Abstract
Background: Supplementation with essential amino acids (EAAs) + arginine is a promising nutritional approach to decrease plasma triglyceride (TG) concentrations, which are an independent risk factor for ischemic heart disease.
Objective: The objective of this study was to examine the effects of 8 wk of EAA supplementation on skeletal muscle basal metabolite concentrations and changes in metabolic response to acute EAA intake, with an emphasis on mitochondrial metabolism, in adults with elevated TGs to better understand the mechanisms of lowering plasma TGs.
Methods: Older adults with elevated plasma TG concentrations were given 22 g EAAs to ingest acutely before and after an 8-wk EAA supplementation period. Skeletal muscle biopsy samples were collected before and after acute EAA intake, both pre- and postsupplementation (4 biopsy samples), and targeted metabolomic analyses of organic acids and acylcarnitines were conducted on the specimens.
Results: Acute EAA intake resulted in increased skeletal muscle acylcarnitine concentrations associated with oxidative catabolism of the supplement components, with the largest increases found in acylcarnitines of branched-chain amino acid oxidative catabolism, including isovaleryl-carnitine (2200%) and 2-methylbutyryl-carnitine (2400%). The chronic EAA supplementation resulted in a 19% decrease in plasma TGs along with accumulation of long-chain acylcarnitines myristoyl- (90%) and stearoyl- (120%) carnitine in skeletal muscle and increases in succinyl-carnitine (250%) and the late-stage tricarboxylic acid cycle intermediates fumarate (44%) and malate (110%).
Conclusions: Supplementation with EAAs shows promise as an approach for moderate reduction in plasma TGs. Changes in skeletal muscle metabolites suggest incomplete fatty acid oxidation and increased anaplerosis, which suggests a potential bottleneck in fatty acid metabolism.
Keywords: acylcarnitines, essential amino acids, TCA intermediates, targeted metabolomics, triglycerides
Introduction
Hypertriglyceridemia is a significant independent risk factor for ischemic heart disease, which is the single largest killer of men and women in the United States (1). Hypertriglyceridemia prevalence increases with age and is associated with obesity, renal disease, and diabetes (2). The treatment of elevated TGs is included in the Adult Treatment Panel III of the National Cholesterol Education Program (3, 4). Revising dietary macronutrient intake is a primary strategy for reducing TGs. Although limiting intake of SFAs, trans FAs, and cholesterol is widely advocated to lower TGs, it is also well established that higher-carbohydrate diets increase plasma TG concentrations (5) and low-carbohydrate diets have been found to reduce plasma TGs more than low-calorie, low-fat diets (6). Because a reduction in dietary carbohydrate is often accompanied by an increase in protein intake, and the independent impact of these 2 factors on plasma TG concentrations may be difficult to differentiate, it is interesting to note that dietary supplementation of a diet provided ad libitum with protein results in a lowering of plasma TGs (7). The influence of amino acid intake on plasma lipid concentrations is largely unknown and is an active area of research (8–10) to better isolate the effects of protein intake on lipid concentrations.
In our previous studies in older adults with impaired glucose tolerance, we observed decreased circulating and tissue TG concentrations after supplementing the diet with a small quantity of essential amino acids (EAAs) + arginine (∼22 g/d) between meals for 16 wk (9). In another study, we found that a combination supplement consisting of EAAs with whey protein and phytosterols reduced plasma cholesterol and TG concentrations in overweight people with mild hyperlipidemia (10). Others have found that obese people exhibit alterations in skeletal muscle metabolism of amino acids and FAs relative to lean people (11), with evidence of increased anaplerosis and limited FA oxidative flux. A recent study reported the coexistence of elevated short- and medium-chain acylcarnitines and reduced branched-chain ketoacids and tricarboxylic acid (TCA) cycle intermediates in skeletal muscle from people with insulin resistance, suggesting that impaired skeletal muscle metabolism of BCAAs and lipids may play a role in the mechanisms underlying reduced insulin sensitivity (12).
This important link between FA and EAA oxidative metabolism is tied to mitochondrial function where the oxidative fate of these classes of molecules ultimately resides through β-oxidation and entrance into the TCA cycle (13, 14). In fact, there is in vitro evidence for a “branched-chain” carnitine-acylcarnitine translocase in the mitochondria implicated in BCAA oxidative metabolism (15). Increasing oxidative flux through BCAA pathways within cultured skeletal muscle cells has been shown to effect a significant increase in FA oxidation, with the implication that obstructions to BCAA oxidation could limit skeletal muscle lipid turnover (12). To better understand this relation and to inform future nutritional interventions in hypertriglyceridemia, we investigated the effects of EAAs + arginine supplementation on mitochondrial metabolism.
Methods
Reagents and chemicals
LC mobile phases consisting of HPLC-grade acetonitrile and the modifiers formic acid and heptafluorobutyric acid were purchased from Sigma-Aldrich, whereas water was generated by using an in-house Milli-Q water purification system. Other chemicals, including O-benzylhydroxylamine (OBHA), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide, ethyl acetate, and methanol, were also purchased from Sigma-Aldrich. Internal standards for targeted metabolomics, including sodium lactate 13C3, citric D4 (2,2,4,4) acid, fumaric 13C4 acid, sodium pyruvate 13C3, α-ketoglutaric D6 acid, butyryl-l-carnitine D3, propionyl-l-carnitine D3, acetyl-l- carnitine D3, decanoyl-l-carnitine D3, hexanoyl-l-carnitine D3, isovaleryl-l-carnitine D9, octadecanoyl-l-carnitine D3, octanoyl-l-carnitine D3, and tetradecanoyl-l-carnitine D3, were purchased from Isotec (Sigma-Aldrich), whereas succinic D6 acid and disodium-2-hydroxy-glutarate D3 (2,3,3) were purchased from CDN Isotopes.
Participant information and experimental protocol
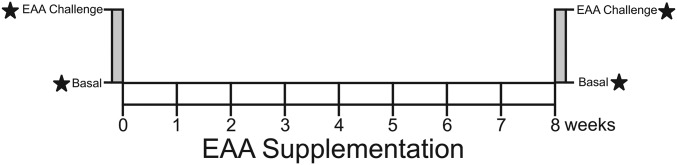
A total of 6 older [mean ± SD age: 69 ± 4 y; BMI (in kg/m2): 35 ± 9] volunteers (4 women, 2 men) with elevated plasma TGs (2.3 ± 0.4 mmol/L) participated in an 8-wk study period (Figure 1). Exclusion criteria included diabetes, evidence of renal or liver disease, bleeding disorders, endocrine disease (excluding well-controlled hypothyroidism), and alcohol or drug abuse. Before beginning the study, the participants were counseled to maintain their typical dietary intake and physical activity pattern. During the participants' study visits and in telephone calls between visits, they were asked about dietary changes and reminded to not make any changes. Before and after an 8-wk EAAs + arginine supplementation phase, participants underwent an acute EAAs + arginine intake experiment wherein they arrived after an overnight fast and a basal muscle biopsy sample was collected from the vastus lateralis as described elsewhere (16), before consuming 1 g of an EAA + arginine supplement dissolved in water every 10 min for the next 3.5 h (22 g EAAs plus arginine in total). The composition of the EAAs was as follows: 3.26% histidine, 8.57% isoleucine, 35.88% leucine, 17.08% lysine-hydrochloride, 3.59% methionine, 4.65% phenylalanine, 9.57% threonine, 7.44% valine, and 9.97% arginine. The EAAs + arginine mixture was provided by Ajinomoto. Upon completion of the acute EAAs + arginine intake, another muscle biopsy was taken. During the chronic supplementation phase, participants ingested two 11-g doses/d in capsule form; one dose between breakfast and lunch and the other dose between lunch and dinner. The participants recorded their doses in a diary and visited the hospital to receive a new supply of supplements every 2 wk. The protocol was approved by the Institutional Review Board at the University of Arkansas for Medical Sciences, and informed consent was obtained from all individuals participating in the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
FIGURE 1.
Overview of EAAs + arginine supplementation experiment. An acute EAAs + arginine-ingestion (3.5 h) study was held before (week 0) and after (week 8) a chronic EAAs + arginine supplementation period. EAA, essential amino acid.
Sample preparation
Skeletal muscle preparation.
A portion of the skeletal muscle biopsy samples were snap-frozen immediately after collection and stored at −80°C until the study was complete. The samples were batch-processed to minimize variation and were processed alongside a set of standard porcine muscle samples to assess the presence of experimental error. A more complete description of sample processing for the skeletal muscles is outlined elsewhere (17). Briefly, ∼15–25 mg frozen skeletal muscle was weighed and then transferred to a prechilled stainless steel homogenization tube loaded with silicon carbide sharp particles (Biospec Products). Prechilled 50:50 methanol:water extraction buffer was added to each tube at a ratio of 13 μL buffer/mg tissue and then a mixture of isotope-labeled standards for organic acids and acylcarnitines was added to each tube (see Supplemental Tables 1 and 2 for isotope standard details). Samples were then homogenized by using a Bead Ruptor 24 (Omni International, Inc.) with the use of two 30-s homogenization steps at 6.3 m/s with a subsequent dry ice cool-down step in between. Samples were centrifuged for 10 min at 14,000 × g at 4°C, and the raw extract supernatant was transferred to a fresh tube and stored at −80°C until subsequent analysis.
Organic acid sample preparation.
An aliquot of the raw skeletal muscle extract was derivatized as described elsewhere (18). Briefly, 100 μL muscle extract was mixed with 50 μL of 1-M OBHA (in pyridine buffer, pH 5.1) followed by the addition of 50 μL of 1-M 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (in pyridine buffer, pH 5.1). The mixtures were shaken at room temperature for 1 h, then extracted with ethyl acetate 3 times. The combined organic layers were dried by SpeedVac (Thermo Fisher) and then reconstituted in mobile phase (as described below) and transferred to a vial for chromatography measurements.
Acylcarnitine sample preparation.
Full details of the acylcarnitine preparation are described elsewhere (19). Briefly, aliquots of the skeletal muscle raw extract were transferred to tubes, dried by SpeedVac, and reconstituted in 0.1% phosphoric acid. Acylcarnitines were then loaded onto Oasis MCX extraction cartridges (Waters Corp.) and activated by methanol immediately before sample loading. Cartridges were washed twice with 0.1% phosphoric acid before samples were eluted with methanol. Eluates were dried by SpeedVac, reconstituted in mobile phase (as described below), and transferred to a vial for chromatography.
Chromatography MS
All LC-MS/MS procedures were conducted with an ABSciex Qtrap 5500 mass spectrometer with an Eksigent Micro 200 LC and controlled by Analyst 1.6.3 software (Sciex, Inc.). Organic acid OBHA derivatives were separated by using a 0.3- × 150-mm ChromXP C18EP column with a 3-μm particle size and 300-Å pore size (Eksigent; Sciex, Inc.). Mobile phases for organic acid analysis consisted of Milli-Q water with 1% formic acid (A) and acetonitrile with 1% formic acid (B). The chromatographic elution program for organic acids was operated with a flow rate of 25 μL/min and began by holding the mobile phase at 25% B for 30 s, followed by a linear gradient to 70% B for 5 min, then a 30 s linear gradient to 100% B, where it was held for a final 30 s. Acylcarnitines were separated on a 0.5- × 150-mm Halo C18 column with a 2.7-μm particle size and a 90-Å pore size (Eksigent; Sciex, Inc.). The mobile phases for acylcarnitine analysis consisted of Milli-Q water with a 0.08% heptafluorobutyric acid ion-pairing agent (A) and acetonitrile (B). The elution program for acylcarnitines was run at 25 μL/min by using a stepped gradient profile from 5% B to 95% B over 10 min (see Supplemental Tables 1 and 2 for more details). MS was conducted by using multiple reaction monitoring (MRM) following the settings as described in the Supplemental Information. Sample concentrations were determined by using MRM peak ratios with internal standards as described in Supplemental Tables 1 and 2.
Data analysis
MRM peak areas were measured by using Analyst 1.6.4 software and MultiQuant and were visually inspected. Muscle sample masses and chromatographic peak areas of analytes and isotope standards were imported into R software for statistical analysis. Mass concentrations of analytes were calculated in terms of analyte mass per milligram of muscle tissue. Grubb's test for outliers (α = 0.05) was performed on participants' metabolite concentration response to both the acute EAAs + arginine challenge and the 8-wk EAAs + arginine supplementation. Nine of 576 metabolic responses measured were determined to be outliers by the Grubb's test and removed from the analysis. Statistical analysis was conducted by using paired t tests (α = 0.05) to compare each participant's basal concentrations for skeletal muscle metabolites before and after the 8-wk supplementation period. Paired t tests were also conducted to compare skeletal muscle metabolite concentrations before and after the periods of acute EAAs + arginine intake.
Results
Plasma TG concentrations
After 8 wk of EAAs + arginine supplementation, fasted plasma TG concentrations were found to decrease from 2.3 ± 0.4 to 1.8 ± 0.3 mmol/L (19%; P < 0.05). This TG plasma decrease is consistent with those reported elsewhere (9, 10).
Short-chain acylcarnitines
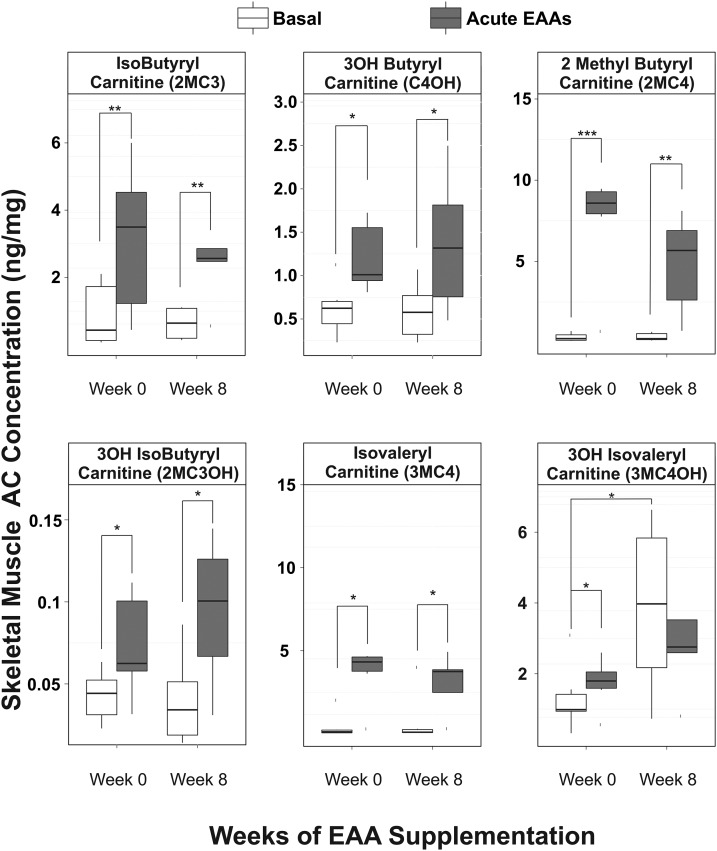
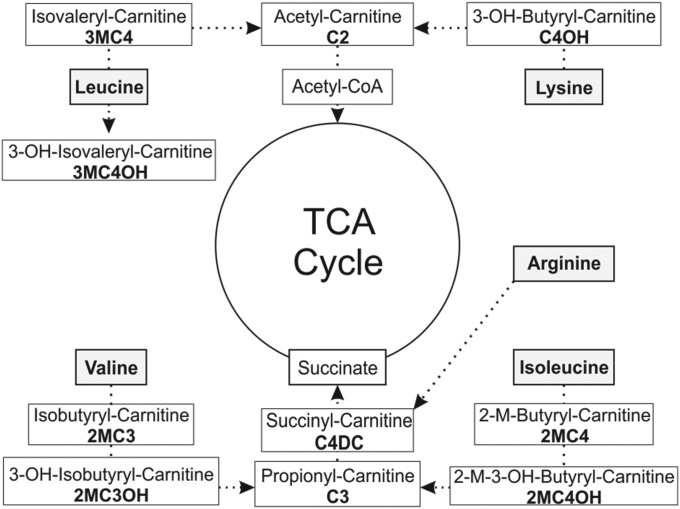
The skeletal muscle acylcarnitine concentrations related to oxidative metabolism of EAAs yielded the most dramatic changes in response to EAAs + arginine challenge (Figure 2). Acylcarnitine metabolites related to oxidation of leucine and isoleucine (i.e., isovaleryl-carnitine and 2-methylbutyryl-carnitine) increased by 2200% (P < 0.04) and 2400% (P < 0.008), respectively, in response to acute EAAs + arginine intake (Figure 3). 3-Hydroxybutyryl-carnitine, a metabolite of lysine metabolism, was also found to increase in response to acute EAAs + arginine intake by 132% (P < 0.04). The metabolic intermediates of valine oxidation, isobutyryl-carnitine (136%; P < 0.01) and 3-hydroxy-isobutyryl-carnitine (95%; P < 0.05), also increased in response to acute EAAs + arginine intake. All of the aforementioned skeletal muscle acylcarnitine basal concentrations were unchanged after 8 wk of EAAs + arginine supplementation. Before chronic supplementation, 3-hydroxy-isovaleryl-carnitine (3MC4OH), an intermediate of oxidative metabolism of leucine, was found to increase in response to acute EAAs + arginine intake (Figure 2; 70%; P < 0.02). Basal concentrations of 3MC4OH increased with supplementation (250%; P < 0.05); however, there was no observable effect of acute EAAs + arginine intake on skeletal muscle 3MC4OH concentrations after the 8-wk supplementation period. It is notable that 3MC4OH does not have a direct route to enter the TCA cycle, which may contribute to its accumulation in muscle tissue (Figure 3).
FIGURE 2.
Box plots showing skeletal muscle AC concentrations before (white boxes) and after (dark-gray boxes) acute EAAs + arginine intake at weeks 0 and 8 of daily EAAs + arginine supplementation; n = 6. ACs shown are intermediates of EAA oxidative metabolism. Solid horizontal lines within the boxes represent the median values. *P < 0.05, **P < 0.01, and ***P < 0.001 (paired t test). AC, acylcarnitine; EAA, essential amino acid; 3OH, 3-hydroxy.
FIGURE 3.
Measured acylcarnitine species within catabolic pathways of amino acids present in EAA supplementation and anaplerotic routes into the TCA cycle through acetyl-CoA or succinyl-CoA. EAA, essential amino acid; TCA, tricaboxylic acid; 3-OH, 3-hydroxy.
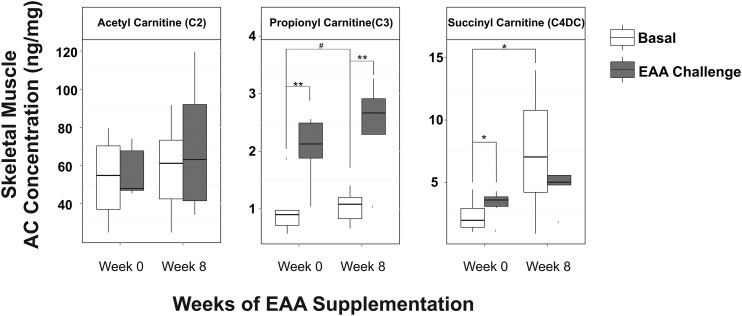
Succinyl-carnitine (C4DC) showed a pattern of changes in response to EAAs + arginine similar to 3MC4OH (Figure 4): increased C4DC concentration in response to the initial acute EAAs + arginine intake (40%; P < 0.05) and increased basal concentrations of C4DC after 8 wk of EAAs + arginine supplementation (250%; P < 0.05), but no increase in response to acute EAAs + arginine intake after the 8-wk supplementation period. C4DC is a metabolic intermediate that is downstream of oxidative metabolism of a number of different amino acids (Figure 3). C4DC is also anaplerotic, acting to restore the TCA intermediate pool through a succinyl-CoA-to-succinate pathway. Propionyl-carnitine (C3) is a precursor to C4DC and is also considered anaplerotic. C3 was found to increase in response to acute EAAs + arginine intake both before (135%; P < 0.005) and after (143%; P < 0.01) the 8-wk supplementation period (Figure 4). Basal concentrations of C3 showed a modest increase (20%) in response to 8 wk of EAAs + arginine supplementation that did not quite reach significance (P < 0.06). Skeletal muscle concentrations of acetyl-carnitine (C2) were not found to be increased either in response to acute EAAs + arginine intake or to 8 wk of supplementation (Figure 4).
FIGURE 4.
Box plots showing skeletal muscle AC concentrations before (white boxes) and after (dark-gray boxes) acute EAAs + arginine intake at weeks 0 and 8 of daily EAAs + arginine supplementation; n = 6. ACs shown are intermediates of EAA oxidative metabolism. Solid horizontal lines within the boxes represent the median values. #P < 0.06, *P < 0.05, and **P < 0.01 (paired t test). AC, acylcarnitine; EAA, essential amino acid.
Medium- and long-chain acylcarnitines
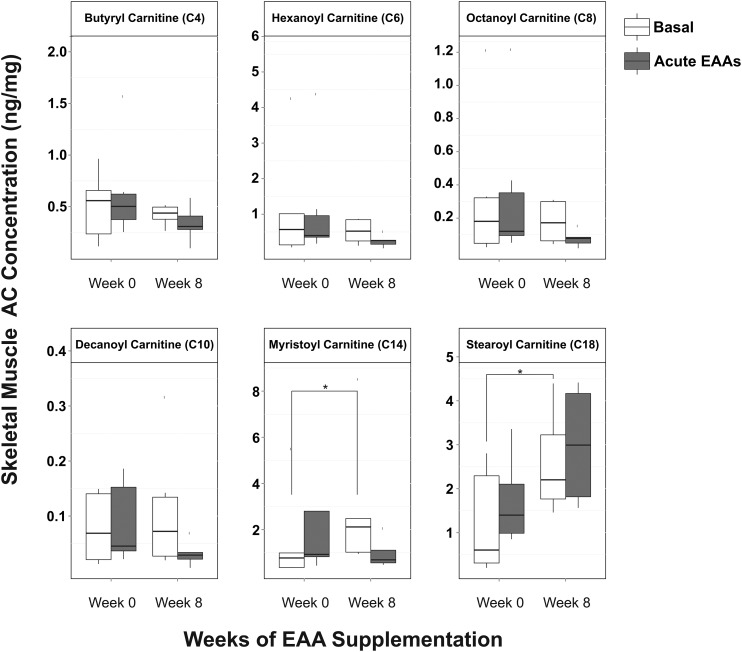
Skeletal muscle medium-chain (C4–C10) and long-chain (C14 and C18) acylcarnitine concentrations did not change significantly in response to acute EAAs + arginine intake either before or after the supplementation period (Figure 5). Basal skeletal muscle medium-chain acylcarnitine concentrations also did not show any changes in response to 8 wk of EAAs + arginine supplementation (Figure 5). The basal concentrations of myristoyl-carnitine (C14; 90% increase; P < 0.05) and stearoyl-carnitine (C18; 120% increase; P < 0.01) were found to increase in response to 8 wk of EAAs + arginine supplementation (Figure 5).
FIGURE 5.
Differential response to acute EAAs + arginine intake before (week 0) and after (week 8) chronic EAAs + arginine supplementation in skeletal muscle concentrations of medium- and long-chain ACs; n = 6. Solid horizontal lines within the boxes represent the median values. *P < 0.05 (paired t test). AC, acylcarnitine; EAA, essential amino acid.
Organic acids
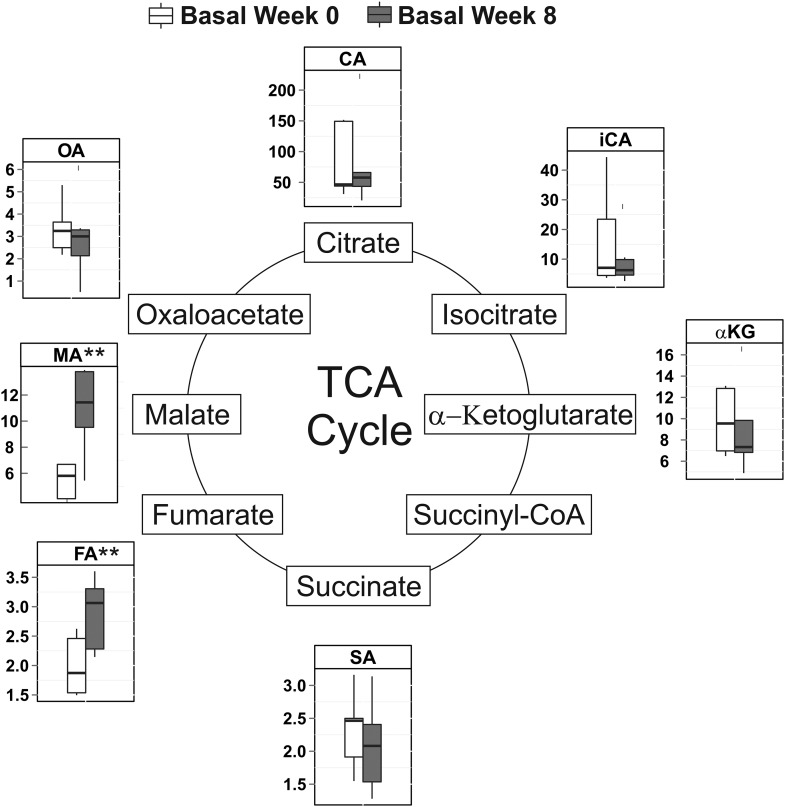
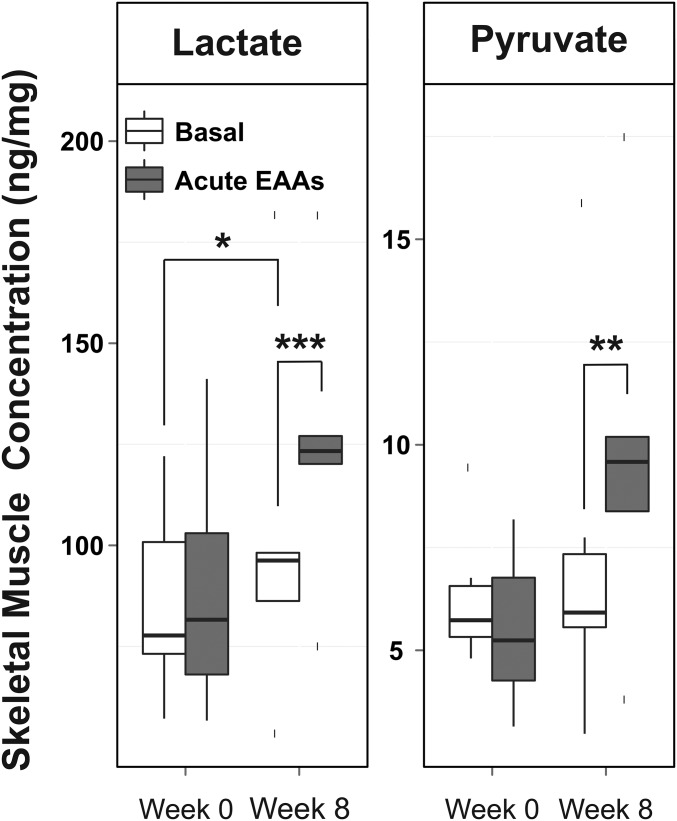
TCA intermediate concentrations in skeletal muscle were unchanged by acute EAAs + arginine intake both before and after the 8-wk EAAs + arginine supplementation period (Supplemental Figure 1). Although the basal skeletal muscle concentrations of fumarate and malate increased by 44% (P < 0.01) and 115% (P < 0.002) (Figure 6), respectively, in response to 8 wk of EAAs + arginine supplementation, there was no increase in the total sum of TCA intermediates (Supplemental Figure 2). A 32% increase in lactate in skeletal muscle (P < 0.04) was observed after the 8-wk supplementation period. Muscle lactate and pyruvate concentrations were only found to increase in response to acute EAAs + arginine intake after, not before, 8 wk of chronic EAAs + arginine supplementation (Figure 7).
FIGURE 6.
Effects of 8 wk of EAAs + arginine supplementation on basal skeletal muscle concentrations of TCA intermediates (in nanograms per milligram); n = 6. Late-stage TCA intermediates malate and fumarate were increased after 8 wk of supplementation (dark-gray boxes) relative to initial concentrations (white boxes). Solid horizontal lines within the boxes represent the median values. **P < 0.01 (paired t test). CA, citrate; EAA, essential amino acid; FA, fumarate; iCA, isocitrate; MA, malate; OA, oxaloacetate; SA, succinate; TCA, tricaboxylic acid; αKG, α-ketoglutarate.
FIGURE 7.
Skeletal muscle lactate and pyruvate concentrations in response to acute EAA intake before (week 0) and after (week 8) chronic EAA supplementation; n = 6. Solid horizontal lines within the boxes represent the median values. *P < 0.05, **P < 0.01, and ***P < 0.001 (paired t test). EAA, essential amino acid.
Discussion
Targeted metabolomic analysis of skeletal muscle biopsy samples obtained from hypertriglyceridemic older adults receiving EAAs + arginine both through acute intake and chronically for 8 wk showed altered acylcarnitine and organic acid profiles. Acute EAAs + arginine intake yielded increases in metabolic acylcarnitine intermediates of metabolism of EAA components, particularly BCAA catabolic intermediates, in skeletal muscle. The leucine metabolite most directly associated with 3MC4OH, β-hydroxy-β-methylbutyrate, has been implicated in the maintenance of muscle mass (20), and the increase in 3MC4OH may be related to a signaling pathway–inhibiting muscle protein breakdown. The acute EAAs + arginine intake–mediated increase in 3-hydroxy-isobutyryl-carnitine is notable for its structural relation to 3-hydroxy-isobutyric acid, a metabolite of valine, which has been found to stimulate endothelial transport of FFAs, leading to lipid accumulation in skeletal muscle in mice (21).
Eight weeks of EAAs + arginine supplementation resulted in increased basal concentrations of long-chain acylcarnitines. Previous in vitro studies found that long-chain acylcarnitine accumulation in muscle tissue elicits an insulin-resistant response (22). In addition, 8-wk EAAs + arginine supplementation resulted in increased skeletal muscle concentrations of anaplerotic acylcarnitines C4DC and C3. Similar observations have been made in mice fed a high-fat diet with BCAAs (23) and are consistent with a hypothesis that increased BCAA catabolism leads to accumulation of FA oxidation intermediates.
Although the total amount of TCA intermediates remained unchanged by 8 wk of supplementation, a shift toward late-stage TCA intermediates was observed. This distribution toward late-stage TCA intermediates is often reflective of anaplerosis (24, 25); and when considered alongside the increases in skeletal muscle anaplerotic acylcarnitines, this suggests that anaplerosis was increased after the 8-wk EAAs + arginine supplementation period. The increased skeletal muscle lactate concentrations after the 8-wk period may arise from increased demand for NAD+ required for increased reliance on β-oxidation (26). Acute EAAs + arginine intake after the 8-wk supplementation period yielded increased skeletal muscle concentrations of lactate and pyruvate only after the 8-wk supplementation period. This suggests that there are alterations in energy metabolism arising from chronic EAAs + arginine supplementation that do not present in the fasted state, but become apparent when amino acids are ingested acutely.
This investigation has several factors that may limit the conclusions. First, there was no placebo control used; therefore, placebo effects could be a possible explanation for our observations. However, the previously published controlled studies give us reason to believe that this is not a placebo effect (9, 10). Second, as a pilot study, the sample size was small (n = 6); thus, the results are at increased risk of arising from type I error. Despite these limitations, we have a high degree of confidence in our results. First, the acute experiment was conducted both before and after the chronic period and the observed effects were confirmed in all but 2 of the 30 metabolites measured (Figure 7), giving us a high degree of confidence that these results do not arise from type I error. The main findings that resulted from 8 wk of chronic supplementation were observed in 2 independent metabolites, increasing our confidence that these did not arise from type I error: the increased long-chain acylcarnitine concentrations were observed in both C14 and C18 and the increased late-stage TCA intermediates were observed in both fumarate and malate. The results described herein are significant and fit within the larger context of our understanding of the physiologic effects of EAAs + arginine supplementation.
Supplementary Material
Acknowledgments
The authors' responsibilities were as follows—BJM, RRW, and EB: designed the research; BJM, NMH, EC, I-YK, SS, and GA: conducted the research; BJM: analyzed the data and had primary responsibility for the final content; BJM and NMH: wrote the manuscript; and all authors: read and approved the final manuscript.
Abbreviations
- C4DC
succinyl-carnitine
- EAA
essential amino acid
- MRM
multiple reaction monitoring
- OBHA
O-benzylhydroxylamine
- TCA
tricaboxylic acid
- 3MC4OH
3-hydroxy-isovaleryl-carnitine
Footnotes
Supported by the Translational Research Institute (grants UL1TR000039 and KL2TR000063 to BJM) through the NIH National Center for Research Resources and the National Center for Advancing Translational Sciences. Also supported by NIH RO1-AG-033761 (award to EB), the Roy and Christine Sturgis Charitable Trust (award to NMH), and pilot grants awarded through the Arkansas Claude Pepper Older Americans Independence Center Pilot Grant Award 5P30AG028718 (BJM, NMH, and EC). NMH, EC, and EB were also funded by the Arkansas Biosciences Institute, the major research component of the Arkansas Tobacco Settlement Proceeds Act of 2000.
References
- 1. Kung HC, Hoyert DL, Xu J, Murphy SL.. Deaths: final data for 2005. Natl Vital Stat Rep 2008;56:1–120. [PubMed] [Google Scholar]
- 2. Jacobson TA, Miller M, Schaefer EJ.. Hypertriglyceridemia and cardiovascular risk reduction. Clin Ther 2007;29:763–77. [DOI] [PubMed] [Google Scholar]
- 3. Cheng AYY, Leiter LA.. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Curr Opin Cardiol 2006;21:400–4. [DOI] [PubMed] [Google Scholar]
- 4. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): final report. Circulation 2002;106:3143–421. [PubMed] [Google Scholar]
- 5. Parks EJ, Hellerstein MK.. Carbohydrate-induced hypertriacylglycerolemia: historical perspective and review of biological mechanisms. Am J Clin Nutr 2000;71:412–33. [DOI] [PubMed] [Google Scholar]
- 6. Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, Williams T, Williams M, Gracely EJ, Stern L.. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med 2003;348:2074–81. [DOI] [PubMed] [Google Scholar]
- 7. Bortolotti M, Maiolo E, Corazza M, Van Dijke E, Schneiter P, Boss A, Carrel G, Giusti V, Lê KA, Quo Chong DG, et al. . Effects of a whey protein supplementation on intrahepatocellular lipids in obese female patients. Clin Nutr 2011;30:494–8. [DOI] [PubMed] [Google Scholar]
- 8. Oda H.. Functions of sulfur-containing amino acids in lipid metabolism. J Nutr 2006;136(Suppl):1666S–9S. [DOI] [PubMed] [Google Scholar]
- 9. Børsheim E, Bui QU, Tissier S, Cree MG, Rønsen O, Morio B, Ferrando AA, Kobayashi H, Newcomer BR, Wolfe RR.. Amino acid supplementation decreases plasma and liver triacylglycerols in elderly. Nutrition 2009;25:281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coker RH, Deutz NE, Schutzler S, Beggs M, Miller S, Wolfe RR, Wei J.. Nutritional supplementation with essential amino acids and phytosterols may reduce risk for metabolic syndrome and cardiovascular disease in overweight individuals with mild hyperlipidemia. J Endocrinol Diabetes Obes 2015;3:1069. [PMC free article] [PubMed] [Google Scholar]
- 11. Baker PR, Boyle KE, Koves TR, Ilkayeva OR, Muoio DM, Houmard JA, Friedman JE.. Metabolomic analysis reveals altered skeletal muscle amino acid and fatty acid handling in obese humans. Obesity (Silver Spring) 2015;23:981–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lerin C, Goldfine AB, Boes T, Liu M, Kasif S, Dreyfuss JM, De Sousa-Coelho AL, Daher G, Manoli I, Sysol JR, et al. . Defects in muscle branched-chain amino acid oxidation contribute to impaired lipid metabolism. Mol Metab 2016;5:926–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guda P, Guda C, Subramaniam S.. Reconstruction of pathways associated with amino acid metabolism in human mitochondria. Genomics Proteomics Bioinformatics 2007;5:166–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Melser S, Lavie J, Bénard G.. Mitochondrial degradation and energy metabolism. Biochim Biophys Acta 2015;1853:2812–21. [DOI] [PubMed] [Google Scholar]
- 15. Roe DS, Roe CR, Brivet M, Sweetman L.. Evidence for a short-chain carnitine-acylcarnitine translocase in mitochondria specifically related to the metabolism of branched-chain amino acids. Mol Genet Metab 2000;69:69–75. [DOI] [PubMed] [Google Scholar]
- 16. Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR.. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab 2006;291:E381–7. [DOI] [PubMed] [Google Scholar]
- 17. Marquis BJ, Louks HP, Bose C, Singh SP.. A new derivatization reagent for high performance liquid chromatography mass spectrometry analysis of biologic al organic acids. Chromatographia. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tan B, Lu ZH, Dong SC, Zhao GS, Kuo MS.. Derivatization of the tricarboxylic acid intermediates with O-benzylhydroxylamine for liquid chromatography-tandem mass spectrometry detection. Anal Biochem 2014;465:134–47. [DOI] [PubMed] [Google Scholar]
- 19. Maeda Y, Ito T, Suzuki A, Kurono Y, Ueta A, Yokoi K, Sumi S, Togari H, Sugiyama N.. Simultaneous quantification of acylcarnitine isomers containing dicarboxylic acylcarnitines in human serum and urine by high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom 2007;21:799–806. [DOI] [PubMed] [Google Scholar]
- 20. Wu H, Xia Y, Jiang J, Du H, Guo X, Liu X, Li C, Huang G, Niu K.. Effect of beta-hydroxy-beta-methylbutyrate supplementation on muscle loss in older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr 2015;61:168–75. [DOI] [PubMed] [Google Scholar]
- 21. Jang C, Oh SF, Wada S, Rowe GC, Liu L, Chan MC, Rhee J, Hoshino A, Kim B, Ibrahim A, et al. . A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat Med 2016;22:421–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aguer C, McCoin CS, Knotts TA, Thrush AB, Ono-Moore K, McPherson R, Dent R, Hwang DH, Adams SH, Harper ME.. Acylcarnitines: potential implications for skeletal muscle insulin resistance. FASEB J 2015;29:336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, et al. . A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009;9:311–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Owen OE, Kalhan SC, Hanson RW.. The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem 2002;277:30409–12. [DOI] [PubMed] [Google Scholar]
- 25. Brunengraber H, Roe CR.. Anaplerotic molecules: current and future. J Inherit Metab Dis 2006;29:327–31. [DOI] [PubMed] [Google Scholar]
- 26. Kane DA.. Lactate oxidation at the mitochondria: a lactate-malate-aspartate shuttle at work. Front Neurosci 2014;8:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.