Abstract
Context:
Possible linkage between biocide and antibiotic resistance in bacteria is a major area of concern.
Aim:
To evaluate the susceptibility of multidrug-resistant (MDR) bacteria to four commonly used biocides.
Settings and Design:
A pilot study was conducted in a tertiary care hospital from April to November 2017.
Materials and Methods:
Fifty-four MDR bacterial isolates, namely Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Staphylococcus aureus, were obtained from various clinical samples of inpatients. These isolates were subjected to tube dilution method for determining minimum inhibitory concentration (MIC) of four commonly used biocides in our hospital, namely 5% w/v povidone iodine, absolute ethanol (99.9%), sodium hypochlorite (4% available chlorine), and quaternary ammonium compounds (QACs) (3.39%). Minimum bactericidal concentration (MBC) of these biocides was determined as per standard guidelines. Similar tests were also performed on corresponding American Type Culture Collection (ATCC) bacterial strains.
Statistical Analysis:
The Fisher exact test.
Results:
Twenty-two MDR bacterial isolates had higher MIC values for QACs than their corresponding ATCC strains. Statistically significant difference in proportion of test isolates exhibiting higher MIC values for QACs and absolute ethanol was observed (P-value = 0.02). Twenty-four MDR bacterial isolates exhibited higher MBC values for sodium hypochlorite than their corresponding ATCC strains. The difference in proportion of test isolates exhibiting higher MBC values for sodium hypochlorite and absolute ethanol, respectively, was statistically significant (P-value <0.0001). The difference in proportion of test isolates exhibiting higher MBC values for absolute ethanol versus QACs and povidone iodine, respectively, was statistically significant (P-values = 0.0003 and 0.0076). Statistically significant differences in susceptibility to biocides among test isolates were also observed.
Conclusion:
Emergence of biocide resistance among MDR bacteria poses a serious threat to our efforts in containing outbreaks of nosocomial infections.
KEYWORDS: Biocides, MBC, MDR bacteria, MIC
INTRODUCTION
Biocides are antiseptics and disinfectants containing active chemical agents, which are extensively used as an integral part of hospital infection control activities.[1] Bacteria vary in their susceptibility to these agents, with bacterial spores being the most resistant.[2] Biocides have different modes of action against various groups of microorganisms. Chlorine-releasing agents such as sodium hypochlorite, which are widely used for disinfection of hard surfaces and blood spills, are highly active oxidizing agents and destroy the cellular activity of proteins. Hypochlorous acid has been considered the active moiety responsible for bacterial inactivation.[3] Similarly, iodine is rapidly bactericidal, fungicidal, virucidal, and sporicidal as it penetrates into proteins and fatty acids of microorganisms, thereby leading to cell death.[4] Quaternary ammonium compounds (QACs) are membrane-active agents, which primarily target the cytoplasmic membrane of bacteria leading to cell damage.[5] Alcohols cause membrane damage and rapid denaturation of proteins, resulting in interference with microbial metabolism leading to cell lysis.[6]
It has often been stated that widespread use of biocides may result in development of microbial resistance.[1] Efflux pumps and cell wall damage have been implicated as possible mechanisms in both biocide- and antibiotic-resistant bacteria. Possibility of common genetic linkage for biocide and antibiotic resistance also exists.[7] Usually “biocide resistance” is interpreted on the basis of minimum inhibitory concentrations (MICs), which reflect their activity against the most sensitive target site. When used at bactericidal concentrations, the concept of efficacy has little or no relevance. It is thus suggested that the term “reduced susceptibility” is relatively better than resistance.[8] MICs are considered as the “gold standard” for determining the susceptibility of organisms to antimicrobials and are therefore used to judge the performance of all other methods of susceptibility testing. MICs are also used in diagnostic laboratories to confirm unusual resistance.[9] However, no standard guidelines exist for testing biocide susceptibility.[10,11]
Antimicrobial resistance is spreading globally, thereby posing a serious threat to humankind. Possible linkage between biocide and antibiotic resistance is now a major topic of discussion and concern. The increased use of disinfectants such as phenolics and QACs has raised some concerns about their overall efficacy.[12,13] There is paucity of literature on the subject of biocide susceptibility in multidrug-resistant (MDR) bacterial isolates.[14,15] This study was conducted to evaluate the susceptibility of MDR bacteria to four commonly used disinfectants/antiseptics, to establish any possible correlation between antibiotic and biocide resistance.
SUBJECTS AND METHODS
A pilot study was conducted in a tertiary care hospital situated in Rishikesh, Uttarakhand, India, from April to November 2017. Fifty-four MDR bacterial isolates (which were identified up to species level as per standard procedures)[16,17] were obtained from various clinical samples of inpatients such as pus, sputum, endotracheal aspirates, drain fluids, and urine. The test isolates included the following: Escherichia coli (n = 26), Klebsiella pneumoniae (n = 8), Pseudomonas aeruginosa (n = 10), and Staphylococcus aureus (n = 10). The antibiotic susceptibility profile of these isolates was generated using modified Kirby–Bauer disk diffusion method as per the 2017 Clinical and Laboratory Standards Institute guidelines.
The test isolates were subjected to tube dilution method by Mazzola et al.[18] for determining MIC of four commonly used biocides in our hospital, namely 5% w/v povidone iodine (0.5% w/v of available iodine), absolute ethanol (99.9%), sodium hypochlorite (4% available chlorine), and QACs (3.39%), respectively.
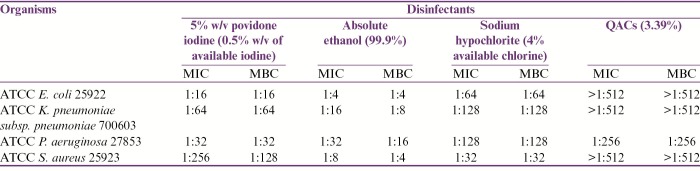
Minimum bactericidal concentration (MBC) of these biocides was determined by subculturing from each of the tubes used for determining MIC onto nutrient agar plates, which were then incubated aerobically at 37°C for 24–48h. The highest dilution of biocide at which no growth was obtained on nutrient agar plates was taken as MBC. Similar tests were also performed on American Type Culture Collection (ATCC) E. coli 25922, ATCC K. pneumoniae subsp. pneumoniae 700603, ATCC P. aeruginosa 27853, and ATCC S. aureus 25923, which were used as control strains.
Categorical variables were presented as proportions. Categorical variables were compared using the Fisher exact test. All statistical tools were two tailed and a significant level (P-value <0.05) was used. All statistical tests were performed using InStat software (GraphPad Software, San Diego, CA).
RESULTS
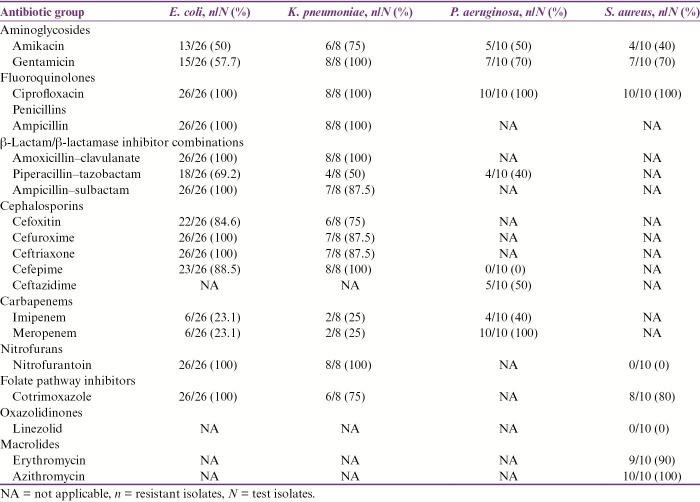
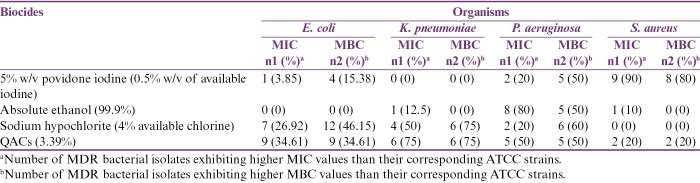
The percentage antibiotic resistance pattern of all 54 MDR bacterial isolates has been shown in Table 1. The MIC and MBC results of ATCC bacterial strains for four commonly used biocides in our hospital have been shown in Table 2. The MIC and MBC results of test isolates for the biocides used in our study have been shown in Table 3 and are summarized as follows:
Table 1.
Percentage antibiotic resistance pattern of MDR bacterial isolates
Table 2.
MIC and MBC results of ATCC bacterial strains for four commonly used biocides in our hospital
Table 3.
Summary of MIC and MBC results of test isolates for four commonly used biocides in our hospital
5% w/v povidone iodine (0.5% w/v of available iodine): Among MDR E. coli isolates, one (3.85%) and four (15.38%) had higher MIC and MBC values, respectively, than their corresponding ATCC strain. All MDR K. pneumoniae isolates exhibited similar MIC and MBC values as those of their control strain. Two (20%) and five (50%) MDR P. aeruginosa isolates showed higher MIC and MBC values, respectively, when compared to their corresponding ATCC strain. Nine (90%) and eight (80%) isolates of MDR S. aureus exhibited higher MIC and MBC values than their corresponding control strain. Statistically significant difference in proportion of MDR S. aureus isolates exhibiting higher MIC values in comparison to MDR E. coli, P. aeruginosa, and K. pneumoniae, respectively, was observed (P-values: <0.0001, 0.005, and 0.0004). The proportion of MDR S. aureus isolates exhibiting higher MBC values was found to be significantly greater than that of MDR E. coli and K. pneumoniae, respectively (P-values: 0.0006 and 0.001). The difference in proportion of MDR S. aureus isolates exhibiting higher MBC values than MDR P. aeruginosa was not found to be statistically significant (P-value: 0.35).
Absolute ethanol (99.9%): All MDR E. coli isolates exhibited similar MIC and MBC values as those of their corresponding ATCC strain. Although one of eight (12.5%) MDR K. pneumoniae isolates exhibited higher MIC, none of these had higher MBC values as compared to their corresponding control strain. Among MDR P. aeruginosa isolates, eight (80%) and five (50%) had higher MIC and MBC values, respectively, with respect to ATCC P. aeruginosa 27853. Although only one (10%) MDR S. aureus isolate had higher MIC, none of these isolates had higher MBC values than the corresponding control strain. The proportion of MDR P. aeruginosa isolates exhibiting higher MIC and MBC values was found to be significantly greater than that of MDR E. coli, S. aureus, and K. pneumoniae, respectively (P-values: <0.0001 and 0.0007, 0.005 and 0.03, and 0.001 and 0.04).
Sodium hypochlorite (4% available chlorine): Seven (26.92%) and twelve (46.15%) MDR E. coli isolates exhibited higher MIC and MBC values, respectively, with respect to their corresponding control strain. Among MDR K. pneumoniae isolates, four (50%) and six (75%), respectively, had higher MIC and MBC values in comparison to their control strain. Two (20%) and six (60%) MDR P. aeruginosa isolates had higher MIC and MBC values, respectively, than the corresponding ATCC strain. All MDR S. aureus isolates had similar MIC and MBC values, respectively, as those of their corresponding ATCC strain. The difference in proportion of MDR E. coli isolates exhibiting higher MIC values as compared to MDR S. aureus, K. pneumoniae, and P. aeruginosa, respectively, was not found to be statistically significant (P-values: 0.15, 0.4, and 1.0). Significantly higher proportion of MDR E. coli isolates had greater MBC values than MDR S. aureus (P-value: 0.01). However, the difference in proportion of MDR E. coli exhibiting higher MBC than MDR K. pneumoniae and P. aeruginosa isolates, respectively, was not found to be statistically significant (P-values: 0.23 and 0.71).
QACs (3.39%): Nine (34.61%), six (75%), five (50%), and two (20%) MDR E. coli, K. pneumoniae, P. aeruginosa, and S. aureus isolates respectively exhibited higher MIC and MBC values in comparison to their corresponding ATCC strains. No statistically significant difference in proportion of MDR E. coli isolates exhibiting higher MIC and MBC values when compared to MDR S. aureus, K. pneumoniae, and P. aeruginosa, respectively, was observed (P-values: 0.68 and 0.68, 0.10 and 0.10, and 0.46 and 0.46).
Overall, 22 (40.7%) MDR bacterial isolates had higher MIC values for QACs (3.9%) than their corresponding ATCC strains. Of the MDR bacterial isolates, 10 (18.5%), 12 (22.2%), and 13 (24.1%) had higher MIC values for absolute ethanol (99.9%), 5% w/v povidone iodine, and sodium hypochlorite (4% available chlorine), respectively, than their corresponding control strains. The difference in proportion of test isolates exhibiting higher MIC values for QACs (3.9%) and 5% w/v povidone iodine, respectively, was not found to be statistically significant (P-value: 0.06; 95% CI of difference: 0.01–0.36). Although the difference in proportion of test isolates exhibiting higher MIC values for QACs (3.9%) and absolute ethanol (99.9%) was found to be statistically significant (P-value: 0.02; 95% CI of difference: 0.05–0.4), it was not so when MIC results of QACs (3.9%) and sodium hypochlorite (4% available chlorine) were compared (P-value: 0.09; 95% CI of difference: −0.01–0.34).
Twenty-four (44.4%) MDR bacterial isolates exhibited higher MBC values for sodium hypochlorite (4% available chlorine) than their corresponding ATCC strains. Of the test isolates, 17 (31.5%), 5 (9.2%), and 22 (40.7%) had higher MBC values for 5% w/v povidone iodine, absolute ethanol (99.9%), and QACs (3.9%), respectively, than their corresponding control strains. While the difference in proportion of test isolates exhibiting higher MBC values for sodium hypochlorite (4% available chlorine) and absolute ethanol (99.9%), respectively, was found to be statistically significant (P-value: <0.0001; 95% CI of difference: 0.31–0.59), it was not so when MBC results of sodium hypochlorite (4% available chlorine) were compared with those of 5% w/v povidone iodine and QACs (3.9%), respectively (P-values: 0.23 and 0.84; 95% CI of difference: −0.05–0.31 and −0.15–0.22). The difference in proportion of test isolates exhibiting higher MBC values for absolute ethanol (99.9%) as compared to QACs (3.9%) and 5% w/v povidone iodine, respectively, was found to be statistically significant (P-values: 0.0003 and 0.0076; 95% CI of difference: 0.27–0.55 and 0.19–0.45).
DISCUSSION
There is no concrete evidence on the subject of biocide resistance in medical literature. However, various recent findings have underlined the importance of biocide resistance as a clinically relevant phenomenon. Nosocomial outbreaks of biocide-resistant organisms have also been described.[19]
In this study, 18.5%, 22.2%, 24.1%, and 40.7% of test isolates respectively showed higher MIC values for absolute ethanol (99.9%), 5% w/v povidone iodine, sodium hypochlorite (4% available chlorine), and QACs (3.9%) than their corresponding ATCC strains. The proportion of test isolates exhibiting higher MIC values for QACs (3.9%) was found to be significantly higher than absolute ethanol (99.9%). Likewise, 9.2%, 31.5%, 40.7%, and 44.4% of these isolates respectively exhibited higher MBC values for absolute ethanol (99.9%), 5% w/v povidone iodine, QACs (3.9%), and sodium hypochlorite (4% available chlorine) as compared to control strains. The proportion of MDR bacterial isolates exhibiting higher MBC values for sodium hypochlorite (4% available chlorine) was found to be significantly higher as compared to absolute ethanol (99.9%). Also, the proportion of test isolates exhibiting higher MBC values for absolute ethanol (99.9%) was significantly higher than that for QACs (3.9%) and 5% w/v povidone iodine, respectively. Statistically significant differences in susceptibility to 5% w/v povidone iodine and absolute ethanol (99.9%) respectively among different MDR bacterial isolates were also observed. In the light of these findings, it can be hypothesized that there exists a possible linkage between antibiotic resistance and reduced biocide susceptibility in bacteria.
Laboratory studies conducted by various researchers have shown that bacteria can become less susceptible to biocides, and cross-resistance may occur to other biocides as well as to antibiotics.[19] Alexander et al.[20] suggested that antibiotic-resistant organisms were generally more disinfectant resistant. Although not correlated, an important observation of his study was that high antibiotic resistance could lead to some biocide-resistant organisms. A study conducted by Stickler et al.[19] provided preliminary evidence on emergence of MDR gram-negative bacteria resistant to chlorhexidine responsible for catheter-associated urinary-tract infections. In a study conducted by Vijayakumar et al.,[21] it was seen that 18.2% and 45.5% of MDR P. aeruginosa isolates exhibited reduced susceptibility to QACs of benzalkonium chloride and cetrimide, respectively. Another important finding of this study was that there was no correlation between reduced susceptibility to biocides and multiple antibiotic resistance in Acinetobacter baumannii and K. pneumoniae, respectively.[21] Naparstek et al.[22] analyzed MICs of chlorhexidine across a large series of 126 isolates of extremely drug resistant K. pneumoniae using agar dilution method. These researchers reported that 90% isolates had high MIC values and reduced susceptibility compared with a control strain.[22] Russell et al.[23] revealed that chlorhexidine gluconate resistance in Pseudomonas stutzeri correlated with resistance to polymyxin B, gentamicin, erythromycin, and ampicillin. Bhatia et al.[24] reported the first case of carbapenem-resistant K. pneumoniae (Cr-Kp) with reduced susceptibility to sodium hypochlorite (4% available chlorine) from India.
Similar findings have been observed among gram-positive cocci also. In a study conducted by Fraise,[25] it was observed that enterococci exhibited intrinsic resistance to chemical agents. However, no clear relationship between antimicrobial resistance and resistance to chemical agents could be established among enterococci. In the same study, it was shown that some strains of methicillin resistant S. aureus, which had indeterminate resistance to glycopeptides, displayed reduced susceptibility to some biocides.[25] A pilot study conducted by Bhatia et al.[26] highlighted that more number of glycopeptide-resistant enterococci exhibited lower susceptibility to 5% w/v povidone iodine in comparison to glycopeptide-sensitive strains.
CONCLUSION
Although inconclusive, the results obtained in our study are pointing toward reduced susceptibility of MDR bacterial isolates to commonly used biocides in hospital settings. The possibility of emergence of biocide resistance among MDR strains of E. coli, K. pneumoniae, P. aeruginosa, and S. aureus poses a serious threat to our efforts in containing outbreaks of nosocomial infections. Studies with larger data sets employing more antibiotics and biocides are needed to confirm or challenge the hypothesis of emerging biocide resistance among MDR bacterial isolates. Time kill studies complemented with genetic analysis of biocide resistance should be encouraged. Also, formulation of standardized testing methodologies for biocide susceptibility is need of the hour.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12:147–79. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ducel G, Fabry J, Nicolle L, editors. WHO. Prevention of hospital-acquired infections: a practical guide. 2nd ed. Geneva, Switzerland: World Health Organization; 2002. [Google Scholar]
- 3.Bloomfield SF. Chlorine and iodine formulations. In: Ascenzi JM, editor. Handbook of disinfectants and antiseptics. New York: Marcel Dekker; 1996. pp. 133–58. [Google Scholar]
- 4.Gottardi W. Iodine and iodine compounds. In: Block SS, editor. Disinfection, sterilization, and preservation. 4th ed. Philadelphia, PA: Lea & Febiger; 1991. pp. 152–66. [Google Scholar]
- 5.Frier M. Derivatives of 4-amino-quinaldinium and 8-hydroxyquinoline. In: Hugo WB, editor. Inhibition and destruction of the microbial cell. London, UK: Academic Press; 1971. pp. 107–20. [Google Scholar]
- 6.Larson EL, Morton HE. Alcohols. In: Block SS, editor. Disinfection, sterilization, and preservation. 4th ed. Philadelphia, PA: Lea & Febiger; 1991. pp. 191–203. [Google Scholar]
- 7.Fraise AP. Biocide abuse and antimicrobial resistance–a cause for concern? J Antimicrob Chemother. 2002;49:11–2. doi: 10.1093/jac/49.1.11. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert P, McBain AJ. Potential impact of increased use of biocides in consumer products on prevalence of antibiotic resistance. Clin Microbiol Rev. 2003;16:189–208. doi: 10.1128/CMR.16.2.189-208.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001;48:5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- 10.Lambert RJ, Joynson J, Forbes B. The relationships and susceptibilities of some industrial, laboratory and clinical isolates of Pseudomonas aeruginosa to some antibiotics and biocides. J Appl Microbiol. 2001;91:972–84. doi: 10.1046/j.1365-2672.2001.01460.x. [DOI] [PubMed] [Google Scholar]
- 11.Sandle T, Vijayakumar R, Saleh Al Aboody M, Saravanakumar S. In vitro fungicidal activity of biocides against pharmaceutical environmental fungal isolates. J Appl Microbiol. 2014;117:1267–73. doi: 10.1111/jam.12628. [DOI] [PubMed] [Google Scholar]
- 12.Levy SB. Antibacterial household products: cause for concern. Emerg Infect Dis. 2001;7:512–5. doi: 10.3201/eid0707.017705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daschner F, Schuster A. Disinfection and the prevention of infectious disease: no adverse effects? Am J Infect Control. 2004;32:224–5. doi: 10.1016/j.ajic.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Russell AD. Introduction of biocides into clinical practice and the impact on antibiotic-resistant bacteria. J Appl Microbiol. 2002;92(Suppl):121–35. [PubMed] [Google Scholar]
- 15.Guo W, Shan K, Xu B, Li J. Determining the resistance of carbapenem-resistant Klebsiella pneumoniae to common disinfectants and elucidating the underlying resistance mechanisms. Patho Glob Health. 2015;109:184–92. doi: 10.1179/2047773215Y.0000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collee JG, Miles RS, Watt B. Tests for the identification of bacteria. In: Collee JG, Fraser AG, Marimon BP, Simmons A, editors. Mackie & Mccartney Practical Medical Microbiology. 14th ed. New Delhi, India: Elsevier; 2007. pp. 131–49. (reprint) [Google Scholar]
- 17.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–81. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 18.Mazzola PG, Jozala AF, Novaes LC, Moriel P, Penna TC. Minimal inhibitory concentration (MIC) determination of disinfectant and/or sterilizing agents. Braz J Pharm Sci. 2009;45:241–8. [Google Scholar]
- 19.Stickler DJ, Clayton CL, Chawla JC. The resistance of urinary tract pathogens to chlorhexidine bladder washouts. J Hosp Infect. 1987;10:28–39. doi: 10.1016/0195-6701(87)90029-6. [DOI] [PubMed] [Google Scholar]
- 20.Alexander DM, Jeawon RH, Persad S. Disinfection resistance in antibiotic resistant organisms. SA J Sci. 1991;87:614–7. [Google Scholar]
- 21.Vijayakumar R, Al-Aboody MS, Meshal KA, Wael AT, Suresh M, Premanathan M, et al. Determination of minimum inhibitory concentrations of common biocides to multidrug-resistant gram-negative bacteria. Appl Med Res. 2016;2:56–62. [Google Scholar]
- 22.Naparstek L, Carmeli Y, Chmelnitsky I, Banin E, Navon-Venezia S. Reduced susceptibility to chlorhexidine among extremely-drug-resistant strains of Klebsiella pneumoniae. J Hosp Infect. 2012;81:15–9. doi: 10.1016/j.jhin.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Russell AD, Tattawasart U, Maillard JY, Furr JR. Possible link between bacterial resistance and use of antibiotics and biocides. Antimicrob Agents Chemother. 1998;42:2151. doi: 10.1128/aac.42.8.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhatia M, Loomba PS, Mishra B, Dogra V, Thakur A. Reduced susceptibility of carbapenem-resistant Klebsiella pneumoniae to biocides: an emerging threat. Indian J Med Microbiol. 2016;34:355–8. doi: 10.4103/0255-0857.188345. [DOI] [PubMed] [Google Scholar]
- 25.Fraise AP. Susceptibility of antibiotic-resistant cocci to biocides. J Appl Microbiol. 2002;92(Suppl):158S–62S. [PubMed] [Google Scholar]
- 26.Bhatia M, Mishra B, Thakur A, Dogra V, Loomba PS. Evaluation of susceptibility of glycopeptide-resistant and glycopeptide-sensitive enterococci to commonly used biocides in a super-speciality hospital: a pilot study. J Nat Sci Biol Med. 2017;8:199–202. doi: 10.4103/0976-9668.210010. [DOI] [PMC free article] [PubMed] [Google Scholar]