Circadian rhythm protects neurons: Although the master clock entrains the whole body rhythm, peripheral tissues also express core clock transcription factors Clock and Bmal1, which regulate expression of clock genes including Period (Per) and Cryptochrome (Cry) proteins. Complexes of Per and Cry proteins repress Bmal1- and Clock-mediated transcription forming a negative feedback loop, which regulates nearly a 24 hours self-sustained rhythm including energy metabolism. Circadian rhythm dysfunction is often observed in patients with Alzheimer’s, Parkinson’s and Huntington’s diseases. Clinical studies and experiments in animal models of neurodegenerative disorders have revealed the progressive nature of circadian dysfunction throughout the course of neurodegeneration. However, the importance of circadian rhythm in the protection of neurons remains to be elucidated. Recent studies suggest that disruption of the circadian rhythm can impair metabolic cooperation between neurons and astrocytes, and thereby enhance oxidative damages in the brain. Thus, understanding the molecular mechanisms by which endogenous antioxidant defense systems are controlled by the circadian rhythm may inform the design of novel therapeutic strategies to protect against neurodegenerative diseases. We recently proposed that neurotrophins activate the redox sensitive transcription factor nuclear factor (erythroid-derived 2)-like 2 (Nrf2), a master regulator of cellular defense against oxidative stress, in a circadian rhythm dependent manner in astrocytes to support neurons in the brain (Ishii et al., 2018).
Neurotrophin receptor p75NTR is a clock component: Neurotrophins such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) play important roles in energy metabolism, survival and the function of neuronal and non-neuronal cells. BDNF facilitates the metabolic cooperation between astrocytes and neurons in the central nervous system. Astrocytes store glycogen as an energy reservoir to provide active neurons with the glycolytic metabolite lactate. BDNF transduces signals through high affinity TrkB receptors and the low affinity p75 receptor (p75NTR). p75NTR is the common receptor for all neurotrophins. Notably, p75NTR is a clock component and its gene expression is directly regulated by the transcription factor Clock:Bmal1 complex like Period 2 (Per2) in tissues (Baeza-Raja et al., 2013). Therefore, neurotrophin signaling through p75NTR is influenced by the circadian rhythm. Per2 expression is highly synchronized among neurons and glial cells in local brain regions with its peak around the Zeitgeber time 1 (ZT1, early light/rest phase) and a sharp nadir around ZT13 (early dark/active phase). However, currently there are no precise data showing the daily variation of p75NTR protein expression levels in brain areas.
P75NTR-mediated signaling induces Nrf2 activation: A notable function of neurotrophins is to activate Nrf2, which upregulates the expression of detoxification enzymes and antioxidants to protect cells from toxic agents and oxidative stress (Itoh et al., 1997; Ishii et al., 2000). Activation of Nrf2 is particularly important in supporting mitochondrial oxidative phosphorylation. Kosaka et al. (2010) showed that NGF activates Nrf2 in rat pheochromocytomous PC12 cells and that Nrf2 upregulates gene expression of NGF, thereby forming a positive feedback loop to stably enhance Nrf2-mediated cell protection by NGF. We recently proposed that neurotrophins may activate Nrf2 via p75NTR-mediated generation of the lipid signal mediator ceramide (Ishii et al., 2018). Dobrowsky et al. (1994) first showed the stimulation of p75NTR activates neutral sphingomyelinase to generate ceramide, but its physiological effect other than apoptosis was not clear. As low levels of ceramide can activate atypical protein kinase Cζ (PKCζ), which phosphorylates and activates casein kinase 2 (CK2), we proposed that neurotrophins activate p75NTR-ceramide-PKCζ-CK2 signaling pathway. Subsequently, CK2 directly phosphorylates and stabilizes/activates Nrf2 (Figure 1A). As p75NTR expression is controlled by the cell clock, this signaling pathway must be altered with time.
Figure 1.
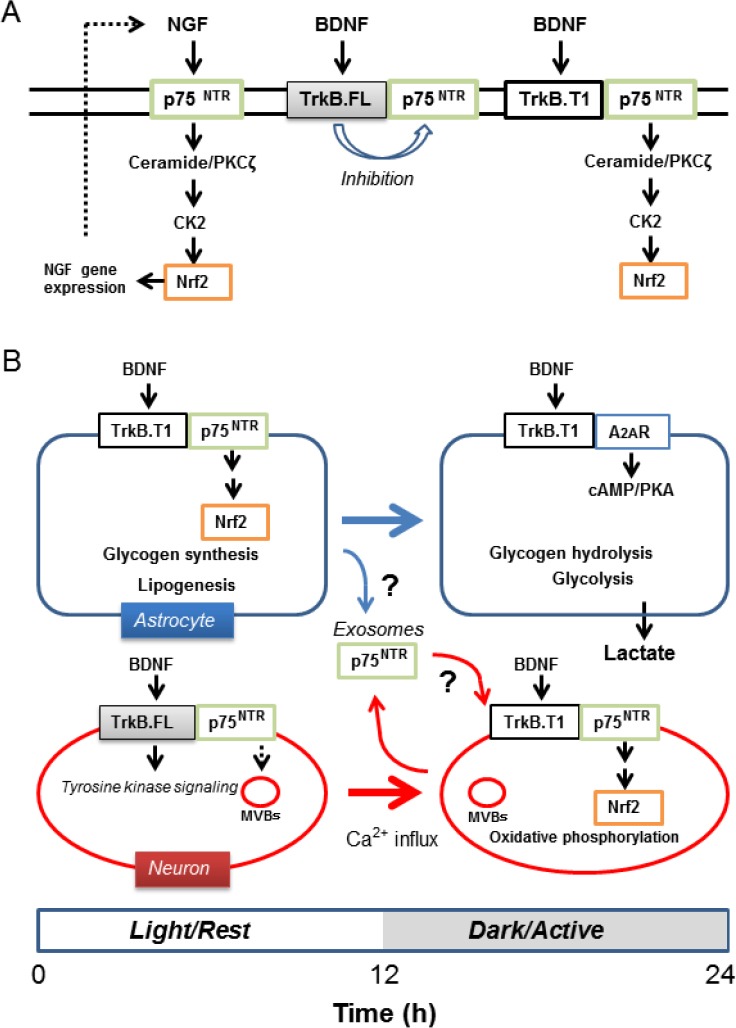
Differential time-dependent regulation of nuclear factor (erythroid-derived 2)-like 2 (Nrf2) through brain-derived neurotrophic factor (BDNF) and its receptors in astrocytes and neurons.
(A) Nerve growth factor (NGF) stimulates neurotrophin receptor p75NTR, which generates ceramide through activation of neutral sphingomyelinase. Ceramide activates protein kinase Cζ (PKCζ) inducing activation of casein kinase 2 (CK2) and Nrf2. BDNF full length receptor TrkB.FL inhibits ceramide generation through p75NTR via its tyrosine kinase activity. However, the BDNF truncated receptor TrkB.T1, which lacks the intracellular tyrosine kinase domain, facilitates ceramide generation following stimulation of TrkB.T1 by BDNF. (B) As expression of p75NTR is controlled by Clock:Bmal1, its protein levels are expected to decline during transition from the light/rest to dark/active phase in astrocytes. However, p75NTR seems to be relatively stable in neurons. It is temporarily stored in multivesicular bodies and can be released in exosomes when neuronal activity increases. Astrocytes may also provide p75NTR via exosomes. Activated neurons downregulate full length TrkB (TrkB.FL) yet upregulate truncated form of TrkB (TrkB.T1) expression. These mechanisms may enable neurons to upregulate Nrf2 through the BDNF-TrkB.T1-p75NTR axis to protect them against oxidative stress generated during the dark/active phase. h: Hour(s).
BDNF activates Nrf2 in a TrkB.T1-p75NTR-dependent manner: Blöchl and Sirrenberg (1996) measured ceramide generation in rat mesencephalic neurons in culture following stimulation either with NGF or BDNF. Treatment of neurons with NGF (100 ng/mL) for 15 minutes generated ceramide levels about 3-fold higher than the control. As neurons hardly express TrkA, these authors concluded that NGF generated ceramide through direct interaction with p75NTR. In contrast, BDNF (100 ng/mL) only marginally increased ceramides under similar culture conditions irrespective of the fact that the neurons express full length TrkB (TrkB.FL). However, pretreatment of neurons with the tyrosine kinase inhibitor K252a increased ceramide generation, suggesting tyrosine kinase activity of TrkB.FL inhibits p75NTR-mediated activation of neural sphingomyelinase. Their study indicates that the BDNF-TrkB.FL-p75NTR axis does not induce ceramide-dependent Nrf2 activation in neurons. However, this study suggested that the truncated form of TrkB (TrkB.T1), which lacks the intracellular receptor tyrosine kinase domain, does not inhibit ceramide generation via p75NTR (Figure 1A). As previous studies established that BDNF can activate CK2 in a p75NTR dependent manner and that astrocytes predominantly express TrkB.T1, we proposed that BDNF-TrkB.T1-p75NTR-mediated generation of ceramide triggers the PKCζ-CK2-Nrf2 signaling pathway (Ishii et al., 2018). When neuronal activity remains relatively low during the rest/light phase, astrocytes preferentially store energy through these p75NTR-dependent signaling pathways. As Clock:Bmal1 regulated Per2 protein peaks in the early light/rest phase and sharply decreases in the early dark/active phase in rodent local brain regions, we speculate the p75NTR-mediated signaling is relatively high during the light phase in astrocytes, when neuronal activity remains low. When neuronal activity increases in the active/dark phase, astrocytes in turn reduce oxygen consumption and increase glycogen hydrolysis and glycolysis through cAMP/PKA-dependent signaling pathways to support neuronal activity. As p75NTR levels decreases, TrkB.T1 changes its functional partner from p75NTR to the adenosine 2A receptor (A2AR) to support cAMP/PKA signaling to facilitate lactate production (Ishii et al., 2018).
Does BDNF activate Nrf2 in neurons? Our model raises the question whether or not BDNF can regulate Nrf2 activation in neurons. Since BDNF-TrkB.FL-p75NTR signaling does not support ceramide-CK2 dependent Nrf2 activation, p75NTR expression may be down-regulated in neurons in the active/dark phase. Notably, neurons require Nrf2-regulated antioxidant defenses during the active/dark phase rather than the rest/light phase. Is it possible that BDNF can upregulate Nrf2 in different time phases in neurons? We here discuss the possibility of such a mechanism by citing two key studies. Firstly, neurons express both TrkB.FL and TrkB.T1 receptors but the ratio of these receptor levels changes depends on neural activity (Gomes et al., 2012). Gomes et al. (2012) observed excitotoxic stimulation of cultured rat hippocampal or striatal neurons downregulated TrkB.FL, while upregulation of TrkB.T1 expression levels caused a significant change in the ratio of the two receptors within a few hours. TrkB.FL downregulation was mediated through Ca2+-sensitive protease calpain and an increase in TrkB.T1 was dependent on transcription and translation. These authors further showed that TrkB.T1 acts as dominant-negative inhibiting TrkB.FL tyrosine kinase-dependent signaling by forming a hetero-dimer, with an increase in the ratio of TrkB.T1 to TrkB.FL protecting neurons from damage during excitotoxic stimulation with glutamate. Their study suggests activated neurons increase the ratio of TrkB.T1 to TrkB.FL, allowing BDNF to induce Nrf2 activation if sufficient levels of p75NTR are expressed in the membrane.
Secondly, another study shows that p75NTR is relatively stable in neurons and can be released from neurons in exosomes (Escudero et al., 2014). Transfer of small vesicles, called exosomes, has been implicated in astrocyte-to-neuron signaling. Exosomes carrying microRNA, mRNA, proteins and lipids generated from endosomal membranes have the potential to modulate the function of the recipient cells. Escudero et al. (2014) characterized the internalization and post-endocytic trafficking of p75NTR in neuronal cells. The authors found that p75NTR evades the lysosomal route and accumulates in two different organelles, Rab11-positive endosomes for recycling back to membrane and CD63-positive multivesicular bodies (MVBs) for exosomes. After stimulation of PC12 cells with NGF for 2 hours, these authors found 43% of p75NTR moved to MVBs different from Rab7-positive MVBs that mature into lysosomes. Similar accumulation of p75NTR in the MVBs was also observed in sympathetic neurons following treatment with BDNF. Notably, release of exosomes can be induced by membrane depolarization. After treatment with KCl, they found full-length p75NTR was enriched in the exosomal fraction released from both cell types. These results indicate BDNF/NGF treatment induces accumulation of p75NTR in the exosome-related MVBs without rapid degradation in neurons, leading to release from cells in exosomes when neuronal activity increases. Neurons may reabsorb exosomes to increase p75NTR levels in the plasma membrane. Astrocytes also secrete exosomes to communicate with other cells including neurons. As exosomes contain sortilin which could act as co-receptor for p75NTR in the proNGF- and proBDNF-induced cell death, and glioblastoma cells release TrkB-containing exosomes (Pinet et al., 2016), it is possible that astrocytes provide p75NTR to neurons via exosomes. This possibility would be of interest to verify in future studies (Figure 1B). Taken together, it is possible that activated neurons retain an ability to activate the BDNF-TrkB.T1-p75NTR-Nrf2 signaling pathway.
Conclusion and future perspective: Ceramide generation may act as a double-edged sword. Overstimulation of p75NTR induces apoptosis due to over-generation of ceramide. TrkB.FL receptor tyrosine kinase activity inhibits neutral sphingomyelinase protecting cells from ceramide toxicity and at the same time restricts p75NTR-mediated signaling. In contrast, the BDNF-TrkB.T1-p75NTR signaling complex is the machinery to generate physiological concentrations of ceramide to activate PKCζ leading to activation of CK2 and Nrf2, thereby modulating energy metabolism and antioxidant capacity. We refer readers to our recent review, which discusses the importance of BDNF receptor-mediated cell shape change via regulation of Rho GTPases (Ishii et al., 2018). We suggest that the BDNF-TrkB.T1-p75NTR-ceramide-PKCζ signaling could modify voltage-activated potassium (Kv)1.5 type channels causing membrane depolarization and influx of Ca2+ leading to activation of ras homolog gene family member A (RhoA) in astrocytes (Ishii et al., 2018). As many neurons express other types of Kv channels, BDNF-TrkB.T1-p75NTR-ceramide-PKCζ signaling may not always induce membrane depolarization and RhoA activation in neurons (Ishii et al., 2013). Differential regulation of the actin cytoskeleton in astrocytes and neurons through BDNF receptors is another important aspect of metabolic cooperation between these two cell types. In summary, in addition to maintaining normal expression of BDNF, preserving circadian control of p75NTR expression and its dynamics in both astrocytes and neurons may serve as therapeutic targets to ameliorate neurodegeneration.
A part of the work (Ishii et al., 2018) was presented at the annual meeting of the Society for the Free Radical Biology & Medicine, on November 30th, 2017 at Baltimore, MD, USA, poster number 91.
We acknowledge the support of JSPS KAKENHI Grant Number 21500386 (TI) and British Heart Foundation (GEM, FS/15/31298; FS/16/67/32548).
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer review reports:
Reviewer 1: Agnès Rioux Bilan, Universite de Poitiers, France.
Comments to authors: This manuscript describing when and how does BDNF activate Nrf2 in astrocytes and neurons is interesting and well documented. Figure 1 allows a better comprehension and appropriately completes the manuscript.
Reviewer 2: Supriya D. Mahajan, State University of New York, USA.
Comments to authors: The current manuscript is an interesting perspective on the role of BDNF and Nrf2 in the interplay between circadian rhythms and cellular redox metabolism. Manuscript is concise and well written and highlights the time dependent regulation of Nrf2 through BDNF and its receptors. The interesting concept is the transcriptional activation of p75NTR is under circadian regulation and also its effects on metabolism. Figure 1 is great and highlights the cross talk between the astrocytes and neurons.
References
- 1.Baeza-Raja B, Eckel-Mahan K, Zhang L, Vagena E, Tsigelny IF, Sassone-Corsi P, Ptacek LJ, Akassoglou K. p75 neurotrophin receptor is a clock gene that regulates oscillatory components of circadian and metabolic networks. J Neurosci. 2013;33:10221–10234. doi: 10.1523/JNEUROSCI.2757-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blöchl A, Sirrenberg C. Neurotrophins stimulate the release of dopamine from rat mesencephalic neurons via Trk and p75Lntr receptors. J Biol Chem. 1996;271:21100–21107. doi: 10.1074/jbc.271.35.21100. [DOI] [PubMed] [Google Scholar]
- 3.Dobrowsky RT, Werner MH, Castellino AM, Chao MV, Hannun YA. Activation of the sphingomyelin cycle through the low-affinity neurotrophin receptor. Science. 1994;265:1596–1599. doi: 10.1126/science.8079174. [DOI] [PubMed] [Google Scholar]
- 4.Escudero CA, Lazo OM, Galleguillos C, Parraguez JI, Lopez-Verrilli MA, Cabeza C, Leon L, Saeed U, Retamal C, Gonzalez A, Marzolo MP, Carter BD, Court FA, Bronfman FC. The p75 neurotrophin receptor evades the endolysosomal route in neuronal cells, favouring multivesicular bodies specialised for exosomal release. J Cell Sci. 2014;127:1966–1979. doi: 10.1242/jcs.141754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomes JR, Costa JT, Melo CV, Felizzi F, Monteiro P, Pinto MJ, Inacio AR, Wieloch T, Almeida RD, Graos M, Duarte CB. Excitotoxicity downregulates TrkB.FL signaling and upregulates the neuroprotective truncated TrkB receptors in cultured hippocampal and striatal neurons. J Neurosci. 2012;32:4610–4622. doi: 10.1523/JNEUROSCI.0374-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishii T, Warabi E, Mann GE. Circadian control of p75 neurotrophin receptor leads to alternate activation of Nrf2 and c-Rel to reset energy metabolism in astrocytes via brain-derived neurotrophic factor. Free Radic Biol Med. 2018 doi: 10.1016/j.freeradbiomed.2018.01.026. doi: 10.1016/j.freeradbiomed.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 7.Ishii T, Warabi E, Siow RC, Mann GE. Sequestosome1/p62: a regulator of redox-sensitive voltage-activated potassium channels, arterial remodeling, inflammation, and neurite outgrowth. Free Radic Biol Med. 2013;65:102–116. doi: 10.1016/j.freeradbiomed.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 8.Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem. 2000;275:16023–16029. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- 9.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 10.Kosaka K, Mimura J, Itoh K, Satoh T, Shimojo Y, Kitajima C, Maruyama A, Yamamoto M, Shirasawa T. Role of Nrf2 and p62/ZIP in the neurite outgrowth by carnosic acid in PC12h cells. J Biochem. 2010;147:73–81. doi: 10.1093/jb/mvp149. [DOI] [PubMed] [Google Scholar]
- 11.Pinet S, Bessette B, Vedrenne N, Lacroix A, Richard L, Jauberteau MO, Battu S, Lalloué F. TrkB-containing exosomes promote the transfer of glioblastoma aggressiveness to YKL-40-inactivated glioblastoma cells. Oncotarget. 2016;7:50349–50364. doi: 10.18632/oncotarget.10387. [DOI] [PMC free article] [PubMed] [Google Scholar]


