Abstract
Suicide is a major global problem, claiming more than 800,000 lives annually. The neurobiological changes that underlie suicidal ideation and behavior are not fully understood. Suicidal patients have been shown to display elevated levels of inflammation both in the central nervous system and the peripheral blood. A growing body of evidence suggests that inflammation is associated with a dysregulation of the kynurenine pathway in suicidal patients, resulting in an imbalance of neuroactive metabolites. Specifically, an increase in the levels of the NMDA receptor agonist quinolinic acid and a simultaneous decrease in neuroprotective metabolites have been observed in suicidal patients, and may contribute to the development of suicidality via changes in glutamate neurotransmission and neuroinflammation. The cause of the dysregulation of kynurenine metabolites in suicidality is not known, but is likely due to differential activity of the involved enzymes in patients. As knowledge in these areas is rapidly growing, targeting the kynurenine pathway enzymes may provide novel therapeutic approaches for managing suicidal behavior.
Keywords: Inflammation, kynurenine pathway, quinolinic acid, glutamate, cytokine, depression
1. Introduction
Suicide is the 14th cause of years of life lost (Mortality and Causes of Death, 2015) with over 800,000 deaths reported globally every year (World Health Organization, 2014). This high death toll is still likely to be under-reported, due to the fact that suicide is stigmatized and illegal in some countries (Varnik, 2012). It is estimated that suicide attempts are 10-20 times more frequent than the number of completed suicides. Both suicide attempts and deaths by suicide result in a great psychological and economic burden for individuals, families and countries. The World Health Organization has recently declared suicide a major public health problem worldwide (World Health Organization, 2014). In the US alone, the economic cost of death by suicide is estimated to be more than $44 billion annually (Centers for Disease Control and Prevention, 2010).
Despite an increase in available clinical treatment options for suicidality, including pharmacological agents as well as electroconvulsive treatment, the incidence rate of suicide is increasing in many countries (World Health Organization, 2014). Even though almost half of the suicidal patients make contact with mental health and primary care providers within one month of their suicide, the health care system fails to accurately detect the risk and prevent suicide (Da Cruz et al., 2011). A difficulty in this area is to correctly identify patients with a clear suicidal intent among patients exhibiting suicidal ideation; not the least because patients with a definite intent may ultimately hide this from family and health care providers. Consequently, there is a critical need to improve suicide risk detection by means of identifying biological risk markers, as well as to develop effective, novel pharmacological interventions.
Suicidal behavior is not confined to a particular diagnostic group of patients, and as such is a transnosological phenomenon (Leenaars, 1992). The majority of patients who complete suicide have an underlying psychiatric disorder, which can range from psychotic disorders, such as schizophrenia, to mood disorders, including depression and bipolar disorder, as well as anxiety and posttraumatic stress disorders. Importantly, it is plausible that the underlying pathological molecular mechanisms, associated with suicidal ideation and behavior, are shared across these diagnostic boundaries. The current treatment options for suicidality tend to depend on the psychiatric diagnosis and often consist of antidepressants and anxiolytics (Wasserman et al., 2012). However, the first-line of choice antidepressant treatments, including SSRIs, and SNRIs, have drawbacks since it can take weeks to develop beneficial mood-enhancing effects, and the risk for suicidality has been reported to increase during the first weeks of treatment, especially in children and adolescents (Wasserman et al., 2012). Pharmacological treatment with lithium in mood disorders (Baldessarini et al., 2006; Guzzetta et al., 2007), clozapine in schizophrenia (Meltzer and Baldessarini, 2003), and electroconvulsive therapy in treatment-resistant depression (Kennedy et al., 2009) have proven effective in decreasing suicidal behavior. In addition, a robust and rapid (within hours) antidepressant and anti-suicidal effect is produced by intravenous injection of ketamine, which is an NMDA-receptor antagonist (Fond et al., 2014; Zarate et al., 2013). The biological mechanisms behind the beneficial effects of these treatments on suicidal behavior are not completely understood.
2. Risk factors of suicide
Suicidal behavior is thought to be triggered by an intricate interplay between genetic predispositions and environmental factors (Roy et al., 2009). The genetic component of suicidal behavior, including attempts and suicide completion, is estimated to be around 40% (McGuffin et al., 2010). Recently, epigenetic changes, including hypermethylation of the brain-derived neurotrophic factor (BDNF) promoter (Kang et al., 2013; Kim et al., 2014), have been proposed to be important in depression and suicidality and may provide the platform for some of the gene-environment interactions (Lockwood et al., 2015). The single most important predictor of death by suicide is previous self-harm (Hawton and van Heeringen, 2009). Psychiatric disorders, especially Major Depressive Disorder (MDD) and bipolar disorder, are present in 90% of individuals who complete suicides (Harris and Barraclough, 1997). Certain personality traits have been proposed to act as additional risk factors in individuals with and without psychiatric disease. These include higher levels of impulsivity and aggression, especially in younger suicide victims (Dumais et al., 2005; Perroud et al., 2011) and hopelessness (David Klonsky et al., 2012). The neurobiological changes implicated in suicidal behavior are not fully understood. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis (Mann, 2003) and serotonergic neurotransmission (Bach and Arango, 2012) are frequently detected in individuals exhibiting suicidal behavior. Inflammation is thought to be a contributing factor as inflammatory mediators, such as cytokines, closely and reciprocally interact with both the HPA axis and serotonin system. Inflammation also causes activation of the kynurenine pathway of tryptophan (TRP) degradation. As is the topic of this review, accumulating evidence suggest that a dysregulation of the enzymes in the kynurenine pathway may contribute to the neurobiological changes observed in suicidal patients.
3. Kynurenine pathway and inflammation
The kynurenine pathway is initiated by the conversion of TRP to N-formylkynurenine by any of these enzymes: indoleamine 2,3-dioxygenase 1 (IDO1), IDO2, or tryptophan 2,3-dioxygenase (TDO) (Figure 1). The resulting N-formylkynurenine is further degraded to kynurenine (KYN), which is a precursor of bioactive compounds, including quinolinic acid (QUIN), kynurenic acid (KYNA), picolinic acid (PIC), and 3-hydroxyanthranilic acid (3-HAA) (Schwarcz et al., 2012). The kynurenine pathway is responsible for over 90% of TRP degradation in the periphery (Leklem, 1971) and many organs and cell types, including liver, intestine, brain and immune cells, express several of the enzymes in this pathway. Pro-inflammatory cytokines, including interferon-γ (IFN-γ), interleukin-1β (IL-1β) and IL-6, can further induce IDO-1 and TDO, and thus activate this pathway (Schwieler et al., 2015; Urata et al., 2014). Since TRP is also a precursor for neurotransmitter serotonin, it has been hypothesized that induction of the kynurenine pathway by inflammation may reduce the availability of TRP and consequently lead to reduced serotonin synthesis (Maes et al., 2011). This could theoretically contribute to the decreased levels of 5-hydroyxyindoleacetic acid, a main metabolite of serotonin, that has been observed in the cerebrospinal fluid (CSF) of suicide attempters (Asberg et al., 1976). However, currently there is no clear evidence demonstrating that inflammation causes a decrease in brain serotonin levels through induction of the kynurenine pathway in depressed and suicidal patients.
Figure 1.
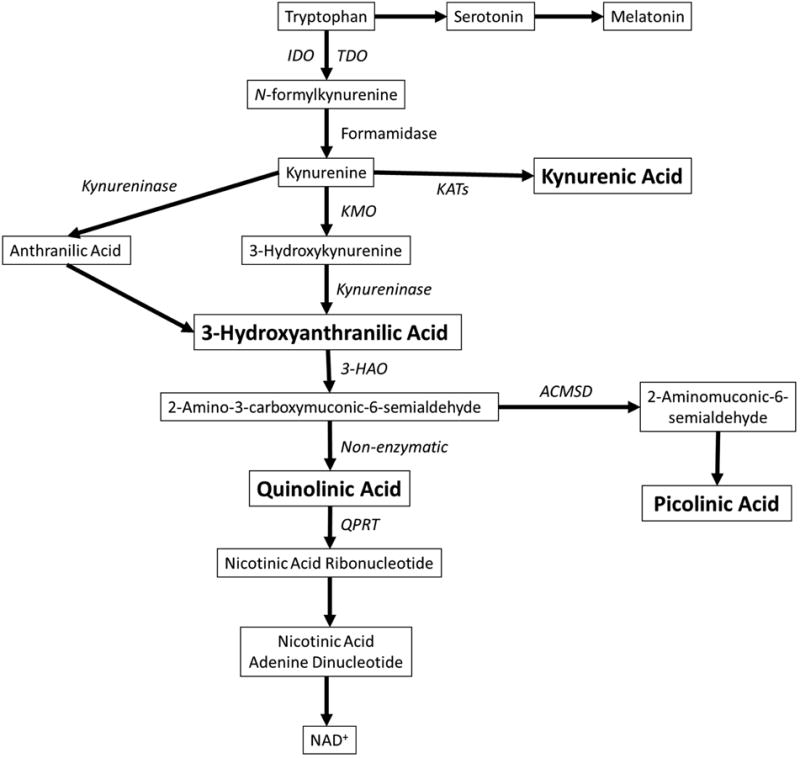
A diagram of the kynurenine pathway. Enzymes are in italics and metabolites are boxed. The metabolites discussed in detail are in bold. Abbreviations: IDO, indoleamine-2,3-dioxygenase; TDO, tryptophan-2,3-dioxygenase; KATs, kynurenine aminotransferases; KMO, kynurenine-3-monooxygenase; 3-HAO, 3-hydroxyanthranilate-3,4-dioxygenase; ACMSD, aminocarboxymuconate-semialdehyde decarboxylase; QPRT, quinolinate phosphoribosyltransferase; NAD, nicotinamide adenine dinucleotide.
4. Kynurenine pathway metabolites in psychiatric disorders and suicidal behavior
In 2011, the first report was published on the relationship between dysregulation of the kynurenine pathway and suicidal behavior (Sublette et al., 2011). In that study, Sublette et al detected elevated plasma KYN levels in suicide attempters with depression, compared to patients with depression but no history of suicidality. A more recent study by Bradley et al found a 40% decrease in plasma TRP levels and a 40% increase in KYN/TRP ratio in suicidal adolescents with MDD, compared to non-suicidal individuals with MDD and healthy controls (Bradley et al., 2015). KYN, like TRP, is able to pass through the blood brain barrier (BBB) into the brain (Schwarcz et al., 2012). In the brain, KYN can be differentially processed by either astrocytes or microglia to produce distinct neuroactive compounds. While the synthesis of 3-hydroxykynurenine (3-HK) and its downstream metabolites, including 3-HAA and QUIN, takes place in microglia and other cells of monocytic origin (Guillemin et al., 2003), the synthesis of KYNA occurs in astrocytes, neurons and oligodendrocytes (Du et al., 1992; Guillemin et al., 2001; Wejksza et al., 2005).
4.1 Quinolinic Acid
QUIN is considered to be an important kynurenine pathway metabolite in terms of its biological activity. It is non-enzymatically produced by spontaneous conversion from the precursor 2-amino-3-carboxymuconic-6-semialdehyde (ACMS) in the absence of the competing enzyme 2-amino-3-carboxymuconic-6-semialdehyde decarboxylase (ACMSD) (Figure 1). ACMSD can convert ACMS to picolinic acid, but when the enzyme is saturated, inactive, or not present, QUIN is instead spontaneously formed. Whereas the detailed regulation of this enzyme is yet to be established, it is known that environmental contaminants (e.g. phthalate esters) and the antituberculosis drug pyrazinamide are capable of inhibiting ACMSD activity, thus increasing the amount of QUIN (Fukuwatari et al., 2004; Fukuwatari et al., 2002). Changes in the nutritional status and the presence of some diseases states, such as diabetes and renal failure, can lead to alteration of ACMSD expression and activity (Egashira et al., 2007; Fukuoka et al., 2003). Saturation of ACMSD occurs when the level of upstream precursor ACMS is elevated, such as under the conditions of increased inflammation, leading to increased QUIN production. The half-life of QUIN is approximately 20 minutes as it gets rapidly broken down by the next enzyme in the kynurenine pathway, quinolinate phosphoribosyl transferase (QPRT), which is found both in the CNS and peripheral organs (Schwarcz et al., 2012). Therefore, the levels and degree of activity of QPRT also regulate QUIN concentration in the tissue of interest (Figure 1).
In the brain, QUIN is primarily produced by microglial cells and infiltrating macrophages. It is considered to be neurotoxic through several mechanisms (Espey et al., 1997; Guillemin et al., 2001; Heyes et al., 1996). One of the mechanisms includes activation of the glutamate N-methyl-D-aspartic acid receptors (NMDARs). QUIN specifically binds to NMDARs containing the NR1 + NR2A and the NR1 + NR2B subunits, which are primarily expressed in the forebrain (de Carvalho et al., 1996). Consequently, the neurons within hippocampus, striatum and neocortex are most vulnerable to QUIN toxicity, while spinal cord and cerebellar neurons are less sensitive (Guillemin, 2012a). A recent study reported that the expression of these NMDAR subunits in dorsolateral prefrontal cortices was higher in suicide victims than controls and most pronounced in females (Gray et al., 2015). Potentially, individuals with a higher expression of these NMDARs would be more vulnerable to increased levels of QUIN - the receptors' agonist. A second mechanism of QUIN neurotoxicity can occur through an increase in glutamate release by neurons and inhibition of its uptake and degradation by astrocytes (Guillemin, 2012b). This leads to elevated extracellular glutamate levels and to overstimulation of the glutamatergic system. In addition, QUIN is capable of forming complexes with iron which leads to formation of reactive oxygen species and can cause lipid peroxidation (Goda et al., 1996; Stipek et al., 1997).
We have found that the QUIN levels in CSF of suicide attempters are around 300% of the levels in healthy controls (Erhardt et al., 2013). In addition, CSF QUIN correlates with CSF IL-6, suggesting that the generation of QUIN is induced by an inflammatory process. We have also found a positive correlation between CSF QUIN and suicidal intent. Athough the highest CSF QUIN levels were at the time of a suicide attempt, levels remain significantly elevated by around 150% over a period of 2 years (Bay-Richter et al., 2015). Theoretically, the elevated levels of QUIN and its neurotoxic effects could contribute to the structural deficits and functional changes previously observed in cortical and subcortical regions of psychiatric patients with suicidal behavior (van Heeringen et al., 2014). In agreement with this hypothesis, Steiner et al reported an increase in density of QUIN-reactive microglia cells in some regions of anterior cingulate cortex (i.e. subgenual anterior cingulate cortex (sACC) and anterior midcingulate cortex (aMCC)) in postmortem brains of depressed patients who died by suicide (Steiner et al., 2011). However, in a recent follow-up study by Steiner's team a decrease in QUIN-immunoreactive microglia was observed in the hippocampal regions of the same suicide victims, suggesting some brain-region differences in the distribution of QUIN-positive microglial cells (Busse et al., 2015).
Our initial interest in studying QUIN in suicidal patients was motivated by several small studies which showed that ketamine, an anesthetic agent that pharmacologically is an NMDAR antagonist, is capable of rapidly inducing anti-suicidal effects (DiazGranados et al., 2010; Larkin and Beautrais, 2011; Price et al., 2009; Zarate et al., 2012). The effect of ketamine on suicidal ideation has proven to occur as early as 40 minutes after the start of infusion and to be long lasting, up to 10 days after infusion (DiazGranados et al., 2010; Ibrahim et al., 2012; Larkin and Beautrais, 2011; Zarate et al., 2012). Later clinical studies assessing the effect of ketamine on depression and suicidal behavior have confirmed the beneficial effects (Price and Mathew, 2015). In addition, a study in an experimental model of depression demonstrated that ketamine could abrogate the development of bacterial lipopolysaccharide (LPS)-induced depressive-like behavior in mice (Walker et al., 2013). Mice intraperitoneally injected with LPS display inflammatory induction of IDO in the brain and develop depressive-like symptoms, which are mediated by NMDAR activation, likely caused by the increased production of QUIN (O'Connor et al., 2009; Walker et al., 2013). Therefore, hyperstimulation of NMDAR may contribute to the pathophysiology of depression and suicidal behavior, although it should be noted that ketamine has additional effects in the brain (Abdallah et al., 2015).
4.2 Kynurenic acid
KYNA is produced from KYN by kynurenine aminotransferase enzymes and, within the central nervous system (CNS), is synthesized in astrocytes, neurons, and oligodendrocytes (Figure 1) (Du et al., 1992; Guillemin et al., 2001; Wejksza et al., 2005). KYNA is a glutamate receptor antagonist capable of inhibiting a range of ionotropic excitatory amino acid receptors, including NMDARs, AMPA receptors (AMPARs) and kainite receptors. In addition, KYNA inhibits α7 nicotinic acetylcholine receptor (α7nAChR) and interacts with the aryl hydrocarbon receptor and an orphan G protein-coupled receptor GPR35 (Stone et al., 2013). It is through activation of GPR35 that KYNA is thought to reduce extracellular brain glutamate levels and prevent the release of pro-inflammatory cytokines in cell lines (Moroni et al., 2012). In addition to its role in receptor binding, KYNA is also an antioxidant capable of scavenging free radicals (Hardeland et al., 1999; Lugo-Huitron et al., 2011).
KYNAs physiological role is neuroprotective and anticonvulsive, however, elevated levels of KYNA correlate with cognitive deficits and psychosis (Erhardt et al., 2009). Patients with schizophrenia spectrum disorder display an approximate 50-70% increase in the CSF KYNA levels compared to healthy controls (Erhardt et al., 2003; Linderholm et al., 2012). Interestingly, a study on suicidal patients with schizophrenia showed that they have significantly lower levels of KYNA compared to non-suicidal patients (Carlborg et al., 2013). We observed that the CSF KYNA levels were decreased by approximately 35% over two years following a suicide attempt, and low levels correlated with more severe depressive and suicidal symptoms (Bay-Richter et al., 2015). As KYNA and QUIN have opposing effects on the NMDAR, the QUIN/KYNA ratio might be a relevant measure suggestive of the overall level of NMDAR stimulation. The ratio is sometimes referred to as a neurotoxic ratio in the literature, although the effects of the metabolites on the receptor are primarily agonistic/antagonistic prior to any neurotoxic effects. The CSF QUIN/KYNA ratio was more than 2-fold elevated in suicide attempters compared to healthy subjects, which suggests an increase in net positive effect on NMDAR agonism in suicidality (Erhardt et al., 2013).
Although several studies, mentioned above, point to suicidal patients as highly relevant in terms of exhibiting pronounced biological changes, such as an exacerbated imbalance of the kynurenine pathway metabolites, scientific studies on this group of patients are relatively sparse. Clinical studies on patients with depression frequently exclude patients with suicidal ideation or behavior, and others include suicidal patients amongst non-suicidal depressive patients without accounting for the presence of suicidality. In agreement with our findings, a previous study on peripheral blood from depressed patients, of which almost 20% had a previous suicide attempt, detected a decrease of 32% in the level of KYNA in the patients (Myint et al., 2007). The study did not attempt to measure QUIN. A recent study by Savitz et al found that both patients with current and remitted MDD (both groups included suicide attempters) had a decreased putative neuroprotective KYNA/QUIN ratio compared to the healthy controls, which indicated the presence of a persistent dysregulation of the kynurenine pathway (Savitz et al., 2015).
4.3 Picolinic Acid
As mentioned in the section related to the production of QUIN, PIC is produced from an unstable precursor metabolite, ACMS, by an enzymatic reaction carried out by the enzyme ACMSD (Figure 1). It has not yet been established whether PIC is able to cross the BBB and enter CNS from the periphery under physiological conditions, but PIC is readily detectable in CSF samples from humans (own unpublished observations) (Coggan et al., 2009; Wang et al., 2013). ACMSD is expressed in the brain, although the expression rate is lower than in kidney and liver (Pucci et al., 2007). Interestingly, in animal models the expression of ACMSD in the brain can be induced or reduced under certain conditions such as streptozocin-induced diabetes or low protein diet, respectively (Fukuoka et al., 2003). Once PIC has been produced, it is not degraded by any enzyme, but is excreted by urine or bile as an end-product of the kynurenine pathway. A study by Guillemin et al found that neuronal and some glial cells in the cortex and hippocampus express ACMSD. Primary neuronal cultures isolated from fetal human brains were also found to constitutively produce PIC (Guillemin et al., 2007). The most firmly established physiological characteristic of PIC is its ability to efficiently chelate iron, copper and other metals (Grant et al., 2009). In addition, PIC is able to antagonize QUIN neurotoxicity in both cell culture and animal models by a currently unknown mechanism, which may involve chelation of endogenous zinc (Beninger et al., 1994; Chen et al., 2011; Cockhill et al., 1992; Jhamandas et al., 1998).
We have recently detected a significant decrease in the CSF PIC levels of suicide attempters compared to healthy controls (in submission). The reduced PIC levels are consistent in several cohorts of suicide attempters and may indicate a decreased function of the enzyme ACMSD in suicidal individuals, which would contribute to the increased production of QUIN and neuroinflammation observed in the patients. Several clinical studies have also discovered promising antidepressant effects of chromium picolinate complex in atypical depression (Davidson et al., 2003; Docherty et al., 2005) and potentiation of pharmacotherapy for dysthymic disorder (McLeod et al., 1999). Administration of chromium picolinate in an animal model of chronic unpredictable mild stress increases the concentration of cortical and cerebellar serotonin levels and decreases plasma corticosterone levels, which could be involved in some observed improvements in depression and anxiety symptoms (Dubey et al., 2015). The observed beneficial effects of chromium picolinate are commonly attributed to the presence of chromium in the complex, while PIC is generally considered to be the non-active ingredient used to solubilize bioactive chromium. Nevertheless, PIC's protective properties against QUIN toxicity in vitro and in animal models, as well as our finding of its low levels in suicide attempters, challenge the notion of PIC as bystander and point to its potential role in managing the depressive symptoms.
4.4 3 Hydroxyanthranilic Acid
In the periphery, 3-hydroxyanthranilic acid (3-HAA) is produced from 3-HK. In the brain, however, anthranilic acid is the preferred precursor for 3-HAA production by kynureninase (Figure 1) (Baran and Schwarcz, 1990). 3-HAA is a highly reactive compound and can be either a pro-oxidant (Goldstein et al., 2000) or anti-oxidant (Christen et al., 1990) depending on the local redox conditions (Darlington et al., 2010). A recent study by Bradley et al did not find significant differences in the plasma levels of 3-HAA between 20 suicidal depressed adolescents, 30 depressed youth without suicidality, and 22 healthy controls (Bradley et al., 2015). No difference was detected in plasma 3-HAA levels or in kynureninase enzyme expression between MDD patients and healthy controls (Hughes et al., 2012). Several studies investigating depression in youth have discovered that only in adolescents that have MDD with melancholic features, and not MDD without melancholic features or healthy controls, there is a positive correlation between plasma 3-HAA/KYN and severity of MDD episode. Moreover, there was also a positive association between the levels of 3-HAA and striatal total choline, a cell membrane breakdown biomarker, suggesting the possibility of the existence of distinct neurobiological characteristics in MDD with melancholic features in adolescence (Gabbay et al., 2010a; Gabbay et al., 2010b).
5. Discussion and conclusions
There is accumulating evidence that dysregulation of the kynurenine pathway, involving changes in the concentration of key metabolites, occurs in some patients with suicidal behavior. The observed changes in levels of bioactive kynurenine pathway metabolites such as QUIN, KYNA, PIC, and 3-HAA could be important in psychiatric symptom generation. QUIN has been shown to affect glutamate neurotransmission via NMDAR agonism, which could be a biological mechanism underlying suicidal behavior. The rapid antidepressant and anti-suicidal effect of ketamine, which is an NMDAR antagonist, could be due to its action on the same biological pathway.
There are some difficulties in analyzing the role of the kynurenine pathway metabolites in suicidality, as well as in other neuropsychiatric conditions, that are important to keep in mind. Importantly, while blood samples are relatively easily obtained from patients, the levels of kynurenine pathway metabolites in these samples may not be reflective of the concentrations of the same metabolites in the brain. On the contrary, it is possible that the regulation and activity of the pathway are separate in the periphery versus the central compartment, as the blood-brain barrier does not allow for free penetrance of several metabolites into the CNS. Only a small number of studies have looked into the correlation between the levels of metabolites in the two compartments, and this area clearly deserves future attention as the kynurenine metabolites are being evaluated as potential candidates of peripheral biomarkers of neuropsychiatric disease. CSF samples from patients are highly valuable in this respect, although these samples still may not reflect the actual conditions within each brain region of interest. In order to fully understand the role of the kynurenine metabolites in neuropsychiatric disease, biosamples from well-characterized patient groups should be paired with detailed animal studies, with the possibility to directly study cause and effect of manipulating the levels of metabolites in specific brain regions.
Another important factor to keep in mind is the handling of biological samples, which may impact the detected levels of metabolites. Once the samples are collected, the storage conditions and the time elapsed between the sample collection and the start of the experiment could differ between various laboratories. Post-mortem samples are even more challenging because of the additional time spent from the time of death until the sample collection. This could potentially influence the results due to the differences in half-life and stability between different metabolites. For example, while PIC and KYNA are stable metabolites that are excreted by the liver and kidney, the half-life of QUIN in blood is only 20 minutes, as this metabolite is degraded by the down-stream enzyme QPRT (Schwarcz et al., 2012). The half-life of individual metabolites is also important to keep in mind for gene expression studies, where a small or temporary increase in the expression of an enzyme producing a stable metabolite can potentially have far greater biological effects than a comparatively large increase in the expression of an enzyme producing a metabolite with a short half-life.
Finally, the psychiatric diagnostic system and the nomenclature regarding suicidality and suicidal behavior have inherent limitations, as the classifications are man-made and may not exactly represent biological disease entities. This should be kept in mind, and as a consequence it may be wise to examine several aspects of psychiatric behavior in a study, rather than focusing only on one outcome measure. For example, in addition to comparing levels of kynurenine metabolites in groups of patients with a diagnosis of depression, the severity of symptoms can be investigated by rating scales that analyze several aspects of depression. Similar clinical assessment scales, investigating several aspects of suicidal behavior, exist and allow a more complete examination of the relation between specific symptoms of suicidality and the biological changes.
A major goal of future studies will be to identify how, in suicidal patients, enzymes in the kynurenine pathway become dysregulated. Why do some metabolites accumulate more than others, and what are the factors that convey vulnerability or resilience to development of suicidal symptoms? It has been proposed that genetic variations in the kynurenine pathway enzymes might be associated with different expression rates or activity of the enzymes, which thus could affect the production of metabolites (Claes et al., 2011). In addition, pro-inflammatory cytokines, which are elevated in suicidal patients, are known to activate the initial step of the kynurenine pathway, however, to what degree certain cytokines may preferentially activate specific enzymes, especially downstream in the pathway, is not currently known. As knowledge regarding the regulation of the kynurenine pathway and the role of its metabolites in suicidality increases, the enzymes of the pathway may become attractive therapeutic targets for managing suicidal behavior.
Highlights.
Increased levels of inflammatory cytokines are present in suicidal patients
Inflammation leads to activation of the kynurenine pathway
The metabolites may induce suicidal symptoms by affecting glutamate neurotransmission
Ketamine may exert anti-suicidal effects by counteracting the kynurenine metabolites
Targeting the kynurenine pathway is a novel therapeutic strategy for suicidal patients
Acknowledgments
This manuscript was supported by NIH 1R01MH104622-01 (LB) and Van Andel Research Institute (LB, EB). The assistance of Keerthi Rajamani and Stan Krzyzanowski in careful proofreading of this manuscript is greatly appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdallah CG, Sanacora G, Duman RS, Krystal JH. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annual review of medicine. 2015;66:509–523. doi: 10.1146/annurev-med-053013-062946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asberg M, Traskman L, Thoren P. 5-HIAA in the cerebrospinal fluid. A biochemical suicide predictor? Archives of general psychiatry. 1976;33:1193–1197. doi: 10.1001/archpsyc.1976.01770100055005. [DOI] [PubMed] [Google Scholar]
- Bach H, Arango V. Neuroanatomy of Serotonergic Abnormalities in Suicide. In: Dwivedi Y, editor. The Neurobiological Basis of Suicide. Boca Raton (FL): 2012. [PubMed] [Google Scholar]
- Baldessarini RJ, Tondo L, Davis P, Pompili M, Goodwin FK, Hennen J. Decreased risk of suicides and attempts during long-term lithium treatment: a meta-analytic review. Bipolar disorders. 2006;8:625–639. doi: 10.1111/j.1399-5618.2006.00344.x. [DOI] [PubMed] [Google Scholar]
- Baran H, Schwarcz R. Presence of 3-hydroxyanthranilic acid in rat tissues and evidence for its production from anthranilic acid in the brain. Journal of neurochemistry. 1990;55:738–744. doi: 10.1111/j.1471-4159.1990.tb04553.x. [DOI] [PubMed] [Google Scholar]
- Bay-Richter C, Linderholm KR, Lim CK, Samuelsson M, Traskman-Bendz L, Guillemin GJ, Erhardt S, Brundin L. A role for inflammatory metabolites as modulators of the glutamate N-methyl-D-aspartate receptor in depression and suicidality. Brain, behavior, and immunity. 2015;43:110–117. doi: 10.1016/j.bbi.2014.07.012. [DOI] [PubMed] [Google Scholar]
- Beninger RJ, Colton AM, Ingles JL, Jhamandas K, Boegman RJ. Picolinic acid blocks the neurotoxic but not the neuroexcitant properties of quinolinic acid in the rat brain: evidence from turning behaviour and tyrosine hydroxylase immunohistochemistry. Neuroscience. 1994;61:603–612. doi: 10.1016/0306-4522(94)90438-3. [DOI] [PubMed] [Google Scholar]
- Bradley KA, Case JA, Khan O, Ricart T, Hanna A, Alonso CM, Gabbay V. The role of the kynurenine pathway in suicidality in adolescent major depressive disorder. Psychiatry research. 2015;227:206–212. doi: 10.1016/j.psychres.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse M, Busse S, Myint AM, Gos T, Dobrowolny H, Muller UJ, Bogerts B, Bernstein HG, Steiner J. Decreased quinolinic acid in the hippocampus of depressive patients: evidence for local anti-inflammatory and neuroprotective responses? European archives of psychiatry and clinical neuroscience. 2015;265:321–329. doi: 10.1007/s00406-014-0562-0. [DOI] [PubMed] [Google Scholar]
- Carlborg A, Jokinen J, Jonsson EG, Erhardt S, Nordstrom P. CSF kynurenic acid and suicide risk in schizophrenia spectrum psychosis. Psychiatry research. 2013;205:165–167. doi: 10.1016/j.psychres.2012.08.021. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Cost of Injury Reports 2010 [Google Scholar]
- Chen Y, Brew BJ, Guillemin GJ. Characterization of the kynurenine pathway in NSC-34 cell line: implications for amyotrophic lateral sclerosis. Journal of neurochemistry. 2011;118:816–825. doi: 10.1111/j.1471-4159.2010.07159.x. [DOI] [PubMed] [Google Scholar]
- Christen S, Peterhans E, Stocker R. Antioxidant activities of some tryptophan metabolites: possible implication for inflammatory diseases. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:2506–2510. doi: 10.1073/pnas.87.7.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes S, Myint AM, Domschke K, Del-Favero J, Entrich K, Engelborghs S, De Deyn P, Mueller N, Baune B, Rothermundt M. The kynurenine pathway in major depression: haplotype analysis of three related functional candidate genes. Psychiatry research. 2011;188:355–360. doi: 10.1016/j.psychres.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Cockhill J, Jhamandas K, Boegman RJ, Beninger RJ. Action of picolinic acid and structurally related pyridine carboxylic acids on quinolinic acid-induced cortical cholinergic damage. Brain research. 1992;599:57–63. doi: 10.1016/0006-8993(92)90852-z. [DOI] [PubMed] [Google Scholar]
- Coggan SE, Smythe GA, Bilgin A, Grant RS. Age and circadian influences on picolinic acid concentrations in human cerebrospinal fluid. Journal of neurochemistry. 2009;108:1220–1225. doi: 10.1111/j.1471-4159.2009.05868.x. [DOI] [PubMed] [Google Scholar]
- Da Cruz D, Pearson A, Saini P, Miles C, While D, Swinson N, Williams A, Shaw J, Appleby L, Kapur N. Emergency department contact prior to suicide in mental health patients. Emergency medicine journal : EMJ. 2011;28:467–471. doi: 10.1136/emj.2009.081869. [DOI] [PubMed] [Google Scholar]
- Darlington LG, Forrest CM, Mackay GM, Smith RA, Smith AJ, Stoy N, Stone TW. On the Biological Importance of the 3-hydroxyanthranilic Acid: Anthranilic Acid Ratio. International journal of tryptophan research : IJTR. 2010;3:51–59. doi: 10.4137/ijtr.s4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David Klonsky E, Kotov R, Bakst S, Rabinowitz J, Bromet EJ. Hopelessness as a predictor of attempted suicide among first admission patients with psychosis: a 10-year cohort study. Suicide & life-threatening behavior. 2012;42:1–10. doi: 10.1111/j.1943-278X.2011.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson JR, Abraham K, Connor KM, McLeod MN. Effectiveness of chromium in atypical depression: a placebo-controlled trial. Biological psychiatry. 2003;53:261–264. doi: 10.1016/s0006-3223(02)01500-7. [DOI] [PubMed] [Google Scholar]
- de Carvalho LP, Bochet P, Rossier J. The endogenous agonist quinolinic acid and the non endogenous homoquinolinic acid discriminate between NMDAR2 receptor subunits. Neurochemistry international. 1996;28:445–452. doi: 10.1016/0197-0186(95)00091-7. [DOI] [PubMed] [Google Scholar]
- DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, Machado-Vieira R, Zarate CA., Jr Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. The Journal of clinical psychiatry. 2010;71:1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty JP, Sack DA, Roffman M, Finch M, Komorowski JR. A double-blind, placebo-controlled, exploratory trial of chromium picolinate in atypical depression: effect on carbohydrate craving. Journal of psychiatric practice. 2005;11:302–314. doi: 10.1097/00131746-200509000-00004. [DOI] [PubMed] [Google Scholar]
- Du F, Schmidt W, Okuno E, Kido R, Kohler C, Schwarcz R. Localization of kynurenine aminotransferase immunoreactivity in the rat hippocampus. The Journal of comparative neurology. 1992;321:477–487. doi: 10.1002/cne.903210313. [DOI] [PubMed] [Google Scholar]
- Dubey VK, Ansari F, Vohora D, Khanam R. Possible involvement of corticosterone and serotonin in antidepressant and antianxiety effects of chromium picolinate in chronic unpredictable mild stress induced depression and anxiety in rats. Journal of trace elements in medicine and biology : organ of the Society for Minerals and Trace Elements. 2015;29:222–226. doi: 10.1016/j.jtemb.2014.06.014. [DOI] [PubMed] [Google Scholar]
- Dumais A, Lesage AD, Alda M, Rouleau G, Dumont M, Chawky N, Roy M, Mann JJ, Benkelfat C, Turecki G. Risk factors for suicide completion in major depression: a case-control study of impulsive and aggressive behaviors in men. The American journal of psychiatry. 2005;162:2116–2124. doi: 10.1176/appi.ajp.162.11.2116. [DOI] [PubMed] [Google Scholar]
- Egashira Y, Sato M, Saito K, Sanada H. Dietary protein level and dietary interaction affect quinolinic acid concentration in rats. International journal for vitamin and nutrition research. Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung. Journal international de vitaminologie et de nutrition. 2007;77:142–148. doi: 10.1024/0300-9831.77.2.142. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Lim CK, Linderholm KR, Janelidze S, Lindqvist D, Samuelsson M, Lundberg K, Postolache TT, Traskman-Bendz L, Guillemin GJ, Brundin L. Connecting inflammation with glutamate agonism in suicidality. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:743–752. doi: 10.1038/npp.2012.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt S, Olsson SK, Engberg G. Pharmacological manipulation of kynurenic acid: potential in the treatment of psychiatric disorders. CNS drugs. 2009;23:91–101. doi: 10.2165/00023210-200923020-00001. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Schwieler L, Engberg G. Kynurenic acid and schizophrenia. Advances in experimental medicine and biology. 2003;527:155–165. doi: 10.1007/978-1-4615-0135-0_18. [DOI] [PubMed] [Google Scholar]
- Espey MG, Chernyshev ON, Reinhard JF, Jr, Namboodiri MA, Colton CA. Activated human microglia produce the excitotoxin quinolinic acid. Neuroreport. 1997;8:431–434. doi: 10.1097/00001756-199701200-00011. [DOI] [PubMed] [Google Scholar]
- Fond G, Loundou A, Rabu C, Macgregor A, Lancon C, Brittner M, Micoulaud-Franchi JA, Richieri R, Courtet P, Abbar M, Roger M, Leboyer M, Boyer L. Ketamine administration in depressive disorders: a systematic review and meta-analysis. Psychopharmacology. 2014;231:3663–3676. doi: 10.1007/s00213-014-3664-5. [DOI] [PubMed] [Google Scholar]
- Fukuoka S, Ishiguro K, Tanabe A, Egashira Y, Sanada H, Fukuwatari T, Shibata K. Identification and expression of alpha cDNA encoding human 2-amino-3-carboxymuconate-6-semialdehyde decarboxylase (ACMSD): a key enzyme for the tryptophan-niacine pathway and quinolinate hypothesis. Advances in experimental medicine and biology. 2003;527:443–453. doi: 10.1007/978-1-4615-0135-0_52. [DOI] [PubMed] [Google Scholar]
- Fukuwatari T, Ohsaki S, Fukuoka S, Sasaki R, Shibata K. Phthalate esters enhance quinolinate production by inhibiting alpha-amino-beta-carboxymuconate-epsilon-semialdehyde decarboxylase (ACMSD), a key enzyme of the tryptophan pathway. Toxicological sciences : an official journal of the Society of Toxicology. 2004;81:302–308. doi: 10.1093/toxsci/kfh204. [DOI] [PubMed] [Google Scholar]
- Fukuwatari T, Sugimoto E, Shibata K. Growth-promoting activity of pyrazinoic acid, a putative active compound of antituberculosis drug pyrazinamide, in niacin-deficient rats through the inhibition of ACMSD activity. Bioscience, biotechnology, and biochemistry. 2002;66:1435–1441. doi: 10.1271/bbb.66.1435. [DOI] [PubMed] [Google Scholar]
- Gabbay V, Klein RG, Katz Y, Mendoza S, Guttman LE, Alonso CM, Babb JS, Hirsch GS, Liebes L. The possible role of the kynurenine pathway in adolescent depression with melancholic features. Journal of child psychology and psychiatry, and allied disciplines. 2010a;51:935–943. doi: 10.1111/j.1469-7610.2010.02245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Liebes L, Katz Y, Liu S, Mendoza S, Babb JS, Klein RG, Gonen O. The kynurenine pathway in adolescent depression: preliminary findings from a proton MR spectroscopy study. Progress in neuro-psychopharmacology & biological psychiatry. 2010b;34:37–44. doi: 10.1016/j.pnpbp.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda K, Kishimoto R, Shimizu S, Hamane Y, Ueda M. Quinolinic acid and active oxygens. Possible contribution of active Oxygens during cell death in the brain. Advances in experimental medicine and biology. 1996;398:247–254. [PubMed] [Google Scholar]
- Goldstein LE, Leopold MC, Huang X, Atwood CS, Saunders AJ, Hartshorn M, Lim JT, Faget KY, Muffat JA, Scarpa RC, Chylack LT, Jr, Bowden EF, Tanzi RE, Bush AI. 3-Hydroxykynurenine and 3-hydroxyanthranilic acid generate hydrogen peroxide and promote alpha-crystallin cross-linking by metal ion reduction. Biochemistry. 2000;39:7266–7275. doi: 10.1021/bi992997s. [DOI] [PubMed] [Google Scholar]
- Grant RS, Coggan SE, Smythe GA. The physiological action of picolinic Acid in the human brain. International journal of tryptophan research : IJTR. 2009;2:71–79. doi: 10.4137/ijtr.s2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray AL, Hyde TM, Deep-Soboslay A, Kleinman JE, Sodhi MS. Sex differences in glutamate receptor gene expression in major depression and suicide. Molecular psychiatry. 2015;20:1057–1068. doi: 10.1038/mp.2015.91. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ. Quinolinic acid, the inescapable neurotoxin. The FEBS journal. 2012a;279:1356–1365. doi: 10.1111/j.1742-4658.2012.08485.x. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ. Quinolinic acid: neurotoxicity. The FEBS journal. 2012b;279:1355. doi: 10.1111/j.1742-4658.2012.08493.x. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Cullen KM, Lim CK, Smythe GA, Garner B, Kapoor V, Takikawa O, Brew BJ. Characterization of the kynurenine pathway in human neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:12884–12892. doi: 10.1523/JNEUROSCI.4101-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin GJ, Kerr SJ, Smythe GA, Smith DG, Kapoor V, Armati PJ, Croitoru J, Brew BJ. Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. Journal of neurochemistry. 2001;78:842–853. doi: 10.1046/j.1471-4159.2001.00498.x. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Smith DG, Smythe GA, Armati PJ, Brew BJ. Expression of the kynurenine pathway enzymes in human microglia and macrophages. Advances in experimental medicine and biology. 2003;527:105–112. doi: 10.1007/978-1-4615-0135-0_12. [DOI] [PubMed] [Google Scholar]
- Guzzetta F, Tondo L, Centorrino F, Baldessarini RJ. Lithium treatment reduces suicide risk in recurrent major depressive disorder. The Journal of clinical psychiatry. 2007;68:380–383. doi: 10.4088/jcp.v68n0304. [DOI] [PubMed] [Google Scholar]
- Hardeland R, Zsizsik BK, Poeggeler B, Fuhrberg B, Holst S, Coto-Montes A. Indole-3-pyruvic and -propionic acids, kynurenic acid, and related metabolites as luminophores and free-radical scavengers. Advances in experimental medicine and biology. 1999;467:389–395. doi: 10.1007/978-1-4615-4709-9_49. [DOI] [PubMed] [Google Scholar]
- Harris EC, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry. 1997;170:205–228. doi: 10.1192/bjp.170.3.205. [DOI] [PubMed] [Google Scholar]
- Hawton K, van Heeringen K. Suicide. Lancet. 2009;373:1372–1381. doi: 10.1016/S0140-6736(09)60372-X. [DOI] [PubMed] [Google Scholar]
- Heyes MP, Achim CL, Wiley CA, Major EO, Saito K, Markey SP. Human microglia convert l-tryptophan into the neurotoxin quinolinic acid. The Biochemical journal. 1996;320(Pt 2):595–597. doi: 10.1042/bj3200595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes MM, Carballedo A, McLoughlin DM, Amico F, Harkin A, Frodl T, Connor TJ. Tryptophan depletion in depressed patients occurs independent of kynurenine pathway activation. Brain, behavior, and immunity. 2012;26:979–987. doi: 10.1016/j.bbi.2012.05.010. [DOI] [PubMed] [Google Scholar]
- Ibrahim L, Diazgranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, Moaddel R, Wainer I, Luckenbaugh DA, Manji HK, Zarate CA., Jr Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:1526–1533. doi: 10.1038/npp.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhamandas KH, Boegman RJ, Beninger RJ, Flesher S. Role of zinc in blockade of excitotoxic action of quinolinic acid by picolinic acid. Amino acids. 1998;14:257–261. doi: 10.1007/BF01345272. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Kim JM, Lee JY, Kim SY, Bae KY, Kim SW, Shin IS, Kim HR, Shin MG, Yoon JS. BDNF promoter methylation and suicidal behavior in depressive patients. Journal of affective disorders. 2013;151:679–685. doi: 10.1016/j.jad.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Milev R, Giacobbe P, Ramasubbu R, Lam RW, Parikh SV, Patten SB, Ravindran AV Canadian Network for Mood and Anxiety Treatments. Canadian Network for Mood and Anxiety Treatments (CANMAT) Clinical guidelines for the management of major depressive disorder in adults. IV. Neurostimulation therapies. Journal of affective disorders 117 Suppl. 2009;1:S44–53. doi: 10.1016/j.jad.2009.06.039. [DOI] [PubMed] [Google Scholar]
- Kim JM, Kang HJ, Bae KY, Kim SW, Shin IS, Kim HR, Shin MG, Yoon JS. Association of BDNF promoter methylation and genotype with suicidal ideation in elderly Koreans. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2014;22:989–996. doi: 10.1016/j.jagp.2014.02.011. [DOI] [PubMed] [Google Scholar]
- Larkin GL, Beautrais AL. A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. Int J Neuropsychopharmacol. 2011;14:1127–1131. doi: 10.1017/S1461145711000629. [DOI] [PubMed] [Google Scholar]
- Leenaars AA. Suicide notes, communication, and ideation. In: M RW, Berman AL, Maltsberger JT, Yufit RI, editors. Assessment and prediction of suicide. The Guilford press; New York: 1992. [Google Scholar]
- Leklem JE. Quantitative aspects of tryptophan metabolism in humans and other species: a review. The American journal of clinical nutrition. 1971;24:659–672. doi: 10.1093/ajcn/24.6.659. [DOI] [PubMed] [Google Scholar]
- Linderholm KR, Skogh E, Olsson SK, Dahl ML, Holtze M, Engberg G, Samuelsson M, Erhardt S. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophrenia bulletin. 2012;38:426–432. doi: 10.1093/schbul/sbq086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood LE, Su S, Youssef NA. The role of epigenetics in depression and suicide: A platform for gene-environment interactions. Psychiatry research. 2015;228:235–242. doi: 10.1016/j.psychres.2015.05.071. [DOI] [PubMed] [Google Scholar]
- Lugo-Huitron R, Blanco-Ayala T, Ugalde-Muniz P, Carrillo-Mora P, Pedraza-Chaverri J, Silva-Adaya D, Maldonado PD, Torres I, Pinzon E, Ortiz-Islas E, Lopez T, Garcia E, Pineda B, Torres-Ramos M, Santamaria A, La Cruz VP. On the antioxidant properties of kynurenic acid: free radical scavenging activity and inhibition of oxidative stress. Neurotoxicology and teratology. 2011;33:538–547. doi: 10.1016/j.ntt.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Maes M, Leonard BE, Myint AM, Kubera M, Verkerk R. The new ‘5-HT’ hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Progress in neuro-psychopharmacology & biological psychiatry. 2011;35:702–721. doi: 10.1016/j.pnpbp.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Mann JJ. Neurobiology of suicidal behaviour. Nature reviews. Neuroscience. 2003;4:819–828. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- McGuffin P, Perroud N, Uher R, Butler A, Aitchison KJ, Craig I, Lewis C, Farmer A. The genetics of affective disorder and suicide. European psychiatry : the journal of the Association of European Psychiatrists. 2010;25:275–277. doi: 10.1016/j.eurpsy.2009.12.012. [DOI] [PubMed] [Google Scholar]
- McLeod MN, Gaynes BN, Golden RN. Chromium potentiation of antidepressant pharmacotherapy for dysthymic disorder in 5 patients. The Journal of clinical psychiatry. 1999;60:237–240. doi: 10.4088/jcp.v60n0406. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Baldessarini RJ. Reducing the risk for suicide in schizophrenia and affective disorders. The Journal of clinical psychiatry. 2003;64:1122–1129. doi: 10.4088/jcp.v64n0920. [DOI] [PubMed] [Google Scholar]
- Moroni F, Cozzi A, Sili M, Mannaioni G. Kynurenic acid: a metabolite with multiple actions and multiple targets in brain and periphery. Journal of neural transmission. 2012;119:133–139. doi: 10.1007/s00702-011-0763-x. [DOI] [PubMed] [Google Scholar]
- Mortality GBD, Causes of Death C. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myint AM, Kim YK, Verkerk R, Scharpe S, Steinbusch H, Leonard B. Kynurenine pathway in major depression: evidence of impaired neuroprotection. Journal of affective disorders. 2007;98:143–151. doi: 10.1016/j.jad.2006.07.013. [DOI] [PubMed] [Google Scholar]
- O'Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Molecular psychiatry. 2009;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perroud N, Baud P, Mouthon D, Courtet P, Malafosse A. Impulsivity, aggression and suicidal behavior in unipolar and bipolar disorders. Journal of affective disorders. 2011;134:112–118. doi: 10.1016/j.jad.2011.05.048. [DOI] [PubMed] [Google Scholar]
- Price RB, Mathew SJ. Does ketamine have anti-suicidal properties? Current status and future directions. CNS drugs. 2015;29:181–188. doi: 10.1007/s40263-015-0232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biological psychiatry. 2009;66:522–526. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucci L, Perozzi S, Cimadamore F, Orsomando G, Raffaelli N. Tissue expression and biochemical characterization of human 2-amino 3-carboxymuconate 6-semialdehyde decarboxylase, a key enzyme in tryptophan catabolism. The FEBS journal. 2007;274:827–840. doi: 10.1111/j.1742-4658.2007.05635.x. [DOI] [PubMed] [Google Scholar]
- Roy A, Sarchiopone M, Carli V. Gene-environment interaction and suicidal behavior. Journal of psychiatric practice. 2009;15:282–288. doi: 10.1097/01.pra.0000358314.88931.b5. [DOI] [PubMed] [Google Scholar]
- Savitz J, Drevets WC, Wurfel BE, Ford BN, Bellgowan PS, Victor TA, Bodurka J, Teague TK, Dantzer R. Reduction of kynurenic acid to quinolinic acid ratio in both the depressed and remitted phases of major depressive disorder. Brain, behavior, and immunity. 2015 doi: 10.1016/j.bbi.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nature reviews. Neuroscience. 2012;13:465–477. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwieler L, Larsson MK, Skogh E, Kegel ME, Orhan F, Abdelmoaty S, Finn A, Bhat M, Samuelsson M, Lundberg K, Dahl ML, Sellgren C, Schuppe-Koistinen I, Svensson C, Erhardt S, Engberg G. Increased levels of IL-6 in the cerebrospinal fluid of patients with chronic schizophrenia--significance for activation of the kynurenine pathway. Journal of psychiatry & neuroscience : JPN. 2015;40:126–133. doi: 10.1503/jpn.140126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J, Walter M, Gos T, Guillemin GJ, Bernstein HG, Sarnyai Z, Mawrin C, Brisch R, Bielau H, Meyer zu Schwabedissen L, Bogerts B, Myint AM. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? Journal of neuroinflammation. 2011;8:94. doi: 10.1186/1742-2094-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipek S, Stastny F, Platenik J, Crkovska J, Zima T. The effect of quinolinate on rat brain lipid peroxidation is dependent on iron. Neurochemistry international. 1997;30:233–237. [PubMed] [Google Scholar]
- Stone TW, Stoy N, Darlington LG. An expanding range of targets for kynurenine metabolites of tryptophan. Trends in pharmacological sciences. 2013;34:136–143. doi: 10.1016/j.tips.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Sublette ME, Galfalvy HC, Fuchs D, Lapidus M, Grunebaum MF, Oquendo MA, Mann JJ, Postolache TT. Plasma kynurenine levels are elevated in suicide attempters with major depressive disorder. Brain, behavior, and immunity. 2011;25:1272–1278. doi: 10.1016/j.bbi.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urata Y, Koga K, Hirota Y, Akiyama I, Izumi G, Takamura M, Nagai M, Harada M, Hirata T, Yoshino O, Kawana K, Fujii T, Osuga Y. IL-1beta increases expression of tryptophan 2,3-dioxygenase and stimulates tryptophan catabolism in endometrioma stromal cells. American journal of reproductive immunology. 2014;72:496–503. doi: 10.1111/aji.12282. [DOI] [PubMed] [Google Scholar]
- van Heeringen K, Bijttebier S, Desmyter S, Vervaet M, Baeken C. Is there a neuroanatomical basis of the vulnerability to suicidal behavior? A coordinate-based meta-analysis of structural and functional MRI studies. Frontiers in human neuroscience. 2014;8:824. doi: 10.3389/fnhum.2014.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnik P. Suicide in the world. International journal of environmental research and public health. 2012;9:760–771. doi: 10.3390/ijerph9030760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AK, Budac DP, Bisulco S, Lee AW, Smith RA, Beenders B, Kelley KW, Dantzer R. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:1609–1616. doi: 10.1038/npp.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Davis I, Liu A, Miller A, Shamsi SA. Improved separation and detection of picolinic acid and quinolinic acid by capillary electrophoresis-mass spectrometry: application to analysis of human cerebrospinal fluid. Journal of chromatography. A. 2013;1316:147–153. doi: 10.1016/j.chroma.2013.09.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman D, Rihmer Z, Rujescu D, Sarchiapone M, Sokolowski M, Titelman D, Zalsman G, Zemishlany Z, Carli V, European Psychiatric A. The European Psychiatric Association (EPA) guidance on suicide treatment and prevention. European psychiatry : the journal of the Association of European Psychiatrists. 2012;27:129–141. doi: 10.1016/j.eurpsy.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Wejksza K, Rzeski W, Okuno E, Kandefer-Szerszen M, Albrecht J, Turski WA. Demonstration of kynurenine aminotransferases I and II and characterization of kynurenic acid synthesis in oligodendrocyte cell line (OLN-93) Neurochemical research. 2005;30:963–968. doi: 10.1007/s11064-005-6178-z. [DOI] [PubMed] [Google Scholar]
- World HealthOrganization. Preventing suicide: A global imperative. Geneva: 2014. [Google Scholar]
- Zarate C, Duman RS, Liu G, Sartori S, Quiroz J, Murck H. New paradigms for treatment-resistant depression. Annals of the New York Academy of Sciences. 2013;1292:21–31. doi: 10.1111/nyas.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V, Luckenbaugh DA. Replication of ketamine's antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biological psychiatry. 2012;71:939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


