Abstract
Objective
Our objective was to characterize physical properties and semivolatile harmful and potentially harmful constituent yields in the mainstream smoke (MSS) of 4 popular little cigars compared to the 3R4F reference cigarette.
Methods
We used the ISO and Canadian Intense Regimen protocols to generate MSS for Cheyenne (Full Flavor and Menthol) and Swisher Sweets (Original and Sweet Cherry) little cigars; and the 3R4F. We examined physical properties such as length, tobacco filler mass, pressure drop, and ventilation for each product. Nicotine, benzo[a]pyrene, and tobacco-specific nitrosamine (TSNA) yields were determined in the MSS.
Results
Little cigars were longer (~15mm), contained more tobacco filler (100–200 mg), and had a higher pressure drop (~1.3X) compared to the 3R4F. Ventilation holes were found only on the filter paper of the 3R4F. Nicotine transmitted to the MSS was similar for all products under the intense smoking protocol. The highest yields of TSNAs and benzo(a)pyrene were measured for the little cigars.
Conclusions
Little cigars may deliver similar levels of nicotine but higher levels of carcinogens to the MSS compared to cigarettes. Thus, previous reports on the toxicity of tobacco smoke based on cigarettes might not apply to little cigar products.
Keywords: little cigar, 3R4F cigarette, tobacco physical properties, tobacco harmful and potentially harmful constituents (HPHCs)
The toxicity of tobacco smoke is the underlying cause of most tobacco related disease, which is the leading cause of preventable death in the United States (US).1–4 Most of the studies that discuss the toxicity, nature, and chemical constituents of tobacco smoke were derived from examining cigarette smoke;2–6 however, there is some evidence that the use of cigar products is associated with increased risk of cancer, including lung, larynx, oral cavity and esophageal cancers.4 Little cigars are comparable to cigarettes with respect to shape, size, filters and packaging,7 but their mainstream smoke (MSS) properties are different.8–10 In part, this might be attributed to different tobacco blends, storage time of the tobacco leaves prior to manufacturing, and differences in the manufacturing processes of little cigars versus cigarettes.10–16 For instance, when compared to cigarettes, the mainstream smoke of little cigars was more basic, showing higher levels of the volatile nicotine fraction,10 the so-called “free-base” nicotine that might be more bio-active.17 Additionally, a previous study in our laboratory showed that the mainstream smoke of little cigars has some unique chemicals and some of the same, but highly concentrated chemicals compared with those measured in mainstream cigarette smoke,9 indicating that little cigars may have different toxicity profiles than cigarettes. Moreover, physical properties and smoking behaviors have been found to impact and change the level of the toxic/carcinogenic chemicals in the MSS of cigarettes;5,10,18–20 but these variables have not been well examined in little cigars yet.
In 2012, the US Food and Drug Administration, Center for Tobacco Products (FDA CTP) established a list of 93 harmful and potentially harmful constituents (HPHCs) in tobacco smoke from cigarettes, pipe, and smokeless tobacco, but not from cigar products.21 The tobacco industry promoted cigar products, including little cigars, as alternatives to cigarettes.7,22 A decrease in the consumption of cigarettes (by about 33%) was observed between 2000–2011, which was concurrent with the increase in the consumption of other non-cigarette products including cigar products (by about 123%).23 This might be to some degree attributed to the fact that these products were not initially placed under FDA’s regulatory jurisdiction as part of the 2009 Family Smoking Prevention and Tobacco Control Act.24
In addition to less stringent regulation, increases in little cigar consumption also may be explained by the lower cost of these products, which can be less than half the price of cigarettes in some states with wide disparities in excise taxes levied for little cigars compared to cigarettes.25 Recently, cigar products have fallen under the FDA’s regulatory authority.26 Studies are needed to understand the chemical constituents and toxicity of these novel tobacco products to inform regulation and protect public health. This study aims to address some of the data gaps surrounding the differences in the physical properties and yields of MSS semivolatile HPHCs between little cigars and cigarettes. To this end, we evaluated the physical properties and MSS chemistry of 4 popular little cigars: Cheyenne Full Flavor (CF), Cheyenne Menthol (CM), Swisher Sweets Original (SO), and Swisher Sweets Sweet Cherry (SC),27,28 and a reference comparator that is representative of a medium-delivery, American-blended cigarette (3R4F, University of Kentucky Reference Cigarette 3R4F).29
METHODS
Selection of Products
Cheyenne [Full Flavor (CF), and Menthol (CM); (Cheyenne International, LLC; Grover, NC)] and Swisher Sweets [Original (SO) and Cherry (SC); (Swisher International, Inc; Jacksonville, FL)] little cigars were purchased in Columbus, OH in February and March 2015. The 3R4F reference cigarette was purchased from the University of Kentucky in 2014.
The selected little cigars in this study have been shown to hold significant shares of the US markets.27,28 Reports show that Swisher Sweets and Cheyenne represented about 30% of the little cigars market.30
All products were stored in their original packaging, refrigerated at 4°C, and conditioned (temperature 22 ± 2°C, and humidity 60 ± 3%, according to ISO 3402:1999(E) protocol)31 prior to machine smoking.
Length and Circumference
The total length of each rod was measured from end to end using a digital caliper (SC-6″C, Mitutoyo). The circumference was measured by slitting each rod longitudinally. The paper was then removed, flattened, and the width was measured with a digital caliper (ISO 4387:2000(E), 2971 protocol).32 Each measurement was made on 10 replicates of each product.
Tobacco Mass and Consumed Tobacco
To measure the tobacco mass, each product was weighed individually in triplicate. The paper and filter of each product was removed and weighed separately, and the weight of the filter plus the paper was then subtracted from the total weight of the product. The weight was determined in a temperature and humidity-controlled environment (22 ± 2°C, and 50 ± 5% relative humidity). To determine the mass of the consumed tobacco, the weight of each little cigar/cigarette was determined before and after smoking by placing the product in a 20 mL glass vial with its corresponding lid and weighing it. The mass was determined based on 3 replicates.
Filter and Filter Paper
The thickness of the paper wrap (around the filter), along with the cross section and longitudinal section of the filters, were examined using Starrett 0–1 inch Micrometers (MS-100) and Flexbar Opti-Flex Vision System (QCZ-2000) (Figures SI5–9, and Table SI3 in the supporting information). Acetone and sulfuric acid were used to test the type of the filter of each product. Filters made of cellulose acetate should dissolve in acetone, whereas filters made of paper dissolve in sulfuric acid.33
Pressure Drop
Pressure drop was measured following the Cores-ta No 41 method.34 Briefly, ambient air was drawn through the rod at 4 defined flow rates (1–4 L/min) (Figure SI1 in the supporting information) using a Thomas pump, and the resulting pressure drop was measured using a Magnehelic Gage (Dwyer Institute Inc, Michigan City, IN). This method was used to measure the pressure drop of the whole rod (little cigar/cigarette), and the filter. The pressure drop of the tobacco part of the cigar/cigarette was then obtained by subtracting the pressure drop of the filter from the whole rod pressure drop (Figure SI2b). Pressure drop was measured and determined based on 3 replicates.
Total Particulate Matter (TPM): Generation and Collection
Total particulate matter (TPM) from each product was generated using a linear, 5-port smoking machine (Hawktech Model FP2000, Tri-City Machine Works, VA) and collected on standard 44 mm quartz fiber filters (QFFs) per the 2 specified methods, International Organization for Standardization/US Federal Trade Commission ISO/FTC (2-second puff duration, 35 mL puff volume, 60-second interval),32 and Canadian Intense Regimen CIR (2-second puff duration, 55 mL puff volume, 30-second interval).35 Before smoking, the product and the QFFs were conditioned at 22 ± 2°C, and 50 ± 5% relative humidity for at least 12 hours. The time between the conditioning and the use of product and QFFs was 7–9 days. One product was smoked per port and TPM was collected onto an individual QFF. The string cut-off method was used as described in ISO 4387:2000E protocol.32 However, the tobacco in each rod was smoked to a butt length of 41.1 – 43.2 mm, or the length of the externally visible cigarette filter over-wrap plus 8 mm. This is a difference of 5.2 ± 0.07 mm longer butt length compared to the ISO methodology.36,37 Filter ventilation holes were (100%) blocked/covered by tape in the case of the CIR protocol. TPM mass was obtained gravimetrically by calculating the weight differences in the QFF before and after the smoking process. The QFFs were weighed using an analytical microbalance in a humidity- and temperature-controlled room (22 ± 2°C and 50 ± 5% relative humidity).
TPM Semivolatile Organic Compound (SVOC) Analysis
Chemical analysis of the collected TPM samples for select semivolatile HPHCs was based on methods previously developed in our laboratories for similar matrices and chemical classes.38–40 Briefly, the collected QFFs were spiked with internal standards and extracted with 50% dichloromethane in acetonitrile for one hour using sonication and an orbital shaker (150 rpm). The extract was then split into 2 aliquots. One aliquot was solvent exchanged into 100 mM ammonium acetate and analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) for the tobacco specific nitrosamines (TSNAs), N′-nitrosonornicotine (NNN) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). The second aliquot was analyzed by automated gas chromatography/mass spectrometry (GC/MS) for the remaining SVOCs including, nicotine, menthol, and benzo[a]pyrene (BaP).
Nicotine and Menthol Content Analysis in the Unburned Tobacco and Paper
To understand the distribution of nicotine and menthol in the selected products, and assess the percent transfer of nicotine and menthol to the mainstream smoke (MSS) of the little cigars compared to the 3R4F cigarette, total nicotine and menthol were measured in the unburned paper wrapper and the tobacco leaves of each product. Nicotine and menthol in the paper and tobacco were determined using methods previously reported by our laboratory.41 Briefly, each product was separated into rod (tobacco and paper) and filter. Tobacco and paper were then weighed, extracted in a solution containing isopropanol, methyl tert-butyl ether, 2N sodium hydroxide, and the internal standard (quinoline) via agitation using an orbital shaker (160 rpm) for 4 hours. The extracts were then analyzed for nicotine and menthol using gas chromatography with flame ionization detection (GC/FID).
RESULTS
Physical Properties of Little Cigars versus 3R4F Cigarette
Filter and wrapper materials
Filters of the 3R4F reference cigarette and the selected little cigars dissolved in acetone, suggesting all filters of the products used in this study are made of cellulose acetate.33 However, different patterns of MSS adsorption were observed in the filter of the 3R4F compared to little cigars after smoking under ISO conditions (Figures SI6–8 in the Supporting Information). Ventilation holes were found in the filter paper of the 3R4F, whereas no holes were detected in any of the filter papers of the selected little cigars (Figure SI5). Additionally, thickness measurements of the filter overwrap paper of the little cigars showed 3 zones of different thicknesses (Figure SI9 and Table SI3), but the 3R4F filter paper showed uniform thickness (Table SI3).
The level of nicotine in the paper wrapper of the 3R4F cigarette was found to be 1.6–3.8X higher than the nicotine levels of the little cigar paper wrappers of Cheyenne (both flavors) and Swisher Sweets-SO, but was comparable to the nicotine levels in the papers of the little cigar Swisher Sweets-SC (Table 1).
Table 1.
Physical Properties (Average ± Standard Error) Measured for the Selected Little Cigars and the 3R4F Reference Cigarette (N = 4)
| Physical Properties | Cheyenne Full Flavor | Cheyenne Menthol | Swisher Sweets Original | Swisher Sweets Sweet Cherry | 3R4F | |
|---|---|---|---|---|---|---|
| Physical Properties | ||||||
|
| ||||||
| Tobacco Mass (mg) | 988 ± 50 | 991 ± 50 | 805 ± 57 | 892 ± 20 | 753 ± 10 | |
|
| ||||||
| Consumed Tobacco (mg) | ISO | 787 ± 32 | 957 ± 3.2 | 641 ± 8.5 | 700 ± 10 | 579 ± 5 |
| CIR | 772 ± 57 | 882 ± 10 | 638 ± 6.3 | 688 ±17 | 568 ± 9 | |
|
| ||||||
| TPM (mg) | ISO | 18.9 ± 0.9 | 22.4 ± 1.1 | 17.8 ± 0.7 | 16.6 ± 0.8 | 7.3 ± 0.3 |
| CIR | 40.6 ± 1.3 | 45.6 ± 2.2 | 42.9 ± 1.5 | 42.9 ± 1.5 | 31.9 ± 1.3 | |
|
| ||||||
| Puff Number (N) | ISO | 11.5 ± 1.3 | 12.5 ± 1.0 | 8.8 ± 0.3 | 9.0 ± 0.05 | 7.0 ± 0.0 |
| CIR | 16.3 ± 1.9 | 15.3 ± 3.3 | 10.3 ± 0.5 | 11.2 ± 0.4 | 9.0 ± 0.0 | |
|
| ||||||
| Circumference (mm) | 24 ± 0.1 | 24 ± 0.1 | 24 ± 0.1 | 24 ± 0.1 | 24.5 ± 0.1 | |
|
| ||||||
| Length (mm) | 99 ± 0.2 | 98.8 ± 0.2 | 99 ± 0.3 | 99 ± 0.2 | 83.9 ± 0.2 | |
|
| ||||||
| Filter Lengtha (mm) | 35.3 ± 0.4 | 35.2 ± 0.2 | 34.9 ± 0.3 | 34.9 ± 0.2 | 26.7 ± 0.0 | |
|
| ||||||
| Nicotine | ||||||
|
| ||||||
| Total nicotine in paper wrapper (mg/g) | 0.9 ± 0.1 | 1.4 ± 0.1 | 0.6 ± 0.1 | 2.2 ± 0.1 | 2.3 ± 0.1 | |
|
| ||||||
| Total nicotine per mass of unburned tobacco (mg/g) | 8.8 ± 0.8 | 10.2 ± 0.6 | 11.7 ± 1.6 | 10.5 ± 0.9 | 16.1 ± 0.4 | |
|
| ||||||
| Mainstream smoke nicotine (mg/rod) | ISO | 0.84 ± 0.02 | 1.18 ± 0.06 | 0.88 ± 0.04 | 0.81 ± 0.03 | 0.60 ± 0.03 |
| CIR | 1.33 ± 0.06 | 2.01 ± 0.07 | 1.52 ± 0.09 | 1.50 ± 0.03 | 1.79 ± 0.06 | |
|
| ||||||
| Transmission of nicotine to smoke % | ISO | 12.2 ± 1.3 | 12.1 ± 1.0 | 11.8 ± 1.7 | 10.9 ± 1.1 | 6.4 ± 0.3 |
| CIR | 19.7 ± 2.5 | 22.4 ± 1.5 | 20.5 ± 3.1 | 20.6 ± 1.9 | 19.5 ± 0.8 | |
|
| ||||||
| Menthol | ||||||
|
| ||||||
| Total menthol per mass of unburned tobacco and paper wrapper (mg/g) | 0.08 | 70.9 | 0.05 | NDb | 0.05 | |
Note.
= Filter material is cellulose acetate for all tested little cigars and 3R4F;
= ND: Not detected
Tobacco mixture/leaves appearance
Different textures of tobacco mixtures were detected between the 2 product classes, as shown in Figure SI4 in the supporting information. The tobacco leaves/mixture of the 3R4F reference cigarette had a more uniform size, color, and texture than the tobacco leaves/mixture of the little cigars. Tobacco leaves/mixtures of the selected little cigars showed different colors, sizes, and textures among the selected flavors and brands (Figure SI4). The Swisher Sweets-SC little cigar showed the most uniform color, size, and texture compared to other little cigars (Figure SI4). Both the 3R4F and the Swisher Sweet-SC tobacco have yellow to light brown color, whereas other products, including Cheyenne (both flavors) and the Swisher Sweets-SO have multi-colored tobacco leaves including yellow, light brown, dark brown, and dark blue (Figure SI4).
Pressure drop
A positive relationship was observed (R2 = 0.88 for little cigars; and R2 = 0.91 for 3R4F) between the flow rate and the pressure drop across all evaluated products (Figure SI1). The pressure drop of the selected little cigars was higher than that of the 3R4F cigarette (Figures SI1). Pressure drop was also measured for both the tobacco rod and filter separately (Figure SI2b). In the 3R4F, the filter accounted for most of the resistance to draw, as the pressure drop that was measured for the filter only was about 16X higher than that of the tobacco part (Figure SI2b). In the little cigars, the tobacco rod and filter both contributed significantly to the resistance to draw. The filter of the little cigars showed a higher (1.5–2X) pressure drop than that of the tobacco (Figure SI2b).
Tobacco mass, consumed tobacco, and TPM
The average tobacco mass of the 3R4F reference cigarette measured in this study (753 ± 10 mg) was like that found in previous reports (755–760 mg)29,42 (Table SI1). The longer butt length used in this study resulted in a smaller (~7%) mass of consumed tobacco.42 Table 1 shows the comparison between results for the 3R4F and the selected little cigars. The tobacco mass of the little cigars was about 100–200 mg higher than that measured for the 3R4F reference cigarette. There was no significant difference between the consumed mass of tobacco under both puffing regimens (ISO and CIR). However, the consumed tobacco mass of Cheyenne (both flavors) was higher than that of Swisher Sweets (both flavors). The consumed mass of tobacco when smoking the 3R4F was lower than all the little cigars (Table 1).
As others have found, approximately 2 times more TPM mass was collected under CIR conditions than with the ISO smoking regimen.43,44 TPM also was evaluated as a percentage of mass of consumed tobacco and is presented in Figure 1. Figure 1 clearly shows that the percentage of TPM per mass of consumed tobacco under the ISO smoking regimen is significantly (p < .001) lower than that determined under the CIR for all products. The little cigars showed significantly higher percentages of TPM per mass of consumed tobacco under the ISO regimen compared with the 3R4F cigarette (p < .01). There were no significant differences observed across all the little cigars (Figure 1). Under CIR, the Swisher Sweets-SO showed a significantly higher percentage of TPM per mass of consumed tobacco compared to other little cigars and 3R4F (p < .05) (Figure 1).
Figure 1. The Total Particulate Matter (TPM) Expressed as a Fraction of the Mass of Tobacco Consumed during Machine Smoking According to ISO and CIR Regimens for the Selected Little Cigars.
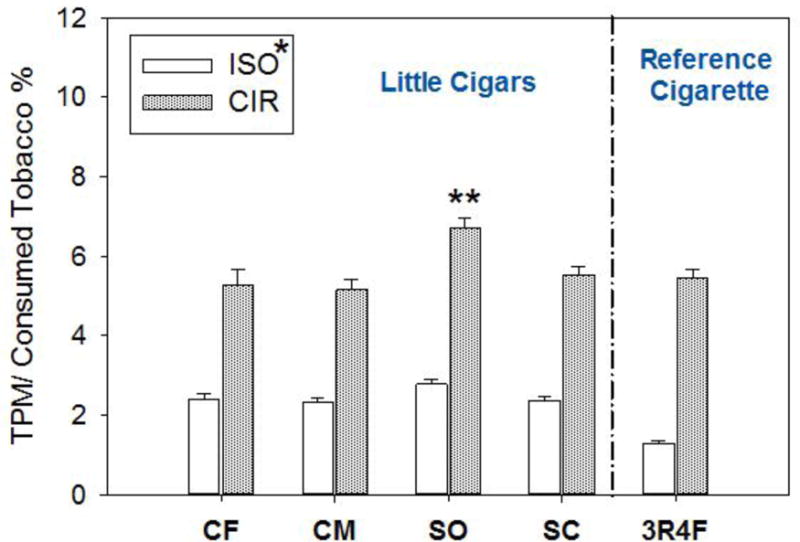
Note.
(CF= Cheyenne Full Flavor; CM = Cheyenne Menthol; SO = Swisher Sweets Original; SC = Swisher Sweets Cherry) and reference cigarette (3R4F); [*]: Statistically significant difference between ISO and CIR (p < .001); [**]: Swisher Sweet Original is statistically significantly higher than other little cigars, as well as the 3R4F (p < .05) under the CIR.
Length, circumference, and puff number
The circumference and length of the 3R4F measured in this study are comparable with previously published data.29,42,43,45 The longer butt length used for this study resulted in fewer puffs taken under both smoking ISO and CIR regimens (Table SI1).43,45 Table 1 shows the length, circumference and number of puffs taken for the 4 little cigars versus the 3R4F. The circumference was similar across all little cigars in this study and comparable with the 3R4F. The length of the whole rod of the little cigars was higher (~15 mm) than the length of the 3R4F, and the length of the filter part of the little cigars was about 8 mm greater than that of 3R4F (Table 1 and Figure SI2a). More puffs were taken for the little cigars compared with the 3R4F under the ISO and CIR regimen (Table 1).
Mainstream Smoke Semivolatile HPHC Yields
Mainstream smoke semivolatile HPHC yields measured under ISO and CIR conditions for the 3R4F were normalized per mass of mainstream TPM and are presented along with previously published data in Table SI1. Our results showed a slightly higher (~10%) average yield per mass of TPM than previous reports.42,43,45 Normalized semivolatile HPHC yields for the 3R4F obtained under ISO conditions were higher (1.4–2X) than those obtained under CIR conditions. These results agreed with the ISO versus CIR differences (1.5–2X) obtained previously43 (Table SI1).
Table 2 shows the semivolatile HPHC yields per mass of TPM for the selected little cigars and 3R4F. When obtained under the ISO regimen, TSNAs (NNN, NNK) and BaP levels per mass of TPM for Cheyenne (both flavors) were comparable to those for the 3R4F. The highest TSNA levels were measured for Swisher Sweet-SO (p < .05) compared to other little cigars and the 3R4F (Table 2 and Figure 2). In contrast to the TSNAs, the lowest yield per mass of TPM for BaP was found for Swisher Sweets-SO and the highest (p < .05) for Cheyenne-CF when compared to other little cigars and the 3R4F under both smoking regimens. Under the CIR, BaP normalized yields for the 3R4F and the little cigars were not significantly different (Figure 2). Under the ISO smoking regimen, nicotine per mass of TPM obtained for 3R4F was significantly (p < .05) higher than both brands of little cigars (Table 2 and Figure 2). Additionally, the percentage of nicotine compared to the mass of TPM for 3R4F was significantly higher than that measured for the selected little cigars when using ISO (p < .001) and CIR (p < .01) (Figure SI3). The percentage of nicotine to the mass of TPM collected under ISO conditions was significantly higher (p < .05) than the percentage found when using CIR across all products (Figure SI3).
Table 2.
Mainstream Smoke Semivolatile HPHCs Yields Expressed as a Fraction of the Total Particulate Matter Generated (Average ± Uncertainty¥) for the Selected Little Cigars and the 3R4F Reference Cigarette (N = 4)
| Chemical Component (Yield/mg TPM) | Cheyenne Full Flavor | Cheyenne Menthol | Swisher Sweets Original | Swisher Sweets Sweet Cherry | 3R4F |
|---|---|---|---|---|---|
| ISO | |||||
| NNN (ng/mg TPM) | 13.6 ± 0.7¥ | 14.8 ± 0.8 | 49.4 ± 2.5 | 22.8 ± 1.2 | 14.3 ± 1.1 |
| NNK (ng/mg TPM) | 14.2 ± 0.9 | 11.3 ± 0.6 | 30.0 ± 1.4 | 17.5 ± 1.2 | 11.9 ± 1.0 |
| BaP (ng/mg TPM) | 0.9 ± 0.06 | 0.8 ± 0.06 | 0.6 ± 0.05 | 0.7 ± 0.04 | 0.8 ± 0.1 |
| Nicotine (μg/mg TPM) | 44.5 ± 2.4 | 52.4 ± 2.4 | 49.2 ± 2.9 | 48.6 ± 3.0 | 80.7 ± 5.1 |
| Menthol (μg/mg TPM) | NDa | 41.7 ± 2.5 | NDa | NDa | NDa |
| CIR | |||||
| NNN (ng/mg TPM) | 10.7 ± 0.6 | 12.4 ± 0.6 | 36.2 ± 1.4 | 18.5 ± 0.6 | 9.2 ± 0.5 |
| NNK (ng/mg TPM) | 10.8 ± 0.9 | 9.6 ± 0.7 | 23.2 ± 0.9 | 15.6 ± 0.6 | 8.7 ± 0.5 |
| BaP (ng/mg TPM) | 0.8 ± 0.05 | 0.6 ± 0.04 | 0.4 ± 0.05 | 0.5 ± 0.04 | 0.4 ± 0.02 |
| Nicotine (μg/mg TPM) | 32.8 ± 1.8 | 44.1 ± 2.6 | 35.5 ± 2.6 | 39.2 ± 1.2 | 55.9 ± 2.9 |
| Menthol (μg/mg TPM) | 0.3 ± 0.01 | 34.0 ± 2.5 | 0.3 ± 0.03 | NDa | NDa |
Note.
= ND: Not detected
Combined Standard Uncertainty = (X value/Y value) *[SQRT (x standard deviation/X value)2 + (y standard deviation/Y value)2]
Figure 2. Levels of Semivolatile HPHCs in the Mainstream Smoke of the Selected Little Cigars.
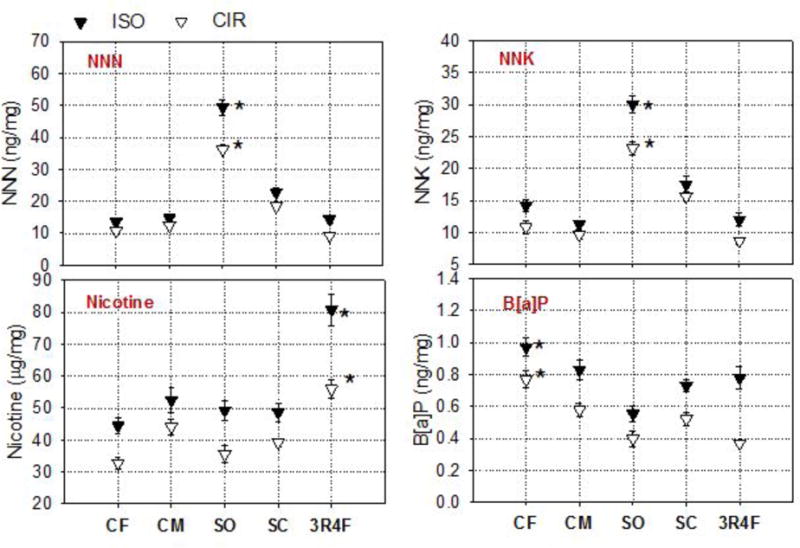
Note.
(CF = Cheyenne Full Flavor; CM = Cheyenne Menthol; SO = Swisher Sweets Original; SC = Swisher Sweets Cherry) and the reference cigarette (3R4F), expressed as a fraction of the mass of mainstream TPM (mg), generated using machine smoking under the ISO and CIR regimens; [*] indicates that the result is statistically significantly (p < .05) higher than other products.
When smoking under ISO conditions, menthol was detected only in Cheyenne-CM (41.7 ± 2.5 μg/mg TPM) (Table 2). But when smoking under CIR, menthol was detected in Cheyenne-CF (0.3 ± 0.01 μg/mg TPM); and Swisher Sweet-SO (0.3 ± 0.03 μg/mg TPM); along with the Cheyenne-CM (34 ± 2.5 μg/mg TPM) (Table 2).
In general, semivolatile HPHCs measured in MSS generated under the ISO regimen for the selected products had the same pattern when compared with the measurements obtained under CIR (Figure 2). Also, semivolatile HPHC yields normalized by mass of TPM were not significantly different between the 2 puffing regimens for a given product (Figure 2).
Yields of nicotine, NNN, NNK, and BaP were also normalized per mass of consumed tobacco and are presented in Figure 3. Under the CIR regimen, like the normalization to mass of TPM results, the highest (p < .05) TSNA normalized yields were obtained for Swisher Sweets-SO. The highest nicotine (p < .05) result was obtained for the 3R4F cigarette; and the highest yield of BaP per mass of consumed tobacco was obtained for the Cheyenne-CF (p < .05) (Figure 3). In general, the yields per mass of consumed tobacco obtained under CIR were significantly (p < .05) higher than those obtained under the ISO smoking regimen (Figure 3).
Figure 3. Levels of Semivolatile HPHCs in the Mainstream Smoke of Selected Little Cigars.
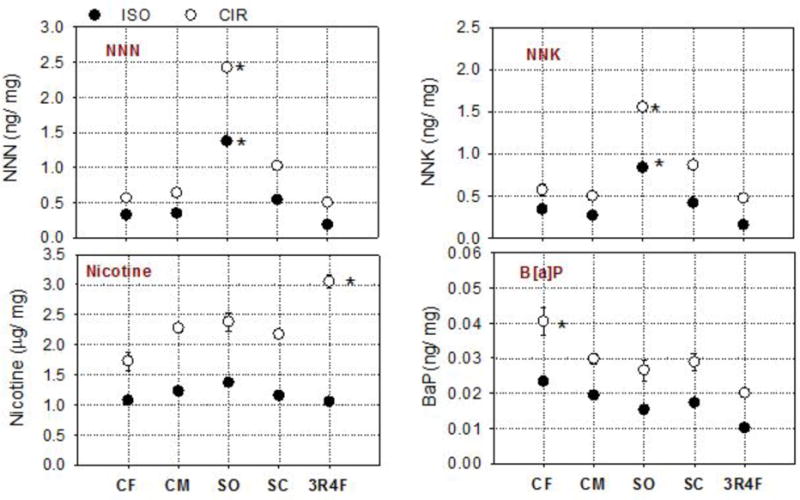
Note.
(CF = Cheyenne Full Flavor; CM = Cheyenne Menthol; SO = Swisher Sweets Original; SC = Swisher Sweets Cherry) and the reference cigarette (3R4F), expressed as a fraction of the tobacco consumed (mg) during machine smoking under the ISO and CIR regimens; [*] indicates that the result is statistically significantly (p < .05) higher than other products.
To understand the amount of semivolatile HPHCs emitted per mass of nicotine that is delivered to the mainstream smoke, compounds measured under both smoking regimens were normalized to MSS nicotine yields. Results, presented in Table SI2, showed that all normalized yields are higher for the selected little cigars compared to 3R4F cigarette. Among the selected little cigars, the highest yields of TSNAs per mass of MSS nicotine were obtained for Swisher Sweets-SO, and the highest BaP result was obtained for the Cheyenne-CF under both smoking regimens (ISO and CIR) (Table SI2).
Nicotine and Menthol Content
Nicotine content of the tobacco filler of the 3R4F cigarette was significantly higher (p < .01) than the nicotine in the tobacco filler of all the selected little cigars (Table 1). In the MSS, nicotine yield per rod (tobacco filler plus paper) was higher for the little cigars under ISO, but lower under the more intense CIR regimen compared to the 3R4F yield. An exception was the nicotine yield of the mentholated little cigar, which yielded significantly higher amounts of nicotine (~1.5X) than the non-mentholated product of the same brand for both regimens (Table 1). The fraction of nicotine that is transmitted from the tobacco filler to the MSS was significantly (p < .01) higher for the little cigars compared to the 3R4F cigarette under ISO conditions, but levels were comparable under CIR conditions (Table 1). Across both product types, the transmission of nicotine from the tobacco filler to the MSS under CIR was significantly higher (p < .01) than that under the ISO regimen (Table 1).
Menthol was detected in the tobacco filler and paper wrapper of all the products, including the 3R4F cigarette, except for the flavored little cigar Swisher Sweets-SC (Table 1).
DISCUSSION
Physical Properties of Little Cigars and 3R4F Cigarette
Physical properties, cigarette design, and smoking behavior may influence the levels of HPHCs in the MSS.5,10,18–20 Previous studies have examined the effect of different physical properties and smoking regimens on the levels and toxicity of the MSS HPHCs across different brands of cigarettes.3,10,19,33,42,43,45,46 To understand how these variables in cigarettes are different from little cigars, we compared the physical properties of the most popular little cigars27,28 with the 3R4F. Our results showed that each of the selected little cigars are longer (~15 mm), and therefore, hold more tobacco mass (100–200 mg), than the 3R4F. Additionally, a higher MSS TPM mass was obtained for the selected little cigars versus the 3R4F (Table 1). Different levels of MSS TPM mass and its associated semivolatile HPHCs might have different toxicity.3,43
Additionally, the selected little cigars showed different tobacco textures and colors implicating different blends that were used for manufacturing these products. The 3R4F that was used in this study is a US blend of tobacco (35.41% of flue cured “bright” tobacco, 21.62% burley, 12.07% oriental, 1.35% Maryland, and 29.55% reconstituted tobacco29). The homogeneous mixture of 3R4F tobacco contrasted with the different colors, textures, and sizes of tobacco leaves of the selected little cigars (Figure SI4). These different mixtures of tobacco, suggesting different types of tobacco leaves and different manufacturing processes, may affect the levels of the MSS HPHCs.14,15,39–41 Thus, studying the role of different tobacco blends on the levels and toxicity of MSS HPHCs of little cigars could help in regulating these products.
Other product design variables that could contribute to the transmission of chemicals from the tobacco filler to the MSS include filter ventilation.13,47,48 Our results showed that the 3R4F cigarette filter paper had ventilation holes, whereas no holes were observed in any of the selected little cigars (Figure SI5). Ventilation holes have been shown to reduce MSS yields from machine smoking by dilution.49 Also, the filter paper manufacturing and design of the 3R4F cigarette were different from the selected little cigars (Table SI3 and Figure SI9). These observations might explain the differences in the MSS adsorption pattern in the filter (Figures SI6–SI8) after smoking and the higher pressure drop of little cigars versus the 3R4F cigarette (Figures SI1 and SI2b).
These results show some distinct differences between the physical properties of a small group of selected little cigars and those of a representative reference cigarette. Rigorous studies of the physical properties of more little cigar products are needed to understand their impact on the MSS HPHC yields. This information is important to the development and implementation of future regulations surrounding little cigars, given FDA CTP’s new authority over these products.
Semivolatile HPHCs of Little Cigars and 3R4F Cigarette
The little cigars had higher yields of NNN, NNK and BaP per mass of TPM than the 3R4F, but the 3R4F cigarette showed the highest nicotine yields (Table 2 and Figure 2). Nicotine in the unburned tobacco of the 3R4F was also higher than in all the selected little cigars (Table 1). Studies have found that tobacco leaves that pass through the fermentation process (an additional aging process applied after curing tobacco) undergo chemical transformation by which the nitrogenous compounds in the tobacco leaves (eg, nicotine) interact with the ambient nitrogen oxides to form TSNAs (ie, NNN and NNK), and thus, nicotine levels decrease.50,54 The tobacco used in little cigars undergoes additional fermentation, whereas that used in cigarettes does not.15,50–53 This additional processing of the little cigar tobacco might explain the lower nicotine levels in the unburned tobacco. Decreased nicotine could influence user behaviors and the addictive nature of little cigars. This processing also may affect TSNA levels in the MSS, although the transmission efficiency of TSNAs was not determined in this study. In fact, when compared amongst the little cigars, MSS TSNA yields that were normalized to the TPM (Figure 2), and to the consumed tobacco (Figure 3), showed high variabilities. For example, Swisher Sweets-SO had the highest levels of TSNAs followed by Swisher Sweets-SC, and Cheyenne (both flavors) had MSS TSNA yields comparable to the 3R4F cigarette. This might implicate other parameters such as the type of curing and the tobacco type/blend,54,55 as well as the storage of the tobacco leaves (before manufacturing the product),45 in the formation of TSNAs in the MSS. Nevertheless, these results imply that tobacco processing may play a role in the HPHCs yields in mainstream smoke from little cigars. These processes may be important to consider in regulatory efforts to reduce the level of toxicant delivery from little cigars.
The delivery of some toxicants may be related. Previous studies on US and non-US brand cigarettes have shown an inverse relationship between MSS BaP and TSNA levels,55 a finding attributed to the decomposition of the PAHs, including BaP, by the nitrogen oxides that result in the formation of TSNAs.54–56 Our data show a similar trend in that the TSNA levels are mirror images of the BaP levels for the 4 little cigar brands tested (Figures 2 and 3).
For all products, our results showed that the transmission of nicotine from the unburned tobacco to the mainstream smoke when smoking under the CIR is higher than when smoking under the ISO regimen (Table 1), ie, more intense puffing and blocked ventilation holes result in a larger fraction of the tobacco’s nicotine content being transferred into the MSS. In addition, the more intense CIR regimen eliminated differences in nicotine transmission between the little cigars and the 3R4F. Under both regimens and per rod, delivery of nicotine for the little cigars was equivalent to or greater than that of the 3R4F cigarette (Table 1), results that concur with those Chen and Pankow10 reported. This finding makes little cigars attractive from a consumer’s viewpoint, in that little cigars deliver just as much nicotine, if not more, per rod than cigarettes, but cost half as much. However, this relationship may be offset by the fact that little cigars also deliver a greater mass of TPM than the 3R4F (Table 1), which is associated with harshness.57 Further studies are needed to investigate the links between the MSS chemistry of little cigars and their growing appeal in the US.23
Menthol has been detected in non-mentholated cigarettes.58 Our results also show the presence of menthol in the 3 non-mentholated little cigars studied. As with cigarettes, this may be done by the manufacturer to reduce the harshness of the “unflavored” little cigar smoke. Studies of cigarettes have found that menthol may be added intentionally to non-menthol cigarettes to make them more appealing, but the addition also may result from contamination/carry-over at the manufacturing facilities, or occur naturally in the tobacco leaves.58,62,63
When compared with previously published data for cigarettes, the menthol level in the Cheyenne Menthol little cigar was about 12X the level of menthol in the cigarettes labeled as mentholated cigarettes.58 We also found that the per rod nicotine delivery of the mentholated Cheyenne little cigar was substantially higher (1.5X) than the non-mentholated little cigar of the same brand. This may impact the product appeal as well as user behaviors and exposures. Menthol reduces the tobacco smoke’s harshness59 and was found to inhibit nicotine metabolism which enhances nicotine delivery and increases exposure.60,61 As these data are only from one little cigar, more work is needed to understand mentholation levels and nicotine delivery across a variety of mentholated little cigars.
The rise in little cigar use, particularly among youth, dictates the need to better understand the potential health implications of these tobacco products, yet, little is currently known. Cigar smoking in general has been linked to multiple serious health consequences,4 and regular cigar smoking, including little cigars, has been shown to account for 9000 premature deaths annually.64 Yet, much of the public believes that cigars are safer than cigarettes.65 Reports have indicated that cigar smoke in general is as toxic as cigarette smoke.4 Our data indicate that under more intense machine smoking regimens, which still may underestimate human smoking behaviors,66 yields of toxic HPHCs from little cigars in particular are 1–4X higher than those found in a representative reference cigarette. In addition, the delivery of nicotine at levels like those found in the reference cigarette indicate the addictive potential and appeal of little cigars. Our studies focused only on laboratory machine smoking HPHC yields across a small number of products. More studies, particularly those based on human puffing topographies and exposures, across a wider range of little cigars are needed to understand the health implications of the increasingly popular little cigars more clearly, and support the FDA’s regulations of these newly deemed products.
IMPLICATIONS FOR TOBACCO REGULATION
The FDA now has broad authority to regulate or restrict the manufacturing of little cigars to protect public health.26 This could include banning or restricting the use of flavors, as with cigarettes, or setting specific product standards that reduce the harm caused by use of little cigars. The FDA relies on objective, science-based evidence to inform its rulemaking. Yet, studies examining the physical properties and mainstream smoke HPHC yields of little cigars are scarce. This report shows that physical properties and MSS semivolatile HPHC yields of the little cigars tested are different from those of the 3R4F reference cigarette. These results indicate that important variables to be considered in the regulation of little cigar products include the tobacco blend, manufacturing processes (curing and fermentation), storage of tobacco leaves before manufacturing, little cigar design, and menthol content and delivery. Although these variables have been well studied in cigarettes, it is important that they be studied in cigar products to understand how the toxicity of little cigars may differ from that of cigarettes.
Supplementary Material
Acknowledgments
The authors thank Ms. Laura Wilson and Ms. Christina Saeger at the Battelle Public Health Center for Tobacco Research for their help in analyzing the samples.
Grant number P50CA180523 from the National Cancer Institute and FDA Center for Tobacco Products (CTP) awarded to the University of Maryland supported the research reported in this paper. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the FDA.
Footnotes
Human Subjects Approval
Research leading to the preparation of this paper did not involve use of human subjects.
Conflict of Interest Statement
The authors have no conflicts of interest to report.
Contributor Information
Samera H. Hamad, Postdoctoral Researcher, Tobacco Center of Regulatory Science, University of Maryland School of Public Health, College Park, MD.
Nathan M. Johnson, Technician, Battelle Public Health Center for Tobacco Research, Battelle Memorial Institute, Columbus, OH.
Margaret E. Tefft, Researcher, Battelle Public Health Center for Tobacco Research, Battelle Memorial Institute, Columbus, OH.
Marielle C. Brinkman, Senior Research Scientist, Battelle Public Health Center for Tobacco Research, Battelle Memorial Institute, Columbus, OH.
Sydney M. Gordon, Research Leader, Battelle Public Health Center for Tobacco Research, Battelle Memorial Institute, Columbus, OH.
Pamela I. Clark, Research Professor, Tobacco Center of Regulatory Science, University of Maryland School of Public Health, College Park, MD.
Stephanie S. Buehler, Principle Research Scientist, Battelle Public Health Center for Tobacco Research, Battelle Memorial Institute, Columbus, OH.
References
- 1.World Health Organization (WHO) WHO report on the global tobacco epidemic, 2011. 2011 Available at: http://www.who.int/tobacco/global_report/2011/en/. Accessed January 29, 2016.
- 2.Corrao MA, Guindon GE, Cokkinides V, Sharma N. Building the evidence base for global tobacco control. Bull World Health Organ. 2000;78(7):884–890. [PMC free article] [PubMed] [Google Scholar]
- 3.Counts ME, Hsu FS, Laffoon SW, et al. Mainstream smoke constituent yields and predicting relationships from a worldwide market sample of cigarette brands: ISO smoking conditions. Regul Toxicol Pharmacol. 2004;39(2):111–134. doi: 10.1016/j.yrtph.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Burns D, Cummings KM, Hoffman D. Cigars: Health Effects and Trends. Bethesda, MD: US Department of Health and Human Services, National Institutes of Health, National Cancer Institute; (Smoking and Tobacco Control Monograph No. 9). Available at: https://cancercontrol.cancer.gov/brp/tcrb/monographs/9/m9_complete.pdf. Accessed July 18, 2017. [Google Scholar]
- 5.Liu C, Feng S, Van Heemst J, McAdam KG. New insights into the formation of volatile compounds in mainstream cigarette smoke. Anal Bioanal Chem. 2010;396(5):1817–1830. doi: 10.1007/s00216-010-3457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vu AT, Taylor KM, Holman MR, et al. Polycyclic aromatic hydrocarbons in the mainstream smoke of popular US cigarettes. Chem Res Toxicol. 2015;28(8):1616–1626. doi: 10.1021/acs.chemrestox.5b00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delnevo CD, Hrywna M. “A whole’nother smoke” or a cigarette in disguise: how RJ Reynolds reframed the image of little cigars. Am J Public Health. 2007;97(8):1368–1375. doi: 10.2105/AJPH.2006.101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann D, Wynder EL. Smoke of cigarettes and little cigars: an analytical comparison. Science. 1972;178(4066):1197–1199. doi: 10.1126/science.178.4066.1197. [DOI] [PubMed] [Google Scholar]
- 9.Klupinski TP, Strozier ED, Friedenberg DA, et al. Identification of new and distinctive exposures from little cigars. Chem Res Toxicol. 2016;29(2):162–168. doi: 10.1021/acs.chemrestox.5b00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen C, Pankow JF. Gas/particle partitioning of two acid-base active compounds in mainstream tobacco smoke: nicotine and ammonia. J Agric Food Chem. 2009;57(7):2678–2690. doi: 10.1021/jf803018x. [DOI] [PubMed] [Google Scholar]
- 11.Frankenburg WG. Chemical changes in the harvested tobacco leaf: part I. Chemical and enzymic conversions during the curing process. Adv Enzymol Relat Areas Mol Biol. 1946;6:309–387. doi: 10.1002/9780470122556.ch8. [DOI] [PubMed] [Google Scholar]
- 12.Bacon CW, Wenger R, Bullock JF. Chemical changes in tobacco during flue-curing. Ind Eng Chem. 1952;44(2):292–296. [Google Scholar]
- 13.Kozlowski LT, O’Connor RJ, Sweeney CT. Cigarette design. Bethesda, MD: US Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 2001. (Smoking and Tobacco Control Monograph No. 13). [Google Scholar]
- 14.Perfetti TA, Coleman WM, Smith WS. Determination of mainstream and sidestream cigarette smoke components for cigarettes of different tobacco types and a set of reference cigarettes. Beitrage zur Tabakforschung International (Contributions to Tobacco Research) 1998;18(3):95–113. [Google Scholar]
- 15.Karl V. Fermentation of Tobacco. Google Patents; 1931. Available at: https://www.google.com/patents/US1812459. Accessed April 6, 2016. [Google Scholar]
- 16.Baker F, Ainsworth SR, Dye JT, et al. Health risks associated with cigar smoking. JAMA. 2000;284(6):735–740. doi: 10.1001/jama.284.6.735. [DOI] [PubMed] [Google Scholar]
- 17.Pankow JF. A consideration of the role of gas/particle partitioning in the deposition of nicotine and other tobacco smoke compounds in the respiratory tract. Chem Res Toxicol. 2001;14(11):1465–1481. doi: 10.1021/tx0100901. [DOI] [PubMed] [Google Scholar]
- 18.Frankenburg WG. Chemical changes in the harvested tobacco leaf. II. Chem Enzym Convers Ferment Aging Adv Enzymol. 1950;10:325–441. doi: 10.1002/9780470122556.ch8. [DOI] [PubMed] [Google Scholar]
- 19.Stedman RL. Chemical composition of tobacco and tobacco smoke. Chem Rev. 1968;68(2):153–207. doi: 10.1021/cr60252a002. [DOI] [PubMed] [Google Scholar]
- 20.Gregg E, Hill C, Hollywood M, et al. The UK smoke constituents testing study. Summary of results and comparison with other studies. Beitrage zur Tabakforschung International (Contributions to Tobacco Research) 2004;21(2):117–138. [Google Scholar]
- 21.Ashley DL, Backinger CL. The Food and Drug Administration’s regulation of tobacco: the Center for Tobacco Products’ Office of Science. Am J Prev Med. 2012;43(5):S255–S263. doi: 10.1016/j.amepre.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 22.O’Connor RJ. Non-cigarette tobacco products: what have we learnt and where are we headed? Tob Control. 2012;21(2):181–190. doi: 10.1136/tobaccocontrol-2011-050281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Centers for Disease Control and Prevention. Consumption of cigarettes and combustible tobacco-United States, 2000-2011. MMWR Morb Mortal Wkly Rep. 2012;61:565–569. [PubMed] [Google Scholar]
- 24.Family Smoking Prevention and Tobacco Control Act. Public Law. 2009. 2009:111–31. HR 1256. Available at: https://www.fda.gov/TobaccoProducts/GuidanceCompli-anceRegulatoryInformation/ucm246129.htm. Accessed July 18, 2017.
- 25.Delnevo CD. Smokers’ choice: what explains the steady growth of cigar use in the US. Public Health Rep. 2006;121(2):116–119. doi: 10.1177/003335490612100203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.US Food and Drug Administration (FDA) Food and Drug Administration-Press Announcements – FDA takes significant steps to protect Americans from dangers of tobacco through new regulation. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm499234.htm. Accessed May 13, 2016.
- 27.CSP Category Management Handbook. Tobacco Cigars. 2014 Available at: http://www.cspnet.com/sites/default/files/magazine-files/Tobacco_Cigars_CMH_2014.pdf Accessed March 30, 2016.
- 28.CSP Category Management Handbook. Tobacco Cigars. 2013 Available at: http://www.cspnet.com/sites/default/files/magazine-files/CMH13_Tobacco_Cigars.pdf. Accessed March 30, 2016.
- 29.University of Kentucky. 3R4F Preliminary Analysis. Available at: https://ctrp.uky.edu/resources/pdf/webdocs/3R4F%20Preliminary%20Analysis.pdf. Accessed January 27, 2016.
- 30.Delnevo CD, Giovenco DP, Miller Lo EJ. Changes in the mass-merchandise cigar market since the tobacco control act. Tob Regul Sci. 2017;3(2):8–16. doi: 10.18001/trs.3.2(suppl1).2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.International Organization for Standardization. ISO 3402:1999 – Tobacco and tobacco products-atmosphere for conditioning and testing. ISO; Available at: http://www.iso.org/iso/catalogue_detail.htm?csnumber=28324. Accessed February 23, 2017. [Google Scholar]
- 32.Standard ISO. Routine analytical cigarette-smoking machine-definitions and standard conditions. 2000 Available at: http://www.standardsbis.in/Gemini/scoperef/SR16022.pdf. Accessed March 31, 2016.
- 33.Borgerding MF, Bodnar JA, Wingate DE. The 1999 Massachusetts benchmark study; final report. Brown & Williamson; 2000. Available at: http://legacy.library.ucsf.edu/tid/yek21c00. Accessed June 24, 2017. [Google Scholar]
- 34.International Organization for Standardization. ISO 6565: Tobacco and tobacco products: Draw resistance of cigarettes and pressure drop of filter rods. Standard conditions and measurement. 2015 Available at: https://www.iso.org/standard/64265.html. Accessed June 24, 2017.
- 35.Canada Government Tobacco Act: Tobacco Reporting Regulations, SOR/2000-273. Regulation June 26, 2000. Schedule 2: Official Methods for Collection of Emission Data on Mainstream Smoke. 2000 Available at: http://laws-lois.justice.gc.ca/eng/regulations/SOR-2000-273/index.html. Accessed June 24, 2017.
- 36.Calafat AM, Polzin GM, Saylor J, et al. Determination of tar, nicotine, and carbon monoxide yields in the mainstream smoke of selected international cigarettes. Tob Control. 2004;13(1):45–51. doi: 10.1136/tc.2003.003673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.University of Kentucky. Center for Tobacco Reference Products. Available at: https://ctrp.uky.edu/. Accessed June 24, 2017.
- 38.Chuang JC, Wilson NK, Lewis RG. Methodology of ambient air monitoring for polycyclic aromatic hydrocarbons. Fresenius Environ Bull. 1999;8(9):547–556. [Google Scholar]
- 39.Chuang JC, Kuhlman MR, Wilson NK. Evaluation of methods for simultaneous collection and determination of nicotine and polynuclear aromatic hydrocarbons in indoor air. Environ Sci Technol. 1990;24(5):661–665. [Google Scholar]
- 40.Chuang JC, Callahan PJ, Lyu CW, Wilson NK. Polycyclic aromatic hydrocarbon exposures of children in low-income families. J Expo Anal Environ Epidemiol. 1999;9(2):85–98. doi: 10.1038/sj.jea.7500003. [DOI] [PubMed] [Google Scholar]
- 41.MacGregor IC, Stanfill SB, Gordon SM, et al. Custom mentholation of commercial cigarettes for research purposes. Toxicol Rep. 2014;1:1068–1075. doi: 10.1016/j.toxrep.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marcilla A, Beltran MI, Gómez-Siurana A, et al. Comparison between the mainstream smoke of eleven RYO tobacco brands and the reference tobacco 3R4F. Toxicol Rep. 2014;1:122–136. doi: 10.1016/j.toxrep.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roemer E, Schramke H, Weiler H, et al. Mainstream smoke chemistry and in vitro and in vivo toxicity of the reference cigarettes 3R4F and 2R4F. Beitrage zur Tabakforschung International (Contributions to Tobacco Research) 2012;25(1):316–335. [Google Scholar]
- 44.Counts ME, Morton MJ, Laffoon SW, et al. Smoke composition and predicting relationships for international commercial cigarettes smoked with three machine-smoking conditions. Regul Toxicol Pharmacol. 2005;41(3):185–227. doi: 10.1016/j.yrtph.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 45.Eldridge A, Betson TR, Gama MV, McAdam K. Variation in tobacco and mainstream smoke toxicant yields from selected commercial cigarette products. Regul Toxicol Pharmacol. 2015;71(3):409–427. doi: 10.1016/j.yrtph.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 46.Pryor WA, Church DF, Evans MD, et al. A comparison of the free radical chemistry of tobacco-burning cigarettes and cigarettes that only heat tobacco. Free Radic Biol Med. 1990;8(3):275–279. doi: 10.1016/0891-5849(90)90075-t. [DOI] [PubMed] [Google Scholar]
- 47.O’Connor RJ, Wilkins KJ, Caruso RV, et al. Cigarette characteristic and emission variations across high-, middle-and low-income countries. Public Health. 2010;124(12):667. doi: 10.1016/j.puhe.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shafer KH, Li S, Parrish M, Plunkett S. Cigarette with Smoke Constituent Attenuator. Google Patents; 2010. Available at: https://www.google.com/patents/US7757699. Accessed April 6, 2016. [Google Scholar]
- 49.Kozlowski LT, Frecker RC, Khouw V, Pope MA. The misuse of ‘less-hazardous’ cigarettes and its detection: hole-blocking of ventilated filters. Am J Public Health. 1980;70(11):1202–1203. doi: 10.2105/ajph.70.11.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hasselbring H. Fermentation of tobacco. Bot Gaz. 1910;49(3):235–236. [Google Scholar]
- 51.Harold BM, Moses HI. Manufacture of Cigarettes. Google Patents; 1939. Available at: https://www.google.com/patents/US2152416. Accessed May 29, 2016. [Google Scholar]
- 52.Thiele W. Manufacturing Cigars. Google Patents; 1929. Available at: https://www.google.com/patents/US1716250. Accessed May 29, 2016. [Google Scholar]
- 53.Fisher B. Curing the TSNA problem: major efforts are underway to eliminate tobacco-specific nitrosamines in US flue-cured tobacco. Tob Report. 2000a;127(8):1–6. [Google Scholar]
- 54.Sophia F, Spiegelhalder B, Preussmann R. Preformed tobacco-specific nitrosamines in tobacco—role of nitrate and influence of tobacco type. Carcinogenesis. 1989;10(8):1511–1517. doi: 10.1093/carcin/10.8.1511. [DOI] [PubMed] [Google Scholar]
- 55.Ding YS, Yan XJ, Jain RB, et al. Determination of 14 polycyclic aromatic hydrocarbons in mainstream smoke from US brand and non-US brand cigarettes. Environ Sci Technol. 2006;40(4):1133–1138. doi: 10.1021/es0517320. [DOI] [PubMed] [Google Scholar]
- 56.Hoffmann D, Wynder EL. The reduction of the tumorigenicity of cigarette smoke condensate by addition of sodium nitrate to tobacco. Cancer Res. 1967;27(1):172–174. [PubMed] [Google Scholar]
- 57.Brunnemann KD, Hoffmann D. The pH of tobacco smoke. Food Cosmet Toxicol. 1974;12(1):115–124. doi: 10.1016/0015-6264(74)90327-7. [DOI] [PubMed] [Google Scholar]
- 58.Ai J, Taylor KM, Lisko JG, et al. Menthol content in US marketed cigarettes. Nicotine Tob Res. 2016;18(7):1575–1580. doi: 10.1093/ntr/ntv162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gardiner P, Clark PI. Menthol cigarettes: moving toward a broader definition of harm. Nicotine Tob Res. 2010;12(Suppl 2):S85–S93. doi: 10.1093/ntr/ntq176. [DOI] [PubMed] [Google Scholar]
- 60.Benowitz NL, Herrera B, Jacob P. Mentholated cigarette smoking inhibits nicotine metabolism. J Pharmacol Exp Ther. 2004;310(3):1208–1215. doi: 10.1124/jpet.104.066902. [DOI] [PubMed] [Google Scholar]
- 61.Ton HT, Smart AE, Aguilar BL, et al. Menthol enhances the desensitization of human α3β4 nicotinic acetylcho-line receptors. Mol Pharmacol. 2015;88(2):256–264. doi: 10.1124/mol.115.098285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Merckel C, Pragst F, Ratzinger A, et al. Application of headspace solid phase microextraction to qualitative and quantitative analysis of tobacco additives in cigarettes. J Chromatogr A. 2006;1116(1):10–19. doi: 10.1016/j.chroma.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 63.Hopp R. Menthol: its origins, chemistry, physiology and toxicological properties. Recent Adv Tob Sci. 1993;19:3–46. [Google Scholar]
- 64.Nonnemaker J, Rostron B, Hall P, et al. Mortality and economic costs from regular cigar use in the United States, 2010. Am J Public Health. 2014;104(9):e86–e91. doi: 10.2105/AJPH.2014.301991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Terchek JJ, Larkin EM, Male ML, Frank SH. Measuring cigar use in adolescents: inclusion of a brand-specific item. Nicotine Tob Res. 2009;11(7):842–846. doi: 10.1093/ntr/ntp074. [DOI] [PubMed] [Google Scholar]
- 66.Koszowski B, Rosenberry ZR, Yi D, et al. Smoking behavior and smoke constituents from cigarillos and little cigars. Tob Regul Sci. 2017;3(2):31–40. doi: 10.18001/trs.3.2(suppl1).4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


