Abstract
Introduction
Type 2 diabetes is a chronic metabolic disorder that has been associated with alterations in the status of trace elements, including zinc, copper, iron and manganese. However, clinical studies reporting statuses of these trace elements in type 2 diabetes patients compared to controls have shown conflicting results.
Objective
This meta-analysis aimed to summarize the existing literature on the statuses of zinc, copper, iron, and manganese in Type 2 diabetes mellitus patients.
Methods
A literature search of Embase, PubMed, EBSCOHost, ScienceDirect, Scopus, Cochrane library and Web of Science electronic databases was conducted to find studies published from 1970 to November 2016 that compared the trace elements of interest between type 2 diabetic patients and healthy controls. Keywords used were type 2 diabetes, diabetes, hyperglycemia, insulin, glucose, HbA1c, trace elements, micronutrients, zinc, manganese, copper, ceruloplasmin, iron and ferritin. The bias corrected Hedges’ g, was utilized as the effect sizes. Due to the biological interaction between trace elements, it is important to collectively evaluate the statuses of these minerals in type 2 diabetes. Thus, the robust variance estimation method was chosen to handle dependency between multiple outcomes.
Results
A total of 52 studies met the inclusion criteria, amounting to 98 effect sizes. Diabetic patients (n=20183) had significantly lower zinc status when compared to controls (effect size = −1.73, p<0.01); whereas copper (effect size = 1.10, p<0.05) and ferritin levels (effect size = 1.05, p<0.01) were significantly higher. Although not significant, ceruloplasmin (effect size = 1.85, p=0.06) and iron (effect size = 1.42, p=0.06) levels were higher, and manganese (effect size = 0.27, p=0.34) was lower in patients.
Conclusion
Results from this meta-analysis indicate lower zinc status accompanied by increased copper and ferritin levels in patients with type 2 diabetes when compared to controls.
INTRODUCTION
Diabetes mellitus is a chronic metabolic disorder that arises due to absolute or relative lack of insulin production by the beta-cells of the pancreas. Impaired secretion of this protein affects glucose metabolism, and consequently, results in hyperglycemia. (1) Unregulated levels of blood glucose can lead to several debilitating conditions such as nephropathy, neuropathy, retinopathy, cardiovascular disease, stroke, and amputations of extremities.(2) About 90 to 95% of the patients are affected by type 2 diabetes (2) which is characterized primarily by insulin resistance, hyperinsulinemia, and beta-cell dysfunction.(3)
Trace elements facilitate numerous biochemical reactions (4), including those related to insulin and glucose metabolism. The transport of zinc into the beta-cells of the pancreas is essential for insulin production and its efficient packaging into vesicles.(5) Moreover, zinc finger protein 407 (6) and zinc-alpha2-glycoprotein (7, 8) have been shown to enhance the expression of the Glucose transporter type 4 (GLUT 4) protein in adipocytes and skeletal muscles, thereby mediating insulin-induced glucose uptake into these cells. Manganese is another trace element involved in carbohydrate metabolism.(9) Animal studies have indicated an association of manganese with optimal insulin synthesis and secretion.(10) Manganese also increases the binding of insulin to its receptor, thereby facilitating the physiological action of this hormone.(11) In a study conducted by Baly et al., manganese deficient rats had diminished insulin-stimulated glucose oxidation in the adipose tissue, and fewer insulin receptors compared to controls.(12) In contrast, elevated levels of copper (13) and iron (14, 15) have been linked to increased oxidative stress, which in turn could lead to insulin resistance, impaired glucose tolerance, and type 2 diabetes.(16) Consistently, greater copper and iron statuses have been associated with increased insulin resistance in humans.(17, 18) Thus, copper and iron also are important while investigating trace element status in type 2 diabetes.
Research comparing zinc, manganese, copper and iron levels between type 2 diabetes patients and healthy individuals have reported inconsistent results. For example, some studies have indicated greater levels of these trace elements in type 2 diabetes patients compared to controls (4, 19, 20); whereas other investigations have demonstrated reduced status. (20–25) Thus, this meta-analysis aimed to summarize the existing literature on difference in zinc, manganese, copper and iron levels between type 2 diabetic and nondiabetic individuals. Ceruloplasmin and ferritin levels were additionally utilized to indicate copper and iron statuses, respectively. It is hypothesized that diabetic patients will have lower zinc and manganese concentrations, and elevated copper and iron statuses compared to nondiabetic controls.
A secondary goal of this research is to explore the influence of age, body mass index (BMI) and gender on the primary outcomes of interest by a moderator analysis. It is well established that age > 45 years, (26) gender (27) and overweight/ obesity (28) may influence trace element concentrations in humans, and are associated with a higher incidence of type 2 diabetes.(29–31) Moreover, the presence of fat depots around organs has been reported to negatively impact the status of minerals when compared to subcutaneous fat. Consequently, gender may influence the development of type 2 diabetes since the incidence of visceral adiposity is higher in men as compared to women.(32)
MATERIAL AND METHODS
Search Strategy
A literature search of Embase, PubMed, EBSCOHost, ScienceDirect, Scopus, Cochrane library and Web of Science electronic databases was conducted to identify studies published between 1970 and November 2016 investigating trace element concentrations in type 2 diabetes mellitus patients and healthy controls. Keywords used were type 2 diabetes, diabetes, hyperglycemia, insulin, glucose, HbA1c, trace elements, micronutrients, zinc, manganese, copper, ceruloplasmin, iron and ferritin.
Data Extraction
Two investigators independently carried out the literature search and recorded the data in the database. The studies recorded by each investigator were then matched to remove duplicate studies. The titles and abstracts were read for each retrieved record to select studies meeting the inclusion criteria. The reference lists of all the retrieved studies also were reviewed by each investigator to minimize the chance of missing relevant studies.
Inclusion and Exclusion Criteria
The inclusion criteria for the studies that were retrieved using the search terms were: a) use of a cross-sectional or case-control design b) reporting of plasma/ serum values of zinc/ copper/ ceruloplasmin/ iron/ ferritin/ manganese of type 2 diabetic patients and healthy controls and b) absence of any diabetes-related complications in patients.
Studies were excluded if: a) they reported statistics jointly for patients with and without complications in type 2 diabetes group; b) the text was not available in English; and c) adequate information was not available in the text to calculate effect sizes.
All articles were assessed based on the inclusion/exclusion criteria and any disagreements were resolved by discussion with a nutrition professor having expertise in field of trace elements.
Statistical analysis
The author, year and country for investigations that met the inclusion criteria were identified. The primary outcome, status of trace elements (zinc, copper, iron and manganese), in the control and diabetic groups were extracted. Other characteristics included for the analysis were type of biomarker (serum/plasma), age, BMI, and percentage of men in the control and diabetic arms. Difference in BMI between the control and diabetic groups was documented. The weighted average of age and proportion of males were determined, with the number of participants in each group representing the weights.
The primary studies reported trace element status in varying units of concentrations. Thus, a standardized mean difference, the bias corrected Hedges’ g, (33) was utilized as the effect size for the meta-analysis. The hypothesis to be tested was that the status of zinc and manganese would be lower in diabetic subjects, whereas that of copper, ceruloplasmin, and iron and ferritin would be higher. Since these trace elements have been shown to interact with each other, the use of a univariate model to determine their effect sizes could inflate Type I error rates. Methods utilized to handle dependency of multiple related outcomes in meta-analysis included robust variance estimation (RVE) method and generalized least squares (GLS) estimation. Unlike GLS estimation, the RVE approach does not require use of the correlations between pairs of multivariate outcomes. Thus, the RVE method was chosen for this meta-analysis due to the lack of reporting of correlations between trace elements in the primary studies.
Computations in the ensuing section were performed using the Statistical Package for Social Sciences (SPSS Version 22, Armonk, NY, 2013). The standard deviations of the trace element biomarker values in the control and diabetic groups, and the associated sample sizes were used to calculate the pooled sample variances. Trace element values of the diabetic subjects were subtracted from the controls when calculating effect sizes. Negative effects indicated that the trace element levels were lower in the diabetic arm as compared to the nondiabetic control. The resulting differences were divided by the pooled sample variances to compute Hedges g, to which the small-sample bias correction was applied. Bias-corrected Hedges g, thus obtained, represented the effect size for each study. The variance and 95% confidence intervals estimates for the effect sizes were calculated using the associated standardized mean difference and sample sizes in each group.
The statistical software, R with macro robumeta, were used to calculate the pooled average effect size and variance using the RVE method to apply Tipton’s (34) small sample bias correction. Statistics reported were 95% CIs, and two-sided Z-statistic to assess the statistical significance of the pooled average effect size. The effects of type of biomarker, age, difference in BMI, and percent males were evaluated by including these variables in the mixed-effects, meta-regression model. Publication bias was assessed by visualization of funnel plots. The I2 statistic was used to assess heterogeneity across studies. Cut-off values of 0, 25, 50 and 75% indicated no, low, moderate and high heterogeneity. A symmetrical funnel plot evidence for lack of publication bias in the meta-analysis. The Egger’s regression test also was performed, with p<0.05 suggesting significant publication bias.
RESULTS
Literature search
Table/Figure 1 illustrates the flow diagram of the screening involved in the selection of articles for the meta-analysis. A total of 97 articles were retrieved, with a final number of 52 case-control studies (1, 4, 19, 20, 22–25, 35–79) with 20183 participants were included in the meta-analysis.
Table 1.
Characteristics of studies included
| First Author | Year | Country | Biomarker | Agea | Proportion of malesa |
Difference in body mass indexb |
Sample size | Effect size | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||
| Control | Diabetic | Zinc | Copper | Ceruloplasmin | Iron | Ferritin | Manganese | |||||||
| Pidduck | 1970 | England | Plasma | 52.95 | 100 | --c | 40 | 36 | 5.71 | 0.18 | -- | -- | -- | -- |
| Pidduck | 1970 | England | Plasma | 57.43 | 0 | -- | 46 | 42 | -4.07 | 1.26 | -- | -- | -- | -- |
| Mateo | 1978 | Spain | Serum | -- | -- | -- | 10 | 49 | 1.2 | 1.29 | 0.39 | -- | -- | -- |
| Raz | 1989 | Israel | Serum | 50.51 | 49.15 | 1.7 | 21 | 38 | 0.58 | -0.9 | -- | -- | -- | -- |
| Car | 1992 | Yugoslavia | Serum | 45.5 | 46.67 | 4.3 | 15 | 15 | −1.42 | −2.86 | -- | -- | -- | -- |
| Williams | 1995 | UK | Plasma | 51.55 | 46.15 | -- | 26 | 26 | −6.09 | −1.99 | -- | -- | -- | -- |
| Hughes | 1998 | Singapore | Serum | -- | 100 | 1.3 | 248 | 72 | -- | -- | -- | -- | 0.44 | -- |
| Hughes | 1998 | Singapore | Serum | -- | 0 | 1.5 | 282 | 54 | -- | -- | -- | -- | 0.46 | -- |
| Daimon | 1998 | Japan | Serum | 57.05 | -- | -- | 158 | 637 | -- | -- | 2.58 | -- | -- | -- |
| Ekmekcioglu | 2000 | Austria | Plasma | 67.05 | -- | 0 | 50 | 53 | 0.31 | 0.2 | -- | --d | -- | 0.2 |
| Kim | 2000 | Korea | Serum | 57.03 | 46.67 | 2.4 | 25 | 50 | -- | -- | -- | -- | 0.32 | -- |
| Olaniyan | 2001 | Nigeria | Serum | 57.03 | 39.81 | -- | 50 | 53 | −0.99 | 0.74 | -- | -- | -- | -- |
| Ekin | 2003 | Turkey | Serum | 56.98 | 0 | 1.2 | 29 | 108 | −4.27 | --d | -- | 5.86 | -- | --d |
| Ekin | 2003 | Turkey | Serum | 56.28 | 100 | 1.6 | 21 | 92 | −4.64 | --d | -- | 4.83 | -- | --d |
| Memisogullari | 2003 | Turkey | Serum | -- | -- | -- | 18 | 21 | -- | -- | 0.86 | -- | -- | -- |
| Memisogullari | 2004 | Turkey | Serum | 54.49 | -- | -- | 21 | 29 | -- | -- | 1.35 | -- | -- | -- |
| Qin | 2004 | China | Serum | -- | -- | -- | 110 | 104 | -- | -- | 2.33 | -- | -- | -- |
| Elis | 2004 | Israel | Serum | 63 | 40.59 | -- | 40 | 29 | -- | -- | -- | −0.04 | 0.15 | -- |
| Jiang | 2004 | USA | Plasma | 56.45 | 0 | 4.1 | 716 | 698 | -- | -- | -- | -- | 0.42 | -- |
| Elsammak | 2005 | Egypt | Serum | -- | -- | -- | 18 | 22 | -- | -- | -- | -- | −1.19 | -- |
| Kaviarasan | 2005 | India | Plasma | 54.24 | -- | 5.21 | 18 | 20 | -- | -- | 3.76 | -- | -- | -- |
| Virgolici | 2005 | Romania | Plasma | 53.4 | 50 | 11.4 | 20 | 20 | -- | -- | 1.02 | -- | -- | -- |
| Al-Maroof | 2006 | Iraq | Serum | 45.73 | -- | 1.69 | 133 | 101 | −1.18 | -- | -- | -- | -- | -- |
| Abou-Shousha | 2006 | Egypt | Serum | 48.52 | 0 | -- | 10 | 20 | -- | -- | -- | -- | 0.65 | -- |
| Lee | 2006 | Korea | Serum | 58.6 | 67.35 | -- | 47 | 48 | -- | -- | -- | 0.15 | 0.86 | -- |
| Adewumi | 2007 | Nigeria | Serum | 46.67 | 100 | 6.25 | 50 | 28 | -- | --d | -- | -- | -- | −0.89 |
| Adewumi | 2007 | Nigeria | Serum | 44.12 | 0 | 3.43 | 40 | 62 | -- | --d | -- | -- | -- | −1.03 |
| Viktorinova | 2009 | Slovak | Plasma | 42.49 | 47.46 | 0.55 | 34 | 25 | −0.49 | 0.53 | -- | -- | -- | -- |
| Rajpathak | 2009 | USA | Serum | 50.3 | 36.4 | 1.5 | 280 | 280 | -- | -- | -- | -- | 0.11 | -- |
| Flores | 2010 | Mexico | Serum | -- | -- | -- | 12 | 76 | 0.04 | 1.05 | -- | -- | -- | 1.15 |
| Sarkar | 2010 | India | Plasma | 54.39 | 62.19 | -- | 78 | 123 | -- | 2.94 | −1.3 | -- | -- | -- |
| Ashourpour | 2010 | Iran | Serum | 53.29 | -- | 0.29 | 53 | 54 | -- | -- | -- | -- | 3.41 | -- |
| Chacko | 2010 | India | Serum | -- | 52.87 | -- | 47 | 40 | -- | -- | 11.13 | -- | -- | -- |
| Nasli-Esfahani | 2011 | Iran | Serum | 50.6 | 34.96 | -- | 151 | 150 | −1.05 | --d | -- | -- | -- | −0.13 |
| Kim | 2011 | Korea | Serum | 51.27 | 100 | 0.8 | 3384 | 727 | -- | -- | -- | -- | 0.3 | -- |
| Kim | 2011 | Korea | Serum | 50.17 | 0 | 2.2 | 3869 | 327 | -- | -- | -- | -- | 0.54 | -- |
| Ferdousi | 2012 | Bangladesh | Serum | -- | -- | 5.15 | 60 | 60 | −0.38 | 0.46 | -- | -- | -- | -- |
| Savu | 2012 | Romania | Plasma | 40.99 | 41 | -- | 15 | 15 | -- | 1.56 | 1.55 | -- | -- | -- |
| Ganesh | 2012 | India | Serum | 42.7 | 38.35 | −1.86 | 30 | 30 | -- | -- | -- | 0.39 | 0.6 | -- |
| Praveeena | 2013 | India | Serum | -- | -- | -- | 40 | 40 | −3.41 | -- | -- | -- | -- | -- |
| Farid | 2013 | Saudi Arabia | Serum | 59.51 | 100 | -- | 55 | 55 | −1.19 | 0.84 | -- | -- | -- | --d |
| Dosa | 2013 | Romania | Plasma | -- | -- | 3.86 | 17 | 30 | −2.53 | 0.44 | -- | -- | -- | -- |
| Kundu | 2013 | India | Serum | -- | -- | 30 | 30 | -- | -- | -- | 0.33 | 2.1 | -- | |
| Santa | 2014 | India | Serum | -- | -- | 5.42 | 40 | 60 | −4.66 | 5.5 | -- | -- | -- | -- |
| Oyedeji | 2014 | Nigeria | Plasma | -- | 0 | -- | 35 | 50 | −2.03 | --d | -- | 2.66 | -- | -- |
| Kumar | 2014 | India | Serum | 48.5 | 74 | -- | 50 | 50 | −1.86 | 2.07 | -- | -- | -- | -- |
| Alam | 2014 | Pakistan | Serum | 40.4 | -- | -- | 44 | 67 | -- | -- | -- | -- | 2.4 | -- |
| Nagarajrao | 2015 | Saudi Arabia | Serum | 49.43 | 55.24 | 0.04 | 58 | 47 | −2.18 | 4.09 | -- | 3.33 | -- | -- |
| Maheshwari | 2015 | India | Serum | 48.28 | 50 | 50 | -- | 2.58 | ||||||
| Badran | 2016 | Egypt | Serum | 52.88 | 57.89 | 0.96 | 36 | 40 | −4.51 | --d | -- | --d | -- | --d |
| Atari-Hajipirlo | 2016 | Iran | Serum | 52.46 | 53.12 | -- | 50 | 46 | −1.25 | 1.21 | -- | 1.35 | -- | -- |
| Wang | 2016 | China | Serum | 67.58 | 100 | 0.87 | 529 | 289 | -- | -- | -- | -- | -- | 0.15 |
| Wang | 2016 | China | Serum | 67.63 | 0 | −2 | 751 | 494 | -- | -- | -- | -- | -- | −0.29 |
| Devi | 2016 | India | Serum | -- | 47.5 | -- | 40 | 40 | −0.67 | 4.06 | -- | -- | -- | -- |
| Goud | 2016 | India | Serum | -- | -- | -- | 20 | 20 | -- | -- | 0.97 | −0.51 | 2.17 | -- |
| Jeppu | 2016 | Malaysia | Serum | -- | -- | -- | 50 | 50 | -- | -- | −2.21 | -- | -- | -- |
| Borah | 2016 | India | Serum | 53.93 | 56.5 | 2.17 | 92 | 92 | -- | -- | -- | -- | 1.87 | -- |
| Eva | 2016 | Banglade | Serum | 47.79 | 54 | 0.36 | 50 | 50 | −0.96 | -- | -- | -- | -- | −0.9 |
| Zhang | 2017 | China | Serum | 55.91 | 34.68 | 1.2 | 1327 | 510 | 0.16 | 0.15 | -- | -- | -- | 0.02 |
Represents the weighted average where the weights are the number of individuals in control and diabetic groups
Represents the difference in the body mass index between diabetic and control groups
Not reported in the primary study
Data did not meet the inclusion criteria
Figure 1.
Flow diagram of the screening process involved in inclusion of articles
Summary of included studies
The characteristics of the included studies are presented in Table/Figure 2. The number of included studies reporting values for zinc, copper, ceruloplasmin, iron, ferritin, and manganese were 25, 20, 12, 9, 16 and 7, respectively. A few investigations reported mean trace element levels separately for men and women, thereby resulting in two effect sizes per study. Thus, the total number of effect sizes for zinc, copper, ceruloplasmin, iron, ferritin and manganese were 27, 21, 12, 10, 19 and 9, respectively.
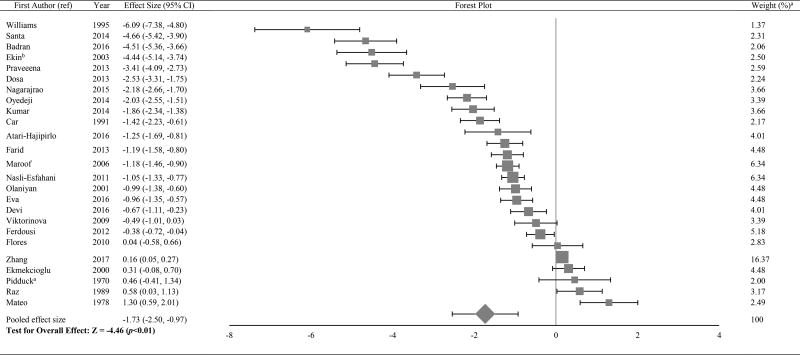
Figure 2.
Forest plot of effect size estimates and its 95% confidence intervals representing difference in zinc levels between healthy and diabetic subjects
aThe weight of each study indicates its influence on the pooled effect size. A higher percentage weight is shown by a larger box and narrower confidence interval for the respective study in the forest plot
bData represented as weighted average of the effect size for men and women
Pooled average effect size for zinc, copper, ceruloplasmin, iron, ferritin and manganese
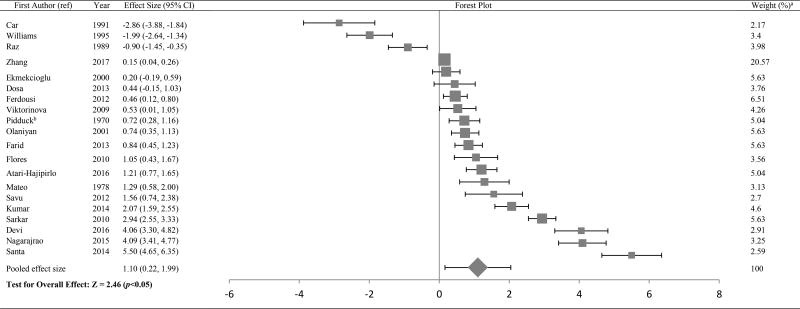
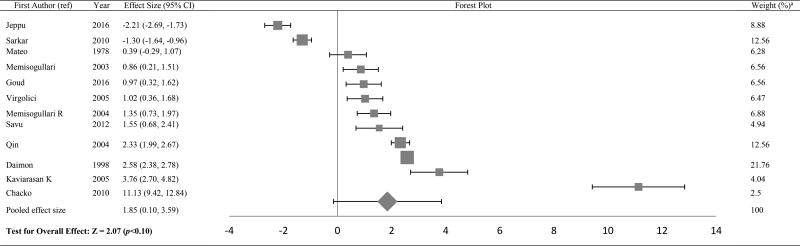
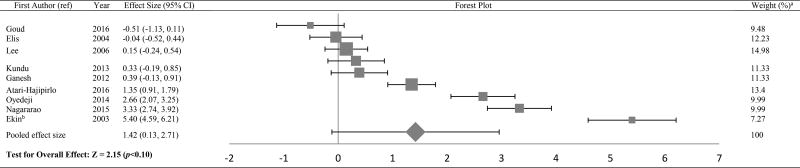
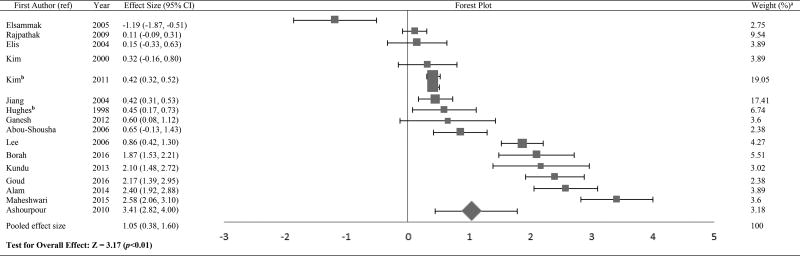
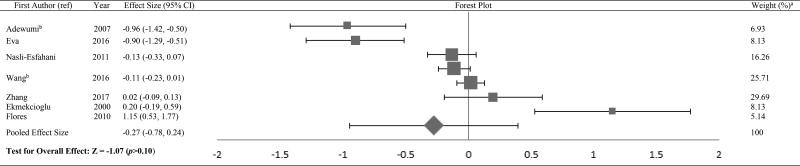
The Forest plots in Tables/Figures 3–8 depict the average pooled effect size, 95% CI and statistical significance for zinc, copper, ceruloplasmin, iron, ferritin and manganese, respectively. For investigations that report separate values for men and women, a weighted average of the effect size was calculated with the number in each gender as weights. Zinc levels were significantly lower in type 2 diabetic subjects when compared to non-diabetic individuals (pooled effect size = −1.73, p<0.01), whereas that for copper (pooled effect size = 1.10, p<0.05) and ferritin (pooled effect size = 1.05, p<0.05) were significantly higher. Patients diagnosed with type 2 diabetes also had higher values for ceruloplasmin (pooled effect size = 1.85, p=0.06) and iron (pooled effect size = 1.42, p=0.06), but lower manganese concentrations (pooled effect size = −0.27, p=0.34) than nondiabetic controls. However, these effect sizes were not significant. The I2 statistic for the meta-analysis was 97.6%.
Figure 3.
Forest plot of effect size estimates and its 95% confidence intervals representing difference in copper levels between healthy and diabetic subjects
aThe weight of each study indicates its influence on the pooled effect size. A higher percentage weight is shown by a larger box and narrower confidence interval for the respective study in the forest plot
bData represented as weighted average of the effect size for men and women
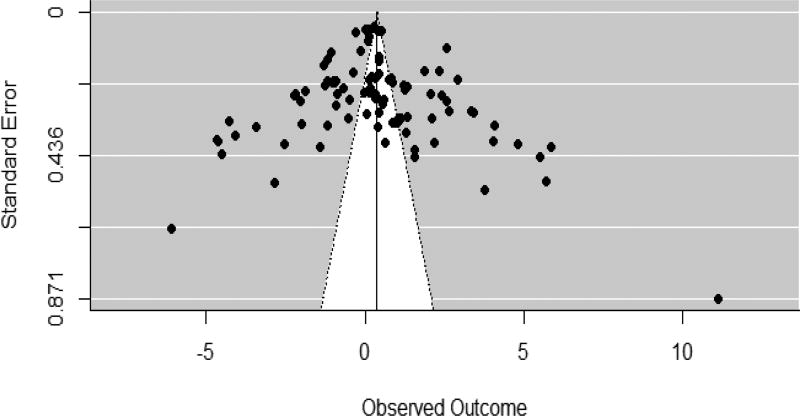
Figure 8.
Funnel plot
Moderator analysis
The influence of biomarker type for quantification of trace element status, age, gender and BMI on effect size were carried out as separate, additional analyses. The weighted average of age and percentage of men were used to represent age and gender, respectively, with the number of individuals in the control and diabetic groups serving as the weights. The weighted mean of the difference in BMI also was used as a moderator.
Z-statistics for testing the type of biomarker, age, gender and BMI as moderator effects were −0.504, −0.219, 0.982, and 0.459 respectively. Furthermore, none of the moderators had a significant influence on any of the effect sizes.
Publication bias
Figure 8 indicates a fairly symmetrical funnel plot. Furthermore, the Egger’s regression showed no evidence for significant publication bias in the included studies.
DISCUSSION
This meta-analysis found that zinc and manganese concentrations were lower in type 2 diabetic individuals compared to nondiabetic controls; while levels of copper, ceruloplasmin, iron and ferritin were higher. However, the bias-corrected Hedges g was not significant for ceruloplasmin, iron and manganese. The inconsistent findings between copper and ceruloplasmin, and iron and ferritin could due to difference in the number of studies investigating levels of these biomarkers. It is important to note that the greatest effect size was observed for ceruloplasmin, followed by iron. The smaller number of studies reporting ceruloplasmin and iron values could have resulted in lower statistical power, and subsequently, non-significant effect sizes. The use of plasma vs serum, age, BMI, and gender did not significantly influence the effect sizes in the present meta-analysis.
The results of this meta-analysis suggest possible zinc deficiencies and elevated copper and iron (as indicated by ferritin) statuses in type 2 diabetic individuals. The findings are comparable to previous research which demonstrates abnormal metabolism of zinc in type 2 diabetes patients, marked by malabsorption and increased urinary losses of this micronutrient.(80) Further, the greater ceruloplasmin (40, 81) and lower transferrin (40) levels observed in type 2 diabetic individuals could result in elevated levels of free copper and iron, respectively. Increased levels of these transition metals have been linked to greater oxidative stress,(82) and subsequently, increased insulin resistance and type 2 diabetes (16). The concomitant decrease in trace elements that function as antioxidants, such as zinc (83) and manganese, (84, 85) may cause an impaired scavenging mechanism of free radicals, and may further exacerbate the prognosis of diabetes. Collectively, these results indicate the need for cohort studies to explore the role of these trace elements in the progression of type 2 diabetes.
The results of this meta-analysis are subject to certain limitations. Since the meta-analysis did not include longitudinal studies, it is not possible to establish causal inferences on the association between altered trace element status and type 2 diabetes. Another limitation is that the results could have been influenced by exclusion of articles that lacked availability of text in English. Further, the high level of heterogeneity in this meta-analysis could have affected the validity of the overall pooled effect size estimate. Nevertheless, the analysis is strengthened by the use of a model that handles dependency between multiple outcomes.
CONCLUSION
In conclusion, this meta-analysis found altered levels of trace minerals in type 2 diabetes patients in comparison with controls. Inadequate zinc and a concurrent excess of copper and iron levels may be associated with an increased level of oxidative stress, and may exacerbate the condition. Longitudinal studies could help in understanding the association between trace element variations and onset and progression of type 2 diabetes.
Figure 4.
Forest plot of effect size estimates and its 95% confidence intervals representing difference in ceruloplasmin levels between healthy and diabetic subjects
aThe weight of each study indicates its influence on the pooled effect size. A higher percentage weight is shown by a larger box and narrower confidence interval for the respective study in the forest plot
Figure 5.
Forest plot of effect size estimates and its 95% confidence intervals representing difference in iron levels between healthy and diabetic subjects
aThe weight of each study indicates its influence on the pooled effect size. A higher percentage weight is shown by a larger box and narrower confidence interval for the respective study in the forest plot
bData represented as weighted average of the effect size for men and women
Figure 6.
Forest plot of effect size estimates and its 95% confidence intervals representing difference in ferritin levels between healthy and diabetic subjects
aThe weight of each study indicates its influence on the pooled effect size. A higher percentage weight is shown by a larger box and narrower confidence interval for the respective study in the forest plot
bData represented as weighted average of the effect size for men and women
Figure 7.
Forest plot of effect size estimates and its 95% confidence intervals representing difference in manganese levels between healthy and diabetic subjects
aThe weight of each study indicates its influence on the pooled effect size. A higher percentage weight is shown by a larger box and narrower confidence interval for the respective study in the forest plot
bData represented as weighted average of the effect size for men and women
Acknowledgments
None.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors. Lead author’s effort was supported in part by the intramural research program of the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
Financial interest disclosure:
None
Conflict of Interest
None
Contributor Information
Namrata Sanjeevi, Postdoctoral Fellow, Health Behavior Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development, 6710B Rockledge Drive Room 3165A, MSC 7004, Bethesda, MD 20817, USA, namratas@utexas.edu.
Jeanne Freeland-Graves, Department of Nutritional Sciences, The University of Texas at Austin, 1 University Station A2703, Austin, TX 78712, USA, jfg@mail.utexas.edu.
S. Natasha Beretvas, Department of Educational Psychology, The University of Texas at Austin, 1 University Station D5800, Austin, TX 78712, USA, tberetvas@austin.utexas.edu.
Prageet K. Sachdev, Department of Nutritional Sciences, The University of Texas at Austin, 1 University Station A2703, Austin, TX 78712, USA, prageet@utexas.edu
References
- 1.S P, Pasula S, Sameera K. Trace elements in diabetes mellitus. J Clin Diagn Res. 2013;7(9):1863–5. doi: 10.7860/JCDR/2013/5464.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Center for Disease Control and Prevention. National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. Atlanta (GA): US Department of Health and Human Services; 2014. 2014. [Google Scholar]
- 3.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365(9467):1333–46. doi: 10.1016/S0140-6736(05)61032-X. [DOI] [PubMed] [Google Scholar]
- 4.Ekmekcioglu C, Prohaska C, Pomazal K, Steffan I, Schernthaner G, Marktl W. Concentrations of seven trace elements in different hematological matrices in patients with type 2 diabetes as compared to healthy controls. Biol Trace Elem Res. 2001;79(3):205–19. doi: 10.1385/BTER:79:3:205. [DOI] [PubMed] [Google Scholar]
- 5.Chistiakov DA, Voronova NV. Zn(2+)-transporter-8: a dual role in diabetes. Biofactors. 2009;35(4):356–63. doi: 10.1002/biof.49. [DOI] [PubMed] [Google Scholar]
- 6.Buchner DA, Charrier A, Srinivasan E, Wang L, Paulsen MT, Ljungman M, et al. Zinc finger protein 407 (ZFP407) regulates insulin-stimulated glucose uptake and glucose transporter 4 (Glut4) mRNA. J Biol Chem. 2015;290(10):6376–86. doi: 10.1074/jbc.M114.623736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miranda ER, Dey CS. Effect of chromium and zinc on insulin signaling in skeletal muscle cells. Biol Trace Elem Res. 2004;101(1):19–36. doi: 10.1385/BTER:101:1:19. [DOI] [PubMed] [Google Scholar]
- 8.May JM, Contoreggi CS. The mechanism of the insulin-like effects of ionic zinc. J Biol Chem. 1982;257(8):4362–8. [PubMed] [Google Scholar]
- 9.Hurley LS, Keen CL, Baly DL. Manganese deficiency and toxicity: effects on carbohydrate metabolism in the rat. Neurotoxicology. 1984;5(1):97–104. [PubMed] [Google Scholar]
- 10.Baly DL, Curry DL, Keen CL, Hurley LS. Effect of manganese deficiency on insulin secretion and carbohydrate homeostasis in rats. J Nutr. 1984;114(8):1438–46. doi: 10.1093/jn/114.8.1438. [DOI] [PubMed] [Google Scholar]
- 11.Ueda M, Robinson FW, Smith MM, Kono T. Effects of divalent cations on the regulation of insulin-sensitive glucose transport and cAMP phosphodiesterase in adipocytes. Insulin-like effects of divalent cations. J Biol Chem. 1984;259(15):9520–5. [PubMed] [Google Scholar]
- 12.Baly DL, Schneiderman JS, Garcia-Welsh AL. Effect of manganese deficiency on insulin binding, glucose transport and metabolism in rat adipocytes. J Nutr. 1990;120(9):1075–9. doi: 10.1093/jn/120.9.1075. [DOI] [PubMed] [Google Scholar]
- 13.Bo S, Durazzo M, Gambino R, Berutti C, Milanesio N, Caropreso A, et al. Associations of dietary and serum copper with inflammation, oxidative stress, and metabolic variables in adults. J Nutr. 2008;138(2):305–10. doi: 10.1093/jn/138.2.305. [DOI] [PubMed] [Google Scholar]
- 14.Wessling-Resnick M. Iron homeostasis and the inflammatory response. Annu Rev Nutr. 2010;30:105–22. doi: 10.1146/annurev.nutr.012809.104804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deb S, Johnson EE, Robalinho-Teixeira RL, Wessling-Resnick M. Modulation of intracellular iron levels by oxidative stress implicates a novel role for iron in signal transduction. Biometals. 2009;22(5):855–62. doi: 10.1007/s10534-009-9214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park K, Gross M, Lee DH, Holvoet P, Himes JH, Shikany JM, et al. Oxidative stress and insulin resistance: the coronary artery risk development in young adults study. Diabetes Care. 2009;32(7):1302–7. doi: 10.2337/dc09-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brudevold R, Hole T, Hammerstrom J. Hyperferritinemia is associated with insulin resistance and fatty liver in patients without iron overload. PLoS One. 2008;3(10):e3547. doi: 10.1371/journal.pone.0003547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HN, Song SW. Concentrations of chromium, selenium, and copper in the hair of viscerally obese adults are associated with insulin resistance. Biol Trace Elem Res. 2014;158(2):152–7. doi: 10.1007/s12011-014-9934-6. [DOI] [PubMed] [Google Scholar]
- 19.Pidduck HG, Wren PJ, Evans DA. Plasma zinc and copper in diabetes mellitus. Diabetes. 1970;19(4):234–9. doi: 10.2337/diab.19.4.234. [DOI] [PubMed] [Google Scholar]
- 20.Ekin S, Mert N, Gunduz H, Meral I. Serum sialic acid levels and selected mineral status in patients with type 2 diabetes mellitus. Biol Trace Elem Res. 2003;94(3):193–201. doi: 10.1385/BTER:94:3:193. [DOI] [PubMed] [Google Scholar]
- 21.McNair P, Christiansen C, Christensen MS, Madsbad S, Faber OK, Binder C, et al. Development of bone mineral loss in insulin-treated diabetes: a 1 1/2 years follow-up study in sixty patients. Eur J Clin Invest. 1981;11(1):55–9. doi: 10.1111/j.1365-2362.1981.tb01765.x. [DOI] [PubMed] [Google Scholar]
- 22.Raz I, Havivi E. Trace elements in blood cells of diabetic subjects. Diabetes Res. 1989;10(1):21–4. [PubMed] [Google Scholar]
- 23.Car N, Car A, Granić M, Skrabalo Z, Momcilović B. Zinc and copper in the serum of diabetic patients. Biol Trace Elem Res. 1992;32:325–9. doi: 10.1007/BF02784618. [DOI] [PubMed] [Google Scholar]
- 24.Williams NR, Rajput-Williams J, West JA, Nigdikar SV, Foote JW, Howard AN. Plasma, granulocyte and mononuclear cell copper and zinc in patients with diabetes mellitus. Analyst. 1995;120(3):887–90. doi: 10.1039/an9952000887. [DOI] [PubMed] [Google Scholar]
- 25.Al-Maroof RA, Al-Sharbatti SS. Serum zinc levels in diabetic patients and effect of zinc supplementation on glycemic control of type 2 diabetics. Saudi Med J. 2006;27(3):344–50. [PubMed] [Google Scholar]
- 26.Sfar S, Jawed A, Braham H, Amor S, Laporte F, Kerkeni A. Zinc, copper and antioxidant enzyme activities in healthy elderly Tunisian subjects. Exp Gerontol. 2009;44(12):812–7. doi: 10.1016/j.exger.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Bates CJ, Prentice A, Finch S. Gender differences in food and nutrient intakes and status indices from the National Diet and Nutrition Survey of people aged 65 years and over. Eur J Clin Nutr. 1999;53(9):694–9. doi: 10.1038/sj.ejcn.1600834. [DOI] [PubMed] [Google Scholar]
- 28.Azab SF, Saleh SH, Elsaeed WF, Elshafie MA, Sherief LM, Esh AM. Serum trace elements in obese Egyptian children: a case-control study. Ital J Pediatr. 2014;40:20. doi: 10.1186/1824-7288-40-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi BC, Shi F. Risk factors for diabetes mellitus by age and sex: results of the National Population Health Survey. Diabetologia. 2001;44(10):1221–31. doi: 10.1007/s001250100648. [DOI] [PubMed] [Google Scholar]
- 30.Kesavadev JD, Short KR, Nair KS. Diabetes in old age: an emerging epidemic. J Assoc Physicians India. 2003;51:1083–94. [PubMed] [Google Scholar]
- 31.Kautzky-Willer A, Harreiter J, Pacini G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr Rev. 2016;37(3):278–316. doi: 10.1210/er.2015-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onat A, Avci GS, Barlan MM, Uyarel H, Uzunlar B, Sansoy V. Measures of abdominal obesity assessed for visceral adiposity and relation to coronary risk. Int J Obes Relat Metab Disord. 2004;28(8):1018–25. doi: 10.1038/sj.ijo.0802695. [DOI] [PubMed] [Google Scholar]
- 33.Cumming G. Understanding the New Statistics: Effect sizes, Confidence Intervals, and Meta-Analysis. New York, NY: Routledge; 2012. [Google Scholar]
- 34.Tipton E. Small sample adjustments for robust variance estimation with meta-regression. Psychol Methods. 2015;20(3):375–93. doi: 10.1037/met0000011. [DOI] [PubMed] [Google Scholar]
- 35.Mateo MC, Bustamante JB, Cantalapiedra MA. Serum, zinc, copper and insulin in diabetes mellitus. Biomedicine. 1978;29(2):56–8. [PubMed] [Google Scholar]
- 36.Hughes K, Choo M, Kuperan P, Ong CN, Aw TC. Cardiovascular risk factors in non-insulin-dependent diabetics compared to non-diabetic controls: a population-based survey among Asians in Singapore. Atherosclerosis. 1998;136(1):25–31. doi: 10.1016/s0021-9150(97)00180-9. [DOI] [PubMed] [Google Scholar]
- 37.Daimon M, Susa S, Yamatani K, Manaka H, Hama K, Kimura M, et al. Hyperglycemia is a factor for an increase in serum ceruloplasmin in type 2 diabetes. Diabetes Care. 1998;21(9):1525–8. doi: 10.2337/diacare.21.9.1525. [DOI] [PubMed] [Google Scholar]
- 38.Kim CH, Kim HK, Bae SJ, Park JY, Lee KU. Association of elevated serum ferritin concentration with insulin resistance and impaired glucose metabolism in Korean men and women. Metabolism. 2011;60(3):414–20. doi: 10.1016/j.metabol.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 39.Olaniyan O, Awonuga MAM, Ajetunmobi AF, Adeleke IA, Fagbolade OJ, Olabiyi KO, Oyekanmi BA, Osadolor HB. Serum copper and zinc levels in Nigerian type 2 diabetic patients. Afr J Diabetes Med. 2012;20(2):36–38. [Google Scholar]
- 40.Memişoğullari R, Bakan E. Levels of ceruloplasmin, transferrin, and lipid peroxidation in the serum of patients with Type 2 diabetes mellitus. J Diabetes Complications. 2004;18(4):193–7. doi: 10.1016/S1056-8727(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 41.Memisoğullari R, Taysi S, Bakan E, Capoglu I. Antioxidant status and lipid peroxidation in type II diabetes mellitus. Cell Biochem Funct. 2003;21(3):291–6. doi: 10.1002/cbf.1025. [DOI] [PubMed] [Google Scholar]
- 42.Qin LX, Zeng X, Huang G. Changes in serum and urine ceruloplasmin concentrations in type 2 diabetes] Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2004;29(2):208–11. [PubMed] [Google Scholar]
- 43.Elis A, Ferencz JR, Gilady G, Livne A, Assia EI, Lishner M. Is serum ferritin high in patients with diabetic retinopathy? A controlled study. Endocr Res. 2004;30(2):141–7. doi: 10.1081/erc-200027354. [DOI] [PubMed] [Google Scholar]
- 44.Jiang R, Manson JE, Meigs JB, Ma J, Rifai N, Hu FB. Body iron stores in relation to risk of type 2 diabetes in apparently healthy women. JAMA. 2004;291(6):711–7. doi: 10.1001/jama.291.6.711. [DOI] [PubMed] [Google Scholar]
- 45.Elsammak M, Refai W, Elsawaf A, Abdel-Fattah I, Abd Elatti E, Ghazal A. Elevated serum tumor necrosis factor alpha and ferritin may contribute to the insulin resistance found in HCV positive Egyptian patients. Curr Med Res Opin. 2005;21(4):527–34. doi: 10.1185/030079905X38141. [DOI] [PubMed] [Google Scholar]
- 46.Kaviarasan K, Arjunan MM, Pugalendi KV. Lipid profile, oxidant-antioxidant status and glycoprotein components in hyperlipidemic patients with/without diabetes. Clin Chim Acta. 2005;362(1–2):49–56. doi: 10.1016/j.cccn.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 47.Vîrgolici B, Mohora M, Stoian I, Lixandru D, Găman L, Paveliu F. A comparative oxidative stress study--obesity with and without diabetes mellitus. Rom J Intern Med. 2005;43(3–4):261–8. [PubMed] [Google Scholar]
- 48.Abou-Shousha S, Abd El-Megeed MH, Sultan HK. Interleukin-8, ferritin and soluble transferrin receptors in type II diabetes mellitus. Egypt J Immunol. 2006;13(1):19–25. [PubMed] [Google Scholar]
- 49.Lee DH, Liu DY, Jacobs DR, Shin HR, Song K, Lee IK, et al. Common presence of non-transferrin-bound iron among patients with type 2 diabetes. Diabetes Care. 2006;29(5):1090–5. doi: 10.2337/diacare.2951090. [DOI] [PubMed] [Google Scholar]
- 50.Adewumi MT, Njoku CH, Saidu Y, Abubakar MK, Shehu RA, Bilbis LS. Serum Chromium, Copper and Manganese Levels of Diabetic Subjects in Katsina, Nigeria. Asian Journal of Biochemistry. 2007;2(4):284–288. [Google Scholar]
- 51.Viktorínová A, Toserová E, Krizko M, Duracková Z. Altered metabolism of copper, zinc, and magnesium is associated with increased levels of glycated hemoglobin in patients with diabetes mellitus. Metabolism. 2009;58(10):1477–82. doi: 10.1016/j.metabol.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 52.Rajpathak SN, Wylie-Rosett J, Gunter MJ, Negassa A, Kabat GC, Rohan TE, et al. Biomarkers of body iron stores and risk of developing type 2 diabetes. Diabetes Obes Metab. 2009;11(5):472–9. doi: 10.1111/j.1463-1326.2008.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flores CR, Puga MP, Wrobel K, Garay Sevilla ME. Trace elements status in diabetes mellitus type 2: possible role of the interaction between molybdenum and copper in the progress of typical complications. Diabetes Res Clin Pract. 2011;91(3):333–41. doi: 10.1016/j.diabres.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 54.Sarkar A, Dash S, Barik BK, Muttigi MS, Kedage V, Shetty JK, et al. Copper and ceruloplasmin levels in relation to total thiols and GST in type 2 diabetes mellitus patients. Indian J Clin Biochem. 2010;25(1):74–6. doi: 10.1007/s12291-010-0015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ashourpour M, Djalali M, Djazayery A, Eshraghian MR, Taghdir M, Saedisomeolia A. Relationship between serum ferritin and inflammatory biomarkers with insulin resistance in a Persian population with type 2 diabetes and healthy people. Int J Food Sci Nutr. 2010;61(3):316–23. doi: 10.3109/09637480903555150. [DOI] [PubMed] [Google Scholar]
- 56.Chacko SK, Cheluvappa R. Increased ceruloplasmin and fibrinogen in type 2 diabetes corresponds to decreased anti-oxidant activity in a preliminary tertiary South Indian hospital study. Exp Clin Endocrinol Diabetes. 2010;118(1):64–67. doi: 10.1055/s-0029-1225647. [DOI] [PubMed] [Google Scholar]
- 57.Nasli-Esfahani E, Faridbod F, Larijani B, Ganjali MR, Norouzi P. Trace element analysis of hair, nail, serum and urine of diabetes mellitus patients by inductively coupled plasma atomic emission spectroscopy. J Diabetes Metab Disord. 2011;10:1–9. [Google Scholar]
- 58.Ferdousi S, Mia AR. Serum levels of copper and zinc in newly diagnosed type-2 diabetic subjects. Mymensingh Med J. 2012;21(3):475–8. [PubMed] [Google Scholar]
- 59.Savu O, Ionescu-Tirgoviste C, Atanasiu V, Gaman L, Papacocea R, Stoian I. Increase in total antioxidant capacity of plasma despite high levels of oxidative stress in uncomplicated type 2 diabetes mellitus. J Int Med Res. 2012;40(2):709–16. doi: 10.1177/147323001204000235. [DOI] [PubMed] [Google Scholar]
- 60.Ganesh S, Dharmalingam M, Marcus SR. Oxidative stress in type 2 diabetes with iron deficiency in Asian Indians. J Med Biochem. 2012;31(2):115–120. [Google Scholar]
- 61.Farid SM, Abulfaraj TG. Trace Mineral Status Related to Levels of Glycated Hemoglobin of Type 2 Diabetic Subjects in Jeddah, Saudi Arabia. Med J Islamic World Acad Sci. 2013;21(2):47–56. [Google Scholar]
- 62.Dosa MD, Adumitresi CR, Hangan LT, Nechifor M. Copper, zinc and magnesium in non-insulin-dependent diabetes mellitus treated with metformin. Diabetes Mellitus - Insights and Perspectives. 2013 [Google Scholar]
- 63.Kundu D, Roy A, Mandal T, Bandyopadhyay U, Ghosh E, Ray D. Relation of iron stores to oxidative stress in type 2 diabetes. Niger J Clin Pract. 2013;16(1):100–3. doi: 10.4103/1119-3077.106776. [DOI] [PubMed] [Google Scholar]
- 64.Santa SR, Swati B, Choudhury KM, Santasmita P, Aruna B, Gargi S, Soma G. Status of serum magnesium, zinc & copper in patients suffering from type 2 diabetes mellitus. J Drug Ther. 2014;4(1):70–72. [Google Scholar]
- 65.Oyedeji SO, Adesina AA, Oke OT, Tijani YO. Evaluation of essential trace metals in female type 2 diabetes mellitus patients in Nigerian population. Afr J Biotechnol. 2014;13(18):1910–1914. [Google Scholar]
- 66.Kumar DA, Priya VS, Jaiprabhu J, Ramalingam K. Serum copper and zinc levels significance in type 2 diabetic patients. J Med Sci Tech. 2014;3(2):79–81. [Google Scholar]
- 67.Alam F, Fatima F, Orakzai S, Iqbal N, Fatima SS. Elevated levels of ferritin and hs-CRP in type 2 diabetes. J Pak Med Assoc. 2014;64(12):1389–91. [PubMed] [Google Scholar]
- 68.Nagarajrao R, Alharbi SA. Evaluation of serum zinc, copper, magnesium and iron levels in type 2 diabetes mellitus patients. Int J Adv Res. 2015;3(2):960–965. [Google Scholar]
- 69.Maheshwari AV, Bhoi BK, Sadariya BR, Javia HN, Gusani JK, Sharma H. Correlation between serum ferritin and glycaemic control in patients of type 2 diabetes mellitus: a case control study. Int J Res Med Sc. 2015;3(9):2327–2330. [Google Scholar]
- 70.Badran M, Morsy R, Soliman H, Elnimr T. Assessment of trace elements levels in patients with Type 2 diabetes using multivariate statistical analysis. J Trace Elem Med Biol. 2016;33:114–9. doi: 10.1016/j.jtemb.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 71.Atari-Hajipirloo S, Valizadeh N, Khadem-Ansari MH, Rasmi Y, Kheradmand F. Altered Concentrations of Copper, Zinc, and Iron are Associated With Increased Levels of Glycated Hemoglobin in Patients With Type 2 Diabetes Mellitus and Their First-Degree Relatives. Int J Endocrinol Metab. 2016;14(2):e33273. doi: 10.5812/ijem.33273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang X, Zhang M, Lui G, Chang H, Liu W, Li Z, et al. Associations of Serum Manganese Levels with Prediabetes and Diabetes among ≥60-Year-Old Chinese Adults: A Population-Based Cross-Sectional Analysis. Nutrients. 2016;8(8) doi: 10.3390/nu8080497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Devi TR, Hijam D, Dubey A, Debnath A, Oinam P, Devi NGT, Singh WG. Study of serum zinc and copper levels in type 2 diabetes mellitus. ICJMR. 2016;3(4):1036–1040. [Google Scholar]
- 74.Goud GKV, Patil S, Rahman MA. A study on antioxidants and iron nutritional status in type 2 diabetes mellitus with and without coronary heart disease. Int J Biomed Res. 2016;7:495–498. [Google Scholar]
- 75.Jeppu AK, Augusthy A, Kumar KA. Plasma glucose and serum ceruloplasmin in metabolic syndrome and diabetes mellitus type 2. RABM. 2016;2:15–19. [Google Scholar]
- 76.Borah M, Goswami RK. Evaluation of serum ferritin in in type II diabetes mellitus: a hospital based observational study from Dibrugarh, Assam, India. Int J Res Med Sc. 2016;4(11):4916–4921. [Google Scholar]
- 77.Eva H, Akhter QS, Alam K. Serum zinc and manganese levels in subjects with type 2 diabetes mellitus. J Bangladesh Soc Physiol. 2016;11(2):50–53. [Google Scholar]
- 78.Zhang H, Yan C, Yang Z, Zhang W, Niu Y, Li X, et al. Alterations of serum trace elements in patients with type 2 diabetes. J Trace Elem Med Biol. 2017;40:91–6. doi: 10.1016/j.jtemb.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 79.Kim NH, Oh JH, Choi KM, Kim YH, Baik SH, Choi DS, et al. Serum ferritin in healthy subjects and type 2 diabetic patients. Yonsei Med J. 2000;41(3):387–92. doi: 10.3349/ymj.2000.41.3.387. [DOI] [PubMed] [Google Scholar]
- 80.Kinlaw WB, Levine AS, Morley JE, Silvis SE, McClain CJ. Abnormal zinc metabolism in type II diabetes mellitus. Am J Med. 1983;75(2):273–7. doi: 10.1016/0002-9343(83)91205-6. [DOI] [PubMed] [Google Scholar]
- 81.Cunningham J, Leffell M, Mearkle P, Harmatz P. Elevated plasma ceruloplasmin in insulin-dependent diabetes mellitus: evidence for increased oxidative stress as a variable complication. Metabolism. 1995;44(8):996–9. doi: 10.1016/0026-0495(95)90095-0. [DOI] [PubMed] [Google Scholar]
- 82.Das TK, Wati MR, Fatima-Shad K. Oxidative Stress Gated by Fenton and Haber Weiss Reactions and Its Association With Alzheimer’s Disease. Archives of Neuroscience. 2015;2(2) [Google Scholar]
- 83.Cruz KJ, de Oliveira AR, Marreiro Ddo N. Antioxidant role of zinc in diabetes mellitus. World J Diabetes. 2015;6(2):333–7. doi: 10.4239/wjd.v6.i2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Coassin M, Ursini F, Bindoli A. Antioxidant effect of manganese. Arch Biochem Biophys. 1992;299(2):330–3. doi: 10.1016/0003-9861(92)90282-2. [DOI] [PubMed] [Google Scholar]
- 85.Aguirre JD, Culotta VC. Battles with iron: manganese in oxidative stress protection. J Biol Chem. 2012;287(17):13541–8. doi: 10.1074/jbc.R111.312181. [DOI] [PMC free article] [PubMed] [Google Scholar]