Abstract
Projected increases in ocean pCO2 levels are anticipated to affect calcifying organisms more rapidly and to a greater extent than other marine organisms. The effects of ocean acidification (OA) have been documented in numerous species of corals in laboratory studies, largely tested using flow-through exposure systems. We developed a recirculating ocean acidification exposure system that allows precise pCO2 control using a combination of off-gassing measures including aeration, water retention devices, venturi injectors, and CO2 scrubbing. We evaluated the recirculating system performance in off-gassing effectiveness and maintenance of target pCO2 levels over an 84-day experiment. The system was used to identify changes in calcification and tissue growth in response to elevated pCO2 (1000 μatm) in three reef-building corals of the Caribbean: Pseudodiploria clivosa, Montastraea cavernosa, and Orbicella faveolata. All three species displayed an overall increase in net calcification over the 84-day exposure period regardless of pCO2 level (control +0.28- 1.12 g, elevated pCO2 +0.18- 1.16 g), and the system was effective at both off-gassing acidified water to ambient pCO2 levels, and maintaining target elevated pCO2 levels over the 3-month experiment.
Keywords: Recirculating system, Ocean acidification, Caribbean coral, Elevated pCO2, Calcification
1 Introduction
Over the past two decades, there have been numerous studies examining the effects of ocean acidification (OA), a term used to describe the reduction of seawater pH in response to oceanic absorption of atmospheric carbon dioxide (CO2) from anthropogenic sources (Caldeira and Wickett, 2003). The general consensus is that OA has detrimental effects on calcifying organisms; the decrease in seawater pH reduces aragonite saturation (Ωarag) which causes dissolution of calcium carbonate (CaCO3) skeletons. The majority of studies have accomplished this research utilizing a flow-through water design, and those studies which utilize a recirculating or static system have produced differing results. Additionally, differences such as methodology of generating projected pCO2 levels and length of exposure can seemingly all have effects on the experimental outcome.
The majority of ocean acidification studies have utilized a flow-through water regime, whereby acidified treatment water is continuously created and replaced, eliminating any issue of compounding acidification effects. Few studies have used a static or recirculating exposure system, due to challenges such as frequent water changes, organismal fouling, and how to effectively restore pCO2 to ambient levels in recirculated waters. Studies that have measured coral calcification rates in response to elevated pCO2 without a flow-through water regime include the Biosphere 2 coral reef biome (recirculating system with water volume of 2650-m3; Langdon et al., 2003), 8-L static mesocosms requiring frequent water changes (Renegar and Riegl, 2005), and an experimental system consisting of three, independent recirculating aquaria (Lunden et al., 2014). Interestingly, the experimental design of a study can seemingly influence the observed response to acidification. Langdon and colleagues (2003) explored the effects of elevated pCO2 for 30- 60 d in Biosphere 2 and reported a detrimental effect in multiple species including Acropora cervicornis, several Porites species, and Pocillopora damicornis. The 16-week exposure of A. cervicornis by Renegar and Riegl (2005) in a closed system indicated increased pCO2 had a negative effect on coral growth. However, other studies have shown that Porites spp, and Pocillopora damicornis are minimally sensitive to OA in flow-through systems (Comeau et al., 2013; Edmunds 2011), and an experiment using a flow-through regime on Acropora cervicornis indicated this species could maintain calcification rates under elevated pCO2 if provided proper supplemental nutrition (Towle et al., 2015). Kurman et al. (2017) demonstrated a net decline in calcification in the cold water coral Lophelia pertusa over a 6-month exposure using a recirculating water regime. However, a 6-month exposure of L. pertusa using a flow-through system showed acclimation to elevated pCO2 and a net calcification increase (Form and Riebesell, 2012).
Laboratory studies that simulate projected pCO2 levels have used two primary methods to acidify treatment waters, both of which have direct implications on carbonate chemistry. First, a strong acid can be added in order to decrease pH levels, which changes total alkalinity (TA) while keeping dissolved inorganic carbon (DIC) levels constant. Alternatively, pure CO2 or a mixture of CO2 and air can be bubbled into treatment waters, keeping TA constant and increasing DIC concentration. Studies have shown different carbonate chemistry results depending on the methodology used, most notably that adding an acid generated pH values well below those anticipated in the year 2100 (Gattuso and Lavigne, 2009). Two studies exposing the coral Stylophora pistillata to increased pCO2 levels showed differing results depending on whether an acid or CO2 was used to acidify treatment waters. Marubini and colleagues (2008) used a strong acid and found a marked decline in calcification rate; however, Reynaud et al. (2003) found no discernable change in calcification rate when using carbon dioxide to acidify treatment water. Bubbling CO2 or a blend of CO2 and atmospheric air is the preferred method in most OA studies as it more accurately mimics anticipated changes due to anthropogenic ocean acidification, and is easily used over long (>30 d) experimental time periods (Atkinson and Cuet, 2008; Gattuso and Lavigne, 2009).
Finally, the length of time an organism is exposed to acidified waters can influence measured calcification rates. Calcification rates of the coral S. pistillata were shown to decline more in a 2.5 h exposure to elevated pCO2 by Gattuso et al. (1998) when compared to a 5-week exposure by Reynaud and colleagues (2003), and Porites compressa calcification rates were two times higher in a 10-week study compared to a 1.5 h exposure (Langdon and Atkinson, 2005; Marubini et al., 2001). More recently, a 95 d study on Siderastrea siderea to extreme pCO2 elevations (2553 μatm) showed an increase in calcification rate up to 60 d, followed by a decline between 61 and 95 d (Castillo et al., 2014).
The purpose of the current study was to develop and test an experimental ocean acidification exposure system that could accurately and precisely create projected pCO2 levels using CO2 and air to acidify treatment water, utilize a recirculating water flow regime, and have the capability of running longer-term experiments (30 d or more). The U.S. Environmental Protection Agency (EPA) Coral Research Laboratory at Gulf Ecology Division (GED) resides on a barrier island; yet, the sound-side location of the facility makes pumping water directly from the Gulf of Mexico impractical. Therefore, all coral cultures at the laboratory function as indoor recirculating systems. As in nature, the culture systems experience drastic changes in pH and pCO2 levels over the course of the day due to photosynthesis and respiration (Manzello et al., 2012; Yates et al., 2007). To complete our experiments, we needed to address this fluctuation in the exposure system, as well as effectively off-gassing treated water before it was returned back into the system. Here, we describe a recirculating OA exposure system that has the ability to maintain projected pCO2 levels indefinitely. A recirculating system capable of off-gassing acidified water will allow other laboratories with limited or no flow-through water capability the opportunity to conduct acidification experiments without building independent treatment systems, as well as eliminate some of the challenges of a static system or microcosm (such as frequent water changes, organismal fouling, and alterations to carbonate chemistry from nutrient build-up). The recirculating system was used to investigate the calcification response of three scleractinian corals species found in the Caribbean Sea and Gulf of Mexico to projected pCO2 levels using the Intergovernmental Panel on Climate Change A1F1 scenario (1000 μatm; IPCC, 2007) over an 84 d exposure period.
2 Materials and methods
2.1 Exposure system
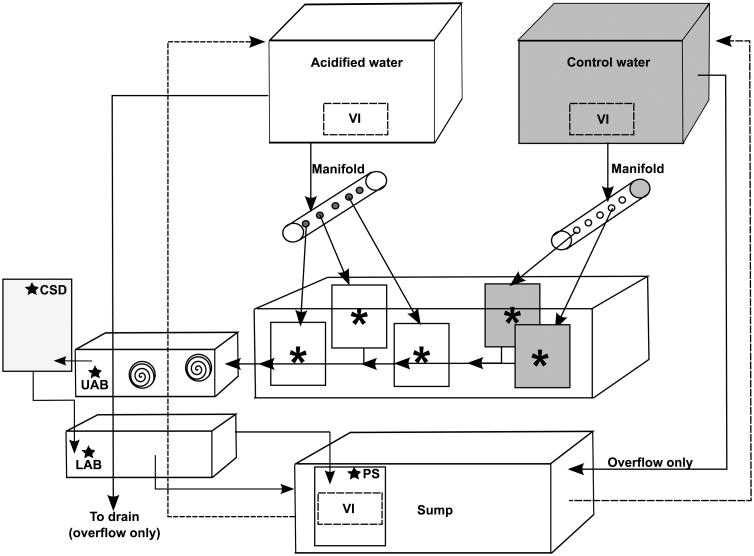
The exposure system consisted of three levels; headboxes which gravity-feed the system, a top water table and a bottom sump (Fig. 1). The fiberglass sump (58.5”L × 30”W; approximately 375-L) maintained water quality through biological filtration (invertebrates, live rock and sand), mechanical filtration (in-line canister with 1 μm filter and ETSS-600 series protein skimmer), and chemical filtration (locally built calcium reactor filled with CaribSea Oolite® aragonite media and a Two Little Fishes PhosBan reactor® filled with a PhosBan media and activated charcoal mixture). Temperature in the sump was maintained within 1.5 °C of target levels by an Aqualogic 1/3 hp chiller (Model CY-4) and a titanium heater (GLO-Quartz model # SLTX-11010R19-FB), controlled by an Aqua Logic NEMA 4× thermostat. An Iwasaki 400 W, 6500 Kelvin metal halide light illuminated the sump on a 10.5/13.5 light/dark cycle. The top portion of the system consisted of a water table (77”L × 41”W × 10.5”H, approximately 520-L) used as a temperature control bath for experimental chambers. Two Iwasaki 400 W, 6500 Kelvin metal halide lights set on a 9.5/14.5 light/dark cycle illuminated the water table.
Fig. 1.
Diagram of the recirculating OA exposure system. Arrows indicate direction of water flow with dashed lines indicating return water from the sump to each headbox. Asterisks denote experimental chambers (n=5 for control and n=5 for elevated pCO2 chambers, only five chambers shown), and spiraled circles denote CO2 scrubbers. UAB: Upper acrylic box housing CO2 scrubbers, LAB: lower acrylic box, CSD: Carlson surge devise, VI: Venturi injector, PS: Protein skimmer. Control water headbox and chambers indicted by grey shading, and a star denotes an off-gassing measure of the system.
2.2 Water quality
Temperature and salinity of the system was determined by a handheld meter (YSI-Pro2030 meter), and recorded twice a day. Temperature was maintained at approximately 26 °C by controlling the temperature of the sump and water bath (described above). Salinity was maintained at approximately 35 by twice-daily additions of deionized (DI) water. Over the course of the 84 d experiment, corals were fed twice a week, during which water flow to the system was shut off for 15 min to allow adequate feeding time. Diet consisted of freeze dried food (Golden Pearls) in three size ranges (5-50, 50-100, and 100-200 μm), and feeding responses (polyps and tentacles extended) were noted in all species. Water changes using Gulf of Mexico water (25% of the sump volume) were performed monthly as preventative maintenance against fouling. To accomplish this, water (salinity of at least 30) was collected 2-3 miles off-shore in the Gulf of Mexico, filtered (1 μm), and transferred to a large reservoir (approximately 2000-L). Water in the reservoir was run through an 80 W UV sterilizer (Aqua Ultraviolet, Model #265749) once a week to eliminate bacteria and microalgae, and aerated continuously to maintain pH levels between 8.0 and 8.1. The reservoir water was allowed to evaporate in order to increase salinity to 35, and was checked for salinity at least once per month. Water quality testing for the presence of calcium and magnesium was performed once a week, and tests for nitrogenous wastes, and inorganic phosphates were performed bi-weekly.
2.3 Water flow and CO2 off-gassing
Water was pumped from the system sump to two 712-L headboxes (labeled acidified water and control water, Fig. 1) by Iwaki 20RZT pumps. These headboxes were used as mixing reservoirs for generating pCO2 levels during experimentation. Each headbox had a PVC standpipe to prevent water over-flow, which drained either directly into the main sump (control water) or down a waste drain (acidified water). Water flow calibrations on experimental chambers and headboxes were performed every other day to ensure that the amount of water coming from the sump was approximately equal to the amount of water returning to the sump. Water was gravity-fed from each headbox to their respective manifold, which in turn supplied acidified or control treatment water to experimental chambers (flow rate of 500 mL/min; Fig. 1). Chamber water drained into two troughs, which then drained into an aerated upper acrylic box housing algal CO2 scrubbers (described below). Water in the upper acrylic box drained into a locally built 30-L Carlson surge device, and then into a lower acrylic box, followed by a compartment in the sump where the intake to the protein skimmer was located (Fig. 1). The sump compartment contained a Danner model 5 powerhead that was equipped with a venturi injector that supplied air via a GAST air compressor pump (Model DOA-P704-AA, GAST Manufacturing Corp.). Acidified treatment water was off-gassed prior to reaching the main body/area of the sump through the following series of steps: a) photosynthesis of Chaetomorpha (small batches of algae which were housed in the upper acrylic box illuminated with a LED light on a 20:4 h light/dark cycle) that served as CO2 scrubbers to reduce dissolved carbon dioxide, b) air stones in the Carlson surge device and acrylic boxes, c) intense aeration of sump compartment, and d) additional bubbling through protein skimmer.
2.4 Carbonate chemistry
We used a modified pCO2 generation system first described by Fangue et al. (2010), and adapted for large-scale use to create desired pCO2 treatment levels (control ∼500 μatm, elevated ∼1000 μatm). Atmospheric air was pumped through a chilled condensing coil, and then fed through columns filled with Drierite® and Sodasorb® to strip out moisture and CO2, respectively. The dry, CO2-free air was then blended with pure CO2 using Mass Flow Controllers (MFC's, Aalborg Instruments) and infused into the acidified water headbox using a venturi injector. Atmospheric air with no CO2 gas addition was metered using a MFC and bubbled into the control water headbox using a venturi injector.
Water samples were collected from two control chambers, two elevated pCO2 chambers, the two headboxes, and the sump every other day at the same time (approximately 8:00am). Once a week, all 10 chambers were sampled on the same day to ensure there was minimal variation between chambers, averaging to each treatment being sampled ∼10× per week. All samples were immediately analyzed for pH (total scale) using the m-cresol purple spectrophotometric method (SOP 6b, Dickson et al., 2007; Shimadzu UV-1700) and total alkalinity using open-celled titration (Metrohm Titrando 905; SOP 3b, Dickson et al., 2007). Titrations were verified by using Certified Reference Materials provided by Andrew Dickson (Scripps Institution of Oceanography). Salinity and barometric pressure were recorded using a YSI-2030 meter and temperature was recorded using a Fluke Thermocouple (Model 51-2B). This information was entered into the program CO2Calc using CO2 constants from Mehrbach (1973), re-fit by Dickson and Millero (1987) in order to calculate pCO2 levels (Robbins et al., 2010). The MFC's were adjusted minimally in order to maintain desired pCO2 levels based upon chemistry results.
2.5 Corals
Fragments of Montastraea cavernosa (Linnaeus, 1766), Orbicella faveolata (Ellis and Solander, 1786), and Pseudodiploria clivosa (Ellis and Solander, 1786) were cut (2 cm2 pieces; n = 20 per species, total of 60 fragments) from 3 to 4 different parent colonies that were initially field-collected, but then maintained in culture systems for at least one year, glued onto acrylic bases and randomly assigned to a control or elevated pCO2 chamber (8-L plastic container with a Hydor koralia pump [model 240] to recirculate water). Two fragments of each species (total of 6 fragments per chamber) were allowed to acclimate in each of the 10 experimental chambers with control seawater recirculating through the system for one week. After this acclimation period, corals were photographed to measure tissue surface area (Fournie et al., 2012) and weighed using a Mettler Toledo PM400 balance using the buoyant weight technique (BWT, Jokiel et al., 1978) to measure calcification, after which acidified water (1000 μatm) flow was turned on to elevated pCO2 chambers (n = 5). Corals were photographed and re-weighed every 30 d until the end of the experiment.
2.6 Calculations and statistics
In an effort to determine how corals were depositing CaCO3 (i.e., if they were extending their skeleton or increasing the density of their current skeleton), percent change in surface area density (SAD) was calculated from measurements of buoyant weight and tissue surface area at each experimental time point using the following equations:
Where: SAD: Surface Area Density (g/mm2)
BWT: Buoyant Weight (g)
TSA: Tissue Surface Area (mm2)
A two-way repeated measures ANOVA was used to investigate the changes in calcification rate, tissue growth rate, and percent change in surface area density seen in M. cavernosa, O. faveolata, and P. clivosa. Exposure period (0-28, 28-56, and 56-84 d) and pCO2 level (∼500 μatm and ∼1000 μatm) were used as main factors for all statistical tests.
3 Results
3.1 Exposure system
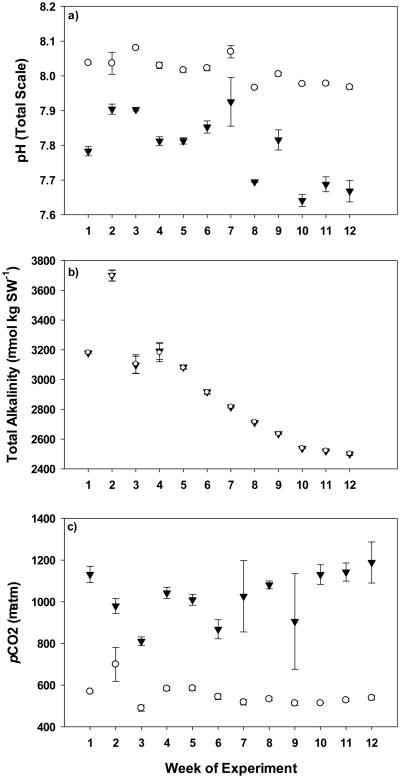
Overall, target control (∼500 μatm) and elevated (∼1000 μatm) pCO2 levels were maintained over the course of the 12-week experiment with minimal variability, with averages of 561 ± 9.44 μatm across control chambers and 1085 ± 27.5 μatm across elevated pCO2 chambers (Fig. 2c). Temperature and salinity were maintained at target levels of 25.76 ± 0.23°C and 35.0 ± 0.06, respectively (Table 1). No discernable levels of nutrients or nitrogenous wastes were found over the course of the experiment (Table 1). Pilot studies indicated that with our experimental system, all 4 off-gassing measures (CO2 scrubbers, intense aeration of both the sump and water retention systems and bubbling through the protein skimmer; Fig. 1) were needed in order to completely de-gas acidified treatment waters to control levels (data not shown). The overall decline in total alkalinity corresponds with a decrease in pH in both control and elevated pCO2 treatments (Fig. 2a and b) illustrating off-gassing effectiveness is dependent upon the degree of acidity of treatment waters.
Fig. 2.
pH (total scale; panel a), total alkalinity (μmol/kg SW; panel b) and pCO2 (μatm; panel c) measurements made over the course of the experiment broken down by week of exposure. Open circles symbolize the average control pCO2 chambers ±SE, and black triangles symbolize the average elevated pCO2 chambers ±SE.
Table 1. Target and average measured values ±SD of temperature (°C), salinity (ppt), Calcium (Ca2+; ppm), Magnesium (Mg; ppm), Phosphates (PO4; ppm), ammonia (NH3; ppm) and Nitrates (NO3; ppm) over the course of the experiment.
| Parameter | Target | Measured |
|---|---|---|
| Temperature (°C) | 26 | 25.8 ± 0.2 |
| Salinity (ppt) | 35.0 | 35.0 ± 0.06 |
| Ca2+ (ppm) | 400-500 | 460.4 ± 18.2 |
| Mg (ppm) | 1250-1500 | 1332.7 ± 43.2 |
| PO4 (ppm) | <0.05 | 0.02 ± 0.02 |
| NH3 (ppm) | <0.1 | 0.0 ± 0.0 |
| NO3 (ppm) | <0.2 | 0.0 ± 0.0 |
3.2 Coral calcification, tissue growth and SAD
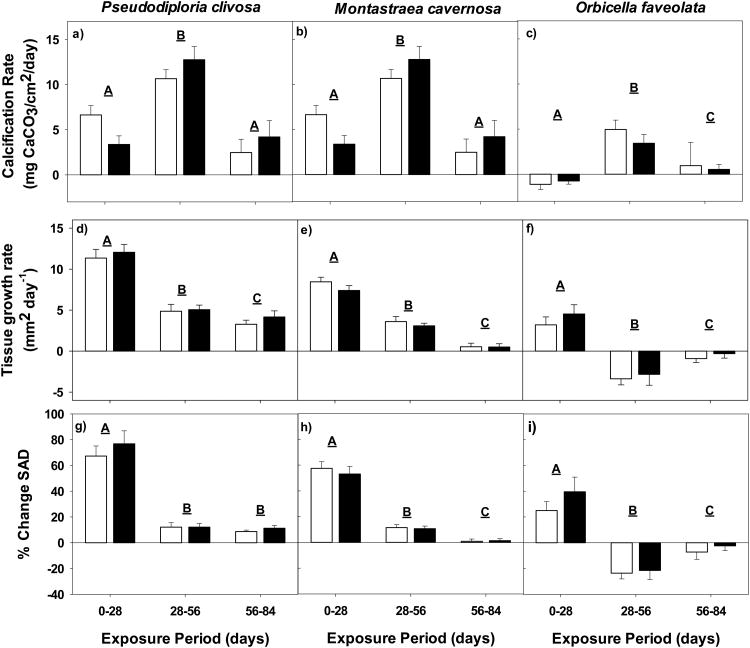
A significant main effect of exposure time with no apparent effect of pCO2 treatment level was found in Pseudodiploria clivosa in regards to calcification rate, tissue growth rate, and percent change in surface area density (Fig. 3a, d and g). Net calcification, tissue surface area and surface area density all increased over the course of the experiment (Fig. 4a-d; Table 2); however tissue growth rates were highest during the first exposure period between 0 and 28 d in both control (11.34 mm2/day) and elevated pCO2 fragments (12.05 mm2/day), slowing over the course of the experiment (Fig. 3d). Calcification rates increased dramatically between the first and second exposure periods, slowing to rates of 2.48 mg CaCO3/cm2/day in control fragments and 4.20 mg CaCO3/cm2/day in elevated pCO2 fragments in the last exposure period (Fig. 3a). The percent change in surface area density declined from 69.5 in control fragments and 77.8 in elevated pCO2 fragments to 14.4 and 14.5 respectively between the first and second exposure periods. The change remained steady through the final 30 d of the experiment, indicating calcification was directed towards skeletal extension rather than increased density (Fig. 3g).
Fig. 3.
Average calcification rate (mg CaCO3/cm2/day), tissue growth rate (mm2/day) and percent change in surface area density of Pseudodiploria clivosa (panels a, d and g, respectively), Montastraea cavernosa (panels b, e, and h, respectively), and Orbicella faveolata (panels c, f and i, respectively) exposed to control pCO2 (white bars; ∼500 μatm), or elevated pCO2 (black bars; ∼1000 μatm) for periods of 0-28, 28-56, and 56-84 days. All errors are reported as average ± SE, with letters indicating significance of each exposure period.
Fig. 4.
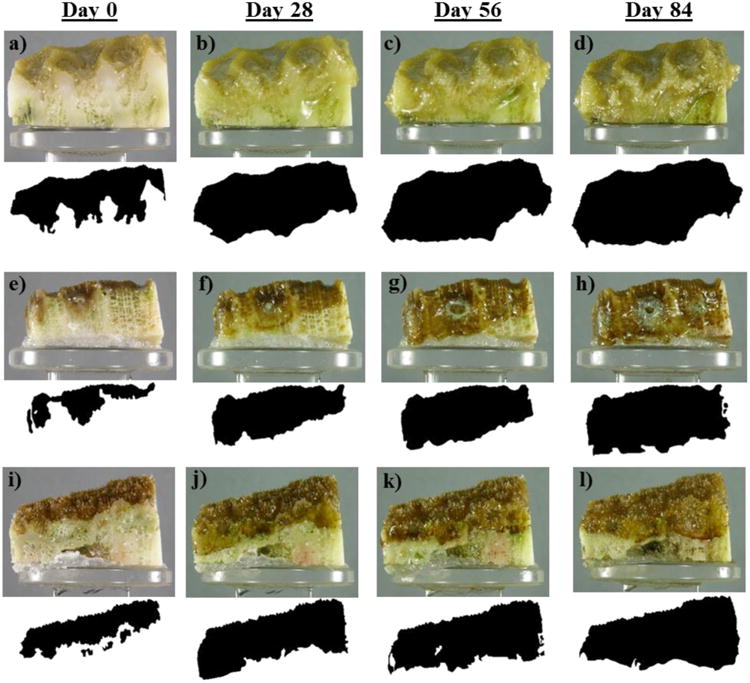
Photographs and masks of tissue growth (beneath each photograph) taken at 0, 28, 56 and 84-days of exposure to elevated pCO2 for Pseudodiploria clivosa (a-d), Montastraea cavernosa (c-h), and Orbicella faveolata (i-l).
Table 2.
Average values for Buoyant Weight (g) Tissue Surface Area (mm2) and Surface Area Density (mm2/g) for Pseudodiploria clivosa, Montastraea cavernosa, and Orbicella faveolata exposed to either control (C) or elevated pCO2 (E; ∼1000μatm) levels for 0, 28, 56, and 84-days.
| Species | 0-day | 28-day | 56-day | 84-day | |
|---|---|---|---|---|---|
| Pseudodiploria clivosa | Buoyant Weight (g) | C: 11.01 ± 0.72 E: 11.32 ± 0.58 |
C: 11.39 ± 0.73 E: 11.51 ± 0.59 |
C: 11.98 ± 0.73 E: 12.23 ± 0.65 |
C: 12.13 ± 0.77 E: 12.48 ± 0.62 |
| Tissue Surface Area (mm2) | C: 455.40 ± 36.67 E: 448.13 ± 32.53 |
C: 773.19 ± 53.84 E: 785.64 ± 44.22 |
C: 909.26 ± 58.00 E: 926.92 ± 39.68 |
C: 1002.37 ± 68.54 E: 1051.51 ± 40.063 |
|
| Surface Area Density (mm2/g) | C: 41.96 ± 2.89 E: 40.15 ± 3.03 |
C: 68.42 ± 2.98 E: 69.05 ± 3.65 |
C: 76.37 ± 3.18 E: 76.91 ± 3.41 |
C: 83.15 ± 3.94 E: 85.42 ± 3.69 |
|
| Montastraea cavernosa | Buoyant Weight (g) | C: 8.92 ± 0.66 E: 8.48 ± 0.66 |
C: 9.04 ± 0.67 E: 8.59 ± 0.66 |
C: 9.34 ± 0.70 E: 8.92 ± 0.70 |
C: 9.41 ± 0.68 E: 8.98 ± 0.67 |
| Tissue Surface Area (mm2) | C: 411.79 ± 26.15 E: 389.62 ± 22.88 |
C: 648.72± 30.59 E: 596.85 ± 22.96 |
C: 749.30 ± 43.61 E: 683.04 ± 24.68 |
C: 765.12 ± 49.86 E: 697.86 ± 25.64 |
|
| Surface Area Density (mm2/g) | C: 49.07 ± 5.41 E: 48.05 ± 3.91 |
C: 76.75 ± 8.09 E: 73.03 ± 5.79 |
C: 86.49 ± 10.22 E: 80.78 ± 6.62 |
C: 88.15 ± 11.23 E: 82.34 ± 7.49 |
|
| Orbicella faveolata | Buoyant Weight (g) | C: 8.26 ± 0.42 E: 8.46 ± 0.47 |
C: 8.20 ± 0.42 E: 8.42 ± 0.46 |
C: 8.48 ± 0.41 E: 8.61 ± 0.51 |
C: 8.54 ± 0.49 E: 8.64 ± 0.50 |
| Tissue Surface Area (mm2) | C: 327.74 ± 19.88 E: 326.91 ± 28.21 |
C: 417.24 ± 45.68 E: 453.44 ± 48.24 |
C: 322.84 ± 33.94 E: 374.04 ± 66.80 |
C: 295.36 ± 31.40 E: 364.86 ± 63.99 |
|
| Surface Area Density (mm2/g) | C: 40.22 ± 2.48 E: 38.77 ± 2.62 |
C: 51.49 ± 5.56 E: 53.56 ± 4.69 |
C: 38.44 ± 3.91 E: 42.35 ± 6.10 |
C: 35.85 ± 4.33 E: 41.29 ± 6.36 |
A significant main effect of exposure time was also seen in Montastraea cavernosa with no main effect of pCO2 level across metrics. Buoyant weight, tissue surface area, and surface area density significantly increased through 56 d of exposure, (Table 2) with no significant changes between 56 d and 84 d. As with P. clivosa, tissue growth rates slowed over the course of the experiment (Fig. 4e-h), and calcification rates increased significantly in the second exposure period followed by a decline to around 1 mg CaCO3/cm2/day in both control and elevated pCO2 treatments (Fig. 3b and e). Percent change in surface area density significantly decreased across time (Fig. 3h), indicating skeletal extension rather than an increase in skeletal density.
A significant main effect of time with no effect of pCO2 level was also seen in Orbicella faveolata. This species was unique among the three species tested, as fragments only significantly increased in buoyant weight between 28 d and 56 d of exposure from 14.9 g to 15.3 g in control treatments and 15.2 g to 15.4 g in elevated pCO2 treatments yet the 28 d time point showed a significant increase in tissue surface area or surface area density (Fig. 4i-l; Table 2). After 28 d of exposure, tissue growth returned to initial levels. Similar to P. clivosa and M. cavernosa, calcification rates significantly increased between the first and second exposure periods, but decreased during the third exposure period (Fig. 3c). However, unlike the other coral species tested, the tissue growth rates in O. faveolata were negative during both the 28-56 d and 56-84 d exposure periods (Fig. 3f). Consequently, the percent change in surface area density was noticeably different compared to M. cavernosa and P. clivosa, declining from 24.7 to -7.5 in control fragments, and 39.3 to -2.2 in elevated pCO2 fragments (Fig. 3i), indicating the possibility that calcification increased skeletal density rather than extension. Visually, there was also quantifiable tissue loss on some of the experimental fragments of this species, noted at the two later exposure periods.
4 Discussion
The recirculating OA exposure system described here maintained the IPCC projected pCO2 level for the year 2100 (∼1000 μatm) for 12-weeks using the preferred method of bubbling CO2 and atmospheric air. We found that, within our system, the combination of intense aeration, water retention, and CO2 scrubbers were key to off-gassing acidified treatment waters, illustrated by the minimal variation in control pH levels over the course of the experiment. The overall decline in total alkalinity over the 12-week exposure was likely due to coral calcification in a system without flow-through water, and has been noted in other studies (Leclercq et al., 2000, Renegar and Riegl, 2005). There is a calcium reactor built into the system, however, it was shut off after week 2 due to the large spike noted in total alkalinity. The reactor was not turned back on during this experiment in order to assess how the exposure system held total alkalinity without outside influence. However, given the overall decline in alkalinity noted over the course of 12-weeks, the reactor will be utilized in future experiments to maintain consistent total alkalinity levels in chambers. With the addition of the calcium reactor, this system can be used for exposures of 12-weeks to over a year. Use of the reactor will also decrease the variability seen in elevated pCO2 chambers in the later weeks of exposure noted in the current study. While a decline in calcification rate was noted in the 56-84 d exposure period compared to the 28-56 d period across species, these rates were not significantly different from the 0-28 d exposure period, indicating the decline in alkalinity was not the cause of reduced calcification rates. The large increase in calcification rate in the 28-56 d exposure period is likely a response to exposed/freshly cut skeleton, which has been noted in other coral studies at GED.
Our experimental system provides evidence that large volumes (∼1850-L) of water can hold stable, elevated pCO2 levels under recirculating conditions while off-gassing and re-using treated water over long exposure periods (>30 d). While other experiments have exposed corals to acidified water for 84 d or longer, the systems in those studies had either a flow-through design (Anthony et al., 2008; Dove et al., 2013; Form and Riebesell, 2012; Leclercq et al., 2002; Takahashi and Kurihara, 2013; Tambutté et al., 2015), treated a large volume of water in a closed system (Biosphere 2, Langdon et al., 2003), treated a very small volume of water in a closed system that required daily water changes (8-L, Renegar and Riegl, 2005), or used a recirculating system with each treatment set up as an independently controlled aquaria, eliminating the need to bring acidified waters down to ambient pCO2 levels before it was used again (Kurman et al., 2017).
With the exception of the work performed by Takahashi and Kurihara (2013), all the studies listed above found that coral species tested showed a negative reaction when exposed to elevated pCO2. However, the studies which used closed or recirculating systems (Kurman et al., 2017; Langdon et al., 2003; Renegar and Riegl, 2005) produced differing results when compared to experiments using the same coral species in flow-through systems (Comeau et al., 2013; Edmunds 2011; Form and Riebesell, 2012; Towle et al., 2015). Therefore, the question is raised if closed or recirculating systems can produce reliable results when examining the calcification response of corals. Our system, however, produced the opposite effect; we saw no decline of calcification rates in response to elevated pCO2 when compared to another study using M. cavernosa, P. clivosa and O. faveolata in a flow-through system.
Okazaki and colleagues (2017) exposed P. clivosa, M. cavernosa, and O. faveolata to 1300 μatm over two months using a flow-through water regime. They noted a decline in calcification rates across all three species in response to acidification. Our calcification rates were double to triple those noted in Okazaki's study. A potential explanation for this difference may be acclimatization, feeding regime used, species-specific tolerance, or the result of different water supply regimes (flow-through versus recirculating). All species used in this study were in culture for over a year, and exposed to pCO2 levels over 500 μatm on a daily basis. Culture systems at GED average pCO2 changes of 200-330 μatm over the course of a day, typically starting at levels between 550-600 μatm (data not shown). Although Florida marine waters have been shown to have large diel fluctuations due to photosynthesis and respiration (change of ∼230 μatm over one day; Yates et al., 2007), pCO2 levels over 500 μatm are rarely documented (Manzello et al., 2012; Yates et al., 2007). The long duration that colonies of P. clivosa, M. cavernosa and O. faveolata were exposed to this elevated pCO2 in culture may have acclimatized the corals and the exposure to 1000 μatm was not a large enough change to elicit a negative response. Fragments in Okazaki's study were field-collected and likely would not have seen the same daily pCO2 changes our species did. Additionally, the feeding regimes in our study were notably different from those used by Okazaki and colleagues; and it is possible corals in our study were able to off-set detrimental effects due to the type of food fed, or the amount of time they were permitted to feed, which has been shown to negate effects of elevated pCO2 in other species (Edmunds 2011; Towle et al., 2015).
An alternative explanation for this difference is P. clivosa, M. cavernosa and O. faveolata are among those coral species not susceptible to elevated pCO2, and perhaps the 2-month exposure in the Okazaki paper was not long enough. Comeau et al. (2013) has shown slow calcifying boulder corals (i.e., those species that do not uptake CO32- as rapidly as other species), are the most tolerant to ocean acidification. Anthony and colleagues (2008) demonstrated that massive Porites species were less sensitive to ocean acidification compared to fast growing, branching Acropora species. Indeed, studies by Dove et al. (2013) and Fabricius et al. (2011) also showed that increased pCO2 levels will shift coral communities to slow-growing corals. Falling into this category are P. clivosa, M. cavernosa and O. faveolata, all predominant reef-building corals in south Florida and the Caribbean (Helmle et al., 2011).
While the corals in our study were relatively unaffected by exposure to acidified waters, evidence in the literature indicates that other factors may drive the response to elevated pCO2 levels. Environmental conditions do not change one at a time, therefore the interaction of these changes is a key driver to organism performance and fitness. Several studies on corals have found increased temperature drives the negative calcification response when combined with acidification (Anthony et al., 2008; Dove et al., 2013; Hoadley et al., 2015; Reynaud et al., 2003). The response of corals to ocean acidification is highly species specific, and may be dependent upon morphology, physiology, life-stage, and interactions with other changing environmental variables. Therefore, a “one size fits all” hypothesis of how coral reefs will respond to ocean acidification appears untenable. The observed tolerance of corals tested here to elevated pCO2 levels is a finding that requires further exploration, particularly in light of the differences of calcification response of corals in flow-through versus closed or recirculating systems. However, the recirculating system developed in this study was successful in maintaining projected pCO2 levels over 84 d by bubbling CO2, was effective at off-gassing acidified waters before they were re-used, and is recommended for future research.
Acknowledgments
There are many people who supported this project. In particular, we thank Beth Moso and Allyn Duffy for their help with day to day maintenance of both organisms and the pCO2 system, and the Facilities staff at the EPA for their assistance in building the recirculating system. All corals used in this study were maintained under permit # FKNMS-2014-162. This work was supported in part by the Oak Ridge Institute for Science and Education (ORISE) Research Program. The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency.
Abbreviations
- BWT
Buoyant Weight
- EPA
Environmental Protection Agency
- GED
Gulf Ecology Division
- SAD
Surface Area Density
- TSA
Tissue Surface Area
Footnotes
Contributions: Laura A. Enzor designed the experiment and recirculating OA exposure system, performed carbonate chemistry measurements, maintained the corals in the exposure system, analyzed the data, and wrote the manuscript. Cheryl Hankins assisted in the design of the recirculating OA exposure system, designed the experiment, maintained corals in the exposure system, took buoyant weight measurements, and reviewed the manuscript. Deborah N. Vivian took photographs of the coral and analyzed them, as well as reviewed the final manuscript. William S. Fisher and Mace G. Barron edited and reviewed the manuscript.\All authors have approved the final version of this manuscript.
References
- Anthony KRN, Kline DI, Diaz-Pulido G, Dove S, Hoegh-Guldberg O. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc Natl Acad Sci. 2008;105:17442–17446. doi: 10.1073/pnas.0804478105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson MJ, Cuet P. Possible effects of ocean acidification on coral reef biogeochemistry: topics for research. Mar Ecol Prog Ser. 2008;373:249–256. [Google Scholar]
- Caldeira K, Wickett ME. Oceanography: anthropogenic carbon and ocean pH. Nature. 2003;425:365. doi: 10.1038/425365a. [DOI] [PubMed] [Google Scholar]
- Castillo KD, Ries JB, Bruno JF, Westfield IT. The reef-building coral Siderastrea siderea exhibits parabolic responses to ocean acidification and warming. Proc R Soc B. 2014;281:20141856. doi: 10.1098/rspb.2014.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comeau S, Edmunds PJ, Spindel NB, Carpenter RC. The responses of eight coral reef calcifiers to increasing partial pressure of CO2 do not exhibit a tipping point. Limnol Oceanogr. 2013;58:388–398. [Google Scholar]
- Dickson AG, Millero FJ. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res A. 1987;34:1733–1743. [Google Scholar]
- Dickson AG, Sabine CL, Christian JR. Guide to best practices for ocean CO2 measurements. Vol. 3 PICES Special Publication; 2007. [Google Scholar]
- Dove SG, Kline DI, Pantos O, Angly FE, Tyson GW, Hoegh-Guldberg O. Future reef decalcification under a business-as-usual CO2 emission scenario. Proc Natl Acad Sci. 2013;110:15342–15347. doi: 10.1073/pnas.1302701110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmunds PJ. Zooplanktivory ameliorates the effects of ocean acidification on the reef coral Porites spp. Limnol Oceanogr. 2011;56:2402–2410. [Google Scholar]
- Fabricius KE, Langdon C, Uthicke S, Humphrey C, Noonan S, De'ath G, Okazaki R, Muehllhener N, Glas MS, Lough JM. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nature Clim Chang. 2011;1:165–169. [Google Scholar]
- Fangue NA, O'Donnell MJ, Sewell MA, Matson PG, MacPherson AC, Hofmann GE. A laboratory-based, experimental system for the study of ocean acidification effects on marine invertebrate larvae. Limno l Oceanogr: Methods. 2010;8:441–452. [Google Scholar]
- Form AU, Riebesell U. Acclimation to ocean acidification during long-term CO2 exposure in the cold-water coral Lophelia pertusa. Glob Change Biol. 2012;18:843–853. [Google Scholar]
- Fournie JE, Vivian DN, Yee SH, Courtney LA, Barron MG. Comparative sensitivity of six scleractinian corals to temperature and solar radiation. Dis Aquat Org. 2012;99:85–93. doi: 10.3354/dao02459. [DOI] [PubMed] [Google Scholar]
- Gattuso JP, Frankignoulle M, Bourge I, Romaine S, Buddemeier RW. Effect of calcium carbonate saturation of seawater on coral calcification. Glob Planet Chang. 1998;18:37–46. [Google Scholar]
- Gattuso JP, Lavigne H. Technical note: Approaches and software tools to investigate the impact of ocean acidification. Biogeosciences. 2009;6:2121–2133. [Google Scholar]
- Helmle KP, Dodge RE, Swart PK, Gladhill DK, Eakin CM. Growth rates of Florida corals from 1937-1996 and their response to climate change. Nat Commun. 2011;2:215. doi: 10.1038/ncomms1222. [DOI] [PubMed] [Google Scholar]
- Hoadley KD, Pettay DT, Grottoli AG, Cai WJ, Melman TF, Schoepf V, Hu X, Li Q, Xu H, Wang Y, Matsui Y, Baumann JH, Warner ME. Physiological response to elevated temperature and pCO2 varies across four Pacific coral species: Understanding the unique host + symbiont response. Sci Rep. 2015;5:18371. doi: 10.1038/srep18371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachuri RK, Reisinger A, editors. Intergovernmental Panel on Climate Change. Climate Change 2007: Synthesis Report Contributions of Working Groups I, II and II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC; Geneva: 2007. [Google Scholar]
- Jokiel PL, Maragos JE, Franzisket L. Coral growth: buoyant weight technique. In: Stoddart DR, Johannes RE, editors. Coral reefs: research methods. UNESCO; Paris: 1978. pp. 529–541. [Google Scholar]
- Kurman MD, Gómez CE, Georgian SE, Lunden JJ, Cordes EE. Intra-specific variation reveals potential for adaptation to ocean acidification in a cold-water coral from the Gulf of Mexico. Front Mar Sci. 2017;4:1–14. [Google Scholar]
- Langdon C, Broecker WS, Hammond DE, Glenn E, Fitzsimmons K, Nelson SG, Peng TH, Hajdas I, Bonani G. Effect of elevated CO2 on the community metabolism of an experimental coral reef. Glob Biogeochem Cycles. 2003;17:1011. [Google Scholar]
- Langdon C, Atkinson MJ. Effect of elevated pCO2 on photosynthesis and calcification of corals and interactions with seasonal change in temperature/irradiance and nutrient enrichment. J Geophys Res. 2005;110:1–16. [Google Scholar]
- Leclercq N, Gattuso JP, Jaubert J. CO2 partial pressure controls the calcification rate of a coral community. Glob Chang Biol. 2000;6:329–334. [Google Scholar]
- Leclercq N, Gattuso JP, Jaubert J. Primary production, respiration and calcification of a coral reef mesocosm under CO2 partial pressure. Limnol Oceanogr. 2002;47(2):558–564. [Google Scholar]
- Lunden JJ, Turner M, McNicholl CG, Glynn CK, Cordes EE. Design, development and implementation of recirculating aquaria for maintenance and experimentation of deep-sea corals and associated fauna. Limnol Oceanogr: Methods. 2014;12:363–372. [Google Scholar]
- Manzello DP, Enochs IC, Neki N, Gledhill DK, Johns EM. Ocean acidification refugia of the Florida Reef tract. PLoS One. 2012;7:e41715. doi: 10.1371/journal.pone.0041715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marubini F, Barnett H, Langdon C, Atkinson MJ. Dependence of calcification on light and carbonate ion concentration for the hermatypic coral, Porites compressa. Mar Ecol Prog Ser. 2001;220:153–162. [Google Scholar]
- Marubini F, Ferrier-Pagès C, Furla P, Allemand D. Coral calcification responds to seawater acidification: a working hypothesis towards a physiological mechanism. Coral Reefs. 2008;27:491–499. [Google Scholar]
- Mehrbach C, Culberson CH, Hawley JE, Pykowicz RM. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol Oceanogr. 1973;18:897–907. [Google Scholar]
- Okazaki RR, Towle EK, van Hooidonk R, Mor C, Winter R, Piggot AM, Cunning R, Baker AC, Klaus JS, Swart PK, Langdon C. Species-specific responses to climate change and community composition determine future calcification rates of Florida Keys reefs. Glob Chang Biol. 2017;23:1023–1035. doi: 10.1111/gcb.13481. [DOI] [PubMed] [Google Scholar]
- Renegar DA, Riegl BM. Effect of nutrient enrichment and elevated CO2 partial pressure on growth rate of Atlantic scleractinian coral Acropora cervicornis. Mar Ecol Prog Ser. 2005;293:69–76. [Google Scholar]
- Reynaud S, Leclercq N, Romaine-Lioud S, Ferrier-Pagès C, Jaubert J, Gattuso JP. Interacting effects of CO2 partial pressure and temperature on photosynthesis and calcification in scleractinian coral. Glob Chang Biol. 2003;9:1660–1668. [Google Scholar]
- Robbins LL, Hansen ME, Kleypas JA, Meylan SC. CO2 Calc: A user-friendly seawater carbon calculator for Windows, Mac OS X and iOS (iPhone) United States Geological Service 2010 [Google Scholar]
- Takahashi A, Kurihara H. Ocean acidification does not affect the physiology of the tropical coral Acropora digitifera during a 5-week experiment. Coral Reefs. 2013;32:305–314. [Google Scholar]
- Tambutté E, Venn AA, Holcomb M, Segonds N, Techer N, Zoccola D, Allemand D, Tambutté S. Morphological plasticity of the coral skeleton under CO2-driven seawater acidification. Nat Commun. 2015;6:7368. doi: 10.1038/ncomms8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towle EK, Enochs IC, Langdon C. Threatened Caribbean coral is able to mitigate the adverse effects of ocean acidification on calcification by increasing feeding rate. PLoS One. 2015;10:e0123394. doi: 10.1371/journal.pone.0123394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates KK, Dufore C, Smiley N, Jackson C, Halley RB. Diurnal variation of oxygen and carbonate system parameters in Tampa Bay and Florida Bay. Mar Chem. 2007;104:110–124. [Google Scholar]