flow cytometry system (BD, Franklin Lakes, NJ, USA).
Quantitative polymerase chain reaction was used to evaluate mRNA expression levels of inflammatory cytokines. After bone marrow-derived macrophages were activated and incubated for 48 hours, cells were collected. Total RNA was isolated with TRIZOL (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions, and then reverse-transcribed into cDNA using the SuperScript II system (Vazyme Biotech, Nanjing, China). GAPDH was used as the internal standard. SYBR Green dye (Vazyme Biotech) was used to monitor amplification. The results are reported as relative quantity values. Relative changes in gene expression levels were analyzed using the 2−ΔΔCT method, as described previously (Schmittgen and Livak, 2008). Gene expression in the control group was normalized to 1, and gene expression in the experimental group was expressed as a ratio to that in the control group (optical density ratio). All assays were performed in triplicate. The primer sequences are shown in Table 1.
Table 1.
Primer sequences
Rat spinal cord neuron culture and apoptosis
Rat spinal cord neuron culture and hydrogen peroxide-induced apoptosis were carried out according to a previous report, with modification (Wang et al., 2016). Briefly, single-cell suspensions were collected from rat dissociated spinal cord 24 hours after birth, and then cultured in poly-D-lysine-coated 24-well plates in Neurobasal-A medium supplemented with 2% B27, 0.5 mM glutamine, 100 U/mL penicillin and streptomycin (Gibco, USA) at a density of 600 cells/mm2. After 7 days, neurons were divided into four groups. In the control group, cells were incubated in neuronal media. In the H2O2 group, cells were incubated in neuronal media for 24 hours, and then exposed to 100 μM H2O2 for 30 minutes. In the Con-M and EPC-CM groups, cells were pretreated with Con-M or EPC-CM for 24 hours, and then exposed to 100 μM H2O2 for 30 minutes. Cells were stained with propidium iodide (10 μg/mL) and Hoechst 33258 (10 μg/mL) for 15 minutes and fixed with 4% paraformaldehyde for 10 minutes. The cells were observed under a fluorescence microscope (Nikon, Tokyo, Japan). Thirty random fields were manually counted. The proportion of double-stained cells to total cells was calculated.
Ex vivo aortic ring assay
Rat aortic rings were cultured in type I rat tail collagen gel as described by Baker, with modification (Baker et al., 2011). Briefly, 100 μL of the gel was added to each well of a pre-cooled 96-well plate, one aortic ring per well was embedded in the middle of the gel. Aortic rings were cultured in 150 μL EPC-CM or Con-M for 7 days. Photomicrographs were taken under a phase contrast microscope (Nikon, Japan). A minimum of three aortic explants was used per experimental condition, and the experiment was repeated at least three times. Sprout length was calculated digitally using Image J software (National Institutes of Health, Rockville, MD, USA).
In vivo experiments
Spinal cord injury and treatment
A total of 30 adult female Sprague-Dawley rats, weighing 200–250 g, were randomly assigned to the EPC-CM, Con-M and PBS groups (n = 10 per group). After intraperitoneal injection of 10% chloral hydrate at a dose of 350 mg/kg and conventional iodine disinfection, complete laminectomy was performed at the T10 level, and SCI was induced using a modified Allen’s method (10 g from a height of 50 mm). After SCI, the rats exhibited the tail reflex and paralysis of the bilateral hind limbs, indicating successful reproduction of the spinal cord contusive injury model (Allen, 1911). A 1-mL volume of EPC-CM, Con-M or PBS was injected intraperitoneally daily until sacrifice. Rats were housed at 22–25°C and 30–40% humidity under a 12/12-hour light/dark cycle, with free access to food and water. The bladder was manually emptied twice daily until recovery of micturition function.
Histopathological examination
Rats were sacrificed at 1 week (n = 5 per group) and 6 weeks (n = 5 per group) post-surgery and used for histopathological analyses. After the tissue was fixed in 4% paraformaldehyde and embedded in paraffin, serial 5-μm-thick coronal sections were cut. The sections were stained with rabbit anti-rat CD86 or rabbit anti-rat CD206 (Abcam, MA, USA). Alexa Fluor 594-conjugated goat anti-rabbit and Alexa Fluor 488-conjugated goat anti-rabbit (Life Technologies, NY, USA) secondary antibodies were used. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole. Immunostained sections were observed under a fluorescence microscope (Nikon, Japan).
Immunohistochemistry was used to detect CD31-positive blood vessel lumens 1 week after injury and neurofilament 200 (NF200) density 6 weeks after injury at the lesion site. Rabbit anti-rat CD31 (Abcam) or rabbit anti-rat NF200 (Proteintech, Cambridge, MA, USA) antibody was incubated overnight at 4°C, and then horseradish peroxidase-labeled goat anti-rabbit IgG (Biosynthesis, Beijing, China) was added. Diaminobenzidine and hematoxylin were used as reagent and counterstain. Results were measured with Image J software.
Serial sections made 6 weeks after injury were stained with hematoxylin and eosin, and examined by phase contrast microscopy. Cystic cavities were analyzed according to a previous study (Cantinieaux et al., 2013), and the amount of cavitation was expressed as cavity extent/spinal cord extent × 100%.
TUNEL assay
Apoptosis was assessed 1 week after injury using the TUNEL kit (Roche, Mannheim, Germany), following the manufacturer’s instructions. Under a high-power objective (× 200; Nikon), the number of apoptotic cells, characterized by TUNEL-stained nuclei, was counted in four randomly selected fields from four sections for each group.
Basso, Beattie and Bresnahan (BBB) score
The BBB Locomotor Rating Scale (Basso et al., 1995; n = 5 per group) was used to assess locomotor function by two independent and blinded observers at 1, 3, 5 and 7 days after surgery, and then every 7 days until sacrifice. The rats were allowed to move freely in an open field apparatus (1 m × 1 m), and their movements were recorded for 5 minutes. Scores were recorded and averaged across both the left and right hind limbs.
Statistical analysis
All data were presented as the mean ± SD and were analyzed with SPSS 17.0 software (SPSS, Chicago, IL, USA). Values were compared using one-way analysis of variance, followed by the least significant difference post hoc analysis for comparisons among multiple groups. P values ≤ 0.05 were considered statistically significant.
Results
Effect of EPC-CM on macrophage phenotype and inflammatory cytokine release in vitro and in vivo
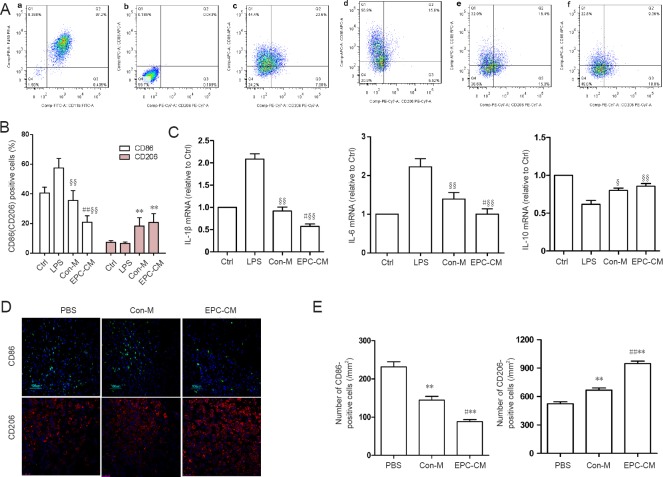
Flow cytometry showed that EPC-CM significantly reduced expression of the classically activated macrophage (M1) marker CD86 in bone marrow-derived macrophages treated with lipopolysaccharide compared with Con-M, while the alternatively activated macrophage (M2) marker CD206 remained relatively unchanged. These data suggest that ECP-CM suppresses macrophage M1 activation (Figure 1A, B).
Figure 1.
Effects of EPC-CM on inflammatory cytokine levels in vitro and in vivo.
(A) Representative flow cytometry data showing the effect of EPC-CM on BMDMs. (a) Mature BMDMs were defined as CD11b+/F4/80+ subpopulations (upper right), with the purity displayed as percentage of the parent population. (b) Control BMDMs were incubated with CD11b and F4/80 antibody and used to set up the gate. (c) Control BMDMs without LPS stimulation were incubated with anti-rat CD11b, F4/80, CD86 and CD206 antibodies. M1 macrophages are CD11b+/F4/80+/CD86+/CD206− (Q1), whereas M2 macrophages are CD11b+/F4/80+/CD86−/CD206+ (Q3). (d) BMDMs treated with LPS. (e) BMDMs cultured with Con-M and simultaneously stimulated with LPS. (f) BMDMs cultured with EPC-CM and stimulated with LPS. (B) Quantitation of M1 and M2 cells among the different groups. Compared with the Con-M group, EPC-CM significantly reduced M1 activation, while M2 cells remained relatively unchanged. (C) mRNA expression levels of inflammatory cytokines (optical density ratio) among groups. (D) Immunofluorescence staining for CD86 (M1 marker) and CD206 (M2 marker) in the epicenter 7 days after SCI (n = 5 per group; green: CD86; red: CD206; blue: DAPI). (E) Quantification of CD86- and CD206-positive cells at 7 days after SCI. **P < 0.01, vs. PBS group; #P < 0.05, ##P < 0.01, vs. Con-M group. §P < 0.05, §§P < 0.01, vs. LPS. Data are presented as the mean ± SD (one-way analysis of variance followed by the least significant difference post hoc test). The experiment was performed at least three times. Ctrl: Control; PBS: phosphate-buffered saline; EPC-CM: endothelial progenitor cell–conditioned medium; Con-M: control medium; BMDMs: bone marrow-derived macrophages; LPS: lipopolysaccharide; IL: interleukin; DAPI: 4′,6-diamidino-2-phenylindole.
We next examined the effect of EPC-CM on the expression of inflammatory cytokines, including IL-1β, IL-6 and IL-10. Quantitative RT-PCR showed that EPC-CM substantially reduced lipopolysaccharide-stimulated expression of the pro-inflammatory cytokines IL-1β and IL-6. In comparison, expression of the anti-inflammatory cytokine IL-10 was not substantially impacted (Figure 1C).
To evaluate the immunomodulatory effect of EPC-CM in vivo, CD86- and CD206-positive cells in the epicenter were counted 1 week after injury. EPC-CM significantly decreased the number of CD86+ cells and markedly increased the number of CD206+ cells (Figure 1D–E). The in vitro and in vivo results indicate that EPC-CM exerts a strong anti-inflammatory effect.
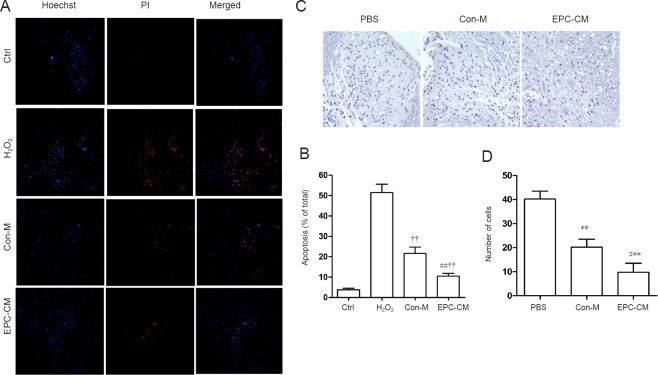
EPC-CM suppressed H2O2-induced neuronal apoptosis in vitro and gray matter neuronal apoptosis in vivo after SCI
The percentage of propidium iodide and Hoechst 33258 double-stained cells were counted in vitro. Apoptotic cells accounted for 51.4 ± 4.1% of total cells in the H2O2 group, while they accounted for 21.6 ± 3.1% of cells in the Con-M group. EPC-CM significantly attenuated the apoptotic rate to 10.5 ± 1.4% (Figure 2A, B). In the gray matter, rats in the EPC-CM group had the fewest TUNEL-positive cells (Figure 2C, D). These data demonstrate that EPC-CM attenuates neuronal injury.
Figure 2.
Effect of EPC-CM on apoptosis in vitro and in vivo.
(A) In vitro representative fluorescence images of Hoechst and PI double staining (original magnification, 200×; blue: Hoechst 33258; red: PI). (B) Quantitative assessment of apoptotic cells in the various groups. EPC-CM significantly reduced H2O2-induced spinal cord neuron apoptosis. The experiment was performed at least three times. (C) Cells in the gray matter in the epicenter stained with TUNEL one week after injury (in vivo). The nuclei of TUNEL-positive (apoptotic) cells are stained a dark color (n = 5 per group; original magnification, 200×). (D) Quantitative analysis of TUNEL-positive cells. Compared with the PBS group, the EPC-CM and Con-M groups had significantly fewer TUNEL-positive cells. **P < 0.01, vs. PBS group; #P < 0.05, ##P < 0.01, vs. Con-M group. ††P < 0.01, vs. H2O2 group. Data are presented as the mean ± SD (one-way analysis of variance followed by the least significant difference post hoc test). The experiment was performed at least three times. Ctrl: Control; PBS: phosphate-buffered saline; PI: propidium iodide; EPC-CM: endothelial progenitor cell-conditioned msedium; Con-M: control medium; TUNEL: terminal deoxynucleotidyl transferase dUTP nick-end labeling.
Effect of EPC-CM on angiogenesis in vitro and in vivo
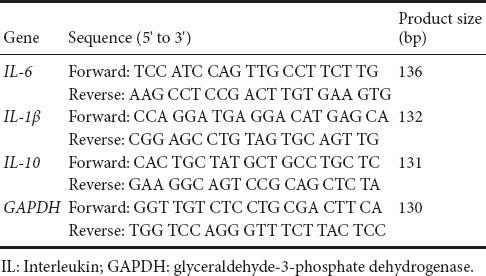
To examine the pro-angiogenic effect of EPC-CM in vitro, we performed the aortic ring assay. EPC-CM had a substantially more robust angiogenic effect, compared with Con-M, evidenced as a significantly wider and clearly denser network of vascular sprouts emerging from the aorta (Figure 3A, B). Furthermore, the EPC-CM group showed the highest number of CD31-positive vascular lumens in the epicenter 7 days after SCI (Figure 3C, D). These results show that EPC-CM promotes angiogenesis.
Figure 3.
Effects of EPC-CM on angiogenesis in rats in vitro and in vivo.
(A) In vitro representative photomicrographs of vessel outgrowth from 1-mm rat aortic rings embedded in type I rat tail collagen gel and incubated with EPC-CM or Con-M. EPC-CM-treated aortic ring shows a wider and denser network of vascular sprouts (original magnification, 40×). Scale bars: 25 μm. (B) Quantitative analysis of sprout length induced by incubation with Con-M or EPC-CM. (C) Representative images of immunostained sections showing CD31-positive lumens in the epicenter in the various groups (in vivo). The typical positive lumens contain a blue nucleus and a brown endothelium. Scale bars: 100 μm. (D) Assessment of the number of CD31+ lumens. *P < 0.05, **P < 0.01, vs. PBS group; #P < 0.05, ##P < 0.01, vs. Con-M group. Data are presented as the mean ± SD (one-way analysis of variance followed by the least significant difference post hoc test). The experiment was performed at least three times. PBS: Phosphate-buffered saline; EPC-CM: endothelial progenitor cell-conditioned medium; Con-M: control medium.
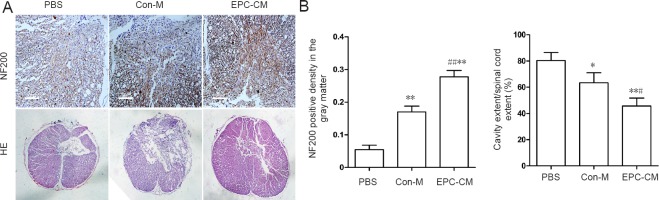
EPC-CM enhanced axonal regeneration, reduced cystic cavities, and promoted functional recovery
To assess the impact of EPC-CM on axonal regrowth, NF200 immunostaining was performed. NF200-positive axonal density was markedly increased in the EPC-CM group compared with the Con-M and PBS groups. Furthermore, the EPC-CM group showed dramatically reduced mean size of cystic cavities compared with the other groups (Figure 4A, B). From day 7 onwards after SCI, EPC-CM-treated animals exhibited better scores than Con-M-treated animals, and this difference was sustained (Figure 5).
Figure 4.
Effects of EPC-CM on the histomorphology of the injured rat spinal cord.
(A) Representative images of NF200 immunostaining in the gray matter at the lesion site (upper panel, scale bar: 100 μm; nuclei are stained blue, and positive cells are stained brown) and cavities by HE staining (lower panel, original magnification, 40×; nuclei are stained blue, and the cytoplasm is stained pink). (B) Quantitation of NF200-positive density and areas of cystic cavities (cavity extent/spinal cord extent × 100%) in the various groups. *P < 0.05, **P < 0.01, vs. PBS group; #P < 0.05, ##P < 0.01, vs. Con-M group. Data are presented as the mean ± SD (one-way analysis of variance followed by the least significant difference post hoc test). The experiment was performed at least three times. PBS: Phosphate-buffered saline; EPC-CM: endothelial progenitor cell-conditioned medium; Con-M: control medium; HE: hematoxylin-eosin; NF200: neurofilament 200.
Figure 5.
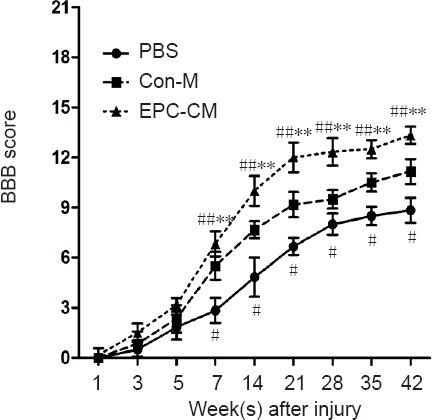
Effects of EPC-CM on motor functional recovery in rats at various time points after spinal cord injury.
The BBB score was evaluated every week after injury (n = 5 per group). There were significant differences among the three groups after surgery from day 7 onwards. #P < 0.05, ##P < 0.01, vs. Con-M group; **P < 0.01, vs. PBS group. Data are presented as the mean ± SD (one-way analysis of variance followed by the least significant difference post hoc test). The experiment was performed at least three times. PBS: Phosphate-buffered saline; EPC-CM: endothelial progenitor cell–conditioned medium; Con-M: control medium; BBB: Basso, Beattie and Bresnahan.
Discussion
In the present study, we found the following: (1) EPC-CM exerts anti-inflammatory effects by reducing M1 macrophage activation and pro-inflammatory cytokine release; (2) EPC-CM promotes angiogenesis in vitro and in vivo; and (3) EPC-CM reduces neuronal apoptosis to provide neuroprotection. Together, these effects of EPC-CM result in tissue sparing and locomotor functional recovery after contusive SCI in the rat.
EPCs release a variety of factors, such as vascular endothelial growth factor, brain-derived neurotrophic factor and hepatocyte growth factor, which inhibit inflammation (Pías-Peleteiro et al., 2017). Wang et al. (2015) reported that vascular endothelial growth factor 165 decreases the levels of IL-1β, IL-10 and tumor necrosis factor-α in the culture medium of lipopolysaccharide-treated spinal neuron–glia co-cultures. Ji et al. (2015) showed that local injection of a lentiviral vector expressing brain-derived neurotrophic factor at the lesion site promotes a shift from the M1 to the M2 phenotype and reduces the inflammatory response after SCI. Hepatocyte growth factor is a potent anti-inflammatory factor that alleviates inflammation and dysfunction in a wide variety of experimental animal models, such as rheumatoid arthritis and autoimmune neuroinflammation (Molnarfi et al., 2015). A previous study provided evidence that EPC transplantation suppresses the expression of pro-inflammatory cytokines, such as tumor necrosis factor-α and IL-6, after SCI (Fujioka et al., 2012). Our present study shows that EPC-CM decreases M1 activation and suppresses mRNA expression of pro-inflammatory cytokines in bone marrow-derived macrophages. The in vivo findings show that CD86+ macrophages are downregulated, while CD206+ macrophages are upregulated. We suspect that the difference between our in vitro and in vivo results might be attributed to differences in the animals used. However, both the in vitro and in vivo findings show that EPC-CM reduces the number of pro-inflammatory macrophages, strongly suggesting, for the first time, that EPC-CM exerts an anti-inflammatory effect. Our results are partially in accordance with those of Cheng et al. (2017) but inconsistent with those of Cantinieaux et al. (2013). In the study by Cheng and colleagues, neural stem cell-conditioned medium significantly downregulated M1 macrophages, while M2 macrophages remained relatively unchanged. Furthermore, IL-10 expression was maintained in vitro, but substantially reduced in vivo. Cantinieaux et al. (2013) reported that bone marrow-derived mesenchymal stem cell-conditioned medium favors a pro-inflammatory state, as indicated by the significant increase in IL-1β secretion and the apparent, but non-significant, increase in IL-6 and tumor necrosis factor-α production. These discrepancies could possibly be ascribed to differences in the conditioned media and model species used.
It is generally thought that increasing angiogenesis and restoring normal vascular perfusion soon after injury is crucial for repair of the spinal cord (Graumann et al., 2011). Angiogenesis is indeed known to induce tissue protection via increased blood flow by supplying many nutritional substances and oxygen that help protect injured spinal tissue from further degradation. Previous studies showed that transplantation of CD133+ cells markedly increases the diameter and number of blood vessels in the epicenter of the injury site (Fujioka et al., 2012; Kamei et al., 2013). Our current findings suggest that EPC-CM enhances angiogenesis at the site of SCI. It is not known which factor in EPC-CM stimulates angiogenesis; however, we speculate that vascular endothelial growth factor plays a major role. Vascular endothelial growth factor is a key mediator of angiogenesis during spinal cord development and SCI (Chung and Ferrara, 2011). Several studies show that vascular endothelial growth factor promotes angiogenesis in central and peripheral regions when delivered directly or via gene therapy (Widenfalk et al., 2003; Yun et al., 2017). For example, exogenous vascular endothelial growth factor 165 delivered locally immediately after SCI increases blood vessel density in the rat.
Neuronal death rapidly followed by oligodendrocyte apoptosis is characteristic of secondary injury in SCI. Here, we found that EPC-CM protects neurons from apoptosis in vitro and in vivo. EPCs cultured under 1.5% oxygen secrete factors related to neuroprotection, such as vascular endothelial growth factor, brain-derived neurotrophic factor and hepatocyte growth factor (Di Santo et al., 2009). Vascular endothelial growth factor reduces apoptosis at the epicenter of injury, as well as 1, 2 and 3 mm distally, as assessed by TUNEL staining (Liu et al., 2010). In addition, vascular endothelial growth factor is a potential neurotrophic factor, promoting neuronal survival in cells cultured in oxygen- and glucose-deprived conditions (Jin et al., 2000). Brain-derived neurotrophic factor rescues neurons after SCI via the TrkB/PI3-kinase/Akt pathway (Weishaupt et al., 2012). Furthermore, hepatocyte growth factor exerts neuroprotective effects after SCI via the hepatocyte growth factor receptor (Met) pathway (Kitamura et al., 2011). A recent study showed that the cytoprotective effects of human EPC-CM against ischemic insult are not vascular endothelial growth factor or IL-8-dependent (Di Santo et al., 2016). The key factors mediating the neuroprotective effects of EPC-CM are unclear and need further investigation.
Here, we observed that EPC-CM significantly promoted axonal regeneration, as evidenced by an increase in NF200 immunoreactivity in the gray matter, and it reduced cystic cavity volume. Importantly, EPC-CM improved BBB scores, suggesting that it promotes functional recovery after SCI.
In summary, the systemic delivery of EPC-CM alleviates inflammation, promotes angiogenesis and provides neuroprotection to improve axonal regeneration and functional recovery after SCI. This novel cell-free therapy circumvents the concerns and restrictions of cell transplantation. However, further studies are needed to identify the factors and signaling pathways underlying the effectiveness of EPC-CM. Nonetheless, EPC-CM may have therapeutic potential for the treatment of SCI.
Footnotes
Conflicts of interest: The authors declare no competing financial interests.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 81171173 and 81672161. The funder had no role study design, data collection, data analysis, data interpretation or writing of the manuscript.
Institutional review board statement: The experiments have been approved by the Animal Ethics Committee of Anhui Medical University of China (approval No. LLSC20150344).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81171173 and 81672161.
(Copyedited by Patel B, Maxwell R, Wang J, Li CH, Qiu Y, Song LP, Zhao M)
References
- 1.Allen AR. Surgery of experimental lesion of spinal cord equivalent to crush injury of fracture dislocation of spinal colum. JAMA. 1911;57:878–880. [Google Scholar]
- 2.Anwar MA, Al Shehabi TS, Eid AH. Inflammogenesis of secondary spinal cord injury. Front cell Neurosci. 2016;10:98. doi: 10.3389/fncel.2016.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker M, Robinson SD, Lechertier T, Barber PR, Tavora B, D’Amico G, Jones DT, Vojnovic B, Hodivala-Dilke K. Use of the mouse aortic ring assay to study angiogenesis. Nat Protoc. 2011;7:89–104. doi: 10.1038/nprot.2011.435. [DOI] [PubMed] [Google Scholar]
- 4.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 5.Brunt KR, Hall SR, Ward CA, Melo LG. endothelial progenitor cell and mesenchymal stem cell isolation, characterization, viral transduction. Methods Mol Med. 2007;139:197–210. doi: 10.1007/978-1-59745-571-8_12. [DOI] [PubMed] [Google Scholar]
- 6.Cantinieaux D, Quertainmont R, Blacher S, Rossi L, Wanet T, Noël A, Brook G, Schoenen J, Franzen R. Conditioned medium from bone marrow-derived mesenchymal stem cells improves recovery after spinal cord injury in rats: an original strategy to avoid cell transplantation. PLoS One. 2013;27:e69515. doi: 10.1371/journal.pone.0069515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng Z, Bosco DB, Sun L, Chen X, Xu Y, Tai W, Didier R, Li J, Fan J, He X, Ren Y. Neural stem cell-conditioned medium suppresses inflammation and promotes spinal cord injury recovery. cell Transplant. 2017;26:469–482. doi: 10.3727/096368916X693473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung AS, Ferrara N. Developmental and pathological angiogenesis. Ann Rev cell Dev Biol. 2011;27:563–584. doi: 10.1146/annurev-cellbio-092910-154002. [DOI] [PubMed] [Google Scholar]
- 9.Cusimano M, Biziato D, Brambilla E, Donegà M, Alfaro-Cervello C, Snider S, Salani G, Pucci F, Comi G, Garcia-Verdugo JM, De Palma M, Martino G, Pluchino S. Transplanted neural stem/precursor cells instruct phagocytes and reduce secondary tissue damage in the injured spinal cord. Brain. 2012;135:447–460. doi: 10.1093/brain/awr339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Santo S, Fuchs AL, Periasamy R, Seiler S, Widmer HR. The cytoprotective effects of human endothelial progenitor cell-conditioned medium against an ischemic insult are not dependent on VEGF and IL-8. Cell Transplant. 2016;25:735–747. doi: 10.3727/096368916X690458. [DOI] [PubMed] [Google Scholar]
- 11.Di Santo S, Yang Z, Wyler von Ballmoos M, Voelzmann J, Diehm N, Baumgartner I, Kalka C. Novel cell-free strategy for therapeutic angiogenesis: in vitro generated conditioned medium can replace progenitor cell transplantation. PLoS One. 2009;21:e5643. doi: 10.1371/journal.pone.0005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Stefano R, Barsotti MC, Armani C, Santoni T, Lorenzet R, Balbarini A, Celi A. Human peripheral blood endothelial progenitor cell synthesize and express functionally active tissue factor. Thromb Res. 2009;123:925–930. doi: 10.1016/j.thromres.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008;209:378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du Y, Zhang S, Yu T, Du G, Zhang H, Yin Z. Wnt3a is critical for endothelial progenitor cell-mediated neural stem cell proliferation and differentiation. Mol Med Rep. 2016;3:2473–2482. doi: 10.3892/mmr.2016.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayta E, Elden H. Acute spinal cord injury: A review of pathophysiology and potential of non-steroidal anti-inflammatory drugs for pharmacological intervention. J Chem Neuroanat. 2017 doi: 10.1016/j.jchemneu.2017.08.001. doi: 10.1016/j.jchemneu.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Fujioka Y, Tanaka N, Nakanishi K, Kamei N, Nakamae T, Izumi B Ohta R, Ochi M. Magnetic field-based delivery of human CD 133+ cells promotes functional recovery after spinal cord injury. Spine (Phila pa 1976) 2012;37:E768–777. doi: 10.1097/BRS.0b013e318246d59c. [DOI] [PubMed] [Google Scholar]
- 17.Garcia E, Aguilar-Cevallos J, Silva-Garcia R, Ibarra A. Cytokine and growth factor activation in vivo and in vitro after spinal cord injury. Mediators Inflamm. 2016 doi: 10.1155/2016/9476020. doi: 10.1155/2016/9476020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu M, Gao Z, Li X, Guo L, Lu T, Li Y, He X. Conditioned medium of olfactory ensheathing cells promotes the functional recovery and axonal regeneration after contusive spinal cord injury. Brain Res. 2017;1654:43–54. doi: 10.1016/j.brainres.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 19.Guest J, Santamaria AJ, Benavides FD. Clinical translation of autologous schwann cell transplantation for the treatment of spinal cord injury. Curr Opin Organ Transplant. 2013;18:682–689. doi: 10.1097/MOT.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo L, Rolfe AJ, Wang X, Tai W, Cheng Z, Cao K, Chen X, Xu Y, Sun D, Li J, He X, Young W, Fan J, Ren Y. Rescuing macrophage normal function in spinal cord injury with embryonic stem cell conditioned media. Mol Brain. 2016 doi: 10.1186/s13041-016-0233-3. doi: 10.1186/s13041-016-0233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graumann U, Ritz MF, Hausmann O. Necessity for re-vascularization after spinal cord injury and the search for potential therapeutic options. Curr Neurovasc Res. 2011;8:334–341. doi: 10.2174/156720211798121007. [DOI] [PubMed] [Google Scholar]
- 22.Himes BT, Neuhuber B, Coleman C, Kushner R, Swanger SA. Recovery of function following grafting of human bone marrow–derived stromal cells into the injured spinal cord. Neurorehabil Neural Repair. 2006;20:278–296. doi: 10.1177/1545968306286976. [DOI] [PubMed] [Google Scholar]
- 23.Ji XC, Dang YY, Gao HY, Wang ZT, Gao M, Yang Y, Zhang HT, Xu RX. Local injection of lenti-BDNF at the lesion site promotes m2 macrophage polarization and inhibits inflammatory response after spinal cord injury in mice. cell Mol Neurobiol. 2015;35:881–890. doi: 10.1007/s10571-015-0182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin KL, Mao XO, Greenberg DA. Vascular endothelial growth factor: direct neuroprotective effect in in vitro ischemia. Proc Nat Acad Sci U S A. 2000;97:10242–10247. doi: 10.1073/pnas.97.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamei N, Kwon SM, Alev C, Nakanishi K, Yamada K, Masuda H, Ishikawa M, Kawamoto A, Ochi M, Asahara T. Ex-vivo expanded human blood-derived CD133+ cells promote repair of injured spinal cord. J Neurol Sci. 2013;328:41–50. doi: 10.1016/j.jns.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Kamei N, Kwon SM, Kawamoto A, Ii M, Ishikawa M, Ochi M, Asahara T. Contribution of bone marrow-derived endothelial progenitor cell to neovascularization and astrogliosis following spinal cord injury. J Neurosci Res. 2012;90:2281–2292. doi: 10.1002/jnr.23113. [DOI] [PubMed] [Google Scholar]
- 27.Kitamura K, Fujiyoshi K, Yamane J, Toyota F, Hikishima K, Nomura T, Funakoshi H, Nakamura T, Aoki M, Toyama Y. Human hepatocyte growth factor promotes functional recovery in primates after Spinal cord injury. PLoS One. 2011;6:e27706. doi: 10.1371/journal.pone.0027706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolar MK, Kingham PJ, Novikova LN, Wiberg M, Novikov LN. The therapeutic effects of human adipose-derived stem cells in a rat cervical spinal cord injury model. Stem cells Dev. 2014;23:1659–1674. doi: 10.1089/scd.2013.0416. [DOI] [PubMed] [Google Scholar]
- 30.Kong X, Gao J. Macrophage polarization: a key event in the secondary phase of acute spinal cord injury. J Cell Mol Med. 2017;21:941–954. doi: 10.1111/jcmm.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Figley S, Spratt SK, Lee G, Ando D, Surosky R, Fehlings MG. An engineered transcription factor which activates VEGF-A enhances recovery after spinal cord injury. Neurobiol Dis. 2010;37:384–393. doi: 10.1016/j.nbd.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 32.Molnarfi N, Benkhoucha M, Funakoshi H, Nakamura T, Lalive PH. Hepatocyte growth factor: a regulator of inflammation and autoimmunity. Autoimmun Rev. 2015;14:293–303. doi: 10.1016/j.autrev.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 33.Montoya GJV, Sutachan JJ, Chan WS, Sideris A, Blanck TJ, Recio-Pinto E. Muscle-conditioned media and cAMP promote survival and neurite outgrowth of adult spinal cord motor neurons. Exp Neurol. 2009;220:303–315. doi: 10.1016/j.expneurol.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Muheremu A, Peng J, Ao Q. Stem cell based therapies for spinal cord injury. Tissue Cell. 2016;4:328–333. doi: 10.1016/j.tice.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Nandoe Tewariem RS, Hurtado A, Bartels RH, Grotenhuis A, Oudega M. Stem cell-based therapies for spinal cord injury. J Spinal Cord Med. 2009;32:105–114. doi: 10.1080/10790268.2009.11760761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norenberg MD, Smith J, Marcillo A. The pathology of human spinal cord injury: defining the problems. J Neurotrauma. 2004;21:429–440. doi: 10.1089/089771504323004575. [DOI] [PubMed] [Google Scholar]
- 37.Oyinbo CA. Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol Exp (Wars) 2011;71:281–299. doi: 10.55782/ane-2011-1848. [DOI] [PubMed] [Google Scholar]
- 38.Pías-Peleteiro J, Campos F, Castillo J, Sobrino T. Endothelial progenitor cells as a therapeutic option in intracerebral hemorrhage. Neural Regen Res. 2017;12:558–561. doi: 10.4103/1673-5374.205085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paul C, Samdani AF, Betz RR, Fischer I, Neuhuber B. Grafting of human bone marrow stromal cells into spinal cord injury: a comparison of delivery methods. Spine. 2009;34:328–333. doi: 10.1097/BRS.0b013e31819403ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quertainmont R, Cantinieaux D, Botman O, Sid S, Schoenen J, Franzen R. Mesenchymal stem cell graft improves recovery after spinal cord injury in adult rats through neurotrophic and pro-angiogenic actions. PLoS One. 2012;7:e39500. doi: 10.1371/journal.pone.0039500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rooney GE, McMahon SS, Ritter T, Garcia Y, Moran C, Madigan NN, Flügel A, Dockery P, O’Brien T, Howard L, Windebank AJ, Barry FP. Neurotrophic factor-expressing mesenchymal stem cells survive transplantation into the contused spinal cord without differentiating into neural cells. Tissue Eng Part A. 2009;15:3049–3059. doi: 10.1089/ten.TEA.2009.0045. [DOI] [PubMed] [Google Scholar]
- 42.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 43.Taoka Y, Okajima K. Role of leukocytes in spinal cord injury in rats. J Neurotrauma. 2000;17:219–229. doi: 10.1089/neu.2000.17.219. [DOI] [PubMed] [Google Scholar]
- 44.Torres-Espin A, Redondo-Castro E, Hernandez J, Navarro X. Bone marrow mesenchymal stromal cells and olfactory ensheathing cells transplantation after spinal cord injury-a morphological and functional comparison in rats. Eur J Neurosci. 2014;39:1704–1717. doi: 10.1111/ejn.12542. [DOI] [PubMed] [Google Scholar]
- 45.Van der Bogt KE, Schrepfer S, Yu J, Sheikh AY, Hoyt G, Govaert JA, Velotta JB, Contag CH, Robbins RC, Wu JC. Comparison of transplantation of adipose tissue-and bone marrow-derived mesenchymal stem cells in the infarcted heart. Transplantation. 2009;87:642–652. doi: 10.1097/TP.0b013e31819609d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang H, Wang Y, Li D, Liu Z, Zhao Z, Han D, Yuan Y, Bi J, Mei X. VEGF inhibits the inflammation in spinal cord injury through activation of autophagy. Biochem Biophys Res Commun. 2015;464:453–458. doi: 10.1016/j.bbrc.2015.06.146. [DOI] [PubMed] [Google Scholar]
- 47.Wang M, Li YJ, Ding Y, Zhang HN, Sun T, Zhang K, Yang L, Guo YY, Liu SB, Zhao MG, Wu YM. Silibinin prevents autophagic cell death upon oxidative stress in cortical neurons and cerebral ischemia-reperfusion injury. Mol Neurobiol. 2016;53:932–943. doi: 10.1007/s12035-014-9062-5. [DOI] [PubMed] [Google Scholar]
- 48.Weishaupt N, Blesch A, Fouad K. BDNF: The career of a multifaceted neurotrophin in spinal cord injury. Exp Neurol. 2012;238:254–264. doi: 10.1016/j.expneurol.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 49.Widenfalk J, Lipson A, Jubran M, Hofstetter C, Ebendal T, Cao Y, Olson L. Vascular endothelial growth factor improves functional outcome and decreases secondary degeneration in experimental spinal cord contusion injury. Neuroscience. 2003;120:951–960. doi: 10.1016/s0306-4522(03)00399-3. [DOI] [PubMed] [Google Scholar]
- 50.Ying W, Cheruku PS, Bazer FW, Safe SH, Zhou B. Investigation of macrophage polarization using bone marrow derived macrophage. J Vis Exp. 2013;23:76. doi: 10.3791/50323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yun Y, Oh J, Kim Y, Kim G, Lee M, Ha Y. Characterization of neural stem cells modified with hypoxia/neuron-specific VEGF expression system forspinal cord injury. Gene Ther. 2017 doi: 10.1038/gt.2017.92. doi: 10.1038/gt.2017.92. [DOI] [PubMed] [Google Scholar]
- 52.Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol cell Cardiol. 2001;33:907–921. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 53.Zhao SX, Hou YN, Zhang ZT, Liu ZP, Nie ZH, Fan GL. Endothelial progenitor cell transplantation combined with early exercise training for spinal cord injury: improvement in hindlimb function and angiogenesis in the injured region. Zhongguo Zuzhi Gongcheng Yanjiu. 2016;20:883–890. [Google Scholar]