Abstract
Background: Environmental enteric dysfunction (EED), frequently seen in rural Malawian children, causes chronic inflammation and increases the risk of stunting. Legumes may be beneficial for improving nutrition and reducing the risk of developing EED in weaning children.
Objective: The objectives of this study were to determine the nutritional value, verify the food safety, and identify metabolite profiles of 3 legume-based complementary foods: common bean (CB), cowpea (CP), and traditional corn-soy blend (CSB).
Methods: Foods were prepared by using local ingredients and analyzed for nutrient composition with the use of Association of Official Analytical Chemists (AOAC) standards (950.46, 991.43, 992.15, 996.06, and 991.36) for macro- and micronutrient proximate analysis. Food safety analysis was conducted in accordance with the Environmental Protection Agency (7471B) and AOAC (2008.02) standards. The metabolite composition of foods was determined with nontargeted ultra-performance LC–tandem mass spectrometry metabolomics.
Results: All foods provided similar energy; CB and CP foods contained higher protein and dietary fiber contents than did the CSB food. Iron and zinc were highest in the CSB and CP foods, whereas CB and CP foods contained higher amounts of magnesium, phosphorus, and potassium. A total of 652 distinct metabolites were identified across the 3 foods, and 23, 14, and 36 metabolites were specific to the CSB, CB, and CP foods, respectively. Among the potential dietary biomarkers of intake to distinguish legume foods were pipecolic acid and oleanolic acid for CB; arabinose and serotonin for CSB; and quercetin and α- and γ-tocopherol acid for CP. No heavy metals were detected, and aflatoxin was measured only in the CSB (5.2 parts per billion).
Conclusions: Legumes in the diet provide a rich source of protein, dietary fiber, essential micronutrients, and phytochemicals that may reduce EED. These food metabolite analyses identified potential dietary biomarkers of legume intake for stool, urine, and blood detection that can be used in future studies to assess the relation between the distinct legumes consumed and health outcomes. This trial was registered at clinicaltrials.gov as NCT02472262 and NCT02472301.
Keywords: corn-soybean blend, Phaseolus vulgaris, Vigna unguiculata, foodomics, metabolomics, malnutrition, enteric disease, environmental enteric dysfunction, complementary foods, infant
Introduction
Environmental enteric dysfunction (EED) is generalized subclinical upper small bowel inflammation and is common among young children living in impoverished settings (1–4). EED is associated with increased intestinal permeability, alterations in gut microbial populations, nutrient malabsorption, poor weight gain, stunting, frequent enteric infections, and decreased response to enteric vaccines (1–4). EED predisposes children to clinically manifest forms of malnutrition, including wasting and stunting, and develops during the first 3 y of life, which is a high-risk period marked by the transition from exclusive breastfeeding, to mixed feeding with complementary foods, to an adult diet (1, 2, 4). In sub-Saharan Africa, common complementary foods include maize, cassava, and sorghum, all of which are staples in an unvaried diet that is high in starch and deficient in protein and micronutrients (5). Alternative and culturally acceptable complementary foods that can supply a better balance of nutrients and can provide anti-inflammatory and other gut barrier–protective effects have the potential to reduce EED and its nutritional comorbidities and might also be of benefit to child health and development.
Legumes are a source of essential amino acids, dietary fiber, lipids, micronutrients, and a myriad of phytochemicals, including multiple antioxidants (6, 7). Corn-soybean blends (CSBs) have been used as complementary foods for decades in food-aid and school feeding programs in an effort to deliver higher-quality protein in comparison to a cereal alone (8, 9). Additional evidence supports that other nonsoy legumes, when compared with CSBs, are a rich source of macronutrients, micronutrients, phytochemicals, and prebiotics that can promote gut health (6, 10–14). These include common beans (Phaseolus vulgaris), which are digestible and well tolerated in young populations (14) and have been shown to reduce markers of inflammation (15, 16). Furthermore, cowpeas (CPs; Vigna unguiculata) are commonly grown in sub-Saharan Africa, where they are considered a staple crop rich in protein, vitamins, and trace minerals including iron and zinc (17, 18). The nutrient profiles of the common bean (CB) and CP, especially their high protein and fiber content, show their potential to improve food and nutrition security in this vulnerable population (13, 19).
Although the CB and CP are readily cultivated in Malawi, they are not commonly utilized as complementary foods. Understanding the nutritional composition and small-molecule profile of foods is crucial in nutrition-based interventions in which this composition will help identify potential dietary biomarkers of intake and disease prevention mechanisms and promote food safety. This methodology, known as food-omics, applies advanced “omics” technologies to identify small compounds in food that can assist in the development of dietary preventive measures against human diseases. The food-omics approach is multifaceted and represents a largely unexploited source in which to identify novel dietary intake biomarkers (20). There have been several studies that reported on the legume metabolome, with a primary focus on improving legume breeding programs (12, 21–24). This includes the Soybean Knowledge Base, an all-inclusive source for soybean translational genomics that provides the integrations of gene, genomics, transcriptomics, proteomics, metabolomics, and phenotype data (22); an investigation on how temperature influences the soybean seed metabolome (23); and an evaluation of diversity among uncooked common beans from 2 centers of domestication (25). In addition, metabolomics has been completed on 17 CP varieties to assess phenolic variations (12).
We conducted a comparative food macro- and micronutrient analysis, a safety assessment, and a metabolomics analysis of 3 legume-based complementary foods—CB, CP, and CSB—collected from Malawi. This analysis will help us assess the future utility of using legume foods as complementary foods for children. We hypothesized that the CB and CP foods would have higher amounts of protein, dietary fiber, and essential micronutrients and FAs than the CSB. In addition, we used our food metabolomics approach to assist in identifying candidate metabolites for use as future dietary biomarkers of intake that are specific to each legume type.
Methods
Identification and preparation of legume foods
Approximately 10 metric tons of CBs and CPs each were purchased from local Malawian markets and wholesalers, generally in 25- or 50-kg sacks. It is estimated that these are aggregates from ∼15 farms. CBs and CPs were prepared by dry-roasting the entire lot of hand-sorted beans between 120°C and 130°C for 45–50 min with the use of local Malawian facilities. After dry-roasting, the beans were milled into flour, and the flour was thoroughly mixed. One-kilogram samples of CBs and CPs were taken from this mixture for analysis. The CSB was prepared commercially by extrusion cooking (Rice Milling). The CSB flour for analysis was taken as a composite sample from 20 sacks of CSB that were prepared locally. These milled legume flours were designed to be consumed by adding them in small quantities to the traditional maize porridges, which have been previously and successfully used for legumes in infant and children dietary feeding trials in Malawi and elsewhere (5, 26–28).
Comparative nutrient analysis
The dietary composition of the foods was measured at Midwest Laboratories. Briefly, 225 g of each legume food was used for proximate analysis (moisture, protein, fat, ash, carbohydrates, and kilocalories), dietary fiber, FA profile, and micronutrient measurements. The methods were based on the Association of Official Analytical Chemists (AOAC) standards as follows: AOAC 950.46 (moisture), AOAC 992.15 (protein), AOAC 996.06 (FA profile), and AOAC 991.36 (fat). For moisture determination, samples were dried in an air oven for 16–18 h at 100–102°C. For protein, samples were digested and distilled to determine total Kjeldahl nitrogen, which was converted into total protein by using a standardized Kjeldahl factor. For fat determination, samples were desiccated and homogenized and fat was extracted by using a solution of petroleum ether, anhydrous sodium sulfate, and defatted cotton. The ash analysis was completed by weighing the sample, heating it to 550°C, and then re-weighing the remaining residue. Carbohydrates and kilocalories were calculated on the basis of the proximate analysis results.
Dietary fiber (soluble and insoluble) was analyzed by using AOAC 991.43. For insoluble dietary fiber, the legume foods were dried and digested with 3 enzymes (protease, amylase, and amyloglycosidase) to break down starch and protein. Ethanol (78% and 95%) was used to precipitate soluble fiber, and insoluble residues were removed with filtration. Residues were weighed to determine insoluble and soluble fiber amounts. Total dietary fiber was the sum of these 2 amounts.
The AOAC 996.06 method was used to analyze the FA profile. Briefly, the methyl ester extracts were injected into a gas chromatograph that used a flame ionization detector. Thirty-nine FAs were screened for and included butyric (4:0), caproic (6:0), caprylic (8:0), capric (10:0), lauric (12:0), tridecanoic (13:0), myristic (14:0), myristoledic (14:1), myristoleic (14:1n–5), pentadecanoic (15:0), palmitic (16:0), palmitelaidic (trans 16:1n–9), palmitoleic (cis 16:1n–9), heptadecanoic (17:0), 10-heptadecanoic (17:1n–10), stearic (18:0), elaidic (trans 18:1n–9), oleic (cis 18:1n–9), linolelaidic (all-trans 18:2n–6,9), linoleic (all-cis 18:2n–9,12), γ-linolenic (all-cis 18:3n–6,9,12), nonadecanoic (19:0), α-linolenic (all-cis 18:3n–9,12,15), arachidic (20:0), 11-eicosenoic (20:1n–11), 11-14-eicosadienoic (20:2n–11,14), homo-γ-linolenic (all-cis 20:3n–8,11,14), 11-14-17-eicosatrienoic (20:3n–11,14,17), arachidonic (20:4n–5,8,11,14), eicosapentaenoic (20:5n–5,8,11,14,17), heneicosanoic (21:0), behenic (21:0), erucic (cis 21:1n–9), docosadienoic (22:2n–13,16), docosapentaenoic (22:5n–4,7,10,13,16), docosahexaenoic (22:6n–4,7,10,13,16,19), tricosanoic (23:0), lignoceric (24:0), and nervonic (24:1n–9) acids. The fat in the legume foods was extracted and saponified, and the FAs were derivatized into FA methyl esters. To quantitate amounts of the identified FAs, the raw chromatographic abundances of each FA in each food sample were compared with those of known standards. Saturated fat, polyunsaturated fat, and monounsaturated fat were totaled and their relative percentages were based on the FA amount measured.
Inductively coupled plasma MS was completed for the following micronutrients: calcium, iron, magnesium, manganese, phosphorus, potassium, sodium, and zinc. Briefly, prepared extracts of each of the legume foods were injected into high-energy plasma that forced the elements in the injected sample to emit light wavelengths that were specific to each micronutrient present. The light intensity was detected and correlated to the amounts of micronutrients in the original legume food sample.
Calculating daily nutrients delivered by 3 legume complementary foods
The daily nutrient intakes for each food were calculated on the basis of serving sizes for weaning children to consume at different ages. The recommendations for the CSB were as follows: 20 g/d (6–8 mo), 30 g/d (9–11 mo), 40 g/d (12–23 mo), and 50 g/d (24–35 mo). The recommendations for CB or CP foods were 21 g/d (6–8 mo), 31.5 g/d (9–11 mo), 42 g/d (12–23 mo), and 52.5 g/d (24–35 mo). The trial registry numbers for clinical trials associated with this research are NCT02472262 and NCT02472301.
Heavy metal and aflatoxin analyses
Heavy metal and aflatoxin analyses were completed by Midwest Laboratories by using methods described previously (29) and included heavy metal (arsenic, cadmium, lead, and mercury) and aflatoxin contents. The heavy metals analysis was based on the Environmental Protection Agency 7471B method (30). Briefly, samples were dissolved in water, digested with potassium permanganate, and then mixed with water, SSC-hydroxyethyl amine sulfate, and stannous sulfate. The resulting solution was subjected to cold-vapor atomic absorption, where concentration values of heavy metals were based on interpolation to a standard curve of absorbance compared with concentration. Amounts of heavy metals detected in the legume food were compared with those defined by the USDA as acceptable for human consumption in bean-family foods (31).
Aflatoxin methods were based on AOAC 2008.02. Briefly, legume foods were finely ground and aflatoxins extracted by using a solution of SSC, methanol, and sodium bicarbonate. The resulting extract was centrifuged and filtered to remove contamination, and aflatoxins were separated by using immunoaffinity column isolation. The presence and concentration of aflatoxins in the column-separated filtrate were determined with targeted LC-MS. Amounts of aflatoxins detected in the legume food were compared with those defined by the FDA as acceptable for human consumption (32).
Comparative nontargeted food metabolomics
The nontargeted food metabolome profile was completed by Metabolon, Inc., as previously published for other whole foods (33). Briefly, each legume food was extracted by using ice-cold 80% methanol for separation and metabolite detection via ultraperformance LC–tandem MS in positive- and negative-ion mode in which samples were quality-controlled within and across assays for individual samples with the use of internal standards. Raw data were extracted and peak-identified by using the Metabolon internal library, and quality-control processed. For each metabolite, peak raw count abundance values were quantified by using AUC analysis, and each metabolite raw abundance was median-scaled by dividing its median raw abundance across the data set into its raw abundance in each legume food. Median-scaled relative abundance z score was further used to visualize specific metabolites in a given chemical class. z Scores are expressed as SDs from the mean of the scaled abundance for each metabolite, and data were calculated by using the following formula: z = (x − µ)/σ, where x was the relative scaled abundance of the metabolite, µ was mean of the scaled relative abundance for the metabolite across the 3 legumes, and σ was the scaled relative abundance SD of the same metabolite across the 3 legume foods. For each food, metabolite profiles were screened for compounds that could serve as biomarkers of legume intake. To be considered a potential biomarker in a given legume food, compounds needed to meet the following criteria: 1) be detected in only one legume food (no others) or 2) have a higher abundance in 1 legume food than in the 2 others, defined as having a z score of ≥1 with the other 2 foods having a z score of ≤−1.
Results
Comparison of targeted nutrients across legume foods
Table 1 provides details on the macronutrient energy content, which was measured in kilocalories per 100 g, and the micronutrient content, which was measured in parts per million (ppm). All of the legume foods provided similar total energy content (374–391 kcal/100 g). Protein content was higher in CP and CB foods (26 and 23 g/100 g, respectively) than in the CSB (13 g/100 g). Similarly, dietary fiber was higher in CP and CB foods (21 and 28 g/100 g, respectively) than in the CSB (8 g/100 g). Insoluble fiber made up the majority of total dietary fiber. The CSB had twice as much fat (4 g/100 g) as CP and CB foods (2 g/100 g each).
TABLE 1.
Quantified macronutrients, micronutrients, FAs, and food safety profile for each legume food fed to children aged 6–36 mo1
| Nutrient | Corn-soybean blend | Common bean | Cowpea |
|---|---|---|---|
| Macronutrients | |||
| Energy, kcal/100 g | 391 | 375 | 374 |
| Carbohydrate, g/100 g | 75 | 66 | 63 |
| Protein, g/100 g | 13 | 23 | 26 |
| Fat, g/100 g | 4 | 2 | 2 |
| Dietary fiber, g/100 g | |||
| Total | 8 | 28 | 21 |
| Insoluble | 8 | 28 | 20 |
| Soluble | ND | ND | 1 |
| Micronutrients, ppm | |||
| Calcium | 2230 | 2030 | 1040 |
| Iron | 133 | 117 | 152 |
| Magnesium | 1000 | 1870 | 1960 |
| Manganese | 10 | 21 | 17 |
| Phosphorus | 3950 | 4840 | 4380 |
| Potassium | 6230 | 15,800 | 15,900 |
| Sodium | 32 | 12 | 38 |
| Zinc | 39 | 27 | 31 |
| FAs,2 % | |||
| SFAs | 16 | 21 | 36 |
| Lauric (12:0) | ND | 0.03 | 0.03 |
| Myristic (14:0) | 0.1 | 0.15 | 0.2 |
| Pentadecanoic (15:0) | 0.02 | 0.15 | 0.1 |
| Palmitic (16:0) | 12 | 16 | 25 |
| Heptadecanoic (17:0) | 0.1 | 0.2 | 0.1 |
| Stearic (18:0) | 3 | 2 | 4 |
| Arachidic (20:0) | 0.5 | 0.5 | 1 |
| Behenic (22:0) | 0.3 | 0.7 | 3 |
| Tricosanoic (23:0) | ND | 0.2 | 0.3 |
| Lignoceric (24:0) | 0.2 | 1 | 2 |
| MUFAs, % | 27 | 9 | 9 |
| Palmitoleic (16:1 cis) | 0.1 | 0.2 | 0.1 |
| 10-Heptadecanoic (17:1) | 0.05 | 0.07 | n.d. |
| Oleic (18:1 cis) | 27 | 8 | 8 |
| 11-Eicosenoic (20:1) | 0.2 | 0.15 | 0.4 |
| PUFAs, % | 57 | 70 | 55 |
| Linoleic (18:2 cis) | 52 | 29 | 34 |
| α-Linolenic (18:3) | 5 | 41 | 21 |
| Heavy metals, ppm | |||
| Arsenic | ND | ND | ND |
| Cadmium | ND | ND | ND |
| Lead | ND | ND | ND |
| Mercury | ND | ND | ND |
| Aflatoxin, ppb | |||
| Aflatoxin B1 | 2.50 | ND | ND |
| Aflatoxin B2 | ND | ND | ND |
| Aflatoxin G1 | 2.67 | ND | ND |
| Aflatoxin G2 | ND | ND | ND |
| Total aflatoxin | 5.17 | ND | ND |
ND, not detected; ppb, parts per billion; ppm, parts per million.
Percentages represent the amount of individual FAs detected in the total amount of FAs identified.
Micronutrient analysis showed that potassium was higher in CP and CB foods (15,900 and 15,800 ppm, respectively) than in the CSB (6230 ppm) food. Phosphorus was also higher in CP and CB foods (4380 and 4840 ppm) than in the CSB (3950 ppm) food. Iron was similar across the 3 groups (CP, CB, and CSB: 152, 117, and 133 ppm, respectively). The CSB food had the highest amounts of calcium (2230 ppm) and zinc (39 ppm) compared with the CP and CB foods. Furthermore, CP and CB foods had more magnesium (CP and CB: 1960 and 1870 ppm, respectively), phosphorus (4380 and 4840 ppm), and potassium (15,900 and 15,800 ppm) than the CSB food (magnesium, phosphorus, and potassium: 1000, 3950, and 6230 ppm, respectively).
An analysis of relative percentages for FA profiles, presented in Table 1, showed that the CP food had the highest amount of saturated fat, at 36%, followed by the CB (21%) and CSB (16%) foods. The SFAs that were highest in the CP food were palmitic acid (25%), stearic acid (4%), behenic acid (3%), and lignoceric acid (2%). The CSB food had the highest amount of MUFAs at 27%, with CB and CP foods both containing 9%. In each legume flour, oleic acid was the largest contributor to MUFA content and represented 8%, 8%, and 27% of total MUFA contents in CP, CB, and CSB foods, respectively. The CB food had the highest amount of PUFAs at 70%, with CSB and CP foods at 57% and 55%, respectively. Among PUFAs, linoleic acid (an n–6 FA) was highest in the CSB food at 52% of the total polyunsaturated fat content (compared with 34% and 29% in CP and CB foods, respectively) and α-linolenic (an n–3 FA) was highest in the CB food at 41% (compared with 21% and 5% in CP and CSB foods, respectively). Butyric, caproic, caprylic, capric, tridecanoic, myristoledic, elaidic, linolelaidic, γ-linolenic, nonadecanoic, 11-14-eicosadienoic, homo-γ-linolenic, 11-14-17-eicosatrienoic, arachidonic, eicosapentaenoic, heneicosanoic, erucic, docosadienoic, docosapentaenoic, docosahexaenoic, and nervonic acids were not detected in the foods.
Comparison of daily nutrient intakes provided by legume foods for children
To evaluate how legumes may provide nutrition as complementary foods, Table 2 estimates the essential nutrients for children during weaning across the 3 legume foods, in grams per day, on the basis of their age in months, as well as the recommended levels of intake from the WHO and the National Academy of Medicine (34, 35). The energy needs from complementary foods for children receiving an average amount of breast milk range between 200 and 550 kcal/d (34). By using these energy recommendations, all of the legume foods provided ∼40% of daily energy requirements for children aged 6–9 mo (78–118 kcal/d) and ∼30–35% for children aged 12–24 mo (156–197 kcal/d).
TABLE 2.
Daily nutrients delivered by 3 legumes as a complementary food on the basis of a weaning child's age and serving size1
| Corn-soybean blend | Common bean | Cowpea | Recommended level of intake2 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nutrient | 6 mo | 9 mo | 12 mo | 24 mo | 6 mo | 9 mo | 12 mo | 24 mo | 6 mo | 9 mo | 12 mo | 24 mo | 6 mo | 9 mo | 12 mo | 24 mo |
| Macronutrients | ||||||||||||||||
| Energy, kcal/d | 78 | 117 | 156 | 196 | 79 | 118 | 158 | 197 | 79 | 118 | 157 | 196 | 2003 | 3003 | 5503 | 5503 |
| Carbohydrate, g/d | 15 | 22 | 30 | 37 | 14 | 21 | 28 | 35 | 13 | 20 | 26 | 33 | 95 | 95 | 130 | 130 |
| Protein, g/d | 3 | 4 | 5 | 7 | 5 | 7 | 10 | 12 | 5 | 8 | 11 | 14 | 11 | 11 | 13 | 13 |
| Fat, g/d | 0.9 | 1.3 | 1.7 | 2.2 | 0.4 | 0.6 | 0.8 | 1.0 | 0.4 | 0.6 | 0.8 | 1.1 | 30 | 30 | 21 | 21 |
| Dietary fiber | ||||||||||||||||
| Total fiber, g/d | 1.7 | 2.5 | 3.4 | 4.2 | 6.0 | 9.0 | 12.0 | 15.0 | 4.3 | 6.5 | 8.6 | 10.8 | ND | ND | 19 | 19 |
| Micronutrients | ||||||||||||||||
| Calcium, mg/d | 45 | 67 | 89 | 112 | 43 | 64 | 85 | 107 | 22 | 33 | 44 | 55 | 260 | 260 | 700 | 700 |
| Iron, mg/d | 2.7 | 4.0 | 5.3 | 6.7 | 2.5 | 3.7 | 4.9 | 6.1 | 3.2 | 4.8 | 6.4 | 8.0 | 11 | 11 | 7 | 7 |
| Potassium, g/d | 0.1 | 0.2 | 0.2 | 0.3 | 0.3 | 0.5 | 0.7 | 0.8 | 0.3 | 0.5 | 0.7 | 0.8 | 0.7 | 0.7 | 3.0 | 3.0 |
| Zinc, mg/d | 0.8 | 1.2 | 1.5 | 1.9 | 0.6 | 0.9 | 1.1 | 1.4 | 0.7 | 1.0 | 1.3 | 1.6 | 3 | 3 | 3 | 3 |
| FAs, g/d | ||||||||||||||||
| Total SFAs | 0.03 | 0.05 | 0.06 | 0.08 | 0.04 | 0.07 | 0.09 | 0.11 | 0.08 | 0.11 | 0.15 | 0.19 | NA | NA | NA | NA |
| Total MUFAs | 0.05 | 0.08 | 0.11 | 0.14 | 0.02 | 0.03 | 0.04 | 0.05 | 0.02 | 0.03 | 0.04 | 0.05 | NA | NA | NA | NA |
| Total PUFAs | 0.11 | 0.17 | 0.23 | 0.28 | 0.15 | 0.22 | 0.30 | 0.37 | 0.12 | 0.17 | 0.23 | 0.29 | 5.1 | 5.1 | 7.7 | 7.7 |
| Linoleic acid | 0.10 | 0.15 | 0.21 | 0.26 | 0.06 | 0.09 | 0.12 | 0.15 | 0.07 | 0.11 | 0.14 | 0.18 | 4.6 | 4.6 | 7 | 7 |
| α-Linolenic acid | 0.01 | 0.02 | 0.02 | 0.03 | 0.09 | 0.13 | 0.17 | 0.22 | 0.04 | 0.07 | 0.09 | 0.11 | 0.5 | 0.5 | 0.7 | 0.7 |
NA, not applicable; ND, not detected.
Based on the WHO and Institute of Medicine DRIs (34, 35). The recommendations for the corn-soybean blend based on age were as follows: 20 g/d (6–8 mo), 30 g/d (9–11 mo), 40 g/d (12–23 mo), and 50 g/d (24–35 mo). The recommendations for the common bean or cowpea foods were 21 g/d (6–8 mo), 31.5 g/d (9–11 mo), 42 g/d (12–23 mo), and 52.5 g/d (24–35 mo).
Recommended energy needs of breastfed children from complementary foods (34).
The CB food provided 45–95% of recommended protein intake (5–12 g/d), 1–5% of recommended fat intake (0.4–1 g/d), and 60–80% of recommended total dietary fiber intake (6–15 g/d). The CP food provided 50–105% of recommended protein intake (5–14 g/d), 1–5% of recommended fat intake (0.4–1.1 g/d), and 45–55% of recommended total dietary fiber intake (4.3–10.8 g/d). The CSB food provided 25–50% of recommended protein intake (3–7 g/d), 3–10% of recommended fat intake (0.9–2.2 g/d), and 20% of recommended total dietary fiber intake (1.7–4.2 g/d).
The total amounts of iron provided by the legume foods were ∼20–45% of the recommended level of 11 mg/d (6–9 mo of age) and increased to >70% of the recommended level of 7 mg/d (12–23 mo of age). At 24 mo, the amount of CP food provided reached the recommended levels of iron (8.0 g/d), with CSB and CB foods at 90% (6.7 and 6.1 g/d, respectively). The intake of zinc between the ages of 6 and 9 mo was ∼20–40% of the recommended level of 3 mg/d and increased to 40–65% between ages 12 and 24 mo. The CSB food provided the highest amount of zinc at 24 mo (1.9 g/d).
Food safety analysis
Table 1 shows amounts of heavy metals and aflatoxins detected in the legume foods. Arsenic, cadmium, lead, and mercury were not detected in any of the legume foods. Aflatoxin was only detected in the CSB food at a cumulative concentration of 5.2 parts per billion (ppb).
Nontargeted food metabolome of legume foods
A total of 652 metabolites were identified collectively across all 3 legume foods and were further organized across 8 classifications, including amino acids, carbohydrates, cofactors and vitamins, energy metabolism, lipids, nucleotides, peptides, and xenobiotics (benzoate metabolism, chemical, drug, and food and plant component). Of the total food metabolome, there were 509 food metabolites identified from CP food, 483 metabolites identified from CB food, and 443 metabolites identified from CSB food (Table 3). Most food metabolites came from amino acid and lipid metabolic pathways (25% and 37%, respectively), and 46–56 metabolites were classified as phytochemicals, indicating they were previously documented as being plant-derived. profile, listing individual metabolites, their associated metabolic pathways, relative abundance, and detection methods, is reported for the 3 foods in Supplemental Table 1.
TABLE 3.
The 652 food metabolites identified from 3 complementary legume foods classified by metabolic pathways
| Number of identified metabolites | |||
|---|---|---|---|
| Corn-soybean blend | Common bean | Cowpea | |
| Metabolic pathways | |||
| Amino acid | 114 | 121 | 128 |
| Carbohydrate | 26 | 26 | 26 |
| Cofactors and vitamins | 24 | 19 | 20 |
| Energy | 12 | 12 | 13 |
| Lipid | 164 | 179 | 183 |
| Nucleotide | 32 | 49 | 47 |
| Peptide | 12 | 16 | 22 |
| Xenobiotic | |||
| Benzoate metabolism | 3 | 4 | 4 |
| Chemical | 7 | 7 | 7 |
| Drug | 3 | 3 | 3 |
| Food and plant components | 46 | 47 | 56 |
| Total number of metabolites | 443 | 483 | 509 |
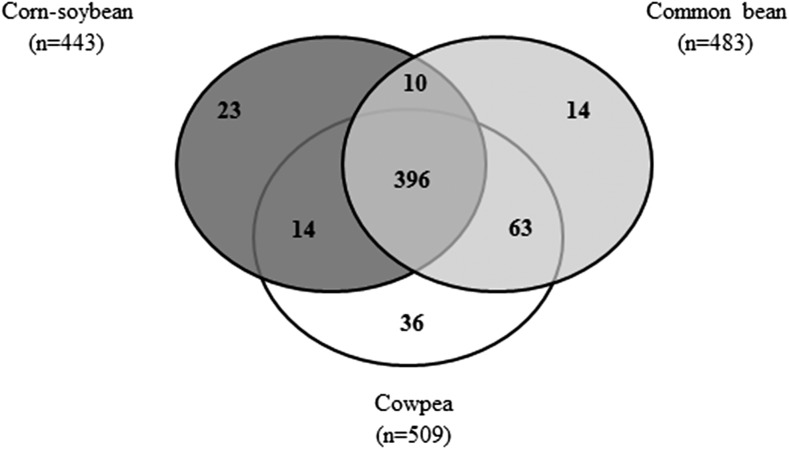
The Venn diagram shown in Figure 1 illustrates the 396 metabolites out of the 652 total metabolites that were identified in all 3 legume-based foods. This figure also shows the number of metabolites detected between 2 foods or that were present only in a single legume food. This included 23 metabolites identified only in CSB food, 14 metabolites distinctly present in CB food, and 36 uniquely present in CP food. The food metabolites unique to each dietary legume are listed in Table 4, which presents potential biomarkers of intake for each food and are clustered by their respective metabolic pathways. For example, arabinose was only identified from the CSB food. Of the metabolites listed in Table 4, those that had evidence of anti-inflammatory activity included putrescine, ribose, riboflavin (vitamin B-2), α-tocotrienol, and γ-tocotrienol in the CSB food; serotonin, dimethylglycine, and oleanolic acid in the CB food; and hypotaurine, dihydroquercetin (taxifolin), eriodictyol, quercetin 3-galactoside, quercetin 3-glucoside, and quercetin in the CP food (36–42, 44–48).
FIGURE 1.
Venn diagram of the total number of metabolites detected across the 3 legume foods.
TABLE 4.
Potential dietary biomarkers of intake by metabolic pathway for 3 complementary legume foods1
| Metabolic pathways | Dietary biom arker | ||
|---|---|---|---|
| Corn-soybean blend | Common bean | Cowpea | |
| Amino acid | Putrescine2 (36), argininosuccinate | 3-Methoxytyramine, serotonin2 (37), tryptophan betaine, dimethylglycine2 (38) | Ophthalmate, S-methylglutathione, 2-methylbutyrylcarnitine (C5), 3-hydroxy-2-ethylpropionate, 3-hydroxyisobutyrate, 3-methyl-2-oxovalerate, hypotaurine2 (39), N-acetyltaurine |
| Carbohydrate | N-acetylglucosamine/N-acetylgalactosamine, arabinose,3 ribose2 (40), ribulose/xylulose | — | N-acetyl-glucosamine-1-phosphate |
| Cofactors and vitamins | Dehydroascorbate, riboflavin (vitamin B-2)2 (41), α-tocopherol acetate, α-tocotrienol2,3 (42, 43), γ-tocotrienol2,3 (42, 43) | — | FMN |
| Energy | Succinylcarnitine | — | — |
| Lipid | Caproate (6:0), glycerophosphoserine, valerate (5:0) | Diacylglycerol (12:0/18:1, 14:0/16:1, 16:0/14:1), propionylcarnitine (C3), 1-pentadecanoylglycerol (15:0) | Palmitoyl-oleoyl-glycerol (16:0/18:1), adipate, nervonate (24:1n–9), 1-myristoylglycerol (14:0), 1-palmitoyl-2-arachidonoyl-GPE (16:0/20:4), docosadienoate (22:2n–6), N-palmitoyl-sphingosine (d18:1/16:0), stearoyl sphingomyelin (d18:1/18:0) |
| Nucleotide | — | 2′-GMP, 3′-CMP, thymine 3′-UMP | 3′-GMP, N2,N2-dimethylguanosine |
| Peptide | — | Leucylalanine, phenylalanylalanine | Alanylleucine, glycylleucine, lysylleucine, valylglutamine, valylleucine, γ-glutamyl-α-lysine, γ-glutamylglycine |
| Xenobiotic | 1,1-Kestotetraose, 2-oxindole-3-acetate, chlorogenate, coumaroylquinates 2–5, feruloylputrescine | Oleanolic acid2,3 (44) | Benzoate, dihydroquercetin (taxifolin)2 (45), eriocitrin, eriodictyol2 (46), galacturonate, quercetin 3-galactoside2,3 (47, 48), quercetin 3-glucoside2,3 (48), quercetin2,3 (48), secoisolariciresinol |
A total of 24 metabolites were solely detected in the corn-soybean blend, 14 metabolites were unique in the common bean, and 36 metabolites in the cowpea.
Metabolite with evidence of anti-inflammatory activity.
Metabolite that has been previously identified in soybean, common bean, or cowpea and considered a strong potential dietary biomarker for these legume foods.
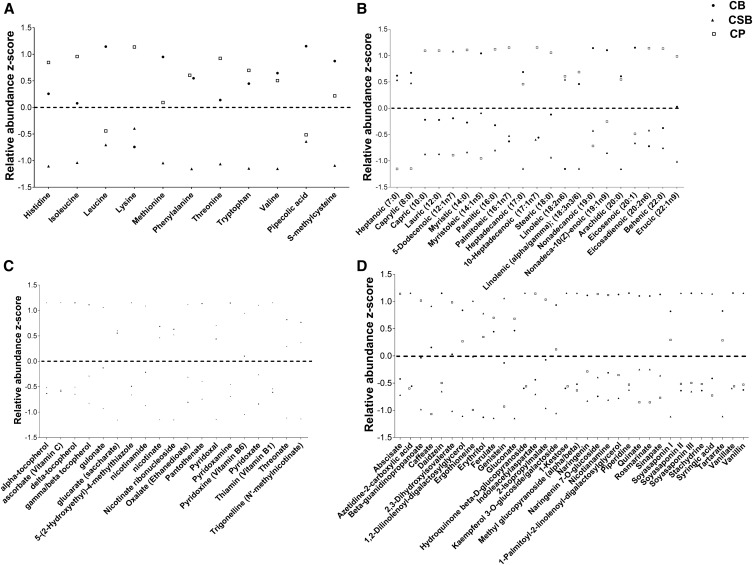
Figure 2 shows the standard distributions of select metabolites across the amino acid, cofactor and vitamin, FA, and phytochemical pathways. A distinct profile of amino acids across the 3 foods is shown in Figure 2A. CB and CP foods had relative abundance z scores above the mean abundance across all legumes for all 9 essential amino acids (histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine), whereas the CSB relative abundance z scores were all below the mean. In addition, pipecolic acid and S-methylcysteine, common nonprotein nitrogen components of Phaseolus and Vigna species (42), were detected in all 3 foods and had the highest scaled relative abundance in the CB food compared with the CSB and CP foods (Figure 2A).
FIGURE 2.
Relative abundance z score distributions of selected metabolites detected in all 3 legume foods to visualize the distinct profiles. These include essential amino acids and common nonprotein nitrogen components in the amino acid pathway (A), FAs in the lipid pathway (B), the cofactors and vitamins pathway (C), and phytochemicals in the xenobiotic pathway (D). Dotted lines represent a relative abundance z score of 0. CB, common bean; CP, cowpea; CSB, corn-soybean blend.
Figure 2B shows the distinct profile of FAs identified from the nontargeted metabolomic analysis. Similar to the amino acid metabolite profile, CB and CP foods had relative abundance z scores above the mean scaled abundance across all legumes for a majority of the FAs. Caprylic acid, capric acid, nonadecanoic acid, and erucic acid were not included in the standard targeted FA analysis, yet were identified by using the nontargeted food metabolome approach. SFAs, including palmitic, stearic, and behenic acids, had the highest relative abundance z scores in the CP food, which was confirmed to match results from the targeted FA analysis (Table 1). Further targeted metabolomic analyses will be needed to quantify FA amounts in these foods for comparison with the FA amounts reported in Tables 1 and 2.
There were 19 cofactor and vitamin metabolites identified by using metabolomics in the 3 foods. The distinct profile of cofactors and vitamins inherent to each food is highlighted in Figure 2C. When compared with CS and CSB foods, the CP food showed the highest relative abundance z scores for the B-vitamin metabolites nicotinate (vitamin B-3), trigonelline, and pantothenate (vitamin B-5). The CSB food had the highest relative abundance z scores for the vitamin B metabolites nicotinamide (vitamin B-3), thiamin (vitamin B-1), and pyridoxine (vitamin B-6) when compared with CP and CB foods. The CSB food also had the highest relative abundance z scores for ascorbate (vitamin C) and α-tocopherol (vitamin E) when compared with CP and CB foods. Other vitamin E components, including δ-tocopherol and γ-tocopherol or β-tocopherol, showed the highest relative abundance z scores in the CP food compared with the CS and CSB foods.
Figure 2D shows the distinct profile of all 34 phytochemicals from all 3 foods. Genistein, an isoflavone found primarily in soybeans, was detected in all 3 foods, and the CSB food had the highest relative abundance z score for genistein compared with the CP and CS foods. Ferulic acid, another abundant phenolic acid in various legumes (12, 49), had the highest relative abundance z score in the CP food when compared with the CB and CSB foods. Across all foods, no additional biomarkers were identified as having a z score ≥1 in 1 food while having a z score of −1 or lower in the other 2 foods.
Discussion
This study assessed the potential nutritional value of 3 dry-roasted, legume-based complementary foods available to children living in rural Malawi via the integration of targeted nutrient and food safety assessments and global, nontargeted food metabolite profiling. Although a CSB is the traditional complementary food recommended to treat childhood malnutrition in Malawi (50, 51), the nutrient, food safety screening, and metabolite analysis presented herein supports future investigation of alternative legumes, specifically CPs and CBs, as complementary foods.
This targeted nutrient analysis showed that all diets provided similar total energy contents, yet CB and CP foods had higher amounts of dietary fiber and protein (Table 1). The CB and CP foods contributed an estimated 45–80% of the DRI for dietary fiber for these children, compared with 20% in the CSB (Table 2). This finding is similar to a previous report on dietary fiber in legumes (11), which supports that CB and CP foods could serve as high-quality sources of dietary fiber in weaning children. Dietary fiber modulates nutrient absorption and increases fermentation by the beneficial colonic microflora, which can lead to improved gut barrier function and decreased inflammation to reduce the risk of developing EED (10). The high amounts of dietary fiber in CB and CP foods, when compared with the CSB food, support their future potential as additional fiber-rich complementary foods to promote gut health in weaning children.
Table 2 further showed how much the 3 legume foods contributed to recommended daily intakes for key nutrients based on the weaning child's age and needs for complementary foods (34, 35). CB and CP foods provided a higher amount of protein per serving than did the CSB food and provided ∼50–75% of the recommended intake in children aged 6–9 mo and 80–100% in children aged 12–24 mo (Table 2). Nontargeted metabolite profiling further showed that the CB and CP foods contained a higher abundance of multiple essential amino acids than the CSB food, including histidine, isoleucine, leucine, methionine, phenylalanine, threonine, tryptophan, and threonine, which supports its utility in balanced nutrition approaches. High-quality proteins with balanced amino acid profiles, such as those found in CB and CP foods, are important for proper gut barrier and immune system development (52). Given that EED both derives from and contributes to a disturbed microbiome and a dysfunctional gut immune system (53), the high protein content of dry-roasted CB and CP proteins merits further investigation for their use as alternative complementary foods in rural Malawian infants.
Micronutrient analyses confirmed that all of the legume foods naturally provided many essential micronutrients that are important to overall child nutritional status and growth. The CSB and CP foods provided a rich source of both iron and zinc compared with the CB food (Table 1) without being additionally fortified. Iron intake is essential for redox reactions, gene regulation, oxygen delivery to tissues, and cell growth (54, 55), and Malawian children and pregnant and breastfeeding mothers are commonly deficient (56). Efforts have been put forth to increase iron intake in rural Malawian children (57), but elemental iron supplementation increased diarrhea risk and did not reduce EED risk or growth stunting (58). Legume foods, such as CSB and CP foods, could potentially serve as novel food-based strategies that can be investigated for their effectiveness in increasing total iron intake. Zinc is an essential micronutrient that is also commonly deficient in rural Malawian children (57). Evidence supports a role for zinc to reduce the duration and severity of diarrhea episodes, and it may protect against EED by restoring gut mucosal barrier integrity and by bolstering antibody production against enteric pathogens (59). Consequently, CP and CSB foods may additionally merit attention as a rich source of zinc to improve deficiencies.
Trypsin inhibitors, amylase inhibitors, phytates, and phytoestrogens are traditionally regarded as antinutrients that naturally occur in legumes (60, 61), and concern has been raised that their presence in the diets of young children may reduce micronutrient absorption (62), interfere with protein and carbohydrate digestion, and disrupt estrogen metabolism (63, 64). However, boiling of whole legume seeds, the most common method of preparation for consumption, reduces the activity of these enzymes by ∼20-fold to amounts below nutritional significance (65). Decreases in these antinutrient enzymes, as well as in phytoestrogens, were also observed during dry-roasting (66), which was performed locally during food preparation. Moreover, a growing body of research supports that chronic exposure by Malawian children to dietary phytic acid does not appreciably alter zinc fecal excretion (67–69), suggesting that when legume diets are appropriately boiled or dry-roasted, other gastrointestinal and dietary factors may be drivers of micronutrient deficiency that can be explored to manage micronutrient deficiencies. In light of these considerations, the high natural amounts of iron and zinc in dry-roasted CSBs and CPs warrant further investigation as accessible and valuable micronutrient sources for complementary feeding.
The targeted FA profile of legume foods showed differences in amounts of bioactive lipids across foods. Specifically, α-linoleic acid, which was higher in CP and CB foods than in the CSB food, has been implicated in reducing colonic inflammation (70), a major contributor to EED pathogenesis. Other bioactive lipids higher in CP and CB foods than in the CSB food included myristic acid, which has been associated with beneficially modulating gut microflora to reduce diarrhea in weaning piglets (71). The naturally high abundance and diversity of these bioactive lipids in CPs and CBs compared with CSBs (72) merit future investigation of their lipid profiles for health promotion as alternative complementary foods in child populations at risk of EED.
Along with macronutrient, micronutrient, and small bioactive compounds, food safety analysis did not detect any heavy metals (arsenic, cadmium, lead, or mercury) in the foods (Table 1). These heavy metals, which are becoming more prevalent in food, continue to have adverse health effects in humans, including gut dysbiosis, mucosal immune dysregulation, and chronic inflammation (73). If individuals are exposed during childhood and development, these risk factors may contribute to EED pathogenesis. Furthermore, aflatoxin was only detected in the CSB at 5.2 ppb (Table 1) and is likely coming from the maize, because this is a known, common aflatoxin source (74). Although there are a number of health risks involved in aflatoxin exposure, including growth faltering and immune suppression in children (74), the FDA has reported acceptable amounts of aflatoxins in human food to be <20 ppb (75). This is ∼4 times more than what was detected in the CSB, which supports that it can be safely consumed by children. Given that these legume foods were harvested and prepared locally, the lack of high amounts of heavy metals and aflatoxins in all of the foods supports their future use as safe complementary foods.
Metabolomic analysis provided an opportunity to quantify, identify, and compare food components and metabolites from the 3 different legume-based foods (Supplemental Table 1, Table 4). Our nontargeted food metabolomics approach successfully identified lipids, amino acids, and other phytochemicals that are established biomarkers or that merit further examination as potential dietary biomarkers of human dietary legume consumption. In all legumes, many of these biomarkers were also associated with intestinal health benefits. Among the 14 potential and identified biomarkers in the CB food was pipecolic acid (Table 4), an established biomarker of legume intake (76). The CB food also contained the potential biomarker oleanolic acid (Table 4), Oleanolic acid, which is the primary MUFA in all legume foods, was isolated in numerous food and medicinal plants and is known to exert anti-inflammatory actions (77). EED is marked by increased oxidative damage to the gut, and by contributing bioactive lipids such as oleanolic acid, CBs may function as a complementary food that could compensate for EED-associated dietary deficiencies and oxidative damage and protect infants against further pathogenesis.
The CP food contained 36 food biomarkers, and among these were quercetin and serotonin (Table 4), which both have established bioactivities in the gut. Quercetin, which was 1 of 14 compounds uniquely detected in the CP food, is a flavonol with natural immunomodulatory and antioxidative properties (78). Quercetin has been shown to control intestinal inflammation via modulation of leukocytes (79) and could therefore be useful in supporting a healthy immune system to lower EED risk. Similarly, serotonin acts on receptors along the intestinal tract to modulate inflammatory responses (37) and thus may be important for maintaining a normal, healthy gut barrier.
Of the 23 biomarkers identified in the CSB, arabinose and α- and γ-tocopherol (Table 4) have been previously reported in soybean literature (43). Arabinose may be converted into short-chain FAs by bacteria to support healthy enterocyte metabolism, bolster gut mucosal immunity, and beneficially modulate the microbiome (80–82). α- and γ-Tocopherol possess broad-spectrum antioxidant and immunomodulatory activities (42). As antioxidants, these compounds scavenge free radicals and help prevent cell membrane oxidation (83). Furthermore, as immune-modulators, α- and γ-tocopherols have been shown to be protective against chronic inflammation, in part via modulation of T helper 2–lymphocyte populations and also by the reduction of the inflammatory mediator PGE2 and the associated enzyme cyclo-oxygenase 2 (42), supporting their use as beneficial immune-modulators in EED protection.
Findings reported herein establish that CP, CB, and CSB legume-based complementary foods are rich in macro- and micronutrients, are safe, and have profiles abundant in bioactive small compounds that may have utility as alternative complementary foods for use during weaning. Incorporating a food-omics approach allowed for an in-depth understanding of the variability between metabolites present in the 3 legume foods, especially in amounts of metabolites that are associated with potential EED prevention in children. This approach also assisted in identifying dietary biomarkers associated with legume foods, which will be useful to assess compliance to different legume food interventions in future dietary clinical trials with these diets. In conclusion, this legume food analysis can guide the future selection of locally available, whole staple foods that are nutritionally adequate and rich in micronutrients and bioactive metabolites that improve health in a population at high risk of EED and stunting.
Supplementary Material
Acknowledgments
We thank Theresa N Ngoma, Ulemu K Chimimba, and Nicole S Benzoni for help in developing the initial recipes used to prepare the legume foods, as well as Iman Zarei for internal review of this manuscript before submission. The authors' responsibilities were as follows—ECB, IT, KMM, MJM, and EPR: designed the research; ECB, LZ, and NJN: analyzed the data; ECB and LZ: wrote the first draft of the manuscript; MJM and EPR: had responsibility for the final content; and all authors: read and approved the final manuscript.
Abbreviations
- AOAC
Association of Official Analytical Chemists
- CB
common bean
- CP
cowpea
- CSB
corn-soybean blend
- EED
environmental enteric dysfunction
- ppb
parts per billion
- ppm
parts per million
Footnotes
Supported by the Feed the Future Food Security Innovation Lab: Collaborative Research on Grain Legumes by the Bureau for Economic Growth, Agriculture, and Trade, the US Agency for International Development under the terms of EDH-A-00-07-00005-00. Additional funding was provided by the Children's Discovery Institute of Washington University and St. Louis Children's Hospital.
References
- 1. Keusch GT, Denno DM, Black RE, Duggan C, Guerrant RL, Lavery JV, Nataro JP, Rosenberg IH, Ryan ET, Tarr PI, et al. . Environmental enteric dysfunction: pathogenesis, diagnosis, and clinical consequences. Clin Infect Dis 2014;59:S207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Crane RJ, Jones KDJ, Berkley JA.. Environmental enteric dysfunction: an overview. Food Nutr Bull 2015;36:S76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prendergast A, Kelly P.. Review: enteropathies in the developing world: neglected effects on global health. Am J Trop Med Hyg 2012;86:756–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Korpe PS, Petri WA.. Environmental enteropathy: critical implications of a poorly understood condition. Trends Mol Med 2012;18:328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trehan I, Benzoni NS, Wang AZ, Bollinger LB, Ngoma TN, Chimimba UK, Stephenson KB, Agapova SE, Maleta KM, Manary MJ.. Common beans and cowpeas as complementary foods to reduce environmental enteric dysfunction and stunting in Malawian children: study protocol for two randomized controlled trials. Trials 2015;16:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Messina MJ.. Legumes and soybeans: overview of their nutritional profiles and health effects. Am J Clin Nutr 1999;70(Suppl):439S–50S. [DOI] [PubMed] [Google Scholar]
- 7. Hayat I, Ahmad A, Masud T, Ahmed A, Bashir S.. Nutritional and health perspectives of beans (Phaseolus vulgaris L.): an overview. Crit Rev Food Sci Nutr 2014;54:580–92. [DOI] [PubMed] [Google Scholar]
- 8. Marchione TJ.. Foods provided through U.S. government emergency food aid programs: policies and customs governing their formulation, selection and distribution. J Nutr 2002;132(Suppl):2104S–11S. [DOI] [PubMed] [Google Scholar]
- 9. de Pee S, Bloem MW.. Current and potential role of specially formulated foods and food supplements for preventing malnutrition among 6- to 23-month-old children and for treating moderate malnutrition among 6- to 59-month-old children. Food Nutr Bull 2009;30(3Suppl):S434–63. [DOI] [PubMed] [Google Scholar]
- 10. Agostoni C, Riva E, Giovannini M.. Dietary fiber in weaning foods of young children. Pediatrics 1995;96:1002–5. [PubMed] [Google Scholar]
- 11. Chen Y, McGee R, Vandemark G, Brick M, Thompson HJ.. Dietary fiber analysis of four pulses using AOAC 2011.25: implications for human health. Nutrients 2016;8:829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cai R, Hettiarachchy NS, Jalaluddin M.. High-performance liquid chromatography determination of phenolic constituents in 17 varieties of cowpeas. J Agric Food Chem 2003;51:1623–7. [DOI] [PubMed] [Google Scholar]
- 13. Moray C, Game ET, Maxted N.. Prioritising in situ conservation of crop resources: a case study of African cowpea (Vigna unguiculata). Sci Rep 2014;4:5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Siddiq M, Uebersax MA.. Dry beans and pulses production and consumption—an overview. In: Siddiq M, Uebersax MA, editors. Dry beans and pulses production, processing and nutrition. Hoboken (NJ): Blackwell Publishing Ltd.; 2012. p. 1–22. [Google Scholar]
- 15. Hartman TJ, Albert PS, Zhang ZY, Bagshaw D, Kris-Etherton PM, Ulbrecht J, Miller CK, Bobe G, Colburn NH, Lanza E.. Consumption of a legume-enriched, low-glycemic index diet is associated with biomarkers of insulin resistance and inflammation among men at risk for colorectal cancer. J Nutr 2010;140:60–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Campos-Vega R, Oomah B, Loarca-Piña G, Vergara-Castañeda H.. Common beans and their non-digestible fraction: cancer inhibitory activity—an overview. Foods 2013;2:374–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ayogu RN, Nnam NM, Mbah M.. Evaluation of two local cowpea species for nutrient, antinutrient, and phytochemical compositions and organoleptic attributes of their wheat-based cookies. Food Nutr Res 2016;60:29600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kyei-Boahen S, Savala CEN, Chikoye D, Abaidoo R.. Growth and yield responses of cowpea to inoculation and phosphorus fertilization in different environments. Front Plant Sci 2017;8:646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abizari AR, Pilime N, Armar-Klemesu M, Brouwer ID.. Cowpeas in Northern Ghana and the factors that predict caregivers' intention to give them to schoolchildren. PLoS One 2013;8:e72087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scalbert A, Brennan L, Manach C, Andres-Lacueva C, Dragsted LO, Draper J, Rappaport SM, van der Hooft JJJ, Wishart DS.. The food metabolome: a window over dietary exposure. Am J Clin Nutr 2014;99:1286–308. [DOI] [PubMed] [Google Scholar]
- 21. Ramalingam A, Kudapa H, Pazhamala LT, Weckwerth W, Varshney RK.. Proteomics and metabolomics: two emerging areas for legume improvement. Front Plant Sci 2015;6:1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Joshi T, Fitzpatrick MR, Chen SY, Liu Y, Zhang HX, Endacott RZ, Gaudiello EC, Stacey G, Nguyen HT, Xu D.. Soybean knowledge base (SoyKB): a web resource for integration of soybean translational genomics and molecular breeding. Nucleic Acids Res 2014;42(D1):D1245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chebrolu KK, Fritschi FB, Ye SQ, Krishnan HB, Smith JR, Gillman JD.. Impact of heat stress during seed development on soybean seed metabolome. Metabolomics 2016;12:28. [Google Scholar]
- 24. Forster GM, Heuberger AL, Broeckling CD, Bauer JE, Ryan EP.. Consumption of cooked navy bean powders modulate the canine fecal and urine metabolome. Curr Metabolomics 2015;3:90–101. [Google Scholar]
- 25. Mensack MM, Fitzgerald VK, Ryan EP, Lewis MR, Thompson HJ, Brick MA.. Evaluation of diversity among common beans (Phaseolus vulgaris L.) from two centers of domestication using 'omics' technologies. BMC Genomics 2010;11:686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Borresen EC, Jenkins-Puccetti N, Schmitz K, Brown DG, Pollack A, Fairbanks A, Wdowik M, Rao S, Nelson TL, Luckasen G, et al. . A pilot randomized controlled clinical trial to assess tolerance and efficacy of navy bean and rice bran supplementation for lowering cholesterol in children. Global pediatric health 2017;4:2333794X17694231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stobaugh HC, Ryan KN, Kennedy JA, Grise JB, Crocker AH, Thakwalakwa C, Litkowski PE, Maleta KM, Manary MJ, Trehan I.. Including whey protein and whey permeate in ready-to-use supplementary food improves recovery rates in children with moderate acute malnutrition: a randomized, double-blind clinical trial. Am J Clin Nutr 2016;103:926–33. [DOI] [PubMed] [Google Scholar]
- 28. LaGrone LN, Trehan I, Meuli GJ, Wang RJ, Thakwalakwa C, Maleta K, Manary MJ.. A novel fortified blended flour, corn-soy blend “plus-plus,” is not inferior to lipid-based ready-to-use supplementary foods for the treatment of moderate acute malnutrition in Malawian children. Am J Clin Nutr 2012;95:212–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Trucksess MW, Weaver CM, Oles CJ, Fry FS, Noonan GO, Betz JM, Rader JI.. Determination of aflatoxins B(1), B(2), G(1), and G(2) and ochratoxin A in ginseng and ginger by multitoxin immunoaffinity column cleanup and liquid chromatographic quantitation: collaborative study. J AOAC Int 2008;91:511–23. [PMC free article] [PubMed] [Google Scholar]
- 30. Environmental Protection Agency Method 7471B: mercury in solid or semisolid waste (manual cold-vapor technique) [Internet]. 1998. [cited 2017 Sep 1]. Available from: https://www.epa.gov/sites/production/files/2015-12/documents/7471b.pdf.
- 31. Hong B.. Technical regulations on mycotoxin and heavy metals MRLs in foods. USDA; 2013. [Google Scholar]
- 32. National Grain and Feed Association Mycotoxin regulatory guidance: FDA. A guide for grain elevators, feed manufacture rs, grain processors and exporters. Arlington (VA): National Grain and Feed Association; 2011. [Google Scholar]
- 33. Zarei I, Brown DG, Nealon NJ, Ryan EP.. Rice bran metabolome contains amino acids, vitamins & cofactors, and phytochemicals with medicinal and nutritional properties. Rice (N Y) 2017;10:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. WHO Complementary feeding of young children in developing countries: a review of current scientific knowledge (WHO/NUT/98.1). Geneva (Switzerland): WHO; 1998. [Google Scholar]
- 35. National Academy of Medicine [Internet]. Dietary Reference Intakes tables and applications [cited 2017 Mar 8]. Available from: http://www.nationalacademies.org/hmd/Activities/Nutrition/SummaryDRIs/DRI-Tables.aspx.
- 36. Lagishetty CV, Naik SR.. Polyamines: potential anti-inflammatory agents and their possible mechanism of action. Indian J Pharmacol 2008;40:121–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bischoff SC, Mailer R, Pabst O, Weier G, Sedlik W, Li ZS, Chen JJ, Murphy DL, Gershon MD.. Role of serotonin in intestinal inflammation: knockout of serotonin reuptake transporter exacerbates 2,4,6-trinitrobenzene sulfonic acid colitis in mice. Am J Physiol Gastrointest Liver Physiol 2009;296:G685–95. [DOI] [PubMed] [Google Scholar]
- 38. Lawson BR, Belkowski SM, Whitesides JF, Davis P, Lawson JW.. Immunomodulation of murine collagen-induced arthritis by N, N-dimethylglycine and a preparation of Perna canaliculus. BMC Complement Altern Med 2007;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hara K, Nakamura M, Haranishi Y, Terada T, Kataoka K, Sata T.. Antinociceptive effect of intrathecal administration of hypotaurine in rat models of inflammatory and neuropathic pain. Amino Acids 2012;43:397–404. [DOI] [PubMed] [Google Scholar]
- 40. Szabó C, Dawson VL.. Role of poly(ADP-ribose) synthetase in inflammation and ischaemia-reperfusion. Trends Pharmacol Sci 1998;19:287–98. [DOI] [PubMed] [Google Scholar]
- 41. Granados-Soto V, Terán-Rosales F, Rocha-González HI, Reyes-García G, Medina-Santillán R, Rodríguez-Silverio J, Flores-Murrieta FJ.. Riboflavin reduces hyperalgesia and inflammation but not tactile allodynia in the rat. Eur J Pharmacol 2004;492:35–40. [DOI] [PubMed] [Google Scholar]
- 42. Reiter E, Jiang Q, Christen S.. Anti-inflammatory properties of α- and γ-tocopherol. Mol Aspects Med 2007;28:668–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dwiyanti MS, Maruyama S, Hirono M, Sato M, Park E, Yoon SH, Yamada T, Abe J.. Natural diversity of seed alpha-tocopherol ratio in wild soybean (Glycine soja) germplasm collection. Breed Sci 2016;66:653–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Singh GB, Singh S, Bani S, Gupta BD, Banerjee SK.. Anti-inflammatory activity of oleanolic acid in rats and mice. J Pharm Pharmacol 1992;44:456–8. [DOI] [PubMed] [Google Scholar]
- 45. Gupta MB, Bhalla TN, Gupta GP, Mitra CR, Bhargava KP.. Anti-inflammatory activity of taxifolin. Jpn J Pharmacol 1971;21:377–82. [DOI] [PubMed] [Google Scholar]
- 46. García-Lafuente A, Guillamón E, Villares A, Rostagno MA, Martínez JA.. Flavonoids as anti-inflammatory agents: implications in cancer and cardiovascular disease. Inflamm Res 2009;58:537–52. [DOI] [PubMed] [Google Scholar]
- 47. Kao ES, Wang CJ, Lin WL, Yin YF, Wang CP, Tseng TH.. Anti-inflammatory potential of flavonoid contents from dried fruit of Crataegus pinnatifida in vitro and in vivo. J Agric Food Chem 2005;53:430–6. [DOI] [PubMed] [Google Scholar]
- 48. Camuesco D, Comalada M, Rodriguez-Cabezas ME, Nieto A, Lorente MD, Concha A, Zarzuelo A, Galvez J.. The intestinal anti-inflammatory effect of quercitrin is associated with an inhibition in iNOS expression. Br J Pharmacol 2004;143:908–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gutiérrez-Uribe JA, Romo-Lopez I, Serna-Saldívar SO.. Phenolic composition and mammary cancer cell inhibition of extracts of whole cowpeas (Vigna unguiculata) and its anatomical parts. J Funct Foods 2011;3:290–7. [Google Scholar]
- 50. Callaghan-Gillespie M, Schaffner AA, Garcia P, Fry J, Eckert R, Malek S, Trehan I, Thakwalakwa C, Maleta KM, Manary MJ, et al. . Trial of ready-to-use supplemental food and corn-soy blend in pregnant Malawian women with moderate malnutrition: a randomized controlled clinical trial. Am J Clin Nutr 2017. Aug 9 (Epub ahead of print; ). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rogers BL, Wilner LB, Maganga G, Walton SM, Suri DJ, Langlois BK, Chui KK, Boiteau JM, Vosti SA, Webb P.. Program changes are effective and cost-effective in increasing the amount of oil used in preparing corn soy blend porridge for treatment of moderate acute malnutrition in Malawi. Matern Child Nutr 2017. Jan 12 (Epub ahead of print; ). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cao KF, Zhang HH, Han HH, Song Y, Bai XL, Sun H.. Effect of dietary protein sources on the small intestine microbiome of weaned piglets based on high-throughput sequencing. Lett Appl Microbiol 2016;62:392–8. [DOI] [PubMed] [Google Scholar]
- 53. Ordiz MI, Stephenson K, Agapova S, Wylie KM, Maleta K, Martin J, Trehan I, Tarr PI, Manary MJ.. Environmental enteric dysfunction and the fecal microbiota in Malawian children. Am J Trop Med Hyg 2017;96:473–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Weiss G.. Iron and immunity: a double-edged sword. Eur J Clin Invest 2002;32(Suppl 1):70–8. [DOI] [PubMed] [Google Scholar]
- 55. Jonker FA, Calis JC, van Hensbroek MB, Phiri K, Geskus RB, Brabin BJ, Leenstra T.. Iron status predicts malaria risk in Malawian preschool children. PLoS One 2012;7:e42670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Titilayo A, Palamuleni ME, Omisakin O.. Sociodemographic factors influencing adherence to antenatal iron supplementation recommendations among pregnant women in Malawi: analysis of data from the 2010 Malawi Demographic and Health Survey. Malawi Med J 2016;28:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang AZ, Shulman RJ, Crocker AH, Thakwalakwa C, Maleta KM, Devaraj S, Manary MJ, Trehan I.. A combined intervention of zinc, multiple micronutrients, and albendazole does not ameliorate environmental enteric dysfunction or stunting in rural Malawian children in a double-blind randomized controlled trial. J Nutr 2017;147:97–103. [DOI] [PubMed] [Google Scholar]
- 58. Paganini D, Uyoga MA, Zimmermann MB.. Iron fortification of foods for infants and children in low-income countries: eff ects on the gut microbiome, gut inflammation, and diarrhea. Nutrients 2016;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lazzerini M, Wanzira H.. Oral zinc for treating diarrhoea in children. Cochrane Database Syst Rev 2016;12:CD005436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lajolo FM, Genovese MI.. Nutritional significance of lectins and enzyme inhibitors from legumes. J Agric Food Chem 2002;50:6592–8. [DOI] [PubMed] [Google Scholar]
- 61. Gibson RS, Bailey KB, Gibbs M, Ferguson EL.. A review of phytate, iron, zinc, and calcium concentrations in plant-based complementary foods used in low-income countries and implications for bioavailability. Food Nutr Bull 2010;31(2Suppl):S134–46. [DOI] [PubMed] [Google Scholar]
- 62. Manary MJ, Hotz C, Krebs NF, Gibson RS, Westcott JE, Broadhead RL, Hambidge KM.. Zinc homeostasis in Malawian children consuming a high-phytate, maize-based diet. Am J Clin Nutr 2002;75:1057–61. [DOI] [PubMed] [Google Scholar]
- 63. Obiro WC, Zhang T, Jiang B.. The nutraceutical role of the Phaseolus vulgaris alpha-amylase inhibitor. Br J Nutr 2008;100:1–12. [DOI] [PubMed] [Google Scholar]
- 64. Sarwar Gilani G, Wu Xiao C, Cockell KA.. Impact of antinutritional factors in food proteins on the digestibility of protein and the bioavailability of amino acids and on protein quality. Br J Nutr 2012;108(Suppl 2):S315–32. [DOI] [PubMed] [Google Scholar]
- 65. Queiroz Kda S, de Oliveira AC, Helbig E, Reis SM, Carraro F.. Soaking the common bean in a domestic preparation reduced the contents of raffinose-type oligosaccharides but did not interfere with nutritive value. J Nutr Sci Vitaminol (Tokyo) 2002;48:283–9. [DOI] [PubMed] [Google Scholar]
- 66. Zaheer K, Humayoun Akhtar M.. An updated review of dietary isoflavones: nutrition, processing, bioavailability and impacts on human health. Crit Rev Food Sci Nutr 2017;57:1280–93. [DOI] [PubMed] [Google Scholar]
- 67. Kennedy G, Hambidge KM, Manary M.. A reduced phytate diet does not reduce endogenous fecal zinc in children on a habitual high-phytate diet. J Pediatr Gastroenterol Nutr 2010;51:678–9. [DOI] [PubMed] [Google Scholar]
- 68. Manary MJ, Krebs NF, Gibson RS, Broadhead RL, Hambidge KM.. Community-based dietary phytate reduction and its effect on iron status in Malawian children. Ann Trop Paediatr 2002;22:133–6. [DOI] [PubMed] [Google Scholar]
- 69. Manary MJ, Hotz C, Krebs NF, Gibson RS, Westcott JE, Arnold T, Broadhead RL, Hambidge KM.. Dietary phytate reduction improves zinc absorption in Malawian children recovering from tuberculosis but not in well children. J Nutr 2000;130:2959–64. [DOI] [PubMed] [Google Scholar]
- 70. Michalak A, Mosinska P, Fichna J.. Polyunsaturated fatty acids and their derivatives: therapeutic value for inflammatory, functional gastrointestinal disorders, and colorectal cancer. Front Pharmacol 2016;7:459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zentek J, Ferrara F, Pieper R, Tedin L, Meyer W, Vahjen W.. Effects of dietary combinations of organic acids and medium chain fatty acids on the gastrointestinal microbial ecology and bacterial metabolites in the digestive tract of weaning piglets. J Anim Sci 2013;91:3200–10. [DOI] [PubMed] [Google Scholar]
- 72. Michaelsen KF, Hoppe C, Roos N, Kaestel P, Stougaard M, Lauritzen L, Mølgaard C, Girma T, Friis H.. Choice of foods and ingredients for moderately malnourished children 6 months to 5 years of age. Food Nutr Bull 2009;30(3Suppl 3):S343–404. [DOI] [PubMed] [Google Scholar]
- 73. Jin Y, Wu S, Zeng Z, Fu Z.. Effects of environmental pollutants on gut microbiota. Environ Pollut 2017;222:1–9. [DOI] [PubMed] [Google Scholar]
- 74. Turner PC, Flannery B, Isitt C, Ali M, Pestka J.. The role of biomarkers in evaluating human health concerns from fungal contaminants in food. Nutr Res Rev 2012;25:162–79. [DOI] [PubMed] [Google Scholar]
- 75. US FDA Guidance for industry: action levels for poisonous or deleterious substances in human food and animal feed. Silver Spring (MD): Food and Drug Administration; 2000. [Google Scholar]
- 76. Perera T, Young MR, Zhang Z, Murphy G, Colburn NH, Lanza E, Hartman TJ, Cross AJ, Bobe G.. Identification and monitoring of metabolite markers of dry bean consumption in parallel human and mouse studies. Mol Nutr Food Res 2015;59:795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Phull AR, Eo SH, Kim SJ.. Oleanolic acid (OA) regulates inflammation and cellular dedifferentiation of chondrocytes via MAPK signaling pathways. Cell Mol Biol (Noisy-le-Grand) 2017;63:12–7. [DOI] [PubMed] [Google Scholar]
- 78. Dueñas M, Fernández D, Hernández T, Estrella I, Muñoz R.. Bioactive phenolic compounds of cowpeas (Vigna sinensis L): modifications by fermentation with natural microflora and with Lactobacillus plantarum ATCC 14917. J Sci Food Agric 2005;85:297–304. [Google Scholar]
- 79. Chirumbolo S.. The role of quercetin, flavonols and flavones in modulating inflammatory cell function. Inflamm Allergy Drug Targets 2010;9:263–85. [DOI] [PubMed] [Google Scholar]
- 80. Tamura M, Kurusu Y, Hori S.. Effect of dietary l-arabinose on the intestinal microbiota and metabolism of dietary daidzein in adult mice. Biosci Microbiota Food Health 2012;31:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kelly GS.. Larch arabinogalactan: clinical relevance of a novel immune-enhancing polysaccharide. Altern Med Rev 1999;4:96–103. [PubMed] [Google Scholar]
- 82. Rivière A, Selak M, Lantin D, Leroy F, De Vuyst L.. Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol 2016;7:979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jan AT, Azam M, Siddiqui K, Ali A, Choi I, Haq QMR.. Heavy metals and human health: mechanistic insight into toxicity and counter defense system of antioxidants. Int J Mol Sci 2015;16:29592–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.