ABSTRACT
Moving the nucleus to a specific position within the cell is an important event during many cell and developmental processes. Several different molecular mechanisms exist to position nuclei in various cell types. In this Commentary, we review the recent progress made in elucidating mechanisms of nuclear migration in a variety of important developmental models. Genetic approaches to identify mutations that disrupt nuclear migration in yeast, filamentous fungi, Caenorhabditis elegans, Drosophila melanogaster and plants led to the identification of microtubule motors, as well as Sad1p, UNC-84 (SUN) domain and Klarsicht, ANC-1, Syne homology (KASH) domain proteins (LINC complex) that function to connect nuclei to the cytoskeleton. We focus on how these proteins and various mechanisms move nuclei during vertebrate development, including processes related to wound healing of fibroblasts, fertilization, developing myotubes and the developing central nervous system. We also describe how nuclear migration is involved in cells that migrate through constricted spaces. On the basis of these findings, it is becoming increasingly clear that defects in nuclear positioning are associated with human diseases, syndromes and disorders.
KEY WORDS: LINC complex, Development, Nuclear envelope, Nuclear migration
Summary: Nuclear migration is a central part of many cell and developmental processes. A surprisingly large number of different molecular mechanisms exist to move nuclei to specific, intracellular locations.
Introduction – nuclear positioning in model organisms
Nuclear migration and positioning are essential to a wide variety of developmental and cellular processes. Our current understanding of the molecular mechanisms of nuclear positioning originates from genetic screens in model organisms. The first screens for nuclear positioning defects were carried out to identify the mutant classes nuclear distribution (nud) and ropy (ro) in the filamentous fungi Aspergillus nidulans and Neurospora crassa, respectively. Mostly, these mutants were shown to possess a disruption of various subunits of cytoplasmic dynein or its regulators, including NudE (NDE1 and NDEL1 in humans) and NudF (LIS1 or PAFAH1B1 in humans) (reviewed in Morris, 2000). In the budding yeast Saccharomyces cerevisiae, nuclei must migrate to the bud neck prior to cell division. Forward genetics and imaging in budding yeast identified that dynein is recruited and anchored to the cortex of the bud tip. From the bud tip, dynein pulls on microtubules to drag the nucleus into the bud neck (reviewed in Moore et al., 2009).
The early studies in fungi set the stage for understanding nuclear positioning in multicellular eukaryotes. Genetic screens in Drosophila melanogaster eye discs and Caenorhabditis elegans embryonic hypodermal precursors led to the discovery of SUN- and KASH-domain proteins, which form LINC (linker of nucleoskeleton and cytoskeleton) complexes across the nuclear envelope (see Box 1) (Starr and Fridolfsson, 2010). The role of LINC complexes in moving nuclei is conserved across eukaryotes. For example, LINC complexes mediate nuclear migration in Arabidopsis thaliana and/or Physcomitrella patens root hairs, trichomes and pollen tubes (Folkers et al., 1997; Mathur et al., 1999; Miki et al., 2015; Tamura et al., 2013; Zhou et al., 2012, 2015; Zhou and Meier, 2014).
Box 1. SUN and KASH proteins span the nuclear envelope, and form the LINC complex.
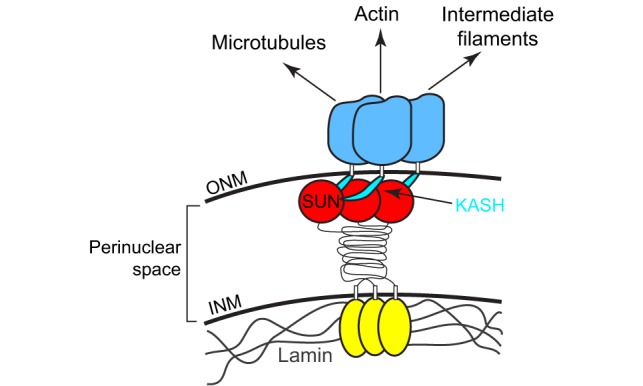
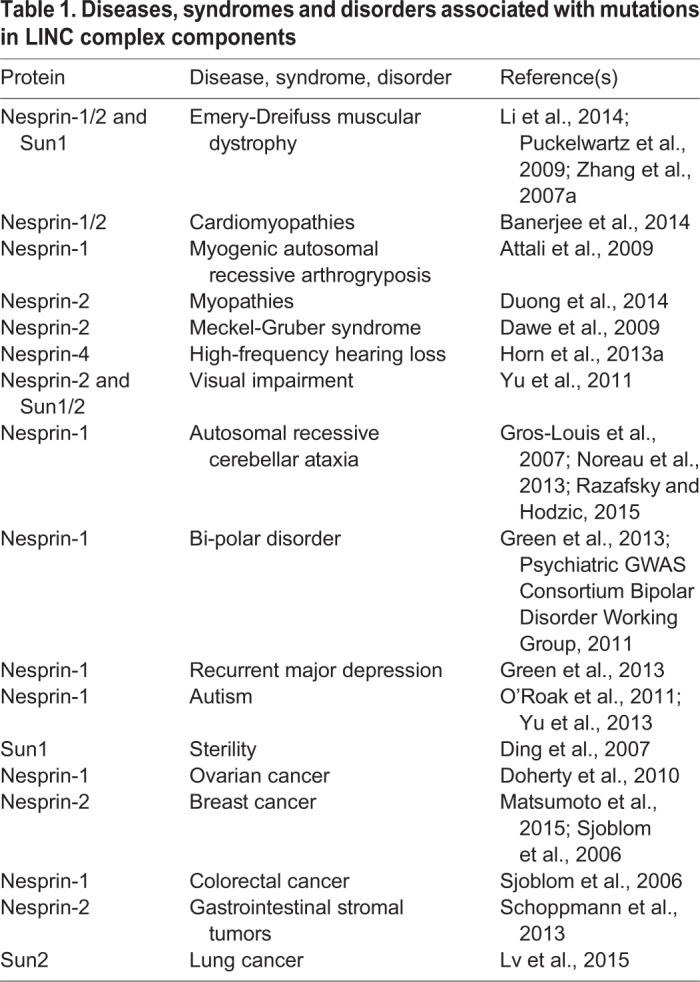
The canonical mechanism for nuclear migration comprises a bridge across the nuclear envelope, termed the linker of the nucleoskeleton and the cytoskeleton (LINC) complex that connects the nucleus to forces generated in the cytoplasm. LINC complexes are conserved across all eukaryotes and are made of two components, a Klarsicht, ANC-1, Syne homology (KASH)-domain protein (blue) in the outer nuclear membrane (ONM) and a Sad1, UNC-84 (SUN)-domain protein (red and yellow) in the inner nuclear membrane (INM) (reviewed in Chang et al., 2015b; Luxton and Starr, 2014; Starr and Fridolfsson, 2010; Tapley and Starr, 2013). The conserved SUN and KASH domains at the C-termini of these proteins interact in the perinuclear space to span the nuclear envelope (Crisp et al., 2006; McGee et al., 2006; Padmakumar et al., 2005). Crystal structures of the SUN–KASH interaction suggest that SUN-domain proteins form a regulated trimer to interact with three KASH-domain peptides (Nie et al., 2016; Sosa et al., 2012; Wang et al., 2012). SUN- and KASH-domain proteins regulate the architecture of the nuclear envelope in cells subjected to mechanical strain (Cain et al., 2014; Crisp et al., 2006). In the cytoplasm, KASH-domain proteins interact with microtubule motors, actin or intermediate filaments to connect the nucleus to a dynamic cytoskeleton (Fridolfsson et al., 2010; Horn et al., 2013a; Meyerzon et al., 2009a; Roux et al., 2009; Starr and Han, 2002; Wilhelmsen et al., 2005; Zhen et al., 2002). Meanwhile, the nucleoplasmic domains of SUN-domain-containing proteins (yellow) interact with structural components in the nucleoplasm, including lamins, to dissipate forces in the nucleoskeleton (Bone et al., 2014; Haque et al., 2006). Besides their role in nuclear positioning discussed here, LINC complexes also function during homolog pairing in meiosis (Chikashige et al., 2006; Ding et al., 2007; Sato et al., 2009), DNA damage repair (Lei et al., 2012; Lottersberger et al., 2015; Swartz et al., 2014) and mechanotransduction (Chambliss et al., 2013; Lombardi et al., 2011). Thus, LINC complexes are required for a variety of cell and developmental functions, such as those described here. Given these functions, it is not surprising that mutations in SUN- and KASH-domain proteins have been linked to a number of human diseases, syndromes and disorders (see Table 1).
However, many nuclear migration events are mediated through mechanisms that are independent of LINC complexes. For example, in Drosophila oocytes, the oocyte nucleus is pushed from behind by the centrosome and growing microtubules (Zhao et al., 2012). Likewise, male pronuclei are pushed away from the cortex by a growing microtubule aster (Reinsch and Gonczy, 1998).
A main take-home message of this Commentary is that a wide variety of molecular mechanisms exist to move nuclei. Moreover, more mechanisms are likely to await discovery. Some of the mechanisms described to date include nuclei that move along microtubules as cargo of kinesins and dynein, nuclei tethered to moving actin filaments, nuclei being pushed or pulled from a distance or passively displaced by other moving cells and, even, nuclei being moved by intracellular pressure. In this Commentary, we focus primarily on mechanisms that move nuclei in several vertebrate developmental contexts. We also discuss the difficulties of nuclear migration through constricted spaces. Given the important developmental processes that depend on nuclear migration, it should come as no surprise that defects in the nuclear migration machinery, especially in SUN- and KASH-domain proteins, have been linked to a variety of diseases (Calvi and Burke, 2015; Cartwright and Karakesisoglou, 2014) (see Table 1 for a brief list and further references). However, it is not yet understood how defects in nuclear migration contribute to disease pathologies. This important area of research will require close collaboration between cell and developmental biologists, engineers and biophysicists, and clinical researchers.
Table 1.
Diseases, syndromes and disorders associated with mutations in LINC complex components
Nuclear migration in polarizing, adherent tissue culture cells
It is challenging to study nuclear migration events within a three-dimensional (3D) in vivo context. It was, therefore, an important breakthrough for the field when a tissue culture model was developed to study nuclear migration. On the edge of a scratched ‘wound’ in an otherwise confluent monolayer, NIH3T3 fibroblasts polarize to place their centrosomes in front of nuclei prior to migration into the wound. Live imaging of fibroblast polarization showed that the centrosome stays in place in the center of the cell while the nucleus actively migrates rearward. This rearward nuclear migration was shown to require actin flow, myosin and Cdc42 (Gomes et al., 2005). Today, this nuclear migration in polarizing fibroblasts is one of the mechanistically best understood nuclear migration events in any system.
Nuclear migration in cultured fibroblasts also helped to uncover a mechanism for nuclear migration that involves transmembrane actin-associated nuclear (TAN) lines (Luxton et al., 2010). TAN lines are found on the apical surface of an adherent, cultured cell and connect to actin cables that orient parallel to the wound edge. The initially identified components of TAN lines are the SUN-domain protein Sun2 and the KASH-domain protein nesprin-2 (expressed from the SYNE2 locus) (Fig. 1A). Here, Sun2 and the giant isoform of nesprin-2, nesprin-2G physically tether the nucleus to actin cables that undergo retrograde flow, thereby moving the nucleus rearward and behind the centrosome (Luxton et al., 2010). At the nucleoplasmic side of TAN lines, lamin A (Lmna) and emerin (Emd) are required to anchor nuclei to TAN lines but are not involved in TAN line formation. Although mutations in lamin A or emerin result in non-moving nuclei, TAN lines still glide along the surface of the nuclear envelope (Chang et al., 2013a; Folker et al., 2011). The inner nuclear membrane protein spindle-associated membrane protein 1 (Samp1; also known as NET5 and officially known as TMEM201) is an additional component of TAN lines and functions, in part, to connect Sun2 to lamin A (Borrego-Pinto et al., 2012). On the cytoplasmic side of TAN lines, the formin FHOD1 plays a structural role. FHOD1 is not necessary for the formation of the actin cables or their retrograde movement but, instead, acts as a second connection between nesprin-2G and actin thus reinforcing the coupling of the nucleus to actin cables (Kutscheidt et al., 2014). On the basis of these data, we have a solid understanding of the molecular mechanism and players, including actin cables and lamins, that connect nuclei to retrograde flow. However, although the importance of TAN lines is quite clear in 3T3 fibroblasts and cultured myoblasts (Chang et al., 2015a; Gundersen and Worman, 2013), their broader in vivo significance remains to be demonstrated. We expect that recent developments in live microscopy, especially lattice light-sheet microscopy (Chen et al., 2014), will enable researchers to test the extent to which TAN lines function or exist in vivo.
Fig. 1.
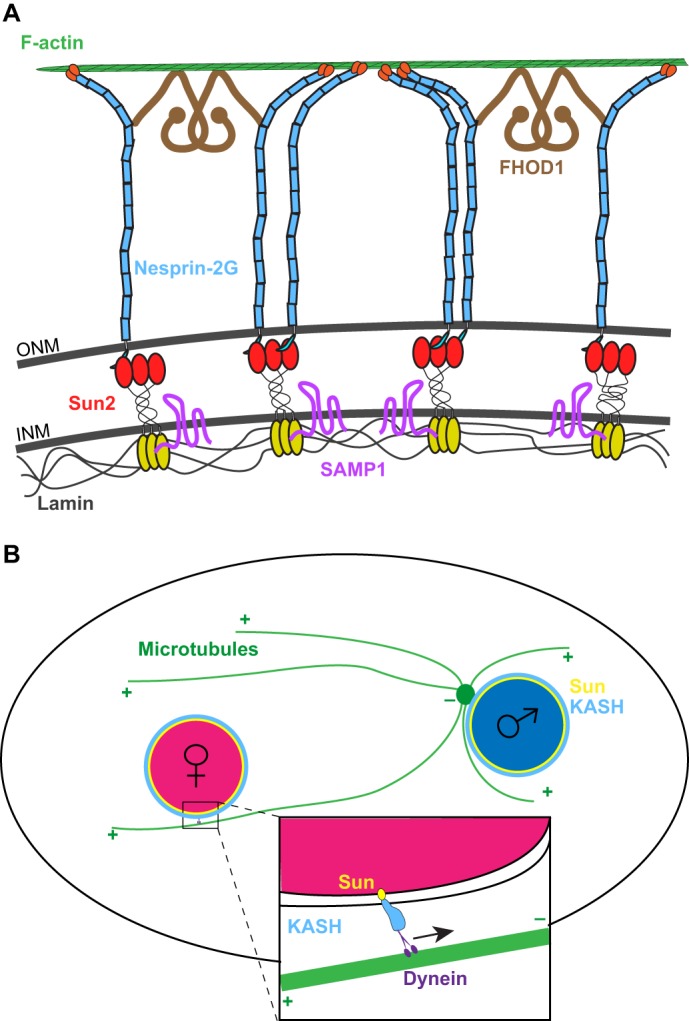
Examples of nuclear migration mediated by LINC complexes. (A) A transmembrane actin-associated nuclear (TAN) line connects the retrograde-moving actin filaments to the nucleoskeleton. F-actin (green) in the cytoplasm interacts with nesprin-2G (blue), an integral outer nuclear membrane (ONM) protein with a large cytoplasmic domain. This interaction is reinforced by the formin FHOD1 (brown). Nesprin-2G interacts in the peri-nuclear space with the inner nuclear membrane (INM) protein Sun2 (red and yellow). Samp1 (pink) in the inner nuclear membrane is also a component of the TAN line. Finally, the TAN line ends in the nucleoplasm, where the nucleoplasmic domain of Sun2 (yellow) interacts with lamins (gray). (B) Schematic of pronuclear migration showing the capturing of the female pronucleus (pink) by microtubules (green) that have nucleated from a centrosome (green circle) attached to the male pronucleus (blue). This process is mediated by a bridge comprising SUN- and KASH-domain proteins on the nuclear envelope (yellow and blue in the inset), which recruits dynein (purple) to the surface of the female pronucleus in order to move it towards the male pronucleus.
Additional insights into the function of nuclear positioning have recently been obtained from studying cultured cells in a 3D-matrix. Fibroblasts can migrate through 3D-matrices in multiple ways (recently reviewed in Petrie and Yamada, 2015), one of which is by using blunt, cylindrical protrusions called lobopodia that are dependent on Rho, but not Rac or Cdc42 (Petrie et al., 2012). Lobopodia migration relies on the existence of a cellular pressure gradient. Forward movement of the nucleus creates high pressure in front of the nucleus and a low-pressure region in the rear of the cell (Petrie et al., 2014). Actomyosin contractions and a RhoA–ROCK–myosin II pathway move nuclei forward. To maintain the pressure gradient, nuclei are attached to the cytoskeleton through the intermediate filament vimentin, which is attached to the nucleus through the KASH-domain protein nesprin-3 (expressed from the SYNE3 locus) (Petrie et al., 2014). Thus, the nucleus acts as a piston to propel fibroblasts through a 3D-matrix (Petrie et al., 2014). The differences between 2D- and 3D-migration of cultured fibroblasts demonstrates that divergent mechanisms of nuclear positioning can contribute to cellular migrations (DeSimone and Horwitz, 2014). Again, a major challenge in this field is to delineate exactly how fibroblasts migrate through an in vivo matrix.
Pronuclear migration in the newly fertilized zygote
A striking example of nuclear migration is when male and female pronuclei must find one another in a newly fertilized zygote. In numerous organisms, from C. elegans to rhesus monkeys, dynein is recruited to the nuclear envelope of pronuclei to mediate their movement. The molecular mechanisms of pronuclear migration are particularly well-characterized in C. elegans. Here, the KASH-domain protein ZYG-12 and the divergent SUN-domain protein SUN-1 (Matefin) are required for centrosome attachment to male pronuclei, and pronuclear migration (Fig. 1B) (Malone et al., 2003; Minn et al., 2009; Zuela and Gruenbaum, 2016). ZYG-12 binds to the dynein light intermediate chain and so recruits dynein to the surface of both male and female pronuclei (Malone et al., 2003). ZYG-12 and SUN-1 are required to keep the centrosome attached to the male pronucleus. Centrosome capture is also dependent on the surface area of pronuclei. Mutants with abnormally small pronuclei lack sufficient surface area to allow dynein to interact with both centrosomes and, therefore, often have a detached centrosome, resulting in pronuclear migration defects (Meyerzon et al., 2009b). Once the centrosome is attached to the male pronucleus, the female pronucleus captures a microtubule from the aster that is associated with the male pronucleus before dynein on the female pronucleus pulls the two pronuclei together (Fig. 1B) (Malone et al., 2003; Rose and Gonczy, 2014).
In zebrafish, pronuclear migration uses a similar mechanism. The KASH-domain protein lymphoid-restricted membrane protein (Lrmp) localizes to the nuclear envelope and centrosomes, and is required for correct pronuclear migration – probably through dynein (Lindeman and Pelegri, 2012). Thus, we speculate that C. elegans ZYG-12 and zebrafish Lrmp are functionally conserved in order to mediate pronuclear migration.
Although model organisms have provided great insight into pronuclear migration, there are fewer mechanistic studies of migration of mammalian pronuclei. This is probably due to the fact that rodents, which serve as the traditional mammalian model, are unique in that they use actin instead of microtubules to position nuclei in oocytes (Almonacid et al., 2015; Schatten, 1994). To better understand pronuclear migration in humans, studies have been conducted with fertilized bovine and rhesus monkey oocytes, in which dynamic microtubules are essential for pronuclear migration (reviewed in Schatten, 1982). Dynein and dynactin both function at the periphery of pronuclei to move the female pronucleus towards the male pronucleus (Payne et al., 2003). The mechanism for the recruitment of dynein to the surface of pronuclei is unknown but, given the role for Lrmp in zebrafish pronuclear migration and the fact that KASH5 (also known as CCDC155) is known to recruit dynein to the nuclear envelope of mouse meiotic cells (Horn et al., 2013b; Morimoto et al., 2012), we hypothesize that KASH5 mediates pronuclear migration in non-rodent mammals. If this is, indeed, the mechanism for the recruitment of dynein to pronuclei through KASH5, Lrmp and/or ZYG-12 to mediate pronuclear migration is likely to be conserved from nematodes to fish to mammals – but not in rodents.
Nuclear movements and muscle development
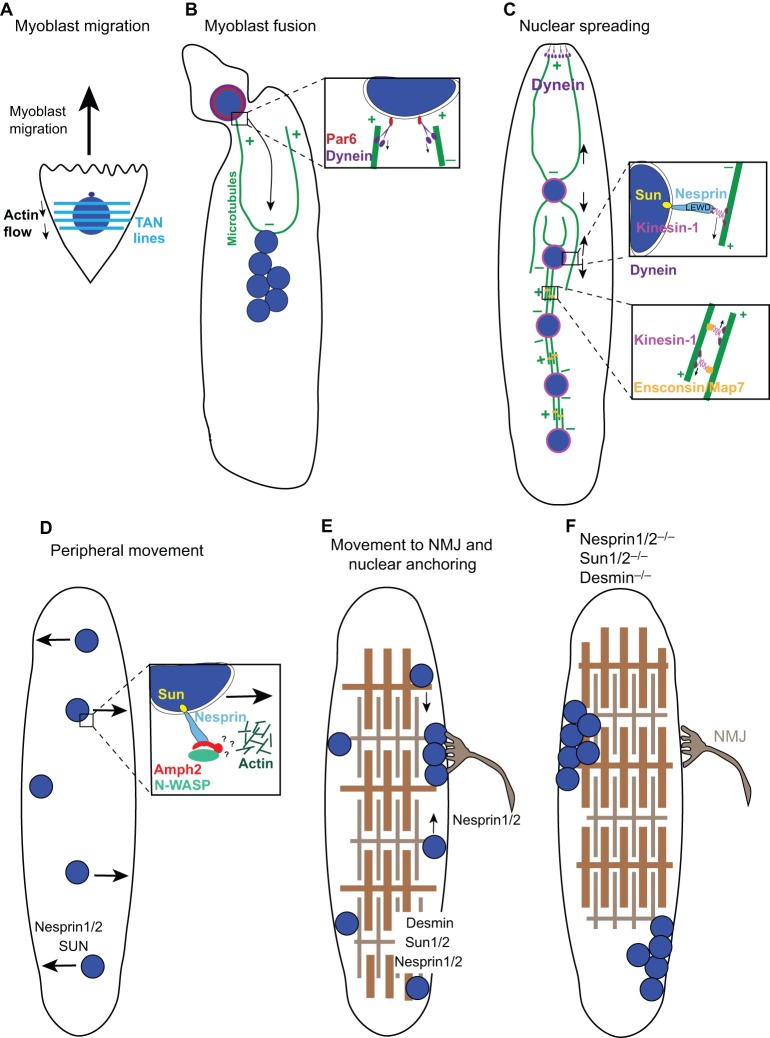
The examples above focus on mechanisms of nuclear positioning in cells with a single nucleus (or two in the case of a fertilized embryo). It is a different challenge to position nuclei in a large syncytium with several or even hundreds of nuclei in a single cell. Here, we examine nuclear positioning in the development of mammalian skeletal muscle as an example. Throughout mammalian muscle development, nuclei undergo at least five mechanistically and temporally distinct nuclear movements, making them an excellent system to study various mechanisms of nuclear migration in syncytia (Cadot et al., 2015). In mammalian skeletal muscle, hundreds of mononucleated myoblasts fuse together to create a giant syncytium that matures into a functional myofiber (Folker and Baylies, 2013). Normally, nuclei are evenly spaced along the length of the syncytium at the periphery of the myofiber to maximize the distance between nuclei (Bruusgaard et al., 2003). Moreover, a group of specialized nuclei localize to and anchor under the synapse at the single neuromuscular junction (Grady et al., 2005; Sanes and Lichtman, 2001). As in other examples of nuclear migration, some nuclear movements during muscle development are dependent on SUN- and KASH-domain proteins, whereas others are not. Further supporting the importance of nuclear positioning in muscle, nuclei are often mis-positioned to the center of the myofiber in wounded and diseased muscles, including in central myopathies (Romero, 2010).
The first nuclear migration event occurs prior to myoblast fusion when migrating cultured myoblasts polarize centrosomes in front of nuclei (Fig. 2A). This process requires actin, myosin, SUN-2 and nesprin-2G to form TAN lines in order to move the nucleus behind the centrosome (Chang et al., 2015a). Thus, myoblasts are polarized in a manner that is analogous to fibroblasts during wound healing.
Fig. 2.
Nuclear migration events in muscle development. During muscle development, numerous mononucleated myoblasts fuse to form a myotube, which further develops into a myofiber. (A) Myoblast nuclei (blue) are polarized with TAN lines (light blue horizontal lines, see Fig. 1A), which harness actin flow during cell migration. (B) Upon the fusion of a myoblast to the end of a myotube, the new nucleus rapidly migrates towards the minus ends of microtubules (green) at the center of the myotube. This migration is mediated by dynein (purple), which recruited to the nuclear envelope through an interaction with Par6 (red). (C) As the myotube develops, nuclei spread out and become evenly spaced throughout the syncytium. Several mechanisms function to spread these nuclei. First, dynein at the cortex of the ends of myotubes pull nuclei towards the poles. Second, as illustrated in the upper inset, kinesin-1 (pink) is directly recruited to the nuclear envelope through a LEWD amino acid motif within nesprins (light blue) to move nuclei towards distal plus ends of microtubules. Last, this process is mediated by kinesin-1 and the microtubule crosslinker Ensconsin/MAP7 (orange), which slide microtubules apart, thereby pushing nuclei away from each other. (D) Subsequently, nuclei move from the center to the periphery of the myotube as sarcomeres begin to develop. This process is likely to utilize a complex between nesprins, amphiphysin-2 (Amph2, red) and N-WASP (green), which connects to a branched actin network (dark green) to move nuclei to the periphery through unknown mechanisms. (E) A few nuclei move to and anchor at the neuromuscular junction (NMJ, gray), whereas the remaining nuclei remain equally spaced at the periphery of the myofiber. (F) In mutant muscles that either lack both nesprin-1 and nesprin-2, both Sun1 and Sun2, or the intermediate filament desmin, nuclei lose their ability to anchor and become clustered, potentially disrupting the function of the muscle.
Then, just after a myoblast fuses with the end of a developing myotube, the nucleus rapidly moves to the center of the myotube in a microtubule-dependent manner (Fig. 2B). This nuclear migration requires Cdc42 and its downstream effectors Par3 and Par6 (also known as Pard3 and Pard6, respectively), within a protein complex that has previously been implicated in spindle positioning and cell polarity (Chen and Zhang, 2013). Par6 functions at the surface of muscle nuclei to recruit dynactin and dynein to nuclei (inset in Fig. 2B). Dynein then pulls the newly added nucleus towards the middle of the myotube. In this model, microtubules are nucleated all around the surface of myotube nuclei with their plus ends extending towards the distal regions of the developing myotube (Fig. 2B) (Cadot et al., 2012). Defects in the function of the factors in this pathway lead to a decreased speed of nuclear migration (Cadot et al., 2012), which is likely to disrupt muscle development in vivo.
After myoblast fusion and before nuclei move to the periphery of the myotube, a third nuclear migration event occurs, the spreading of syncytial nuclei (Bruusgaard et al., 2006). Nuclear spreading in the developing myotube is microtubule dependent, and both dynein and kinesin-1 motors are required (Fig. 2C) (Folker et al., 2012; Metzger et al., 2012; Wilson and Holzbaur, 2012, 2015). Here, the microtubule-binding protein known as Ensconsin in Drosophila (microtubule-associated protein 7, MAP7, in mammals) binds to the tail of kinesin-1, which then walks towards the plus end of a second microtubule. Because microtubules are nucleated at the surface of nuclei in myotubes, the Ensconsin/MAP7–kinesin-1 complex crosslinks microtubules and slides them apart (inset in Fig. 2C) (Metzger et al., 2012). Two independent dynein pathways function in Drosophila muscle to space nuclei (Folker et al., 2012). In the first, cytoplasmic linker protein 190 (CLIP-190), dynactin and dynein connect microtubules to the cortex and so pull nuclei away from each another. The second pathway involves Lis1 and is necessary for localization of dynein to the poles of muscle cells to establish proper muscle length. Impairment of either pathway results in locomotion defects, demonstrating the importance of nuclear positioning and appropriate muscle length in muscle function (Folker et al., 2012). In addition to its role in mediating microtubule sliding, kinesin-1 also functions on the surface of nuclei. Kinesin-1 is recruited to mammalian myotube nuclei through nesprin-2 or nesprin-1 (encoded by the SYNE1 locus). LEWD amino acid motifs in the nesprins directly interact with the kinesin light chain to target kinesin-1 to the nuclear surface (inset in Fig. 2C) (Wilson and Holzbaur, 2015). From its position at the nuclear envelope, kinesin-1 is then thought to walk towards the plus ends of microtubules, thereby helping to maximize the distance between nuclei (Wilson and Holzbaur, 2015).
The fourth nuclear migration event in developing myotubes is towards the periphery of the syncytium, similar to how nuclei move in myofibers that recover from injury (Falcone et al., 2014; Romero, 2010) (Fig. 2D). This nuclear movement is dependent on the correct spreading of the nuclei in the previous step, as well as a complex formed between the F-BAR protein amphiphysin-2 and the actin-nucleator neuronal Wiskott–Aldrich Syndrome protein (N-WASP) (Falcone et al., 2014). Furthermore, amphiphysin-2 is recruited to the surface of nuclei through an interaction with nesprin-2 (D'Alessandro et al., 2015). Nesprins are required for the timely nuclear movement to the periphery of myotubes (Falcone et al., 2014; Zhang et al., 2007a). Because N-WASP and amphiphysin-2 are also required to form normal triads, i.e. structures in which T-tubules are associated with the sarcoplasmic reticulum, it has been difficult to determine whether nuclear migration to the periphery directly depends on actin or only requires the correct formation of triads. However, because nesprins directly interact with amphiphysin-2 in other tissues, it is reasonable to propose that a complex between nesprin and amphiphysin-2 is responsible for nuclear movement to the periphery of myotubes (Fig. 2D). It remains to be determined how amphiphysin-2 and N-WASP regulate a local actin network that can generate the mechanical forces to move the nucleus.
The final nuclear migration event involves the localization of a subset of myonuclei to immediately underneath the neuromuscular junction (NMJ) (Fig. 2E) (Sanes and Lichtman, 2001), whereas the remaining nuclei move to the myofiber surface so they are closely associated with blood vessels (Ralston et al., 2006). LINC components are required for localization of myonuclei under the NMJ (Grady et al., 2005; Lei et al., 2009; Zhang et al., 2007a), as is lamin A (Méjat et al., 2009). However, it is unknown whether LINC complexes or lamins are required for initial movements or – more likely – whether they anchor nuclei after their arrival at the NMJ. It has been suggested that LINC complexes function redundantly with intermediate filaments to anchor nuclei in mature myofibers. For instance, in mice, knockouts of Sun1 and Sun2, nesprin-1 and nesprin-2, or the main muscle intermediate filament desmin lead to defects in nuclear anchorage (Fig. 2F) (Chapman et al., 2014; Lei et al., 2009; Ralston et al., 2006; Zhang et al., 2007a).
Many questions remain about the molecular mechanisms for how nuclei migrate in various steps of mammalian skeletal muscle development. First, do TAN lines function in vivo during myoblast migration? Second, how is the function of MAP7 in sliding microtubules apart coordinated with the nucleus becoming a kinesin and dynein cargo to position nuclei throughout the length of the developing myotube (Fig. 2C) (Folker et al., 2012, 2014; Metzger et al., 2012; Wilson and Holzbaur, 2012, 2015)? Third, how do LINC complexes and amphaphysin-2 connect nuclei to actin networks to mediate nuclear migration to the periphery? Finally, because some NMJs that completely lack associated myonuclei appear normal (Grady et al., 2005), what is the relevance of myonuclei aggregating under the NMJ?
The most crucial, remaining questions in the field focus on how defects in nuclear positioning in muscles contribute to diseases. Mutations in several SUN-, KASH-domain and lamin proteins have been correlated with muscular dystrophies (see Table 1) (Meinke et al., 2014; Puckelwartz et al., 2009; Tapley and Starr, 2013; Zhang et al., 2007a). Furthermore, presence of SUN-domain proteins enhance dystrophies that are associated with mutations of lamin A, because Sun1-knockout and lamin A double-mutant mice have less severe pathologies than those carrying lamin A single mutants (Chen et al., 2012; Meinke et al., 2014; Starr, 2012). In another example, knockdown of nesprin-2 in migrating myoblasts leads to a partial defect in the fusion of developing myotubes (Chang et al., 2015a), which is predicted to disrupt muscle development or repair and, thus, could lead to muscular dystrophies. Yet, it remains unclear how nuclear positioning defects relate to disease. For example, in one nesprin-1 knockout mouse line nuclear positioning in adult skeletal muscle is completely disrupted, yet the strain displays no overt pathology (Zhang et al., 2007b). An independent nesprin-1 knockout line gives rise to mice with an Emery–Dreifuss muscular dystrophy-like phenotype (Puckelwartz et al., 2009). In both lines, the last exon encoding the transmembrane and KASH domains of nesprin-1 is removed, suggesting that something in the genetic background of these two strains contributes to the difference in phenotypes. In addition, it is unclear whether centrally located nuclei are a cause or an effect of centronuclear myopathies (Romero and Bitoun, 2011). Finally, how the presence of normal SUN proteins enhances phenotypes that are caused by defects in lamin A is unknown. We propose that the presence of functional LINC complexes allows for forces to be transmitted to the weak, lamin-A-deficient nucleoskeleton, thereby resulting in mechanical damage to the integrity of the nucleus (Starr, 2012). Clearly, much work remains before we fully understand how LINC complexes and nuclear positioning contribute to the severity of muscular dystrophy symptoms.
Nuclear migration in developing epithelia
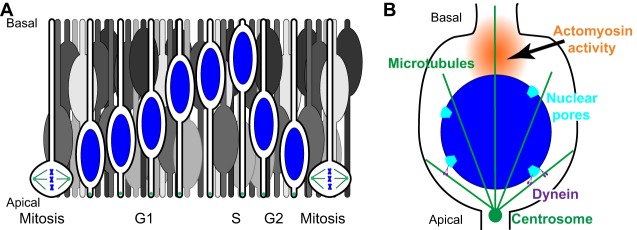
Dramatic and developmentally crucial nuclear migration events take place during proliferative cell cycles of pseudostratified epithelia, so-called interkinetic nuclear migration (reviewed in Baye and Link, 2008; Spear and Erickson, 2012b). A single cell of pseudostratified epithelia has extensions that reach all the way from the basal surface to the apical surface of the tissue, but nuclei are scattered throughout the entire width of the epithelia to help to accommodate a maximum number of cells in the proliferative epithelium (Fig. 3A). However, mitotic divisions in pseudostratified epithelia take place at the apical surface where the centrosome resides throughout the cell cycle. After mitosis, nuclei migrate basally during G1 and S phase, and back again apically during G2, linking nuclear migration with the cell cycle. Interkinetic nuclear migration was first described in pig and chick neural tubes in 1935 (Sauer, 1935), and is conserved across vertebrates, insects and cnidaria (Meyer et al., 2011). In vertebrates, interkinetic nuclear migration is crucial for the development of neuroepithelia, including the retina, neural tube and neocortex. During the past decade, numerous studies have imaged interkinetic nuclear migration in a variety of systems and provided significant insights into the underlying molecular mechanisms. Yet, the literature is full of seemingly contradictory findings with regard to the possible mechanisms. Much of the confusion stems from the use of divergent model systems that greatly differ in size. For example, the widths of pseudostratified epithelia in Drosophila discs or zebrafish retina are about 50 µm, whereas mammalian neocortexes are much thicker. The mouse neocortex is 250 µm thick and primates have neocortexes that are millimeters thick. Below, we discuss three mechanisms that take place in different phases of interkinetic nuclear migration: the role of LINC complexes in recruiting microtubule motors to the surface of nuclei, passive nuclear movements basally during G1/S and G2-specific recruitment of dynein to the cytoplasmic surface of nuclear pore complexes that mediate apical movement.
Fig. 3.
Interkinetic nuclear migration. (A) Illustrated here are nuclei (blue) that undergo interkinetic nuclear migration within a pseudostratified epithelium. Cells with blue nuclei are organized chronologically to show one cell cycle. Other cells (in gray) illustrate the crowded nature of this epithelium. As G2-phase nuclei actively move towards the apical side, they passively push G1-phase nuclei out of the way, resulting in their migration towards the basal surface. (B) Active apical movement during G2 phase; dynein (purple) is recruited to the nuclear surface at nuclear pores (light blue) to move nuclei on microtubules (green). Actomyosin contracts in a zone (orange) behind the nucleus to push the nucleus to the apical surface of the epithelium just prior to mitosis.
LINC complexes have been implicated early on in mediating interkinetic nuclear migration. Indeed, Drosophila Klarsicht (providing the K in the KASH acronym) was originally identified because klarsicht mutants disrupt nuclear migration in proliferating eye discs (Fischer-Vize and Mosley, 1994; Mosley-Bishop et al., 1999; Patterson et al., 2004). Klarsicht, its SUN partner Klaroid, lamin, dynactin and dynein work together to move nuclei in the model pseudostratified epithelium of Drosophila eye discs (Fan and Ready, 1997; Kracklauer et al., 2007; Patterson et al., 2004). Likewise, in the developing mouse neocortex or retina, mutations in the LINC complex components disrupt interkinetic nuclear migration (Razafsky et al., 2012; Zhang et al., 2009). As nesprin-2 interacts with both dynein and kinesin-1, this suggests that KASH-domain proteins function here by coordinating microtubule motors at the surface of migrating nuclei (Zhang et al., 2009). Alternatively, kinesin-3 might mediate basal movements (Tsai et al., 2010). Finally, overexpression of the KASH domain of nesprin-2 in zebrafish retina results in mislocalized basal nuclei (Tsujikawa et al., 2007). Together, these findings lead to a model whereby KASH-domain proteins recruit dynein to the nuclear envelope for the rapid apically directed nuclear migration during G2 phase. KASH-domain proteins might also be involved in kinesin-mediated basal movements during G1/S phase. However, nuclear positioning is not completely disrupted by mutations within LINC complexes in developing eye epithelia of fly, zebrafish or mouse (Kracklauer et al., 2007; Patterson et al., 2004; Razafsky et al., 2012; Tsujikawa et al., 2007; Yu et al., 2011), suggesting that other mechanisms are sufficient for at least some nuclear migration events in these tissues. Furthermore, it is worth noting that, in other systems discussed below, LINC complexes appear dispensable for interkinetic nuclear migration.
Multiple mechanisms independent of the LINC complex that mediate nuclear migration events in pseudostratified epithelia have been described in the literature. For example, many basal nuclear migrations during G1/S phase are passive, cell non-autonomous movements, during which G1/S nuclei are forced basally when G2 nuclei of neighboring cells actively migrate apically and, therefore, push G1/S nuclei out of the apical regions (Fig. 3A) (Kosodo et al., 2011). LINC-independent mechanisms that underlie apical movements during G2 are better understood. Dynein, which is required for the rapid apical movement of nuclei within the mouse neocortex (Hu et al., 2013), is recruited to the surface of nuclei through interactions with nuclear pore components (Fig. 3B) (Bolhy et al., 2011; Splinter et al., 2010). Dynein is specifically recruited during G2, just in time for the apical aspect of interkinetic nuclear migration (Baffet et al., 2015). Occasionally, a nucleus does not complete nuclear migration to the apical surface before the onset of mitosis. In this case, centrosomes are pulled away from the apical surface towards the nucleus to initiate mitosis (Spear and Erickson, 2012a; Strzyz et al., 2015). Shortly thereafter, before mitosis is completed, actomyosin contraction is required to complete the apical migration of the nucleus (Norden et al., 2009; Spear and Erickson, 2012a) (Fig. 3B). In summary, a variety of microtubule- and actin-based mechanisms function together to ensure interkinetic nuclear migration and development of pseudostratified epithelia. Future studies will focus on understanding the differences of the mechanisms in different models. What works for one interkinetic nuclear migration might not be sufficient for a different event in larger epithelia.
Nuclear positioning also plays a role in columnar epithelia. Polarized intestinal epithelial cells contain highly organized and polarized microtubules, essential for the correct positioning of nuclei and other organelles within the cell (Toya et al., 2016). A better-characterized nuclear migration event in columnar epithelia cells is that of hair cells in the inner ear. In these hair cells, nuclei migrate to a basal position within the cell in a kinesin-1-dependent mechanism. Nesprin-4 (encoded by the SYNE4 locus) and its partners (Sun1 or Sun2) recruit kinesin-1 to the surface of nuclei (Roux et al., 2009). Knockouts of either nesprin-4 or Sun1 in mice result in misposition of nuclei in inner hair cells, degeneration of outer hair cell, and hearing loss of high frequencies (Horn et al., 2013a). However, further studies are required to elucidate how exactly nuclear mispositioning contributes to the hearing loss. Interestingly, in certain Iraqi-Jewish families, mutations in the KASH-domain protein nesprin-4 are also associated with hearing loss at high frequencies (Horn et al., 2013a). Thus, mispositioning of nuclei in epithelia is likely to contribute to a variety of conditions in humans.
Nuclear movements through constricted spaces
In the examples above, nuclei remain relatively rounded throughout migration, suggesting that only minimal mechanical strains exist to deform nuclei during movements. In contrast, many cells migrate through narrow openings between other cells or in the extra-cellular matrix (ECM), thereby experiencing extensive external mechanical forces. For example, hematopoietic cells must migrate through a basement membrane to exit the bone marrow and enter capillaries (Junt et al., 2007; Shin et al., 2013). The nucleus is, typically, the largest organelle of a cell and is five to ten times stiffer than the surrounding cytoplasm (Dahl et al., 2004; Friedl et al., 2011). Therefore, nuclear deformability limits the ability of cells to migrate through constricted spaces (Fu et al., 2012; Wolf et al., 2013). In fact, pathologists have indirectly appreciated this for decades by using lobulated nuclei, which are indicative of a flexible and softer nucleus, as an indication for cancer cells (Chow et al., 2012).
The relative expression levels between different nuclear lamins have been shown to regulate nuclear stiffness and its deformability in a variety of cells, including neutrophils (Rowat et al., 2013), metastatic cells (Fu et al., 2012; Harada et al., 2014; Wolf et al., 2013), mouse embryonic fibroblasts (MEFs) (Davidson et al., 2014) and hematopoietic cells (Shin et al., 2013). Indeed, overexpression of lamin A increases the transit time through constrictions, whereas lamin A knockout cells migrate faster (Davidson et al., 2014; Rowat et al., 2013) (Fig. 4). Thus, lamin A plays a crucial role in increasing the stiffness and in reducing the deformability of migrating nuclei. Similarly, in bone marrow exit assays, cells with high ratios of lamin A to lamin B are retained in the bone marrow, suggesting a reduced ability of the nucleus to deform (Shin et al., 2013). Furthermore, an excess of lamin A has also been shown to impede matrix-metalloproteinase-independent migration, for which nuclear deformability is the main determinant in migration through the ECM (Wolf et al., 2013). These results are consistent with previous findings that levels of lamin A positively correlate with nuclear stiffness, as demonstrated by using micropipette aspiration (Dahl et al., 2006) or resistance to mechanical strain (Broers et al., 2004; Lammerding et al., 2006, 2004). In general, increasing the amount of lamin A makes the nucleus stiffer and less deformable, thereby resulting in slower migration of a cell through a constricted space.
Fig. 4.
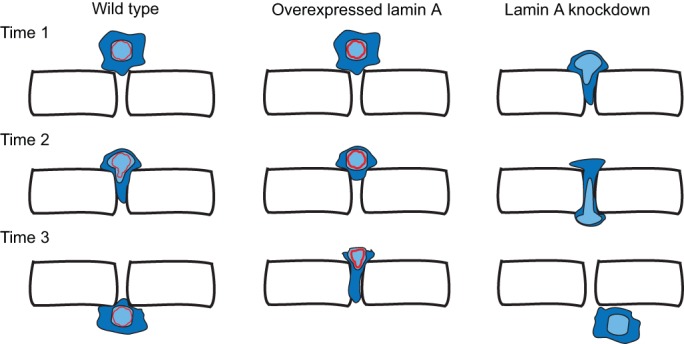
Nuclei limit cell migration through constricted spaces. Schematic of an experiment that uses fabricated constrictions and in which a cell is induced to migrate towards a chemoattractant through a constricted space. (Left) Wild-type cells (blue) migrate through constricted spaces at a given rate that is influenced by the composition and stiffness of the nucleus (light blue) . (Middle) Upon overexpression of lamin A (red), nuclei become stiffer and less deformable, resulting in slower migration of cells through constricted spaces. (Right) By contrast, knockdown of lamin A reduces nuclear stiffness and increases their deformability, allowing cells to squeeze more easily through a constricted space than wild-type cells (right panel).
In addition to what has been learned about the role of lamin A, future experiments following single cells migrating through constrictions are likely to yield additional mechanistic insights into nuclear migration. Recent data point to at least three areas that warrant additional studies. The first is the role of chromatin compaction during nuclear constriction. Condensed heterochromatin has been implicated in migration through constricted spaces, because blocking the formation of heterochromatin in breast cancer cells results in reduced migration (Fu et al., 2012). The second is the poorly understood aspect by how actin filaments in the nucleoplasm regulate nuclear stiffness. The small GTPase Rac1 has recently been implicated in nuclear stiffness because its enrichment in COS cell nuclei induced the formation of actin filament formation, resulting in nuclear deformations (Navarro-Lérida et al., 2015). The third one relates to events that occur in the cytoplasm at the rear of the nucleus such as actomyosin-based contraction, similar to those that take place during interkinetic nuclear migration. Non-muscle myosin IIB is crucial for generating forces that allow nuclei to squeeze through a narrow constricted space (Thomas et al., 2015).
As cell biologists and engineers more broadly adapt and develop assays to image cell and nuclear migrations through narrow spaces, we expect additional factors involved in nuclear deformability, stiffness and shape changes to be identified and characterized. Although substantial insights have been made in the past five years by imaging individual cells migrating through constrictions, much less is understood about how groups of cells migrate collectively. Researchers are likely to need to utilize in vivo assays to study collective migrations, such as those developed for studying Drosophila border cells or zebrafish lateral line cells (Friedl and Gilmour, 2009). Of particular interest are future studies on how nuclear stiffness is related to metastasis of individual or groups of cancer cells, which then have the potential to lead to the development of cancer treatments.
Conclusions and future directions
In the past 15 years, substantial progress in describing and understanding the molecular mechanisms for the movement of nuclei has been made. The diversity of molecular mechanisms that mediate different nuclear migration events has been particularly surprising. Studies that used model organisms have identified the LINC complex, at the core of which is the interaction between KASH- and SUN-domain proteins that span the nuclear envelope. In order to move nuclei, LINC complexes connect them to microtubule motors or a dynamic actin network. However, there are a number of nuclear migration events that do not depend on LINC complexes. Alternative mechanisms include utilization of microtubules to push a nucleus, dynein recruitment to the nuclear envelope by the nuclear pore, and actomyosin contractions at the rear of the nucleus, among others (Bolhy et al., 2011; Norden et al., 2009; Splinter et al., 2010; Zhao et al., 2012). The crucial nature of several of these nuclear migration events in development, and the small number of phenotypes observed upon mutation of LINC and other known nuclear migration components suggests roles for partially redundant pathways that are yet to be identified.
Despite the recent progress, a number of questions remain with regard to nuclear migration in a variety of developing mammalian tissues. For instance, the differences in interkinetic nuclear migration observed for different pseudostratified epithelia warrant further investigation. Likewise, the consequences of mispositioning nuclei during muscle function remain poorly understood. Moving back to the initial stages of development, even the mechanisms of pronuclear migration in humans remain poorly explored, especially, because in this case rodents are not useful models because they lack microtubule involvement for pronuclear migration (reviewed in Schatten, 1994). Furthermore, many details of the better-understood mechanisms of nuclear migration remain unknown. For example, some KASH-domain proteins recruit both dynein and kinesin to the surface of the nucleus (Fridolfsson and Starr, 2010), but it remains an open question how a single cargo regulates the switch between different molecular motors (Hancock, 2014).
It is likely that multiple mechanisms remain to be identified for moving nuclei. Recent studies in traditional models, such as Drosophila oocytes or cultured fibroblasts, have led to the discovery of entirely unexpected mechanisms for nuclear movements, including one, in which the nucleus is used as a piston to propel cells through 3D matrices, and another in which microtubules and centrosomes are pushing a nucleus from behind (Petrie et al., 2014; Zhao et al., 2012). Moreover, dramatic nuclear migrations take place in many ciliates and their mechanisms are almost completely unexplored (Mikami, 2000). Thus, additional, new models for nuclear migration need to be developed and studied.
Finally, more research is required to better understand how nuclear migration is related to human disease. Nuclear migration is often the rate-limiting step of metastasizing cells (Wolf et al., 2007), but how metastatic cell nuclei remodel to squeeze into narrow openings is poorly understood. Many exciting recent findings in this field come from studies of nuclei squeezing through constricted spaces in a variety of in vitro contexts. Since the in vitro nature of these studies is limiting it is essential to study nuclear squeezing events in vivo. One possible model is nuclear migration in C. elegans larval P-cells, in which the nucleus migrates from lateral to ventral through a constricted space of about 200 nm between the muscle and the cuticle (Chang et al., 2013b; Sulston, 1976). Such future in vivo studies are likely to expand our understanding of disease. Mutations in SUN- or KASH-domain proteins have been linked to a wide variety of human diseases, syndromes and disorders (Table 1). However, the underlying relationships between nuclear positioning and disease pathologies are almost completely unknown. In the near future, we anticipate that many of the molecular details on how nuclear migration contributes to human diseases will be uncovered. Such findings are expected to lead to translational studies for the treatment of associated diseases.
Acknowledgements
We thank members of D.A.S.’s lab for insightful discussions and the anonymous referees for their helpful comments.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
Research in the lab of D.A.S. is supported by the National Institutes of Health [grant numbers: R01 GM073874 to D.A.S. and T32 GM007377 to C.R.B.]. Deposited in PMC for release after 12 months.
References
- Almonacid M., Ahmed W. W., Bussonnier M., Mailly P., Betz T., Voituriez R., Gov N. S. and Verlhac M.-H. (2015). Active diffusion positions the nucleus in mouse oocytes. Nat. Cell Biol. 17, 470-479. 10.1038/ncb3131 [DOI] [PubMed] [Google Scholar]
- Attali R., Warwar N., Israel A., Gurt I., McNally E., Puckelwartz M., Glick B., Nevo Y., Ben-Neriah Z. and Melki J. (2009). Mutation of SYNE-1, encoding an essential component of the nuclear lamina, is responsible for autosomal recessive arthrogryposis. Hum. Mol. Genet. 18, 3462-3469. 10.1093/hmg/ddp290 [DOI] [PubMed] [Google Scholar]
- Baffet A. D., Hu D. J. and Vallee R. B. (2015). Cdk1 activates pre-mitotic nuclear envelope dynein recruitment and apical nuclear migration in neural stem cells. Dev. Cell 33, 703-716. 10.1016/j.devcel.2015.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee I., Zhang J., Moore-Morris T., Pfeiffer E., Buchholz K. S., Liu A., Ouyang K., Stroud M. J., Gerace L., Evans S. M. et al. (2014). Targeted ablation of nesprin 1 and nesprin 2 from murine myocardium results in cardiomyopathy, altered nuclear morphology and inhibition of the biomechanical gene response. PLoS Genet. 10, e1004114 10.1371/journal.pgen.1004114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baye L. M. and Link B. A. (2008). Nuclear migration during retinal development. Brain Res. 1192, 29-36. 10.1016/j.brainres.2007.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhy S., Bouhlel I., Dultz E., Nayak T., Zuccolo M., Gatti X., Vallee R., Ellenberg J. and Doye V. (2011). A Nup133-dependent NPC-anchored network tethers centrosomes to the nuclear envelope in prophase. J. Cell Biol. 192, 855-871. 10.1083/jcb.201007118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone C. R., Tapley E. C., Gorjanacz M. and Starr D. A. (2014). The C. elegans SUN protein UNC-84 interacts with lamin to transfer forces from the cytoplasm to the nucleoskeleton during nuclear migration. Mol. Biol. Cell 25, 2853-2865. 10.1091/mbc.E14-05-0971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrego-Pinto J., Jegou T., Osorio D. S., Aurade F., Gorjanacz M., Koch B., Mattaj I. W. and Gomes E. R. (2012). Samp1 is a component of TAN lines and is required for nuclear movement. J. Cell Sci. 125, 1099-1105. 10.1242/jcs.087049 [DOI] [PubMed] [Google Scholar]
- Broers J. L. V., Peeters E. A. G., Kuijpers H. J. H., Endert J., Bouten C. V. C., Oomens C. W. J., Baaijens F. P. T. and Ramaekers F. C. S. (2004). Decreased mechanical stiffness in LMNA−/− cells is caused by defective nucleo-cytoskeletal integrity: implications for the development of laminopathies. Hum. Mol. Genet. 13, 2567-2580. 10.1093/hmg/ddh295 [DOI] [PubMed] [Google Scholar]
- Bruusgaard J. C., Liestøl K., Ekmark M., Kollstad K. and Gundersen K. (2003). Number and spatial distribution of nuclei in the muscle fibres of normal mice studied in vivo. J. Physiol. 551, 467-478. 10.1113/jphysiol.2003.045328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruusgaard J. C., Liestøl K. and Gundersen K. (2006). Distribution of myonuclei and microtubules in live muscle fibers of young, middle-aged, and old mice. J. Appl. Physiol. 100, 2024-2030. 10.1152/japplphysiol.00913.2005 [DOI] [PubMed] [Google Scholar]
- Cadot B., Gache V., Vasyutina E., Falcone S., Birchmeier C. and Gomes E. R. (2012). Nuclear movement during myotube formation is microtubule and dynein dependent and is regulated by Cdc42, Par6 and Par3. EMBO Rep. 13, 741-749. 10.1038/embor.2012.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadot B., Gache V. and Gomes E. R. (2015). Moving and positioning the nucleus in skeletal muscle - one step at a time. Nucleus 6, 373-381. 10.1080/19491034.2015.1090073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain N. E., Tapley E. C., McDonald K. L., Cain B. M. and Starr D. A. (2014). The SUN protein UNC-84 is required only in force-bearing cells to maintain nuclear envelope architecture. J. Cell Biol. 206, 163-172. 10.1083/jcb.201405081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvi A. and Burke B. (2015). LINC complexes and their role in human disease. In eLS. John Wiley & Sons, Ltd; 10.1002/9780470015902.a0025970 [DOI] [Google Scholar]
- Cartwright S. and Karakesisoglou I. (2014). Nesprins in health and disease. Semin. Cell Dev. Biol. 29, 169-179. 10.1016/j.semcdb.2013.12.010 [DOI] [PubMed] [Google Scholar]
- Chambliss A. B., Khatau S. B., Erdenberger N., Robinson D. K., Hodzic D., Longmore G. D. and Wirtz D. (2013). The LINC-anchored actin cap connects the extracellular milieu to the nucleus for ultrafast mechanotransduction. Sci. Rep. 3, 1087 10.1038/srep01087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W., Folker E. S., Worman H. J. and Gundersen G. G. (2013a). Emerin organizes actin flow for nuclear movement and centrosome orientation in migrating fibroblasts. Mol. Biol. Cell 24, 3869-3880. 10.1091/mbc.E13-06-0307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y.-T., Dranow D., Kuhn J., Meyerzon M., Ngo M., Ratner D., Warltier K. and Starr D. A. (2013b). toca-1 is in a novel pathway that functions in parallel with a SUN-KASH nuclear envelope bridge to move nuclei in Caenorhabditis elegans. Genetics 193, 187-200. 10.1534/genetics.112.146589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W., Antoku S., Östlund C., Worman H. J. and Gundersen G. G. (2015a). Linker of nucleoskeleton and cytoskeleton (LINC) complex-mediated actin-dependent nuclear positioning orients centrosomes in migrating myoblasts. Nucleus 6, 77-88. 10.1080/19491034.2015.1004947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W., Worman H. J. and Gundersen G. G. (2015b). Accessorizing and anchoring the LINC complex for multifunctionality. J. Cell Biol. 208, 11-22. 10.1083/jcb.201409047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman M. A., Zhang J., Banerjee I., Guo L. T., Zhang Z., Shelton G. D., Ouyang K., Lieber R. L. and Chen J. (2014). Disruption of both nesprin 1 and desmin results in nuclear anchorage defects and fibrosis in skeletal muscle. Hum. Mol. Genet. 23, 5879-5892. 10.1093/hmg/ddu310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. and Zhang M. (2013). The Par3/Par6/aPKC complex and epithelial cell polarity. Exp. Cell Res. 319, 1357-1364. 10.1016/j.yexcr.2013.03.021 [DOI] [PubMed] [Google Scholar]
- Chen C.-Y., Chi Y.-H., Mutalif R. A., Starost M. F., Myers T. G., Anderson S. A., Stewart C. L. and Jeang K.-T. (2012). Accumulation of the inner nuclear envelope protein sun1 is pathogenic in progeric and dystrophic laminopathies. Cell 149, 565-577. 10.1016/j.cell.2012.01.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B.-C., Legant W. R., Wang K., Shao L., Milkie D. E., Davidson M. W., Janetopoulos C., Wu X. S., Hammer J. A. III, Liu Z. et al. (2014). Lattice light-sheet microscopy: imaging molecules to embryos at high spatiotemporal resolution. Science 346, 1257998 10.1126/science.1257998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikashige Y., Tsutsumi C., Yamane M., Okamasa K., Haraguchi T. and Hiraoka Y. (2006). Meiotic proteins bqt1 and bqt2 tether telomeres to form the bouquet arrangement of chromosomes. Cell 125, 59-69. 10.1016/j.cell.2006.01.048 [DOI] [PubMed] [Google Scholar]
- Chow K.-H., Factor R. E. and Ullman K. S. (2012). The nuclear envelope environment and its cancer connections. Nat. Rev. Cancer 12, 196-209. 10.1038/nrc3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisp M., Liu Q., Roux K., Rattner J. B., Shanahan C., Burke B., Stahl P. D. and Hodzic D. (2006). Coupling of the nucleus and cytoplasm: role of the LINC complex. J. Cell Biol. 172, 41-53. 10.1083/jcb.200509124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl K. N., Kahn S. M., Wilson K. L. and Discher D. E. (2004). The nuclear envelope lamina network has elasticity and a compressibility limit suggestive of a molecular shock absorber. J. Cell Sci. 117, 4779-4786. 10.1242/jcs.01357 [DOI] [PubMed] [Google Scholar]
- Dahl K. N., Scaffidi P., Islam M. F., Yodh A. G., Wilson K. L. and Misteli T. (2006). Distinct structural and mechanical properties of the nuclear lamina in Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. USA 103, 10271-10276. 10.1073/pnas.0601058103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alessandro M., Hnia K., Gache V., Koch C., Gavriilidis C., Rodriguez D., Nicot A.-S., Romero N. B., Schwab Y., Gomes E. et al. (2015). Amphiphysin 2 orchestrates nucleus positioning and shape by linking the nuclear envelope to the actin and microtubule cytoskeleton. Dev. Cell 35, 186-198. 10.1016/j.devcel.2015.09.018 [DOI] [PubMed] [Google Scholar]
- Davidson P. M., Denais C., Bakshi M. C. and Lammerding J. (2014). Nuclear deformability constitutes a rate-limiting step during cell migration in 3-D environments. Cell. Mol. Bioeng. 7, 293-306. 10.1007/s12195-014-0342-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe H. R., Adams M., Wheway G., Szymanska K., Logan C. V., Noegel A. A., Gull K. and Johnson C. A. (2009). Nesprin-2 interacts with meckelin and mediates ciliogenesis via remodelling of the actin cytoskeleton. J. Cell Sci. 122, 2716-2726. 10.1242/jcs.043794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSimone D. W. and Horwitz A. R. (2014). Many modes of motility. Science 345, 1002-1003. 10.1126/science.1259176 [DOI] [PubMed] [Google Scholar]
- Ding X., Xu R., Yu J., Xu T., Zhuang Y. and Han M. (2007). SUN1 is required for telomere attachment to nuclear envelope and gametogenesis in mice. Dev. Cell 12, 863-872. 10.1016/j.devcel.2007.03.018 [DOI] [PubMed] [Google Scholar]
- Doherty J. A., Rossing M. A., Cushing-Haugen K. L., Chen C., Van Den Berg D. J., Wu A. H., Pike M. C., Ness R. B., Moysich K., Chenevix-Trench G. et al. (2010). ESR1/SYNE1 polymorphism and invasive epithelial ovarian cancer risk: an Ovarian Cancer Association Consortium study. Cancer Epidemiol. Biomarkers Prev. 19, 245-250. 10.1158/1055-9965.EPI-09-0729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong N. T., Morris G. E., Lam Le T., Zhang Q., Sewry C. A., Shanahan C. M. and Holt I. (2014). Nesprins: tissue-specific expression of epsilon and other short isoforms. PLoS ONE 9, e94380 10.1371/journal.pone.0094380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone S., Roman W., Hnia K., Gache V., Didier N., Laine J., Aurade F., Marty I., Nishino I., Charlet-Berguerand N. et al. (2014). N-WASP is required for Amphiphysin-2/BIN1-dependent nuclear positioning and triad organization in skeletal muscle and is involved in the pathophysiology of centronuclear myopathy. EMBO Mol. Med. 6, 1455-1475. 10.15252/emmm.201404436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan S. S. and Ready D. F. (1997). Glued participates in distinct microtubule-based activities in Drosophila eye development. Development 124, 1497-1507. [DOI] [PubMed] [Google Scholar]
- Fischer-Vize J. A. and Mosley K. L. (1994). Marbles mutants: uncoupling cell determination and nuclear migration in the developing Drosophila eye. Development 120, 2609-2618. [DOI] [PubMed] [Google Scholar]
- Folker E. S. and Baylies M. K. (2013). Nuclear positioning in muscle development and disease. Front. Physiol. 4, 363 10.3389/fphys.2013.00363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folker E. S., Ostlund C., Luxton G. W. G., Worman H. J. and Gundersen G. G. (2011). Lamin A variants that cause striated muscle disease are defective in anchoring transmembrane actin-associated nuclear lines for nuclear movement. Proc. Natl. Acad. Sci. USA 108, 131-136. 10.1073/pnas.1000824108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folker E. S., Schulman V. K. and Baylies M. K. (2012). Muscle length and myonuclear position are independently regulated by distinct Dynein pathways. Development 139, 3827-3837. 10.1242/dev.079178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folker E. S., Schulman V. K. and Baylies M. K. (2014). Translocating myonuclei have distinct leading and lagging edges that require kinesin and dynein. Development 141, 355-366. 10.1242/dev.095612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkers U., Berger J. and Hulskamp M. (1997). Cell morphogenesis of trichomes in Arabidopsis: differential control of primary and secondary branching by branch initiation regulators and cell growth. Development 124, 3779-3786. [DOI] [PubMed] [Google Scholar]
- Fridolfsson H. N. and Starr D. A. (2010). Kinesin-1 and dynein at the nuclear envelope mediate the bidirectional migrations of nuclei. J. Cell Biol. 191, 115-128. 10.1083/jcb.201004118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridolfsson H. N., Ly N., Meyerzon M. and Starr D. A. (2010). UNC-83 coordinates kinesin-1 and dynein activities at the nuclear envelope during nuclear migration. Dev. Biol. 338, 237-250. 10.1016/j.ydbio.2009.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P. and Gilmour D. (2009). Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 10, 445-457. 10.1038/nrm2720 [DOI] [PubMed] [Google Scholar]
- Friedl P., Wolf K. and Lammerding J. (2011). Nuclear mechanics during cell migration. Curr. Opin. Cell Biol. 23, 55-64. 10.1016/j.ceb.2010.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Chin L. K., Bourouina T., Liu A. Q. and VanDongen A. M. (2012). Nuclear deformation during breast cancer cell transmigration. Lab Chip. 12, 3774-3778. 10.1039/c2lc40477j [DOI] [PubMed] [Google Scholar]
- Gomes E. R., Jani S. and Gundersen G. G. (2005). Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell 121, 451-463. 10.1016/j.cell.2005.02.022 [DOI] [PubMed] [Google Scholar]
- Grady R. M., Starr D. A., Ackerman G. L., Sanes J. R. and Han M. (2005). Syne proteins anchor muscle nuclei at the neuromuscular junction. Proc. Natl. Acad. Sci. USA 102, 4359-4364. 10.1073/pnas.0500711102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green E. K., Grozeva D., Forty L., Gordon-Smith K., Russell E., Farmer A., Hamshere M., Jones I. R., Jones L., McGuffin P. et al. (2013). Association at SYNE1 in both bipolar disorder and recurrent major depression. Mol. Psychiatry 18, 614-617. 10.1038/mp.2012.48 [DOI] [PubMed] [Google Scholar]
- Gros-Louis F., Dupré N., Dion P., Fox M. A., Laurent S., Verreault S., Sanes J. R., Bouchard J.-P. and Rouleau G. A. (2007). Mutations in SYNE1 lead to a newly discovered form of autosomal recessive cerebellar ataxia. Nat. Genet. 39, 80-85. 10.1038/ng1927 [DOI] [PubMed] [Google Scholar]
- Gundersen G. G. and Worman H. J. (2013). Nuclear positioning. Cell 152, 1376-1389. 10.1016/j.cell.2013.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock W. O. (2014). Bidirectional cargo transport: moving beyond tug of war. Nat. Rev. Mol. Cell Biol. 15, 615-628. 10.1038/nrm3853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque F., Lloyd D. J., Smallwood D. T., Dent C. L., Shanahan C. M., Fry A. M., Trembath R. C. and Shackleton S. (2006). SUN1 interacts with nuclear lamin A and cytoplasmic nesprins to provide a physical connection between the nuclear lamina and the cytoskeleton. Mol. Cell. Biol. 26, 3738-3751. 10.1128/MCB.26.10.3738-3751.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T., Swift J., Irianto J., Shin J.-W., Spinler K. R., Athirasala A., Diegmiller R., Dingal P. C. D. P., Ivanovska I. L. and Discher D. E. (2014). Nuclear lamin stiffness is a barrier to 3D migration, but softness can limit survival. J. Cell Biol. 204, 669-682. 10.1083/jcb.201308029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn H. F., Brownstein Z., Lenz D. R., Shivatzki S., Dror A. A., Dagan-Rosenfeld O., Friedman L. M., Roux K. J., Kozlov S., Jeang K.-T. et al. (2013a). The LINC complex is essential for hearing. J. Clin. Invest. 123, 740-750. 10.1172/JCI66911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn H. F., Kim D. I., Wright G. D., Wong E. S. M., Stewart C. L., Burke B. and Roux K. J. (2013b). A mammalian KASH domain protein coupling meiotic chromosomes to the cytoskeleton. J. Cell Biol. 202, 1023-1039. 10.1083/jcb.201304004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D. J.-K., Baffet A. D., Nayak T., Akhmanova A., Doye V. and Vallee R. B. (2013). Dynein recruitment to nuclear pores activates apical nuclear migration and mitotic entry in brain progenitor cells. Cell 154, 1300-1313. 10.1016/j.cell.2013.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junt T., Schulze H., Chen Z., Massberg S., Goerge T., Krueger A., Wagner D. D., Graf T., Italiano J. E. Jr., Shivdasani R. A. et al. (2007). Dynamic visualization of thrombopoiesis within bone marrow. Science 317, 1767-1770. 10.1126/science.1146304 [DOI] [PubMed] [Google Scholar]
- Kosodo Y., Suetsugu T., Suda M., Mimori-Kiyosue Y., Toida K., Baba S. A., Kimura A. and Matsuzaki F. (2011). Regulation of interkinetic nuclear migration by cell cycle-coupled active and passive mechanisms in the developing brain. EMBO J. 30, 1690-1704. 10.1038/emboj.2011.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kracklauer M. P., Banks S. M. L., Xie X., Wu Y. and Fischer J. A. (2007). Drosophila klaroid encodes a SUN domain protein required for Klarsicht localization to the nuclear envelope and nuclear migration in the eye. Fly 1, 75-85. 10.4161/fly.4254 [DOI] [PubMed] [Google Scholar]
- Kutscheidt S., Zhu R., Antoku S., Luxton G. W. G., Stagljar I., Fackler O. T. and Gundersen G. G. (2014). FHOD1 interaction with nesprin-2G mediates TAN line formation and nuclear movement. Nat. Cell Biol. 16, 708-715. 10.1038/ncb2981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammerding J., Schulze P. C., Takahashi T., Kozlov S., Sullivan T., Kamm R. D., Stewart C. L. and Lee R. T. (2004). Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J. Clin. Invest. 113, 370-378. 10.1172/JCI200419670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammerding J., Fong L. G., Ji J. Y., Reue K., Stewart C. L., Young S. G. and Lee R. T. (2006). Lamins A and C but not lamin B1 regulate nuclear mechanics. J. Biol. Chem. 281, 25768-25780. 10.1074/jbc.M513511200 [DOI] [PubMed] [Google Scholar]
- Lei K., Zhang X., Ding X., Guo X., Chen M., Zhu B., Xu T., Zhuang Y., Xu R. and Han M. (2009). SUN1 and SUN2 play critical but partially redundant roles in anchoring nuclei in skeletal muscle cells in mice. Proc. Natl. Acad. Sci. USA 106, 10207-10212. 10.1073/pnas.0812037106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K., Zhu X., Xu R., Shao C., Xu T., Zhuang Y. and Han M. (2012). Inner nuclear envelope proteins SUN1 and SUN2 play a prominent role in the DNA damage response. Curr. Biol. 22, 1609-1615. 10.1016/j.cub.2012.06.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Meinke P., Huong Le T. T., Wehnert M. and Noegel A. A. (2014). Contribution of SUN1 mutations to the pathomechanism in muscular dystrophies. Hum. Mutat. 35, 452-461. 10.1002/humu.22504 [DOI] [PubMed] [Google Scholar]
- Lindeman R. E. and Pelegri F. (2012). Localized products of futile cycle/lrmp promote centrosome-nucleus attachment in the zebrafish zygote. Curr. Biol. 22, 843-851. 10.1016/j.cub.2012.03.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi M. L., Jaalouk D. E., Shanahan C. M., Burke B., Roux K. J. and Lammerding J. (2011). The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton. J. Biol. Chem. 286, 26743-26753. 10.1074/jbc.M111.233700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lottersberger F., Karssemeijer R. A., Dimitrova N. and de Lange T. (2015). 53BP1 and the LINC complex promote microtubule-dependent DSB mobility and DNA repair. Cell 163, 880-893. 10.1016/j.cell.2015.09.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luxton G. W. G. and Starr D. A. (2014). KASHing up with the nucleus: novel functional roles of KASH proteins at the cytoplasmic surface of the nucleus. Curr. Opin. Cell Biol. 28, 69-75. 10.1016/j.ceb.2014.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luxton G. W. G., Gomes E. R., Folker E. S., Vintinner E. and Gundersen G. G. (2010). Linear arrays of nuclear envelope proteins harness retrograde actin flow for nuclear movement. Science 329, 956-959. 10.1126/science.1189072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv X.-B., Liu L., Cheng C., Yu B., Xiong L., Hu K., Tang J., Zeng L. and Sang Y. (2015). SUN2 exerts tumor suppressor functions by suppressing the Warburg effect in lung cancer. Sci. Rep. 5, 17940 10.1038/srep17940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone C. J., Misner L., Le Bot N., Tsai M.-C., Campbell J. M., Ahringer J. and White J. G. (2003). The C. elegans hook protein, ZYG-12, mediates the essential attachment between the centrosome and nucleus. Cell 115, 825-836. 10.1016/S0092-8674(03)00985-1 [DOI] [PubMed] [Google Scholar]
- Mathur J., Spielhofer P., Kost B. and Chua N. (1999). The actin cytoskeleton is required to elaborate and maintain spatial patterning during trichome cell morphogenesis in Arabidopsis thaliana. Development 126, 5559-5568. [DOI] [PubMed] [Google Scholar]
- Matsumoto A., Hieda M., Yokoyama Y., Nishioka Y., Yoshidome K., Tsujimoto M. and Matsuura N. (2015). Global loss of a nuclear lamina component, lamin A/C, and LINC complex components SUN1, SUN2, and nesprin-2 in breast cancer. Cancer Med. 4, 1547-1557. 10.1002/cam4.495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee M. D., Rillo R., Anderson A. S. and Starr D. A. (2006). UNC-83 IS a KASH protein required for nuclear migration and is recruited to the outer nuclear membrane by a physical interaction with the SUN protein UNC-84. Mol. Biol. Cell 17, 1790-1801. 10.1091/mbc.E05-09-0894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinke P., Mattioli E., Haque F., Antoku S., Columbaro M., Straatman K. R., Worman H. J., Gundersen G. G., Lattanzi G., Wehnert M. et al. (2014). Muscular dystrophy-associated SUN1 and SUN2 variants disrupt nuclear-cytoskeletal connections and myonuclear organization. PLoS Genet. 10, e1004605 10.1371/journal.pgen.1004605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méjat A., Decostre V., Li J., Renou L., Kesari A., Hantaï D., Stewart C. L., Xiao X., Hoffman E., Bonne G. et al. (2009). Lamin A/C-mediated neuromuscular junction defects in Emery-Dreifuss muscular dystrophy. J. Cell Biol. 184, 31-44. 10.1083/jcb.200811035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger T., Gache V., Xu M., Cadot B., Folker E. S., Richardson B. E., Gomes E. R. and Baylies M. K. (2012). MAP and kinesin-dependent nuclear positioning is required for skeletal muscle function. Nature 484, 120-124. 10.1038/nature10914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer E. J., Ikmi A. and Gibson M. C. (2011). Interkinetic nuclear migration is a broadly conserved feature of cell division in pseudostratified epithelia. Curr. Biol. 21, 485-491. 10.1016/j.cub.2011.02.002 [DOI] [PubMed] [Google Scholar]
- Meyerzon M., Fridolfsson H. N., Ly N., McNally F. J. and Starr D. A. (2009a). UNC-83 is a nuclear-specific cargo adaptor for kinesin-1-mediated nuclear migration. Development 136, 2725-2733. 10.1242/dev.038596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerzon M., Gao Z., Liu J., Wu J.-C., Malone C. J. and Starr D. A. (2009b). Centrosome attachment to the C. elegans male pronucleus is dependent on the surface area of the nuclear envelope. Dev. Biol. 327, 433-446. 10.1016/j.ydbio.2008.12.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami K. (2000). Fertilization in protozoa. In Fertilization in Protozoa and Metazoan Animals: Cellular and Molecular Aspects (ed. Tarin J. J. and Cano A.), pp. 1-25. Berlin; Heidelberg; New York: Springer-Verlag. [Google Scholar]
- Miki T., Nishina M. and Goshima G. (2015). RNAi screening identifies the armadillo repeat-containing kinesins responsible for microtubule-dependent nuclear positioning in Physcomitrella patens. Plant Cell Physiol. 56, 737-749. 10.1093/pcp/pcv002 [DOI] [PubMed] [Google Scholar]
- Minn I. L., Rolls M. M., Hanna-Rose W. and Malone C. J. (2009). SUN-1 and ZYG-12, mediators of centrosome-nucleus attachment, are a functional SUN/KASH pair in Caenorhabditis elegans. Mol. Biol. Cell 20, 4586-4595. 10.1091/mbc.E08-10-1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. K., Stuchell-Brereton M. D. and Cooper J. A. (2009). Function of dynein in budding yeast: mitotic spindle positioning in a polarized cell. Cell Motil. Cytoskeleton 66, 546-555. 10.1002/cm.20364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto A., Shibuya H., Zhu X., Kim J., Ishiguro K.-I., Han M. and Watanabe Y. (2012). A conserved KASH domain protein associates with telomeres, SUN1, and dynactin during mammalian meiosis. J. Cell Biol. 198, 165-172. 10.1083/jcb.201204085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris N. R. (2000). Nuclear migration. From fungi to the mammalian brain. J. Cell Biol. 148, 1097-1102. 10.1083/jcb.148.6.1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley-Bishop K. L., Qinghong L., Patterson K. and Fischer J. A. (1999). Molecular analysis of the klarsicht gene and its role in nuclear migration within differentiating cells of the Drosophila eye. Curr. Biol. 9, 1211-1220. 10.1016/S0960-9822(99)80501-6 [DOI] [PubMed] [Google Scholar]
- Navarro-Lérida I., Pellinen T., Sanchez S. A., Guadamillas M. C., Wang Y., Mirtti T., Calvo E. and Del Pozo M. A. (2015). Rac1 nucleocytoplasmic shuttling drives nuclear shape changes and tumor invasion. Dev. Cell 32, 318-334. 10.1016/j.devcel.2014.12.019 [DOI] [PubMed] [Google Scholar]
- Nie S., Ke H., Gao F., Ren J., Wang M., Huo L., Gong W. and Feng W. (2016). Coiled-coil domains of SUN proteins as intrinsic dynamic regulators. Structure 24, 80-91. 10.1016/j.str.2015.10.024 [DOI] [PubMed] [Google Scholar]
- Norden C., Young S., Link B. A. and Harris W. A. (2009). Actomyosin is the main driver of interkinetic nuclear migration in the retina. Cell 138, 1195-1208. 10.1016/j.cell.2009.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noreau A., Bourassa C. V., Szuto A., Levert A., Dobrzeniecka S., Gauthier J., Forlani S., Durr A., Anheim M., Stevanin G. et al. (2013). SYNE1 mutations in autosomal recessive cerebellar ataxia. JAMA Neurol. 70, 1296-1231. [DOI] [PubMed] [Google Scholar]
- O'Roak B. J., Deriziotis P., Lee C., Vives L., Schwartz J. J., Girirajan S., Karakoc E., MacKenzie A. P., Ng S. B., Baker C. et al. (2011). Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat. Genet. 43, 585-589. 10.1038/ng.835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmakumar V. C., Libotte T., Lu W., Zaim H., Abraham S., Noegel A. A., Gotzmann J., Foisner R. and Karakesisoglou I. (2005). The inner nuclear membrane protein Sun1 mediates the anchorage of Nesprin-2 to the nuclear envelope. J. Cell Sci. 118, 3419-3430. 10.1242/jcs.02471 [DOI] [PubMed] [Google Scholar]
- Patterson K., Molofsky A. B., Robinson C., Acosta S., Cater C. and Fischer J. A. (2004). The functions of Klarsicht and nuclear lamin in developmentally regulated nuclear migrations of photoreceptor cells in the Drosophila eye. Mol. Biol. Cell 15, 600-610. 10.1091/mbc.E03-06-0374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne C., Rawe V., Ramalho-Santos J., Simerly C. and Schatten G. (2003). Preferentially localized dynein and perinuclear dynactin associate with nuclear pore complex proteins to mediate genomic union during mammalian fertilization. J. Cell Sci. 116, 4727-4738. 10.1242/jcs.00784 [DOI] [PubMed] [Google Scholar]
- Petrie R. J. and Yamada K. M. (2015). Fibroblasts lead the way: a unified view of 3D cell motility. Trends Cell Biol. 25, 666-674. 10.1016/j.tcb.2015.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie R. J., Gavara N., Chadwick R. S. and Yamada K. M. (2012). Nonpolarized signaling reveals two distinct modes of 3D cell migration. J. Cell Biol. 197, 439-455. 10.1083/jcb.201201124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie R. J., Koo H. and Yamada K. M. (2014). Generation of compartmentalized pressure by a nuclear piston governs cell motility in a 3D matrix. Science 345, 1062-1065. 10.1126/science.1256965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychiatric GWAS Consortium Bipolar Disorder Working Group. (2011). Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat. Genet. 43, 977-983. 10.1038/ng.943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puckelwartz M. J., Kessler E., Zhang Y., Hodzic D., Randles K. N., Morris G., Earley J. U., Hadhazy M., Holaska J. M., Mewborn S. K. et al. (2009). Disruption of nesprin-1 produces an Emery Dreifuss muscular dystrophy-like phenotype in mice. Hum. Mol. Genet. 18, 607-620. 10.1093/hmg/ddn386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston E., Lu Z., Biscocho N., Soumaka E., Mavroidis M., Prats C., Lømo T., Capetanaki Y. and Ploug T. (2006). Blood vessels and desmin control the positioning of nuclei in skeletal muscle fibers. J. Cell. Physiol. 209, 874-882. 10.1002/jcp.20780 [DOI] [PubMed] [Google Scholar]
- Razafsky D. and Hodzic D. (2015). A variant of Nesprin1 giant devoid of KASH domain underlies the molecular etiology of autosomal recessive cerebellar ataxia type I. Neurobiol. Dis. 78, 57-67. 10.1016/j.nbd.2015.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razafsky D., Blecher N., Markov A., Stewart-Hutchinson P. J. and Hodzic D. (2012). LINC complexes mediate the positioning of cone photoreceptor nuclei in mouse retina. PLoS ONE 7, e47180 10.1371/journal.pone.0047180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinsch S. and Gonczy P. (1998). Mechanisms of nuclear positioning. J. Cell Sci. 111, 2283-2295. [DOI] [PubMed] [Google Scholar]
- Romero N. B. (2010). Centronuclear myopathies: a widening concept. Neuromuscul. Disord. 20, 223-228. 10.1016/j.nmd.2010.01.014 [DOI] [PubMed] [Google Scholar]
- Romero N. B. and Bitoun M. (2011). Centronuclear myopathies. Semin. Pediatr. Neurol. 18, 250-256. 10.1016/j.spen.2011.10.006 [DOI] [PubMed] [Google Scholar]
- Rose L. and Gonczy P. (2014). Polarity establishment, asymmetric division and segregation of fate determinants in early C. elegans embryos. WormBook 1-43. 10.1895/wormbook.1.30.2 [DOI] [PubMed] [Google Scholar]
- Roux K. J., Crisp M. L., Liu Q., Kim D., Kozlov S., Stewart C. L. and Burke B. (2009). Nesprin 4 is an outer nuclear membrane protein that can induce kinesin-mediated cell polarization. Proc. Natl. Acad. Sci. USA 106, 2194-2199. 10.1073/pnas.0808602106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowat A. C., Jaalouk D. E., Zwerger M., Ung W. L., Eydelnant I. A., Olins D. E., Olins A. L., Herrmann H., Weitz D. A. and Lammerding J. (2013). Nuclear envelope composition determines the ability of neutrophil-type cells to passage through micron-scale constrictions. J. Biol. Chem. 288, 8610-8618. 10.1074/jbc.M112.441535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J. R. and Lichtman J. W. (2001). Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat. Rev. Neurosci. 2, 791-805. 10.1038/35097557 [DOI] [PubMed] [Google Scholar]
- Sato A., Isaac B., Phillips C. M., Rillo R., Carlton P. M., Wynne D. J., Kasad R. A. and Dernburg A. F. (2009). Cytoskeletal forces span the nuclear envelope to coordinate meiotic chromosome pairing and synapsis. Cell 139, 907-919. 10.1016/j.cell.2009.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer F. C. (1935). Mitosis in the neural tube. J. Comp. Neurol. 62, 377-405. 10.1002/cne.900620207 [DOI] [Google Scholar]
- Schatten G. (1982). Motility during fertilization. Int. Rev. Cytol. 79, 59-163. 10.1016/S0074-7696(08)61673-3 [DOI] [PubMed] [Google Scholar]
- Schatten G. (1994). The centrosome and its mode of inheritance: the reduction of the centrosome during gametogenesis and its restoration during fertilization. Dev. Biol. 165, 299-335. 10.1006/dbio.1994.1256 [DOI] [PubMed] [Google Scholar]
- Schoppmann S. F., Vinatzer U., Popitsch N., Mittlbock M., Liebmann-Reindl S., Jomrich G., Streubel B. and Birner P. (2013). Novel clinically relevant genes in gastrointestinal stromal tumors identified by exome sequencing. Clin. Cancer Res. 19, 5329-5339. 10.1158/1078-0432.CCR-12-3863 [DOI] [PubMed] [Google Scholar]
- Shin J.-W., Spinler K. R., Swift J., Chasis J. A., Mohandas N. and Discher D. E. (2013). Lamins regulate cell trafficking and lineage maturation of adult human hematopoietic cells. Proc. Natl. Acad. Sci. USA 110, 18892-18897. 10.1073/pnas.1304996110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoblom T., Jones S., Wood L. D., Parsons D. W., Lin J., Barber T. D., Mandelker D., Leary R. J., Ptak J., Silliman N. et al. (2006). The consensus coding sequences of human breast and colorectal cancers. Science 314, 268-274. 10.1126/science.1133427 [DOI] [PubMed] [Google Scholar]
- Sosa B. A., Rothballer A., Kutay U. and Schwartz T. U. (2012). LINC complexes form by binding of three KASH peptides to domain interfaces of trimeric SUN proteins. Cell 149, 1035-1047. 10.1016/j.cell.2012.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. C. and Erickson C. A. (2012a). Apical movement during interkinetic nuclear migration is a two-step process. Dev. Biol. 370, 33-41. 10.1016/j.ydbio.2012.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. C. and Erickson C. A. (2012b). Interkinetic nuclear migration: a mysterious process in search of a function. Dev. Growth Differ. 54, 306-316. 10.1111/j.1440-169X.2012.01342.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splinter D., Tanenbaum M. E., Lindqvist A., Jaarsma D., Flotho A., Yu K. L., Grigoriev I., Engelsma D., Haasdijk E. D., Keijzer N. et al. (2010). Bicaudal D2, dynein, and kinesin-1 associate with nuclear pore complexes and regulate centrosome and nuclear positioning during mitotic entry. PLoS Biol. 8, e1000350 10.1371/journal.pbio.1000350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr D. A. (2012). Laminopathies: too much SUN is a bad thing. Curr. Biol. 22, R678-R680. 10.1016/j.cub.2012.06.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr D. A. and Fridolfsson H. N. (2010). Interactions between nuclei and the cytoskeleton are mediated by SUN-KASH nuclear-envelope bridges. Annu. Rev. Cell Dev. Biol. 26, 421-444. 10.1146/annurev-cellbio-100109-104037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr D. A. and Han M. (2002). Role of ANC-1 in tethering nuclei to the actin cytoskeleton. Science 298, 406-409. 10.1126/science.1075119 [DOI] [PubMed] [Google Scholar]
- Strzyz P. J., Lee H. O., Sidhaye J., Weber I. P., Leung L. C. and Norden C. (2015). Interkinetic nuclear migration is centrosome independent and ensures apical cell division to maintain tissue integrity. Dev. Cell 32, 203-219. 10.1016/j.devcel.2014.12.001 [DOI] [PubMed] [Google Scholar]
- Sulston J. E. (1976). Post-embryonic development in the ventral cord of Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 275, 287-297. 10.1098/rstb.1976.0084 [DOI] [PubMed] [Google Scholar]
- Swartz R. K., Rodriguez E. C. and King M. C. (2014). A role for nuclear envelope-bridging complexes in homology-directed repair. Mol. Biol. Cell 25, 2461-2471. 10.1091/mbc.E13-10-0569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Iwabuchi K., Fukao Y., Kondo M., Okamoto K., Ueda H., Nishimura M. and Hara-Nishimura I. (2013). Myosin XI-i links the nuclear membrane to the cytoskeleton to control nuclear movement and shape in Arabidopsis. Curr. Biol. 23, 1776-1781. 10.1016/j.cub.2013.07.035 [DOI] [PubMed] [Google Scholar]
- Tapley E. C. and Starr D. A. (2013). Connecting the nucleus to the cytoskeleton by SUN-KASH bridges across the nuclear envelope. Curr. Opin. Cell Biol. 25, 57-62. 10.1016/j.ceb.2012.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. G., Yenepalli A., Denais C. M., Rape A., Beach J. R., Wang Y.-L., Schiemann W. P., Baskaran H., Lammerding J. and Egelhoff T. T. (2015). Non-muscle myosin IIB is critical for nuclear translocation during 3D invasion. J. Cell Biol. 210, 583-594. 10.1083/jcb.201502039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toya M., Kobayashi S., Kawasaki M., Shioi G., Kaneko M., Ishiuchi T., Misaki K., Meng W. and Takeichi M. (2016). CAMSAP3 orients the apical-to-basal polarity of microtubule arrays in epithelial cells. Proc. Natl. Acad. Sci. USA 113, 332-337. 10.1073/pnas.1520638113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai J.-W., Lian W.-N., Kemal S., Kriegstein A. R. and Vallee R. B. (2010). Kinesin 3 and cytoplasmic dynein mediate interkinetic nuclear migration in neural stem cells. Nat. Neurosci. 13, 1463-1471. 10.1038/nn.2665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujikawa M., Omori Y., Biyanwila J. and Malicki J. (2007). Mechanism of positioning the cell nucleus in vertebrate photoreceptors. Proc. Natl. Acad. Sci. USA 104, 14819-14824. 10.1073/pnas.0700178104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Shi Z., Jiao S., Chen C., Wang H., Liu G., Wang Q., Zhao Y., Greene M. I. and Zhou Z. (2012). Structural insights into SUN-KASH complexes across the nuclear envelope. Cell Res. 22, 1440-1452. 10.1038/cr.2012.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsen K., Litjens S. H. M., Kuikman I., Tshimbalanga N., Janssen H., van den Bout I., Raymond K. and Sonnenberg A. (2005). Nesprin-3, a novel outer nuclear membrane protein, associates with the cytoskeletal linker protein plectin. J. Cell Biol. 171, 799-810. 10.1083/jcb.200506083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. H. and Holzbaur E. L. F. (2012). Opposing microtubule motors drive robust nuclear dynamics in developing muscle cells. J. Cell Sci. 125, 4158-4169. 10.1242/jcs.108688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. H. and Holzbaur E. L. F. (2015). Nesprins anchor kinesin-1 motors to the nucleus to drive nuclear distribution in muscle cells. Development 142, 218-228. 10.1242/dev.114769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K., Wu Y. I., Liu Y., Geiger J., Tam E., Overall C., Stack M. S. and Friedl P. (2007). Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat. Cell Biol. 9, 893-904. 10.1038/ncb1616 [DOI] [PubMed] [Google Scholar]
- Wolf K., te Lindert M., Krause M., Alexander S., te Riet J., Willis A. L., Hoffman R. M., Figdor C. G., Weiss S. J. and Friedl P. (2013). Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J. Cell Biol. 201, 1069-1084. 10.1083/jcb.201210152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Lei K., Zhou M., Craft C. M., Xu G., Xu T., Zhuang Y., Xu R. and Han M. (2011). KASH protein Syne-2/Nesprin-2 and SUN proteins SUN1/2 mediate nuclear migration during mammalian retinal development. Hum. Mol. Genet. 20, 1061-1073. 10.1093/hmg/ddq549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T. W., Chahrour M. H., Coulter M. E., Jiralerspong S., Okamura-Ikeda K., Ataman B., Schmitz-Abe K., Harmin D. A., Adli M., Malik A. N. et al. (2013). Using whole-exome sequencing to identify inherited causes of autism. Neuron 77, 259-273. 10.1016/j.neuron.2012.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Bethmann C., Worth N. F., Davies J. D., Wasner C., Feuer A., Ragnauth C. D., Yi Q., Mellad J. A., Warren D. T. et al. (2007a). Nesprin-1 and -2 are involved in the pathogenesis of Emery Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Hum. Mol. Genet. 16, 2816-2833. 10.1093/hmg/ddm238 [DOI] [PubMed] [Google Scholar]
- Zhang X., Xu R., Zhu B., Yang X., Ding X., Duan S., Xu T., Zhuang Y. and Han M. (2007b). Syne-1 and Syne-2 play crucial roles in myonuclear anchorage and motor neuron innervation. Development 134, 901-908. 10.1242/dev.02783 [DOI] [PubMed] [Google Scholar]
- Zhang X., Lei K., Yuan X., Wu X., Zhuang Y., Xu T., Xu R. and Han M. (2009). SUN1/2 and Syne/Nesprin-1/2 complexes connect centrosome to the nucleus during neurogenesis and neuronal migration in mice. Neuron 64, 173-187. 10.1016/j.neuron.2009.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T., Graham O. S., Raposo A. and St Johnston D. (2012). Growing microtubules push the oocyte nucleus to polarize the Drosophila dorsal-ventral axis. Science 336, 999-1003. 10.1126/science.1219147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen Y. Y., Libotte T., Munck M., Noegel A. A. and Korenbaum E. (2002). NUANCE, a giant protein connecting the nucleus and actin cytoskeleton. J. Cell Sci. 115, 3207-3222. [DOI] [PubMed] [Google Scholar]
- Zhou X. and Meier I. (2014). Efficient plant male fertility depends on vegetative nuclear movement mediated by two families of plant outer nuclear membrane proteins. Proc. Natl. Acad. Sci. USA 111, 11900-11905. 10.1073/pnas.1323104111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Graumann K., Evans D. E. and Meier I. (2012). Novel plant SUN-KASH bridges are involved in RanGAP anchoring and nuclear shape determination. J. Cell Biol. 196, 203-211. 10.1083/jcb.201108098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Groves N. R. and Meier I. (2015). Plant nuclear shape is independently determined by the SUN-WIP-WIT2-myosin XI-i complex and CRWN1. Nucleus 6, 144-153. 10.1080/19491034.2014.1003512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuela N. and Gruenbaum Y. (2016). Matefin/SUN-1 phosphorylation on serine 43 is mediated by CDK-1 and required for its localization to centrosomes and normal mitosis in C. elegans embryos. Cells 5, 8 10.3390/cells5010008 [DOI] [PMC free article] [PubMed] [Google Scholar]