Abstract
Purpose
To prospectively determine sexual function, bother, and potency preservation in men treated with prostate brachytherapy and twice weekly tadalafil.
Methods
From 2005–2011, men treated with low dose rate prostate brachytherapy were treated on a prospective registration study. All patients were prescribed tadalafil 10 mg twice weekly. The expanded prostate cancer index composite (EPIC) questionnaire was administered prior to treatment and at each follow-up. A subgroup analysis of men with sexual potency at baseline was performed
Results
Two hundred thirty-seven men were analyzed. Median age was 64 years (range 44–86). Median follow-up was 24.8 months (range 1–60). At baseline, 175 (74%) reported erections firm enough for sexual activity and 148 (62%) were potent (erections firm enough for intercourse). Statistically significant changes in sexual function/bother were appreciated from baseline throughout the analysis period, though absolute changes were relatively small and did not meet criteria for clinical significance. At 24-month follow-up 72% reported erections firm enough for sexual activity and 56% were potent. Of men with potency at baseline, 89% had erections firm enough for sexual activity and 76% remained potent 24 months post-treatment.
Conclusions
Peri-procedural tadalafil and prostate brachytherapy resulted in high rates of sexual potency preservation and no clinically significant effect on sexual quality of life.
Introduction
Low dose rate (LDR) permanent prostate brachytherapy is a safe and highly effective treatment for men with organ confined prostate cancer.(1–3) Treatment involves the placement of small radioactive sources into the prostate gland in order to deliver radiation directly to the prostate. Because radiation dose delivery is inversely and exponentially related to the distance from the radiation source, radiation dose to the surrounding bladder, rectum, and connective tissue is minimized. LDR-prostate brachytherapy is more convenient than alternative treatments for prostate cancer as it can be delivered in a single outpatient visit. Erectile dysfunction (ED) is a common side effect of prostate cancer treatment, inclusive of prostate brachytherapy,(4) with affected patients suffering significant health related quality of life (HRQOL) consequences.(5–7) In men who are fully potent prior to prostate cancer treatment, sexual quality of life impairment is perhaps the strongest predictor of overall patient satisfaction.(5) Due in part to the complexity of sexual function, varying definitions of ED, and the historical absence of a standardized sexual quality of life instrument, high quality data on sexual function after prostate brachytherapy is limited.(8)
The term penile rehabilitation describes the use of medications or devices after definitive prostate cancer treatment delivered with the intent of preserving erectile tissue integrity and maximizing recovery of erectile function. The fundamental principle is to minimize post-treatment fibrosis of penile tissue through optimizing oxygenation and protecting endothelial cells.
Use of 5-phosphodiesterase inhibitors (PDE5i) with the intention of sexual quality of life preservation after prostate cancer treatment has been successfully implemented in men treated by radical prostatectomy.(9–11) While the primary mechanism of ED following prostatectomy is thought to be predominantly neurogenic(12), ED following radiation therapy is thought to be primarily vasogenic.(13) Following radiation therapy, sensitive endothelial cells lining penile arteries and the sinusoids of the corpora cavernosa can be damaged, leading to luminal stenosis, arterial insufficiency, and induction of fibrosis in the corporal tissue months to years after radiation treatment.(14,15) Hypoxia in the erectile tissue microvasculature appears to mediate the process of post-radiation fibrosis.(16,17) Achieving erections on a regular basis may prevent up-regulation of TGF-β, a cytokine believed to play a central role in post-radiation fibrosis.(18) PDE5i use facilitates periodic oxygenation of erectile tissues, promotes endothelial cell/smooth muscle vitality, and allows many patients to achieve increased erection rigidity.(19–23) Delayed initiation of PDE5i therapy in men with ED after prostate brachytherapy results in worse erectile function compared to early medical intervention.(24) All of the above suggest a role for peri-procedural PDE5i therapy in men treated with prostate brachytherapy. The current study reports the results of a prospective cohort of men treated with prostate brachytherapy and peri-procedural tadalafil for penile rehabilitation.
Materials and Methods
Patient Selection and Treatment
The overseeing institutional review board approved patient data collection and analysis. All participants provided written informed consent for treatment. Patients treated with prostate brachytherapy monotherapy were eligible for analysis. Criteria for prostate brachytherapy monotherapy during the analysis period were generally restricted to the following: AJCC 7th edition clinical stage T1c-T2c, presenting PSA <15 ng/ml, and Gleason score ≤ 7. Men with contra-indications to PDE5is were excluded from participation. Men endorsing any of the following were also excluded from analysis: any prior PDE5i use, use of other sexual medications including intra-cavernousal injections, use of sexual devices for erection enhancement, receipt of androgen deprivation therapy with therapeutic or cytoreductive intent, or receipt of supplemental external beam radiation therapy. Men with penile prostheses were also specifically excluded.
All patients were prescribed tadalafil 10 mg po twice weekly starting 2 weeks prior to prostate brachytherapy. The dosing and schedule of tadalafil was based on the intention of encouraging spontaneous erections twice weekly. No specific duration of therapy was pre-determined, however, continuation of tadalafil was encouraged for at least 6 months after the prescribed radiation dose was delivered (approximately 6 isotope half-lives). All patients were treated with iodine-125, palladium-103, or cesium-131 brachytherapy seeds to prescribed doses of 145 Gray (Gy), 125 Gy, and 115 Gy respectively. A standard ultra-sound guided, trans-perineal technique utilizing pre-loaded brachytherapy needles was used for all procedures. No patient received supplemental external beam radiation or androgen deprivation therapy.
Assessment of sexual function and bother
The expanded prostate cancer index composite (EPIC) and companion utilization of sexual medications and devices questionnaire are validated prostate cancer specific instruments for assessment of patient reported outcomes.(25) All patients completed the EPIC questionnaires prior to initiation of study treatment (baseline) and at regular follow-up intervals (1, 4, 8, 12 months post-brachytherapy and every 6 months thereafter). Sexual function, bother, and summary scores were tabulated according to instrument guidelines scaled from 0–100; where lower scores represent worse sexual function and more bother. The companion utilization of sexual medications and devices questionnaire was co-administered at each assessment time-point and is inclusive of patients reported sexual potency in the absence of pharmaceutical or mechanical enhancement.
Definition of potency
Data regarding potency was derived from patient responses to EPIC-26 question #18 “How would you describe the quality of your erections during the last four weeks” with possible responses limited to the following: 1) None at all 2) Not firm enough for any sexual activity 3) Firm enough for masturbation and foreplay only 4) Firm enough for intercourse. Potency was defined as erections firm enough for sexual intercourse. Men reporting erections firm enough for sexual intercourse, masturbation, or foreplay were defined as having erections firm enough sexual activity. Preservation of sexual potency and sexual activity was determined through subgroup analysis at baseline and subsequent follow-up.
Statistical Analysis
A paired t-test was used for statistical comparisons to baseline with statistical significance defined as p <0.05. The scores were plotted against assessment time as a graphical aid to visualize changes over time. Clinical significance as used in this study represents a meaningful change in the patient’s reported quality of life defined objectively as a change that is greater than half the standard deviation (SD) of the baseline score. This definition of clinical significance is consistent with statistical principles for psychometric testing. (26)
Results
Patient Characteristics
Two hundred thirty-seven men were eligible for analysis. The median age was 64 years (range 44–86). The median follow-up was 24.8 months (range, 1–60). One hundred seventy-five men (74%) reported erection firm enough for sexual activity at baseline. Of these men, 148 (62% of total) were potent with “erections firm enough for intercourse”, 27 (11% of total) reported erections “firm enough for masturbation and foreplay only”, 23 (10% of total) reported erections “not firm enough for any sexual activity”, and 39 (17% of total) reported “no erections” at baseline. Demographic and clinical characteristics of the study sample are summarized in Table 1.
Table 1.
Baseline demographics and clinical features for the study population
| Characteristic | Value | |
|---|---|---|
|
| ||
| Median Follow-Up (Range) | 24.8 (1–60) months | |
|
| ||
| Median Age (Range) | 64 (441–86) years | |
|
| ||
| Race (%) | ||
| White | 187 (81) | |
| Black | 28 (12) | |
| Hispanic | 12 (5) | |
| Other | 4 (2) | |
|
| ||
| Clinical Stage (%) | ||
| T1c | 197 (86) | |
| T2a | 28 (12) | |
| T2b | 4 (2) | |
| T3b | 1 (0.4) | |
|
| ||
| Gleason Score (%) | ||
| 6 | 94 (41) | |
| 7 | 136 (59) | |
| 9 | 1 (0.4) | |
|
| ||
| Mean PSA (Range) | 5.51 (0.101–67.00) ng/mL | |
|
| ||
| Mean Baseline EPIC Sexual Score Total (Standard Deviation) | ||
| Function | 50.9 (27.9) | |
| Bother | 72.8 (31.8) | |
| Summary | 57.5 (26.7) | |
|
| ||
| Hypertension (%) | ||
| Yes | 135(59) | |
| No | 95 (41) | |
|
| ||
| Tobacco Smoking (%) | ||
| Yes | 56 (25) | |
| No | 171 (75) | |
|
| ||
| Diabetes Mellitus (%) | ||
| Yes | 21 (9) | |
| No | 210 (91) | |
Preservation of sexual potency
For all respondents, 70% and 72% reported erections firm enough for sexual activity at 12 and 24 months post-treatment demonstrating a net change from baseline of −4% and −2% respectively. Potency was reported by 48% and 56% of respondents at 12 and 24 months representing a net change of −14% and −6% respectively. The change in potency over time for all patients is represented in Figure 1.
Figure 1.
Sexual potency for all respondents treated with prostate brachytherapy and twice weekly tadalafil. (A) Erection quality over time (B) Percentage of all patients reporting erections firm enough for sexual activity over time.
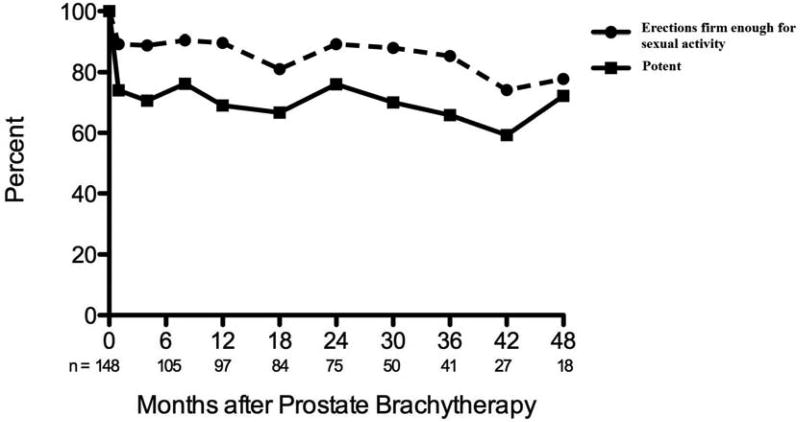
Men with potency at baseline were likely to preserve erections firm enough for sexual activity. Of the 148 men reporting erections firm enough for sexual activity at baseline, 90% and 89% reported preservation of this function at 12 and 24-months post-treatment, respectively. However, potency was observed to decline following therapy, as 31% and 24% experienced a loss of potency at 12 and 24 months following treatment. (Figure 2).
Figure 2.
Potency preservation following prostate brachytherapy and twice weekly tadalafil.
Sexual function and bother
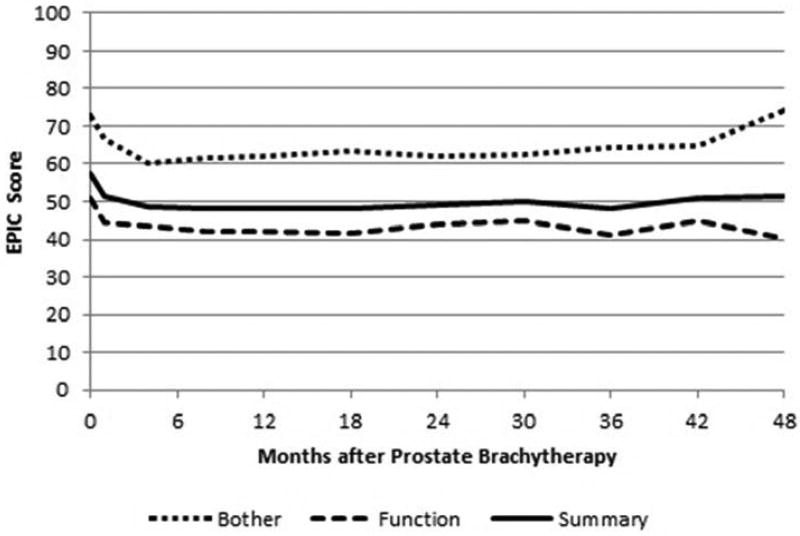
The mean ± standard deviation sexual summary, function, and bother scores at baseline were 57.5 ± 26.7, 50.9 ± 27.9, and 72.8 ± 31.8 respectively. There were statistically significant changes in sexual summary scores at each follow-up assessment (all patients). At 12 months the sexual summary change was −8.5 (p<0.0001), and at 24 months the sexual summary change was −9.4 (p<0.0001). None of the post-treatment changes were clinically significant. For the subset of potent patients at baseline, the change at 12 months was −6.3 (p=0.006) and the change at 24 months was −5.5 (p=0.013).
Statistically significant changes in sexual function were appreciated at each follow-up time point. The mean change in sexual function score at 12 and 24 month follow-up was −7.5 (p < 0.001) and −8.7 (p < 0.001). Neither of these changes was clinically significant. Statistically significant changes in sexual bother were also appreciated at each post-treatment assessment. The mean change in sexual bother score at 12 and 24 month follow-up was −10.5 (p < 0.001) and −11.1 (p < 0.0001). No clinically significant changes were appreciated at any time-point. Figure 3 illustrates the change in sexual summary, function, and bother scores over time.
Figure 3.
Expanded prostate cancer index composite sexual domain scores following prostate brachytherapy and twice weekly tadalafil.
Significantly more patients reported worse erection frequency, sexual function, distress from poor erections, and overall sexual distress at both 12-months and 24-months following prostate brachytherapy compared with baseline. More patients reported poor erection quality compared to baseline at the 12-month and 24-month follow-up; however this difference was statistically significant at 12-months only (p = 0.01 at 12-months; p = 0.26 at 24-months). There were no statistically significant differences in the percent of patients who expressed distress from an inability to reach orgasm at 12 or 24 months compared to baseline. Fifty-two percent and 47 percent of respondents reported continued use of tadalafil at 12 and 24 month follow up respectively. Table 2 summarizes the levels of sexual distress and dysfunction over time.
Table 2.
Percentage of patients reporting sexual dysfunction or distress after prostate brachytherapy and twice weekly tadalafil
| Months After Brachytherapy |
0 | 4 | 8 | 12 | 18 | 24 |
|---|---|---|---|---|---|---|
| Poor erection quality | 38 | 51 | 50 | 52* | 52 | 44 |
| Poor erection frequency | 34 | 49 | 51 | 55* | 58 | 54* |
| Poor sexual function | 30 | 42 | 44 | 46* | 47 | 42* |
| Distress from poor erections† | 22 | 34 | 32 | 35* | 37 | 35* |
| Distress from inability to reach orgasm† | 22 | 30 | 31 | 25 | 27 | 31 |
| Overall sexual distress† | 18 | 28 | 27 | 28* | 26 | 25* |
Responses were dichotomized on the basis of the response that the quality-of-life concern was “a moderate or big problem”
p <0.05 compared to baseline. Comparisons performed at 12 and 24 months only.
Discussion
To our knowledge, this study is the first ever prospective assessment of peri-procedural PDE5i use with radiation therapy using a validated instrument for measurement of sexual function and bother. In this study evaluating the application of low dose tadalafil as penile rehabilitation following prostate brachytherapy, no clinically significant differences in patient reported sexual function or bother were appreciated. The sexual function preservation was relatively high with approximately 90% of men with erections firm enough for any sexual activity at baseline maintaining this ability and 76% of men with potency at baseline experiencing no detrimental effect on their sexual potency 2-years after prostate brachytherapy. Despite statistically significant differences in sexual function at all time-periods post-treatment compared to baseline, the absolute change from baseline was relatively small and did not result in worse sexual bother two years post-treatment.
Sexual function and potency preservation using the study regimen is modestly superior to other contemporary brachytherapy series using the EPIC to record patient quality of life. The Prostate Cancer Outcomes and Satisfaction with Treatment Quality Assessment (PROSTQA) trial is a prospective, longitudinal, multi-center cohort of men with previously untreated localized prostate cancer who elected prostatectomy, external beam radiation therapy, or brachytherapy as primary treatment. Data were collected via telephone interview using the EPIC survey and companion utilization of sexual medications and devices. Sanda et al. reported the initial urinary, bowel, sexual and hormonal quality of life changes over time following prostate cancer treatment.(5) Alemozaffer et al. recently published an additive secondary analysis through development of a multivariate model to predict ED after prostate cancer treatment using the EPIC data from PROSTQA with external validation using patients from the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) database.(27,28) In the patients from PROSTQA treated with prostate brachytherapy (n = 247), 43% reported the ability to attain functional erections suitable for intercourse at 2-years; this compares to 56% in the current study using low-dose tadalafil.
Our study is unique from prior series in that patients who used sexual medications or devices at baseline were excluded. The use of PDE5is is common in the demographic of men diagnosed with prostate cancer.(28,29) For example, 31% of patients treated with prostate brachytherapy in PROSTQA reported use of sexual medications or devices at baseline. Initiation of PDE5is after radiation treatment for prostate cancer is also common and highly effective.(30–35) In PROSTQA, 47% of men treated with prostate brachytherapy used sexual medications or devices 24-months following treatment with a higher incidence (54%) of use in men who reported optimal potency at baseline. PDE5is were the most common intervention for erectile dysfunction; with intra-urethral alprostadil, penile injections, vacuum erection devices, and penile prostheses used less frequently. As a result, one might make the argument that comparing the current study to PROSTQA represents a comparison of early versus delayed intervention. Interestingly, while the incidence of sexual medication and device use over time increased in PROSTQA, almost half of the men treated on the current study discontinued supportive measures over time. This finding suggests early intervention with PDE5is may reduce the need for erectile dysfunction support long-term.
Our study has several limitations including the following: 1) The optimal methodology for assessing a treatment regimen such as this is a randomized, placebo controlled trial. Although we entertain a comparison of our results to the PROSTQA study as a historical control, the treatment period of the current cohort occurred predominantly prior to reporting of PROSTQA. Therefore, our analysis was neither designed nor powered for direct comparison to PROSTQA. Furthermore, the absence of a control group invalidates our ability to adjust for an increasing prevalence of ED with age. Considering the complexity of sexual function and satisfaction, our results are likely influenced by extrinsic and/or intrinsic variables best controlled through randomization. Our results should be interpreted cautiously as they are likely influenced by differences between patient groups and selection bias. Thus, these results should be considered hypotheses generating as opposed to conclusive. 2) Although sexual function and bother appear to stabilize after 24-months post treatment, the median follow-up of the current study is relatively short when considering fibrotic effect post-radiation, which can occur several years following therapy. Longer follow-up of this cohort is desirable. 3) The dosing and schedule of tadalafil for this study was determined based upon the concept of promoting spontaneous erections at least twice per week. A conservative dose of 10 mg twice weekly was chosen to minimize cross-reactivity with other commonly prescribed medications following prostate brachytherapy (ie. selective alpha-blockers). While conventional dosing of daily tadalafil (5 mg) has been shown to prevent corporal fibrosis and veno-occlusive dysfunction following cavernousal nerve resection, it is unclear whether the same physiologic response occurs with the dose/schedule used in our cohort. The Radiation Therapy Oncology Group (RTOG) recently completed accrual of a phase III randomized, placebo controlled trial comparing daily tadalafil (5 mg) versus placebo in men treated with radiation therapy for prostate cancer. The results of this trial are pending and should clarify the role of PDE5is as penile rehabilitation after radiation therapy.
In conclusion, men with prostate cancer treated with prostate brachytherapy and low-dose tadalafil twice weekly were highly likely to maintain their sexual potency. Early treatment with PDE5is may reduce the need for subsequent therapeutic use of sexual medications and devices after prostate brachytherapy. The optimal method for penile rehabilitation after radiation treatment has yet to be defined and requires further investigation.
Acknowledgments
This work was supported in part by the Cancer Center Support Grant (NCI Grant P30 CA016672).
LIST OF ABBREVIATIONS
- LDR
low dose rate
- ED
erectile dysfunction
- HRQOL
health related quality of life
- PSA
prostate specific antigen
- PDE5i
5-phosphodiesterase inhibitors
- Gy
Gray
- EPIC
expanded prostate index composite
- RTOG
Radiation Therapy Oncology Group
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: none
References
- 1.Crook J, Borg J, Evans A, Toi A, Saibishkumar EP, Fung S, et al. 10-year experience with I-125 prostate brachytherapy at the Princess Margaret Hospital: results for 1,100 patients. Int. J. Radiat. Oncol. Biol. Phys. 2011 Aug 1;80(5):1323–9. doi: 10.1016/j.ijrobp.2010.04.038. [DOI] [PubMed] [Google Scholar]
- 2.Blasko JC, Mate T, Sylvester JE, Grimm PD, Cavanagh W. Brachytherapy for carcinoma of the prostate: techniques, patient selection, and clinical outcomes. Semin Radiat Oncol. 2002 Jan;12(1):81–94. doi: 10.1053/srao.2002.28667. [DOI] [PubMed] [Google Scholar]
- 3.Potters L, Morgenstern C, Calugaru E, Fearn P, Jassal A, Presser J, et al. 12-year outcomes following permanent prostate brachytherapy in patients with clinically localized prostate cancer. J. Urol. 2008 May;179(5 Suppl):S20–24. doi: 10.1016/j.juro.2008.03.133. [DOI] [PubMed] [Google Scholar]
- 4.Frank SJ, Pisters LL, Davis J, Lee AK, Bassett R, Kuban DA. An assessment of quality of life following radical prostatectomy, high dose external beam radiation therapy and brachytherapy iodine implantation as monotherapies for localized prostate cancer. J. Urol. 2007 Jun;177(6):2151–2156. doi: 10.1016/j.juro.2007.01.134. discussion 2156. [DOI] [PubMed] [Google Scholar]
- 5.Sanda MG, Dunn RL, Michalski J, Sandler HM, Northouse L, Hembroff L, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N. Engl. J. Med. 2008 Mar 20;358(12):1250–61. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 6.Litwin MS, Gore JL, Kwan L, Brandeis JM, Lee SP, Withers HR, et al. Quality of life after surgery, external beam irradiation, or brachytherapy for early-stage prostate cancer. Cancer. 2007 Jun 1;109(11):2239–47. doi: 10.1002/cncr.22676. [DOI] [PubMed] [Google Scholar]
- 7.Gore JL, Kwan L, Lee SP, Reiter RE, Litwin MS. Survivorship beyond convalescence: 48-month quality-of-life outcomes after treatment for localized prostate cancer. J. Natl. Cancer Inst. 2009 Jun 16;101(12):888–92. doi: 10.1093/jnci/djp114. [DOI] [PubMed] [Google Scholar]
- 8.Incrocci L, Slob AK, Levendag PC. Sexual (dys)function after radiotherapy for prostate cancer: a review. Int. J. Radiat. Oncol. Biol. Phys. 2002 Mar 1;52(3):681–93. doi: 10.1016/s0360-3016(01)02727-4. [DOI] [PubMed] [Google Scholar]
- 9.McCullough AR, Hellstrom WG, Wang R, Lepor H, Wagner KR, Engel JD. Recovery of erectile function after nerve sparing radical prostatectomy and penile rehabilitation with nightly intraurethral alprostadil versus sildenafil citrate. J. Urol. 2010 Jun;183(6):2451–6. doi: 10.1016/j.juro.2010.01.062. [DOI] [PubMed] [Google Scholar]
- 10.Mulhall J, Land S, Parker M, Waters WB, Flanigan RC. The use of an erectogenic pharmacotherapy regimen following radical prostatectomy improves recovery of spontaneous erectile function. J Sex Med. 2005 Jul;2(4):532–540. doi: 10.1111/j.1743-6109.2005.00081_1.x. discussion 540–542. [DOI] [PubMed] [Google Scholar]
- 11.Montorsi F, Guazzoni G, Strambi LF, Da Pozzo LF, Nava L, Barbieri L, et al. Recovery of spontaneous erectile function after nerve-sparing radical retropubic prostatectomy with and without early intracavernous injections of alprostadil: results of a prospective, randomized trial. J. Urol. 1997 Oct;158(4):1408–10. [PubMed] [Google Scholar]
- 12.Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol. Clin. North Am. 2005 Nov;32(4):379–395. doi: 10.1016/j.ucl.2005.08.007. v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zelefsky MJ, Eid JF. Elucidating the etiology of erectile dysfunction after definitive therapy for prostatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 1998 Jan 1;40(1):129–33. doi: 10.1016/s0360-3016(97)00554-3. [DOI] [PubMed] [Google Scholar]
- 14.Mulhall J, Ahmed A, Parker M, Mohideen N. The hemodynamics of erectile dysfunction following external beam radiation for prostate cancer. J Sex Med. 2005 May;2(3):432–7. doi: 10.1111/j.1743-6109.2005.20362.x. [DOI] [PubMed] [Google Scholar]
- 15.Fajardo LF. The pathology of ionizing radiation as defined by morphologic patterns. Acta Oncol. 2005;44(1):13–22. doi: 10.1080/02841860510007440. [DOI] [PubMed] [Google Scholar]
- 16.Müller A, Tal R, Donohue JF, Akin-Olugbade Y, Kobylarz K, Paduch D, et al. The effect of hyperbaric oxygen therapy on erectile function recovery in a rat cavernous nerve injury model. J Sex Med. 2008 Mar;5(3):562–70. doi: 10.1111/j.1743-6109.2007.00727.x. [DOI] [PubMed] [Google Scholar]
- 17.Moreland RB, Traish A, McMillin MA, Smith B, Goldstein I, Saenz de Tejada I. PGE1 suppresses the induction of collagen synthesis by transforming growth factor-beta 1 in human corpus cavernosum smooth muscle. J. Urol. 1995 Mar;153(3 Pt 1):826–34. [PubMed] [Google Scholar]
- 18.Westbury CB, Yarnold JR. Radiation fibrosis - current clinical and therapeutic perspectives. Clin Oncol (R Coll Radiol) 2012 Dec;24(10):657–72. doi: 10.1016/j.clon.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Lysiak JJ, Yang S-K, Klausner AP, Son H, Tuttle JB, Steers WD. Tadalafil increases Akt and extracellular signal-regulated kinase 1/2 activation, and prevents apoptotic cell death in the penis following denervation. J. Urol. 2008 Feb;179(2):779–85. doi: 10.1016/j.juro.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 20.Ferrini MG, Davila HH, Kovanecz I, Sanchez SP, Gonzalez-Cadavid NF, Rajfer J. Vardenafil prevents fibrosis and loss of corporal smooth muscle that occurs after bilateral cavernosal nerve resection in the rat. Urology. 2006 Aug;68(2):429–35. doi: 10.1016/j.urology.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 21.De Young LX, Domes T, Lim K, Carson J, Brock GB. Endothelial rehabilitation: the impact of chronic PDE5 inhibitors on erectile function and protein alterations in cavernous tissue of diabetic rats. Eur. Urol. 2008 Jul;54(1):213–20. doi: 10.1016/j.eururo.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 22.Kovanecz I, Rambhatla A, Ferrini MG, Vernet D, Sanchez S, Rajfer J, et al. Chronic daily tadalafil prevents the corporal fibrosis and veno-occlusive dysfunction that occurs after cavernosal nerve resection. BJU Int. 2008 Jan;101(2):203–10. doi: 10.1111/j.1464-410X.2007.07223.x. [DOI] [PubMed] [Google Scholar]
- 23.Mulhall JP, Müller A, Donohue JF, Mullerad M, Kobylarz K, Paduch DA, et al. The functional and structural consequences of cavernous nerve injury are ameliorated by sildenafil citrate. J Sex Med. 2008 May;5(5):1126–36. doi: 10.1111/j.1743-6109.2008.00794.x. [DOI] [PubMed] [Google Scholar]
- 24.Schiff JD, Bar-Chama N, Cesaretti J, Stock R. Early use of a phosphodiesterase inhibitor after brachytherapy restores and preserves erectile function. BJU Int. 2006 Dec;98(6):1255–8. doi: 10.1111/j.1464-410X.2006.06441.x. [DOI] [PubMed] [Google Scholar]
- 25.Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000 Dec 20;56(6):899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 26.Cohen J. Statistical Power Ananlysis for Behavioral Sciences. New York, NY: Academic Press; 1977. [Google Scholar]
- 27.Lubeck DP, Litwin MS, Henning JM, Stier DM, Mazonson P, Fisk R, et al. The CaPSURE database: a methodology for clinical practice and research in prostate cancer. CaPSURE Research Panel. Cancer of the Prostate Strategic Urologic Research Endeavor. Urology. 1996 Nov;48(5):773–7. doi: 10.1016/s0090-4295(96)00226-9. [DOI] [PubMed] [Google Scholar]
- 28.Alemozaffar M, Regan MM, Cooperberg MR, Wei JT, Michalski JM, Sandler HM, et al. Prediction of erectile function following treatment for prostate cancer. JAMA. 2011 Sep 21;306(11):1205–14. doi: 10.1001/jama.2011.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whaley JT, Levy LB, Swanson DA, Pugh TJ, Kudchadker RJ, Bruno TL, et al. Sexual function and the use of medical devices or drugs to optimize potency after prostate brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2012 Apr 1;82(5):e765–771. doi: 10.1016/j.ijrobp.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kedia S, Zippe CD, Agarwal A, Nelson DR, Lakin MM. Treatment of erectile dysfunction with sildenafil citrate (Viagra) after radiation therapy for prostate cancer. Urology. 1999 Aug;54(2):308–12. doi: 10.1016/s0090-4295(99)00146-6. [DOI] [PubMed] [Google Scholar]
- 31.Weber DC, Bieri S, Kurtz JM, Miralbell R. Prospective pilot study of sildenafil for treatment of postradiotherapy erectile dysfunction in patients with prostate cancer. J. Clin. Oncol. 1999 Nov;17(11):3444–9. doi: 10.1200/JCO.1999.17.11.3444. [DOI] [PubMed] [Google Scholar]
- 32.Zelefsky MJ, McKee AB, Lee H, Leibel SA. Efficacy of oral sildenafil in patients with erectile dysfunction after radiotherapy for carcinoma of the prostate. Urology. 1999 Apr;53(4):775–8. doi: 10.1016/s0090-4295(98)00594-9. [DOI] [PubMed] [Google Scholar]
- 33.Merrick GS, Butler WM, Lief JH, Stipetich RL, Abel LJ, Dorsey AT. Efficacy of sildenafil citrate in prostate brachytherapy patients with erectile dysfunction. Urology. 1999 Jun;53(6):1112–6. doi: 10.1016/s0090-4295(99)00048-5. [DOI] [PubMed] [Google Scholar]
- 34.Valicenti RK, Choi E, Chen C, Lu JD, Hirsch IH, Mulholland GS, et al. Sildenafil citrate effectively reverses sexual dysfunction induced by three-dimensional conformal radiation therapy. Urology. 2001 Apr;57(4):769–73. doi: 10.1016/s0090-4295(00)01104-3. [DOI] [PubMed] [Google Scholar]
- 35.Incrocci L, Hop WCJ, Slob AK. Efficacy of sildenafil in an open-label study as a continuation of a double-blind study in the treatment of erectile dysfunction after radiotherapy for prostate cancer. Urology. 2003 Jul;62(1):116–20. doi: 10.1016/s0090-4295(03)00133-x. [DOI] [PubMed] [Google Scholar]