Abstract
Stroke can lead to sensory deficits that impair functional control of arm movements. Here we describe a simple test of arm motion detection (AMD) that provides an objective, quantitative measure of movement perception related proprioceptive capabilities in the arm. Seven stroke survivors and thirteen neurologically intact control subjects performed the AMD test. In a series of ten trials that took less than 15 minutes to complete, participants used a two-button user interface to adjust the magnitude of hand displacements produced by a horizontal planar robot until the motions were just perceptible (i.e. on the threshold of detection). The standard deviation of movement detection threshold was plotted against the mean and a normative range was determined from the data collected with control subjects. Within this normative space, subjects with and without intact proprioception could be discriminated on a ratio scale that is meaningful for ongoing studies of degraded motor function. Thus, the AMD test provides a relatively fast, objective and quantitative measure of upper extremity proprioception of limb movement (i.e. kinesthesia).
I. INTRODUCTION
Each Year, nearly 800,000 Americans experience a new or recurrent stroke [1]. Up to half of survivors experience persistent sensory impairments severe enough to impair quality of life [2]. The kinesthetic sense of limb position and motion (i.e. proprioception) is known to be important in planning and controlling limb posture and movement [3–7]. However, clinical tests evaluating impairments in proprioception suffer from poor reliability [8–9]. Thus, efforts are currently being made to design standardized tests [10–11] and automated procedures [12–13] to quantify somatosensory deficits.
Although there are many quick clinical tests for impairment of proprioceptive sensation, most provide only a coarse assessment of the degree of impairment. Many of the automated procedures, though very sensitive in their assessment of the degree of impairment, can take up to an hour to complete which renders them impractical in most scenarios. Here, we propose a robotic technique that provides a quick evaluation of the integrity of upper extremity proprioception on a continuous, normative ratio scale.
II. METHODS
A. Subjects
Thirteen neurologically intact control subjects (NI; 18–86 years; 7 females) and seven unilateral, hemiparetic stroke survivors (SS; 51–64 years; 4 males, 4 right hand more-affected) gave written, informed consent to participate in this study in compliance with policies established by the Marquette University Institutional Review Board. All SS were in the chronic stage of recovery (>6 months post-stroke). SS were excluded from the study if unable to give informed consent, follow 2-step instructions, or raise the arm to the test position of 75° to 90° shoulder abduction. NI control subjects had no history of neurological disorder and were able to achieve the test position without discomfort. NI control subjects participated in one session lasting approximately 15 minutes and SS subjects participated in two experimental sessions lasting not more than three hours each.
B. Clinical Assessments
All SS participated in a clinical evaluation session consisting of the:
Modified Ashworth Scale (MAS) to assess muscle tone. MAS scores are graded from “0” for normal muscle tone to “4,” for muscle tone sufficient to render the arm rigid.
Upper extremity motor portion of the Fugl-Meyer (FM) Assessment of Physical Performance. The upper extremity motor portion assesses motor impairment of the arm where a maximal score of “66” indicates that the subject retains normal reflexes, can move outside of motor synergies, and has a variety of intact grasps.
Upper extremity sensation portion of the FM Assessment, which includes a version of the “up/down” test [14], wherein proprioception at the shoulder, elbow, wrist and thumb are evaluated by passively moving the tested joint back and forth in a plane of movement. When the joint stops moving, the subject is asked to indicate segment orientation (“up or down?”). Six repetitions are performed at each joint. If response is 100% accurate, proprioception is rated “intact” and that joint is given a numerical score of “2”; if the subject is unable to respond with confidence (i.e. one error), proprioception is rated as “impaired” and the joint is given a score of “1”; if the subject is unable to determine joint orientation reliably (two or more errors), proprioception is rated “absent” and the joint is given a score of “0.” A maximal score for intact proprioception at all joints tested is “8,” wherein the subject’s proprioception is considered intact (+), a lower score indicates the subject has impaired proprioception (−). This assessment also evaluates light touch in the arm and hand in a similar manner with the maximal score of “4” indicating intact light touch sensation and a score of “0” indicating its absence.
Thirteen-item Chedoke Arm and Hand Activities Inventory (CAHAI) which includes bimanual tasks of daily living to assess functional ability of the affected hand to successfully stabilize or manipulate objects. A minimum score of “13” on the CAHAI indicates that the subject performed all tasks without using the involved hand, could not complete the tasks, or was deemed unsafe to try the tasks. A maximal score of “91” indicates that the subject performed all of the tasks using both the hands efficiently.
Montreal Cognitive Assessment (MoCA) to assess cognitive impairments involving attention and concentration, executive functions, memory, language, visuo-constructional skills, conceptual thinking, mental calculations, and orientation. A score of “26” or greater out of a maximal score of “30” on the MoCA indicates normal cognitive function.
C. Experimental Setup and Procedures
Subjects sat in a high-backed chair and grasped the handle of a horizontal planar robot (Fig. 1) equipped with sensors that monitored instantaneous handle position and hand reaction forces. The handle location during testing was approximately 30 cm from the sternum in the sagittal plane. The “more affected” (SS) or dominant (NI) upper arm was supported between 75° and 90° abduction by a lightweight, chair-mounted support or ceiling mounted sling. The tested arm and hand were occluded from view. Subjects were instructed to relax their arm muscles during testing.
Figure 1.
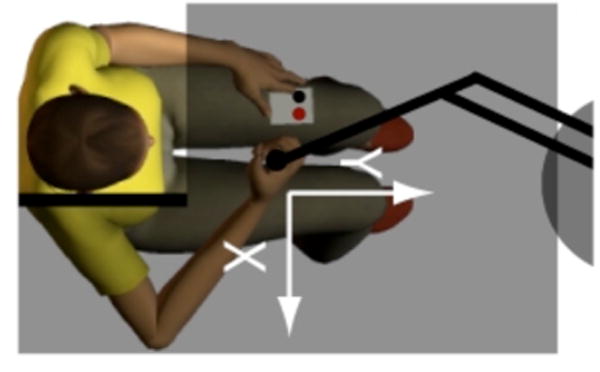
Experimental set-up with participant seated in front of the robot. The subject holds a spherical or cylindrical handle mounted at the end of the robot arm and holds a two-button response box in the lap. Adjustable opaque screens block view of the arm and the robot.
D. Proprioceptive Detection of Limb Movement
Subjects performed ten, sixty-second trials of a stimulus detection task. During the trials, the robot generated complex, two-dimensional sum-of-sinusoids force perturbations (4 N peak-to-peak max). The set of perturbation frequencies differed along the X-axis (1.75 and 1.2 Hz) and Y-axis (1.65 and 1.1 Hz) such that for any given perturbation magnitude, the induced shoulder and elbow joint torques were approximately equal in magnitude and frequency at the testing location.
The ten trials alternated between two types: ascending and descending. Throughout the experiment, subjects were continually asked, “do you feel the robot moving your hand?” The experimenter used a two-button response box to adjust the size of the robotic perturbations based on the subject’s response until the subject answered ‘no’ on a descending trial, indicating that s/he had just ceased to feel motion, or ‘yes’ on ascending trials indicating that s/he had just begun to feel the motion. One button increased the perturbation magnitude by ~ 0.5% of full scale (where full scale = 4 N) with each press; the other button decreased the perturbation magnitude by the same amount. We used the final value of the perturbation magnitude in each trial as an estimate of the motion detection threshold. By approaching the motion detection threshold from both directions, we sought to minimize the effect of response bias on the estimated threshold. Initial perturbation amplitudes alternated between 0 N and 4 N on consecutive trials for ascending and descending trial types, respectively (Fig. 2).
Figure 2.
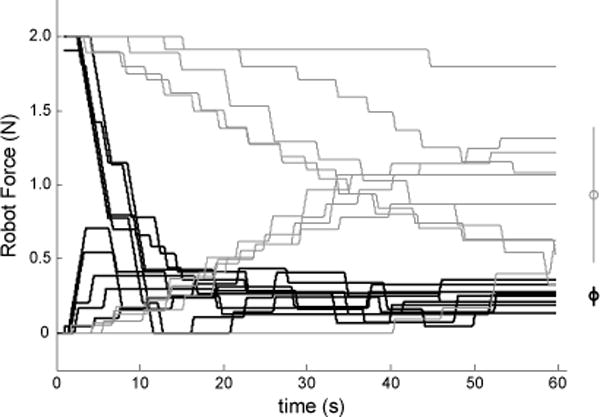
Successive adjustments in perturbation magnitude during each individual trial for selected subjects (gray traces: SS05, who had impaired proprioception (−); black: subject NI06). Right margin: mean and standard deviation of the final values for these two subjects.
E. Data Analysis
Clinical test scores were tabulated for each SS. MAS scores were averaged across the shoulder, elbow and wrist joints. Up/down test scores at the shoulder, elbow, wrist and thumb were summed.
Kinesthetic detection threshold was defined as the across-trial mean (μf) of the final commanded force perturbation magnitudes chosen by each subject (cf. Fig. 2, right). The standard deviation (σf) of the detection threshold was considered to be an estimate of the subject’s uncertainty in their psychometric assessment of threshold. We used Spearman Rank Correlation to assess the relationship between the results from the five clinical tests (MAS, FM, CAHAI, MoCA and “up or down?”) and μf and σf from the AMD test.
We defined a space within which kinesthetic sensation can be compared between stroke survivors and neurologically-intact individuals by plotting parameters σf against μf. Under the assumption that μf and σf are uncorrelated within individuals, we estimated the probability of intact proprioception as the product of the and cumulative likelihood functions from the NI group. By plotting AMD test performance for a given limb within the normative {μf, σf} space, we obtain both an estimate of the likelihood of intact proprioceptive sensation in that limb and an estimate of the magnitude of impairment in terms of detection threshold μf and choice uncertainty σf.
III. RESULTS
Table 1 shows descriptive statistics and clinical test scores for all SS participants. MoCA scores averaged 21.9±7.01, with three subjects exhibiting expressive aphasia. Our sample of stroke survivors was a heterogeneous group with regards to motor impairment (FMM scores ranged from 9 to 66), functional arm use (CAHAI scores ranged from 14 to 91) and clinical assessment of proprioceptive integrity (ranging from intact at the shoulder, elbow, wrist and thumb to absent in at least one joint).
TABLE I.
Functional Testing Scores for SS participants.
| ID | Age | MoCA | FMM | FMprop. | FMLT | C-13 | MAS | AMD |
|---|---|---|---|---|---|---|---|---|
| 1 | 55 | 27 | 27 | 2S, 1E, 0W, 0T | 2 | 18 | 0.375 | 1.29±0.68 |
| 2 | 61 | 10* | 27 | 1S, 1E, 0W, 0T | 2 | 15 | 1.0 | 1.30±0.44 |
| 3 | 51 | 23* | 21 | 2S, 2E, 2W, 2T | 2 | 23 | 1.375 | 0.45±0.11 |
| 4 | 61 | 28 | 9 | 2S, 2E, 0W, 1T | 2 | 14 | 1.125 | 0.48±0.39 |
| 5 | 63 | 14* | 45 | 2S, 1E, 1W, 0T | 1 | 32 | 0.75 | 0.93±0.45 |
| 6 | 64 | 26 | 30 | 2S, 2E, 2W, 2T | 4 | 23 | 2.75 | 0.28±0.07 |
| 7 | 61 | 25 | 66 | 1S, 1E, 1W, 1T | 2 | 91 | 0 | 0.75±0.06 |
Abbreviations. ID: Subject identifier; MoCA: Montreal Cognitive Assessment Test; FMM: upper extremity motor portion of the Fugl-Meyer Assessment of Physical Performance; FMprop: “up or down?” test from the upper extremity sensory portion of the Fugl-Meyer Assessment of Physical Performance; FMLT: light touch test from upper extremity sensory portion of the Fugl-Meyer Assessment of Physical Performance; C-13: Thirteen item version of the Chedoke Arm and Hand Activities Inventory; MAS: Modified Ashworth Scale; AMD: our robotic assessment of kinesthetic detection of arm motion, μf ±σf.
Asterisks indicate participants with expressive aphasia, which can negatively impact MoCA score.
The scores of the clinical tests were generally poorly correlated, suggesting that the tests evaluated relatively independent aspects of function. In contrast, a correlation was found between the FM and the CAHAI (ρ=0.836; p=0.019).
The sequence of perturbation adjustments from all ten trials of two selected subjects are shown in Fig. 2, with the gray traces representing a SS with impaired proprioception and the black traces representing a NI control subject. The force applied to the hand at the end of each trial was considered an estimate of that subject’s kinesthetic threshold. The variability of the ten threshold determinations was considered an estimate of the subject’s uncertainty in assessment of threshold. For all SS, the threshold estimated using ascending trials was not different from the threshold estimated using descending trials (t6=1.430, p=0.203). Average force threshold and related motion magnitude were as follows: NI Group: 0.18±0.10 N and 0.24±0.13 cm; SS with clinically intact proprioception {SS(+) Group}: 0.37±0.12 N and 0.22±0.11 cm; SS with impaired and/or absent proprioception {SS(−) Group}: 0.95±0.35 N and 0.73±0.33 cm. Testing time was less than 15 minutes for each participant.
When plotted within the {μf, σf} space (Fig. 3), NI AMD scores (pink squares) were tightly grouped within the lower left quadrant with mean detection thresholds, μf < 0.5 N and threshold variabilities, σf < 0.2 N. Moving from the origin outward, the color map depicts the cumulative likelihood of intact proprioception decreasing as values of μf and σf increase. Contour lines, derived from the bivariate distribution of NI performance data, provide an intuitive visual indicator of the likelihood of intact proprioception for a given arm.
Figure 3.
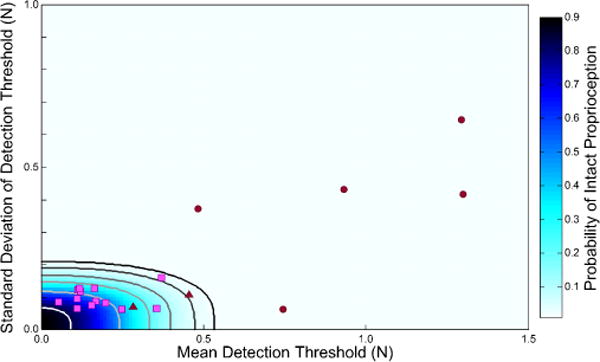
Relationship between mean detection threshold (μf, x-axis) and variability of the detection threshold (σf, y-axis). NI control subject data (pink squares) were used to determine confidence interval bounds for intact proprioception (contours). The 99.9% CI bound is shown in black. Five SS(−) (maroon circles) had a combination of thresholds and variability that put them outside the bounds of the 99.9 % CI. Two SS(+) had a combination of thresholds and variability that put them inside the 99.9 % CI (maroon triangles).
SS(+) (maroon triangles in Fig. 3) had AMD performance values {μf, σf} similar to those generated by NI control subjects. SS(−) (maroon circles) demonstrated markedly elevated μf and/or σf values.
Table 2 shows the Spearman rank correlation between the mean AMD scores, μf, and the up/down test, FMprop, for all SS was significant (ρ=−0.982; p<0.001) suggesting that the AMD test is at least as sensitive to proprioceptive integrity in the arm as the standard clinical test. The mean AMD scores were not related to the scores on the MoCA (ρ=-0.393; p=0.383) indicating that the AMD test was not dependent on cognitive function within this group of SS.
TABLE II.
Correlations between AMD and functional measures
| MoCA | FMM | C-13 | MAS | FMProp | μf | σf | |
|---|---|---|---|---|---|---|---|
| MoCA | 1 | ||||||
| FMM | −0.342 | 1 | |||||
| C-13 | −0.324 | 0.836* | 1 | ||||
| MAS | −0.036 | −0.109 | −0.027 | 1 | |||
| FMProp | 0.327 | −0.202 | 0.183 | 0.615 | 1 | ||
| μf | −0.393 | 0.126 | −0.198 | −0.559 | −0.982* | 1 | |
| σf | −0.071 | −0.252 | −0.414 | −0.018 | −0.582 | −0.679 | 1 |
Abbreviations. MoCA: Montreal Cognitive Assessment Test; FMM: upper extremity motor portion of the Fugl-Meyer Assessment of Physical Performance; C-13: Thirteen-item version of the Chedoke Arm and Hand Activities Inventory; MAS: Modified Ashworth Scale; FMprop: “up or down?” test from the upper extremity sensory portion of the Fugl-Meyer Assessment of Physical Performance; μf: mean AMD score; σf: variation in AMD score.
Asterisks indicate significance at the 0.05 level.
The variability, σf, about the AMD score was not significantly correlated with the results from the up/down test (ρ=−0.582, p=0.170) indicating that the variability about the decision is less indicative of proprioceptive status. This measure was also not related to the results from the MoCA (ρ=−0.071; p=0.879).
IV. DISCUSSION AND CONCLUSIONS
Results of the AMD test generally concurred with the findings of the clinical “up or down?” test, which is part of the sensory portion of the FM Assessment. NI control subjects and SS(+) with intact proprioception typically had low AMD performance values {μf, σf}, whereas AMD performance values were markedly increased for SS(−) with impaired proprioception in the arm and hand (Fig. 3).
Current clinical tests of proprioception are useful because they are easy to administer and can give clinicians a quick, rough estimate of a patient’s proprioceptive status. However, the clinical test administered here – the up/down test - is limited in that there are only three possible grades of proprioception: intact, impaired and absent. By collapsing a continuum of impairment to just three ordinal classification groups, this clinical test sacrifices measurement resolution for speed of administration. By contrast, the AMD test yields a pair of ratiometric performance variables {μf, σf} that can indicate the likelihood and magnitude of proprioceptive impairment in the tested limb when plotted within the NI subjects’ normative performance space.
Another limitation of the up/down test is its susceptibility to a ceiling effect due to the production of secondary sensory cues. When the clinician moves a limb segment, shearing forces are produced by the examiner’s fingers, which can indicate the direction of movement (and thus the final limb posture) for individuals retaining tactile sensation. In addition, manipulating the limb posture of the arm about a relaxed shoulder can affect the posture of the trunk, cause clothing to shift against the skin, or can cause the head to move slightly. Each of these secondary cues could be used to infer limb segment orientation. As a result of these cues, many subjects who claim to have impaired proprioceptive perception can accurately and reliably report the spatial orientation of their elbow and shoulder joints [15]. By contrast, the AMD test applies very small perturbations to the hand, which are not likely to cause obvious shearing forces or significant motion of the trunk, head or clothing.
While the AMD is a highly effective measure of movement-related proprioception, it is limited in that it does not test the subject’s ability to estimate limb position or the direction of displacement. The AMD also occurs with the arm in a single posture; it is unknown if movement detection thresholds vary as arm posture changes. In addition, the AMD may potentially prove useful as a measure to detect changes in kinesthetic acuity, though this study does not provide adequate rigor (i.e., comparison of the measure against multiple psychometric tests of kinesthetic acuity) to make that claim at present.
A final advantage of the AMD test over current clinical tests of proprioceptive integrity is that the AMD is specifically designed to quantify kinesthetic sensitivity to horizontal planar hand perturbations similar to those currently used in many studies of robotic interventions for the promotion of functional arm movement post-stroke. Future studies seeking to understand the impact of proprioceptive deficits in the control of limb posture and movement post-stroke should quantify kinesthetic sensitivity on a scale commensurate with the environmental perturbations used to challenge sensorimotor performance. While no current test is flawless, the AMD is a suitable test of kinesthetic integrity for certain instances (e.g. for research purposes, where it may be useful to distinguish between environmental perturbations with magnitudes above vs. below the threshold of perception).
Acknowledgments
We thank Dr. Lior Botzer for insightful suggestions on a previous version of this test and for robot programming efforts.
This work was supported by NIH RO1HD053727.
Contributor Information
Maria C. Bengtson, Marquette University, Milwaukee, WI 53233 USA.
Leigh A. Mrotek, Marquette University, Milwaukee, WI 53233 USAUniversity of Wisconsin, Oshkosh, Oshkosh, WI, 54901-8630 USA
Tina Stoeckmann, Marquette University, Milwaukee, WI 53233 USA.
Claude Ghez, Columbia University College of Physicians and Surgeons, New York NY 10032 USA.
Robert A. Scheidt, Marquette University, Milwaukee, WI 53233 USANorthwestern University, Evanston, IL 60208 USA.
References
- 1.Roger VL, et al. Heart Disease and Stroke Statistics–2012 Update: A Report From the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carey LM. Somatosensory Loss after Stroke. Critical Reviews in Physical and Rehabilitation Medicine. 1995;7:51–91. [Google Scholar]
- 3.Scheidt RA, Conditt MA, Secco EL, Mussa-Ivaldi FA. Interaction of Visual and Proprioceptive Feedback During Adaptation of Human Reaching Movements. Journal of Neurophysiology. 2005;93:3200–3213. doi: 10.1152/jn.00947.2004. [DOI] [PubMed] [Google Scholar]
- 4.Ghez C, Krakauer JW, Sainburg RL, Ghilardi MF. Spatial representations and internal models of limb dynamics in motor learning. In: Gazzaniga M, editor. The New Cognitive Neurosciences. 2. Cambridge, MA: MIT Press; 2000. pp. 501–514. [Google Scholar]
- 5.Sainburg RL, Ghilardi MF, Poizner H, Ghez C. Control of Limb Dynamics in Normal Subjects and Patients Without Proprioception. Journal of Neurophysiology. 1995;72:820–835. doi: 10.1152/jn.1995.73.2.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sober S, Sabes P. Multisensory integration during motor planning. J Neurosci. 2003;23:6982–6992. doi: 10.1523/JNEUROSCI.23-18-06982.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarlegna FR, Sainburg RL. The roles of vision and proprioception in the planning of reaching movements. In: Sternad D, editor. Progress in Motor Control. New York: Springer Science; 2009. pp. 317–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bohannon RW, Smith MB. Interrater Reliability of a Modified Ashworth Scale of Muscle Spasticity. Physical Thearapy. 1987;67:206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 9.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The Post-Stroke Hemiplegic Patient. Scandinavian Journal of Rehabilitation Medicine. 1975;7:13–31. [PubMed] [Google Scholar]
- 10.Barreca S, Stratford P, Masters LM, Lambert CL, Griffiths J. Comparing 2 Versions of the Chedoke Arm and Hand Activity Inventory With the Action Research Arm Test. Physical Thearapy. 2006;86:245–252. [PubMed] [Google Scholar]
- 11.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assesment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. Journal of the American Geriatrics Society. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 12.Dukelow SP, Herter TM, Moore KD, Demers MJ, Glasgow JI, Bagg SD, et al. Quantitative Assessment of LImb Postion Sense Following Stroke. Neurorehabilitation and Neural Repair. 2010;24:178–187. doi: 10.1177/1545968309345267. [DOI] [PubMed] [Google Scholar]
- 13.Simo LS, Ghez C, Botzer L, Scheidt RA. A Robotic Test of Proprioception Within the Hemiparetic Arm Post-Stroke. Journal of Neuroengineering and Rehabilitation. 2014;11:77. doi: 10.1186/1743-0003-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeGowin RL, Brown DD, Christensen J, DeGowin EL. DeGowin & DeGowin’s diagnostic examination. 6. New York: McGraw-Hill, Health Professions Division; 1994. [Google Scholar]
- 15.Lin J-H, Hsueh I-P, Sheu C-F, Hsieh C-L. Psychometric properties of the sensory scale of the Fugl-Meyer assessment in stroke patients. Clinical Rehabilitation. 2004;18:391–397. doi: 10.1191/0269215504cr737oa. [DOI] [PubMed] [Google Scholar]


