Abstract
Background: Quinoa (Chenopodium quinoa) is a pseudo-cereal originally cultivated in the Andean region. The popularity of its seeds has increased in recent years due to the claims of health benefits and superfood qualities. Studies to date on the health benefits of quinoa have been restricted to animal models, and the results provide weak to moderate evidence to support improved plasma lipid profiles. Clinical trials in humans to examine the claims of health benefits of quinoa are limited to a few prospective studies and one randomized trial carried out in postmenopausal women. To our knowledge, no studies have been conducted in the general population.
Objective: The objective of this randomized clinical trial was to investigate the effect of different quinoa doses (25 and 50 g/d) on body composition, serum lipids and hormones, and nutrient intakes in overweight and obese humans.
Methods: This was a dose-response randomized, controlled, single-blind trial with a parallel design (1 control and 2 treatment groups) that compared the effect of 25 and 50 g quinoa/d in 50 overweight and obese participants over a 12-wk intervention period.
Results: Body composition, nutrient intake, and total, LDL, and HDL cholesterol were not significantly altered by quinoa consumption (P > 0.05). Mean serum triglyceride (TG) concentration was reduced significantly in the 50-g quinoa group from 1.14 to 0.72 mmol/L at 12 wk (P < 0.05). The prevalence of metabolic syndrome (MetS) was also reduced in this group by 70%. No significant changes in TGs were observed in the control and 25-g quinoa groups. The prevalence of MetS was reduced by 40% (from n = 7 at baseline to n = 4 at 12 wk) in the 25-g group.
Conclusions: The consumption of 50 g quinoa/d lowers serum TGs in overweight and obese participants and reduces the prevalence of MetS. This trial was registered at clinicaltrials.gov as UTN U1111-1175-470.
Keywords: quinoa seeds, triglycerides, dose response, overweight and obese subjects, obesity, metabolic syndrome
Introduction
Quinoa (Chenopodium quinoa) is a pseudo-cereal originally cultivated in the Andean region between Bolivia and Peru. In recent years, the popularity of quinoa seeds has increased due to claims of health benefits and superfood properties. Research has shown that quinoa seeds have an improved macronutrient profile (1), including gluten-free characteristics, a particularly beneficial essential amino acid ratio (2), and a superior phytochemical composition (3) compared with other cereals and grains.
Despite quinoa's composition and properties, scientific evidence supporting health claims such as weight loss, antidiabetic effects, and appetite suppression in in vivo models is limited and the evidence is restricted to a few animal studies. Among this body of evidence, it can be observed that a possible health benefit of quinoa consumption may be linked to a potential lipid-lowering effect (4–6). Human clinical studies have been limited to prospective studies on the effect of cereal bars containing quinoa on cardiovascular disease (CVD) markers and immunologic responses in a cohort of celiac patients after the consumption of quinoa flakes (7, 8). Both studies showed changes in total cholesterol and in TGs. In addition, LDL-cholesterol changes were reported in the study that used cereal bars (8). Furthermore, a randomized controlled trial was carried out in postmenopausal women analyzing the effect of quinoa consumption on lipid profiles and oxidative stress markers (9). The results of this trial were in line with previous data that reported favorable changes in TGs and total and LDL cholesterol.
Obesity has reached epidemic proportions according to the WHO in 2016, whereby ∼1.9 billion adults were overweight and 600 million of these were considered obese (10). A cluster of metabolic factors, including abdominal fat measured as waist circumference, reduced HDL cholesterol, increased TGs, augmented fasting plasma glucose, and elevated blood pressure, have been termed the metabolic syndrome (MetS) (11, 12); and MetS has been linked to an increased risk of chronic disease states such as obesity, coronary artery disease, and type 2 diabetes. MetS has a substantive impact on the economy reflected in the uses of health care resources and the decrease in productivity. In the United States, the estimated annual cost for 2011 was $320.1 billion for CVD and stroke, with $195.6 billion associated with direct costs and $124.5 billion linked to loss of productivity due to premature deaths (13). Addressing the components of the MetS will lead to an increase in health and a potential reduction in costs to the economy.
To our knowledge, no previous studies have been conducted that analyzed the effect of quinoa consumption in people who are overweight and obese with regard to MetS risk factors and health benefits. The aim of this research was to investigate a dose-response effect of quinoa seed consumption in a randomized controlled trial design on body composition, circulating lipids, hormones, and nutrient intakes in overweight and obese humans.
Methods
Design
A dose-response randomized, controlled, single-blind trial that used a parallel design including 1 control and 2 treatment groups was undertaken. Researchers who assessed study outcomes were blinded from participant interventions and codes.
Participants
Overweight and obese adults were recruited via advertisements at La Trobe University by using flyers, social media, and e-mails after ethical approval was obtained. Participants were included if they were aged between 18 and 65 y and had a BMI (in kg/m2) >25. Exclusion criteria were pregnancy, diagnosis of diabetes or heart disease, and current use of lipid-lowering medication. Participants gave written consent before the commencement of the study after being briefed about study procedures and expectations.
Food samples
White organic quinoa seeds were purchased from an Australia-based company (Quilla Foods Pty.) dedicated to the importation of Bolivian quinoa. Quinoa bags were carefully weighed and packed into transparent sachets of either 25 or 50 g and packaged in a box of 42 sachets, which constituted a 6-wk supply to each participant in the treatment groups.
Randomization
Participants were randomly allocated into 1 of 3 treatment groups (control and 25 and 50 g quinoa seeds/d). This randomization was carried out following a block randomization protocol stratified by sex with the use of a computer-generated numbering program retrieved from the website random.org. Allocation sequences and assignment of participant interventions were carried out by an external researcher before the appointments.
Study protocol
Participants attended appointments at La Trobe University's Bundoora campus at baseline and at 6 and 12 wk of the intervention period. Participants allocated to the 25- and 50-g quinoa groups were given a quinoa supply for 6 wk at baseline and again at their 6-wk appointment and were advised to consume 1 quinoa sachet/d. Participants were instructed on cooking methods for quinoa consumption, and recipes to incorporate quinoa were given to participants who requested them. Participants were not instructed on how and when to consume the quinoa and were advised to retain their normal lifestyle and diet throughout the study. Participants were given a calendar to self-report quinoa consumption for compliance analysis. Those in the control arm were advised to continue their normal routine and to avoid the consumption of quinoa meals during the study period.
Study outcomes
Primary outcomes included lipid profile, and secondary outcomes such as body composition and dietary intake were measured at baseline and at 6 and 12 wk. Hormone concentrations were also measured at baseline and at 12 wk.
Anthropometric measures
Anthropometric measures such as body weight and height were recorded by using a stadiometer (Wedderburn) and waist-to-hip ratio were assessed by using a measuring tape according to WHO criteria (14). Body composition was analyzed by using a DXA scan (Discovery QDR series; Hologic, Inc.) calibrated daily before measurement.
Serum lipids, hormones, and glucose
Fasting blood samples were collected in an 8.5-mL serum separation tube. After 30 min of incubation time at room temperature, blood was centrifuged at 1300 × g for 10 min at room temperature, and the separated serum was stored at −80°C until analysis. Lipid profile (total, HDL, and LDL cholesterol and TGs) and glucose concentrations were measured by using enzymatic assays in a chemistry analyzer (Indiko; Thermo Scientific) as per the manufacturer's protocol. Adiponectin, leptin, insulin, and C-peptide were determined by multiplex ELISA on a Bio-Plex 200 system (Bio-Rad Laboratories Pty. Ltd.) following the manufacturer's instructions. Acquired fluorescence data were analyzed by the Bio-Plex Manager software version 6.1 (Bio-Rad Laboratories Pty. Ltd.).
Dietary intake
Participants recorded their food intake with the use of a 3-d food diary. They were provided with templates and were advised to record intake for 3 d after the first appointment and for 3 d before the second (6 wk) and final (12 wk) appointments. Data were analyzed by using FoodWorks version 8 (Xyris).
Compliance
Participants were advised to complete a compliance checklist calendar every time they consumed a quinoa sachet. In addition, leftover sachets were returned at the following appointment.
Prevalence of MetS
Analysis of abnormalities, including those related to MetS, were reported by using the criteria stated in the Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults [Adult Treatment Panel III (ATP III)] (12), including waist circumference (men: >102 cm; women: >88 cm), circulating TGs (≥1.7 mmol/L), circulating HDL cholesterol (men: <1.04 mmol/L; women: <1.3 mmol/L), and fasting glucose (≥5.6 mmol/L).
Statistical analysis
Data were analyzed for normality by using Kolmogorov-Smirnov test. Baseline characteristics were assessed by 1-factor ANOVA for parametric data and Kruskal-Wallis test for nonparametric data. Results are presented as means ± SEMs, with the exception of lipid profile and hormones, which are reported as medians (IQRs). Treatment differences were adjusted for baseline values and analyzed by using a linear mixed model with fixed factors (baseline of the variable, treatment and time, and interaction between treatment and time) and a subject-specific intercept as a random effect. Non-normally distributed variables, such as adiponectin, glucose, HDL cholesterol, and TGs were log-transformed in order to fit the model. When significant interactions were observed, pairwise comparisons with Bonferroni were carried out. Subgroup analysis was performed by a linear mixed model with the above considerations. The prevalence of MetS was analyzed by using 1-factor ANOVA for baseline values and repeated-measures ANOVA for between- and within-group comparisons. All data were analyzed by using SPSS, Inc., software (version 24.0). The level of significance was chosen as 5%.
The study was approved by the La Trobe University Human Ethics Committee (HEC 14- 065) and registered with the Australian New Zealand Clinical Trials Registry (ANZCTR http://www.anzctr.org.au/) as UTN U1111-1175-470.
Results
Participants
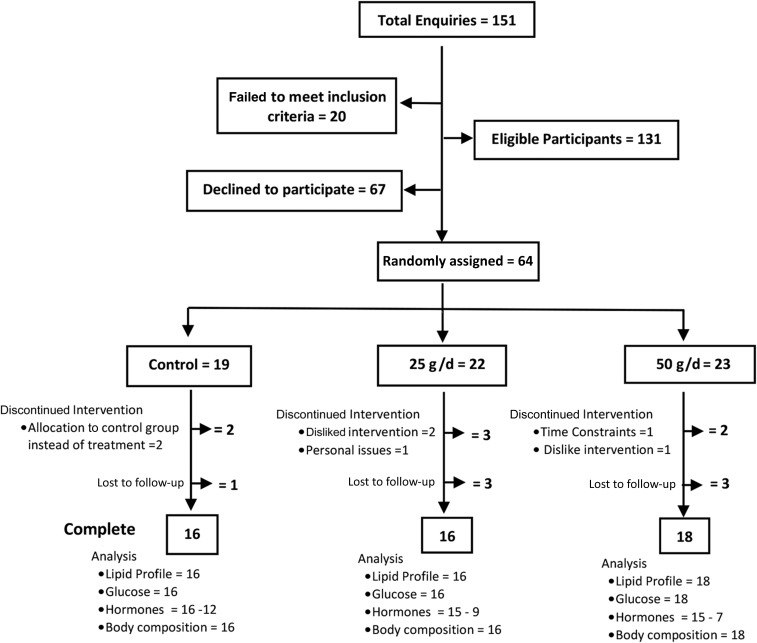
One hundred fifty-one participants expressed interest in the study. After screening, 131 met the inclusion criteria and were deemed eligible to participate. Sixty-seven eligible participants declined involvement, and 64 participants were randomly assigned. Nine participants discontinued the study after the baseline appointment (2 in the control group, 3 in the 25-g group, and 4 in the 50-g group) and 5 after the 6-wk appointment (1 in the control group, 3 in the 25-g group, and 1 in the 50-g group). Fifty participants successfully completed the 12-wk intervention period. Figure 1 shows an overview of the recruitment randomization process and further analysis of the data. Seventy-one percent of participants were women (n = 53), and the study group was aged between 20 and 64 y (mean ± SEM: 37.96 ± 1.43 y). According to the WHO criteria for obesity classification (10), 42% (n = 31) were considered overweight (BMI >25), 28% (n = 21) were obese type I (BMI between 30 and 34.9), 15% (n = 11) were obese type II (BMI between 35 and 39.9), and 15% (n = 11) were obese type III (BMI ≥40). There were no significant differences between groups at baseline (control and interventions) (Table 1). Participants were randomly assigned from February 2015 to May 2016, and the follow-up period ended August 2016.
FIGURE 1.
Participant flowchart: CONSORT guidelines. CONSORT, Consolidated Standards of Reporting Trials.
TABLE 1.
Baseline characteristics of overweight and obese participants in the control and treatment groups
| Treatment group | ||||
|---|---|---|---|---|
| Characteristics | Control group | 25 g quinoa/d | 50 g quinoa/d | P 1 |
| Participants, n | 16 | 16 | 18 | |
| Sex (M/F), n (%) | 5 (31.52)/11 (68.75) | 7 (43.75)/9 (56.25) | 4 (21.05)/14 (78.94) | |
| Age,2 y | 38 (22–59) | 35 (21–61) | 40 (21–64) | 0.447 |
| BMI,2 kg/m2 | 29.91 (25.3–41.2) | 32.84 (25.9–46.1) | 31.49 (25–39.5) | 0.287 |
| Body composition | ||||
| Body weight, kg | 81.28 ± 4.033 | 89.91 ± 4.31 | 85.85 ± 2.80 | 0.286 |
| Body fat, % | 40.21 ± 2.0 | 41.41 ± 1.73 | 41.25 ± 1.72 | 0.894 |
| Total lean tissue, % | 61.22 ± 2.36 | 59.79 ± 2.14 | 58.75 ± 1.72 | 0.862 |
| Bone mineral density, g/cm2 | 2.42 ± 0.12 | 2.46 ± 0.10 | 2.48 ± 0.09 | 0.927 |
| Waist circumference, cm | 102.97 ± 3.19 | 109.32 ± 3.64 | 106.63 ± 2.07 | 0.268 |
| Lipid profile and glucose,4 mmol/L | ||||
| Total cholesterol | 4.69 (3.49–5.38) | 4.45 (3.32–5.49) | 4.36 (3.51–5.58) | 0.729 |
| LDL cholesterol | 3.93 (2.90–4.45) | 3.90 (3.10–4.69) | 3.25 (2.87–3.53) | 0.221 |
| HDL cholesterol | 1.20 (0.80–1.43) | 1.04 (0.89–1.23) | 1.28 (0.79–1.56) | 0.749 |
| TGs | 1.07 (0.84–1.21) | 1.36 (0.90–2.38) | 1.14 (0.611–1.73) | 0.345 |
| Fasting glucose | 4.71 (4.58–5.07) | 4.85 (4.58–6.63) | 4.65 (4.19–5.09) | 0.481 |
| Hormones4 | ||||
| Adiponectin, µg/mL | 15.33 (9.74–27.10) | 10.73 (4.83–15.27) | 13.91(10.28–21.50) | 0.176 |
| Leptin, ng/mL | 11.08 (3.85–17.18) | 10.86 (5.52–15.82) | 6.64 (3.45–20.4) | 0.947 |
| C-peptide, ng/mL | 1.00 (0.71–1.12) | 1.04 (0.88–1.72) | 0.99 (0.84–1.43) | 0.398 |
| Insulin, mIU/L | 11.31 (4.97–16.69) | 11.60 (3.21–15.89) | 11.46 (7.65–19.34) | 0.551 |
| Nutrient intake | ||||
| Energy, kJ | 8066.71 ± 691.32 | 7908.55 ± 774.10 | 7618.25 ± 595.96 | 0.885 |
| Protein, g | 87.68 ± 11.14 | 85.37 ± 13.38 | 84.53 ± 6.13 | 0.972 |
| Total fat, g | 75.86 ± 7.54 | 73.65 ± 8.07 | 79.64 ± 7.38 | 0.851 |
| Carbohydrates, g | 201.96 ± 16.92 | 193.52 ± 14.59 | 167.23 ± 17.25 | 0.296 |
| Dietary fiber, g | 23.60 ± 2.42 | 24.20 ± 3.85 | 22.52 ± 2.26 | 0.908 |
| Protein, % of energy | 18.30 ± 1.32 | 17.57 ± 1.27 | 19.51 ± 1.27 | 0.559 |
| Total fat, % of energy | 34.80 ± 1.72 | 34.40 ± 1.51 | 38.54 ± 1.81 | 0.171 |
| Carbohydrates, % of energy | 41.64 ± 1.21 | 42.07 ± 2.15 | 39.64 ± 1.53 | 0.610 |
| Fiber, % of energy | 2.46 ± 0.27 | 2.43 ± 0.20 | 2.53 ± 0.22 | 0.948 |
| Other nutrients, % of energy | 0.94 ± 0.16 | 1.23 ± 0.20 | 1.04 ± 0.17 | 0.531 |
Derived by using 1-factor ANOVA for body composition and nutrient intake and Kruskal-Wallis test for lipid profile, glucose, and hormones.
Values are means (ranges).
Values are means ± SEMs.
Values are medians (IQRs).
Effect of quinoa on anthropometric measures and body composition
As shown in Table 2, no significant differences were observed for body-composition measurements after the 6- and 12-wk intervention time periods between and within treatment groups. In the control group, body weight varied from a 3.6% decrease (−2.92 kg) at 6 wk to a 4.1% increase (3.19 kg) at 12 wk. In both intervention groups, changes in body weight were reported as a 0.5% increase (0.43 kg) at 6 wk, whereas a 1.5% (−1.32 kg) and a 1.7% (−1.43 kg) decrease at 12 wk in the 25- and 50-g groups, respectively, was observed. There were no differences in the percentage of body fat and total lean tissue. There was a decrease in waist circumference of 2.8 cm in the control group at 6 wk, but an increase of 3.3 cm at 12 wk. In the 25-g group, waist circumference increased by 0.6 cm at 6 wk and decreased by 1 cm at 12 wk, whereas in the 50-g group there was a reduction of 0.2 and 1.3 cm at 6 and 12 wk, respectively.
TABLE 2.
Effect of quinoa on body composition in overweight and obese participants at 6 and 12 wk of intervention1
| Body-composition characteristics | Control | 25 g/d | 50 g/d | P (treatment)2 | |||
|---|---|---|---|---|---|---|---|
| 6 wk | 12 wk | 6 wk | 12 wk | 6 wk | 12 wk | ||
| BMI, kg/m2 | 28.45 ± 0.72 | 29.85 ± 1.19 | 33.45 ± 1.38 | 32.81 ± 1.43 | 30.86 ± 1.11 | 30.20 ± 1.16 | 0.968 |
| Body weight, kg | 78.43 ± 2.80 | 81.62 ± 4.31 | 90.34 ± 4.19 | 89.02 ± 4.24 | 86.05 ± 2.80 | 84.59 ± 2.83 | 0.775 |
| Body fat, % | 39.47 ± 2.0 | 40.29 ± 1.99 | 41.28 ± 1.73 | 41.41 ± 1.93 | 40.96 ± 1.71 | 40.25 ± 1.87 | 0.380 |
| Total lean tissue, % | 61.86 ± 2.29 | 61.09 ± 2.31 | 59.85 ± 1.98 | 59.74 ± 2.15 | 59.04 ± 1.71 | 59.75 ± 1.86 | 0.341 |
| Bone mineral density, g/cm2 | 1.87 ± 0.11 | 2.42 ± 0.13 | 1.95 ± 0.09 | 2.44 ± 0.11 | 1.84 ± 0.08 | 2.49 ± 0.10 | 0.477 |
| Waist circumference, cm | 100.15 ± 3.62 | 103.26 ± 3.62 | 109.88 ± 3.61 | 108.86 ± 3.54 | 106.34 ± 2.20 | 105.03 ± 2.07 | 0.265 |
Values are means ± SEMs.
Indicates the main effect of treatment observed between groups (baseline adjusted on the basis of a linear mixed model).
Serum lipids, hormones, and glucose
The interaction effects between lipids, lipoproteins, and fasting glucose with quinoa intake is reported in Table 3. No significant effect was observed for total, HDL, and LDL cholesterol and fasting glucose between and within groups at 6 and 12 wk of quinoa consumption. However, a significant difference was noted for TGs in the between-group analysis. A pairwise comparison adjusted with a Bonferroni correction showed that TGs were significantly lower in the 50-g group compared with the control group (0.72 and 1.25 mmol/L, respectively; P = 0.022). Furthermore, within-group comparison showed that the 50-g group had significantly lower TG concentrations at the 12-wk time point compared with baseline (1.14 and 0.72 mmol/L at baseline and 12 wk, respectively; P = 0.001). No significant difference was observed at 6 wk compared with baseline. These results indicate that there is a dose-response effect with daily consumption of 50 g quinoa, which reduced serum TGs by ∼28.1% after 6 wk of a dietary intervention and by 36.8% after 12 wk. TG changes in the 25-g group were not significant at either 6 or 12 wk, although concentrations showed a slight reduction when compared with baseline values (1.36, 1.33, and 1.27 mmol/L at baseline, 6 wk, and 12 wk, respectively).
TABLE 3.
Effect of quinoa intake on fasting biochemical measures in overweight and obese participants in the control and treatment groups at 6 and 12 wk of intervention1
| Control group | 25-g/d group | 50-g/d group | P (treatment)2 | ||||
|---|---|---|---|---|---|---|---|
| Characteristics | 6 wk | 12 wk | 6 wk | 12 wk | 6 wk | 12 wk | |
| Lipid profile and glucose, mmol/L | |||||||
| Total cholesterol | 4.65 (3.70–5.56) | 4.71 (4.03–5.35) | 4.69 (3.48–5.43) | 4.27 (3.30–4.88) | 4.38 (3.58–5.38) | 4.63 (3.41–5.59) | 0.787 |
| LDL cholesterol | 3.73 (3.06–4.86) | 3.58 (2.81–4.70) | 3.78 (2.93–4.46) | 4.15 (3.62–4.61) | 3.48 (2.96–4.56) | 3.42 (2.64–4.02) | 0.838 |
| HDL cholesterol | 1.41 (1.10–1.48) | 1.20 (1.08–1.62) | 1.05 (0.89–1.55) | 1.02 (0.93–1.37) | 1.26 (0.81–1.70) | 1.48 (0.86–1.71) | 0.350 |
| TGs | 1.22 (0.64–1.34) | 1.25 (1.11–1.33) | 1.33 (0.85–2.07) | 1.27 (0.92–1.80) | 0.82 (0.59–1.26) | 0.72 (0.56–1.01) | 0.025 |
| Fasting glucose | 4.72 (4.54–5.07) | 4.96 (4.39–5.20) | 4.85 (4.61–5.32) | 5.04 (4.98–5.56) | 4.65 (4.20–5.09) | 4.90 (4.26–5.40) | 0.111 |
| Hormones | |||||||
| Adiponectin, µg/mL | NM | 18.15 (11.80–27.01) | NM | 13.08 (5.49–22.89) | NM | 14.76 (10.92–19.44) | 0.446 |
| Leptin, ng/mL | NM | 8.84 (3.77–15.57) | NM | 11.06 (5.65–17.68) | NM | 6.41 (2.93–19.80) | 0.973 |
| C-peptide, ng/mL | NM | 0.79 (0.69–1.35) | NM | 1.16 (0.74–1.58) | NM | 0.95 (0.69–1.33) | 0.629 |
| Insulin, mLU/L | NM | 12.72 (5.15–22.68) | NM | 11.75 (6.20–15.57) | NM | 9.86 (5.82–16.01) | 0.688 |
Values are medians (IQRs). NM, value not measured.
Indicates the main effect of treatment, observed between groups (baseline adjusted on the basis of a linear mixed model).
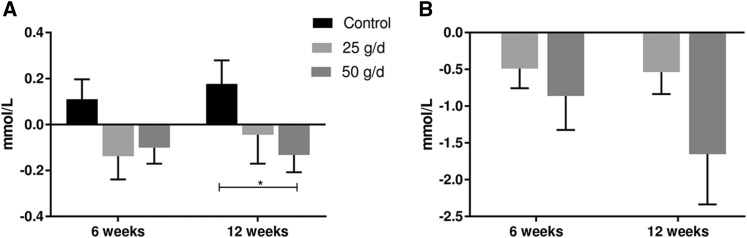
Further analysis showed that 15.8% of all of the participants in the study had TGs exceeding optimum concentrations of 1.7 mmol/L, the cutoff according to the ATP III MetS criteria (12). Analysis of the effect of quinoa on normo- and hypertriglyceridemic groups showed a significant change (P = 0.048) in participants with normal TGs who consumed 50 g quinoa/d compared with controls at 12 wk (Figure 2). Reductions were also observed within the 50-g group from 0.86 mmol/L at baseline to 0.76 mmol/L at 6 wk and 0.70 mmol/L at 12 wk, although these were nonsignificant (P = 0.336). This represents a reduction of 12.7% and 18.4% at 6 and 12 wk, respectively. No significant differences were observed in TGs in participants who consumed 25 g quinoa/d. In the hypertriglyceridemic group, a significant decrease was observed at 12 wk (P = 0.001) in participants who consumed 50 g quinoa/d. TGs were reduced by 30.4% at 6 wk (from 2.83 mmol/L at baseline to 1.97 mmol/L) and by 58% at 12 wk (from 2.83 mmol/L at baseline to 1.18 mmol/L). Participants who consumed 25 g quinoa showed a reduction of 18.5% at 6 wk (2.7 mmol/L at baseline to 2.20 mmol/L) and 23.7% at 12 wk (from 2.7 mmol/L at baseline to 2.06 mmol/L). No significant difference was observed in the between-group comparison. Of note, TG concentrations were normalized to within the healthy range (<1.7 mmol/L) in the hypertriglyceridemic group who consumed 50 g quinoa/d.
FIGURE 2.
Changes in subgroup TG concentrations in participants with normal TGs (<1.7 mmol/L) (A) and those with high TGs (≥1.7 mmol/L) (B). Values were calculated as the difference between 6 and 12 wk and baseline and were compared by using a linear mixed-effects model with time as a within-subject factor and intervention group as a between-subject factor. Values are means ± SEMs. *Significant changes during the 12-wk intervention, P < 0.05.
Circulating hormones were not significantly affected by quinoa consumption. Adiponectin increased in the control group by 18% and by 21% and 6% in the 25-g and 50-g quinoa groups, respectively, during the intervention period. Leptin and C-peptide decreased by 21% in the control group and increased by 11% in the 25-g group. In addition, leptin decreased by 3%, whereas C-peptide increased in the 50-g group. Insulin concentrations remained stable in the control and 25-g groups and showed a decrease in the 50-g group (14%). Results are reported in Table 3.
Prevalence of MetS
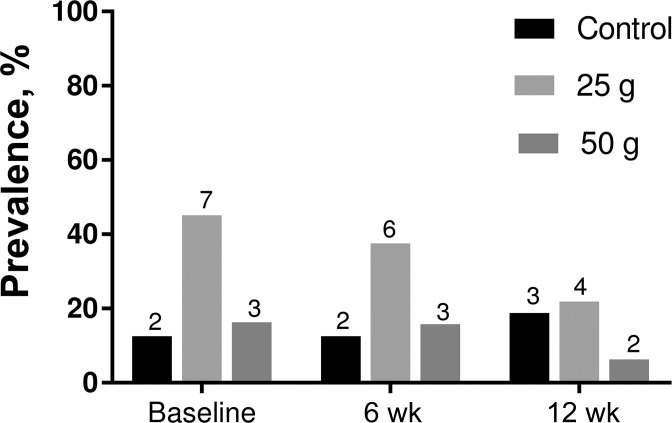
The prevalence of MetS as defined by the ATP III criteria in the treatment groups is reported in Figure 3. According to the criteria for MetS, 24% (n = 12) of total participants had ≥3 risk factors, including high waist circumference (men >102 cm and women >88 cm), reduced HDL cholesterol (men <1.04 mmol/L and women <1.3 mmol/L), high TGs (≥1.7 mmol/L), and higher fasting blood glucose (≥5.6 mmol/L) at baseline. No changes in prevalence were observed at 6 wk for the control and 50-g groups, whereas the 25-g group showed a reduction of 14% in prevalence at this time point. At the completion of the study, there was a decrease in the occurrence of MetS in the 25-g group of 41% (n = 3 compared with n = 7 at baseline) and in the 50-g group of 70% (n = 2 compared with n = 3 at baseline). On the contrary, the prevalence of MetS in the control group increased by 6.8%. The reduction in the prevalence of MetS was a result of the increase in HDL cholesterol and the decrease in TG concentration. No changes were observed in waist circumference and fasting glucose among the groups. Mean differences between and within groups were not significant.
FIGURE 3.
The prevalence of MetS in participants from the control and treatment groups across intervention periods. The number of participants is specified above each bar. MetS, metabolic syndrome.
Dietary intake
As summarized in Table 4, quinoa consumption did not significantly alter nutrient intake during the intervention time. There was an increase in reported energy intake in the control group of 359 kJ at 6 wk and a reduction of 252 kJ at 12 wk. In the 25-g group there was a reduction of 364 kJ at 6 wk and a further increase of 385 kJ at 12 wk. Conversely, the 50-g group showed no difference at 6 wk, followed by a decrease of 585 kJ at 12 wk. There were no reported trends in changes in macronutrients among all treatment groups. In the control group, protein and fiber intakes increased, whereas total fat intake decreased at both 6 and 12 wk; and carbohydrate intake increased at 6 wk but decreased at 12 wk. For the 25-g group there was an increase in protein and carbohydrate intakes and a reduction in total fat intake at 6 and 12 wk, and dietary fiber intake increased at 6 wk but decreased at 12 wk. Finally, in the 50-g group there was a decrease in total fat and dietary fiber intakes and an increase in carbohydrate intake at 6 and 12 wk, whereas protein intake showed an increase at 6 wk but a reduction at 12 wk.
TABLE 4.
Effect of quinoa on nutrient intake outcomes of overweight and obese participants in the control and treatment groups at 6 and 12 wk of intervention1
| Nutrient intake | Control group | 25-g/d group | 50-g/d group | P (treatment)2 | |||
|---|---|---|---|---|---|---|---|
| 6 wk | 12 wk | 6 wk | 12 wk | 6 wk | 12 wk | ||
| Energy, kJ | 8425.26 ± 670.43 | 8173.67 ± 577.70 | 7543.71 ± 657.56 | 7929.18 ± 541.45 | 7638.19 ± 414.77 | 7053.39 ± 623.47 | 0.305 |
| Protein, g | 90.68 ± 12.08 | 95.39 ± 11.52 | 95.21 ± 13.11 | 92.30 ± 11.31 | 90.35 ± 8.06 | 82.24 ± 7.98 | 0.460 |
| Total fat, g | 72.72 ± 8.44 | 74.24 ± 8.22 | 64.48 ± 6.27 | 69.31 ± 10.92 | 70.45 ± 6.39 | 65.51 ± 9.13 | 0.654 |
| Carbohydrates, g | 225.04 ± 17.43 | 199.70 ± 9.08 | 194.41 ± 19.04 | 198.87 ± 13.67 | 187.78 ± 13.13 | 175.15 ± 14.60 | 0.299 |
| Dietary fiber, g | 24.93 ± 2.23 | 24.92 ± 2.23 | 20.27 ± 2.34 | 24.86 ± 2.78 | 23.76 ± 2.19 | 25.78 ± 3.18 | 0.330 |
| Protein, % of energy | 17.71 ± 1.17 | 19.47 ± 1.55 | 21.17 ± 1.78 | 20.04 ± 2.31 | 19.98 ± 1.55 | 20.11 ± 1.59 | 0.537 |
| Total fat, % of energy | 31.05 ± 2.29 | 33.02 ± 2.12 | 32.12 ± 1.83 | 31.29 ± 3.13 | 33.87 ± 2.16 | 33.57 ± 1.76 | 0.794 |
| Carbohydrates, % of energy | 45.26 ± 2.35 | 41.34 ± 2.06 | 42.31 ± 2.24 | 41.98 ± 2.57 | 40.65 ± 1.69 | 41.57 ± 1.90 | 0.459 |
| Fiber, % of energy | 2.59 ± 0.37 | 2.53 ± 0.33 | 2.18 ± 0.24 | 2.52 ± 0.24 | 2.52 ± 0.24 | 2.95 ± 0.29 | 0.489 |
| Other nutrients, % of energy | 1.24 ± 0.37 | 1.20 ± 0.31 | 1.30 ± 0.26 | 1.24 ± 0.31 | 0.87 ± 0.13 | 1.08 ± 0.15 | 0.466 |
Values are means ± SEMs.
Indicates the main effect of treatment observed between groups (baseline adjusted on the basis of a linear mixed model).
Compliance
According to self-report diaries, participants in the 25-g group showed compliance to the prescribed quinoa consumption of >90.0% at 6 wk and 89.6% at 12 wk, whereas participants in the 50-g group showed compliance of >90.7% at 6 wk and 82.3% at 12 wk.
Discussion
The aim of this dose-response randomized controlled study was to assess the relation between quinoa consumption and anthropometric, biochemical, and dietary data in participants who are overweight or obese. There was no effect of quinoa on anthropometric measures or body composition, circulating hormones, glucose, or total, HDL, or LDL cholesterol. There was also no effect observed on dietary intake data. However, our results showed that the consumption of 50 g quinoa seeds/d for 12 wk reduced serum TGs in overweight and obese adults.
TGs have been extensively linked to CVDs as an independent risk marker. Early evidence showed that in individuals who survive cardiovascular events, serum TGs are augmented compared with healthy controls, even after controlling for other lipoproteins such as LDL (15, 16). Some authors also argue that the relative risk of a cardiovascular event may increase by ∼32% in men and 14% in women for each millimole per liter increase in circulating TGs (17). Furthermore, recent evidence suggests that TGs are not only a risk marker but also have an active pathogenic role in the development of cardiovascular events. Mutations in the intermediates of lipid metabolism lead to changes in cardiovascular risk. Modifications in proteins such as APO-CIII (18, 19) result in a reduction in the risk of coronary artery calcification. Conversely, alterations in enzymes such as lipoprotein lipase (LPL) (20) cause an increase in coronary artery disease. This confirms that the reduction in TGs achieved in this study may potentially reduce CVD risk.
Circulating TGs are the result of a complex network involving synthesis in the liver, absorption of chylomicrons by the intestine, peripheral lipolysis mediated by the action of LPL, and hepatic clearance of the remnant molecules. Early evidence showed that in obese participants (21), hypertriglyceridemia is the consequence of the increase in hepatic VLDL production due to an increased flux of FFAs from adipose tissue (22); this overproduction causes a further impairment in LPL activity in adipose tissue and muscle, which thus increases the circulating TG concentrations.
The reduction of 36% after the consumption of 50 g quinoa for 12 wk observed in our study is greater than the 16% reduction observed in previous reports in healthy participants who consumed 19.5 g quinoa for 4 wk (8) and the 4% reduction in overweight postmenopausal women who consumed 25 g quinoa for 4 wk (9). The mechanism by which quinoa consumption results in a reduction in TGs is not fully understood. A reduction in intestinal dietary fat absorption observed through the increase in lipid content in the feces was reported in rodents fed a diet containing quinoa protein extract and a quinoa extract enriched with 20-hydroxyecdysone (4, 23). A possible underlying mechanism for this quinoa effect may be based on bile acid activity. In an in vitro essay it was shown that quinoa proteins have a higher bile acid–binding capacity affecting the absorption of lipids (4). Bile acid emulsification of fats constitutes an essential part of intestinal lipid absorption.
Other approaches, such as increased dietary fiber intake, have been associated with improvement in lipid profile in a number of randomized clinical trials (24–26). Although human studies are inconclusive, some authors argued that dietary fiber may have an important role in hepatic cholesterol synthesis linked to bile acid regulation (27). Although quinoa has an enhanced content of soluble and insoluble fiber compared with other cereals and grains (28, 29), our results do not support these findings.
The reduction in TGs in our study is comparable to the reduction evidenced in pharmacologic therapy that used 40% nicotinic acid (30), 35% fibrates (31), and 20% statins (32). In addition, the consumption of 3.4 g omega-3 FAs/d reduced TGs by 23% in healthy participants with mild hypertriglyceridemia (33). Each of these treatments has a very different mechanism of action and cannot be compared with the possible TG-lowering mechanism of quinoa seed intake.
Quinoa consumption has increased steadily over recent years. In 2011, the per capita consumption of quinoa in Bolivia was reported to be ∼1.11 kg/y; and in 2012, it escalated to 2.37 kg/y. In contrast, in nonproducer countries such as Canada and Australia, quinoa was not consumed in reported per capita quantities in 2011 and it reached just 227 g/y in 2014 in Canada and 81 g/y in Australia. Therefore the reduction in TGs observed in our study would require a significantly higher amount of quinoa consumption than shown in the current data. However, quinoa is a suitable replacement for grain consumption, whose intake was in the range of 112–175 g/d for the Australian population in 2011–2012 (34). In addition to providing a higher amount of nutrients such as protein and micronutrients, quinoa may have an important role in the reduction in the risk of CVDs.
Incorporating quinoa into the diet did not alter the overall nutrient intake of participants. There was a positive change with respect to a reduction in the prevalence of MetS due to the positive changes in HDL cholesterol and circulating TGs. Although the numbers were small, making small substitutions to diet quality without concomitant weight loss can improve the metabolic profile of overweight and obese participants.
Study limitations
This study showed a diminution of circulating TGs when quinoa seeds were consumed as part of the daily diet. However, due to the small sample size, further investigation must be conducted to determine the role of quinoa seed intake in participants with high circulating TGs. In addition, a compliance biomarker should be established in order to have an independent measure of quinoa consumption.
Conclusions
In our study, we showed that the consumption of 50 g quinoa seeds/d for 12 wk reduced serum TGs and therefore the prevalence of MetS in overweight and obese individuals. Further studies are needed to elucidate a clear mechanism of action by which quinoa seeds lower circulating TG concentrations.
Acknowledgments
The authors' responsibilities were as follows—DN-P, JR, and MJ: designed the project; DN-P: conducted the clinical trial and carried out laboratory experiments; DN-P and MJ: performed the statistical analysis; and all authors: wrote the manuscript and read and approved the final manuscript.
Abbreviations
- ATP III
Adult Treatment Panel III
- CVD
cardiovascular disease
- LPL
lipoprotein lipase
- MetS
metabolic syndrome
References
- 1. Vega-Gálvez A, Miranda M, Vergara J, Uribe E, Puente L, Martinez EA.. Nutrition facts and functional potential of quinoa (Chenopodium quinoa willd.), an ancient Andean grain: a review. J Sci Food Agric 2010;90:2541–7. [DOI] [PubMed] [Google Scholar]
- 2. Escuredo O, González Martín MI, Wells Moncada G, Fischer S, Hernández Hierro JM.. Amino acid profile of the quinoa (Chenopodium quinoa Willd.) using near infrared spectroscopy and chemometric techniques. J Cereal Sci 2014;60:67–74. [Google Scholar]
- 3. Ryan E, Galvin K, O'Connor TP, Maguire AR, O'Brien NM.. Phytosterol, squalene, tocopherol content and fatty acid profile of selected seeds, grains, and legumes. Plant Foods Hum Nutr 2007;62:85–91. [DOI] [PubMed] [Google Scholar]
- 4. Takao T, Nakamichi W, Kana Y, Syoko I, Saori S, Yukari T, Kenichi N, Yotaro K.. Hypocholesterolemic effect of protein isolated from quinoa (Chenopodium quinoa Willd.) seeds. Food Sci Technol Res 2005;11:161–7. [Google Scholar]
- 5. Paśko P, Zagrodzki P, Barton H, Chlopicka J, Gorinstein S.. Effect of quinoa seeds (Chenopodium quinoa) in diet on some biochemical parameters and essential elements in blood of high fructose-fed rats. Plant Foods Hum Nutr 2010;65:333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Foucault AS, Mathe V, Lafont R, Even P, Dioh W, Veillet S, Tome D, Huneau JF, Hermier D, Quignard-Boulange A.. Quinoa extract enriched in 20-hydroxyecdysone protects mice from diet-induced obesity and modulates adipokines expression. Obesity (Silver Spring) 2012;20:270–7. [DOI] [PubMed] [Google Scholar]
- 7. Zevallos VF, Herencia LI, Chang F, Suzanne D, Ellis J, Ciclitira PJ.. Gastrointestinal effects of eating quinoa (Chenopodium quinoa Willd.) in celiac patients. Am J Gastroenterol 2014;109:270–8. [DOI] [PubMed] [Google Scholar]
- 8. Farinazzi-Machado FMV, Barbalho SM, Oshiiwa M, Goulart R, Junior OP.. Use of cereal bars with quinoa (Chenopodium quinoa W.) to reduce risk factors related to cardiovascular diseases. Food Sci Technol (Campinas) 2012;32:239–44. [Google Scholar]
- 9. De Carvalho FG, Payao OP, Joao PG, Afonso JJA, Sergio MJ, Marliere NA.. Metabolic parameters of postmenopausal women after quinoa or corn flakes intake—a prospective and double-blind study. Int J Food Sci Nutr 2014;65:380–5. [DOI] [PubMed] [Google Scholar]
- 10. WHO Fact sheet 311—obesity and overweight [Internet]. 2016. [cited 2016 May 10]. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/.
- 11. Alberti KG, Zimmet PZ.. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539–53. [DOI] [PubMed] [Google Scholar]
- 12. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486–97. [DOI] [PubMed] [Google Scholar]
- 13. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 2015;131:e29–322. [DOI] [PubMed] [Google Scholar]
- 14. WHO Waist circumference and waist-hip ratio: report of a WHO expert consultation. Geneva (Switzerland): WHO; 2008. [Google Scholar]
- 15. Brunner D, Altman S, Loebl K, Schwartz S, Levin S.. Serum cholesterol and triglycerides in patients suffering from ischemic heart disease and in healthy subjects. Atherosclerosis 1977;28:197–204. [DOI] [PubMed] [Google Scholar]
- 16. Hamsten A, Walldius G, Dahlén G, Johansson B, De Faire U.. Serum lipoproteins and apolipoproteins in young male survivors of myocardial infarction. Atherosclerosis 1986;59:223–35. [DOI] [PubMed] [Google Scholar]
- 17. Austin MA, Hokanson JE, Edwards KL.. Hypertriglyceridemia as a cardiovascular risk factor. Am J Cardiol 1998;81(4A):7B–12B. [DOI] [PubMed] [Google Scholar]
- 18. Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjærg-Hansen A.. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med 2014;371:32–41. [DOI] [PubMed] [Google Scholar]
- 19. Crosby J, Peloso GM, Auer PL, Crosslin DR, Stitziel NO, Lange LA, Lu Y, Tang ZZ, Zhang H, Hindy G, et al. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med 2014;371:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wittrup HH, Tybjærg-Hansen A, Nordestgaard BG.. Lipoprotein lipase mutations, plasma lipids and lipoproteins, and risk of ischemic heart disease: a meta-analysis. Circulation 1999;99:2901–7. [DOI] [PubMed] [Google Scholar]
- 21. Grundy SM, Mok HYI, Zech L, Steinberg D, Berman M.. Transport of very low density lipoprotein triglycerides in varying degrees of obesity and hypertriglyceridemia. J Clin Invest 1979;63:1274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nikkilä EA, Kekki M.. Plasma triglyceride transport kinetics in diabetes mellitus. Metabolism 1973;22:1–22. [DOI] [PubMed] [Google Scholar]
- 23. Foucault AS, Even P, Lafont R, Dioh W, Veillet S, Tome D, Huneau JF, Hermier D, Quignard-Boulange A.. Quinoa extract enriched in 20-hydroxyecdysone affects energy homeostasis and intestinal fat absorption in mice fed a high-fat diet. Physiol Behav 2014;128:226–31. [DOI] [PubMed] [Google Scholar]
- 24. Chen J, He J, Wildman RP, Reynolds K, Streiffer RH, Whelton PK.. A randomized controlled trial of dietary fiber intake on serum lipids. Eur J Clin Nutr 2006;60:62–8. [DOI] [PubMed] [Google Scholar]
- 25. Hashizume C, Kishimoto Y, Kanahori S, Yamamoto T, Okuma K, Yamamoto K.. Improvement effect of resistant maltodextrin in humans with metabolic syndrome by continuous administration. J Nutr Sci Vitaminol (Tokyo) 2012;58:423–30. [DOI] [PubMed] [Google Scholar]
- 26. Hu X, Gao J, Zhang Q, Fu Y, Li K, Zhu S, Li D.. Soy fiber improves weight loss and lipid profile in overweight and obese adults: a randomized controlled trial. Mol Nutr Food Res 2013;57:2147–54. [DOI] [PubMed] [Google Scholar]
- 27. Garcia-Diez F, Garcia-Mediavilla V, Bayon JE, Gonzalez-Gallego J.. Pectin feeding influences fecal bile acid excretion, hepatic bile acid and cholesterol synthesis and serum cholesterol in rats. J Nutr 1996;126:1766–71. [DOI] [PubMed] [Google Scholar]
- 28. Lamothe LM, Srichuwong S, Reuhs BL, Hamaker BR.. Quinoa (Chenopodium quinoa W.) and amaranth (Amaranthus caudatus L.) provide dietary fibres high in pectic substances and xyloglucans. Food Chem 2015;167:490–6. [DOI] [PubMed] [Google Scholar]
- 29. Repo-Carrasco-Valencia RA-M, Serna LA.. Quinoa (Chenopodium quinoa, Willd.) as a source of dietary fiber and other functional components. Food Sci Technol (Campinas) 2011;31:225–30. [Google Scholar]
- 30. Vogt A, Kassner U, Hostalek U, Steinhagen-Thiessen E.. Prolonged-release nicotinic acid for the management of dyslipidemia: an update including results from the NAUTILUS study. Vasc Health Risk Manag 2007;3:467–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P, Huttunen JK, Kaitaniemi P, Koskinen P, Manninen V, et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. N Engl J Med 1987;317:1237–45. [DOI] [PubMed] [Google Scholar]
- 32. Bradford RH, Shear CL, Chremos AN, Dujovne C, Downton M, Franklin FA, Gould AL, Hesney M, Higgins J, Hurley DP, et al. Expanded Clinical Evaluation of Lovastatin (EXCEL) study results. I. Efficacy in modifying plasma lipoproteins and adverse event profile in 8245 patients with moderate hypercholesterolemia. Arch Intern Med 1991;151:43–9. [DOI] [PubMed] [Google Scholar]
- 33. Skulas-Ray AC, Kris-Etherton PM, Harris WS, Vanden Heuvel JP, Wagner PR, West SG.. Dose-response effects of omega-3 fatty acids on triglycerides, inflammation, and endothelial function in healthy persons with moderate hypertriglyceridemia. Am J Clin Nutr 2011;93:243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Australian Bureau of Statistics Australian Health Survey: nutrition first results—foods and nutrients, 2011-12 [Internet]. 2014. [cited 2017 May 18]. Available from: http://www.abs.gov.au/ausstats/abs@.nsf/Lookup/by%20Subject/4364.0.55.007∼2011-12~Main%20Features~Cereals%20and%20cereal%20products∼720.