Abstract
Background: Dietary diversity is a key element of diet quality, but diets of women of reproductive age (WRA; aged 15–49 y) in resource-poor settings are often deficient in a range of micronutrients. Previous work showed associations between simple food-group diversity indicators (FGIs) and micronutrient adequacy among WRA. For operational and advocacy purposes, however, there is strong demand for a dichotomous indicator reflecting an acceptable level of dietary diversity.
Objective: The aim of the study was to develop a dichotomous indicator of dietary diversity in WRA.
Methods: We performed a secondary analysis of 9 data sets containing quantitative dietary data from WRA in resource-poor settings (total n = 4166). From the raw dietary data, we calculated an individual “mean probability of adequacy” (MPA) across 11 micronutrients. Several candidate FGIs were constructed. Indicator performance in predicting an MPA >0.60 was assessed within each data set by using receiver-operating characteristic analysis and sensitivity and specificity analysis at various FGI cutoffs. The analysis was performed separately for nonpregnant and nonlactating (NPNL) women and for lactating women.
Results: We identified 2 “best candidate” dichotomous indicators on the basis of 9- or 10-point food-group scores (FGI-9 and FGI-10) with a cutoff of ≥5 food groups. Both were significantly correlated to MPA in each site (P < 0.001). Areas under the curve were moderate, ranging from 0.62 to 0.82 among NPNL women and from 0.56 to 0.90 among lactating women. Comparisons of results slightly favored FGI-10 for all women.
Conclusions: When resource-intensive dietary methods are not feasible, a simple dichotomous indicator based on a cutoff of ≥5 of 10 defined food groups reflects “minimum dietary diversity for women of reproductive age.” According to the conclusions of a consensus meeting of experts, this indicator is well suited for population-level assessment, advocacy, and possibly also for tracking of change in dietary diversity across time.
Keywords: women of reproductive age, dietary diversity, food groups, developing countries, resource-poor settings, indicator, diet quality, nutrition-sensitive interventions
Introduction
Women of reproductive age (WRA) are nutritionally vulnerable and their diets frequently fall short of needs, particularly in resource-poor settings (1–3). In such contexts, diets are often monotonous, dominated by starchy staple foods, and unbalanced, and do not provide sufficient micronutrients. Poor nutrition before and during pregnancy and lactation compromises the health of mothers and their infants (4). For these reasons, the first “1000 days” have been targeted in global efforts to improve nutrition for vulnerable groups (5), and there are also renewed efforts to tackle pre- and periconceptual nutrition for girls and women (6). Nutrition-sensitive interventions outside the health sector (e.g., in agriculture, education, hygiene and sanitation, and social protection) also increasingly focus on improving the nutrition of WRA, among other vulnerable groups (7).
With a wide range of policy and programmatic interventions and country programs now aiming to improve diets and nutrition of WRA, there is increased demand for a range of metrics to track progress. At the same time, “gold standard” methods that provide nationally representative and quantitative individual dietary data remain out of reach for many countries, and in other countries such surveys are too infrequent to meet needs for assessment and tracking. In this context, the Women's Dietary Diversity Project (WDDP) undertook to develop a simple feasible indicator as a proxy for micronutrient adequacy, a universally recognized dimension of diet quality. Dietary diversity is implicit in all evidence-based healthy diet patterns (8) and is advocated in all food-based dietary guidelines (9) and in guidance from the WHO (10). More specifically, recent antenatal care guidance from the WHO also affirms the importance of diverse diets during pregnancy (11). Although dietary diversity alone is not sufficient to ensure diet quality, which also requires adequate and balanced macronutrient intake and moderation in intake of free sugars, salt, and certain fats, diversity is necessary to achieve a high-quality diet.
Dietary diversity has been operationalized variously as the sum of individual foods or food groups consumed across varying time periods (12). Early work under the WDDP showed consistent associations between several simple food-group diversity indicators (FGIs) and micronutrient adequacy of WRA, and concluded that simple food-group scores, based on 1-d recalls of food groups consumed, could be used for population-level assessment (1). Subsequently, several organizations used a 9-point (9 food groups) Women's Dietary Diversity Score in surveys and programs (13, 14). However, particularly in the context of advocacy efforts and for cross-sectoral communication, demand increased for an indicator that can be expressed in terms of prevalence meeting or not meeting a minimally acceptable level of diversity. This article reports on efforts by the WDDP team to respond to the demand and develop a dichotomous indicator of “Minimum Dietary Diversity for WRA.”
Methods
Study design and participants
Participants were WRA (ages 15–49 y) from 9 data sets from low-income countries. Five data sets from Africa and Asia were analyzed during the first phase of the WDDP, as described in Arimond et al. (1), including data collected in Ouagadougou, Burkina Faso, in 2006 (hereafter, abbreviated BF1), in Bamako, Mali, in 2007 (Mali), in rural Mozambique in 2006 (Moz), in rural Bangladesh in 1996 (Ban1), and in peri-urban Cebu, Philippines, in 2005 (Phi). During the current phase, these were reanalyzed and were supplemented with 4 additional data sets obtained from the HarvestPlus Project, from rural Bangladesh in 2008 (Ban2), rural Burkina Faso in 2010 (BF2), rural Uganda in 2007 (Ug1), and urban and rural Uganda in 2008 (Ug2). Study sites, sample size calculation, and sample designs depended on the primary study objectives and are fully described elsewhere (15–23). Briefly, the primary study objectives were often to help refine a program strategy (Ban1 and Mali), to help determine the potential impact of food fortification (Ban2, BF2, and Ug2), or to serve as a baseline for the impact evaluation of a biofortification intervention (Moz and Ug1). One data set was extracted from 1 round of a longitudinal prospective health and nutrition survey (Phi), and 1 study was undertaken with the WDDP primary objective of validating simple dietary diversity indicators as a measure of micronutrient adequacy among WRA (BF1). Study sites were selected on the basis of the study objectives. Study areas from rural regions were therefore either poor and food-insecure regions (Ban 1, Moz, and Ug1) or regions where targeted crops were present [Ban2, BF2, and Ug2 (rural part)]. Urban or peri-urban studies were not representative of the whole-country urban population but either of a homogenic ethnic group (Mali) or to represent the variety of living conditions in the setting [BF1, Phi, and Ug2 (urban part)]. A minimum sample size of 100 WRA was required for inclusion. All of the sampling procedures involved either randomization or an invitation to all eligible women in the study area; however, some studies targeted households with infants (Ban2, BF2, Moz, and Ug1) rather than all WRA (Ban1, BF1, Mali, Phi, and Ug2). The representativeness of each sample is discussed in the original articles, and primary study protocols for all sites were approved by ethical review committees or institutional review boards (15–23).
Dietary data collection and management
Data sets had dietary data consisting of 24-h recalls, with the exception of one, Ban2, in which 12-h direct-weighed records were complemented by a 12-h recall. In most studies, the 24-h recalls were performed by using the multiple-pass method, adapted from widely acknowledged guidelines (24–26). Recipes were usually collected from the female household member who was responsible for cooking, and standard recipes were used for dishes consumed outside the home. For estimating and measuring quantities, the methods used were those best suited to local foods and conditions (e.g., direct weighing, volume equivalent using beakers with marked volumes, playdough models, photographs, etc.). The study team carefully checked that dietary data were collected in all studies with the use of well-documented and sound methods. There was at least a second recall on nonconsecutive days for a subsample of ≥40 women for each data set. Raw dietary data were transformed into nutrient intakes by using food-composition tables developed by the original researchers at each site (15–23). For the current analysis, women with energy intakes either <0.9 times estimated basal metabolic rate or >3.0 times basal metabolic rate were excluded from the analyses (27). More details on dietary data collection and treatment can be found in Martin-Prevel et al. (28).
We aimed to summarize micronutrient adequacy across a range of micronutrients with known public health relevance at the outset of the WDDP (1). Most of the available information relates to potential impacts on pregnancy outcomes (29) and breast-milk content (30). We also considered the availability of nutrient data in the processed data sets and in available food-composition tables. The following 11 micronutrients were selected: vitamin A, thiamin, riboflavin, niacin, vitamin B-6, folate, vitamin B-12, vitamin C, calcium, iron, and zinc. We used the probability approach to estimate micronutrient adequacy (31). This approach is based on information or assumption about both the distribution of nutrient requirements in the population (interindividual) and the day-to-day variation (intraindividual) in nutrient intakes (32). By using the repetition of the 24-h recalls in a subsample of participants, the probability of adequacy (PA) associated with “usual intake” was calculated for each micronutrient and each woman. We compared distributions of estimated usual intakes to WHO and FAO requirement distributions for vitamins (33). Where Estimated Average Requirements (EARs) are not provided by WHO or FAO, we back-calculated an EAR from Recommended Nutrient Intakes by using the CV from the WHO or FAO, if available (33), or otherwise from the Institute of Medicine at the US National Academy of Sciences (31). For iron requirements, which are known to be skewed for nonpregnant and nonlactating (NPNL) women, we used Institute of Medicine tables (34), but adjusted for absorption of 5% (BF1, BF2, Moz, Ug1, and Ug2) or 10% (Ban1, Ban2, Mali, and Phi) on the basis of diet patterns, according to WHO and FAO guidance (33). For zinc, we used the International Zinc Nutrition Consultative Group's EARs and CVs (35), assuming low absorption (25%) in BF1, BF2, Moz, Ug1, and Ug2 and intermediate absorption (34%) in Ban1, Ban2, Mali, and Phi. The EARs used for assessing the PA for each nutrient are presented in Supplemental Table 1.
We used a Box-Cox transformation to estimate approximate Gaussian nutrient intake distributions, then calculated the best linear unbiased predictor of the usual intake for each nutrient before calculating PA for each micronutrient. The mean PA for a population group is equivalent to the population prevalence of adequacy for a particular micronutrient (31). We also averaged all 11 PAs to form a summary variable for micronutrient adequacy, mean probability of adequacy (MPA). Like individual micronutrient PAs, the MPA has a possible range of 0–1. The distribution of MPA was also transformed when necessary to approximate normality for use in analyses.
Choice of FGIs
With an aim of examining candidate indicators that could potentially be generated from large-scale survey data, we selected a set of FGIs that 1) were based on food groups, not individual food items, 2) varied in level of aggregation of foods into groups, 3) varied in the minimum quantity of consumption required for a food group to “count” in the score (1 or 15 g), and 4) were based on recall of a single day. This set of FGIs allowed us to explore the effect of aggregation and minimum quantities on indicator performance. In the first phase of the WDDP we examined indicators when foods were aggregated into 6, 9, 13, or 21 food groups and concluded that the 9- and 13-group indicators with the 15-g minimum provided the best balance between performance and feasibility (1).
The second phase, with additional data sets, provided an opportunity to examine a broader range of indicators, on the basis of additional analyses and screening, with the objective of identifying an indicator with the strongest and most consistent association with micronutrient adequacy. We took as a starting point the 9-group indicator noted above (hereafter denoted FGI-9). We then examined the contribution to micronutrient adequacy of various combinations of subgroups of the most disaggregated (21-group) indicator. On the basis of these contributions, we identified 4 disaggregations and 1 “re-aggregation” of the FGI-9 that might strengthen associations to micronutrient adequacy. The “re-aggregation” was to regroup “organ meat” with “all meat, poultry and fish” the 4 disaggregations were to count separately “other fruits” and “other vegetables,” “meat and poultry” and “fish,” “nuts and seeds” and “pulses,” and “grains” and “other starchy staples.” We also examined the percentages of WRA who had consumed various subgroups of these disaggregations and re-aggregations on the same day. Finally, we screened a series of 9-, 10-, 11-, and 12-group indicators with various combinations of food groups and with, and without, the 15-g minimum consumption criterion and examined whether they strengthened the association with micronutrient adequacy. In this article we report results for the 9-group indicator already in use (FGI-9) and compare it with a “best choice” 10-group indicator (FGI-10) (Table 1). The latter was selected after screening of the larger set because it maximized the trade-off between good performance in terms of association with micronutrient adequacy and alignment with nutrition messages with regard to good-quality diets. Full details on this second-phase analyses of all candidate indicators are provided in Martin-Prevel et al. (28). Both candidate indicators (FGI-9 and FGI-10) incorporate the 15-g minimum criterion.
TABLE 1.
Food groups as aggregated in 2 candidate indicators1
| 21 Most-disaggregated food groups | 9-Group indicator (FGI-9) | 10-Group indicator (FGI-10) |
| 1. Grains and grain products | 1. Grains, white roots and tubers, and plantains | 1. Grains, white roots and tubers, and plantains |
| 2. All other starchy staples | ||
| 3. Cooked dry beans and peas | 2. All legumes and nuts | 2. Pulses (beans, peas, and lentils) |
| 4. Soybeans and soy products | ||
| 5. Nuts and seeds | 3. Nuts and seeds | |
| 6. Milk and yogurt | 3. Dairy | 4. Dairy |
| 7. Cheese | ||
| 8. Organ meat | 4. Organ meat | 5. Meat, poultry, and fish |
| 9. Large or small wild or domesticated mammals, reptiles, and amphibians | 5. All other meat, poultry, and fish | |
| 10. Wild or domesticated birds | ||
| 11. Small fish eaten whole with bones | ||
| 12. Large whole fish, dried fish, shellfish, other seafood, and mollusks | ||
| 13. Insects and grubs | ||
| 14. Eggs | 6. Eggs | 6. Eggs |
| 15. Vitamin A–rich dark-green leafy vegetables | 7. Dark-green leafy vegetables | 7. Dark-green leafy vegetables |
| 16. Vitamin A–rich deep-yellow/orange/red vegetables | 8. Other vitamin A–rich fruit and vegetables | 8. Other vitamin A–rich fruit and vegetables |
| 17. Vitamin A–rich fruit | ||
| 18. Vitamin C–rich vegetables | 9. Other fruit and vegetables | 9. Other vegetables |
| 19. All other vegetables | ||
| 20. Vitamin C–rich fruit | 10. Other fruit | |
| 21. All other fruit | ||
FGI, food-group indicator.
Statistical analysis
Data were analyzed by using Stata version 12 (36), accounting for sample design characteristics as appropriate. Most statistical analyses were performed within data sets. P values <0.05 were considered significant for all tests. Data are presented as means ± SDs or medians (IQRs). Except for PA and MPA, statistics reflect observations from a single day (the first of 2 d in most sites, but the second of 3 observation days in BF1). We used Pearson's correlations and simple linear regressions to describe associations between FGIs and MPA, with and without controlling for energy, by using the best linear unbiased predictor of estimated usual energy intake. Regression diagnostics included assessment of normality of residuals and heteroscedasticity tests. Untransformed values of MPA are presented in descriptive Figure 1, and the transformed variable was used in correlation and regression analyses.
FIGURE 1.
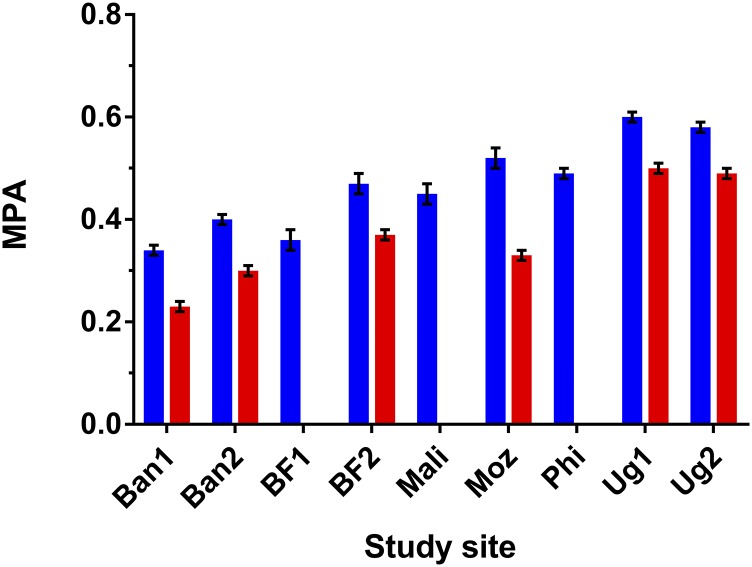
MPAs over 11 micronutrients for nonpregnant, nonlactating women (blue bars) and lactating women (red bars). In the BF1, Mali, and Phi sites there were <100 lactating women and no separate analysis was performed. Values are means ± SEs. Ban1, first site in rural Bangladesh; Ban2, second site in rural Bangladesh; BF1, Burkina Faso urban site (Ouagadougou); BF2, Burkina Faso rural site; Moz, rural site in Mozambique; MPA, mean probability of adequacy; Phi, site in peri-urban Cebu, Philippines; Ug1, rural Ugandan site; Ug2, urban and rural areas in Uganda.
We used receiver-operating characteristic analysis to assess FGI prediction of MPA >0.60. Although MPA theoretically ranges ≤1, which would be the desirable level of adequacy for all women, we estimated the MPA among a nationally representative sample of German women, who were assumed to have few economic constraints on access to a high-quality diet, and estimated an average MPA of 0.83. On the basis of this, a threshold of 0.70 would have been a reasonable choice to define a “positive” indicator (i.e., the level of adequacy to reach to consider it as satisfactory) in resource-poor contexts. In contrast, there is no basis for defining a “negative” indicator (i.e., the level under which adequacy is clearly unacceptable). The MPA cutoff of 0.60 was eventually selected because distribution of MPA in the samples did not allow the use of higher cutoffs and we judged a cutoff of 0.60 to be a reasonable cutoff for a “better” MPA. The AUC summarizes the predictive power of each indicator across all possible FGI cutoffs. We also assessed indicator characteristics in terms of sensitivity, specificity, and misclassification. For our purposes, unlike in some clinical applications, we did not aim to maximize only sensitivity or only specificity but looked for a balance between the 2, and hence considered the sum of them to minimize misclassification.
We assessed indicator performance separately for different physiologic groups because of the impact of elevated requirements on MPA, and because during pregnancy in particular it is extremely difficult to meet iron and folate requirements without supplements (11). Therefore, although we present descriptive results for all women, results comparing the performance of the 2 candidate indicators are presented separately for NPNL women in the main text and for lactating women in Supplemental Tables 2 and 3 when subsample sizes allowed (≥100 women); however, no site reported a sufficient number of pregnant women for separate analysis. Finally, for the selected “best candidate” dichotomous indicator (FGI-10), for descriptive purposes we performed weighted analyses across all sites to assess several characteristics of women's diets above and below the FGI cutoff of 5 food groups.
Results
General characteristics of women and their diets
Data were available for 4166 women, with sample size varying by site (Table 2). The median rate of exclusion from original samples due to implausible energy intake according to the Goldberg criteria was 11.2% [range: 0–18.0% for all data sets but one (Phi) in which the exclusion rate was 61.3%, which will be discussed later]. The proportion of lactating women was particularly high in some sites due to study designs targeting mothers of infants and young children. The average age of the women was similar across sites, but BMIs varied widely. Median energy intakes ranged from 1671 kcal in Phi to 2439 kcal in Ug1 (Table 2). Macronutrient intakes were imbalanced in Ban1, Ban2, and Moz, with carbohydrate intakes exceeding and fat intakes below the WHO ranges for population intake goals. Protein intakes slightly exceeded the WHO range in BF2 and in Phi. For NPNL women, MPAs ranged from 0.34 in Ban1 to 0.60 in Ug1, whereas corresponding values for lactating women were 0.23 and 0.50, respectively (Figure 1). PAs for specific micronutrients varied widely across sites. Looking across sites, the most consistent “problem nutrients” for NPNL women (with PAs <0.50 in at least half the sites) were riboflavin, folate, vitamin B-12, calcium, and iron (Table 3), whereas for lactating women 9 of 11 nutrients were “problem nutrients” (Supplemental Table 4).
TABLE 2.
Characteristics of women and their diets1
| Data set | n | General characteristics | Anthropometric measures | Dietary intakes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Repeated, 2n (%) | Pregnant, n (%) | Lactating, n (%) | Mean age, y | NPNL, n (%) | Mean height, cm | BMI (kg/m2) < 18.5, % | BMI ≥25.0, % | Energy,3 kcal | Carbohydrate, % | Protein, % | Fat, % | ||
| Ban1 | 412 | 148 (35.9) | 0 (0.0) | 111 (26.9) | 31.3 | 301 (73.1) | 150.3 | 47.8 | 1.7 | 2163 (746) | 84 | 10 | 6 |
| Ban2 | 422 | 397 (94.1) | 0 (0.0) | 221 (52.4) | 26.9 | 201 (47.8) | 149.9 | 36.3 | 4.5 | 1905 (691) | 82 | 10 | 8 |
| BF1 | 178 | 173 (97.2) | 13 (7.3) | 35 (19.7) | 31.1 | 130 (73.0) | 163.1 | 9.2 | 29.1 | 2176 (1105) | 67 | 10 | 22 |
| BF2 | 407 | 138 (33.9) | 45 (11.1) | 228 (56.0) | 31.2 | 134 (32.9) | 162.4 | 14.3 | 4.6 | 2185 (911) | 66 | 17 | 17 |
| Mali | 102 | 96 (94.1) | 0 (0.0) | 0 (0.0) | 31.4 | 102 (100) | 166.0 | 17.2 | 28.1 | 2019 (895) | 59 | 11 | 30 |
| Moz | 391 | 90 (23.0) | 52 (13.3) | 242 (61.9) | 28.8 | 97 (24.8) | 153.7 | 7.1 | 7.2 | 2029 (961) | 82 | 11 | 7 |
| Phi | 848 | 848 (100) | 37 (4.4) | 88 (10.4) | 30.8 | 723 (85.2) | 151.0 | 20.5 | 22.5 | 1671 (828) | 59 | 16 | 25 |
| Ug1 | 452 | 122 (27.0) | 57 (12.6) | 198 (43.8) | 32.4 | 197 (43.6) | 157.8 | 6.8 | 29.2 | 2439 (948) | 75 | 10 | 15 |
| Ug2 | 954 | 95 (10.0) | 0 (0.0) | 344 (36.1) | 28.5 | 610 (63.9) | — | — | — | 2298 (1239) | 74 | 10 | 16 |
After exclusion of women with reported energy intakes of either <0.9 times the estimated BMR or >3.0 times the BMR. Ban1, Bangladesh rural data set (1996); Ban2, Bangladesh rural data set (2008); BF1, Burkina Faso urban data set; BF2, Burkina Faso rural data set; BMR, basal metabolic rate; Mali, Mali urban data set; Moz, Mozambique rural data set; NPNL, nonpregnant and nonlactating; Phi, Philippines peri-urban data set; Ug1, Uganda rural data set; Ug2, Uganda urban and rural data set.
Women in the sample with more than one 24-h recall.
Values are medians (IQRs).
TABLE 3.
Probability of adequacy of individual micronutrients in each data set1
| Data set | Thiamin | Riboflavin | Niacin | Vit. B-6 | Folate | Vit. B-12 | Vit. C | Vit. A | Calcium | Iron | Zinc |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ban12 | 0.09 ± 0.23 | 0.15 ± 0.29 | 0.30 ± 0.32 | 0.82 ± 0.31 | 0.02 ± 0.10 | 0.20 ± 0.38 | 0.52 ± 0.46 | 0.53 ± 0.45 | 0.04 ± 0.16 | 0.10 ± 0.16 | 0.93 ± 0.19 |
| Ban22 | 0.62 ± 0.36 | 0.01 ± 0.05 | 1.00 ± 0.00 | 1.00 ± 0.01 | 0.00 ± 0.00 | 0.01 ± 0.12 | 0.74 ± 0.40 | 0.38 ± 0.44 | 0.00 ± 0.00 | 0.09 ± 0.14 | 0.60 ± 0.34 |
| BF1 | 0.45 ± 0.40 | 0.11 ± 0.24 | 0.19 ± 0.29 | 0.64 ± 0.39 | 0.15 ± 0.31 | 0.08 ± 0.27 | 0.66 ± 0.45 | 0.73 ± 0.39 | 0.03 ± 0.11 | 0.16 ± 0.18 | 0.77 ± 0.33 |
| BF2 | 0.61 ± 0.44 | 0.67 ± 0.40 | 0.79 ± 0.32 | 0.59 ± 0.42 | 0.36 ± 0.44 | 0.03 ± 0.17 | 0.33 ± 0.44 | 0.32 ± 0.40 | 0.18 ± 0.33 | 0.37 ± 0.28 | 0.95 ± 0.16 |
| Mali2 | 0.60 ± 0.38 | 0.28 ± 0.38 | 0.31 ± 0.38 | 0.67 ± 0.38 | 0.00 ± 0.00 | 0.17 ± 0.33 | 0.88 ± 0.28 | 0.50 ± 0.43 | 0.04 ± 0.12 | 0.53 ± 0.29 | 0.96 ± 0.14 |
| Moz | 0.68 ± 0.41 | 0.45 ± 0.42 | 0.49 ± 0.38 | 0.90 ± 0.26 | 0.45 ± 0.45 | 0.23 ± 0.42 | 0.90 ± 0.28 | 0.86 ± 0.31 | 0.01 ± 0.06 | 0.01 ± 0.03 | 0.76 ± 0.36 |
| Phi2 | 0.29 ± 0.40 | 0.23 ± 0.36 | 0.89 ± 0.24 | 0.74 ± 0.37 | 0.71 ± 0.38 | 0.84 ± 0.33 | 0.22 ± 0.40 | 0.60 ± 0.40 | 0.01 ± 0.08 | 0.23 ± 0.21 | 0.60 ± 0.38 |
| Ug1 | 0.91 ± 0.25 | 0.50 ± 0.44 | 0.83 ± 0.30 | 0.99 ± 0.06 | 0.53 ± 0.43 | 0.21 ± 0.39 | 0.98 ± 0.14 | 0.82 ± 0.34 | 0.05 ± 0.18 | 0.04 ± 0.08 | 0.76 ± 0.35 |
| Ug2 | 0.83 ± 0.33 | 0.65 ± 0.43 | 0.76 ± 0.36 | 0.89 ± 0.29 | 0.76 ± 0.38 | 0.04 ± 0.18 | 0.87 ± 0.33 | 0.85 ± 0.34 | 0.06 ± 0.20 | 0.07 ± 0.14 | 0.61 ± 0.42 |
Values are means ± SDs in NPNL women. Sample sizes are the same as those given in Table 2 (“NPNL” column). Ban1, Bangladesh rural data set (1996); Ban2, Bangladesh rural data set (2008); BF1, Burkina Faso urban data set; BF2, Burkina Faso rural data set; Mali, Mali urban data set; Moz, Mozambique rural data set; NPNL, nonpregnant and nonlactating; Phi, Philippines peri-urban data set; Ug1, Uganda rural data set; Ug2, Uganda urban and rural data set; Vit., vitamin.
Using medium bioavailability for iron and zinc; otherwise, low bioavailability.
Correlation of indicators with MPA and indicator performance
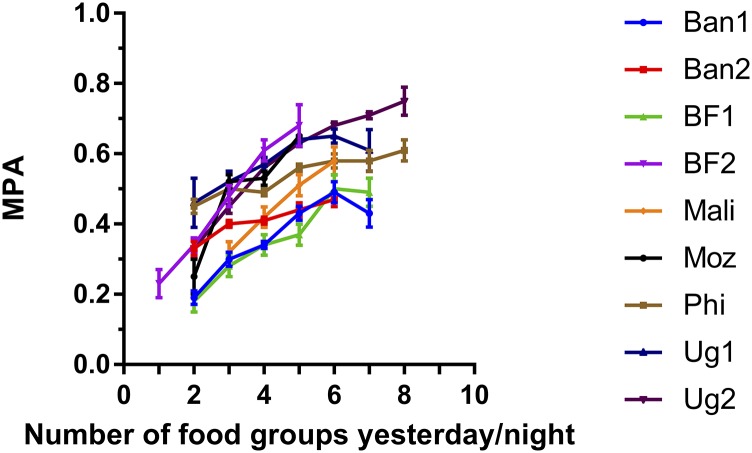
Both the 9- and the 10-group indicators were significantly correlated to MPA at each site (all P < 0.001) (Table 4). Correlations ranged from 0.25 to 0.56 for NPNL women and from 0.26 to 0.52 for lactating women (Supplemental Table 2). In models that controlled for energy intake, correlations were attenuated in most sites but remained significant. Figure 2 shows the association between FGI-10 and MPA for NPNL women when not controlling for energy intake, and shows an increase in MPA as the number of food groups increases. Results for lactating women (Supplemental Figure 1) and results for FGI-9 (data not shown) were visually similar.
TABLE 4.
Linear correlations of FGIs with MPA and AUCs for an MPA cutoff of 0.60 in NPNL women1
| FGI-9 | FGI-10 | FGI-9 | FGI-10 | Women reaching the MPA cutoff, n (%) | |||
|---|---|---|---|---|---|---|---|
| Data set | NC | C | NC | C | AUC | AUC | |
| Ban1 | 0.51*** | 0.45*** | 0.50*** | 0.44*** | 0.818 | 0.811 | 19 (6.4) |
| Ban2 | 0.34*** | 0.32*** | 0.36*** | 0.33*** | 0.673 | 0.695 | 4 (2.0) |
| BF1 | 0.41*** | 0.38*** | 0.44*** | 0.39*** | 0.709 | 0.702 | 16 (12.2) |
| BF2 | 0.46*** | 0.40*** | 0.55*** | 0.46*** | 0.743* | 0.794* | 41 (31.3) |
| Mali | 0.47*** | 0.49*** | 0.45*** | 0.48*** | 0.710 | 0.700 | 21 (20.6) |
| Moz | 0.42*** | 0.26** | 0.42*** | 0.31** | 0.659* | 0.679* | 42 (40.8) |
| Phi | 0.26*** | 0.25*** | 0.25*** | 0.23*** | 0.624 | 0.617 | 248 (34.3) |
| Ug1 | 0.27*** | 0.32*** | 0.31*** | 0.29*** | 0.620* | 0.669* | 112 (56.9) |
| Ug2 | 0.49*** | 0.29*** | 0.56*** | 0.31*** | 0.729*** | 0.768*** | 336 (55.1) |
Sample sizes are the same as those given in Table 2 (“NPNL” column). FGI-9 and FGI-10 are FGIs summing 9 and 10 food groups, respectively. FGI scores are from 1 observation day only. *P < 0.05; **P < 0.01; ***P < 0.001; for NC and C these indications refer to P values for tests of the significance of the Pearson's correlation between the indicator and MPA; for AUC these indications refer to P values for tests of the significance of differences between AUCs for the 2 indicators. Note that for the Philippines site, the difference in AUC was 0.007 but was significant due to the large sample size. Ban1, Bangladesh rural data set (1996); Ban2, Bangladesh rural data set (2008); BF1, Burkina Faso urban data set; BF2, Burkina Faso rural data set; C, controlled for energy intake; FGI, food-group indicator; Mali, Mali urban data set; Moz, Mozambique rural data set; MPA, mean probability of adequacy; NC, not controlled for energy intake; NPNL, nonpregnant and nonlactating; Phi, Philippines peri-urban data set; Ug1, Uganda rural data set; Ug2, Uganda urban and rural data set.
FIGURE 2.
Associations between 10-food-group diversity score (FGI-10) and MPAs averaged across 11 micronutrients for nonpregnant, nonlactating women. Error bars represent ± SEMs. Data points representing <5 women are not shown. Ban1, first site in rural Bangladesh; Ban2, second site in rural Bangladesh; BF1, Burkina Faso urban site (Ouagadougou); BF2, Burkina Faso rural site; FGI, food-group indicator; Moz, rural site in Mozambique; MPA, mean probability of adequacy; Phi, site in peri-urban Cebu, Philippines; Ug1, rural Ugandan site; Ug2, urban and rural areas in Uganda.
AUCs were moderate, ranging from 0.62 to 0.82 for NPNL women (Table 4). AUCs significantly differed between the 2 indicators in 6 sites, but differences were small. AUCs were 0.02–0.05 higher for FGI-10 in 4 sites and ≤0.01 higher for FGI-9 in 2 sites. For lactating women, AUCs ranged from 0.56 to 0.90 and comparisons between the 2 indicators showed a significant difference in only 1 of 6 sites, favoring FGI-10 (difference of 0.05) (Supplemental Table 2).
In sensitivity and specificity analyses, we evaluated indicator performance in detecting MPAs >0.60 with the food-group cutoff of ≥5 food groups, because this cutoff provided the best balance between sensitivity and specificity across sites for both FGI-9 and FGI-10 (results not shown). Sensitivity and specificity results (Table 5) also showed moderate performance. Considering the sum of sensitivity and specificity, differences between the indicators were small (<5.0) in 4 sites, but in the remaining 5 sites the large differences were all in favor of FGI-10. Positive predictive value was similar between the 2 indicators; the 2 large differences were also in favor of FGI-10. For lactating women, considering the sum of sensitivity and specificity, results again favored FGI-10. For positive predictive value, most differences were very small, but 1 large difference favored each indicator (Supplemental Table 3).
TABLE 5.
Comparison of performance of 2 indicators in predicting an MPA >0.60 in NPNL women1
| Women reaching the cutoff of ≥5 food groups, n (%) | Se | Sp | Se + Sp | FGI10 − FGI9 (Se + Sp) | PPV | FGI10 − FGI9 (PPV) | |
|---|---|---|---|---|---|---|---|
| Ban1 | |||||||
| FGI-9 | 63 (21) | 68.4 | 82.3 | 150.7 | 3.6 | 20.6 | −2.7 |
| FGI-10 | 84 (28) | 78.9 | 75.4 | 154.3 | 17.9 | ||
| Ban2 | |||||||
| FGI-9 | 37 (18) | 0.0 | 81.2 | 81.2 | 21 | 0.0 | 2.2 |
| FGI-10 | 46 (23) | 25.0 | 77.2 | 102.2 | 2.2 | ||
| BF1 | |||||||
| FGI-9 | 56 (43) | 62.5 | 59.6 | 122.1 | −4.3 | 17.9 | −1.5 |
| FGI-10 | 61 (47) | 62.5 | 55.3 | 117.8 | 16.4 | ||
| BF2 | |||||||
| FGI-9 | 2 (1) | 2.4 | 98.9 | 101.3 | 11.1 | 50.0 | 25 |
| FGI-10 | 8 (6) | 14.6 | 97.8 | 112.4 | 75.0 | ||
| Mali | |||||||
| FGI-9 | 43 (42) | 66.7 | 64.2 | 130.9 | −2.5 | 32.6 | −1.5 |
| FGI-10 | 45 (44) | 66.7 | 61.7 | 128.4 | 31.1 | ||
| Moz | |||||||
| FGI-9 | 9 (9) | 14.3 | 95.1 | 109.4 | 20.8 | 66.7 | 16.6 |
| FGI-10 | 18 (17) | 35.7 | 94.5 | 130.2 | 83.3 | ||
| Phi | |||||||
| FGI-9 | 307 (42) | 58.1 | 65.7 | 123.8 | −2.7 | 46.9 | −1.4 |
| FGI-10 | 308 (42) | 56.5 | 64.6 | 121.1 | 45.5 | ||
| Ug1 | |||||||
| FGI-9 | 62 (31) | 37.5 | 76.5 | 114 | 14.2 | 67.7 | 1.7 |
| FGI-10 | 108 (55) | 67.0 | 61.2 | 128.2 | 69.4 | ||
| Ug2 | |||||||
| FGI-9 | 226 (25) | 50.3 | 79.2 | 129.5 | 11.1 | 75.2 | 0.8 |
| FGI-10 | 317 (52) | 69.5 | 71.1 | 140.6 | 76.0 |
Sample sizes are the same as those given in Table 2 (“NPNL” column). FGI-9 and FGI-10 are FGIs summing 9 and 10 food groups, respectively. The analyses of sensitivity and specificity used a threshold of ≥5 food groups for both FGI-9 and FGI-10. Ban1, Bangladesh rural data set (1996); Ban2, Bangladesh rural data set (2008); BF1, Burkina Faso urban data set; BF2, Burkina Faso rural data set; FGI, food-group indicator; Mali, Mali urban data set; Moz, Mozambique rural data set; MPA, mean probability of adequacy; NPNL, nonpregnant and nonlactating; Phi, Philippines peri-urban data set; PPV, positive predictive value; Se, sensitivity (which, in this context, means the proportion of all those who truly have better MPA who are identified by the indicator); Sp, specificity (which, in this context, means the proportion of those who truly have a lower MPA who are identified by the indicator); Ug1, Uganda rural data set; Ug2, Uganda urban and rural data set.
Proportion of women consuming ≥5 food groups and comparison of diets above and below this cutoff
For FGI-10, the proportion of NPNL women who consumed ≥5 food groups varied from 6% (BF2) to >50% in the 2 Ugandan sites. In weighted analyses summarizing across all sites, NPNL women who achieved the cutoff of ≥5 food groups had an MPA of 0.55, compared with 0.39 for women who consumed ≤4 food groups. In relative terms, the group of women above the cutoff had an MPA ∼40% higher than those below the cutoff. Women who consumed ≥5 of the FGI-10 food groups were highly likely to have consumed ≥1 animal-source food (84%); legumes, nuts, or seeds (84%); and ≥2 distinct fruit and vegetable groups (98%).
Discussion
Simple indicators that reflect diet quality are needed for global assessment, monitoring, and advocacy, and dietary diversity is one necessary—although not sufficient—component of diet quality. We previously showed reasonably strong and consistent associations between food-group diversity scores and the probability of micronutrient adequacy for WRA (1), but for certain policy and programmatic uses, the demand for a dichotomous indicator capturing “minimum dietary diversity” for WRA has increased. We report here on efforts to meet this demand.
Building on previous work, we extended analysis to a larger number of data sets and considered additional candidate indicators that aggregated food groups differently. Balancing performance and complexity, we narrowed the selection to 2 candidate indicators, one reflecting a 9-food-group score that had already been adopted by some groups and a second, new 10-food-group indicator, both with a cutoff of 5 food groups for indicating “minimum dietary diversity.” The 2 indicators, FGI-9 and FGI-10, performed similarly on several criteria, but the sensitivity and specificity analysis favored FGI-10. There was inevitably some subjectivity attached to the choice of presenting results for only these 2 “best choice” candidate indicators and not for the full set tested. However, we judged it useful to include FGI-9 because the underlying 9-point score was already in fairly wide use and we further selected FGI-10 because it showed performance equal to or better than all other candidates. In addition, although these types of indicators should not be equated with or used to generate dietary guidance, we considered alignment with guidance as a qualitative criterion for evaluating candidate indicators. We considered that by separating nuts and seeds from pulses, and by splitting “other fruit and vegetables” into 2 groups, FGI-10 aligns better with current priorities for improving diets through increased intakes of these food groups (37–39).
The full set of analyses and other considerations were presented to a group of academic experts and other key stakeholders in a meeting to reach consensus on a global dietary diversity indicator for women (40). Participants unanimously selected FGI-10 with a cutoff of ≥5 food groups, and affirmed its usefulness for population-level assessment, advocacy, and possibly tracking (41). In parallel with the WHO “minimum dietary diversity” indicator for infants and young children (42), the indicator name “minimum dietary diversity for women of reproductive age” (MDD-W) was adopted, and subsequently, a manual for operationalizing the indicator was developed and published (43). Page 2 of this manual gives a clear definition of the indicator, indicating how the cutoff of 5 food groups should be interpreted: “The proportion of women 15–49 years of age who reach this minimum in a population can be used as a proxy indicator for higher micronutrient adequacy.”
Our analysis and the resulting indicator have several limitations. First, although efforts were made to obtain data sets from urban, rural, and geographically diverse contexts, the 6 African and 3 Asian data sets do not represent all global diet patterns. We acknowledge that most of the studies were not performed in samples that were representative of the whole population living in each particular country or context, but this was deemed not to be a strong limitation for the main purpose of our analysis. In addition, all samples involved randomization and some checking was performed to verify that they correctly represented typical situations of each context. Because the study sites were generally in resource-poor areas, however, the indicator may be most relevant in similar settings. In one case (Phi), the rate of exclusion from the original sample was very high, because reported energy intakes were very low. This case was carefully considered by the study team and it was eventually kept in the analysis because representativeness was not the main concern for the primary purpose of this work. In addition, because the shape of the distributions of energy and micronutrient intakes, PAs, and FGIs is very similar in the analysis subsample and the full sample, we judged that it would not bias the results. More details can be found in chapter 6 of Martin-Prevel et al. (28). Second, in our analysis, the FGIs were derived from the quantitative 24-h recall and weighed-record data, whereas in real-life applications, the MDD-W will be derived from simple qualitative food-group recalls, which tend to be less accurate, in particular because it is more difficult to apply the 15-g minimum criterion (44). Third, the indicator was developed specifically for situations in which simplicity during data collection and processing is a very high priority and where, for example, it is not considered feasible to collect multiple days of recalls or information on quantities consumed. All diet indicators based on a single day of recall lack precision due to normal day-to-day variability in intakes, and all recall-based indicators are subject to measurement error and possibly bias (45). In addition, lack of information on quantities means that there is inevitable variability in the association between MDD-W and the underlying dimension for which it is a proxy, micronutrient adequacy. These sources of variability, added to the variability in the study contexts, explain why there is substantial variation in the performance of the dichotomous score. They also contribute to the moderate sensitivity and specificity of the indicator, and make it poorly suited for many research applications, because associations (e.g., to predictors or outcomes) will be attenuated (46). Finally, we were not able to assess the performance of the candidate indicators for pregnant women.
Despite these limitations and the resulting issue of attenuation, similar simple, recall-based FGIs have shown meaningful associations with micronutrient adequacy (47), household food security (48), season (49), and women's decision-making autonomy (50) and have shown responsiveness—for example, to changes in food prices (51) and to interventions (52). When measured repeatedly across pregnancy, a simple FGI was prospectively associated with maternal anemia and birth outcomes (53). Because the MDD-W itself is a new indicator, there are no published studies reporting on its use as a dichotomous indicator. However, several of the studies just noted used food-group scores similar to the underlying 10-point score. The use of the 10-point score may be preferred both in research and in some programmatic contexts when, for example, a baseline measure shows extremely low diversity, such that improvements will not be readily detected with an indicator of consumption of ≥5 food groups.
The MDD-W is best suited for the purpose for which it was designed, which is population-level assessment and possibly tracking of change across time in one critical dimension of diet quality. For this purpose, the MDD-W was proposed as 1 of the 8 priority indicators for measuring progress in actions to improve nutrition and other development outcomes in the framework of the Sustainable Development Goals (54). According to the conclusions of a consensus meeting of experts, the indicator is also suited for use in advocacy with diverse audiences, and particularly in settings in which presentation of a mean food-group score would not serve communication needs. In these contexts, it may begin to fill a gap in very simple, yet valid indicators of diet quality, particularly in the poorest population groups.
Supplementary Material
Acknowledgments
In addition to the current authors, the WDDP study group includes: Elodie Becquey (International Food Policy Research Institute), Inge D Brouwer (Wageningen University), Alicia Carriquiry (Iowa State University), Melissa C Daniels (University of North Carolina at Chapel Hill), Nadia Fanou-Fogny (Université d'Abomey Calavi), Elaine Ferguson (London School of Hygiene and Tropical Medicine), Maria L Joseph (General Dynamics Information Technology), Marie T Ruel (International Food Policy Research Institute), and Liv Elin Torheim (Akershus University College).
We thank participants at the “FAO Working Group Meeting on Deriving a Global Indicator for Defining Adequacy of Women's Diets” held in Rome, 19–20 March 2014: Catherine Leclercq (FAO), Queenie Mak (FAO), Astrid Mathiassen [World Food Programme (WFP)], Giorgia Fiorella Nicolò (FAO), Verena Nowak (FAO), Marian Amaka Odenigbo [International Fund for Agricultural Development (IFAD)], Viviana Panetti (FAO), and Michele Rude (FAO). The authors also thank people who attended the “Meeting to Reach Consensus on a Global Dietary Diversity Indicator for Women” held in Washington, DC, 15–16 July 2014: Jennifer Coates (Tufts University), Katherine Dennison (USAID), Leslie Elder (World Bank), Agnès Guyon (The Strengthening Partnerships, Results, and Innovations in Nutrition Globally project), Anna Herforth (consultant), Patrick Hoang-Vu Eozenou (World Bank), Elizabeth Jordan-Bell (USAID), Lynnda Kiess (WFP), Monica Kothari (Demographic and Health Survey/PATH), Julia Krasevec (UNICEF), Jef Leroy (International Food Policy Research Institute), Chessa Lutter (Pan American Health Organization), Lisa Maniscalco (USAID), Lynnette Neufeld (Global Alliance for Improved Nutrition), Marian Amaka Odenigbo (IFAD), Anne Peniston (USAID), Abigail Perry (Department For International Development), Ellen Piwoz (Bill & Melinda Gates Foundation), Sandra Remancus (Food And Nutrition Technical Assistance), Anne Swindale (USAID), Nadra Franklin (meeting facilitator), Zeina Maalouf-Manasseh (notes), and Juliana Arias Kluge and Gianluca Bangara (assistants). The authors' responsibilities were as follows—YM-P, MA, GK, TJB, MCD, MM, MD, and DW: developed the research concept and, together with PA, WTKL, and AL, designed the analysis plan; PA and DW: analyzed data under the guidance of YM-P; MA and YM-P: wrote the manuscript and had primary responsibility for the final content; and all authors: contributed to the interpretation of results, were involved in the analyses and had access to the data, had final responsibility for the decision to submit for publication, commented on drafts, and read and approved the final manuscript. Other members of the WDDP study group took part in data analysis and interpretation of results.
Abbreviations
- Ban1
data collected in rural Bangladesh in 1996
- Ban2
data collected in rural Bangladesh in 2008
- BF1
data collected in Ouagadougou, Burkina Faso, in 2006
- BF2
data collected in rural Burkina Faso in 2010
- EAR
Estimated Average Requirement
- FGI
food group indicator
- Mali
data collected in Bamako, Mali, in 2007
- MDD-W
minimum dietary diversity for women of reproductive age
- Moz
data collected in rural Mozambique in 2006 MPA, mean probability of adequacy
- NPNL
nonpregnant and nonlactating
- PA
probability of adequacy
- Phi
data collected in peri-urban Cebu, Philippines, in 2005
- Ug1
data collected in rural Uganda in 2007
- Ug2
data collected in urban and rural Uganda in 2008
- WDDP
Women's Dietary Diversity Project
- WRA
women of reproductive age
Contributor Information
Women's Dietary Diversity Project (WDDP) Study Group:
Yves Martin-Prevel, Mary Arimond, Pauline Allemand, Doris Wiesmann, Terri J Ballard, Megan Deitchler, Marie Claude Dop, Gina Kennedy, Anna Lartey, Warren TK Lee, and Mourad Moursi
References
- 1. Arimond M, Wiesmann D, Becquey E, Carriquiry A, Daniels MC, Deitchler M, Fanou-Fogny N, Joseph ML, Kennedy G, Martin-Prevel Y, et al. Simple food group diversity indicators predict micronutrient adequacy of women's diets in 5 diverse, resource-poor settings. J Nutr 2010;140(Suppl):2059S–69S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee SE, Talegawkar SA, Merialdi M, Caulfield LE.. Dietary intakes of women during pregnancy in low- and middle-income countries. Public Health Nutr 2013;16:1340–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Torheim LE, Ferguson EL, Penrose K, Arimond M.. Women in resource-poor settings are at risk of inadequate intakes of multiple micronutrients. J Nutr 2010;140(Suppl):2051S–8S. [DOI] [PubMed] [Google Scholar]
- 4. Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorell R, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013;382:427–51. [DOI] [PubMed] [Google Scholar]
- 5. Branca F, Piwoz E, Schultink W, Sullivan LM.. Nutrition and health in women, children, and adolescent girls. BMJ 2015;351:h4173. [DOI] [PubMed] [Google Scholar]
- 6. WHO. Essential nutrition actions: improving maternal, newborn, infant and young child health and nutrition. WHO guidelines approved by the guidelines review committee. Geneva (Switzerland): WHO; 2013. [PubMed] [Google Scholar]
- 7. Ruel MT, Alderman H.. Nutrition-sensitive interventions and programmes: how can they help to accelerate progress in improving maternal and child nutrition? Lancet 2013;382:536–51. [DOI] [PubMed] [Google Scholar]
- 8. McCullough ML.. Diet patterns and mortality: common threads and consistent results. J Nutr 2014;144:795–6. [DOI] [PubMed] [Google Scholar]
- 9. FAO. Food-based dietary guidelines [Internet]. [cited 2017 Feb 28]. Available from: http://www.fao.org/nutrition/nutrition-education/food-dietary-guidelines/en/.
- 10. WHO. Healthy diet—fact sheet N°394 [Internet]. [cited 2017 Feb 28]. Available from: http://www.who.int/mediacentre/factsheets/fs394/en/.
- 11. WHO. WHO recommendations on antenatal care for a positive pregnancy experience. Geneva (Switzerland): WHO; 2016. [PubMed] [Google Scholar]
- 12. Ruel MT.. Operationalizing dietary diversity: a review of measurement issues and research priorities. J Nutr 2003;133(11Suppl 2):3911S–26S. [DOI] [PubMed] [Google Scholar]
- 13. FAO. Guidelines for measuring household and individual dietary diversity. Rome (Italy): FAO; 2013. Available from: http://www.fao.org/3/a-i1983e.pdf. [Google Scholar]
- 14. US Agency for International Development. Multi-sectoral nutrition strategy 2014-2025. Washington (DC): US Agency for International Development; 2014. [Google Scholar]
- 15. Arimond M, Torheim L, Wiesmann D, Joseph M, Carriquiry A.. Dietary diversity as a measure of the micronutrient adequacy of women's diets: results from rural Bangladesh Site [Internet]. 2009. [cited 2016 Nov 20]. Available from: http://www.fantaproject.org/research/womens-dietary-diversity-project.
- 16. Arsenault JE, Yakes EA, Hossain MB, Islam MM, Ahmed T, Hotz C, Lewis B, Rahman AS, Jamil KM, Brown KH.. The current high prevalence of dietary zinc inadequacy among children and women in rural Bangladesh could be substantially ameliorated by zinc biofortification of rice. J Nutr 2010;140:1683–90. [DOI] [PubMed] [Google Scholar]
- 17. Becquey E, Capon G, Martin-Prevel Y.. Dietary diversity as a measure of the micronutrient adequacy of women's diets: results from Ouagadougou, Burkina Faso Site [Internet]. 2009. [cited 2016 Nov 20]. Available from: http://www.fantaproject.org/research/womens-dietary-diversity-project.
- 18. Daniels M.. Dietary diversity as a measure of the micronutrient adequacy of women's diets: results from metropolitan Cebu, Philippines Site [Internet]. 2009. [cited 2016 Nov 20]. Available from: http://www.fantaproject.org/research/womens-dietary-diversity-project.
- 19. Harvey P, Rambeloson Z, Dary O.. The 2008 Uganda food consumption survey: determining the dietary patterns of Ugandan women and children. The USAID Micronutrient and Child Blindness Project [Internet]. 2010. [cited 2016 Nov 20]. Available from: https://www.spring-nutrition.org/publications/projects/a2z/2008-uganda-food-consumption-survey-determining-dietary-patterns-ugandan.
- 20. Hotz C, Loechl C, de Brauw A, Eozenou P, Gilligan D, Moursi M, Munhaua B, van Jaarsveld P, Carriquiry A, Meenakshi JV.. A large-scale intervention to introduce orange sweet potato in rural Mozambique increases vitamin A intakes among children and women. Br J Nutr 2012;108:163–76. [DOI] [PubMed] [Google Scholar]
- 21. Hotz C, Loechl C, Lubowa A, Tumwine JK, Ndeezi G, Nandutu Masawi A, Baingana R, Carriquiry A, de Brauw A, Meenakshi JV, et al. Introduction of beta-carotene-rich orange sweet potato in rural Uganda resulted in increased vitamin A intakes among children and women and improved vitamin A status among children. J Nutr 2012;142:1871–80. [DOI] [PubMed] [Google Scholar]
- 22. Kennedy G, Fanou N, Seghieri C, Brouwer I.. Dietary diversity as a measure of the micronutrient adequacy of women's diets: results from Bamako, Mali site [Internet]. 2009. [cited 2016 Nov 20]. Available from: http://www.fantaproject.org/research/womens-dietary-diversity-project.
- 23. Martin-Prevel Y, Allemand P, Nikiema L, Ayassou KA, Ouedraogo HG, Moursi M, De Moura FF.. Biological status and dietary intakes of iron, zinc and vitamin A among women and preschool children in rural Burkina Faso. PLoS One 2016;11:e0146810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gibson RS, Ferguson EL.. An interactive 24-hour recall for assessing the adequacy of iron and zinc intakes of developing countries. Washington (DC): US Agency for International Development and International Life Sciences Insitute; 1999. [Google Scholar]
- 25. Gibson RS, Ferguson EL.. An interactive 24-hour recall for assessing the adequacy of iron and zinc intakes in developing countries. HarvestPlus Technical Monograph Series 8. Washington (DC), Cali (Colombia): International Food Policy Research Institute (IFPRI) and International Center for Tropical Agriculture (CIAT); 2008. [Google Scholar]
- 26. Raper N, Perloff B, Ingwersen L, Steinfeldt L, Anand J.. An overview of USDA's dietary intake data system. J Food Compos Anal 2004;17:546–55. [Google Scholar]
- 27. Goldberg GR, Black AE, Jebb SA, Cole TJ, Murgatroyd PR, Coward WA, Prentice AM.. Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur J Clin Nutr 1991;45:569–81. [PubMed] [Google Scholar]
- 28. Martin-Prevel Y, Allemand P, Wiesmann D, Arimond M, Ballard T, Deitchler M, Dop M, Kennedy G, Lee W, Moursi M.. Moving forward on choosing a standard operational indicator of women's dietary diversity. Rome (Italy): FAO; 2015. [Google Scholar]
- 29. Allen LH.. Multiple micronutrients in pregnancy and lactation: an overview. Am J Clin Nutr 2005;81(Suppl):1206S–12S. [DOI] [PubMed] [Google Scholar]
- 30. Bartley KA, Underwood BA, Deckelbaum RJ.. A life cycle micronutrient perspective for women's health. Am J Clin Nutr 2005;81:1188S–93S. [DOI] [PubMed] [Google Scholar]
- 31. Institute of Medicine. Dietary Reference Intakes: applications in dietary assessment. Washington (DC): National Academies Press; 2000. [PubMed] [Google Scholar]
- 32. Barr SI, Murphy SP, Poos MI.. Interpreting and using the dietary references intakes in dietary assessment of individuals and groups. J Am Diet Assoc 2002;102:780–8. [DOI] [PubMed] [Google Scholar]
- 33. WHO; FAO. Human vitamin and mineral requirements. Report of a joint FAO and WHO expert consultation held in Bangkok, Thailand, 21-30 September 1998. 2nd ed.Geneva (Switzerland): WHO; 2004. [Google Scholar]
- 34. Institute of Medicine. Dietary Reference Intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington (DC): National Academies Press; 2000. [PubMed] [Google Scholar]
- 35. Brown KH, Rivera JA, Bhutta Z, Gibson RS, King JC, Lonnerdal B, Ruel MT, Sandtrom B, Wasantwisut E, Hotz C; International Zinc Nutrition Consultative Group . Assessment of the risk of zinc deficiency in populations and options for its control. IZiNCG Technical Document 1. Food Nutr Bull 2004;25(1Suppl 2):S99–203. [PubMed] [Google Scholar]
- 36. StataCorp. Stata statistical software: release 12. College Station (TX): StataCorp LP; 2011. [Google Scholar]
- 37. Imamura F, Micha R, Khatibzadeh S, Fahimi S, Shi P, Powles J, Mozaffarian D.. Dietary quality among men and women in 187 countries in 1990 and 2010: a systematic assessment. Lancet Glob Health 2015;3:e132–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miller V, Yusuf S, Chow CK, Dehghan M, Corsi DJ, Lock K, Popkin B, Rangarajan S, Khatib R, Lear SA, et al. Availability, affordability, and consumption of fruits and vegetables in 18 countries across income levels: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet Glob Health 2016;4:e695–703. [DOI] [PubMed] [Google Scholar]
- 39. WHO. Diet, nutrition and the prevention of chronic diseases. Report of a joint WHO and FAO expert consultation held in Geneva, 2002. World Health Organ Tech Rep 2003;916:139#x201340. [PubMed]
- 40. Food and Nutrition Technical Assistance (FANTA III). Consensus meeting on a global indicator to measure women's dietary diversity [Internet]. 2015. [cited 2017 Feb 28]. Available from: http://www.fantaproject.org/news-and-events/2014-consensus-meeting-on-mddw.
- 41. FAO. Summary report of a meeting to reach consensus on a global dietary diversity indicator for women. Rome (Italy): FAO; 2014. Available from: https://www.fantaproject.org/sites/default/files/resources/WDDP-Meeting-Report-Oct2014.pdf. [Google Scholar]
- 42. WHO. Indicators for assessing infant and young child feeding practices: conclusions of a consensus meeting held 6–8 November 2007 in Washington D.C., USA. Geneva (Switzerland): WHO; 2008. [Google Scholar]
- 43. FAO. FHI 360. Minimum dietary diversity for women: a guide for measurement [Internet].2016. [cited 2017 Feb 28]. Available from: http://www.fao.org/3/a-i5486e.pdf.
- 44. Martin-Prevel Y, Becquey E, Arimond M.. Food group diversity indicators derived from qualitative list-based questionnaire misreported some foods compared to same indicators derived from quantitative 24-hour recall in urban Burkina Faso. J Nutr 2010;140(Suppl):2086S–93S. [DOI] [PubMed] [Google Scholar]
- 45. Gibson RS.. Principles of nutritional assessment. 2nd ed.New York: Oxford University Press; 2005. [Google Scholar]
- 46. Agogo GO, van der Voet H, van't Veer P, Ferrari P, Leenders M, Muller DC, Sanchez-Cantalejo E, Bamia C, Braaten T, Knuppel S, et al. Use of two-part regression calibration model to correct for measurement error in episodically consumed foods in a single-replicate study design: EPIC case study. PLoS One 2014;9:e113160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Henjum S, Torheim LE, Thorne-Lyman AL, Chandyo R, Fawzi WW, Shrestha PS, Strand TA.. Low dietary diversity and micronutrient adequacy among lactating women in a peri-urban area of Nepal. Public Health Nutr 2015;18:3201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Na M, Mehra S, Christian P, Ali H, Shaikh S, Shamim AA, Labrique AB, Klemm RD, Wu LS, West KP Jr.. Maternal dietary diversity decreases with household food insecurity in rural Bangladesh: a longitudinal analysis. J Nutr 2016;146:2109–16. [DOI] [PubMed] [Google Scholar]
- 49. Stevens B, Watt K, Brimbecombe J, Clough A, Judd J, Lindsay D.. The role of seasonality on the diet and household food security of pregnant women living in rural Bangladesh: a cross-sectional study. Public Health Nutr 2017;20:121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Amugsi DA, Lartey A, Kimani E, Mberu BU.. Women's participation in household decision-making and higher dietary diversity: findings from nationally representative data from Ghana. J Health Popul Nutr 2016;35:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Martin-Prevel Y, Becquey E, Tapsoba S, Castan F, Coulibaly D, Fortin S, Zoungrana M, Lange M, Delpeuch F, Savy M.. The 2008 food price crisis negatively affected household food security and dietary diversity in urban Burkina Faso. J Nutr 2012;142:1748–55. [DOI] [PubMed] [Google Scholar]
- 52. Olney DK, Bliznashka L, Pedehombga A, Dillon A, Ruel MT, Heckert JA.. 2-Year integrated agriculture and nutrition program targeted to mothers of young children in Burkina Faso reduces underweight among mothers and increases their empowerment: a cluster-randomized controlled trial. J Nutr 2016;146:1109–17. [DOI] [PubMed] [Google Scholar]
- 53. Zerfu TA, Umeta M, Baye K.. Dietary diversity during pregnancy is associated with reduced risk of maternal anemia, preterm delivery, and low birth weight in a prospective cohort study in rural Ethiopia. Am J Clin Nutr 2016;103:1482–8. [DOI] [PubMed] [Google Scholar]
- 54. UN Standing Committee on Nutrition. Priority nutrition indicators for the post-2015 sustainable development goals: a policy brief (2014) [Internet]. [cited 2016 Dec 27]. Available from: https://www.unscn.org/files/Publications/Policy_brief_Priority_Nutrition_Indicators_for_the_Post-2015_SDGs.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.