Abstract
Background
Gait and balance disorders are common among individuals who have experienced a mild to moderate traumatic brain injury (TBI). However, little is known about how the neuromuscular control of gait is altered following a TBI.
Research Question
Investigate the relationship between lower limb muscle activation patterns and chronic gait deficits in individuals who previously experienced a mild to moderate TBI.
Methods
Lower extremity electromyographic (EMG) signals were collected bilaterally during treadmill and overground walking in 44 ambulatory individuals with a TBI >1 year prior and 20 unimpaired controls. Activation patterns of TBI muscles were cross-correlated with normative data from control subjects to assess temporal phasing of muscle recruitment. Clinical assessments of gait and balance were performed using dynamic posturography, the dynamic gait index, six-minute walk test, and preferred walking speed.
Results
TBI subjects exhibited abnormal activation patterns in the tibialis anterior, medial gastrocnemius, and rectus femoris muscles during both overground and treadmill walking. Activation patterns of the vastus lateralis and soleus muscles did not differ from normal. There was considerable heterogeneity in performance on clinical balance and gait assessments. Abnormal muscle activation patterns were significantly correlated with variations in the dynamic gait index among the TBI subjects.
Significance
Individuals who have experienced a prior TBI do exhibit characteristic changes in the temporal coordination of select lower extremity muscles, which may contribute to impairments during challenging walking tasks.
Introduction
Individuals with prior mild to moderate traumatic brain injury (TBI) often report difficulties with balance and walking. Unfortunately following the first few months of recovery, there is often little improvement in walking ability [1]. It is generally believed that motor patterns become relatively fixed after this period, such that residual gait deficits become chronic [2–4]. Among TBI survivors, these chronic gait deficits have been linked to falls, a loss of mobility, and decreased quality of life [1,5,6]. Thus, there is a need to understand the motor patterns underlying chronic TBI gait. This understanding could help in identifying targets for treatment and evaluating the efficacy of emerging neurorehabilitation protocols, e.g. noninvasive neuromodulation [7], that are designed to induce fundamental shifts in the sensorimotor control of gait.
Prior studies have used clinical and quantitative gait analysis protocols to describe chronic TBI gait. Such studies consistently observe marked heterogeneity among the degree of balance and gait impairment present [3,8–12]. This heterogeneity has been linked to both self-reported balance problems and falls [13,14]. For example, individuals with a prior TBI who exhibited diminished scores on the Dynamic Gait Index (DGI) [15], a clinical assessment of an individual’s ability to walk under challenging conditions, were identified as being at risk for falls [14]. Spatiotemporal aspects of gait also differ, with TBI being linked to a tendency to walk more slowly, take shorter steps and exhibit greater mediolateral sway [8,16,17]. Joint kinematics of the lower extremity has also been shown to accurately classify a range of TBI-related gait disorders [3,8,18]. Thus, there seems to be a role for quantitative gait analysis to provide insights into gait deficits associated with prior TBI. However, to our knowledge, previous studies have not analyzed the underlying muscle coordination patterns that give rise to observed gait dynamics following TBI.
The purpose of this study was to investigate lower limb muscle activation patterns during walking in individuals who experienced a TBI more than one year prior. Muscle activation patterns were compared to those of healthy controls [19] to identify muscles which most often exhibit deviations from normal temporal phasing. We also assessed if abnormal muscle activation patterns were associated with clinical assessments of gait and balance.
Methods
Participants
Forty-four people with a balance disorder as a result of a traumatic brain injury (age: 53.4 ± 8.5 years, range: 28–64 years; 28 females; time since injury: 6.3±7.6 years, range: 1–33 years) and twenty control subjects (25.3 ± 3.3 years, 10 females) participated in the study. This protocol was approved by the University of Wisconsin–Madison Health Sciences Institutional Review Board, and all subjects provided written informed consent before participating. We only recruited individuals with a mild to moderate non-penetrative TBI, as confirmed by a “non-remarkable” neurological report from their medical records (i.e. no loss of brain matter or evidence of refractory hematoma, etc.), with a loss of consciousness < 24 hours. Each individual was at least one year post-injury, had completed a focused physical rehabilitation program for their TBI, and felt that he/she had reached a plateau in recovery. Participants were community dwelling, and had normal gait and balance prior to their brain injury. Further inclusion for the TBI group were: ambulatory and able to walk independently for 20 minutes; a sensory organization test (SOT) [20] composite score at least 8 points below the normal limit of normal (i.e. mean – 1.67*SD, adjusted for height and weight); absence of any other neurological disorder besides those attributed to their TBI, and no changes in health or medications in the 3 months prior to participation. Unimpaired control subjects had no history of neurological or musculoskeletal disorder or injury, had no contraindication to exercise, and were not at-risk for cardiovascular events. For complete inclusion/exclusion criteria, see supplementary information.
Clinical Assessment
Sensory Organization Test (SOT)
Computerized dynamic posturography assessed standing balance under six conditions challenging the visual, vestibular, and proprioceptive systems (NeuroCom Intl., Clackamas, OR). Three consecutive 20-s trials were performed while standing barefoot on a force platform under each condition: (1) eyes open; (2) eyes closed; (3) eyes open, sway-referenced visual surround; (4) eyes open, sway-referenced platform; (5) eyes closed, sway-referenced platform; (6) eyes open, sway-referenced platform and visual surround. An overall composite score (0–100) was calculated from body sway.
Dynamic Gait Index (DGI)
Gait ability was assessed using the Dynamic Gait Index [15]. Subjects performed eight functional walking tasks that included normal overground walking, changing gait speeds, walking with head turns, walking while turning, walking over and around obstacles, and stair climbing. Each task was scored 0–3 by a trained physical therapist, where 3 indicates normal. DGI scores ≤19 have been linked to falls for individuals with TBI [14].
Six-Minute Walk Test (6MWT)
Walking capacity was assessed using the 6-minute walk test [21,22]. Subjects were instructed to walk along a quiet, level hallway as fast as they could safely for six minutes. The hallway formed a continuous circular path such that no sharp turns were required. The test administrator followed each subject’s path with a measuring wheel, and recorded the total distance walked.
Preferred Walking Speed (PWS) and Treadmill Speed (PTS)
Each subject’s preferred overground walking speed was measured as subjects walked two times down a six-meter walkway at a ‘normal, comfortable pace’. Speed was calculated from the average time to cross the middle four meters of the walkway. Preferred walking speed has shown excellent test-retest reliability among TBI participants [23]. TBI subjects were put on the treadmill (Bronze Basic Treadmill, PaceMaster, Logan, UT) and the speed was initially set to match their PWS. Some subjects were unable to comfortably match their PWS on the treadmill. For these subjects, we lowered their treadmill speed to the highest speed where they felt they could walk comfortably. This speed was defined as the preferred treadmill speed (PTS).
Electromyographic Collections during Overground and Treadmill Walking
Lower limb electromyographic (EMG) activities were recorded bilaterally while subjects performed two 60-second walking trials: one on a treadmill at their PTS, and another while walking at a comfortable pace down a level hallway with no sharp turns. Two subjects exhibited difficulty walking on the treadmill even at very slow speeds and were allowed to hold on to the handrails for support. Healthy subjects walked on the treadmill at their preferred walking speed (1.2±0.2 m/s), and at a slower speed (1.0 m/s) that more closely matched the average PTS of the TBI subjects.
Surface electrodes (Trigno™ Wireless EMG: 4 bar contacts, 99.9% Ag, 5 × 1 mm, 10 mm inter-electrode distance, CMMR >80 dB, signal-to-noise ratio <0.75 µV) were placed over the tibialis anterior (TA), medial gastrocnemius (MG), soleus (SL), vastus lateralis (VL), rectus femoris (RF), and medial hamstrings (MH) muscles. After the skin was shaved and cleansed with an isopropyl alcohol swab, electrodes were coated with conductive gel and placed over the muscle bellies in line with fiber orientation. The standard EMG electrode locations were determined by the same investigator for each subject. EMG activities were recorded using a wireless system (Trigno™ Personal Monitor; Delsys Inc., Boston, MA). Heel-strike events were detected using the accelerometers of two Trigno sensors positioned at the ankle over the Achilles tendons [24,25]. All sensors were secured with elastic wrap. EMG and accelerometer signals were digitally sampled at 1926 and 148 Hz, respectively.
The EMG and accelerometery signals were processed using a custom MATLAB script (R2017a, MathWorks Inc., Natick, MA). Accelerometery data was low-pass filtered at 25 Hz using a 3rd order Butterworth filter, and heel strikes were identified from magnitude peaks in the filtered data [26,27]. EMG data was band-pass filtered (1–350 Hz) using a 4th order Butterworth filter, then full-wave rectified and low-pass filtered at 10 Hz to obtain the linear envelopes of muscle activation. Muscle activity over each gait cycle were extracted and time normalized to 101 points per stride. Muscle activation patterns were then ensemble averaged over all consecutive strides in the 60-sec trials. Ensemble averaged muscle activation patterns were amplitude normalized to their root-mean-squared to represent the underlying fixed muscle activation present in each subject. We also assessed the EMG variability over consecutive strides using the coefficient of variation (CV), defined as the root-mean-square of the EMG standard deviation over the gait cycle, divided by the mean ensemble average [28]. The control subject EMG activities were processed similarly and averaged across subjects to create a comparative activation profile for each muscle.
Comparing Activation Patterns Between TBI and Control Subjects
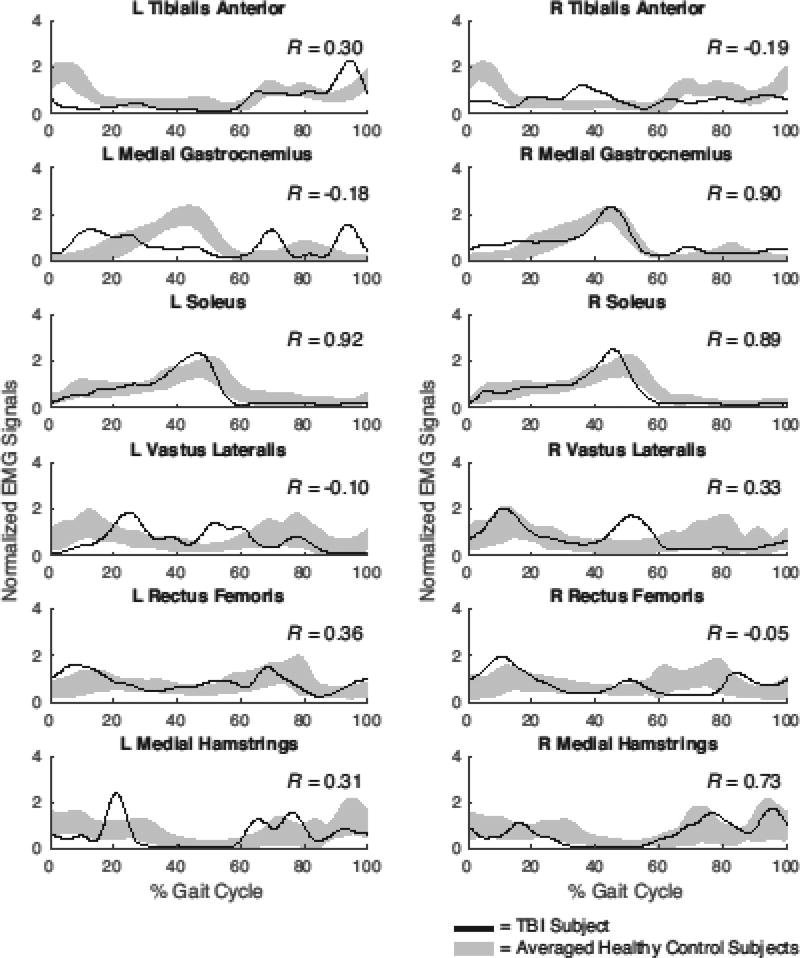
The timing and shape of individual muscle activation patterns were compared to normal using an EMG cross-correlation method [19]. Specifically, for each muscle, we computed the Pearson correlation coefficient (R) between a subject’s muscle activation pattern and the ensemble average pattern of the control subjects. Correlation coefficients closer to 1 meant the muscle activation timing was consistent with normal phasing over the gait cycle, while correlation closer to −1 indicated out of phase activity (Fig. 1). For each muscle, correlation coefficients were averaged across the two limbs of an individual.
Figure 1.
Muscle activations during treadmill walking for a representative TBI subject and normative patterns from twenty control subjects (shaded curves represent mean ±1 s.d.). Pearson cross-correlations (R-values) provide a measure of the agreement between the TBI and average control data.
Statistical Analysis
Mann–Whitney U tests were run to assess the significance of differences between TBI and control subjects in: (1) overground preferred walking speed; (2) muscle activation cross-correlations; (3) coefficient of variation across strides. We examined the association between clinical metrics and muscle cross-correlations for both treadmill and overground walking using a Spearman’s rank-order correlation. We controlled for variations in speed of the TBI subjects by using PWS as a covariate. We also used a Spearman’s rank-order correlation to investigate the potentially confounding effect of age on all the outcome measures of the TBI subjects. A p value <0.05 was considered statistically significant. All of the statistical analyses were performed using SPSS (v. 24, IBM Corp., Armonk, NY).
Results
Balance and Gait Assessments
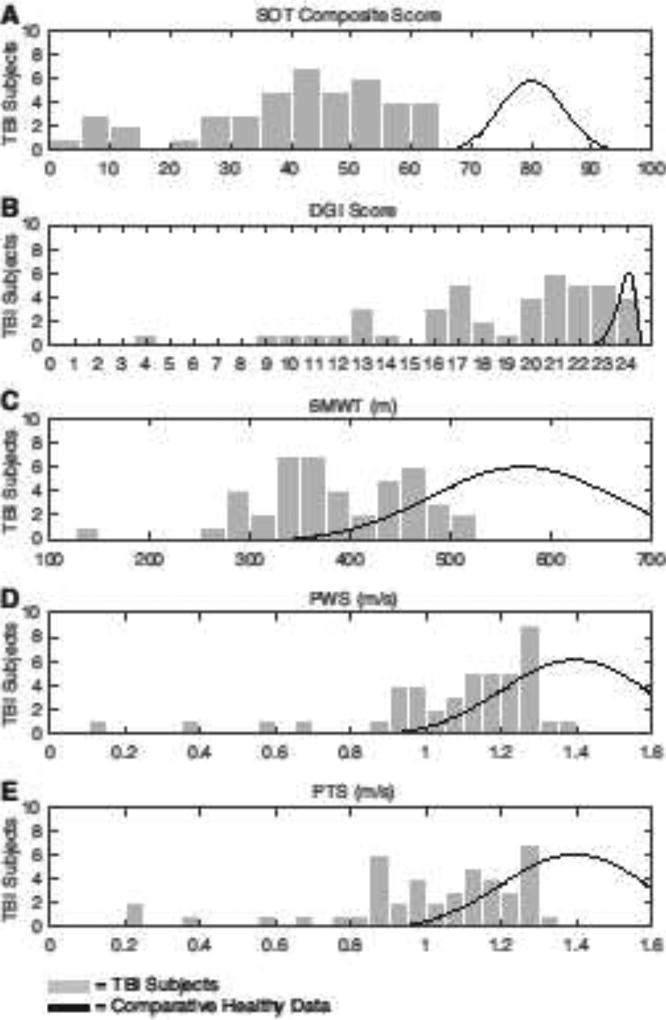
TBI subjects exhibited wide variability in preferred walking speed and performance on clinical balance and gait assessments (Fig. 2). The average PWS was 1.1±0.2 m/s, with the average PTS being 1.0±0.3 m/s. Median PWS was significantly lower for the TBI group (1.14 m/s) than the healthy controls (1.21 m/s), p = 0.036. For the TBI subjects, the SOT average composite score was 39±17. 36% of TBI subjects had an SOT composite score < 38, which is a threshold linked with an increased risk for falls [29]. The TBI subjects had an average DGI of 19±5. 45% of the TBI subjects had a DGI score ≤ 19, which is a threshold associated with an increased risk for falls. The 6MWT had an average distance of 386±78 m, which on average is outside of the normal range from 400 to 700 m measured in healthy adult controls of similar age [22,30]. For all these assessments, the age of the TBI subjects was not associated with their measured performance.
Figure 2.
Substantial heterogeneity is seen in the performance of the TBI group on the: A) sensory organization test (SOT), B) dynamic gait index (DGI), C) six-minute walk test (6MWT), D) preferred walking speed (PWS), E) and preferred treadmill speeds (PTS). For comparison, plotted are distributions of normative data for healthy individuals within similar age ranges as the TBI subjects: SOT (79.8±5.6, age: 20–59 years [48]), DGI (23.9±0.4, age: 50–69 [49]), 6MWT (571±90 m, age: 42–76 [30]), PWS and PTS (1.39±0.19 m/s, age: 50–69 [50]). Note that we screened for TBI subjects to have a Sensory Organization Test (SOT) composite score >8 points below normal, after adjusting for height and weight.
Preferred walking speeds, DGI and SOT assessments were not normally distributed, as assessed by Shapiro–Wilk’s test (p > 0.05). When controlling for PWS, the clinical assessments were not associated with each other. DGI vs 6MWT: rs = 0.252, p = 0.103. DGI vs SOT: rs = 0.238, p = 0.124. SOT vs 6MWT: rs = 0.223, p = 0.151. Similar results were obtained when controlling for walking speed by including PTS as a covariate.
Electromyography
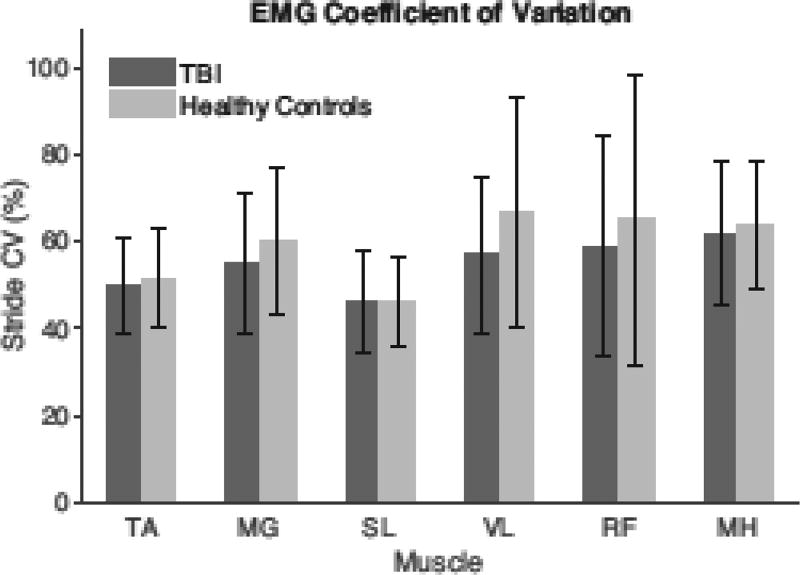
The TBI and healthy control group showed no significant differences (p > 0.05) in EMG variability about the ensemble averaged activation patterns for the muscles recorded (Fig. 3). Additionally, the relative EMG variability across different muscles was consistent with previous observations [28].
Figure 3.
The EMG variability about ensemble average muscle activation patterns was assessed using the coefficient of variation (CV), which can be considered a measure of the variability-to-signal ratio for each muscle [28]. There were no significant differences in inter-stride variability between the TBI and healthy control group for the muscles recorded.
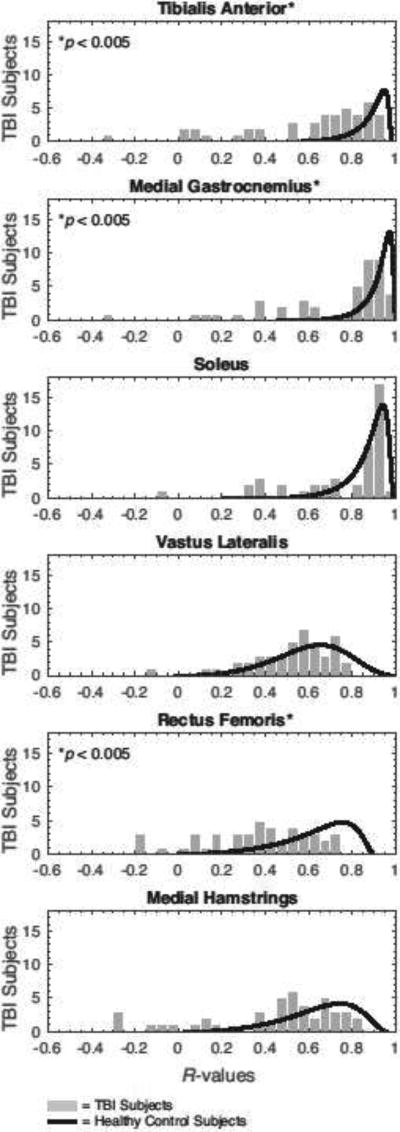
Select muscles exhibited variations from normative temporal patterns during gait in the TBI subjects (Fig. 4). During treadmill gait, median cross-correlations (R-values) were significantly lower in the TBI group for the tibialis anterior (TA, p < 0.001), medial gastrocnemius (MG, p = 0.001), and rectus femoris (RF, p = 0.002) muscles. There was also a tendency toward significance in the medial hamstrings (MH, p=0.06), while non-significant differences were found for the soleus (SL, p = 0.253) and vastus lateralis (VL, p = 0.241) muscles. Similar results were observed during overground gait, where median cross-correlations were significantly lower in TBI group for the TA (p < 0.001), MG (p = 0.020), and RF (p = 0.006) muscles. Correlations for the SL (p = 0.844), VL (p = 0.395), and MH (p = 0.102) did not differ between groups in the overground walking conditions. For all muscles, these correlations were not associated with the age of the TBI subjects during both overground and treadmill walking.
Figure 4.
Distributions of the muscle cross-correlations (R-values) for the TBI group compared with those of the healthy controls. Pearson cross-correlations were determined from treadmill walking data, and averaged between the left and right leg. Mann–Whitney U tests identified significant intergroup differences in the correlations exhibited by the tibialis anterior, medial gastrocnemius, and rectus femoris muscles (p<0.05). Similar distribution patterns were observed during overground walking.
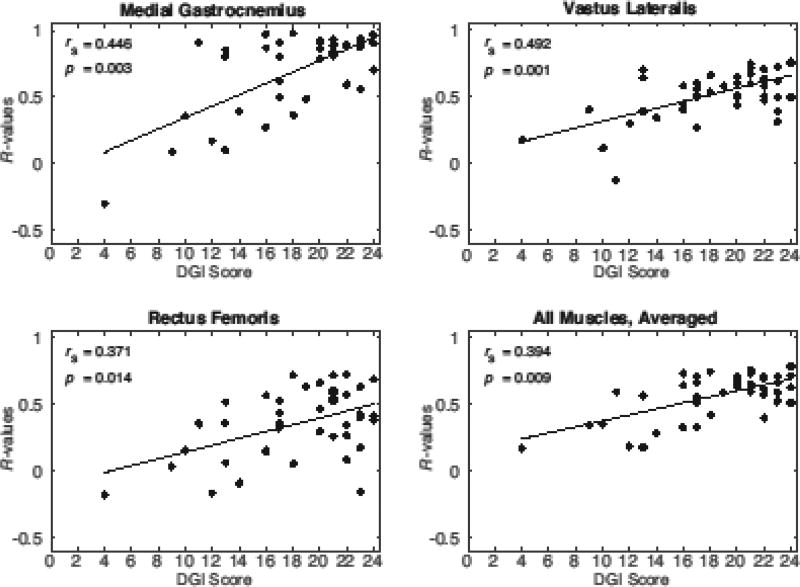
Correlations with Clinical Assessments
Among the TBI subjects, DGI scores were significantly associated with variations in the muscle cross-correlations (R-values) for both the MG and VL muscles (Table 1). DGI was also significantly associated with the RF correlations when walking on the treadmill, but not overground (Fig. 5). There was also a significant relationship between DGI and the average cross-correlation across all muscles for both overground (rs = 0.416, p = 0.010) and treadmill walking (rs = 0.394, p = 0.009). There was no relationship between the SOT or 6MWT and any muscle cross-correlations.
Table 1.
Spearman’s rank-order correlations (rs) between clinical metrics and muscle cross-correlations (average R-value between left and right leg) for both treadmill and overground walking, controlling for the preferred walking speed of the TBI subjects.
| Muscle cross- correlations (R-values) |
SOT | DGI | 6MWT | |||
|---|---|---|---|---|---|---|
| Treadmill | Overground | Treadmill | Overground | Treadmill | Overground | |
| Tibialis Anterior | ||||||
| rs | −0.146 | −0.303 | 0.134 | 0.080 | 0.065 | 0.000 |
| p | 0.352 | 0.068 | 0.391 | 0.640 | 0.679 | 1.000 |
| Medial Gastrocnemius | ||||||
| rs | 0.034 | −0.125 | 0.446 | 0.511 | 0.135 | 0.040 |
| p | 0.829 | 0.461 | 0.003 | 0.001 | 0.387 | 0.814 |
| Soleus | ||||||
| rs | 0.167 | −0.036 | 0.184 | 0.304 | 0.069 | −0.034 |
| p | 0.284 | 0.833 | 0.238 | 0.067 | 0.661 | 0.843 |
| Vastus Lateralis | ||||||
| rs | −0.062 | −0.185 | 0.492 | 0.427 | 0.119 | 0.006 |
| p | 0.691 | 0.272 | 0.001 | 0.008 | 0.448 | 0.973 |
| Rectus Femoris | ||||||
| rs | 0.038 | −0.174 | 0.371 | 0.092 | 0.238 | −0.114 |
| p | 0.811 | 0.304 | 0.014 | 0.587 | 0.124 | 0.503 |
| Medial Hamstrings | ||||||
| rs | −0.094 | 0.039 | 0.005 | 0.225 | 0.003 | −0.111 |
| p | 0.549 | 0.820 | 0.977 | 0.18 | 0.983 | 0.512 |
| All Muscles, averaged | ||||||
| rs | −0.037 | −0.092 | 0.394 | 0.416 | 0.078 | −0.117 |
| p | 0.816 | 0.588 | 0.009 | 0.010 | 0.618 | 0.492 |
Abbreviations: SOT = sensory organization test composite score; DGI = dynamic gait index; 6MWT = six-minute walk test.
Figure 5.
The cross-correlations (R-values) of the medial gastrocnemius, vastus lateralis, and rectus femoris muscles in the TBI group were significantly correlated with the Dynamic Gait Index (DGI), controlling for preferred walking speed. When R-values for all muscles recorded on a subject were averaged together, this composite R-value was also significantly correlated with DGI. Values shown are from the treadmill walking condition.
Discussion
The study aimed to assess altered muscular coordination of gait in a cross-section of individuals who sustained a traumatic brain injury (TBI) more than one year ago. Overall, we found that the TBI group exhibited abnormal activation patterns of select muscles during both treadmill and overground walking. We also found that abnormal muscle activation patterns were associated with poor performance on the Dynamic Gait Index (DGI). However, muscle activation patterns were unrelated to clinical assessments of standing balance (SOT) and six-minute walking tests (6MWT). These results suggest that quantitative EMG analysis could potentially be useful to track subtle changes in the coordination of gait that arise with TBI, and provide a potential avenue to quantitatively track meaningful changes in muscular coordination with neurorehabilitation.
We evaluated the disruption of temporal muscle activity by cross-correlating muscle activation patterns during gait with normative patterns collected on healthy control subjects. Thus, a high cross-correlation represents normal temporal phasing of a muscle’s activity level throughout the gait cycle. This cross-correlation method has previously been shown to be consistent across different walking trials, testing sessions, and examiners [19]. The TBI subjects in this study had R-values ranging from -0.32 to 0.98, representing a substantial range in variations from normative muscle activation patterns. This heterogeneity is a hallmark of chronic TBI gait [3,8,9], and likely reflects the highly variable nature of gait deficits seen in this population.
There was consistency in the muscles that exhibited deviations from the normal temporal activation patterns. In particular, the TBI subjects exhibited marked variations in the temporal activation patterns of the ankle dorsiflexors, and biarticular gastrocnemius and rectus femoris muscles. There was also a strong tendency for the hamstrings to exhibit abnormal muscle activity during treadmill walking. The dorsiflexor and biarticular muscles have also been shown to exhibit abnormal temporal phasing in other individuals with neurological injury due to stroke, Parkinson’s disease, and spinal cord injury [31– 33]. Interestingly, TBI subjects exhibited vastus lateralis (VL) and soleus (SL) muscle activation patterns that did not differ significantly from that seen in healthy controls. VL and SL are uniarticular extensor muscles that are normally active during load acceptance and push-off, respectively [34]. The major role of these extensor muscles is to provide vertical support of the body [35]. Thus, it is possible that mechanical constraints of walking generally dictate VL and SL during specific phases, imposing relative normative phasing of the gait cycle. In contrast, the dorsiflexors and biarticular muscles are believed to be used to fine-tune joint motion, modulate stiffness and facilitate inter-joint coordination [36,37], which may not necessitate the same mechanical constraints on activation timing.
It is interesting that abnormal muscle activation patterns could discriminate inter-individual differences in performance on the dynamic gait index (DGI). DGI is a clinical battery of eight locomotor tasks that challenge sensorimotor control processes during normal walking, walking while turning head, navigating obstacles, and climbing stairs. The range of DGI scores seen in this study are consistent with those previously reported in chronic TBI [14,38], with nearly half (45%) having a DGI score ≤ 19, which is defined as a threshold for an increased risk for falls [14]. A relationship between activation patterns in overground and treadmill gait with the DGI (Fig. 5) suggests that subtle abnormalities underlying normal walking may be amplified when sensory processing tasks are challenged in walking. Consistent with this idea, a prior study showed that an obstacle-crossing task could reveal gait instabilities within TBI subjects that were previously undetected via qualitative assessments [17]. The control of walking involves integration of neural activity arising from both spinal cord and cortical levels. The rhythmic activation patterns underlying gait are believed to arise in part from neural circuits within the spinal cord that can induce cyclic stepping, as has been shown in both infants and spinal cord injured individuals [39,40]. These rhythmic muscle activities are theorized to be supplemented with cortically modulated muscle activity that can enhance the robustness of gait in the presence of internal and external perturbations [41]. Thus, TBI may alter cortical modulation of the motor neuron pools [1], resulting in both mistimed activation patterns and challenges with dynamic balance, as seen in the DGI scores. A lack of relationship between abnormal gait activation patterns and performance on the SOT supports the idea that static and dynamic balance challenge different aspects of sensorimotor control [42,43].
There are limitations that should be noted with this study. First, the healthy controls were not matched for age, height, or weight and we cannot exclude the possibility that these differences may account for some of the observed differences between the TBI and healthy control subjects. For example, the TBI group was on average much older than the controls (+28 years, although the ages spanned a range of 28–64 years). However, we found that the age of the TBI subjects was not associated with their measures of balance and gait, nor was it associated with cross-correlations of muscle activation, suggesting that the group differences we observed were not explained by differences in age. Further, a prior study found that age-related changes in muscle activation patterns generally only emerge at faster speeds [44], where the current study was focused at speeds that would be classified as slow and normal in healthy controls. We also acknowledge that the wide variability of performance observed in the TBI subjects might further be explained by differences within the TBI subjects, such as the type and severity of trauma sustained or differences in body dimensions, which we did not accounted for in this study. Second, we used the ensemble average muscle activation obtained over multiple strides, rather than individual stride data. However, we did not observe significant differences in inter-stride variance, suggesting that the activation patterns were relatively stable for each group. Even though several subjects found walking on a treadmill to be considerably challenging, we found that the significant abnormal activation we reported was consistent between the treadmill and overground walking. Additionally, although we time-normalized each subject’s muscle activation pattern to 100% of the gait cycle, we did not account for altered temporal gait parameters within the gait cycle, such as single limb support time. As altered temporal parameters are characteristic of TBI gait [12], we acknowledge this may influence the correlation between individual muscle activation patterns. Lastly, our study analyzed the activation of each muscle independently. In future studies, we plan to use synergy analysis to better understand both the complexity and inter-muscle coordination of muscles throughout the lower limbs [7,45,46].
In summary, we have shown that individuals who have experienced a prior TBI do exhibit changes in activation patterns of characteristic muscles during gait. Abnormal muscle activation patterns were associated with observable deficits in challenging walking tasks, suggesting that the abnormal patterns could reflect a profound and permanent impairment in the neurological control of walking. In continuing studies, we will assess the potential to use neuromodulation protocols [7,47] to induce fundamental shifts in the coordination of gait among individuals exhibiting gait deficits due to a prior TBI.
Supplementary Material
Highlights.
Investigated EMG activation patterns during gait following a traumatic brain injury
TBI individuals exhibited abnormal temporal activation in select muscles
Abnormal activation patterns were associated with scores on the dynamic gait index
Quantitative EMG analysis could be used to track shifts in the coordination of gait
Acknowledgments
Development of the TBI training and testing methods used in this study were supported by the Department of Defense [W81XWH-13-1-0081]. Samuel Acuña's participation in the EMG collection and analysis was supported by a National Institutes of Health training grant [R25GM08325]. The authors would like to thank the staff of the Tactile Communication and Neurorehabilitation Laboratory, Holly Shoenberg, Michael Schmidt, Kristen Rasske, and Emily Keuler for assistance in data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contribution statement:
MT, YD, and DG conceived and designed the study; SA, MT, and YD acquired data; SA and DG analyzed and interpreted data; SA and DG prepared manuscript; Each of the authors has read and concurs with the content in the final manuscript.
Each of the authors has read and concurs with the content in the final manuscript. The material within has not been and will not be submitted for publication elsewhere except as an abstract
Conflict of Interest:
Danilov and Tyler have financial interests in Advanced NeuroRehabilitation, LLC and NeuroHabilitation Corp., which both have intellectual property rights to technology reported in this article.
References
- 1.Kozlowski DA, Leasure JL, Schallert T. The control of movement following traumatic brain injury. Compr. Physiol. 2013;3:121–139. doi: 10.1002/cphy.c110005. [DOI] [PubMed] [Google Scholar]
- 2.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 2006;21:375–8. doi: 10.1097/00001199-200609000-00001. doi:00001199-200609000-00001 [pii] [DOI] [PubMed] [Google Scholar]
- 3.Williams G, Lai D, Schache A, Morris ME. Classification of Gait Disorders Following Traumatic Brain Injury. J. Head Trauma Rehabil. 2015;30:E13–E23. doi: 10.1097/HTR.0000000000000038. [DOI] [PubMed] [Google Scholar]
- 4.Sosnoff JJ, Broglio SP, Shin S, Ferrara MS. Previous mild traumatic brain injury and postural-control dynamics. J. Athl. Train. 2011;46:85–91. doi: 10.4085/1062-6050-46.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olver JH, Ponsford JL, Curran CA. Outcome following traumatic brain injury: a comparison between 2 and 5 years after injury. Brain Inj. 1996;10:841–848. doi: 10.1080/026990596123945. [DOI] [PubMed] [Google Scholar]
- 6.Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: A public health perspective. J. Head Trauma Rehabil. 1999;14:602–15. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Danilov Y, Kaczmarek K, Skinner K, Tyler M. Cranial Nerve Noninvasive Neuromodulation: New Approach to Neurorehabilitation. In: Kobeissy FH, editor. Brain Neurotrauma Mol. Neuropsychol. Rehabil. Asp. CRC Press; Boca Raton, FL: 2015. pp. 605–628. [DOI] [PubMed] [Google Scholar]
- 8.Williams G, Morris ME, Schache A, McCrory PR. Incidence of Gait Abnormalities After Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2009;90:587–593. doi: 10.1016/j.apmr.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Niechwiej-Szwedo E, Inness EL, Howe JA, Jaglal S, McIlroy WE, Verrier MC. Changes in gait variability during different challenges to mobility in patients with traumatic brain injury. Gait Posture. 2007;25:70–77. doi: 10.1016/j.gaitpost.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Williams G, Morris ME, Schache A, McCrory P. Observational gait analysis in traumatic brain injury: Accuracy of clinical judgment. Gait Posture. 2009;29:454–459. doi: 10.1016/j.gaitpost.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Williams G, Galna B, Morris ME, Olver J. Spatiotemporal deficits and kinematic classification of gait following a traumatic brain injury: a systematic review. J. Head Trauma Rehabil. 2010;25:366–74. doi: 10.1097/HTR.0b013e3181cd3600. [DOI] [PubMed] [Google Scholar]
- 12.Chow JW, Yablon Sa, Horn TS, Stokic DS. Temporospatial characteristics of gait in patients with lower limb muscle hypertonia after traumatic brain injury. Brain Inj. 2010;24:1575–84. doi: 10.3109/02699052.2010.523053. [DOI] [PubMed] [Google Scholar]
- 13.Kaufman KR, Brey RH, Chou L-S, Rabatin A, Brown AW, Basford JR. Comparison of subjective and objective measurements of balance disorders following traumatic brain injury. Med. Eng. Phys. 2006;28:234–239. doi: 10.1016/j.medengphy.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Medley A, Thompson M, French J. Predicting the probability of falls in community dwelling persons with brain injury: a pilot study. Brain Inj. 2006;20:1403–8. doi: 10.1080/02699050601082057. [DOI] [PubMed] [Google Scholar]
- 15.Herman T, Inbar-Borovsky N, Brozgol M, Giladi N, Hausdorff JM. The Dynamic Gait Index in healthy older adults: the role of stair climbing, fear of falling and gender. Gait Posture. 2009;29:237–241. doi: 10.1016/j.gaitpost.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basford JR, Chou LS, Kaufman KR, Brey RH, Walker A, Malec JF, Moessner AM, Brown AW. An assessment of gait and balance deficits after traumatic brain injury. Arch. Phys. Med. Rehabil. 2003;84:343–349. doi: 10.1053/apmr.2003.50034. [DOI] [PubMed] [Google Scholar]
- 17.Chou L, Kaufman KR, Walker-Rabatin AE, Brey RH, Basford JR. Dynamic instability during obstacle crossing following traumatic brain injury. Gait Posture. 2004;20:245–254. doi: 10.1016/j.gaitpost.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Williams G, Schache A, Morris ME. Running abnormalities after traumatic brain injury. Brain Inj. 2013;27:434–443 10p. doi: 10.3109/02699052.2012.750754. [DOI] [PubMed] [Google Scholar]
- 19.Wren TaL, Patrick Do K, Rethlefsen Sa, Healy B. Cross-correlation as a method for comparing dynamic electromyography signals during gait. J. Biomech. 2006;39:2714–2718. doi: 10.1016/j.jbiomech.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Wrisley DM, Stephens MJ, Mosley S, Wojnowski A, Duffy J, Burkard R. Learning Effects of Repetitive Administrations of the Sensory Organization Test in Healthy Young Adults. Arch. Phys. Med. Rehabil. 2007;88:1049–1054. doi: 10.1016/j.apmr.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Crapo RO, Casaburi R, Coates AL, Enright PL, MacIntyre NR, McKay RT, Johnson D, Wanger JS, Zeballos RJ, Bittner V, Mottram C. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002;166:111–117. doi: 10.1164/rccm.166/1/111. [DOI] [PubMed] [Google Scholar]
- 22.Enright PL. The Six-Minute Walk Test Introduction Standards and Indications 6-Minute Walk Test Versus Shuttle Walk Test Safety Variables Measured Conducting the Test Ensuring Quality Factors That Influence 6-Minute Walk Distance Interpreting the Results Improving the. 2003:783–785. [Google Scholar]
- 23.van Loo MA, Moseley AM, Bosman JM, de Bie RA, Hassett L. Test-re-test reliability of walking speed, step length and step width measurement after traumatic brain injury: a pilot study. Brain Inj. 2004;18:1041–1048. doi: 10.1080/02699050410001672314. [DOI] [PubMed] [Google Scholar]
- 24.Meinert I, Brown N, Alt W. Effect of footwear modifications on oscillations at the Achilles tendon during running on a treadmill and over ground: A cross-sectional study. PLoS One. 2016;11 doi: 10.1371/journal.pone.0152435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grech C, Formosa C, Gatt A. Shock attenuation properties at heel strike: Implications for the clinical management of the cavus foot. J. Orthop. 2016;13:148–151. doi: 10.1016/j.jor.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jasiewicz JM, Allum JHJ, Middleton JW, Barriskill A, Condie P, Purcell B, Li RCT. Gait event detection using linear accelerometers or angular velocity transducers in able-bodied and spinal-cord injured individuals. Gait Posture. 2006;24:502–509. doi: 10.1016/j.gaitpost.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 27.Selles RW, Formanoy MAG, Bussmann JBJ, Janssens PJ, Stam HJ. Automated estimation of initial and terminal contact timing using accelerometers; development and validation in transtibial amputees and controls. IEEE Trans. Neural Syst. Rehabil. Eng. 2005;13:81–88. doi: 10.1109/TNSRE.2004.843176. [DOI] [PubMed] [Google Scholar]
- 28.Winter DA, Yack HJ. EMG profiles during normal human walking: stride-to-stride and intersubject variability. Electroencephalogr. Clin. Neurophysiol. 1987;67:402–411. doi: 10.1016/0013-4694(87)90003-4. [DOI] [PubMed] [Google Scholar]
- 29.Whitney SL, Marchetti GF, Schade AI. The relationship between falls history and computerized dynamic posturography in persons with balance and vestibular disorders. Arch. Phys. Med. Rehabil. 2006;87:402–407. doi: 10.1016/j.apmr.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Casanova C, Celli BR, Barria P, Casas A, Cote C, De Torres JP, Jardim J, Lopez MV, Marin JM, Montes De Oca M, Pinto-Plata V, Aguirre-Jaime A. The 6-min walk distance in healthy subjects: Reference standards from seven countries. Eur. Respir. J. 2011;37:150–156. doi: 10.1183/09031936.00194909. [DOI] [PubMed] [Google Scholar]
- 31.Den Otter AR, Geurts ACH, Mulder T, Duysens J. Abnormalities in the temporal patterning of lower extremity muscle activity in hemiparetic gait. Gait Posture. 2007;25:342–352. doi: 10.1016/j.gaitpost.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 32.Wang P, Low KH, McGregor AH, Tow A. Detection of abnormal muscle activations during walking following spinal cord injury (SCI) Res. Dev. Disabil. 2013;34:1226–1235. doi: 10.1016/j.ridd.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Albani G, Sandrini G, Künig G, Martin-Soelch C, Mauro A, Pignatti R, Pacchetti C, Dietz V, Leenders KL. Differences in the EMG pattern of leg muscle activation during locomotion in Parkinson’s disease. Funct. Neurol. 2003;18:165–170. [PubMed] [Google Scholar]
- 34.Neptune RR, Sasaki K, Kautz SA. The effect of walking speed on muscle function and mechanical energetics. Gait Posture. 2008;28:135–143. doi: 10.1016/j.gaitpost.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson FC, Pandy MG. Individual muscle contributions to support in normal walking. Gait Posture. 2003;17:159–169. doi: 10.1016/S0966-6362(02)00073-5. [DOI] [PubMed] [Google Scholar]
- 36.Jacobs R, Bobbert MF, van Ingen Schenau GJ. Function of mono- and biarticular muscles in running. Med. Sci. Sports Exerc. 1993;25:1163–1173. [PubMed] [Google Scholar]
- 37.van Ingen Schenau GJ, Pratt CA, Macpherson JM. Differential use and control of monoand biarticular muscles. Hum. Mov. Sci. 1994;13:495–517. doi: 10.1016/0167-9457(94)90051-5. [DOI] [Google Scholar]
- 38.Buster TW, Chernyavskiy P, Harms NR, Kaste EG, Burnfield JM. Computerized dynamic posturography detects balance deficits in individuals with a history of chronic severe traumatic brain injury. Brain Inj. 2016;9052:1–7. doi: 10.1080/02699052.2016.1183822. [DOI] [PubMed] [Google Scholar]
- 39.Calancie B, Needham-Shropshire B, Jacobs P, Willer K, Zych G, Green BA. Involuntary stepping after chronic spinal cord injury Evidence for a central rhythm generator for locomotion in man. Brain. 1994;117:1143–1159. doi: 10.1093/brain/117.5.1143. [DOI] [PubMed] [Google Scholar]
- 40.Forssberg H. Ontogeny of human locomotor control I. Infant stepping, supported locomotion and transition to independent locomotion. Exp. Brain Res. 1985;57:480–493. doi: 10.1007/BF00237835. [DOI] [PubMed] [Google Scholar]
- 41.Ijspeert AJ. Central pattern generators for locomotion control in animals and robots: A review. Neural Networks. 2008;21:642–653. doi: 10.1016/j.neunet.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 42.Winter DA. Human blance and posture control during standing and walking. Gait Posture. 1995;3:193–214. doi: 10.1016/0966-6362(96)82849-9. [DOI] [Google Scholar]
- 43.Winter DA. Biomechanics of Normal and Pathological Gait. J. Mot. Behav. 1989;21:337–355. doi: 10.1080/00222895.1989.10735488. [DOI] [PubMed] [Google Scholar]
- 44.Schmitz A, Silder A, Heiderscheit B, Mahoney J, Thelen DG. Differences in lower-extremity muscular activation during walking between healthy older and young adults. J. Electromyogr. Kinesiol. 2009;19:1085–1091. doi: 10.1016/j.jelekin.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwartz MH, Rozumalski A, Steele KM. Dynamic motor control is associated with treatment outcomes for children with cerebral palsy. Dev. Med. Child Neurol. 2016;58:1139–1145. doi: 10.1111/dmcn.13126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steele KM, Rozumalski A, Schwartz MH. Muscle synergies and complexity of neuromuscular control during gait in cerebral palsy. Dev. Med. Child Neurol. 2015;57:1176–1182. doi: 10.1111/dmcn.12826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tyler ME, Kaczmarek KA, Rust KL, Subbotin AM, Skinner KL, Danilov YP. Non-invasive neuromodulation to improve gait in chronic multiple sclerosis: a randomized double blind controlled pilot trial. J. Neuroeng. Rehabil. 2014;11:79. doi: 10.1186/1743-0003-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.