Abstract
Background: Polyphenols offer high antioxidant potential that may protect against chronic diseases. Epidemiologic evidence documenting their influence on body composition and obesity risk is limited, particularly among Hispanics/Latinos who are disproportionately prone to obesity.
Objectives: The aims of this study were to evaluate cross-sectional associations of urinary polyphenols with body mass index (BMI) and body fat percentage (%BF) in a diverse Hispanic/Latino population and to assess the reliability of polyphenol measurements.
Methods: Participants were 442 adults from the Study of Latinos/Nutrition and Physical Activity Assessment Study (SOLNAS) aged 18–74 y. Doubly labeled water was used as an objective recovery biomarker of energy. Polyphenol excretion from 24-h urine samples was assessed. Measures were repeated in a subsample (n = 90) to provide a reliability measure. Anthropometric measures were obtained by trained personnel, and %BF was measured by 18O dilution. Linear regression models were used to evaluate multivariable associations between body composition and polyphenols. Spearman correlation coefficients between BMI and %BF with polyphenols and intraclass correlation coefficients (ICCs) between polyphenol measures were computed.
Results: A weak correlation was observed for resveratrol and %BF (r = −0.11, P = 0.02). In multivariable-adjusted regression models, weak inverse associations were observed for resveratrol and urolithin A with %BF [β ± SE: −0.010 ± 0.004 (P = 0.007) and −0.004 ± 0.002 (P = 0.03), respectively]. For every 50% increase in these urinary polyphenols, there was a 1% and 0.4% decrease in %BF. Urolithin A was inversely associated with BMI (β ± SE: −0.004 ± 0.002; P = 0.02) and with 5% lower odds of obesity in models not adjusted for total energy expenditure (TEE; OR: 0.95; 95% CI: 0.91, 0.99; P = 0.02). For every 50% increase in urolithin A, there was a 0.4-unit decrease in BMI. Associations were attenuated after adjustment for TEE. Reliability study findings were indicative of weak to moderate correlations (ICCs: 0.11–0.65), representing a degree of within-person variation in polyphenol biomarkers.
Conclusions: Although associations were weak, resveratrol and urolithin A were inversely associated with obesity. Repeated polyphenol urine measures could clarify their long-term impact on body adiposity.
Keywords: polyphenols, obesity, body fat, doubly labeled water, Hispanic/Latino
Introduction
Polyphenols are non-nutritive plant secondary metabolites found in fruit, vegetables, legumes, cereals, cocoa and chocolate, coffee, tea, and wine (1). Due to their high antioxidant potential, interest in their potential role as agents for preventing and treating chronic diseases, including cardiovascular disease (CVD) and type 2 diabetes, has emerged (2). More than 500 dietary polyphenol compounds with varying distributions in foods have been identified and classified as 1) flavonoids, 2) phenolic acids, 3) lignans, and 4) stilbenes (3). Absorption is dependent on their diverse structure, but most polyphenols, once absorbed, are rapidly excreted in the urine and bile as glucuronides and sulfate esters (4). Polyphenols that are not absorbed in addition to those excreted in bile are extensively metabolized by the gut microbiota to produce a range of simple phenolic compounds (4). For example, enterolactone and enterodiol are enterolignans formed by intestinal microbiota from lignan precursors, which are contained mainly in vegetables, whole-grain products, berries, and flaxseeds (5). Similarly, urolithin A is an active microbial metabolite derived from ellagitannins contained in pomegranates, berries, and nuts (6).
Cell culture and animal studies suggest that dietary polyphenols may have a pronounced antiobesity effect associated with lower body weight and fat mass (7, 8), but few preclinical and clinical studies have been conducted. These have shown that polyphenols found in foods such as green and white teas containing catechins, fruit such as blueberries containing anthocyanins, red grapes and wine containing resveratrol, and spices such as turmeric, which contains curcumin, may reduce the risk of adiposity and obesity (9–14). Polyphenols appear to alter lipid and energy metabolism, thereby facilitating weight loss, preventing weight gain, and reducing the risk of excess body adiposity and obesity (7). Potential mechanisms underlying these associations include the suppression of fat absorption from the gut, anabolic pathways, angiogenesis in adipose tissues, and differentiation of preadipocytes to adipocytes (7, 13, 15). Other possible mechanisms include the stimulation of catabolic pathways in adipose tissues, liver, and other tissues and apoptosis of mature adipocytes (7).
Although these associations are biologically plausible, epidemiologic studies are limited and inconsistent in findings, ranging from a null association to a lower risk of excess adiposity and obesity with higher polyphenol intakes (7, 8). Observational studies within the United States report only dietary intakes of a limited number of polyphenols or their food sources (3). Because a range of genetic, anthropometric, medical, and physiologic factors influence polyphenol metabolism and bioavailability (6, 16), biomarkers of polyphenol intake may represent more objective indicators of exposure than existing dietary assessment methods. Previous biomarker studies have examined a limited range of blood and urinary polyphenols, primarily isoflavones and lignans (1, 3), but have not included measures of body composition and obesity as primary outcomes. In the United States, a recent cross-sectional study that used nationally representative data from 2003 to 2010 reported that high urinary enterolactone concentrations were inversely associated with obesity (17), but only one urinary polyphenol biomarker was assessed and analyses of body composition were not stratified by race or ethnicity.
To address this important knowledge gap, this study aimed to examine 8 polyphenols (enterolactone, enterodiol, resveratrol, daidzein, urolithin A, naringenin, hesperetin, and quercetin) and their cross-sectional associations with BMI and body fat percentage (%BF) among a diverse Hispanic/Latino population enrolled in the Study of Latinos/Nutrition and Physical Activity Assessment Study (SOLNAS) (n = 485) (20). These 8 polyphenols were selected on the basis of previous evidence of likely predominance of occurrence in urine based on foods consumed by the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) participants and accuracy of analytical techniques (18, 19). The half-lives of polyphenols are short (i.e., 4.4 h for enterodiol and 12.6 h for enterolactone), so a single measurement reflects relatively short-term exposure (21). Therefore, a secondary aim was to assess the reliability of the polyphenol measurements on the basis of measures of polyphenol concentrations repeated 6–12 mo apart among a subset of participants (n = 90). We hypothesized that higher concentrations of urinary polyphenols would be associated with lower BMI and %BF and that there would be moderate intraclass correlations between the repeated biomarker measurements.
Methods
Study population
As described elsewhere (22, 23), the HCHS/SOL is a population-based cohort study in self-identified Hispanic/Latino adults aged 18–74 y (n = 16,415), representing individuals with origins from Cuba, the Dominican Republic, Mexico, Puerto Rico, and Central and South America. Recruitment and sampling design are described in detail elsewhere (22, 23). Briefly, participants were recruited between 2008 and 2011 by using a probability sampling design to select households from census blocks close to a clinic center in Chicago, Illinois; Miami, Florida; Bronx, New York; and San Diego, California.
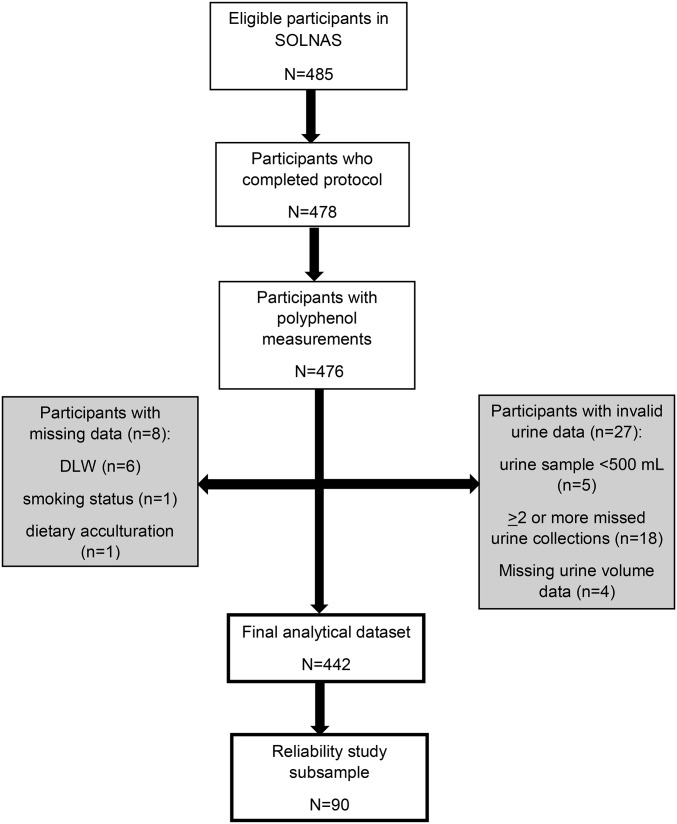
The analytic sample was derived from an ancillary study, SOLNAS, of the HCHS/SOL conducted in 2011–2012 (n = 478) (20) with primary aims to calibrate energy, protein, sodium, and potassium intakes. For the current study, in total there were 476 participants with polyphenol measurements available (Figure 1). Participants who provided a urine sample of <500 mL (n = 5); those with ≥2 missed urine collections (n = 18) or with missing urine volume (n = 4) were excluded. Of the 449 remaining participants, those with missing data for doubly labeled water (DLW) (n = 6), smoking status (n = 1), and dietary acculturation (n = 1) were also excluded for a final analytic sample of 442 participants. Ninety of the 442 participants (final analytic sample) repeated the entire protocol ∼6 mo later to provide reliability information. The HCHS/SOL parent study and SOLNAS protocol and procedures were approved by the institutional review boards for human subjects research of each field center and the coordination and reading centers.
FIGURE 1.
Creation of analytical data set from the SOLNAS cohort. DLW, doubly labeled water; SOLNAS, Study of Latinos/Nutrition and Physical Activity Assessment Study.
Assessment of urinary polyphenols
At the baseline visit, SOLNAS participants were provided with 24-h urine collection and storage instructions (i.e., refrigerate specimens), containers including boric acid as a preservative, and ice packs for transport. The 24-h urine samples were returned at the second clinic visit, which took place 12 d later. Urinary polyphenols were measured according to well-established protocols (24, 25). In brief, urine pH was adjusted for optimal enzymatic hydrolysis followed by the addition of internal standards, incubation with β-glucuronidase and arylsulfatase for 1 h at 37°C, and repeated extraction of the deconjugated analytes with ethyl acetate. The extracts were dried and re-dissolved in the mobile phase of the LC-MS system (TSQ Ultra; Thermo Scientific), separated by LC with the use of C18 reverse-phase columns and detected by tandem MS after electrospray ionization in negative mode. Limits of quantification for the 8 polyphenols of interest varied between 0.5 nM/L for urolithin A and 5 nM/L for resveratrol. Among a 10% blinded quality-control (QC) sample (n = 49), the CVs, excluding nondetectable values, were 8.7% for enterolactone, 9.8% for daidzein, 14.7% for hesperetin, 14.0% for enterodiol, 23.0% for quercetin, 24.0% for urolithin A, and 29.0% for resveratrol (Supplemental Table 1). The results of the laboratory's internal QC (n = 69) indicate CVs ranging from 10% for daidzein to 38% for resveratrol.
To test the reliability of the study measures, ∼20% of the SOLNAS study participants repeated the study protocol. The reliability subsample consisted of 98 participants who repeated the baseline visit measurements during a third visit, which took place 6–12 mo after the SOLNAS baseline visit. Twelve days after SOLNAS visit 3, this subsample returned to repeat the procedures of the SOLNAS second visit, including a second collection of 24-h urine. These urine samples were used to assess the reliability of the obtained polyphenol measurements. Eight participants were excluded from this analysis due to small urine volume (total 24-h urine volume ≤500 mL) or ≥2 missed urine collections; therefore, the final reliability study sample consisted of 90 participants.
Assessment of BMI and %BF
The primary dependent variables in this study were BMI and %BF as measures of body composition. Anthropometric measurements were consistent with the procedures and QC guidelines established in the parent study, and all of the field technicians were centrally trained and certified. Standing height was measured by using a wall-mounted stadiometer, whereas body weight was measured with a Tanita Body Composition Analyzer (model TBF-300A; Tanita Corporation of America, Inc.). BMI was calculated as follows: BMI (kg/m2) = weight (kg)/height (m2). As described previously (26), the %BF of participants was measured by the 18O dilution method. Briefly, pre-dose spot urine samples were initially collected. Then, each participant ingested 1.38 g of 10 atom percent 18O-labeled water (Sigma-Aldrich Corporation) per kilogram of body weight followed by the collection of 2 additional spot urine samples at 3 and 4 h postdose and again on day 12 (20). Participants aged ≥60 y also provided a blood sample 3 h and 12 d later to adjust for age-related postvoid urine retention (27). The 18O content of the urine and plasma samples was subsequently measured by using gas-isotope-ratio MS (28). The 18O dilution space (NO) was calculated from the zero-time intercept of the 18O turnover rate by using the back-extrapolation method and was converted to total body water (TBW) after correcting for the 1% overestimation of TBW due to isotope exchange with nonaqueous exchangeable oxygen in the body (29). TBW was converted to lean body mass (LBM) by using an LBM hydration of 73% (26). Body fat was calculated as the difference between body weight and LBM in this study.
Assessment of other measures
Demographic, medical, lifestyle, and acculturation data collected at the HCHS/SOL parent study baseline visit were used for these analyses. Dietary data were collected by using 24-h dietary recalls conducted in-person during the baseline visits of HCHS/SOL and SOLNAS and over the phone during subsequent visits (19). Recalls were administered by using Nutrition Data System for Research version 11 software developed by the Nutrition Coordinating Center, University of Minnesota. Dietary recalls were conducted by bilingual interviewers, most of whom were native Spanish speakers and certified in the use of Nutrition Data System for Research software. Interviewers used the language preferred by the respondent (19). For these analyses, the means of the baseline SOLNAS 24-h recall, obtained in person, and the second HCHS/SOL 24-h recall, obtained over the phone, were used. Participants with unreliable recalls (e.g., who reported energy intakes <500 kcal/d in either the SOLNAS or HCHS/SOL 24-h recalls) were excluded. In addition, dietary acculturation was assessed by asking whether types of foods were from Hispanic/Latino or American origin with the use of a 5-level Likert scale ranging from “mainly Hispanic/Latino” to “mainly American foods.”
Habitual physical activity during a typical week was also self-reported at the baseline visit by using a modified Global Physical Activity Questionnaire (30). Total energy expenditure (TEE) over a period of ∼2 wk was evaluated by using the DLW recovery biomarker as previously described (20). TEE was calculated from the carbon dioxide production rate by using the modified Weir equation (31), and a standard respiratory quotient or food quotient of 0.86 for populations consuming a Western diet (based on a high-fat diet) was used for these calculations (32). TEE provides an estimate of energy intake in weight-stable individuals (20), and the calculation was therefore used to approximate energy intake for these analyses.
Statistical analyses
Descriptive statistics were used to summarize the distribution of participant characteristics that were clinically relevant to the hypothesized polyphenol and cardiometabolic risk factor associations, such as age, sex, ethnicity, language preference, education, income, medical history, alcohol assessed by food-propensity questionnaire, smoking, physical activity, and dietary acculturation pattern (mainly Hispanic/Latino foods, mostly Hispanic/Latino foods and some American foods, equal amounts of Hispanic/Latino foods and American foods, mostly American foods and some Hispanic/Latino foods, or mainly American foods) in the overall sample. Participant characteristics were also examined across BMI categories: normal (18.5–24.9), overweight (25–29.9), and obese (≥30) by using chi-square tests for most categorical variables and Fisher's exact test for diabetes, cancer, income, dietary acculturation, and hormone use. For total energy intake, Wilcoxon's test was used to compare the median values across the BMI categories. The distribution of urinary polyphenol concentrations was examined, and a Wilcoxon's Signed Rank test was used to determine whether their mean ranks significantly differed between men and women. For nondetectable concentrations, half of the lower level of detection was used. Allowing for a nonlinear relation, Spearman correlation coefficients were computed to measure rank correlation between urinary polyphenols and both BMI and %BF.
The exposures of interest were urinary polyphenols and the primary dependent variables BMI and %BF. Polyphenol concentrations were multiplied by 24-h urine volume to obtain daily excretions of polyphenols. Both exposure and dependent variables were log-transformed due to skewed distribution. Individuals with log (polyphenol) outside of mean ± 3 SDs of the distribution by sex were considered outliers and excluded from these analyses. Linear regression models for BMI and %BF regressed on urinary polyphenols were fitted. P values were determined by Wald tests with robust variance, as estimated by generalized estimating equations. Logistic regression models were used to estimate the odds of obesity (BMI >30) in the highest compared with the lowest quartile of urinary polyphenols. For both linear and logistic regression models, estimates unadjusted for TEE in addition to multivariable-adjusted estimates were reported. These models were adjusted for age, sex, Hispanic background, language preference, history of diabetes, history of CVD, hypertension status, elevated total cholesterol, educational level, income level, smoking status, alcohol consumption level, self-reported physical activity, dietary acculturation, and TEE. Dietary intake was estimated by computing the mean intake from both collected 24-h recalls, and TEE estimated with the DLW method was used as a reference assessment of energy intake.
To assess the reliability of the urinary polyphenol measurements, intraclass correlation coefficients (ICCs) were computed for the reliability subsample of 90 participants who repeated the entire protocol ∼6 mo later. In exploratory analyses, Spearman correlation coefficients were computed to measure the rank correlation between the polyphenol food sources and urinary polyphenols, and subsequently between the polyphenol food sources and BMI and %BF. For urinary polyphenol measurements below the detection limit, half of the minimum was imputed. Statistical procedures were conducted by using SAS version 9.4 software (SAS Institute, Inc.) and R version 3.2.4 software (R Foundation for Statistical Computing). All of the significance tests were conducted at the 2-sided 0.05 level.
Results
Study population characteristics
Baseline demographic and lifestyle characteristics of the SOLNAS study population are shown in Table 1 for the overall sample and by BMI category. Overall, 29.9% of participants were Mexican, 26.0% Puerto Rican, 14.2% Cuban, 11.1% Central American, 10.4% Dominican, and 8.4% South American. Almost one-third were aged 18–39 y, 43.4% aged 40–54 y, and 28.8% aged 55–75 y. With regard to their medical history, 39.4% of participants reported a history of hypercholesterolemia, 27.4% reported hypertension, 26.7% reported CVD, 8.6% reported diabetes, and 2.7% reported a history of cancer. More than half (61.5%) of SOLNAS participants were female, and 76.7% indicated that Spanish was their language of preference. Forty-three percent reported an educational level greater than high school, whereas approximately one-third (31.9%) had less than a high school education and one-quarter (24.7%) had a high school diploma as their highest level of formal education. More than half of SOLNAS participants had an annual income <$20,000 (52.4%), with 31.9% reporting an income between $20,001 and $40,000, and only 2.5% of the study sample reported an annual income >$75,000.
TABLE 1.
Participant characteristics in the SOLNAS study, overall and by BMI category1
| BMI (kg/m2) | |||||
|---|---|---|---|---|---|
| Overall | <24.9 | 25–29.9 | ≥30 | P | |
| Participants | 442 (100) | 89 (20.1) | 175 (39.6) | 178 (40.3) | |
| Age, y | 0.005 | ||||
| 18–39 | 123 (27.8) | 39 (43.8) | 43 (24.6) | 41 (23.0) | |
| 40–54 | 192 (43.4) | 31 (34.8) | 76 (43.4) | 85 (47.8) | |
| 55–74 | 127 (28.8) | 19 (21.4) | 56 (32.0) | 52 (29.2) | |
| Sex | 0.96 | ||||
| Male | 170 (38.5) | 35 (39.3) | 68 (38.9) | 67 (37.6) | |
| Female | 272 (61.5) | 54 (60.7) | 107 (61.1) | 111 (62.4) | |
| Ethnicity | 0.81 | ||||
| Dominican | 46 (10.4) | 11 (12.4) | 18 (10.3) | 17 (9.5) | |
| Central American | 49 (11.1) | 10 (11.2) | 20 (11.4) | 19 (10.7) | |
| Cuban | 63 (14.2) | 12 (13.5) | 27 (15.4) | 24 (13.5) | |
| Mexican | 132 (29.9) | 22 (24.7) | 52 (29.7) | 58 (32.6) | |
| Puerto Rican | 115 (26.0) | 27 (30.3) | 39 (22.3) | 49 (27.5) | |
| South American | 37 (8.4) | 7 (7.9) | 19 (10.9) | 11 (6.2) | |
| Spanish language preference | 339 (76.7) | 63 (70.8) | 139 (79.4) | 137 (77.0) | 0.29 |
| Medical history | |||||
| Cancer | 12 (2.7) | 0 (0) | 6 (3.4) | 6 (3.4) | 0.21 |
| Diabetes | 38 (8.6) | 3 (3.4) | 12 (6.9) | 23 (12.9) | 0.02 |
| CVD composite2 | 118 (26.7) | 16 (18.0) | 42 (24.0) | 60 (33.7) | 0.01 |
| Hypertension | 121 (27.4) | 15 (16.9) | 43 (24.6) | 63 (35.4) | 0.003 |
| Hypercholesterolemia | 174 (39.4) | 28 (31.5) | 67 (38.3) | 79 (44.4) | 0.12 |
| Education | 0.42 | ||||
| Less than high school | 141 (31.9) | 28 (31.5) | 48 (27.4) | 65 (36.5) | |
| High school | 109 (24.7) | 21 (23.6) | 49 (28.0) | 39 (21.9) | |
| More than high school | 192 (43.4) | 40 (44.9) | 78 (44.6) | 74 (41.6) | |
| Income3 | 0.05 | ||||
| <$20,000 | 210 (52.4) | 49 (59.0) | 75 (48.1) | 86 (53.1) | |
| $20,001–$40,000 | 128 (31.9) | 22 (26.5) | 47 (30.1) | 59 (36.4) | |
| $40,001–$75,000 | 53 (13.2) | 12 (14.5) | 28 (18.0) | 13 (8.0) | |
| >$75,000 | 10 (2.5) | 0 (0) | 6 (3.9) | 4 (2.5) | |
| Smoking | 0.25 | ||||
| Current | 90 (20.4) | 21 (23.6) | 39 (22.3) | 30 (16.9) | |
| Past | 88 (19.9) | 15 (16.9) | 29 (16.6) | 44 (24.7) | |
| Never | 264 (59.7) | 53 (59.6) | 107 (61.1) | 104 (58.4) | |
| Alcohol | 0.42 | ||||
| No current use | 320 (72.4) | 59 (66.3) | 126 (72.0) | 135 (75.8) | |
| 1–7 drinks/wk | 84 (19.0) | 22 (24.7) | 35 (20.0) | 27 (15.2) | |
| >7 drinks/wk | 38 (8.6) | 8 (9.0) | 14 (8.0) | 16 (9.0) | |
| Physical activity | 0.15 | ||||
| Low | 37 (8.4) | 9 (10.1) | 12 (6.9) | 16 (9.0) | |
| Moderate | 199 (45.0) | 45 (50.6) | 86 (49.1) | 68 (38.2) | |
| High | 206 (46.6) | 35 (39.3) | 77 (44.0) | 94 (52.8) | |
| Dietary acculturation | 0.66 | ||||
| Mainly Hispanic/Latino foods | 182 (41.2) | 35 (39.3) | 76 (43.4) | 71 (39.9) | |
| Mostly Hispanic/Latino foods and some American foods | 138 (31.2) | 28 (31.5) | 55 (31.4) | 55 (30.9) | |
| Equal amounts of both Hispanic/Latino and American foods | 101 (22.9) | 19 (21.4) | 35 (20.0) | 47 (26.4) | |
| Mostly American foods and some Hispanic/Latino foods | 17 (3.9) | 6 (6.7) | 7 (4.0) | 4 (2.3) | |
| Mainly American foods | 4 (0.9) | 1 (1.1) | 2 (1.1) | 1 (0.6) | |
| Energy intake,4 kcal/d (estimated from doubly labeled water) | 2322 (2053, 2715) | 2066 (1808, 2314) | 2246 (2034, 2583) | 2542 (2235, 2997) | <0.0001 |
| Hormone therapy | 8 (1.9) | 3 (3.4) | 4 (2.3) | 1 (0.6) | 0.21 |
Values are n (%) unless otherwise indicated. Chi-square test was used to compute P values for most categorical variables; Fisher's exact test was used to compute P values for diabetes, cancer, income, dietary acculturation, and hormone therapy use. CVD, cardiovascular disease; SOLNAS, Study of Latinos/Nutrition and Physical Activity Assessment Study.
CVD composite included coronary artery disease, stroke, transient ischemic attack, peripheral artery disease, and heart failure.
Income missing for n = 41, grouped as 1 category in regression models.
Values for total energy intake are medians (IQRs); Wilcoxon test was used to compute P values for total energy intake.
Sixty percent of participants were never smokers, whereas 20% were current and past smokers, respectively. Most participants (72.4%) did not consume alcohol, 19.0% consumed 1–7 drinks/wk, and 8.6% consumed >7 drinks/wk. Seventy-two percent of participants reported consumption of more Hispanic/Latino foods than American foods. Twenty-three percent consumed equal amounts of Hispanic/Latino and American foods, whereas ∼5% of participants consumed more American foods. In general, the median energy intake estimated from the DLW method in this population was 2322 kcal/d. More than 90% of participants reported engaging in moderate to high levels of physical activity, whereas only 8.4% reported low physical activity levels.
In this study population, 20% of participants had a normal BMI, whereas 40% were overweight and 40% were obese. Overweight and obese participants were more likely to report a history of diabetes, CVD, and hypertension (P ≤ 0.02). Finally, although no significant differences in dietary acculturation were noted, there were significant differences in energy intake (P < 0.0001). Obese and overweight participants had generally higher mean energy intakes than did those with a normal BMI (2542, 2246, and 2066 kcal/d, respectively). Finally, polyphenol distributions in the overall sample indicated that naringenin was the most abundant polyphenol in urine (median: 554.7 nm/L), whereas urolithin A was the least abundant (median: 1.9 nm/L) (Table 2). There was a high proportion of nondetectable resveratrol (69.0%).
TABLE 2.
Distribution of urinary polyphenol concentrations in SOLNAS1
| Polyphenol | Not detectable, % | Urinary polyphenol concentration, nmol/L | ||
|---|---|---|---|---|
| 25th percentile | Median | 75th percentile | ||
| Enterolactone | 0.7 | 101.2 | 373.3 | 889.5 |
| Enterodiol | 8.4 | 9.7 | 25.1 | 55.0 |
| Resveratrol | 69.0 | 2.5 | 2.5 | 8.0 |
| Daidzein | 0 | 43.6 | 149.6 | 545.0 |
| Urolithin A | 25.8 | 0.5 | 1.9 | 9.8 |
| Naringenin | 0 | 248.0 | 554.7 | 1282.7 |
| Hesperetin | 0.5 | 17.7 | 46.5 | 351.6 |
| Quercetin | 7.0 | 4.4 | 7.3 | 14.5 |
n = 442. For nondetectable concentrations, half of the mean lower level of detection was used. SOLNAS, Study of Latinos/Nutrition and Physical Activity Assessment Study.
Associations between polyphenol urinary biomarkers and measures of body composition
Table 3 summarizes Spearman correlations between BMI and %BF with polyphenols. There were no significant correlations between any of the examined polyphenols and BMI. Resveratrol was the only polyphenol significantly associated with %BF (r = −0.11, P = 0.02). The regression coefficients of the equations to regress log %BF and log BMI from log polyphenol biomarker concentrations in multivariable-adjusted models not adjusted for TEE and fully adjusted with the exclusion of outliers are presented in Table 4. Findings were generally indicative of a null association between most urinary polyphenols and measures of body composition. Only urolithin A was significantly associated with BMI in models not adjusted for TEE (β ± SE: −0.004 ± 0.002; P = 0.02), such that for every 50% increase in urinary urolithin A, there was 0.4-unit decrease in BMI. Likewise, an inverse association was observed for urolithin A in relation to obesity risk in models not adjusted for TEE, because higher urinary concentrations were associated with a 5% lower risk of obesity (OR: 0.95; 95% CI: 0.91, 0.99; P = 0.02). However, this association was attenuated with adjustment for TEE (OR: 0.97; 95% CI: 0.92, 1.01; P = 0.15) (Table 4). When urinary polyphenol concentrations were examined in relation to %BF, weak inverse associations were observed for resveratrol and urolithin A in models not adjusted for TEE [β ± SE: −0.01 ± 0.004 (P = 0.007) and −0.005 ± 0.002 (P = 0.01), respectively] and in fully adjusted models [β ± SE: −0.010 ± 0.004 (P = 0.007) −0.004 ± 0.002 (P = 0.03), respectively]. For every 50% increase in urinary resveratrol and urolithin A, there was a 1% (95% CI: −1.8%, −0.2%) and 0.4% (95% CI: −0.9, −0.01) decrease in %BF, respectively.
TABLE 3.
Spearman correlations between daily excretions of polyphenol biomarkers and body-composition measures in SOLNAS1
| BMI | %BF | |||
|---|---|---|---|---|
| Polyphenol | Correlation coefficient | P | Correlation coefficient | P |
| Enterolactone | −0.02 | 0.72 | −0.01 | 0.90 |
| Enterodiol | −0.01 | 0.87 | 0.01 | 0.78 |
| Resveratrol | −0.02 | 0.70 | −0.11 | 0.02 |
| Daidzein | 0.09 | 0.05 | −0.04 | 0.37 |
| Urolithin A | −0.09 | 0.05 | 0.02 | 0.61 |
| Naringenin | 0.03 | 0.59 | −0.06 | 0.18 |
| Hesperetin | 0.02 | 0.61 | −0.02 | 0.69 |
| Quercetin | 0.05 | 0.28 | −0.01 | 0.80 |
n = 442. SOLNAS, Study of Latinos/Nutrition and Physical Activity Assessment Study; %BF, body fat percentage.
TABLE 4.
Multivariable associations between polyphenol biomarker concentrations and risk of obesity1
| Model 1 | Model 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | β ± SE2 | % Change (95% CI)3 | P | R 2 | β ± SE2 | ">% Change (95% CI)3 | P | R 2 | |
| BMI | |||||||||
| Enterolactone | 442 | −0.001 ± 0.002 | −0.1 (−0.5, 0.3) | 0.61 | 0.13 | 0.0007 ± 0.002 | 0.1 (−0.3, −0.5) | 0.13 | 0.40 |
| Enterodiol | 437 | −0.002 ± 0.003 | −0.2 (−0.8, 0.4) | 0.47 | 0.13 | 0.001 ± 0.002 | 0.1 (−0.3, 0.5) | 0.54 | 0.40 |
| Resveratrol | 433 | −0.003 ± 0.004 | −0.3 (−1.1, 0.5) | 0.39 | 0.14 | −0.003 ± 0.003 | −0.3 (−0.9, 0.3) | 0.13 | 0.41 |
| Daidzein | 442 | 0.003 ± 0.002 | 0.3 (−0.1, 0.7) | 0.20 | 0.13 | 0.003 ± 0.002 | 0.3 (−0.1, 0.7) | 0.13 | 0.40 |
| Urolithin A | 439 | −0.004 ± 0.002 | −0.4 (−0.8, −0.01) | 0.02 | 0.14 | −0.002 ± 0.002 | −0.2 (−0.6, 0.2) | 0.25 | 0.40 |
| Naringenin | 441 | −0.001 ± 0.003 | −0.1 (−0.7, 0.5) | 0.72 | 0.13 | −0.001 ± 0.003 | −0.1 (−0.7, 0.5) | 0.64 | 0.40 |
| Hesperetin | 442 | 0.0004 ± 0.002 | 0.04 (−0.4, 0.4) | 0.80 | 0.13 | 0.001 ± 0.001 | 0.1 (−0.1, 0.3) | 0.30 | 0.40 |
| Quercetin | 438 | 0.005 ± 0.004 | 0.5 (−0.3, 1.3) | 0.17 | 0.14 | 0.005 ± 0.003 | 0.5 (−0.1, 1.1) | 0.13 | 0.40 |
| Obesity [BMI (kg/m2) ≥30] | |||||||||
| Enterolactone | 442 | — | 0.98 (0.93, 1.04) | 0.52 | 0.13 | — | 1.00 (0.94, 1.06) | 0.99 | 0.28 |
| Enterodiol | 437 | — | 0.97 (0.91, 1.03) | 0.33 | 0.14 | — | 1.01 (0.94, 1.08) | 0.88 | 0.28 |
| Resveratrol | 433 | — | 1.02 (0.93, 1.12) | 0.62 | 0.14 | — | 1.03 (0.93, 1.14) | 0.58 | 0.28 |
| Daidzein | 442 | — | 1.05 (0.99, 1.10) | 0.07 | 0.14 | — | 1.06 (1.01, 1.12) | 0.03 | 0.28 |
| Urolithin A | 439 | — | 0.95 (0.91, 0.99) | 0.02 | 0.14 | — | 0.97 (0.92, 1.01) | 0.15 | 0.28 |
| Naringenin | 441 | — | 0.99 (0.92, 1.07) | 0.82 | 0.13 | — | 1.00 (0.92, 1.08) | 0.92 | 0.27 |
| Hesperetin | 442 | — | 1.00 (0.97, 1.04) | 0.86 | 0.13 | — | 1.02 (0.97, 1.06) | 0.50 | 0.28 |
| Quercetin | 438 | — | 1.02 (0.94, 1.12) | 0.60 | 0.14 | — | 1.04 (0.94, 1.14) | 0.49 | 0.29 |
| %BF | |||||||||
| Enterolactone | 442 | −0.004 ± 0.002 | −0.4 (−0.8, 0) | 0.08 | 0.44 | −0.004 ± 0.002 | −0.4 (−0.8, 0) | 0.11 | 0.45 |
| Enterodiol | 437 | −0.002 ± 0.003 | −0.2 (−0.8, 0.4) | 0.55 | 0.43 | −0.001 ± 0.003 | −0.1 (−0.7, 0.5) | 0.72 | 0.44 |
| Resveratrol | 433 | −0.01 ± 0.004 | −1.0 (−1.8, −0.2) | 0.007 | 0.49 | −0.01 ± 0.004 | −1.0 (−1.8, −0.2) | 0.007 | 0.50 |
| Daidzein | 442 | 0.001 ± 0.002 | 0.1 (−0.3, 0.5) | 0.55 | 0.44 | 0.001 ± 0.002 | 0.1 (−0.3, 0.5) | 0.55 | 0.44 |
| Urolithin A | 439 | −0.005 ± 0.002 | −0.5 (−0.9, −0.1) | 0.01 | 0.45 | −0.004 ± 0.002 | −0.4 (−0.9, −0.01) | 0.03 | 0.45 |
| Naringenin | 441 | −0.0006 ± 0.003 | −0.1 (−0.7, 0.5) | 0.84 | 0.44 | −0.0007 ± 0.003 | −0.1 (−0.7, 0.5) | 0.84 | 0.44 |
| Hesperetin | 442 | 0.001 ± 0.002 | 0.1 (−0.3, 0.5) | 0.52 | 0.44 | 0.001 ± 0.002 | 0.1 (−0.3, 0.5) | 0.44 | 0.45 |
| Quercetin | 438 | 0.003 ± 0.004 | 0.3 (−0.5, 1.1) | 0.51 | 0.44 | 0.003 ± 0.004 | 0.3 (−0.5, 1.1) | 0.51 | 0.44 |
Polyphenols were ln-transformed. Model 1 was adjusted for age, sex, Hispanic background, language preference, history of diabetes, history of cardiovascular diseases, hypertension status, elevated total cholesterol, educational level, income level, smoking status, alcohol consumption, self-reported physical activity, and dietary acculturation. Model 2 was additionally adjusted for total energy expenditure measured by using doubly labeled water. Individuals with log(polyphenol) outside the mean ± 3 SDs of the distribution by sex were considered outliers and were excluded from analyses. %BF, body fat percentage.
Change in log(BMI) or log(%BF) per 50% change in polyphenol daily excretion.
Percent change in BMI or %BF or ORs of obesity per 50% change in polyphenol daily excretion. Percent change = exp(β) − 1.
Reliability of the urinary polyphenol measurements
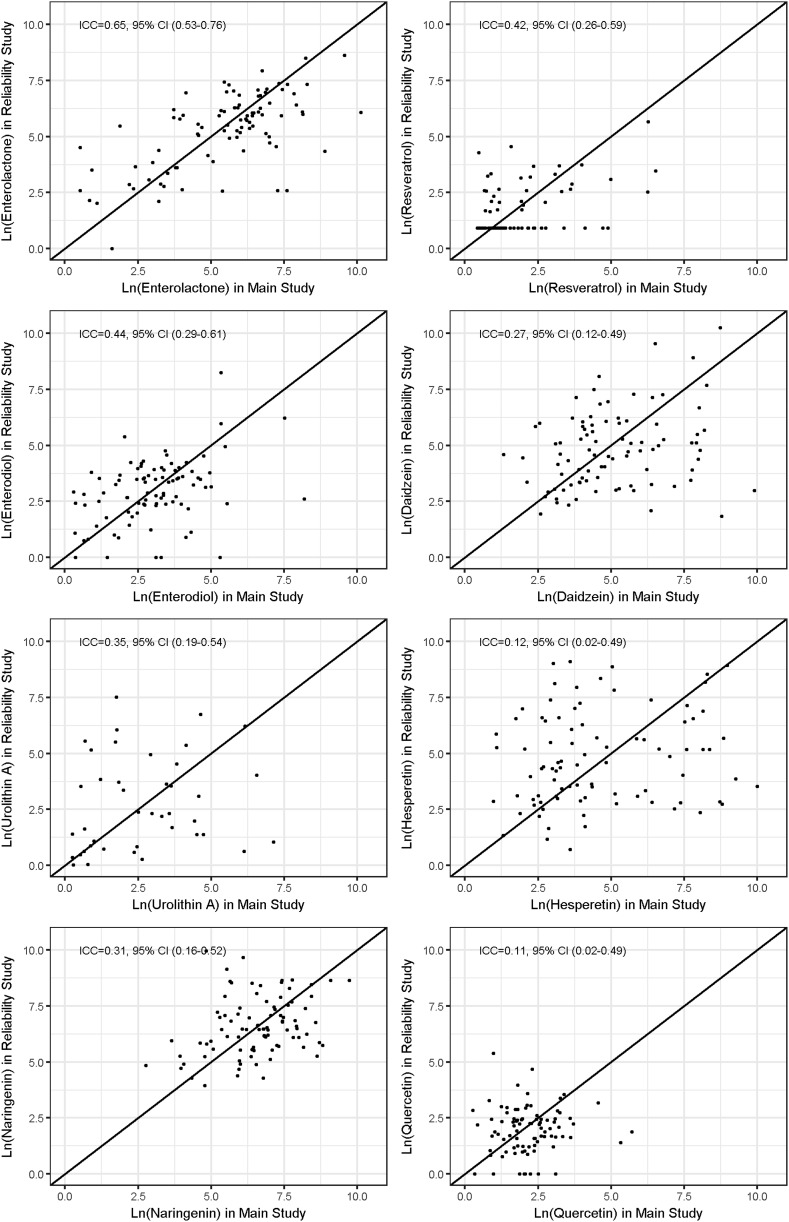
Figure 2 shows the ICCs between polyphenol measures. The ICCs were indicative of weak to moderate correlations between the urinary polyphenol concentrations measured at 2 time points, suggesting that the degree of within-person variation for urinary polyphenols varies among types of polyphenol biomarkers. In general, all of the ICCs were positive and their magnitude was weak for daidzein, hesperetin, and quercetin (ICCs ranging from 0.11 to 0.27) and moderate for resveratrol, enterodiol, urolithin A, and naringenin (ICCs ranging from 0.31 to 0.65). The strongest ICC was observed for enterolactone (0.65), whereas the weakest was observed for quercetin (0.11).
FIGURE 2.
ICCs to measure the reliability of urinary polyphenol measurements. Circles are observations of natural log transformed measurements of polyphenols. Diagonal lines are X = Y correlation. ICC, intraclass correlation coefficient.
Discussion
Within a large, diverse sample of Hispanic/Latino adults across the United States, most urinary polyphenols were not associated with BMI or %BF. A weak negative correlation suggested an inverse association between resveratrol and %BF, and this association persisted in multivariable-adjusted regression models. Likewise, weak significant inverse associations were observed between urolithin A and %BF, suggesting that these polyphenols may have a modest protective impact on measures of body composition. Urolithin A was additionally associated with lower BMI and risk of obesity in models not adjusted for TEE, but associations were attenuated in models adjusted for TEE. These weak associations may also be attributed to the fact that 24-h urinary polyphenols may only be reflective of polyphenols consumed within 48 h of the urine collection. The reliability study findings were indicative of weak to moderate correlations between urinary polyphenols measured at 2 time points, which is suggestive of a varying degree of within-person variation in polyphenol biomarkers.
Previous evidence investigating the impact of polyphenol biomarkers on measures of body composition and the risk of obesity is limited (17, 33). One previous analysis (17) in a nationally representative US sample that used 2003–2010 NHANES data reported a null association between enterodiol and obesity risk, which is consistent with the findings of the present study. However, in that study (17), higher urinary enterolactone concentrations were associated with a 56% lower obesity risk and a 42% lower risk of abdominal obesity. In contrast, another analysis (33) that used 1999–2004 NHANES data showed that the excretion of enterolignans, including enterolactone and enterodiol, and isoflavones, including daidzein, was not associated with waist circumference, which is consistent with the null findings in the present study. Inconsistencies between our study findings and NHANES data may be attributed to differences in the metabolism of polyphenols between the study populations due to variations in gut microbiota, for example, or due to methodologic differences in the measurement of polyphenols (spot urine in NHANES compared with 24-h urine collection in SOLNAS).
Hispanic/Latinos have higher prevalence rates of obesity than do non-Hispanic whites (34). In fact, a recent analysis within the HCHS/SOL reported that elevated BMI is common in Hispanic/Latino adults and is associated with a considerable excess of CVD risk factors (35). In addition, Hispanics/Latinos have unique dietary habits that are reflective of their cultural heritage, which may result in differential intakes of polyphenols (19). Given the measurement error associated with dietary assessment of polyphenol intakes, this study sought to examine whether a biomarker of polyphenols, which may provide a more robust measure than intake, is associated with body adiposity in this population group to determine whether polyphenol intakes may be of public health relevance for addressing the overweight and obesity burden in this highly affected population group.
Our study suggested a potential protective effect of urinary urolithin A on %BF, BMI, and the risk of obesity. Urolithin A is a metabolite produced by the colon microbiota from ellagitannin and ellagic acid, which occur naturally in some fruit, such as berries (strawberries and raspberries), nuts (walnuts, pistachios, cashews, acorns, and pecans), pomegranates, and grapes (36). In vitro evidence suggests that urolithin A may exert its antiobesity effects by reducing de novo lipogenesis and differentiation of adipocytes while enhancing FA oxidation via the activation of AMP-activated protein kinase, which has been shown to regulate energy homeostasis (36). These findings are corroborated by rodent studies in which the consumption of ellagic acid–rich fruit extracts from pomegranates, blueberries, and chestnuts was associated with a significant reduction in body weight and white adipose tissue mass (37–40), but little information is available in humans. Urolithin A may also play a role in regulating epigenetic factors to control white adipose tissue formation, thereby promoting metabolic activities against diet-induced obesity (36). However, because different urolithins are produced in response to a host's metabolic health, each urolithin may augment or attenuate the obesity-related benefits of ellagic acid, and urolithin A shows a large interindividual variability associated with differences in gut microbial ecology (36), human studies should consider microbiome composition in investigations of the role of this polyphenol in obesity.
Resveratrol was also significantly, inversely associated with %BF in this study. This association is biologically plausible, because in vivo and in vitro evidence has shown that resveratrol enhances lypolytic activity in adipocytes, promotes FA β-oxidation, inhibits adipogenesis, induces apoptosis, decreases lipogenesis, and reduces fat depot size and total body fat (7, 8). However, epidemiologic evidence on the role of resveratrol in the etiology of obesity is limited and inconsistent (7, 8). Most existing studies are short-term clinical trials that reported conflicting results on the potential antiobesity effects of resveratrol, ranging from mimicking the metabolic consequences of energy restriction (12) to no association (41). However, these studies had short durations and limited sample sizes and focused on dietary supplementation of resveratrol. In exploratory analyses, we found that wine intake was weakly but significantly correlated with urinary resveratrol concentratons (r = 0.15, P = 0.001), and a weak inverse correlation was observed with both BMI (r = −0.14, P = 0.003) and %BF (r = −0.13, P = 0.006) (Supplemental Tables 2 and 3).
It is notable that most studies on polyphenols and obesity risk have focused primarily on dietary intakes of polyphenols [reviewed in (7, 8)]. In exploratory analyses, we computed correlation coefficients between the examined urinary polyphenols and their food sources. We found that there was a weak positive correlation (r = 0.07–0.18) between most urinary polyphenols and their food sources (Supplemental Table 2). A previous analysis within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort (3) reported weak to strong correlations between urinary polyphenols and their food sources, with moderate correlations primarily observed for resveratrol with red wine and hesperetin and naringenin with citrus fruit intake. We were unable to assess correlations between soy and daidzein, berries and urolithin A, or resveratrol and red wine in this cohort due to the unavailability of dietary data on soy, berry, or red wine intake, so we used intakes of legumes, fruit and vegetables, and total wine as respective proxies. This may explain, at least in part, the weaker correlations observed in this cohort. These differences between our study and previous findings can also be attributed to variations in dietary patterns between European and Latino populations and suggest that there may be racial differences in polyphenol metabolism.
The weak correlations between urinary polyphenols and their food sources observed in our study may also point toward the limited utility of using a single measure of urinary polyphenol concentrations as a dietary biomarker within the Hispanic/Latino population. Findings from the reliability study showed significant within-person variations in urinary polyphenol concentrations, highlighting the need for multiple urine measurements to better capture polyphenol status, particularly for certain polyphenols for which weak ICCs were observed (quercetin, hesperetin, and daidzein). In addition, a number of factors that have been shown to affect between-person variability should be taken into account (3). Previous evidence indicates that total alcohol consumption is a relevant source of variability in urinary polyphenols and that excretion concentrations may vary by sex and educational levels (3). Individuals who had higher alcohol consumption (particularly for resveratrol) tended to be male (particularly for hesperetin, naringenin) and had higher educational levels (particularly for enterolactone and daidzein), and therefore these groups tended to have higher urinary polyphenol concentrations (3). In this study population, we also observed sex differences in urinary polyphenol excretion. The majority of our sample were nonconsumers of alcohol and reported educational levels less than high school, which may account for the lower urinary polyphenol concentrations than previously reported. Furthermore, it is notable that CVs were high for quercetin, resveratrol, and urolithin A. Possible explanations include measurements being less robust at lower concentrations or interference during analyte measurement.
Future studies capturing usual intakes of polyphenols should also account for gut microbial ecology to explain potential differences in polyphenol status beyond the effects of diet. In addition, further work is warranted, potentially with the use of animal models, to evaluate etiologic associations between polyphenols and body adiposity, including physiologic influence through phytoestrogenic activities, alterations in energy and lipid metabolism, inhibition of angiogenesis in adipose tissues, adipocyte differentiation and apoptosis, antioxidant properties, or factors associated with particular gut microbial profiles. Translational studies from animal models to human studies are needed to confirm the potential antiobesity benefits of polyphenols and their food sources.
Strengths of this study include the use of a biomarker measure to evaluate associations between polyphenols in relation to body composition and risk of obesity, because these objective measures incorporate factors such as absorption, metabolism, and bioavailability and avoid measurement error associated with self-reported dietary intake. The 24-h urine samples are a further strength of this study compared with previous studies, which often relied on spot urine measures. Additional strengths include an ethnically diverse cohort of Hispanics/Latinos in the United States with a wide age range, representation of both sexes, rigorous measurement of exposure and outcome variables with the use of well-established protocols, anthropometric measures obtained by trained personnel, and %BF assessed by using the 18O dilution method. TEE was evaluated by using the DLW recovery biomarker, which provides an objective measure of energy intake.
Limitations include a smaller number of some Hispanic/Latino subgroups, such as Central Americans, Dominicans, and South Americans, particularly among men, thereby preventing the examination of associations within these subgroups and differences in dietary polyphenol intake (19). Additional research with larger numbers of men and women from these ethnic groups is warranted. Potential errors associated with body-composition techniques including the 18O dilution method require consideration, but these should have minimal effect on our interpretation of the body-composition data.
In conclusion, these results can help inform future studies of polyphenol biomarkers in relation to measures of body composition and the risk of obesity that may seek to further explore the previous findings in experimental animal models. The observed suggestion of a potential protective impact for certain polyphenol biomarkers, albeit weak, and the inconsistency of findings and dearth of epidemiologic literature should serve as an impetus for additional studies in diverse populations that stratify by race/ethnicity to identify possible etiologic associations between urinary polyphenols and cardiometabolic risk factors. Given that polyphenols are absorbed and excreted relatively quickly after ingestion, the substantial within-person variability of polyphenol metabolites in urine samples renders urinary concentrations less likely to represent long-term intakes. Therefore, establishing both a more reliable marker of longer-term polyphenol intake as well as methods combining high sensitivity and coverage to quantify the many polyphenols present in human biospecimens represents an important research frontier in this area. Finally, due to the low bioavailability of polyphenols in humans, clinical trials and community intervention studies that account for ethnicity, genetics, lifestyle, or factors associated with gut microbial profiles may better clarify or confirm the potential antiobesity benefits of ingesting polyphenol-containing foods.
Supplementary Material
Acknowledgments
We thank the investigators and staff of the HCHS/SOL for their valuable contributions: a complete list of staff and investigators can be found elsewhere (23). The authors' responsibilities were as follows—JMB, YM-R, and AAF: conceived this project, developed the overall research plan, and oversaw the study activities; NM and JMB: wrote the manuscript and assisted with the analytic approach; SH: performed the statistical analysis; MLD, AAF, MDG, RCK, YM-R, DS-A, LVH, and WWW: advised on the research plan and provided insights into the review and revision of the manuscript for important intellectual content; JMB: had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Abbreviations
- CVD
cardiovascular disease
- DLW
doubly labeled water
- HCHS/SOL
Hispanic Community Health Study/Study of Latinos
- ICC
intraclass correlation coefficient
- LBM
lean body mass
- QC
quality control
- SOLNAS
Study of Latinos/Nutrition and Physical Activity Assessment Study
- TBW
total body water
- TEE
total energy expenditure
- %BF
body fat percentage
Footnotes
The Study of Latinos Nutrition and Physical Activity Assessment Study (SOLNAS) was supported by R01HL095856 from the National Heart, Lung, and Blood Institute (NHLBI). The Hispanic Community Health Study/Study of Latinos (HCHS/SOL) was carried out as a collaborative study supported by contracts from the NHLBI to the University of North Carolina (N01-HC65233), the University of Miami (N01-HC65234), Albert Einstein College of Medicine (N01-HC65235), Northwestern University (N01-HC65236), and San Diego State University (N01-HC65237). The following institutes, centers, or offices contribute to the HCHS/SOL through a transfer of funds to the NHLBI: the National Institute on Minority Health and Health Disparities, the National Institute on Deafness and Other Communication Disorders, the National Institute of Dental and Craniofacial Research, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Neurological Disorders and Stroke, and the NIH–Office of Dietary Supplements. Funding for the assays was supported by the Sackler Institute and the Feldstein Medical Foundation.
References
- 1. Pérez-Jiménez J, Hubert J, Hooper L, Cassidy A, Manach C, Williamson G, Scalbert A.. Urinary metabolites as biomarkers of polyphenol intake in humans: a systematic review. Am J Clin Nutr 2010;92:801–9. [DOI] [PubMed] [Google Scholar]
- 2. Li AN, Li S, Zhang YJ, Xu XR, Chen YM, Li HB.. Resources and biological activities of natural polyphenols. Nutrients 2014;6:6020–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zamora-Ros R, Touillaud M, Rothwell JA, Romieu I, Scalbert A.. Measuring exposure to the polyphenol metabolome in observational epidemiologic studies: current tools and applications and their limits. Am J Clin Nutr 2014;100:11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Manach C, Williamson G, Morand C, Scalbert A, Rémésy C.. Bioavailability and bioefficacy of polyphenols in humans: review of 97 bioavailability studies. Am J Clin Nutr 2005;81(Suppl):230S–42S. [DOI] [PubMed] [Google Scholar]
- 5. Sun Q, Wedick NM, Pan A, Townsend MK, Cassidy A, Franke AA, Rimm EB, Hu FB, van Dam RM.. Gut microbiota metabolites of dietary lignans and risk of type 2 diabetes: a prospective investigation in two cohorts of U.S. women. Diabetes Care 2014;37:1287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Williamson G, Clifford MN.. Colonic metabolites of berry polyphenols: the missing link to biological activity? Br J Nutr 2010;104(Suppl 3):S48–66. [DOI] [PubMed] [Google Scholar]
- 7. Meydani M, Hasan ST.. Dietary polyphenols and obesity. Nutrients 2010;2:737–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang S, Moustaid-Moussa N, Chen L, Mo H, Shastri A, Su R, Bapat P, Kwun I, Shen CL.. Novel insights of dietary polyphenols and obesity. J Nutr Biochem 2014;25:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu CH, Lu FH, Chang CS, Chang TC, Wang RH, Chang CJ.. Relationship among habitual tea consumption, percent body fat, and body fat distribution. Obes Res 2003;11:1088–95. [DOI] [PubMed] [Google Scholar]
- 10. Hughes LA, Arts IC, Ambergen T, Brants HA, Dagnelie PC, Goldbohm RA, van den Brandt PA, Weijenberg MP.. Higher dietary flavone, flavonol, and catechin intakes are associated with less of an increase in BMI over time in women: a longitudinal analysis from the Netherlands Cohort Study. Am J Clin Nutr 2008;88:1341–52. [DOI] [PubMed] [Google Scholar]
- 11. Nagao T, Hase T, Tokimitsu I.. A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obesity (Silver Spring) 2007;15:1473–83. [DOI] [PubMed] [Google Scholar]
- 12. Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, et al. . Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab 2011;14:612–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alappat LA, Awad AB.. Curcumin and obesity: evidence and mechanisms. Nutr Rev 2010;68:729–38. [DOI] [PubMed] [Google Scholar]
- 14. Li PL, Lu M, Nguyen MT, Bae EJ, Chapman J, Feng D, Hawkins M, Pessin JE, Sears DD, Nguyen AK, et al. . Functional heterogeneity of CD11c-positive adipose tissue macrophages in diet-induced obese mice. J Biol Chem 2010;285:15333–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Timmers S, Hesselink MK, Schrauwen P.. Therapeutic potential of resveratrol in obesity and type 2 diabetes: new avenues for health benefits? Ann N Y Acad Sci 2013;1290:83–9. [DOI] [PubMed] [Google Scholar]
- 16. Zamora-Ros R, Rabassa M, Cherubini A, Urpi-Sarda M, Llorach R, Bandinelli S, Ferrucci L, Andres-Lacueva C.. Comparison of 24-h volume and creatinine-corrected total urinary polyphenol as a biomarker of total dietary polyphenols in the Invecchiare in Chianti study. Anal Chim Acta 2011;704:110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frankenfeld CL.. Cardiometabolic risk factors are associated with high urinary enterolactone concentration, independent of urinary enterodiol concentration and dietary fiber intake in adults. J Nutr 2014;144:1445–53. [DOI] [PubMed] [Google Scholar]
- 18. Sun Q, Wedick NM, Tworoger SS, Pan A, Townsend MK, Cassidy A, Franke AA, Rimm EB, Hu FB, van Dam RM.. Urinary excretion of select dietary polyphenol metabolites is associated with a lower risk of type 2 diabetes in proximate but not remote follow-up in a prospective investigation in 2 cohorts of US women. J Nutr 2015;145:1280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Siega-Riz AM, Sotres-Alvarez D, Ayala GX, Ginsberg M, Himes JH, Liu K, Loria CM, Mossavar-Rahmani Y, Rock CL, Rodriguez B, et al. . Food-group and nutrient-density intakes by Hispanic and Latino backgrounds in the Hispanic Community Health Study/Study of Latinos. Am J Clin Nutr 2014;99:1487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mossavar-Rahmani Y, Shaw PA, Wong WW, Sotres-Alvarez D, Gellman MD, Van Horn L, Stoutenberg M, Daviglus ML, Wylie-Rosett J, Siega-Riz AM, et al. . Applying recovery biomarkers to calibrate self-report measures of energy and protein in the Hispanic Community Health Study/Study of Latinos. Am J Epidemiol 2015;181:996–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hullar MA, Lancaster SM, Li F, Tseng E, Beer K, Atkinson C, Wähälä K, Copeland WK, Randolph TW, Newton KM, et al. . Enterolignan-producing phenotypes are associated with increased gut microbial diversity and altered composition in premenopausal women in the United States. Cancer Epidemiol Biomarkers Prev 2015;24:546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lavange LM, Kalsbeek WD, Sorlie PD, Avilés-Santa LM, Kaplan RC, Barnhart J, Liu K, Giachello A, Lee DJ, Ryan J, et al. . Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol 2010;20:642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sorlie PD, Avilés-Santa LM, Wassertheil-Smoller S, Kaplan RC, Daviglus ML, Giachello AL, Schneiderman N, Raij L, Talavera G, Allison M, et al. . Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol 2010;20:629–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Franke AA, Custer L, Wilkens LR, Le Marchand L, Nomura AY, Goodman MT, Kolonel LN.. LC/PDA/MS analysis of dietary phytoestrogens from human urine and blood. J Chromatogr B Analyt Technol Biomed Sci 2002;777:45–59. [DOI] [PubMed] [Google Scholar]
- 25. Franke AA, Halm BM, Kakazu K, Li X, Custer LJ.. Phytoestrogenic isoflavonoids in epidemiologic and clinical research. Drug Test Anal 2009;1:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wong WW, Strizich G, Heo M, Heymsfield SB, Himes JH, Rock CL, Gellman MD, Siega‐Riz AM, Sotres‐Alvarez D, Davis SM, Arredondo EM.. Relationship between body fat and BMI in a US Hispanic population‐based cohort study: results from HCHS/SOL. Obesity (Silver Spring) 2016;24:1561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blanc S, Colligan AS, Trabulsi J, Harris T, Everhart JE, Bauer D, Schoeller DA.. Influence of delayed isotopic equilibration in urine on the accuracy of the (2)H(2)(18)O method in the elderly. J Appl Physiol 2002;92:1036–44. [DOI] [PubMed] [Google Scholar]
- 28. Wong WW, Lee LS, Klein PD.. Deuterium and oxygen-18 measurements on microliter samples of urine, plasma, saliva, and human milk. Am J Clin Nutr 1987;45:905–13. [DOI] [PubMed] [Google Scholar]
- 29. Schoeller D, van Santen E, Peterson DW, Dietz W, Jaspan J, Klein PD.. Total body water measurement in humans with 18O and 2H labeled water. Am J Clin Nutr 1980;33:2686–93. [DOI] [PubMed] [Google Scholar]
- 30. Bull F, Maslin TS, Armstrong T.. Global Physical Activity Questionnaire (GPAQ): nine country reliability and validity study. J Phys Act Health 2009;6:790–804. [DOI] [PubMed] [Google Scholar]
- 31. Weir JB.. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949;109:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Black AE, Prentice AM, Coward WA.. Use of food quotients to predict respiratory quotients for the doubly-labelled water method of measuring energy expenditure. Hum Nutr Clin Nutr 1986;40:381–91. [PubMed] [Google Scholar]
- 33. Struja T, Richard A, Linseisen J, Eichholzer M, Rohrmann S.. The association between urinary phytoestrogen excretion and components of the metabolic syndrome in NHANES. Eur J Nutr 2014;53:1371–81. [DOI] [PubMed] [Google Scholar]
- 34. Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL.. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2016;315:2284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaplan RC, Avilés-Santa ML, Parrinello CM, Hanna DB, Jung M, Castañeda SF, Hankinson AL, Isasi CR, Birnbaum-Weitzman O, Kim RS, Daviglus ML.. Body mass index, sex, and cardiovascular disease risk factors among Hispanic/Latino adults: Hispanic Community Health Study/Study of Latinos. J Am Heart Assoc 2014;3:e000923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kang I, Buckner T, Shay NF, Gu L, Chung S.. Improvements in metabolic health with consumption of ellagic acid and subsequent conversion into urolithins: evidence and mechanisms. AdvNutr 2016;7:961–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lei FZ, Zhang XN, Wang W, Xing DM, Xie WD, Su H, Du LJ.. Evidence of anti-obesity effects of the pomegranate leaf extract in high-fat diet induced obese mice. Int J Obes (Lond) 2007;31:1023–9. [DOI] [PubMed] [Google Scholar]
- 38. Neyrinck AM, Van Hée VF, Bindels LB, De Backer F, Cani PD, Delzenne NM.. Polyphenol-rich extract of pomegranate peel alleviates tissue inflammation and hypercholesterolaemia in high-fat diet-induced obese mice: potential implication of the gut microbiota. Br J Nutr 2013;109:802–9. [DOI] [PubMed] [Google Scholar]
- 39. Song Y, Park H, Kang SN, Jang SH, Lee SJ, Ko YG, Kim GS, Cho JH.. Blueberry peel extracts inhibit adipogenesis in 3T3-L1 cells and reduce high-fat diet-induced obesity. PLoS One 2013;8:e69925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Noh JR, Gang GT, Kim YH, Yang K, Hwang JH, Lee H, Oh WK, Song KS, Lee CH.. Chestnut (Castanea crenata) inner shell extract inhibits development of hepatic steatosis in C57BL/6 mice fed a high-fat diet. Food Chem Toxicol 2012;121:437–42. [Google Scholar]
- 41. Poulsen MM, Vestergaard PF, Clasen BF, Radko Y, Christensen LP, Stødkilde-Jørgensen H, Møller N, Jessen N, Pedersen SB, Jørgensen JO.. High-dose resveratrol supplementation in obese men: an investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes 2013;62:1186–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.