Abstract
Background: Repeated phytic acid consumption leads to iron absorption adaptation but, to the best of our knowledge, the impact of repeated tannin consumption has not yet been established. Salivary proline-rich proteins (PRPs) may improve iron absorption by precipitating tannins.
Objectives: This study aimed to determine the effect of long-term, dose-response condensed tannin supplementation on iron bioavailability and status and to assess the effect of salivary proteins on iron bioavailability during prolonged condensed tannin consumption. A secondary objective was to assess astringency as a potential marker for adaptation to tannins and iron bioavailability.
Methods: Eleven nonanemic women were enrolled in a double-blind 3-dose crossover trial. Three (1.5, 0.25, or 0.03 g) condensed tannin supplements were consumed 3 times/d for 4 wk in random order, with 2-wk washouts in between. Meal challenges were employed before and after supplementation to assess iron bioavailability, iron status, salivary PRP changes, and astringency.
Results: Tannin supplementation in any dose did not change iron bioavailability at any dose (P > 0.82) from weeks 0 to 4. Hemoglobin (P = 0.126) and serum ferritin (P = 0.83) were unchanged by tannin dose from weeks 0 to 4. There were significant correlations among tannin supplementation and iron bioavailability, basic proline-rich proteins (bPRPs) (r = 0.366, P = 0.003), and cystatin production (r = 0.27, P = 0.03). Astringency ratings did not change significantly within or between tannin doses (P > 0.126), but there were negative relations among bPRP (r < −0.32, P < 0.21), cystatin production (r < −0.2, P < 0.28), and astringency ratings.
Conclusions: Condensed tannin consumption did not affect iron bioavailability or status regardless of the supplementation period in premenopausal nonanemic women. Correlation analyses suggest that bPRPs and cystatins are associated with improved iron bioavailability and that lower ratings of astringency may predict improved iron absorption with repeated tannin consumption.
Keywords: tannins, iron bioavailability, salivary proline-rich proteins, adaptation, antinutritional factors, proanthocyanidins, iron deficiency anemia
Introduction
An estimated 1 billion people suffer from iron deficiency anemia worldwide (1). Iron deficiency most commonly occurs in women, children, vegetarians, and people with insufficient iron intake (1). Despite multiple initiatives aimed at reducing iron deficiency anemia in the past 20 y, an estimated 29% of nonpregnant women were anemic in 2011 (a 4% reduction since 1995) (2).
Research has shown that tannins negatively affect iron bioavailability (3–8) by forming insoluble antinutrient-mineral complexes (9), which has deterred tannin-rich foods (e.g., sorghum) from being used within food aid for regions that are largely undernourished (10). Previous studies suggest that long-term tannin consumption may not inhibit iron bioavailability as much as single-meal studies predict (11, 12). For example, long-term antinutritional factor consumption in animals (13–15) and humans (16, 17) resulted in improved nonheme iron bioavailability compared with single-meal studies. In these studies, the negative effects (reduced iron status or bioavailability) of antinutritional factor intake over time were not sustained, and these findings are proposed to be attributable to adaptation to antinutritional factors over time. In studies that showed reductions in iron bioavailability with tannin consumption, individual iron absorption was highly variable (18, 19) and many individuals consuming diets with large concentrations of tannins maintained normal iron stores (20).
Many studies that demonstrate reduced iron bioavailability with tannin consumption have used hydrolyzable tannic acid (which is not commonly consumed) or tea tannins (which may be metabolized differently than condensed tannins that are commonly found in food) (11). To the best of our knowledge, no studies have yet determined the long-term effects of condensed tannin consumption apart from other antinutritional factors such as fiber or phytates on iron bioavailability or status. In addition, whether long-term condensed tannin consumption results in iron absorption adaptation, or what mechanisms underlie this adaptation if it does occur, has not been determined.
Mechanistically, iron absorption adaptation to tannins may start in the mouth (21). Saliva contains 6 main classes of salivary proteins [histatins, cystatins, statherins, acidic proline-rich proteins (aPRPs), basic proline-rich proteins (bPRPs), and glycosylated proline-rich proteins (gPRPs)] that may exert independent effects on tannins (22). The binding of proline-rich proteins (PRPs) to condensed tannins may also prevent condensed tannins from chelating iron, thereby improving iron bioavailability (21, 22). Tannin-PRP complexes are insoluble within the gastrointestinal tract (23, 24), preventing tannin-iron chelation throughout digestion. A variety of PRP subtypes make up different salivary profiles, which may be largely genetically determined (25, 26). Genetic determination of salivary profiles favoring effective tannin precipitation may explain why some individuals have a greater capacity to consume tannins without negative impacts on iron status than others. Upregulation of PRP secretion during tannin consumption was shown to improve protein (27) and iron bioavailability in animal studies (12, 14), and animals that do not upregulate PRP synthesis in response to tannin consumption have poor growth outcomes (28).
PRP-tannin binding was previously identified in sensory studies because it causes an oral astringency sensation (22). Theoretically, identification of PRP “adapters” may then be possible through simple, inexpensive astringency testing (22). Furthermore, changes in astringency sensation may indicate an upregulation of PRP production over time with repeated tannin consumption (29).
The primary objectives of the current tannin dose-response crossover trial were to determine the effect of 4-wk multimeal dose-response condensed tannin supplementation on iron bioavailability and status and to understand the effect of salivary proteins on iron bioavailability during prolonged condensed tannin consumption. A secondary objective was to assess astringency as a potential marker of adaptation to tannins and iron bioavailability.
In this study, we hypothesized that 1) condensed tannin supplementation would not change iron bioavailability (determined by iron absorption) or status (determined by hemoglobin and ferritin) regardless of dose over 4 wk, 2) salivary PRP production would be induced by tannin consumption over time and by higher tannin doses than lower doses, and 3) PRP production would be positively associated with improved iron bioavailability after tannin consumption. Secondary hypotheses were that 1) astringency perception would be changed with tannin consumption over time, 2) salivary PRP production would predict astringency with tannin consumption, and 3) astringency could be used as a surrogate marker for iron bioavailability with tannin consumption.
Methods
Inclusion and exclusion criteria
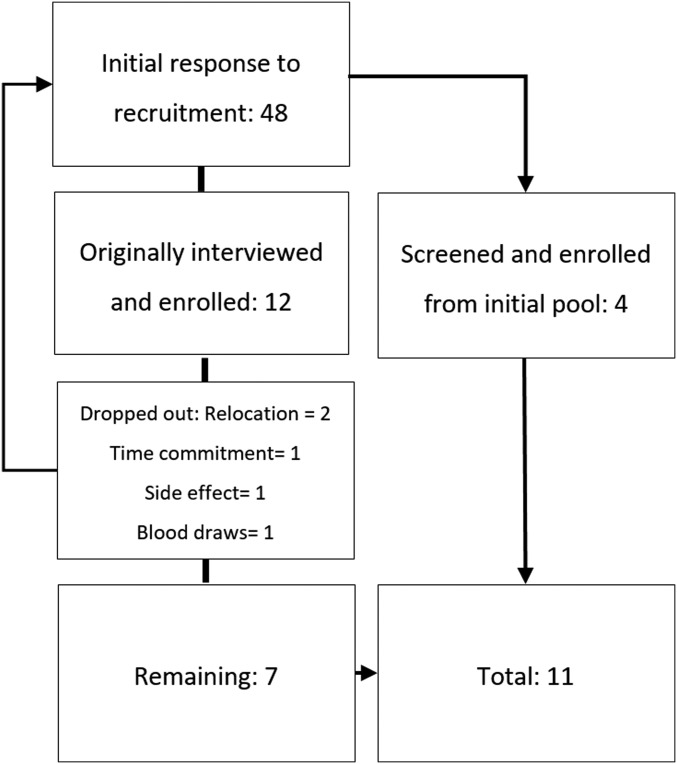
The Kansas State University Institutional Review Board approved the study protocol (no. 8121). Because of the study length, enrollment was on a rolling basis and is outlined in Figure 1. An announcement requesting participants was sent to faculty and students through a university e-mail digest and was disseminated through department social media channels. In total, 48 women responded, and potential participants were screened in person or via phone (Figure 1). Before screening, participants were required to read and sign an informed consent document, and all study procedures, risks, and benefits were reviewed verbally. During screening, participants were asked to complete a medical history questionnaire. Premenopausal women (aged 18–35 y) who were nonobese [BMI (in kg/m2) ≤30.0], had no history of oral or gastrointestinal disease, consumed moderate (≤1 drink/d) or no alcohol, and did not use tobacco were eligible for participation. To minimize losses as a result of potential exacerbated anemia during the study, we included nonanemic women who had normal (n = 2; ferritin range: 88–100 ng/mL) or marginal (n = 9; ferritin range: 7–30 ng/mL) iron stores because antinutritional factor supplementation significantly decreases iron absorption in iron-replete individuals. Iron absorption has been significantly antinutritional supplementation in iron-replete individuals (6, 30). Additional exclusion criteria included pregnancy, breastfeeding, blood disorders affecting iron status or absorption, current supplementation or medication that would impair iron status, or food allergies to supplements. No participants consumed iron supplements before or during the study period (see the Supplemental Methods for the screening questionnaire and exclusion criteria). Participants were compensated for completing study activities.
FIGURE 1.
Enrollment allocation. Forty-eight potential participants responded to the study call and were screened; the study was conducted on a rolling basis. Twelve participants were initially enrolled. During the study duration, 5 participants dropped out after the first study supplementation period was completed (2 because of relocation, 1 because of the study time commitment, 1 because of intolerance to blood draws, and 1 because of supplement intolerance). Another 4 participants were recruited on a rolling basis from the initially screened pool of 48.
Study design
Blinding and randomization.
Participants were assigned identification numbers, and a researcher (NMF) not involved in data collection randomly assigned each participant number to a tannin supplement order (SAS Studio). The study participants, principal investigator, and project coordinator were blinded to the dose order.
Supplementation periods.
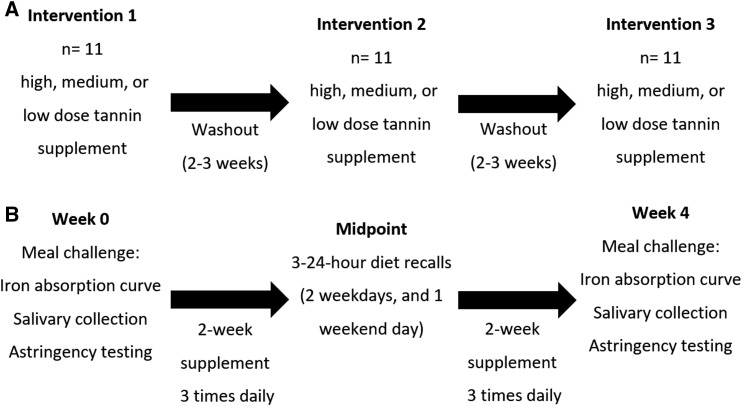
Supplementation periods consisted of week 0 and week 4 meal challenges, with 4 wk of tannin supplementation in between (Figure 2). Four-week supplementation periods were chosen as completed previously (31) to assess for iron status changes with inhibited iron absorption at each meal and to allow for time to adapt to tannins. Each participant consumed a powdered, condensed tannin (Grape seed extract, 95% condensed proanthocyanidins; NuSci, Walnut, California) supplement mixed in an opaque bottle with water and a noncaloric flavor enhancer and sweetener (MiO Original) to improve its palatability, 3 times/d for 4 wk. Supplements were prepared weekly by an outside researcher; participants returned weekly to pick up supplements and were questioned about supplement adherence. In addition, supplement bottles were checked for total supplement consumption, and adherence issues were noted. High (1.5 g), medium (0.25 g), or low (0.03 g) condensed tannin doses were provided for consumption with 3 daily meals. These doses represented the amount of condensed tannins from 100 g high tannin red sorghum (32–34), 1 cup of tea (6–8), or the lowest inhibitory vegetable meals cited previously (3, 35). Each week 4 meal challenge was followed by a 2-wk washout period to allow normalization of iron absorption and potentially PRPs to the participant's usual diet (36). Two participants had a single washout period of 3 wk instead of 2 wk because of participant availability for meal challenges.
FIGURE 2.
Supplementation periods (A) and supplementation period activities (B). The study consisted of 3 supplementation periods for each participant, and a high (1.5 g), medium (0.25 g), or low (0.03 g) condensed tannin supplement was provided for 4 wk. There were 2- to 3-wk washout periods between supplementation periods, which aimed to stabilize salivary protein and iron biomarkers. Supplementation periods consisted of week 0 and week 4 meal challenges, salivary collection, and astringency testing. At the midpoint of each supplementation period, three 24-h dietary recalls (2 weekday and 1 weekend day) were collected from each participant.
Tannin meal challenges.
At weeks 0 and 4 of each supplementation period, participants completed meal challenges at the Kansas State University Physical Activity and Nutrition Clinical Research Consortium. Participants were asked to arrive fasted (≥8 h) at 0700, having abstained from teeth cleaning (2 h) and exercise (24 h) to minimize diurnal variations or other confounding factors in salivary production (37) and iron uptake (38) (Figure 2B). Premeal saliva was collected with the passive drool technique (2 mL total) in cryovials, and samples were immediately stored at −80°C in a freezer. A 20-gauge indwelling peripheral intravenous catheter was placed in either the median cubital, cephalic, or basilic vein for multiple blood samples, and the catheter was flushed and saline locked (no intravenous fluids were provided during the meal challenges except for flushes) with 10 mL 0.9% isotonic saline between blood collections. From the fasting blood draw, 2 separate samples were collected in 5 mL serum separator and 3-mL EDTA vacutainer tubes to measure serum iron (by spectrophotometry), C-reactive protein (CRP) (by nephelometry; sensitivity: 0.2 mg/dL), ferritin (by immunoassay; sensitivity: 0.1 ng/mL), and whole-blood hemoglobin concentrations (by electronic cell cytometry). After fasted blood collection, participants consumed a challenge meal including a 95-g bagel with 12 g sugar-free strawberry jam, with half sprinkled with 15 mg anhydrous ferrous sulfate (39) and the other half with 75 mg ascorbic acid (16, 40), and a 90-g banana simultaneously with their assigned supplement dose (1.5, 0.25, or 0.03 g in 8 fl oz). Ferrous sulfate and ascorbic acid were weighed with a scale to the nanogram, and weights were recorded for percentage of maximum iron absorption calculations. To determine salivary protein stimulation after tannin consumption, salivary samples were collected 15 min after participants consumed their final bite of the meal (22). Subsequent blood samples were collected in tubes and analyzed for serum iron at 180 and 240 min. After collection, serum samples were centrifuged at 2500 × g for 15 min after clotting for 20 min and were kept at room temperature for analysis. All blood samples were analyzed by a certified laboratory (Quest Diagnostics) within 24 h.
Regression analysis of the percentage of maximum iron absorption determined with a blood draw system with 3 time points.
To minimize blood draws, we determined the validity of a blood draw system with 3 time points to determine iron bioavailability by serum iron. In the first supplementation period, 4 participants had blood drawn every 30 min for 4 h to establish the correlation with a previously proposed draw system with 3 time points (0, 180, and 210 min) (40). Regression of polynomial lines from data points was calculated on a computer data system (Microsoft Excel, 2013), and R2 values were calculated for goodness of fit. From these data, we verified that the time points proposed were representative of the full models previously used (16, 40). Correlations >0.99 were seen; thus, blood samples were also drawn for serum iron at these 3 time points to determine the percentage of maximum iron absorption and the incremental AUC (iAUC) of serum iron (40).
Calculation of iAUC and the maximum percentage of iron absorption.
Serum iron data were used to calculate maximum iron recovery percentage and iAUC for iron bioavailability analysis. The iron recovery percentage was calculated as shown in Equation 1 (40):
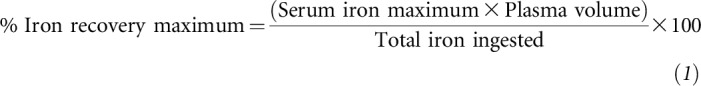 |
where iron maximum is expressed in μmol/L, plasma volume is expressed in L, and the amount of iron is expressed in μmol.
Plasma volume was calculated as shown in Equation 2:
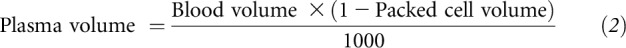 |
where plasma volume is expressed in L, blood volume is expressed in mL and equals 69.6 mL/kg body weight, and packed cell volume is given as a decimal.
iAUC values were calculated by trapezoidal integration as shown in Equation 3:
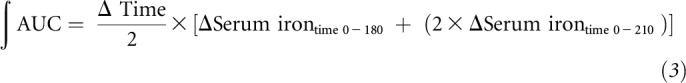 |
Astringency testing.
After the peripheral indwelling intravenous catheter was removed after each meal challenge, participants were asked to complete an astringency test (41, 42). Each participant was given 4 different concentrations of alum powder in 10 mL distilled water (0.20%, 0.15%, 0.07%, and 0.03%) in random order to sip. They were first given a verbal description of the sensation of astringency and were asked to rate each solution based on their perception of astringency on a 5-point Likert scale (with 1 indicating not astringent and 5 indicating extremely astringent). Participants waited for 30 s before testing the next sample.
Dietary analysis in supplementation periods.
Within each supplementation period, 24-h dietary recalls were collected on 3 different days (2 weekdays and 1 weekend day) (43). At the beginning of week 2 of each 4-wk supplementation period, participants were e-mailed a unique username and password to complete 24-h dietary recalls for 2 weekdays and 1 weekend day on the Automated Self-Administered 24-h recall. After all recalls were collected in each supplementation period, dietary data were extracted and the following means calculated: total caloric intake (in kilocalories), protein (in grams), fat (in grams), carbohydrate (in grams), iron (in milligrams), ascorbic acid (in milligrams), meat protein (in ounces), sugar (in grams), fiber (in grams), zinc (in milligrams), and copper (in milligrams). Food intake logs were downloaded from the Automated Self-Administered 24-h recall for manual calculation of proanthocyanidins and polyphenols. During this process, a research assistant used an electronic spreadsheet (Microsoft Excel) to review all dietary data for each participant. Food items were referenced from USDA tables pulled into an electronic spreadsheet, and total proanthocyanidin (condensed tannin) (44) and polyphenol (45) amounts were calculated and summated for each recall. From these summations, means were calculated. All assessments and calculations were reviewed by the project coordinator before analyses were completed.
Salivary PRP measurement using acidified saliva sample preparation.
Frozen salivary samples were thawed overnight in a refrigerator. Before sample analysis, consistency in chromatogram output with duplicate samples was verified, and samples were analyzed in a single run. For PRP extraction, 900 µL saliva was mixed with 10 µL 10% trifluoroacetic acid (TFA) in water and centrifuged for 5 min at 8000 × g, and the supernatant was filtered through a 0.2-µm polyvinylidene difluoride syringe filter as described previously (46). Before syringe filters were employed we verified that there was no PRP peak loss with their use. The supernatant was then analyzed with HPLC.
HPLC parameters and equipment.
All reagents were of analytic grade. Acetonitrile, TFA, and HPLC-grade water were purchased from Fisher Scientific. We injected 90 µm salivary supernatant into a Fisher BioBasic C8 analytic column (2.1 × 150 mm, 5 µm) at a flow rate of 0.3 mL/min for 49 min at 40°C with an autosampler (Shimadzu SIL) on a HPLC system containing an LC20AB pump (Shimadzu) and a Shimadzu SPD-M20A PDA system. Detection of PRPs was carried out at 214 nm (22, 46). The mobile phase consisted of 0.2% TFA in HPLC-grade water (A) and 0.2% TFA in 80:20 acetonitrile and HPLC-grade water (B) (22, 46, 47). A linear gradient was applied from 0 to 39 min from 0% to 54% (B) and then from 39 to 49 min at 54%–100% B to elute late proteins (22, 46, 47). After each run, the column was washed and stabilized by increasing the linear gradient back to 100% A and held for 10 min.
Statistical analyses
Data were analyzed using SAS statistical software (version 3.6; SAS Studio), and statistical significance was set at P < 0.05. All data are presented as means ± SDs. Before analysis, all data were analyzed for normality and homogeneity of data in Q-Q plots and with Levene's tests. Variables that were non-normal (proanthocyanidin monomers, dimers, total proanthocyanidins, ascorbic acid, sugar, and iron intake) were log transformed and were determined to be normal before further analysis. Log-transformed variables were included in stepwise variable selection in adjusted model building (described below). All log-transformed data were back transformed for results presentation.
Sample size.
A paired t-test sample size calculation (version 3.6; SAS Studio) determined that 4 participants would be needed to detect a change in iAUC of 41%, which was observed in a similarly designed antinutritional factor adaptation study (16) as statistically significant with 80% power and an α level of 0.05.
Demographic data, washout, and randomization order analysis.
Week 0 demographic and nutritional intake data were analyzed with ANOVA by supplementation period. Randomization order and previous dose effect were analyzed with chi-square testing to assess for bias in supplementation period order or a previous effect of supplementation period. ANOVA was used to analyze changes between the previous and next supplementation period during washout for hemoglobin, ferritin, iAUC for serum iron, and percentage of maximum iron absorption.
Regression analysis of hematologic outcomes.
Linear regression of raw outcomes data was used to determine whether 4 wk of multiple-daily tannin supplementation would change iron absorption or status within or between supplementation periods. In regression analysis, differences between supplementation periods were analyzed for ferritin, hemoglobin, and percentage of maximum iron absorption at weeks 0 and 4 (to analyze for within-dose responses). Multiple regression was used to adjust models for repeated (participant) and random (ferritin, CRP, dietary intake, weight, and age) covariates after stepwise selection for significant (P < 0.05) variables. To maximize analysis of individual iron bioavailability and status within different supplementation periods, individual movements (increase, decrease, or maintain) in dose responses (hemoglobin, ferritin, percentage of maximum iron absorption, and serum iron iAUC) were analyzed with chi-square testing and Fisher's exact tests.
PRP and astringency outcomes analysis.
PRP changes with tannin supplementation and correlations with iron bioavailability.
To determine whether salivary PRP production would be inducible by tannin consumption both over time and in a dose-dependent manner, salivary proteins were divided into type by retention times (22, 46, 47), peak milli-absorbance units values were recorded for each, and protein subtypes were aggregated to quantify total salivary proteins and PRPs. Salivary protein subtypes were further analyzed by proportion to total milli-absorbance units as shown in Equations 4 and 5:
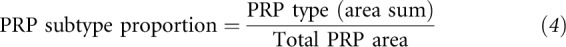 |
 |
Differences in salivary protein production from weeks 0 to 4 within doses were analyzed with multiple-factor ANOVA. To determine whether PRP production would impact iron bioavailability with tannin consumption, Pearson's product-moment correlations were used to determine correlations between the percentage of maximum iron absorption, iAUC for serum iron, randomization order, and PRP types.
Astringency perception, connections to salivary protein production, and iron bioavailability.
We determined whether astringency perception was changed within or between tannin doses using chi-square testing and Fisher's exact tests by allocated and previous dose. Connections between salivary protein production, iron bioavailability, and astringency were analyzed with Pearson's product-moment correlations.
Results
Week 0 demographics
The mean participant age was 26 ± 1.2 y (range: 20–35 y). All participants were occasional (2–3 drinks/mo) or moderate (2–3 drinks/wk) alcohol consumers. Except for 1 participant who consumed a vegan diet and took vitamin B-12 supplements, no participants took vitamin or mineral supplements during the study period. The mean BMI of participants was 24 ± 2.4 (range: 18.2–28.9). Participant weights (in kilograms) did not significantly change between tannin doses from weeks 0 to 4 of each supplementation period.
Supplementation order and outcome measures
With our randomization procedure, 6 of the 11 participants were randomly assigned to 1.5-g tannin doses during the first supplementation period and to 0.03-g doses during the second supplementation period. iAUC values for serum iron (P = 0.118), hemoglobin (P = 0.87), and ferritin (P = 0.15) were not different by order of tannin dose in any supplementation period. Supplementation order significantly positively affected the percentage of maximum iron absorption after the 1.5-g tannin dose when taken in the third supplementation order compared with the first (P = 0.046), meaning that lower doses taken before the 1.5-g dose led to significantly improved iron bioavailability during that intervention period. There were no significant differences in week 0 to week 4 dose responses for hemoglobin, ferritin, serum iron iAUC, or percentage of maximum iron absorption when accounting for previous dose by chi-square testing (results are shown in the Supplemental Results). There were no significant changes in hemoglobin (P = 0.993), ferritin (P = 0.982), iAUC for serum iron (P = 0.984), or percentage of maximum iron absorption (P = 0.998) at each week 0 time point, and previous tannin dose did not affect outcomes changes during washout for hemoglobin (P = 0.68), ferritin (P = 0.511), percentage of maximum iron absorption (P = 0.735), or iAUC for serum iron (P = 0.137). No salivary protein measurements were significantly correlated with tannin dose order (P > 0.62).
Study dietary intake
There was wide variability in nutrient consumption during supplementation periods, but there were no significant differences in total calorie, macronutrient, meat, fiber, or micronutrient consumption between tannin doses (Table 1). Despite wide variability in nutrient consumption, individual macronutrient and micronutrient intake was not different between tannin doses. Iron intake was 7–18% less than the RDA of 18 mg in all supplementation periods, and ascorbic acid exceeded the RDA by 15%–80%. Although it was not significant, dietary proanthocyanidin intake (apart from supplements) trended toward lower amounts in the 0.03-g (69.1 ± 78.9 mg) and 0.25-g (82.3 ± 85.1 mg) doses than in the 1.5-g dose (123.2 ± 136.6 mg; P > 0.09). Mean 0.03-, 0.25-, and 1.5-g tannin supplements constituted 2-, 8-, and 35-fold of higher dietary proanthocyanidin intake for their respective supplementation period.
TABLE 1.
Dietary intake of calories, macronutrients, micronutrients, and proanthocyanidins by supplementation period (n = 11)1
| Supplementation period, g | |||
|---|---|---|---|
| Parameter | 0.03 | 0.25 | 1.5 |
| Total caloric intake, kcal/d | 2186.2 ± 570.9 | 2230.5 ± 640.6 | 1957.8 ± 348 |
| Protein, g/d | 80.8 ± 27.2 | 79.7 ± 21.6 | 71.6 ± 16.6 |
| Fat, g/d | 90.3 ± 30 | 93.5 ± 27.9 | 71.1 ± 19.5 |
| Carbohydrates, g/d | 259.3 ± 96.2 | 268.4 ± 109.9 | 252.1 ± 126.2 |
| Meat, oz/d | 3.61 ± 2.5 | 3.81 ± 2.6 | 3.6 ± 1.9 |
| Sugar, g/d | 122.8 ± 57.9 | 127.7 ± 69.3 | 120.0 ± 99.8 |
| Fiber, g/d | 21.2 ± 12 | 19.2 ± 10 | 21.6 ± 14.3 |
| Iron, mg/d | 15.1 ± 6.6 | 15.7 ± 7.2 | 14.7 ± 6.2 |
| Ascorbic acid, mg/d | 109.7 ± 87.9 | 80.9 ± 66.9 | 110.4 ± 142.8 |
| Zinc, mg/d | 12.6 ± 5 | 12.8 ± 5.6 | 10.2 ± 2.5 |
| Copper, mg/d | 1.5 ± 0.69 | 1.5 ± 0.91 | 1.4 ± 0.61 |
| Monomers, mg/d | 8.0 ± 7.5 | 18.2 ± 23.4 | 16.1 ± 21.8 |
| Dimers, mg/d | 8.6 ± 6.6 | 13.5 ± 15.7 | 14.8 ± 17.3 |
| Trimers, mg/d | 5.3 ± 4.5 | 6.7 ± 8.0 | 8.9 ± 12.5 |
| 4–6 mers, mg/d | 15 ± 16.3 | 16.6 ± 19.4 | 27.5 ± 36.3 |
| 7–10 mers, mg/d | 9.6 ± 12.7 | 9.0 ± 9.8 | 15.7 ± 18.7 |
| Polymers, mg/d | 22.7 ± 39.5 | 18.4 ± 25.7 | 40.3 ± 51.3 |
| Total proanthocyanidin intake, mg/d | 69.1 ± 78.9 | 82.3 ± 85.1 | 123.2 ± 136.6 |
| Total polyphenol intake, mg/d | 1106.6 ± 531.1 | 1139.6 ± 647.3 | 1108.9 ± 590 |
Values are means ± SDs. No significant differences were noted (P > 0.05). oz, ounce.
Supplementation period iron absorption and hematologic indices of iron status
Unadjusted regression outcomes.
Individual-level data are included in the Supplemental Table 1. There were no changes in unadjusted iron bioavailability (by iAUC and percentage of maximum iron absorption) within or between tannin supplementation periods (Table 2). In addition, there were no differences in week 0 (P = 0.82) or week 4 (P = 0.92) unadjusted serum iron iAUC or in week 0 (P = 0.82) or week 4 (P = 0.62; Table 2) percentages of maximum iron absorption between tannin doses. Hemoglobin (P = 0.838 and 0.68) and ferritin values (P = 0.855 and 0.575) were not different at week 0 or week 4 for any tannin dose, respectively (Table 2). There were no significant differences in hemoglobin (P = 0.90), ferritin (P = 0.81), percentage of maximum iron absorption (P = 0.39), or serum iron iAUC (P = 1.0) for improvement, deterioration, or maintenance by any tannin dose through chi-square testing (Table 3).
TABLE 2.
Unadjusted iron bioavailability, status, and inflammatory markers at weeks 0 and 4 of each supplementation period1
| Supplementation period, g | ||||||
|---|---|---|---|---|---|---|
| 0.03 | 0.25 | 1.5 | ||||
| Parameter | Week 0 | Week 4 | Week 0 | Week 4 | Week 0 | Week 4 |
| Maximum iron absorption, % | 12.7 (7.5, 17.9) | 10.7 (5.4, 15.9) | 12.1 (6.9, 17.3) | 12.4 (7.2, 17.6) | 11.2 (6.0, 16.5) | 10.3 (5.0, 15.5) |
| iAUC for serum iron, µg/dL ⋅ h | 2155 (612, 3696) | 2269 (727, 3810) | 2461 (919, 4003) | 2769 (1228, 4311) | 2237 (696, 3779) | 2277 (735, 3819) |
| Hemoglobin, g/dL | 13.2 (13.0, 13.4) | 13.3 (13.1, 13.5) | 13.3 (13.1, 13.5) | 13.4 (13.2, 13.6) | 13.4 (13.1, 13.5) | 13.3 (13.2, 13.6) |
| Ferritin, ng/mL | 35.4 (28.4, 42.4) | 42.3 (35.3, 49.3) | 35.8 (28.8, 42.8) | 37.3 (30.3, 44.3) | 40.0 (33.0, 47.0) | 44.5 (37.5, 51.5) |
| CRP, mg/dL | 0.2 (0.0, 0.5) | 0.3 (0.0, 0.5) | 0.3 (0.1, 0.5) | 0.2 (0.0, 0.4) | 0.2 (0, 0.4) | 0.3 (0.1, 0.6) |
Values are means (95% CIs). No significant differences were noted (P > 0.05). CRP, C-reactive protein; iAUC, incremental AUC.
TABLE 3.
Comparison of improvement, maintenance, or deterioration of iron bioavailability and status within each supplementation period1
| Parameter within each supplementation period, g | Iron bioavailability and status | P 2 | ||
|---|---|---|---|---|
| Improvement | Maintenance | Deterioration | ||
| Maximum iron absorption, % | ||||
| 0.03 | 5 | 0 | 6 | 0.394 |
| 0.25 | 8 | 0 | 3 | |
| 1.5 | 5 | 0 | 6 | |
| Total | 18 | 0 | 15 | |
| iAUC serum iron, µg/dL ⋅ h | ||||
| 0.03 | 6 | 0 | 5 | 1.0 |
| 0.25 | 6 | 0 | 5 | |
| 1.5 | 6 | 0 | 5 | |
| Total | 18 | 0 | 15 | |
| Hemoglobin, g/dL | ||||
| 0.03 | 6 | 2 | 3 | 0.896 |
| 0.25 | 5 | 2 | 4 | |
| 1.5 | 4 | 4 | 3 | |
| Total | 15 | 8 | 10 | |
| Ferritin, ng/dL | ||||
| 0.03 | 7 | 1 | 3 | 0.816 |
| 0.25 | 5 | 1 | 5 | |
| 1.5 | 6 | 0 | 5 | |
| Total | 18 | 2 | 13 | |
No significant differences were noted (P > 0.05). iAUC, incremental AUC.
Fisher's exact test.
Stepwise linear regression analysis and adjusted regression models.
To test the impact of dietary and individual physiologic differences (iron status, anthropometric, salivary protein) on iron bioavailability and status, we employed stepwise regression analysis to establish significant covariates to build an adjusted model for hematologic outcomes. Covariates that were significantly positively associated with serum iron iAUC and percentage of maximum iron absorption included bPRP and cystatin production (Table 4). Significant covariates that were negatively associated with serum iron iAUC and percentage of maximum iron absorption included aPRP and total salivary protein production, a higher rating of 0.2 mg/dL astringency testing, and total meat consumption. Significant covariates positively associated with ferritin concentrations included bPRP production and zinc consumption.
TABLE 4.
Estimation of iron bioavailability and status attributable to supplementation period, time, and significant covariates1
| Parameter | B | SE B | β | t | P |
|---|---|---|---|---|---|
| Maximum iron absorption, % | |||||
| Model | 6.92 | <0.0001 | |||
| Constant | 48.9 | 22.7 | 0 | 2.16 | 0.004 |
| Supplementation period | −0.36 | 1.5 | −0.023 | 0.81 | 0.82 |
| Week 0 | 1.3 | 1.9 | 0.07 | 0.70 | 0.49 |
| Hemoglobin | −4.2 | 1.7 | −0.20 | −1.54 | 0.034 |
| Ferritin | −0.06 | 0.03 | −0.23 | −2.00 | 0.023 |
| CRP | −26.1 | 3.7 | −0.74 | −5.91 | <0.0001 |
| bPRP | 10.22 | 3.02 | 0.25 | 2.00 | 0.023 |
| aPRP | −16.9 | 6.5 | −0.21 | 1.86 | 0.012 |
| Cystatin | 0.0016 | 0.0004 | 0.06 | 2.51 | 0.0008 |
| iAUC serum iron, µg/dL ⋅ h | |||||
| Model | 9.81 | <0.0001 | |||
| Constant | 10281 | 1869.2 | 0 | 5.5 | |
| Supplementation period | 65.94 | 299.6 | 0.022 | 0.22 | 0.83 |
| Week 0 | −210.6 | 379.4 | −0.05 | −0.56 | 0.58 |
| CRP | −2091.3 | 514.5 | −0.40 | −4.06 | 0.0002 |
| bPRP | 0.0042 | 0.001 | 0.31 | 3.12 | 0.003 |
| Total salivary protein | −6606 | 1731 | −0.38 | −3.82 | 0.0004 |
| Meat | −185.7 | 78.3 | −0.23 | −2.37 | 0.022 |
| 0.2 astringency | −237.3 | 238.4 | −0.31 | −2.83 | 0.0066 |
| Hemoglobin, g/dL | |||||
| Model | 5.54 | <0.0001 | |||
| Constant | 12.4 | 0.27 | 0 | 41.55 | <0.0001 |
| Supplementation period | 0.17 | 0.11 | 0.14 | 1.55 | 0.126 |
| Week 0 | −0.05 | 0.13 | −0.03 | −5.12 | <0.0001 |
| CRP | −0.97 | 0.19 | −0.47 | 4.14 | 0.0005 |
| Ferritin | 0.007 | 0.001 | 0.36 | 3.32 | 0.0001 |
| Fat | 0.008 | 0.002 | 0.32 | −3.82 | 0.002 |
| Ferritin, ng/dL | |||||
| Model | 6.47 | <0.0001 | |||
| Constant | −213.7 | 69.5 | 0 | −3.33 | 0.0016 |
| Supplementation period | −5.44 | 5.64 | −0.11 | −0.97 | 0.83 |
| Week 0 | −0.54 | 7.06 | −0.01 | −0.08 | 0.58 |
| Hemoglobin | 22.95 | 4.49 | 0.50 | 4.60 | 0.0002 |
| bPRP | 38.76 | 14.4 | 0.29 | 2.69 | 0.003 |
| Maximum iron, % | −0.70 | 0.40 | −0.19 | −1.72 | 0.0004 |
| Zinc | −2.24 | 0.85 | −0.32 | −2.63 | 0.022 |
Significance was determined at P < 0.05. aPRP, acidic proline-rich protein; bPRP, basic proline-rich protein; CRP, C-reactive protein; iAUC, incremental AUC.
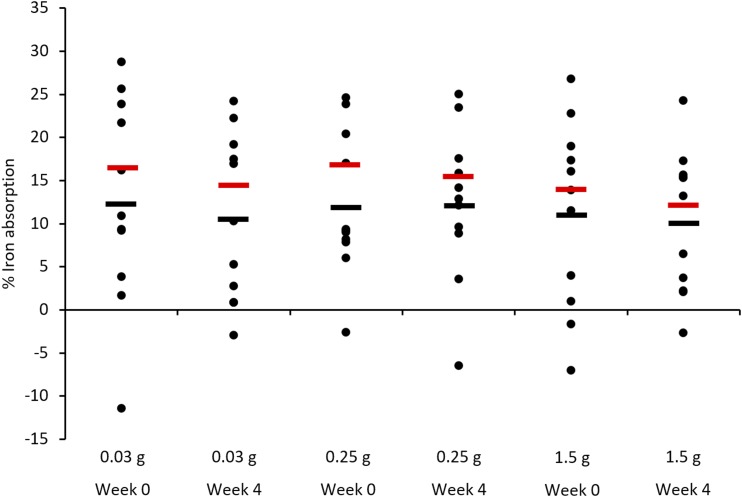
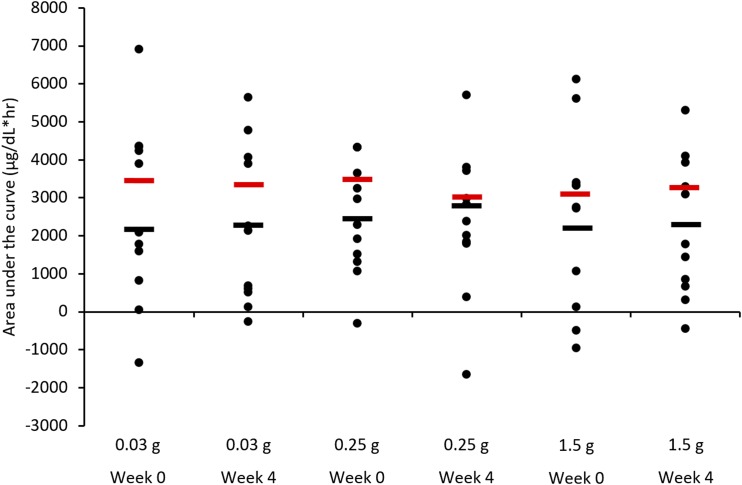
Significant covariates for each outcome measure were added to the linear regression for adjusted outcomes analysis. After the full adjustment for significant covariates, neither serum iron iAUC nor percentage of maximum iron absorption was statistically different between or within each tannin dose (Figures 3 and 4; Table 4). There were no significant differences in adjusted hemoglobin or ferritin values within or between tannin supplementation periods (Table 4).
FIGURE 3.
Mean adjusted and unadjusted individual-level iron absorption at weeks 0 and 4 of each supplementation period. Values adjusted for hemoglobin, ferritin, C-reactive protein, basic proline-rich proteins, and acidic proline-rich proteins are shown in red, whereas unadjusted values are shown in black. There were no significant differences (P > 0.05) in iron absorption at any dose of condensed tannins before or after the supplementation periods.
FIGURE 4.
Individual-level incremental AUC for serum iron at weeks 0 and 4 of each supplementation period. Mean regression-adjusted values for C-reactive protein, basic proline-rich protein, total salivary protein, meat consumption, and rating of highest level of astringency are shown in red, whereas unadjusted values are shown in black. There were no significant differences (P > 0.05) in iron absorption within the tannin supplementation periods.
Correlations between salivary protein production and iron absorption with tannin supplementation.
There were no significant correlations between total salivary protein production and iron absorption (by percentage of maximum iron absorption and iAUC for serum iron) during the study. In all tannin doses, and when combining all data from 4-wk supplementation periods, bPRP production was significantly and positively correlated with percentage of maximum iron absorption at weeks 0 and 4 (Table 5). There were more positive correlations between maximum iron absorption and bPRP values with week 4 (0.03 g) and 0.25-g dose than the 1.5-g dose (Table 5), suggesting that bPRP production was potentially important to enhance iron bioavailability for lower, but not higher, tannin doses. Week 0 and week 4 aPRP production was significantly negatively correlated with iron absorption in each supplementation period (Table 5). Total gPRP production was significantly negatively correlated with iron bioavailability at week 4 in the 1.5-g supplementation period (Table 5). Statherin production was not correlated with iron absorption, whereas cystatin was overall significantly positively correlated with iron absorption (Table 5).
TABLE 5.
Correlations between percentage of maximum iron absorption and salivary proteins at weeks 0 and 4 of each supplementation period (n = 11)1
| bPRP | aPRP | gPRP | Statherin | Cystatin | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose, g | R | P | R | P | R | P | R | P | R | P | R | P |
| 0.03 | ||||||||||||
| Week 0 | 0.218 | 0.518 | −0.18 | 0.596 | −0.131 | 0.70 | −0.241 | 0.475 | 0.290 | 0.387 | −0.078 | 0.82 |
| Week 4 | 0.605 | 0.049* | −0.198 | 0.56 | 0.184 | 0.587 | −0.055 | 0.872 | −0.007 | 0.985 | 0.09 | 0.793 |
| 0.25 | ||||||||||||
| Week 0 | 0.25 | 0.46 | −0.645 | 0.03* | −0.204 | 0.547 | −0.245 | 0.469 | 0.326 | 0.328 | 0.047 | 0.892 |
| Week 4 | 0.489 | 0.07 | 0.057 | 0.876 | 0.111 | 0.76 | 0.112 | 0.757 | 0.138 | 0.704 | 0.158 | 0.66 |
| 1.5 | ||||||||||||
| Week 0 | 0.297 | 0.438 | 0.075 | 0.861 | 0.391 | 0.298 | 0.01 | 0.80 | 0.201 | 0.60 | 0.46 | 0.182 |
| Week 4 | 0.173 | 0.611 | −0.483 | 0.133 | −0.595 | 0.05* | 0.10 | 0.767 | 0.114 | 0.739 | −0.076 | 0.825 |
| Total | 0.366 | 0.003* | −0.20 | 0.028* | −0.23 | 0.06 | 0.07 | 0.57 | 0.27 | 0.03* | 0.20 | 0.11 |
*P < 0.05. aPRP, acidic proline-rich protein; bPRP, basic proline-rich protein; gPRP, glycosylated proline-rich protein.
Astringency testing
Astringency ratings with tannin consumption.
There were no significant differences in astringency ratings due to tannin dose order in chi-square testing (P > 0.09), except for 0.03 g following 1.5-g dose supplementation, which significantly reduced astringency ratings (P = 0.047; Table 6). There were no significant effects of tannin dose on changes in ratings of astringency (P > 0.126); however, astringency ratings were lower for the 0.15-mg/dL astringency dose after supplementation with the 1.5-g compared with the 0.03-g tannin dose (P = 0.013).
TABLE 6.
Mean astringency ratings, and changes from weeks 0 to 4 of the supplementation periods1
| Supplementation period, g | |||||||
|---|---|---|---|---|---|---|---|
| 0.03 | 0.25 | 1.5 | |||||
| Dose, mg/dL | Week 0 | Week 4 | Week 0 | Week 4 | Week 0 | Week 4 | P 2 |
| 0.03 | 1.4 (1.1, 1.6) | 1.1 (0.9, 1.3) | 1.2 (0.9, 1.4) | 1.3 (1.0, 1.5) | 1.2 (0.9, 1.4) | 1 (0.8, 1.2)* | 0.31 |
| 0.07 | 2.2 (1.8, 2.5) | 2.3 (1.9, 2.6) | 2 (1.7, 2.4) | 1.9 (1.6, 2.3) | 2.1 (1.7, 2.4) | 1.8 (1.5, 2.2)* | 0.50 |
| 0.15 | 3.3 (2.8, 3.7) | 3.4 (2.9, 3.8) | 3.2 (2.7, 3.6) | 3.5 (3, 3.9) | 2.4 (1.9, 2.8) | 3.3 (2.8, 3.7)** | 0.126 |
| 0.25 | 4.2 (3.7, 4.7) | 4.4 (3.9, 4.8) | 4.3 (3.8, 4.7) | 4.5 (4, 5) | 3.3 (2.9, 3.8) | 4.4 (3.9, 4.8) | 0.55 |
Values are means (95% CIs). A value of 1 indicates not astringent, whereas 5 indicates extremely astringent. *P < 0.05 (week 4 compared with week 0). **P < 0.05 (0.03-g compared with 1.5-g supplementation period).
Fisher's exact test for weeks 0–4.
Astringency ratings with PRP production.
Cystatin and bPRP production correlated with a lower astringency sensation in all alum doses. In correlations from individual participants with astringency ratings at the highest alum concentration (0.2 mg/dL), there were significant positive correlations between total salivary proteins (7 of 11 participants; r > 0.49, P < 0.05) and astringency and there were negative relations between bPRPs (9 of 11 participants; r = −0.32 to −0.81, P = 0.001–0.21), cystatins (9 of 11 participants, r = −0.2 to −0.76, P = 0.03–0.28), and astringency.
Discussion
The primary objectives of this trial were to determine the effect of long-term dose-response condensed tannin supplementation on iron bioavailability and status and to understand the effect of salivary proteins on iron bioavailability during prolonged condensed tannin consumption. A secondary objective was to assess astringency as a potential marker for adaptation to tannins and iron bioavailability.
Hematologic outcomes and tannin supplementation periods
Overall, our results support the hypotheses of no significant reductions in iron bioavailability or status with 3 supplementation periods of long-term, multiple-daily tannin supplements over 4 wk. Despite nonsignificant negative trends in week 0 iron absorption with the 1.5-g (highest) dose compared with 0.25- and 0.03-mg (lowest) doses, hemoglobin and ferritin were maintained in all groups throughout the supplementation periods (Table 2). There were no differences in ferritin or CRP measurements within individuals or among tannin doses throughout the study (Table 2). To the best of our knowledge, this is the first study to quantify the effects of long-term, dose-response condensed tannin effects on iron bioavailability and status.
Our findings of no significant changes in iron bioavailability or status within or among tannin doses are in contrast with previous single-meal studies using black tea (6, 8, 48), which contains theaflavins and thearubigins (49), or in trials using tannic acid (3, 4, 48). Tannic acid and tea tannins may bind to salivary proteins and chelate iron differently than condensed tannins (proanthocyanidins), which are typically larger in size and consumed within a complex food matrix (50, 51). Condensed tannin consumption in humans and rats (52–55) also resulted in no changes in iron bioavailability or status. In contrast, dose-dependent inhibition of grape seed extract on iron bioavailability has been reported in Caco-2 cells (56). Similar discrepancies in in vivo and in vitro models were observed previously. Iron status in pigs consuming red and white beans (which are higher and lower in tannins, respectively) resulted in no difference in iron status outcomes, whereas the Caco-2 cell model showed higher iron bioavailability from white beans than red (57). Inconsistencies between long-term in vivo and in vitro studies may be partially a result of the complexity of factors contributing to human and animal digestion, including salivary proteins, which likely are not accounted for in simulated digestion. In addition, the single-meal digestion simulation used in Caco-2 cells might have the same limitations as short-term bioavailability studies. The discrepancies between long-term consumption studies compared with short-term bioavailability studies and Caco-2 findings may suggest that caution must be exercised when using the evidence from the latter types of research to predict chronic consumption in vivo iron outcomes.
To the best of our knowledge, this study is the first to quantify the effects of multiple-daily, multidose condensed proanthocyanidins on iron bioavailability or status. A similar study showed that 4-wk tea supplementation, similar to this study's 0.25-g supplement dose, resulted in significantly lower ferritin concentrations in nonanemic and anemic women (31), suggesting that there may be differences in the impact of tannin type on iron status. Interestingly, in a study observing effects from green leafy vegetables on hemoglobin, significant improvements were seen after only 3 wk (58). Although supplementation amounts in our study at 1.5 g were 50 × >0.03-g and 10 × >0.25-g tannin doses, iron absorption was only modestly reduced in the 1.5-g supplementation period (equivalent to consuming 100 g high tannin sorghum 3 times/d), and there were no significant reductions in iron status over time. There were no changes in iron absorption, ferritin, or hemoglobin over time in any adjusted or unadjusted models, suggesting that condensed tannin intake at any dose did not affect iron absorption. Although other studies have noted a reduction in iron bioavailability with condensed tannin intake (4, 59), our study is the first (to the best of our knowledge) to isolate supplementation of proanthocyanidins outside of other antinutritional factors commonly consumed concurrently in vivo.
PRP production and iron bioavailability
To the best of our knowledge, this study is the first to assess correlations between salivary protein production and iron bioavailability and to investigate long-term tannin supplementation effects on salivary profiles in humans. Overall, our hypotheses that salivary PRP production would be inducible in higher tannin doses than in lower doses and that PRP production would impact iron bioavailability with tannin consumption were partially supported. There were no significant changes in PRP or salivary protein production within or among tannin doses; however, there were significant correlations between PRPs, non-PRP salivary proteins, and iron bioavailability, suggesting that participants producing higher quantities of total salivary proteins, bPRPs, and cystatins improved iron absorption with condensed tannin intake. Correlations between bPRPs, cystatins, and iron absorption tended to be stronger at week 4 in lower doses, suggesting that salivary protein subtypes may change with regular tannin consumption to improve iron bioavailability but are likely not the only physiologic adaptation when higher tannin doses are consumed. In Caco-2 cells, bPRPs inhibited uptake of small tannin molecules through formation of insoluble complexes, but this process was mediated in part by sodium-glucose transporter-1 and multidrug resistance protein 2 (60). It may be that bPRPs signal changes in these receptors that mediate tannin absorption and iron-related sequelae.
Binding of bPRP to polyphenols may be preferential compared with other PRP subtypes (61), and production of larger bPRPs that would efficiently bind to tannins is most likely genetically determined (61–63). This idea may help to explain the wide variability in iron absorption among participants and age-related changes in iron absorption with tannin consumption. For example, in preterm infants, salivary protein profiles vary widely from adults (47), and bPRPs are almost nonexistent, which may affect tolerance of the former population to tannins.
In contrast with findings showing that bPRPs supported iron bioavailability with tannin consumption, gPRP and aPRP production (especially at week 0 for each tannin dose) was significantly negatively correlated with iron bioavailability. Negative impacts of these PRP subtypes on iron bioavailability could mean that individuals producing higher amounts of aPRP or gPRP proteins absorb iron less efficiently, especially when initially exposed to increased concentrations of tannins and until other homeostatic protective mechanisms are employed. This is the first time, to the best of our knowledge, that aPRP and gPRP interactions with tannins over time have been determined in vivo. It may be that aPRP and gPRP are upregulated with tannin consumption but do not bind to condensed tannins effectively, thereby increasing protein-iron chelation. Furthermore, aPRP and gPRP may be effectively inhibited by carbohydrate consumption (64) compared with bPRP, meaning that individuals producing more of these proteins may less effectively prevent tannin-iron chelation.
Astringency as a predictor of iron bioavailability with tannin consumption
Our findings partially supported our secondary hypotheses that 1) astringency perception would be changed with tannin consumption over time, 2) salivary PRP production would predict astringency, and 3) astringency could be used as a surrogate marker for iron bioavailability, based on PRP expression with consumption. Astringency ratings did not change within or among tannin doses throughout the study, except for the highest tannin dose (1.5 g). Astringency ratings were lower with higher tannin concentrations and were also significantly negatively correlated with bPRP and cystatin production, suggesting that reductions in ratings of very astringent, or bitter, foods may help predict iron bioavailability with tannin exposure. Despite this, we did not find consistent associations between iron bioavailability and astringency ratings within or between supplementation periods.
Limitations
There are several important limitations that must be considered when interpreting results from our study. It must be acknowledged that tannin supplementation limits the generalizability of these findings to tannins within foods, which commonly coexist with other antinutritional factors such as phytic acid. Tannin-rich food commodities may also confer different effects with antinutritional-food matrix interactions. It is also possible that several factors, including above-RDA ascorbic acid intake and challenge meal ascorbic acid supplementation, may have inhibited the tannin effects on iron bioavailability seen previously (59), although iron bioavailability has been inhibited with similar doses of ascorbic acid in test meals (16). In addition, the population assessed in this study consisted of nonanemic premenopausal adult women with a sufficient and varied diet. Given that women were nonanemic and their week 0 ferritin stores ranged from 7 to 100 ng/mL, it is possible that 4 wk may not have been long enough for multimeal supplementation to affect iron status; however, antinutritional factors have been shown to change serum ferritin and hemoglobin in as little as 2–4 wk (31, 65–68). Iron bioavailability in our study was less than the 9% suggested previously for the model employed here (40). Although our study findings are consistent with many studies testing effects of antinutritional factors (3–8, 16, 40), low iron bioavailability may have affected the sensitivity of iron absorption curves between supplements (40). Similarly, variability in iron absorption limited the power of our sample size and may have diminished the small impact of tannins on iron absorption observed here. Despite this, it is important to consider the lack of a concentration-dependent effect from tannins on individual study participants who had limited variability in iron absorption throughout the supplementation periods. Individual results from the crossover design support our findings overall (Supplemental Table 1). It may be problematic to generalize these findings to a clinical population, such as anemic women and children, who may have a different response to tannin exposure.
Participants noted that they experienced increased salivary flow rates during the 1.5-g tannin doses compared with the 0.03-g tannin doses, although flow rates were not measured quantitatively. It is also important to note that although concentrations of PRPs themselves did not change through the study, subjective experiences of salivary flow rates among participants were greater at week 4 in higher (0.25 and 1.5 g) supplementation periods than at week 0. Previous research findings indicate that salivary flow and PRP concentration provide more accurate estimates of total production than concentration alone (69). A previous study showed that the salivary flow rate is an independent factor in reducing ratings of astringency along with salivary protein concentration (70), suggesting that total PRP production in our study may have been increased with increasing salivary flow rates. Lack of measurement of the salivary flow rate is a limitation in PRP-iron outcomes analysis because we were not able to assimilate total PRP quantification from a predetermined 2-mL salivary sample (which was obtained over varying time spans). Finally, we grouped salivary types based on elution times. This method has been employed previously (46) but is not an accurate representation of salivary protein quantification.
Future directions
Foremost, better characterization of proanthocyanidin-phytic acid interactions on iron bioavailability and salivary protein production must be explored, including the effects of mixed antinutritional factor outcomes regarding iron bioavailability over time. Mixed diets have conferred different findings in the past (71) than those presented in this research study, and understanding nutrient interactions may be key to understanding these discrepancies. In addition, effects of tannins in anemic populations, who may have disease burden or dietary deficiencies, must be explored.
Because of the complexity of PRP subtypes, determining which specific bPRP and cystatins improve iron bioavailability with tannin challenge may enable diet specification in both children and adults (61). Determination of PRP genetic makeup in anemic and nonanemic tannin consumers may help to determine those with tannin-binding subtypes, and protein production could later be determined based on findings. More studies are needed to determine the effects of tannin supplementation on iron bioavailability in infants and the effects of different tannin types (tannic acid, theaflavins, and thearubigins) on salivary proteins. Further comparison of oral and enteric tannin exposure may help to determine nonsalivary determinants of physiologic tannin mediation.
Long-term condensed tannin supplementation did not impair iron bioavailability, ferritin, or hemoglobin concentrations in nonanemic premenopausal women. Iron absorption after tannin supplementation was positively correlated with bPRP and cystatin production, and tannin supplementation was associated with significantly reduced ratings of astringency over time. These findings suggest that individual physiology may need to be accounted for when considering the nutritional impact on iron bioavailability and status. Given the lack of impact of condensed tannins on iron status over time, these results suggest that efforts to remove condensed tannins from the diet to increase iron bioavailability and status may need to be reconsidered.
Supplementary Material
Acknowledgments
The authors' responsibilities were as follows—NMD: conceived and conducted the experiments, analyzed the data, and wrote the manuscript; NMF: randomly assigned participants to the supplementation periods, prepared the supplements throughout the study, and edited the manuscript; KAK: analyzed the salivary proteins on HPLC and edited the manuscript; SKR: conceived the experiment and edited the manuscript; MDH: conceived and edited the manuscript; BLL: conceived and oversaw the experiment, analyzed the data, and edited the manuscript; and all authors: read and approved the final manuscript.
Abbreviations
- aPRP
acidic proline-rich protein
- bPRP
basic proline-rich protein
- CRP
C-reactive protein
- gPRP
glycosylated proline-rich protein
- iAUC
incremental AUC
- PRP
proline-rich protein
- TFA
trifluoroacetic acid
Footnotes
Supported by the USDA Foreign Agricultural Service under the Micronutrient Fortified Food Aid Products Pilot program (contract FFE-621-2012/033-00). Publication of this article was funded in part by the Kansas State University Open Access Publishing Fund.
References
- 1. Camaschella C.. Iron-deficiency anemia. N Engl J Med 2015;372:1832–43. [DOI] [PubMed] [Google Scholar]
- 2. Stevens GA, Finucane MM, De-Regil LM, Paciorek CJ, Flaxman SR, Branca F, Peña-Rosas JP, Bhutta ZA, Ezzati M; Nutrition Impact Model Study Group (Anaemia) . Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Glob Health 2013;1:e16–e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gillooly M, Bothwell TH, Torrance JD, MacPhail AP, Derman DP, Bezwoda WR, Mills W, Charlton RW, Mayet F.. The effects of organic acids, phytates and polyphenols on the absorption of iron from vegetables. Br J Nutr 1983;49:331–42. [DOI] [PubMed] [Google Scholar]
- 4. Tuntawiroon M, Sritongkul N, Brune M, Rossander-Hulten L, Pleehachinda R, Suwanik R, Hallberg L.. Dose-dependent inhibitory effect of phenolic compounds in foods on nonheme-iron absorption in men. Am J Clin Nutr 1991;53:554–7. [DOI] [PubMed] [Google Scholar]
- 5. Gorczyca D, Prescha A, Szeremeta K, Jankowski A.. Iron status and dietary iron intake of vegetarian children from Poland. Ann Nutr Metab 2013;62:291–7. [DOI] [PubMed] [Google Scholar]
- 6. Disler PB, Lynch SR, Charlton RW, Torrance JD, Bothwell TH, Walker RB, Mayet F.. The effect of tea on iron absorption. Gut 1975;16:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zijp IM, Korver O, Tijburg LBM.. Effect of tea and other dietary factors on iron absorption. Crit Rev Food Sci Nutr 2000;40:371–98. [DOI] [PubMed] [Google Scholar]
- 8. Thankachan P, Walczyk T, Muthayya S, Kurpad AV, Hurrell RF.. Iron absorption in young Indian women: the interaction of iron status with the influence of tea and ascorbic acid. Am J Clin Nutr 2008;87:881–6. [DOI] [PubMed] [Google Scholar]
- 9. Scalbert A, Mila I, Expert D, Marmolle F, Albrecht AM, Hurrell R, Huneau JF, Tomé D.. Polyphenols, metal ion complexation and biological consequences. Basic Life Sci 1999;66:545–54. [DOI] [PubMed] [Google Scholar]
- 10. Webb P, Rogers BL, Rosenberg I, Schlossman N, Wanke C, Bagriansky J, Sadler K, Johnson Q, Tilahun J, Reese Masterson A, et al. . Improving the nutritional quality of US food aid: recommendations for changes to products and programs. Boston (MA): Tufts University; 2011. [Google Scholar]
- 11. Delimont NM, Haub MD, Lindshield BL.. The impact of tannin consumption on iron bioavailability and status: a narrative review. Curr Dev Nutr 2017;1:e000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Beverly AB, Zhu L, Fish TL, Thannhauser T, Rutzke MA, Miller DD.. Green tea ingestion by rats does not affect iron absorption but does alter the composition of the saliva proteome. J Food Sci 2012;77:H96–104. [DOI] [PubMed] [Google Scholar]
- 13. Wauben IPM, Atkinson SA.. Calcium does not inhibit iron absorption or alter iron status in infant piglets adapted to a high calcium diet. J Nutr 1999;129:707–11. [DOI] [PubMed] [Google Scholar]
- 14. Kim HS, Miller DD.. Proline-rich proteins moderate the inhibitory effect of tea on iron absorption in rats. J Nutr 2005;135:532–7. [DOI] [PubMed] [Google Scholar]
- 15. Lopez HW, Coudray C, Bellanger J, Younes H, Demigne C, Remesy C.. Intestinal fermentation lessens the inhibitory effects of phytic acid on mineral utilization in rats. J Nutr 1998;128:1192–8. [DOI] [PubMed] [Google Scholar]
- 16. Armah SM, Boy E, Chen D, Candal P, Reddy MB.. Regular consumption of a high-phytate diet reduces the inhibitory effect of phytate on nonheme-iron absorption in women with suboptimal iron stores. J Nutr 2015;145:1735–9. [DOI] [PubMed] [Google Scholar]
- 17. Hunt JR, Roughead ZK.. Adaptation of iron absorption in men consuming diets with high or low iron bioavailability. Am J Clin Nutr 2000;71:94. [DOI] [PubMed] [Google Scholar]
- 18. Hunt JR, Roughead ZK.. Nonheme-iron absorption, fecal ferritin excretion, and blood indexes of iron status in women consuming controlled lactoovovegetarian diets for 8 wk. Am J Clin Nutr 1999;69:944–52. [DOI] [PubMed] [Google Scholar]
- 19. Jaramillo Á, Briones L, Andrews M, Arredondo M, Olivares M, Brito A, Pizarro F.. Effect of phytic acid, tannic acid and pectin on fasting iron bioavailability both in the presence and absence of calcium. J Trace Elem Med Biol 2015;30:112–7. [DOI] [PubMed] [Google Scholar]
- 20. Mennen L, Hirvonen T, Arnault N, Bertrais S, Galan P, Hercberg S.. Consumption of black, green and herbal tea and iron status in French adults. Eur J Clin Nutr 2007;61:1174–9. [DOI] [PubMed] [Google Scholar]
- 21. Delimont NM, Rosenkranz SR, Haub M, Lindshield BL.. Salivary proline-rich proteins may reduce tannin-iron chelation: a systematic narrative review. Nutr Metab (Lond) 2017;14:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brandão E, Soares S, Mateus N, De Freitas V.. In vivo interactions between procyanidins and human saliva proteins: effect of repeated exposures to procyanidins solution. J Agric Food Chem 2014;62:9562–8. [DOI] [PubMed] [Google Scholar]
- 23. Shimada T.. Salivary proteins as a defense against dietary tannins. J Chem Ecol 2006;32:1149–63. [DOI] [PubMed] [Google Scholar]
- 24. Skopec MM, Hagerman A, Karasov W.. Do salivary proline-rich proteins counteract dietary hydrolyzable tannin in laboratory rats? J Chem Ecol 2004;30:1679–92. [DOI] [PubMed] [Google Scholar]
- 25. Mennella JA, Pepino MY, Reed DR.. Genetic and environmental determinants of bitter perception and sweet preferences. Pediatrics 2005;115: e216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bachmanov AA, Beauchamp GK.. Taste receptor genes. Annu Rev Nutr 2007;27:389–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mehansho H, Hagerman A, Clements S, Butler L, Rogler J, Carlson DM.. Modulation of proline-rich protein biosynthesis in rat parotid glands by sorghums with high tannin levels. Proc Natl Acad Sci USA 1983;80:3948–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mehansho H, Ann DK, Butler LG, Rogler J, Carlson DM.. Induction of proline-rich proteins in hamster salivary glands by isoproterenol treatment and an unusual growth inhibition by tannins. J Biol Chem 1987;262:12344–50. [PubMed] [Google Scholar]
- 29. Torregrossa AM, Nikonova L, Bales M, Leal M, Smith J, Contreras R, Eckel LA.. Induction of salivary proteins modifies measures of both orosensory and postingestive feedback during exposure to a tannic acid diet. PLoS One 2014;9:e105232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brune M, Rossander L, Hallberg L.. Iron absorption: no intestinal adaptation to a high phytate diet. Am J Clin Nutr 1989;49:542–5. [DOI] [PubMed] [Google Scholar]
- 31. Schleiser K, Kühn B, Kiehntopf M, Winnefeld K, Roskos M, Bitsh R, Böhm V.. Comparative evaluation of green and black tea consumption on the iron status of omnivorous and vegetarian people. Food Res Int 2012;46:522–7. [Google Scholar]
- 32. Radhakrishnan MR, Sivaprasad J.. Tannin content of sorghum varieties and their role in iron bioavailability. J Agric Food Chem 1980;28:55–7. [DOI] [PubMed] [Google Scholar]
- 33. Wu Y, Li X, Xiang W, Zhu C, Lin Z, Wu Y, Li J, Pandravada S, Ridder DD, Bai G, et al. . Presence of tannins in sorghum grains is conditioned by different natural alleles of tannin. Proc Natl Acad Sci USA 2012;109:10281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nyachoti C, Atkinson J, Leeson S.. Sorghum tannins: a review. World Poult Sci J 1997;53:5–21. [Google Scholar]
- 35. Brune M, Rossander L, Hallberg L.. Iron absorption and phenolic compounds: importance of different phenolic structures. Eur J Clin Nutr 1989;43:547–57. [PubMed] [Google Scholar]
- 36. Kloepfer K, Schmid P, Wuillemin WA, Rüfer A.. Reference values for oral iron absorption of bivalent iron in healthy volunteers. Swiss Med Wkly 2015;145:w14063. [DOI] [PubMed] [Google Scholar]
- 37. Messana I, Cabras T, Inzitari R, Lupi A, Zuppi C, Olmi C, Fadda MB, Cordaro M, Giardina B, Castagnola M.. Characterization of the human salivary basic proline-rich protein complex by a proteomic approach. J Proteome Res 2004;3:792–800. [DOI] [PubMed] [Google Scholar]
- 38. Pattini A, Schena F, Guidi G.. Serum ferritin and serum iron changes after cross-country and roller ski endurance races. Eur J Appl Physiol Occup Physiol 1990;61:55–60. [DOI] [PubMed] [Google Scholar]
- 39. Hoppe M, Hulthen L, Hallberg L.. The validation of using serum iron increase to measure iron absorption in human subjects. Br J Nutr 2004;92:485–8. [DOI] [PubMed] [Google Scholar]
- 40. Conway RE, Geissler C, Hider R, Thompson R, Powell J.. Serum iron curves can be used to estimate dietary iron bioavailability in humans. J Nutr 2006;136:1910–4. [DOI] [PubMed] [Google Scholar]
- 41. Lee J, Chambers DH.. A lexicon for flavor descriptive analysis of green tea. J Sens Stud 2007;22:256–72. [Google Scholar]
- 42. Chanadang S, Chambers E IV, Alavi S.. Tolerance testing for cooked porridge made from a sorghum based fortified blended food. J Food Sci 2016;81:S1210–21. [DOI] [PubMed] [Google Scholar]
- 43. National Cancer Institute Choosing an approach for dietary assessment [Internet]. Bethesda (MD): National Cancer Institute (US) 2016. [cited 2017 Apr 23]. Available from: https://dietassessmentprimer.cancer.gov/approach/. [Google Scholar]
- 44. Bhagwat S, Haytowitz D, Prior R, Gu L, Hammerstone J, Gebhardt S, et al. . USDA database for proanthocyanidin content of selected foods Beltsville (MD): USDA. 2004. [cited 2017 Sep 21]. Available from: https://www.ars.usda.gov/northeast-area/beltsville-md/beltsville-human-nutrition-research-center/nutrient-data-laboratory/docs/usda-database-for-the-proanthocyanidin-content-of-selected-foods-2004/.
- 45. US Department of Agriculture Agricultural Research Service USDA database for the flavonoid content of selected foods, release 3.0 [Internet]. Beltsville (MD): USDA. 2011. [cited 2017 Sep 21]. Available from: https://www.ars.usda.gov/northeast-area/beltsville-md/beltsville-human-nutrition-research-center/nutrient-data-laboratory/docs/usda-database-for-the-flavonoid-content-of-selected-foods-release-31-december-2013/.
- 46. Soares S, Vitorino R, Osório H, Fernandes A, Venâncio A, Mateus N, Amado F, de Freitas V.. Reactivity of human salivary proteins families toward food polyphenols. J Agric Food Chem 2011;59:5535–47. [DOI] [PubMed] [Google Scholar]
- 47. Castagnola M, Inzitari R, Fanali C, Iavarone F, Vitali A, Desiderio C, Vento G, Tirone C, Romagnoli C, Cabras T, et al. . The surprising composition of the salivary proteome of preterm human newborn. Mol Cell Proteomics 2011;10:M110.003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Derman D, Sayers M, Lynch SR, Charlton RW, Bothwell TH, Mayet F.. Iron absorption from a cereal-based meal containing cane sugar fortified with ascorbic acid. Br J Nutr 1977;38:261–9. [DOI] [PubMed] [Google Scholar]
- 49. Menet MC, Sang S, Yang CS, Ho C, Rosen RT.. Analysis of theaflavins and thearubigins from black tea extract by MALDI-TOF mass spectrometry. J Agric Food Chem 2004;52:2455–61. [DOI] [PubMed] [Google Scholar]
- 50. Canon F, Ployon S, Mazauric J, Sarni-Manchado P, Refregiers M, Giuliani A, Cheynier V.. Binding site of different tannins on a human salivary proline-rich protein evidenced by dissociative photoionization tandem mass spectrometry. Tetrahedron 2015;71:3039–44. [Google Scholar]
- 51. Canon F, Ballivian R, Chirot F, Antoine R, Sarni-Manchado P, Lemoine J, Dugourd P.. Folding of a salivary intrinsically disordered protein upon binding to tannins. J Am Chem Soc 2011;133:7847–52. [DOI] [PubMed] [Google Scholar]
- 52. Yun S, Zhang T, Li M, Chen B, Zhao G.. Proanthocyanidins inhibit iron absorption from soybean (Glycine max) seed ferritin in rats with iron deficiency anemia. Plant Foods Hum Nutr 2011;66:212–7. [DOI] [PubMed] [Google Scholar]
- 53. Garcia-Lopez JS, Erdman JW Jr., Sherman AR.. Iron retention by rats from casein-legume test meals: Effect of tannin level and previous diet. J Nutr 1990;120:760–6. [DOI] [PubMed] [Google Scholar]
- 54. Hamdaoui MH, Chabchoub S, Hedhili A.. Iron bioavailability and weight gains to iron-deficient rats fed a commonly consumed Tunisian meal ‘bean seeds ragout’ with or without beef and with green or black tea decoction. J Trace Elem Med Biol 2003;17:159–64. [DOI] [PubMed] [Google Scholar]
- 55. Welch RM, House WA, Beebe S, Cheng Z.. Genetic selection for enhanced bioavailable levels of iron in bean (Phaseolus vulgaris L.) seeds. J Agric Food Chem 2000;48:3576–80. [DOI] [PubMed] [Google Scholar]
- 56. Ma Q, Kim E, Lindsay EA, Han O.. Bioactive dietary polyphenols inhibit heme iron absorption in a dose-dependent manner in human intestinal Caco-2 cells. J Food Sci 2011;76:H143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tan SY, Yeung CK, Tako E, Glahn RP, Welch RM, Lei X, Miller DD.. Iron bioavailability to piglets from red and white common beans (Phaseolus vulgaris). J Agric Food Chem 2008;56:5008–14. [DOI] [PubMed] [Google Scholar]
- 58. Agte V, Jahagirdar M, Chiplonkar S.. GLV supplements increased plasma β-carotene, vitamin C, zinc, and hemoglobin in young healthy adults. Eur J Nutr 2006;45:29–36. [DOI] [PubMed] [Google Scholar]
- 59. Cercamondi CI, Egli IM, Zeder C, Hurrell RF.. Sodium iron EDTA and ascorbic acid, but not polyphenol oxidase treatment, counteract the strong inhibitory effect of polyphenols from brown sorghum on the absorption of fortification iron in young women. Br J Nutr 2014;111:481–9. [DOI] [PubMed] [Google Scholar]
- 60. Cai K, Hagerman AE, Minto RE, Bennick A.. Decreased polyphenol transport across cultured intestinal cells by a salivary proline-rich protein. Biochem Pharmacol 2006;71:1570–80. [DOI] [PubMed] [Google Scholar]
- 61. Levine M.. Susceptibility to dental caries and the salivary proline-rich proteins. Int J Dent 2011;2011:953412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stubbs M, Chan J, Kwan A, So J, Barchynsky U, Rassouli-Rahsti M, Robinson R, Bennick A.. Encoding of human basic and glycosylated proline-rich proteins by the PRB gene complex and proteolytic processing of their precursor proteins. Arch Oral Biol 1998;43:753–70. [DOI] [PubMed] [Google Scholar]
- 63. Lyons KM, Azen EA, Goodman PA, Smithies O.. Many protein products from a few loci: assignment of human salivary proline-rich proteins to specific loci. Genetics 1988;120:255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Soares S, Mateus N, de Freitas V.. Carbohydrates inhibit salivary proteins precipitation by condensed tannins. J Agric Food Chem 2012;60:3966–72. [DOI] [PubMed] [Google Scholar]
- 65. Blunden RW, Lloyd JV, Rudzki Z, Kimber RJ.. Changes in serum ferritin levels after intravenous iron. Ann Clin Biochem 1981;18:215–7. [DOI] [PubMed] [Google Scholar]
- 66. Siimes MA, Koerper MA, Ličiko V, Dallman PR.. Ferritin turnover in plasma: an opportunistic use of blood removed during exchange transfusion. Pediatr Res 1975;9:127–9. [DOI] [PubMed] [Google Scholar]
- 67. Johnson-Wimbley T, Graham DY.. Diagnosis and management of iron deficiency anemia in the 21st century. Therap Adv Gastro 2011;4:177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wheby MS.. Effect of iron therapy on serum ferritin levels in iron-deficiency anemia. Blood 1980;56:138–40. [PubMed] [Google Scholar]
- 69. Jensen JL.. Salivary acidic proline‐rich proteins in rheumatoid arthritis. Ann N Y Acad Sci 1998;842:209–11. [DOI] [PubMed] [Google Scholar]
- 70. Dinnella C, Recchia A, Fia G, Bertuccioli M, Monteleone E.. Saliva characteristics and individual sensitivity to phenolic astringent stimuli. Chem Senses 2009;34:295–304. [DOI] [PubMed] [Google Scholar]
- 71. Hunt JR.. High-, but not low-bioavailability diets enable substantial control of women's iron absorption in relation to body iron stores, with minimal adaptation within several weeks. Am J Clin Nutr 2003;78:1168–77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.