Abstract
Traumatic brain injury (TBI) produces neuronal dysfunction and cellular loss that can culminate in lasting impairments in cognitive and motor abilities. Therapeutic agents that promote repair and replenish neurons post-TBI hold promise in improving recovery of function. Insulin-like growth factor-1 (IGF-1) is a neurotrophic factor capable of mediating neuroprotective and neuroplasticity mechanisms. Targeted overexpression of IGF-1 enhances the generation of hippocampal newborn neurons in brain-injured mice; however, the translational neurogenic potential of exogenously administered IGF-1 post-TBI remains unknown. In a mouse model of controlled cortical impact, continuous intracerebroventricular infusion of recombinant human IGF-1 (hIGF) for 7 days, beginning 15 min post-injury, resulted in a dose-dependent increase in the number of immature neurons in the hippocampus. Infusion of 10 μg/day of IGF-1 produced detectable levels of hIGF-1 in the cortex and hippocampus and a concomitant increase in protein kinase B activation in the hippocampus. Both motor function and cognition were improved over 7 days post-injury in IGF-1–treated cohorts. Vehicle-treated brain-injured mice showed reduced hippocampal immature neuron density relative to sham controls at 7 days post-injury. In contrast, the density of hippocampal immature neurons in brain-injured mice receiving acute onset IGF-1 infusion was significantly higher than in injured mice receiving vehicle and equivalent to that in sham-injured control mice. Importantly, the neurogenic effect of IGF-1 was maintained with as much as a 6-h delay in the initiation of infusion. These data suggest that central infusion of IGF-1 enhances the generation of immature neurons in the hippocampus, with a therapeutic window of at least 6 h post-injury, and promotes neurobehavioral recovery post-TBI.
Keywords: : cognition, hippocampus, IGF-1, neurogenesis, TBI
Introduction
The connection between traumatic brain injury (TBI) and the development of cognitive dysfunction has been well described. Many TBI survivors report that persistent cognitive dysfunction contributes to reduced quality of life in the years after their injury.1–4 Observations from magnetic resonance imaging or post-mortem studies implicate disrupted hippocampal function as a likely contributor to cognitive dysfunction post-TBI.5–10
Impaired motor and cognitive function are well-characterized consequences of the controlled cortical impact (CCI) rodent model of TBI.11–17 In addition to widespread neuronal death in the contused cortex, CCI is associated with cell loss in the dentate gyrus granular cell layer, hilus, CA-1, and CA-3.12–15,18,19 The population of immature neurons in the subgranular zone (SGZ) of the dentate gyrus is particularly vulnerable to injury, with greater than 50% reduction in this population in the days after moderate to severe CCI.20–22 In the wake of the substantial immature neuron loss, the injured hippocampus exhibits a compensatory response that increases the generation of newborn neurons in the weeks after injury through proliferation and differentiation of neural stem cells in the SGZ.21–25 However, only a small portion of newly proliferated cells adopt a neuronal phenotype, and few post-trauma–born neurons survive to incorporate into the granular layer.21,26,27 Immature neurons appear to have unique roles in hippocampal-dependent learning and memory,28–30 and mice subjected to CCI after targeted immature neuron ablation showed worse cognitive performance than CCI-injured mice with intact immature neurons at the time of injury.31 Thus, therapeutic agents that promote the generation, survival, or integration of immature neurons hold promise for the treatment of TBI.
Insulin-like growth factor-1 (IGF-1) is a potent neurotrophic factor that promotes cell survival and enhances the generation of new neurons both in vitro and in vivo.32–36 IGF-1 promotes brain growth during development, contributes to neurogenesis in the adult hippocampus and subventricular zone of naive and hypophysectomized rodents,34,36–40 and mediates exercise-induced increases in hippocampal neurogenesis.41–44 Reductions in IGF-1 are associated with impairments in brain development45 and have been linked to pathology associated with aging and neurodegenerative conditions.46–49 Conditional astrocyte-specific overexpression of IGF-1 in the context of TBI improves neurobehavioral function50 and promotes the generation and dendritic growth of hippocampal immature neurons post-CCI.22 Systemic administration of IGF-1 has been shown to improve neurobehavioral function and stimulate downstream IGF-1 signaling in the brain,51,52 but its effects on neurogenesis have not been evaluated to date.
To this end, we evaluated the efficacy of centrally infused IGF-1 to enhance neurogenesis and improve neurobehavioral function in a mouse model of CCI. We first evaluated a range of doses (0.3, 1, 3, and 10 μg/day) to select a dose with maximal benefit. Using this dose, we measured brain levels of IGF-1 and confirmed activation of protein kinase B (Akt), a downstream signaling protein. The ability of early onset, continuous IGF-1 infusion to improve motor and cognitive function and increase immature neuron density in the first week after injury was tested. Last, onset of IGF-1 infusion was delayed 6 h post-injury to evaluate the clinical relevance of this approach targeting hippocampal plasticity.
Methods
Animals
All experimental protocols were approved by the University of Kentucky Institutional Animal Care and Use Committee in accord with the established guidelines from the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health. Animals were group housed in the University of Kentucky Medical Center animal vivarium with a 14:10 h light/dark photoperiod and provided food and water ad libitum. Adult male C57BL/6J mice 8–10 weeks of age, weighing 25–30 g, were purchased from Jackson Laboratories (Bar Harbor, ME) for experiments.
Controlled cortical impact
Mice were anesthetized using 3% isoflurane for preparation of the scalp and placement in the stereotaxic frame (David Kopf Instruments, Tujunga, CA) and maintained by 2% isoflurane by nose cone for the duration of the surgery. An incision was made on the midline of the scalp to expose the skull. A 5-mm craniotomy was placed midway between bregma and lambda, lateral to the sagittal suture on the left hemisphere of the skull, leaving the dura intact. CCI injury was performed with a computer-controlled pneumatically driven piston with a 3-mm rounded impactor tip to rapidly (3.5 m/s) and transiently (500 msec) produce a 1-mm depth impact to the exposed dura of the brain (TBI-0310 Impactor; Precision Systems and Instrumentation, Versailles, KY). Sham-injured animals received anesthesia and a craniotomy. Animals were randomly assigned to either sham injury or brain injury and treatment with either vehicle or recombinant human IGF-1 (hIGF-1).
Central infusion of insulin-like growth factor-1
Mice were randomized for injury and treatment status. While anesthetized, a cannula was placed in the contralateral ventricle (anteroposterior AP −0.5 mm bregma, mediolateral ML −1.0 mm, dorsoventral DV −3.0 mm53) 15 min after sham injury or CCI. The cannula (brain infusion kit 3; Alzet, Cupertino, CA) was attached to an osmotic minipump (model 1007D, 0.5 μL/h; Alzet) for continuous intracerebroventricular (ICV) infusion for a period of 7 days. Minipumps were primed in sterile USP-grade phosphate-buffered saline (PBS) in a 37°C water bath for at least 6 h before surgical implantation, with the exception of the delayed treatment study, in which an approximate 6-h delay in treatment initiation was achieved by implantation of a nonprimed minipump. The base of the cannula was secured to the skull using an instant adhesive (Loctite 454; Alzet) and a set screw (MX-0090-01F-C; AmazonSupply.com, Seattle, WA) was placed adjacent to the base of the cannula with a small amount of dental acrylic. The minipump was positioned in the subcutaneous space over the scapula, lateral to the spine. Mice exhibited no observable impairment in mobility resulting from minipump implantation. The minipumps were filled with vehicle (pH 7.4, 24 mM of acetic acid diluted in USP-grade PBS) or 0.026, 0.085, 0.26, or 0.85 mg/mL of hIGF-1 (National Hormone and Peptide Program, Torrance, CA) to deliver 0.3, 1, 3, or 10 μg/day. IGF-1 dosing was extrapolated from previous studies demonstrating efficacy of IGF-1 to attenuate somatosensory impairment and reduce cell loss after cerebral hypoxic-ischemia,54–57 reduce cell loss in a model of Huntington's disease,58 and promote hippocampal neurogenesis.59 After cannula implantation, a small circular disk of dental acrylic was adhered to the skull over the craniotomy site, the scalp was sutured, and the mouse was placed on a heating pad to maintain normal body temperature. All mice received a subcutaneous injection of 1 mL of sterilized saline to prevent dehydration from the surgical procedure and implantation of the minipump. Once ambulatory, mice were returned to their home cage.
Study 1 (dose response for human insulin-like growth factor-1)
Mice subjected to CCI injury were randomized to receive infusion of 0.3, 1, 3, or 10 μg/day of IGF-1 (n = 5/dose) or vehicle (n = 4) beginning 15 min post-injury for a period of 7 days. Histological assessment and quantification of immature neuron density was performed as the primary outcome measure at 7 days post-injury. No animals were excluded. Although histology was the primary outcome for this study, cognitive function was assessed at 3 and 7 days post-injury using the novel object recognition (NOR) task, as described below, in order to provide pilot data to inform later studies.
Study 2 (acute onset infusion of 10 μg/day of human insulin-like growth factor-1)
Based on results from study 1, larger cohorts of mice were generated to more comprehensively evaluate the effects of acute-onset 10-μg/day hIGF-1 infusion. Sham-injured (n = 10/treatment group) and CCI-injured mice (n = 19/treatment group) received central infusion of 10 μg/day of hIGF-1 or vehicle for 7 days post-injury at a flow rate of 0.5 μL/h. Blood from nonfasted mice was collected onto test strips by tail prick by a 25-G needle and glucose levels measured using a handheld glucometer (OneTouch Ultra®; LifeScan Canada Ltd., Burnaby, BC, Canada) before anesthesia, at 90 min, 1 and 7 days post-injury. Motor and cognitive deficits were assessed during the week post-injury by a modified neurological severity score (NSS) and the NOR task, respectively, as described below. At 7 days post-injury, mice received an overdose of Fatal-Plus® (65 mg/kg of sodium pentobarbital; Vortech Pharmaceuticals, Dearborn, MI), and blood was collected by intracardial puncture for measurement of serum hIGF-1. Mice were randomized into one of two groups to be utilized for 1) quantification of hIGF-1 and Akt activation by enzyme-linked immunosorbent assay (ELISA) and western blot, respectively (n = 5 sham-injured per treatment group and n = 8–9 CCI-injured per treatment group) or 2) cresyl violet histological and immature neuron immunohistochemical assessments (n = 4 sham-injured/treatment group and n = 8–10 brain-injured/treatment group) described below. Accurate placement of the cannula was determined by the visual verification of the needle track in cresyl violet–stained coronal sections. A small number of animals were excluded based on improper placement of the cannula (n = 1 sham + vehicle; n = 1 sham + IGF-1) or because of obstruction or damage to cannulation tubing (n = 1 sham + IGF-1; n = 2 CCI + IGF-1). An additional cohort of mice received CCI brain injury and either no cannulation, vehicle infusion, or 10 μg/day of IGF-1 infusion (n = 6/group) over 7 days, at which time they were euthanized for measurement of brain water content as described below.
Study 3 (delayed onset infusion of human insulin-like growth factor-1)
Mice were subjected to CCI and infused with either 10 μg/day of IGF-1 (n = 5) or vehicle (n = 3) beginning at approximately 6 h post-injury by implantation of a nonprimed minipump at 15 min post-injury. Histological assessment and quantification of immature neuron density were performed at 7 days post-injury. No animals were excluded.
Motor function assessment using a modified neurological severity score
A previously described modification of the NSS task60 was used to assess motor dysfunction at 3 h and 1, 3, 5, and 7 days post-injury. One day pre-injury, mice were acclimated to four Plexiglas beams of varying widths (3, 2, 1, and 0.5 cm) and a 0.5-cm diameter wooden rod. The beams and rod were 60 cm in length and elevated 47 cm above a table top. Three points were given for successful crossing of the beam with normal position and usage of the forelimb and hindlimb within 30 sec. Two points were given for successful crossing of the beam despite either a forelimb or hindlimb hanging below the beam, and 1 point was given for crossing the beam despite inverting below the beam one or more times. The mouse was righted and allowed to continue across the beam if it became inverted on the beam. A score of zero was given if the mouse did not cross in the allotted 30 sec or fell off the beam. For the rod, two points were given for successful crossing of the beam in the allotted 30 sec. A score of 1 was given for crossing despite inverting more than three times. A score of 0 was given if the mouse did not traverse in the allotted time or fell off the rod. A cumulative score for each animal was recorded on each day of testing. Scoring was performed by an evaluator blinded to injury and treatment conditions of the mice.
Cognitive performance evaluation using a novel object recognition paradigm
One day pre-injury, each mouse was habituated to a dedicated, clear plastic cage (32 × 20 × 14 cm) with an open top for a period of 1 h. Several hours later, each mouse was returned to its dedicated cage, now containing two identical Lego®Duplo® figures placed in opposite corners, and exploration was monitored for 5 min (familiarization trial). At 3 days post-injury, the familiarization trial was repeated using the two original objects, recording the time spent exploring each object over a 5-min period. Mice were then returned to their home cage. After a 4-h delay, the mouse was returned to the test cage where one of the original objects had been replaced with a novel object. The time spent exploring each object was recorded for the 5-min testing period. On day 7 post-injury, mice were tested for 5 min using the same familiar object and a new novel object. A recognition index was calculated as the time spent exploring the novel object as a percentage of total object exploration time. Testing was performed by an evaluator blinded to injury and treatment conditions of the mice.
Tissue collection
In study 2, blood was collected at the terminal time point to measure hIGF-1 in serum. After overdose with Fatal-Plus, as described above, blood was collected intracardially, transferred to an Eppendorf tube, and allowed to coagulate for 30 min. To extract serum, coagulated blood was centrifuged at 4500g for 10 min at room temperature. Serum was transferred to a new tube and stored at −80°C.
For ELISA and western blotting, mice received an overdose of Fatal-Plus before decapitation. The brain was rapidly removed from the skull and placed onto an ice-cold dissection plate. To concentrate collection of tissue on the injury site, 3 mm of the anterior brain was blocked in the coronal plane and the remaining ipsilateral and contralateral cortical and hippocampal regions were dissected and separately placed in Eppendorf tubes and stored at −80°C.
For histology and immunohistochemistry, mice received an overdose of Fatal-Plus and were transcardially perfused with heparinized saline to clear blood from the vasculature followed by 10% neutral-pH buffered formalin. Mice were decapitated and their heads placed in formalin for 24 h. The brain was dissected from the head and placed in formalin for an additional 24 h. Brains were then placed in 30% sucrose for 24 h for cryoprotection before freezing in −30°C isopentanes cooled on dry ice. Brains were cut into 40-μm-thick coronal sections on a freezing sliding microtome (Microm; Dolbey-Jamison, Pottstown, PA) at −20°C (Physitemp Instruments, Inc., Clifton, NJ).
For edema measurements, mice received an overdose of Fatal-Plus before decapitation. The brain was rapidly removed from the skull and placed onto an ice-cold dissection plate. The ipsilateral and contralateral cortical and hippocampal regions were dissected as described for ELISA and western blot analysis. To calculate water weight using the wet/dry weight method,61,62 tissue was immediately weighed, desiccated in an oven at 70°C for 24 h, and reweighed to acquire the dry weight. The percent water content was calculated as (wet weight – dry weight) / wet weight × 100.
Preparation of tissue for enzyme-linked immunosorbent assay and western blotting
Cortical and hippocampal samples were homogenized in cold lysis buffer (1% triton X-100, 20 mM of Tris-HCl, 150 mM of NaCl, 5 mM of EGTA, 10 mM of EDTA, 10% glycerol, and proteinase inhibitor cocktail; Roche, Indianapolis, IN) and centrifuged for 30 min at 4°C at a speed of 10,000g. Supernatants were collected and utilized for analysis. Protein concentrations were determined using a BCA assay kit (Pierce Biotechnology, Rockford, IL).
Quantification of insulin-like growth factor-1 by enzyme-linked immunosorbent assay
By using recombinant hIGF-1, exogenously infused IGF-1 could be distinguished from endogenous mouse IGF-1 using an ELISA kit for hIGF-1. hIGF-1 was quantified using the highly specific Quantikine® human IGF-1 ELISA kit (DG100; R&D Systems Inc., Minneapolis, MN) according to the manufacturer's instructions. hIGF-1 was not detected in serum or brain samples of vehicle-treated mice. Serum and brain samples were pretreated to dissociate IGF-1 from the binding proteins. hIGF-1 standards (0.094–6.000 ng/mL) and pre-treated samples were pipetted in duplicate into a 96-well plate coated with monoclonal antibody specific to hIGF-1. Absorbance of each well was measured at 450 and 540 nm (background subtraction) wavelengths.
Western blot
Western blot analyses were performed as previously described.50 Supernatant samples from ipsilateral hippocampi were loaded in triplicate (30 μg) and electrophoresed through a 3–8% Tris-HCl gel at 150 V. After transfer onto nitrocellulose membranes, membranes were blocked for 1 h in 5% dry milk dissolved in 0.1% Tween 20 in Tris-buffered saline (TBS), and incubated overnight with a primary antibody for phosphorylated Akt (pAkt) ser473 (rabbit monoclonal, 1:2000; Cell Signaling Technology, Danvers, MA). Membranes were rinsed and incubated for 1 h with a secondary antibody recognizing rabbit immunoglobulin G (IgG; 1:5000, IRDye800CW®; Rockland Instruments, Inc., Gilbertsville, PA). Membranes were rinsed and imaged with the Li-Cor Odyssey Infrared Imaging System (Li-Cor Biosciences, Lincoln, NE). After pAkt development, membranes were probed for the control protein actin using an anti-β-actin primary antibody (mouse monoclonal, 1:5000; Calbiochem Inc., San Diego, CA) and secondary antibody conjugated to an infrared dye (1:10,000; Rockland). For quantification, optical density (OD) of each pAkt band was divided by its respective actin OD and normalized to the mean relative OD for vehicle-treated sham-injured mice.
Histological (cresyl violet) staining
For each brain, every tenth coronal section was mounted and air-dried on gelatin-coated slides. Slides were hydrated in graded ethanol solutions, stained with 0.5% cresyl violet (Acros Organics, Somerville, NJ), rinsed in water, dehydrated through graded ethanol solutions, cleared in xylenes (Fisher Scientific, Fair Lawn, NJ), and mounted with Permount (Fisher Scientific).
Immunohistochemistry
In an additional set, every tenth coronal brain section (n = 6–7 per animal), centered with respect to the injury epicenter, was immunolabeled using standard free-floating immunohistochemistry protocols for doublecortin (DCX) as previously described.22 Sections were washed in TBS and blocked with 5% normal horse serum with 0.1% Triton-X-100 in TBS for 30 min. DCX primary antibody (rabbit polyclonal, 1:500; Abcam, Cambridge, MA) was diluted in blocking solution and placed on the tissues for overnight incubation at 4°C. Sections were rinsed with TBS and incubated with secondary antibody (anti-rabbit IgG conjugated with Alexa Fluor® 488; Jackson ImmunoResearch, West Grove, PA) diluted in blocking solution for 1 h and rinsed with TBS. Sections were incubated with Hoechst (1:10,000; Invitrogen, Carlsbad, CA) for 1.5 min to label all nuclei and rinsed with TBS. Labeled sections were mounted on gelatin-coated slides, cover-slipped with Fluoromount (Southern Biotech, Birmingham, AL), and stored at 4°C.
Image acquisition
Representative images of cresyl violet–stained sections were acquired at 4 × magnification using an AX80 Olympus microscope (Olympus, Center Valley, PA). Representative images of DCX immunoreactivity were acquired at 10 × or 20 × magnification using a C2+ TiE Nikon confocal microscope (Nikon, Melville, NY).
Cellular quantification
Quantification of DCX-positive cells was performed for each section in the ipsilateral dentate gyrus granular layer for all studies, and the contralateral granular layer in study 1. All positively labeled cells were manually counted live through the microscope eyepieces by assessing DCX immunoreactivity through all focal planes within the dentate gyrus granular layer at 40 × magnification on an epifluorescent microscope (Olympus BX51) equipped with a fluorescein isothiocyanate filter (41001; Chroma Technology, Bellows Falls, VT). Live quantification at 40 × magnification was utilized to enhance resolution of immature neurons. Cellular counts were normalized to the volume of the dentate gyrus granular layer of each section to control for differences in dentate gyrus size across sections. The Hoechst-labeled dentate gyrus granular layer was imaged (AX80; Olympus) and its area measured using ImagePro (MediaCybernetics, Rockville, MD). The thickness of each section was measured, in microns, using an epifluorescent scope (Olympus BX51) equipped with a stereology stage. Granular layer area and thickness measurements were used to calculate granular layer volume. Volumetric density measurements (1000 cells/mm3) represent the entire granular layer, inclusive of the subgranular, inner, and outer granular layers. A mean cell density was calculated for all quantified sections for each animal. Sections from animals with representative cell densities, based upon analysis of cell counts, were selected for imaging and included in the figure panels. Quantification of all sections was performed by an investigator blinded to the injury and treatment conditions of each animal.
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM). In study 1, cellular densities were statistically compared by one-way analysis of variance (ANOVA) followed by a Dunnett's multiple comparison test. NOR recognition indices for each group were compared to chance performance (50%) using a one-sample t-test. In study 2, serum levels of IGF-1 were compared using a Student's t-test and blood glucose levels were compared by a repeated measures two-way ANOVA. Akt activation, edema measurements for each region, and cellular densities were compared by one-way ANOVA followed by Tukey's post-hoc tests. NOR recognition indices for each group were compared to chance performance (50%) using a one-sample t-test. Relative differences in NOR recognition indices across groups were analyzed at each time point by one-way ANOVA, followed by Tukey's multiple comparisons tests. Brain levels of IGF-1 and NSS motor function scores were compared by repeated measures one-way ANOVA with Tukey's post-hoc tests. In study 3, cellular densities were compared using an unpaired two-tailed t-test. Statistical tests were completed using Graphpad Prism software (Graphpad Software Inc., La Jolla, CA). A p value less than 0.05 was considered statistically significant.
Results
Insulin-like growth factor-1 infusion produces dose-dependent enhancement of hippocampal neurogenesis
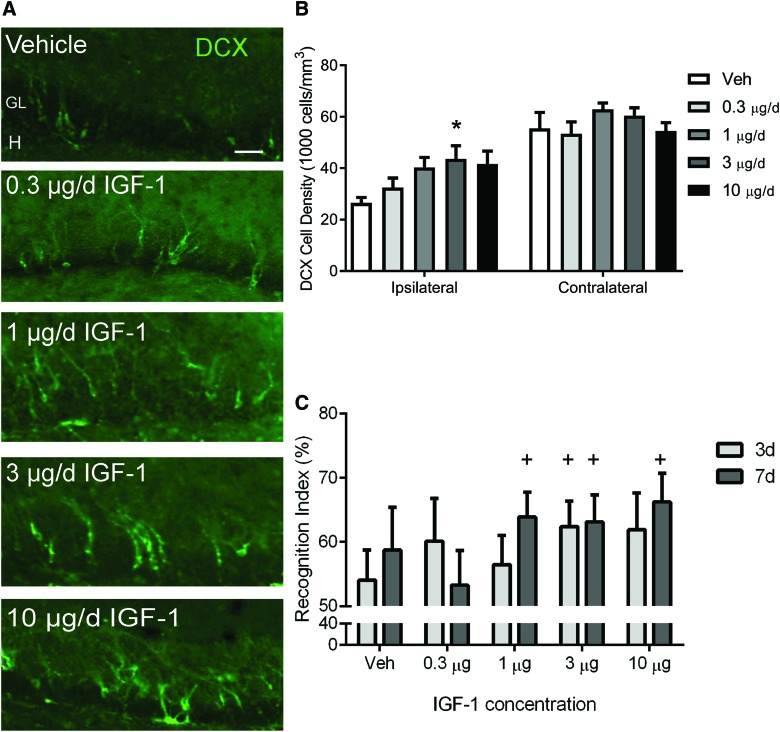
For study 1, a dose escalation was initially completed to guide future studies utilizing IGF-1 therapy. To evaluate the effect of IGF-1 on immature neurons, the number of cells labeled with DCX, a marker of immature neurons, was quantified in CCI-injured mice treated for 7 days with vehicle or 0.3, 1, 3, or 10 μg/day of hIGF-1 by continuous ICV infusion beginning at 15 min post-injury. CCI brain injury resulted in the loss of approximately 50% of DCX immunoreactive neurons in the ipsilateral hippocampus in vehicle-treated mice (Fig. 1A,B). IGF-1 treatment resulted in an apparent dose-dependent enhancement of hippocampal immature neuron density in the ipsilateral hippocampus at 7 days post-injury (Fig. 1B), with 3 μg/day of hIGF-1 resulting in a significantly greater density of immature neurons than vehicle treatment (p < 0.05).
FIG. 1.
Dose-dependent effects of insulin-like growth factor (IGF-1) delivered by central infusion on hippocampal neurogenesis and memory retention after controlled cortical impact (CCI). (A) Representative images of doublecortin (DCX; green) immunoreactivity in the ipsilateral hippocampus of vehicle (Veh) and IGF-1–infused CCI-injured mice at 7 days post-injury. CCI + Veh mice exhibited a robust loss of DCX immunoreactivity in the dentate gyrus granular layer. DCX immunoreactivity appeared to increase in a dose-dependent manner in CCI + IGF-1 mice. Granular layer (GL) and hilus (H). Scale bar represents 50 μm. (B) In the hippocampus ipsilateral to impact, immature neuron density increased as a function of IGF-1 infusate concentration, peaking at 3 μg/day of IGF-1, which produced a significantly higher density of DCX+ cells compared to vehicle infusion (*p < 0.05). Immature neuron density in the hippocampus contralateral to impact was not significantly changed. (C) Recognition indices (% time exploring novel object) at 3 and 7 days post-CCI. Brain-injured mice receiving vehicle or 0.3 μg/day of IGF-1 performed at chance levels, whereas CCI mice receiving 1, 3, or 10 μg/day of IGF-1 performed above chance at either 3 days, 7 days, or both time points (+p < 0.05 compared to 50%). Data are presented as mean + SEM (n = 4 vehicle-treated CCI-injured and n = 5/dose IGF-1–treated CCI-injured mice). SEM, standard error of the mean. Color image is available online at www.liebertpub.com/neu
In the same cohort of mice, the NOR task was completed at 3 and 7 days to provide initial insights into cognitive effects of IGF-1 infusion. Statistically, the performance of brain-injured mice receiving either vehicle solution or the lowest dose of IGF-1 was equivalent to chance (50%), consistent with a trauma-induced loss of memory of the familiar object. In contrast, brain-injured mice that received 1, 3, or 10 μg/day achieved recognition scores significantly above chance at 3 and/or 7 days post-injury, suggesting that IGF-1 infusion improved memory retention (Fig. 1C). Together, these behavioral and histological data suggest that doses within the range of 1–10 μg/day may be efficacious. Therefore, a dose of 10 μg/day of IGF-1 was selected for subsequent studies.
Insulin-like growth factor-1 signaling is enhanced in the brain after intracerebroventricular administration
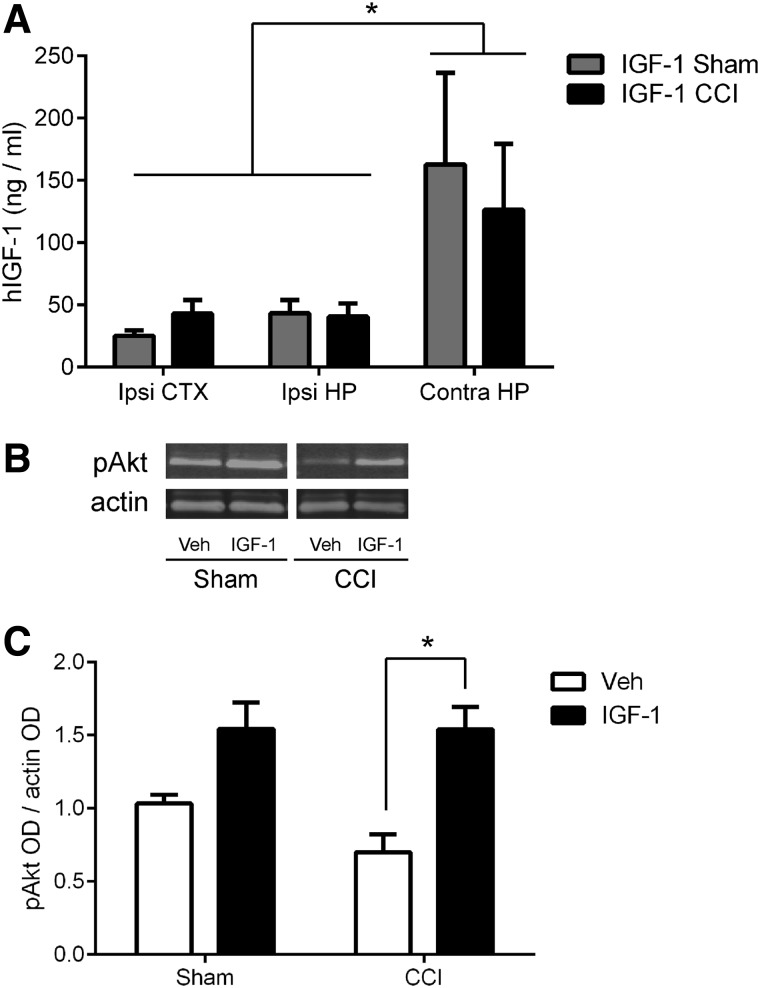
Study 2 was designed to confirm the neurogenic effect of 10 μg/day of hIGF-1 in a larger cohort, expand and replicate the neurobehavioral evaluation, and measure central nervous system (CNS) levels of IGF-1 and potential downstream signaling molecules. A 7-day ICV infusion of 10 μg/day of hIGF-1 into the contralateral ventricle beginning at 15 min post-injury resulted in measurable hIGF-1 in the ipsilateral (impacted) hemisphere, with equivalent abundance in the cortex and hippocampus, suggesting widespread delivery. The hippocampus contralateral to the impact injury (same hemisphere as the cannula) exhibited significantly higher concentrations of hIGF-1 compared to the ipsilateral cortex or hippocampus (region effect, F2,22 = 6.151; p < 0.01; Tukey's post-hoc, p < 0.05; Fig. 2A). Hippocampal levels of pAkt measured by western blot varied significantly across groups (ANOVA, F3,23 = 9.695; p < 0.0005; Fig. 2B,C). In vehicle-treated mice, pAkt levels at 7 days after brain injury were approximately 70% those after sham injury, but this injury effect was not statistically significant. Infusion of IGF-1 significantly enhanced phosphorylation of Akt in the ipsilateral hippocampus of brain-injured mice (Tukey's post-hoc test, p < 0.0005 compared to vehicle; Fig. 2C). Together, these data demonstrate that ICV infusion of IGF-1 produced a measurable and widespread increase of IGF-1 in the brain, and increased the activation of a prosurvival pathway in the injured hippocampus.
FIG. 2.
Central infusion of 10 μg/day human insulin-like growth factor-1 (hIGF-1) over 7 days elevates brain levels of hIGF-1 and enhances Akt activation in the hippocampus after controlled cortical impact (CCI). (A) hIGF-1 was detected in the ipsilateral (ipsi) cortex (CTX), and ipsilateral and contralateral (contra) hippocampus (HP) in sham and CCI mice at 7 days post-injury (*p < 0.05, contra HP compared to ipsi CTX or HP). (B) Representative western blot images of phosphorylated Akt (pAkt) and actin, as a control protein, for vehicle (Veh) and IGF-1–treated sham and CCI mice at 7 days post-injury. (C) Infusion of IGF-1 in CCI-injured mice resulted in a significant increase in Akt phosphorylation within the hippocampus at 7 days post-injury, compared to vehicle infusion (*p < 0.005). Optical density (OD) for each pAkt band was normalized to its respective control protein actin band OD; the mean of the triplicate samples for each mouse was normalized to the mean OD value of the vehicle-treated sham group. The results are presented as mean + SEM (n = 5 sham-injured/treatment and n = 9 CCI-injured/treatment). Akt, protein kinase B; SEM, standard error of the mean.
IGF-1 is cleared from cerebrospinal fluid (CSF) and enters the systemic circulation.63 Thus, we also quantified systemic concentrations of IGF-1. hIGF-1 was not detected in serum of vehicle-treated mice. CCI + IGF-1 mice exhibited somewhat lower serum hIGF-1 concentrations (41.8 ± 5.0 ng/mL) compared to sham + IGF-1 mice (58.2 ± 10.8 ng/mL), but the difference was not significant. Because of findings from our previous work showing acute hypoglycemia at 1 day post-injury with systemic infusion of high concentrations of IGF-1,51 blood glucose levels were measured at 90 min and 1 and 7 days post-CCI to monitor for hypoglycemia after central infusion. Blood glucose concentrations were not different among treatment groups (Table 1). Anesthesia and surgical procedures induced a significant elevation in blood glucose concentrations at 90 min post-injury, independent of injury and treatment condition (p < 0.05 compared to all other time points), but blood glucose returned to baseline at 1 and 7 days post-injury (Table 1).
Table 1.
Blood Glucose Levels after Central Infusion
| Blood glucose measurements after central infusion (mg/dL) | ||||
|---|---|---|---|---|
| Baseline | 90 min | 1 day | 7 days | |
| Sham + Veh | 176 ± 7 | 233 ± 11* | 183 ± 8 | 200 ± 11 |
| Sham + IGF-1 | 165 ± 6 | 210 ± 10* | 171 ± 11 | 163 ± 7 |
| CCI + Veh | 187 ± 9 | 229 ± 9* | 203 ± 17 | 192 ± 16 |
| CCI + IGF-1 | 179 ± 9 | 231 ± 12* | 170 ± 9 | 189 ± 11 |
Blood glucose was measured before sham or controlled cortical impact (CCI) injury, and at several time points post-injury in nonfasted mice. Blood glucose levels were significantly higher at 90 min after surgical procedures compared to all other time points, independent of treatment and injury status (*p < 0.05). Data are presented as mean ± SEM (n = 9 sham-injured/treatment and n = 19 CCI-injured/treatment).
Veh, vehicle; IGF-1, insulin-like growth factor-1; SEM, standard error of the mean.
Insulin-like growth factor-1 improves neurobehavioral performance after controlled cortical impact
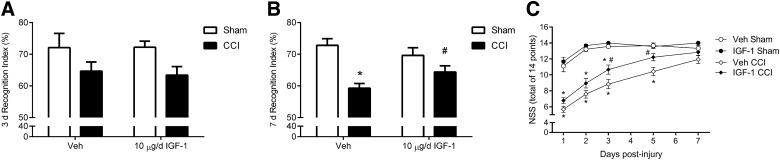
To evaluate cognitive performance in this larger cohort, we again utilized the NOR task at 3 and 7 days post-injury. An ANOVA detected significant differences among the groups at 3 days post-injury (ANOVA, F3,49 = 3.245; p < 0.05; Fig. 3A). Although the mean recognition indices of injured mice were somewhat lower than their respective sham cohorts, no post-hoc comparisons were significant. Further, all groups at both 3 and 7 days performed at a level above chance (50%), suggesting that overall the injury effect was milder than in study 1. Nonetheless, at 7 days post-injury, vehicle-treated, brain-injured mice exhibited significantly lower recognition indices than sham mice receiving vehicle (ANOVA, F3,48 = 12.84; p < 0.0001; post-hoc, p < 0.05; Fig. 3B). Infusion of IGF-1 resulted in significantly higher recognition indices in brain-injured mice (p < 0.05 compared to vehicle-treated, CCI mice; Fig. 3B), such that NOR performance was statistically equivalent to that of shams receiving IGF-1. Recognition indices of the two sham groups were not different at either time point.
FIG. 3.
Central infusion of 10 μg/day of insulin-like growth factor-1 (IGF-1) attenuates cognitive and motor impairment after controlled cortical impact (CCI). (A) At 3 days post-injury, novel object recognition (NOR) task performance varied across groups (ANOVA, p < 0.05), but memory retention of injured cohorts was not significantly less than sham cohorts in post-hoc testing. (B) At 7 days post-injury, CCI + Veh mice exhibited a significant reduction in the recognition index compared to either sham group (*p < 0.005). In contrast, memory function of CCI + IGF-1 mice was significantly improved when compared to CCI + Veh mice (#p < 0.05) and was not statistically different from that of sham groups. (C) CCI + Veh mice exhibited significant impairment in motor function at 1, 2, 3, and 5 days post-injury as assessed by a modified neurological severity score (NSS) (*p < 0.001, compared to sham + Veh mice). CCI + IGF-1 mice also exhibited a significant motor impairment at 1, 2, and 3 days post-injury when compared to sham + IGF-1 (*p < 0.001). However, CCI + IGF-1 mice showed improved motor function at 3 and 5 days post-injury compared to CCI + Veh mice (#p < 0.01). Data are presented as mean ± SEM (n = 9 sham-injured/treatment and n = 19 CCI-injured/treatment). ANOVA, analysis of variance; SEM, standard error of the mean; Veh, vehicle.
Assessment of motor performance revealed that brain injury impaired motor function acutely post-injury, with substantial recovery of function by 7 days (ANOVA group effect, F3,51 = 28.9; p < 0.0001; time effect, F4,204 = 74.9; p < 0.0001; interaction, F12,204 = 7.4; p < 0.0001; Fig. 3C). CCI mice receiving vehicle showed a significant reduction in motor function at 1, 2, 3, and 5 days post-injury, compared to sham-injured mice (p < 0.001). Conversely, CCI mice treated with IGF-1 exhibited motor function impairment at only 1, 2, and 3 days post-injury (p < 0.001 compared to sham + IGF-1 mice). IGF-1 infusion significantly improved motor function of brain-injured mice at 3 and 5 days post-injury (p < 0.05 compared to CCI + Veh). Central infusion of IGF-1 reduced cognitive impairment and accelerated motor recovery post-CCI.
Acute-onset insulin-like growth factor-1 infusion increases immature neuron density after controlled cortical impact
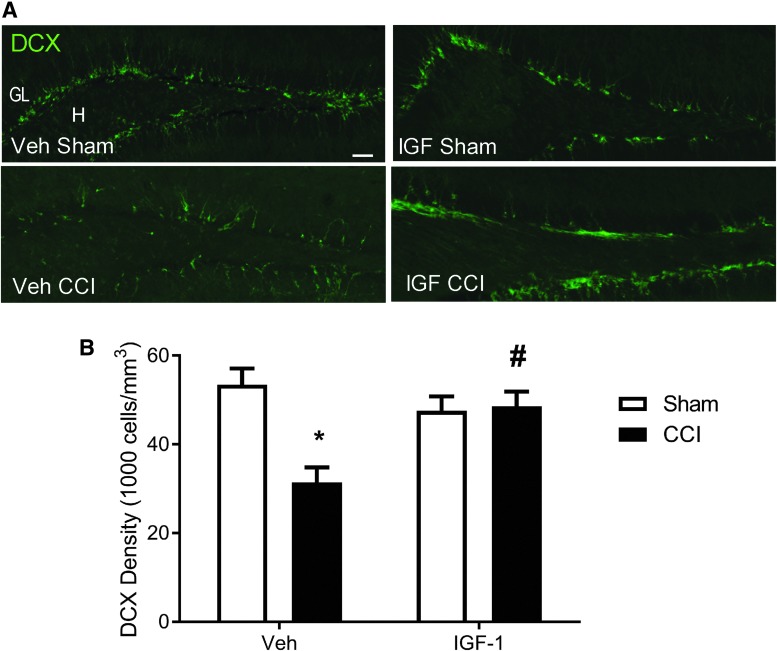
Compared to sham-injured mice, mice receiving CCI followed by vehicle treatment exhibited a marked loss of DCX immunolabeling in the dentate gyrus ipsilateral to the impact (Fig. 4A). In contrast, DCX labeling was better preserved in IGF-1–treated CCI mice. Quantification of DCX+ cell numbers revealed significant differences in the density of immature neurons across experimental groups (ANOVA, F3,22 = 8.04; p < 0.001). In vehicle-treated mice, CCI produced a significant reduction in DCX+ immature neuron density at 7 days post-injury (post-hoc, p < 0.005 compared to sham injury; Fig. 4B). IGF-1 infusion after CCI brain injury resulted in significantly higher DCX+ cell densities compared to vehicle infusion (p < 0.005). Notably, the density of immature neurons in CCI + IGF-1 mice was equivalent to that in sham-injured mice (vehicle or IGF-1 infused), indicating that IGF-1 treatment effectively increased the immature neuron population to baseline levels at 7 days post-injury.
FIG. 4.
Central infusion of 10 μg/day of insulin-like growth factor-1 (IGF-1) increases immature neuron density in the injured hippocampus after controlled cortical impact (CCI). (A) Representative images of doublecortin (DCX; green) immunoreactivity in the ipsilateral hippocampus of vehicle (Veh) and IGF-1–infused sham- and CCI-injured mice at 7 days post-injury. Scale bar represents 50 μm. Granular layer (GL) and Hilus (H). (B) Brain injury resulted in a significant decrease in DCX+ cell density in vehicle-treated mice (*p < 0.005, compared to sham + Veh), but not in CCI mice infused with IGF-1. DCX+ cell density was significantly greater in IGF-1–treated brain-injured mice than in vehicle-treated counterparts at 7 days after CCI injury (#p < 0.005, compared to CCI + Veh mice). Immature neuron counts obtained from the ipsilateral granular layer were normalized to the volume of the ipsilateral granular layer to calculate cellular density (1000/mm3). Data are expressed as mean + SEM (n = 4 sham-injured/treatment, n = 10 CCI + Veh, and n = 9 CCI + IGF-1). SEM, standard error of the mean. Color image is available online at www.liebertpub.com/neu
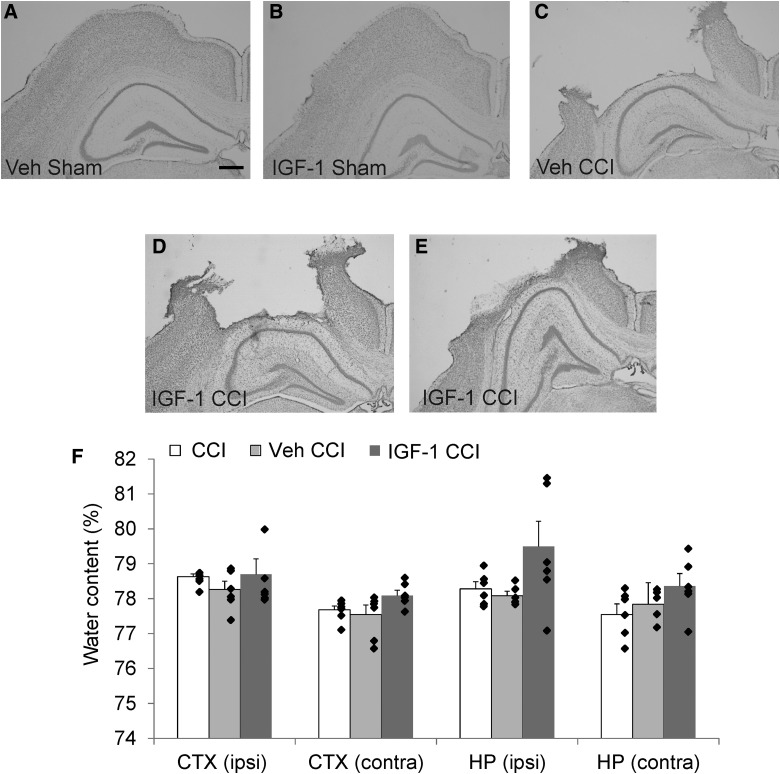
Histological examination of brain tissue from sham mice revealed evidence of cortical swelling near the craniotomy site after 7 days of ICV infusion, despite the absence of swelling or dural damage at the completion of surgery (Fig. 5A,B). CCI injury produced cortical cell loss and cavitation, subcortical white matter loss, and granular cell layer thinning in the hippocampus typical of moderate CCI (Fig. 5C). Qualitative assessment revealed that the majority of injured mice infused with IGF-1 exhibited gross pathology similar to vehicle-treated CCI-injured mice (Fig. 5C,D). However, a small subset of mice (3 of 10) showed swelling in subcortical regions and hippocampal distortion post-CCI (Fig. 5E). In animals exhibiting cortical brain swelling, midline tissue shift was noted, although overt ventricle enlargement was not appreciated in either hemisphere. Based on these qualitative observations, we sought to determine whether ICV infusion prolonged post-traumatic edema. Analysis of regional water content in the cortex and hippocampus revealed no statistically significant changes in water content among the experimental groups (Fig. 5F). Water content percentages observed in these regions (77–80%) were comparable to those reported after sham injury.64–66 A modest increase in mean water content in the ipsilateral hippocampus of brain-injured mice infused with 10 μg/day of IGF-1 appeared to be driven by a subset of mice (2 of 6) that exhibited a higher than normal water content (81–82%). Differences in age or body weight were not found to correlate with the incidence of brain swelling (data not shown).
FIG. 5.
Gross histopathology and regional water content at 7 days after controlled cortical impact (CCI). (A–E) Representative images of cresyl violet staining of the cortex (CTX) and hippocampus (HP) ipsilateral (ipsi) to impact at 7 days post-injury from vehicle (Veh) and IGF-1–treated mice subjected to sham or CCI injury. Sham-injured mice infused with either (A) vehicle or (B) IGF-1 exhibited brain swelling at the craniotomy site, which was not present at the time of surgery. (C) Injured mice treated with vehicle exhibited cortical cavitation and hippocampal cell loss consistent with CCI. (D) The majority of IGF-1–infused brain-injured mice (7 of 10) exhibited characteristic histopathology with minimal or no brain swelling. (E) However, a subset of IGF-1–infused CCI-injured mice (3 of 10) showed distortion of the hippocampus at 7 days post-injury, raising concerns that central infusion of IGF-1 could exacerbate cerebral edema (n = 4 sham-injured/treatment and n = 10 CCI-injured/treatment). Scale bar represents 500 μm. (F) Water content was quantified in mice subjected to CCI injury without cannulation or infusion (CCI), CCI injury with central infusion of vehicle (Veh CCI), or CCI injury with central infusion of 10 μg/day of IGF-1 (IGF-1 CCI; n = 6/group). Comparison by one-way ANOVA revealed infusion of IGF-1 did not result in significant regional cerebral edema; however, a small subset of mice (2 of 6) had markedly increased water content in the ipsilateral (ipsi) hippocampus. Contralateral (contra). Individual data points are superposed with bars representing mean + SEM. ANOVA, analysis of variance; SEM, standard error of the mean.
Delayed administration of insulin-like growth factor-1 increases immature neuron density
Considering the initiation of treatment in clinical trials typically occurs between 3 and 8 h after a TBI,67 a delayed IGF-1 infusion paradigm is highly clinically relevant. In study 3, we used a strategy to delay IGF-1 treatment by approximately 6 h by implantation of a nonprimed minipump. Immature neurons were quantified, confirming a reduction in DCX+ cell density in the ipsilateral dentate gyrus granular layer of vehicle-treated injured mice (Fig. 6A). Delayed onset, continuous infusion of hIGF-1 in CCI-injured mice significantly enhanced immature neuron density at 7 days post-injury compared to vehicle infusion (p < 0.05; Fig. 6B). Interestingly, compared to the acute infusion paradigm used in studies 1 and 2, delayed infusion of IGF-1 produced as great, if not greater, enhancement of immature neuron recovery. Delayed infusion of 10 μg/day yielded a 75% increase in immature neuron density over vehicle treatment whereas acute infusion of 10 μg/day of IGF-1 increased DCX+ cell density 58% over vehicle treatment in study 1 (Fig. 1B) and 55% over vehicle treatment in study 2 (Fig. 4B).
FIG. 6.
Delayed infusion of 10 μg/day of insulin-like growth factor-1 (IGF-1) increased hippocampal immature neuron density 1 week after controlled cortical impact (CCI). (A) Compared to CCI + Veh mice, CCI + IGF-1 mice showed increased doublecortin (DCX; green) immunoreactivity. Scale bar represents 100 μm. (B) Infusion of IGF-1 post-CCI resulted in significantly increased immature neuron density at 7 days post-injury, compared to vehicle treatment (#p < 0.05). Data are presented as mean + SEM (n = 3 CCI + Veh and n = 5 CCI + IGF-1). SEM, standard error of the mean. Color image is available online at www.liebertpub.com/neu
Discussion
Systemic administration of IGF-1 and conditional overexpression of IGF-1 have been shown to improve neurobehavioral function in rodents subjected to experimental TBI,50–52 but the efficacy of centrally infused IGF-1 after TBI has not been evaluated. In the current study, we demonstrate, for the first time, that central administration of IGF-1 improves cognitive and motor function and enhances hippocampal immature neuron density in mice after experimental TBI.
Radiolabeled IGF-1 injected ICV into adult rats can be detected throughout the brain parenchyma, including the cortex, white matter tracts, and the hippocampus within 30 min,68–70 highlighting that ICV administration is applicable for broad delivery of IGF-1 throughout the brain after experimental TBI. We confirmed that continuous ICV infusion resulted in measurable levels of exogenous IGF-1 in the cortex and bilaterally in the hippocampus at 7 days post-injury. A 7-day infusion duration was chosen in order to achieve sustained levels of IGF-1 during the period of peak cellular proliferation in the hippocampus post-TBI.21,24 It is not known whether a shorter duration of IGF-1 infusion would be sufficient to produce comparable effects or whether a longer duration would result in greater benefit or diminished efficacy. Further optimization of IGF-1 treatment duration in TBI models is needed. In a developmental hippocampal organotypic slice culture model of epileptogenesis, acute (3-day) IGF-1 treatment was neuroprotective, but long-term (14-day) treatment contributed to aberrant electrophysiological function.71
Although it is well established that IGF-1 in the systemic circulation can pass into the brain through receptor-mediated endocytosis,72 the converse is also true: Centrally infused IGF-1 can be cleared from CSF and enter the systemic circulation, likely through the lymphatic system and cranial and/or spinal nerve roots.63 IGF-1 infused into the lateral ventricle was detected at concentrations of approximately 40–60 ng/mL in the systemic circulation at the cessation of infusion. Central infusion was selected to minimize systemic hypoglycemia, as a result of binding of IGF-1 with the insulin receptor at high systemic concentrations, considering that this was previously observed at 24 h after moderate TBI in rats administered IGF-1 subcutaneously.51 Even though central infusion resulted in elevated serum IGF-1, it did not produce systemic hypoglycemia. This finding highlights that central infusion of IGF-1 eliminated a potential side effect of systemic administration of IGF-1 after experimental TBI.
It is important to note that ICV cannulation possesses inherent risks, but this route of delivery may provide an advantage for therapeutic administration in moderately to severely injured TBI patients in whom an intracranial pressure monitor and shunt has been implanted. Our findings highlight that ICV infusion of 10 μg/day of IGF-1 resulted in improved neurobehavioral function, similar to our previous findings of improved motor and cognitive function after systemic administration of a much higher dose of 4 mg/kg/day.51 In the current study, central infusion of IGF-1 resulted in measurable levels of hIGF-1 in both the brain and in serum. In the systemic circulation, IGF-1 can be found free or bound to one of multiple binding proteins, shown to modulate the actions and presentation of IGF-1 to the IGF-1 receptor.73 Subcutaneous injections of escalating doses of 0.1–30.0 mg/kg of IGF-1 results in increasing circulating levels of total and free IGF-1 at 30 min post-injection.74 Previous studies evaluating systemic administration of IGF-1 post-TBI did not evaluate serum or brain levels of IGF-1,51,52,75 preventing direct comparison of local levels of IGF-1 in the damaged brain for central versus systemic dosing. The current work is the first study to evaluate both systemic and brain levels of exogenous IGF-1 administered post-TBI. We show that central infusion results in increased brain IGF-1 levels, with detectable IGF-1 in systemic circulation; however, we did not assess whether this is free or bound IGF-1. Future work is warranted to understand the interplay between pools of IGF-1 in the brain and systemic circulation, and whether the method of administration can influence the ratio of free and bound IGF-1 in circulation.
Central infusion of IGF-1 produced beneficial effects on neurobehavioral function post-TBI. IGF-1 significantly accelerated recovery of motor function at 3 and 5 days post-injury compared to vehicle treatment. Central infusion of IGF-1 also reduced impairment of memory retention associated with CCI as evaluated with an NOR test. The NOR task relies on a rodent's innate behavior to explore novel objects as a way to assay the duration or strength of memory for a familiar, or previously explored, object.76 A decrease in the relative exploration of a novel object compared to a familiar object reflects a decline in residual memory of the familiar object.77–79 Brain injury caused a decline in memory function and, in both the initial dose study and in the subsequent study with 10 μg/day of recombinant hIGF-1 infusion, memory retention was improved in IGF-1–treated, brain-injured mice compared to CCI mice receiving vehicle. These data corroborate previous findings that conditional, astrocyte-specific overexpression of IGF-1 attenuated motor and cognitive deficits in an NOR task at 7 days post-CCI.50 Subsequent to mild weight drop injury, systemic injections of 4 μg/kg of IGF-1 at 24 and 48 h post-injury improved performance in a Y-maze assessment of cognitive function at 7 days post-injury.52 Continuous systemic infusion of 4 mg/kg/day of IGF-1 improved spatial learning and memory and motor function at 2 weeks post-injury in rats subjected to moderate TBI.51 Collectively, previous reports and our current findings highlight that administration of IGF-1 promotes recovery of motor and cognitive function in rats and mice in multiple models and across the severity spectrum of TBI. It is not yet known whether improved recovery of the immature neuron population contributes to behavioral recovery and what other cellular mechanisms may underlie these functional effects.
The IGF-1 receptor is expressed by immature and mature neurons and by astrocytes, and in the vasculature, choroid plexus, and hippocampus throughout adulthood.80–82 Binding of IGF-1 to its cognate receptor elicits activation of Akt and the subsequent inhibition of proapoptotic factors, including caspase-9, Bad, and NF-κB.83,84 Enhanced Akt activation is associated with increased neuronal survival after CNS injury; conversely, low levels of Akt activation have been linked to increased cellular susceptibility to damage and death.85,86 CCI results in an acute and transitory rise in endogenous levels of IGF-1 in the brain87 and a transient increase in hippocampal Akt phosphorylation for hours after experimental TBI, with a return to baseline levels by 24 h post-injury.52,87,88 In a conditional astrocytic-specific IGF-1–overexpressing mouse model, elevated IGF-1 increased Akt phosphorylation in the hippocampus at 24 and 72 h post-CCI,50 suggesting that supplementation of IGF-1 may extend the duration of increased IGF-1 signaling cascades post-TBI. IGF-1-treated mice in this study exhibited a significant increase in Akt phosphorylation in the hippocampus after 7 days of IGF-1 infusion, suggesting that IGF-1 infusion effectively prolonged Akt activity post-TBI. IGF-1 is a multifaceted growth factor and may elicit multiple actions in the brain. IGF-1 may alter endoplasmic reticulum stress by increasing CHOP expression,75 and may increase IGF-1 receptor and ERK phosphorylation.52 We have previously shown that IGF-1 overexpression promotes survival of neurons in the hippocampus.50 In the current study, we highlight that central infusion of IGF-1 enhances hippocampal immature neuron density, but it is plausible that IGF-1 activates or enhances multiple mechanisms in the week post-TBI that may contribute to improved functional recovery. Future investigation into the potency of IGF-1 to exert long-lasting protective and reparative effects after the cessation of treatment will be an important step in understanding and optimizing effective therapeutic treatment paradigms for IGF-1 in the context of TBI.
Because cortical brain swelling and hippocampal distortion in brain-injured mice were more pronounced in a subset of IGF-1 infused mice, we investigated whether IGF-1 might potentiate or prolong regional cerebral edema. Quantification of water content revealed no overt increase in edema with ICV infusion, although a small number of IGF-1–infused animals did exhibit elevated water content. Parameters of body weight, age, and blood glucose did not predict the sporadic occurrence of infusion-related swelling. CSF turnover occurs approximately every 6 h with a total volume of approximately 40 μL of fluid present in the adult mouse brain.89,90 In the current study, an osmotic minipump with a 0.5-μL/h flow rate results in the infusion of 3 μL in a 6-h period. It is not known whether ICV infusion altered post-traumatic intracranial pressure. The cannula base utilized in the present study prevented the implantation of an intracranial pressure–monitoring device into the lateral ventricle, but our findings highlight that future studies should examine the effect of continuous ICV infusion on CSF fluid dynamics in rodent models of TBI. In clinical trials, systemic coadministration of IGF-1 and growth hormone for a period of 14 days to moderate to severely brain-injured patients did not alter fluid retention or increase intracranial pressure,91 suggesting that systemic infusion of IGF-1 does not disrupt CSF balance in the context of human contusion TBI.
Immature neurons are particularly vulnerable to CCI, as demonstrated by a marked reduction in their numbers within days post-injury.20–22,92,93 We observed a similar reduction in DCX+ cells in the granular layer of the injured dentate gyrus at 7 days post-CCI, a time point when immature neurons loss is being partially offset by neuronal differentiation of newly proliferated cells in the subgranular zone.21,24 In the current study, central infusion of IGF-1 post-CCI increased hippocampal immature neuron density at 7 days post-injury. This response showed a dose-dependent trend over the range of 0.3–10.0 μg/day of hIGF-1.
Increases in immature neuron density at 7 days post-TBI may reflect improved survival of existing immature neurons or enhanced generation of new immature neurons. IGF-1 is a potent neurotrophic factor that promotes cell survival of cultured neurons and reduces cell loss after acute CNS injury,54,55,94–96 but can also increase cellular proliferation and promote neuronal differentiation.32–34 We previously showed that astrocyte-specific overexpression of IGF-1 did not protect against acute immature neuron loss at 3 days, but enhanced the generation of new neurons in the injured brain, resulting in increased immature neuron density by 10 days.22 IGF-1 enhances the generation of newborn neurons in the hippocampus by enhancing neuronal differentiation of newly proliferated cells in the uninjured34,42 and traumatically injured22 brain. In the current study, IGF-1 infusion resulted in an increased immature neuron density in brain-injured, but not sham-injured, mice. Whereas immature hippocampal neurons express IGF-1 receptor82 and IGF-1 stimulates neurogenesis under physiological conditions such as exercise,43,44 our data suggest that IGF-1 is far more potent in increasing neurogenesis in the damaged hippocampus. Because CCI elicits a larger increase in proliferation in the damaged (ipsilateral) hippocampus than the contralateral hippocampus,21,23 IGF-1 may act to increase the proportion of newly born cells that commit to a neuronal fate, thereby selectively increasing immature neuron numbers in the injured hippocampus. Future studies will need to determine whether central infusion of IGF-1 promotes long-term survival, maturation, and functional incorporation of the newly generated immature neurons into the hippocampus post-CCI.
In the clinical setting, the average administration window of therapeutic agents in clinical trials is 3–8 h post-TBI for a variety of reasons, including transportation, stabilization, and enrollment of the patient into the trial.67 Using a delayed administration paradigm, we demonstrate that infusion of IGF-1 initiated 6 h post-CCI was effective at increasing immature neuron density. These data provide critical support for the clinical utility of IGF-1 to promote posttraumatic hippocampal plasticity. The duration of the therapeutic window for IGF-1 enhancement of neurogenesis post-TBI has not yet been established. Given that post-traumatic proliferation is elevated for several days post-TBI,21,23,24 IGF-1 may possess an extended window for stimulating differentiation of new neurons.
Conclusions
In summary, we demonstrate that prolonged ICV infusion of IGF-1 increased levels of pAkt in the hippocampus and improved hippocampal immature neuron density after brain injury. The neurogenic effect of centrally delivered IGF-1 was dose dependent and was retained with a clinically relevant 6-h delayed infusion onset. IGF-1 infusion also significantly attenuated motor and cognitive dysfunction after CCI in mice. This study adds to pre-clinical literature supporting beneficial effects of IGF-1 in treating TBI, but highlights the need for careful consideration of the administration paradigm.
Acknowledgments
This work was supported by Kentucky Spinal Cord and Head Injury Research Trust grants KSCHIRT 7-20 and 14-12A and National Institutes of Health grants R01 NS072302, P30 NS051220, and F32 NS090748. The authors thank Sindhu K. Madathil, PhD, and Jennifer Brelsfoard for their assistance in these experiments.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Truelle J.L., Koskinen S., Hawthorne G., Sarajuuri J., Formisano R., Von Wild K., Neugebauer E., Wilson L., Gibbons H., Powell J., Bullinger M., Hofer S., Maas A., Zitnay G., and Von Steinbuechel N.; Qolibri Task Force. (2010). Quality of life after traumatic brain injury: the clinical use of the QOLIBRI, a novel disease-specific instrument. Brain Inj. 24, 1272–1291 [DOI] [PubMed] [Google Scholar]
- 2.Lundin A., de Boussard C., Edman G., and Borg J. (2006). Symptoms and disability until 3 months after mild TBI. Brain Inj. 20, 799–806 [DOI] [PubMed] [Google Scholar]
- 3.Levin H.S. (1998). Cognitive function outcomes after traumatic brain injury. Curr. Opin. Neurol. 11, 643–646 [DOI] [PubMed] [Google Scholar]
- 4.Theadom A., Barker-Collo S., Jones K., Kahan M., Te Ao B., McPherson K., Starkey N., Feigin V., and Group, B.I.y.R. (2017). Work limitations 4 years following mild traumatic brain injury: a cohort study. Arch. Phys. Med. Rehabil. 98, 1560–1566 [DOI] [PubMed] [Google Scholar]
- 5.Lynch D.R., and Dawson T.M. (1994). Secondary mechanisms in neuronal trauma. Curr. Opin. Neurol. 7, 510–516 [DOI] [PubMed] [Google Scholar]
- 6.Tate D.F., and Bigler E.D. (2000). Fornix and hippocampal atrophy in traumatic brain injury. Learn. Mem. 7, 442–446 [DOI] [PubMed] [Google Scholar]
- 7.Bigler E.D., Blatter D.D., Anderson C.V., Johnson S.C., Gale S.D., Hopkins R.O., and Burnett B. (1997). Hippocampal volume in normal aging and traumatic brain injury. AJNR. Am. J. Neuroradiol. 18, 11–23 [PMC free article] [PubMed] [Google Scholar]
- 8.Ariza M., Serra-Grabulosa J.M., Junque C., Ramirez B., Mataro M., Poca A., Bargallo N., and Sahuquillo J. (2006). Hippocampal head atrophy after traumatic brain injury. Neuropsychologia 44, 1956–1961 [DOI] [PubMed] [Google Scholar]
- 9.Kotapka M.J., Graham D.I., Adams J.H., Doyle D., and Gennarelli T.A. (1993). Hippocampal damage in fatal paediatric head injury. Neuropathol. Appl. Neurobiol. 19, 128–133 [DOI] [PubMed] [Google Scholar]
- 10.Serra-Grabulosa J.M., Junque C., Verger K., Salgado-Pineda P., Maneru C., and Mercader J.M. (2005). Cerebral correlates of declarative memory dysfunctions in early traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 76, 129–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamm R.J., Dixon C.E., Gbadebo D.M., Singha A.K., Jenkins L.W., Lyeth B.G., and Hayes R.L. (1992). Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J. Neurotrauma 9, 11–20 [DOI] [PubMed] [Google Scholar]
- 12.Fox G.B., and Faden A.I. (1998). Traumatic brain injury causes delayed motor and cognitive impairment in a mutant mouse strain known to exhibit delayed Wallerian degeneration. J. Neurosci. Res. 53, 718–727 [DOI] [PubMed] [Google Scholar]
- 13.Saatman K.E., Feeko K.J., Pape R.L., and Raghupathi R. (2006). Differential behavioral and histopathological responses to graded cortical impact injury in mice. J. Neurotrauma 23, 1241–1253 [DOI] [PubMed] [Google Scholar]
- 14.Smith D.H., Soares H.D., Pierce J.S., Perlman K.G., Saatman K.E., Meaney D.F., Dixon C.E., and McIntosh T.K. (1995). A model of parasagittal controlled cortical impact in the mouse: cognitive and histopathologic effects. J. Neurotrauma 12, 169–178 [DOI] [PubMed] [Google Scholar]
- 15.Hannay H.J., Feldman Z., Phan P., Keyani A., Panwar N., Goodman J.C., and Robertson C.S. (1999). Validation of a controlled cortical impact model of head injury in mice. J. Neurotrauma 16, 1103–1114 [DOI] [PubMed] [Google Scholar]
- 16.Dixon C.E., Flinn P., Bao J., Venya R. and Hayes R.L. (1997). Nerve growth factor attenuates cholinergic deficits following traumatic brain injury in rats. Exp. Neurol. 146, 479–490 [DOI] [PubMed] [Google Scholar]
- 17.Carlson S.W., Yan H., and Dixon C.E. (2017). Lithium increases hippocampal SNARE protein abundance after traumatic brain injury. Exp. Neurol. 289, 55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dixon C.E., Clifton G.L., Lighthall J.W., Yaghmai A.A., and Hayes R.L. (1991). A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Methods 39, 253–262 [DOI] [PubMed] [Google Scholar]
- 19.Hall E.D., Sullivan P.G., Gibson T.R., Pavel K.M., Thompson B.M., and Scheff S.W. (2005). Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J. Neurotrauma 22, 252–265 [DOI] [PubMed] [Google Scholar]
- 20.Gao X., Deng-Bryant Y., Cho W., Carrico K.M., Hall E.D. and Chen J. (2008). Selective death of newborn neurons in hippocampal dentate gyrus following moderate experimental traumatic brain injury. J. Neurosci. Res. 86, 2258–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rola R., Mizumatsu S., Otsuka S., Morhardt D.R., Noble-Haeusslein L.J., Fishman K., Potts M.B., and Fike J.R. (2006). Alterations in hippocampal neurogenesis following traumatic brain injury in mice. Exp. Neurol. 202, 189–199 [DOI] [PubMed] [Google Scholar]
- 22.Carlson S.W., Madathil S.K., Sama D.M., Gao X., Chen J., and Saatman K.E. (2014). Conditional overexpression of insulin-like growth factor-1 enhances hippocampal neurogenesis and restores immature neuron dendritic processes after traumatic brain injury. J. Neuropathol. Exp. Neurol. 73, 734–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dash P.K., Mach S.A., and Moore A.N. (2001). Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J. Neurosci. Res. 63, 313–319 [DOI] [PubMed] [Google Scholar]
- 24.Sun D., Colello R.J., Daugherty W.P., Kwon T.H., McGinn M.J., Harvey H.B., and Bullock M.R. (2005). Cell proliferation and neuronal differentiation in the dentate gyrus in juvenile and adult rats following traumatic brain injury. J. Neurotrauma 22, 95–105 [DOI] [PubMed] [Google Scholar]
- 25.Bye N., Carron S., Han X., Agyapomaa D., Ng S.Y., Yan E., Rosenfeld J.V., and Morganti-Kossmann M.C. (2011). Neurogenesis and glial proliferation are stimulated following diffuse traumatic brain injury in adult rats. J. Neurosci. Res. 89, 986–1000 [DOI] [PubMed] [Google Scholar]
- 26.Sun D., McGinn M.J., Zhou Z., Harvey H.B., Bullock M.R., and Colello R.J. (2007). Anatomical integration of newly generated dentate granule neurons following traumatic brain injury in adult rats and its association to cognitive recovery. Exp. Neurol. 204, 264–272 [DOI] [PubMed] [Google Scholar]
- 27.Emery D.L., Fulp C.T., Saatman K.E., Schutz C., Neugebauer E., and McIntosh T.K. (2005). Newly born granule cells in the dentate gyrus rapidly extend axons into the hippocampal CA3 region following experimental brain injury. J. Neurotrauma 22, 978–988 [DOI] [PubMed] [Google Scholar]
- 28.Deng W., Saxe M.D., Gallina I.S., and Gage F.H. (2009). Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J. Neurosci. 29, 13532–13542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu Y., Arruda-Carvalho M., Wang J., Janoschka S.R., Josselyn S.A., Frankland P.W., and Ge S. (2012). Optical controlling reveals time-dependent roles for adult-born dentate granule cells. Nat. Neurosci. 15, 1700–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodman T., Trouche S., Massou I., Verret L., Zerwas M., Roullet P. and Rampon C. (2010). Young hippocampal neurons are critical for recent and remote spatial memory in adult mice. Neuroscience 171, 769–778 [DOI] [PubMed] [Google Scholar]
- 31.Blaiss C.A., Yu T.S., Zhang G., Chen J., Dimchev G., Parada L.F., Powell C.M. and Kernie S.G. (2011). Temporally specified genetic ablation of neurogenesis impairs cognitive recovery after traumatic brain injury. J. Neurosci. 31, 4906–4916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brooker G.J., Kalloniatis M., Russo V.C., Murphy M., Werther G.A., and Bartlett P.F. (2000). Endogenous IGF-1 regulates the neuronal differentiation of adult stem cells. J. Neurosci. Res. 59, 332–341 [DOI] [PubMed] [Google Scholar]
- 33.Arsenijevic Y., and Weiss S. (1998). Insulin-like growth factor-I is a differentiation factor for postmitotic CNS stem cell-derived neuronal precursors: distinct actions from those of brain-derived neurotrophic factor. J. Neurosci. 18, 2118–2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aberg M.A., Aberg N.D., Hedbacker H., Oscarsson J., and Eriksson P.S. (2000). Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J. Neurosci. 20, 2896–2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aberg N.D., Brywe K.G., and Isgaard J. (2006). Aspects of growth hormone and insulin-like growth factor-I related to neuroprotection, regeneration, and functional plasticity in the adult brain. TheScientificWorldJournal 6, 53–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCurdy R.D., Feron F., McGrath J.J., and Mackay-Sim A. (2005). Regulation of adult olfactory neurogenesis by insulin-like growth factor-I. Eur. J. Neurosci. 22, 1581–1588 [DOI] [PubMed] [Google Scholar]
- 37.Bozyczko-Coyne D., Glicksman M.A., Prantner J.E., McKenna B., Connors T., Friedman C., Dasgupta M., and Neff N.T. (1993). IGF-I supports the survival and/or differentiation of multiple types of central nervous system neurons. Ann. N. Y. Acad. Sci. 692, 311–313 [DOI] [PubMed] [Google Scholar]
- 38.Sara V.R., and Carlsson-Skwirut C. (1988). The role of the insulin-like growth factors in the regulation of brain development. Prog. Brain Res. 73, 87–99 [DOI] [PubMed] [Google Scholar]
- 39.Carson M.J., Behringer R.R., Brinster R.L., and McMorris F.A. (1993). Insulin-like growth factor I increases brain growth and central nervous system myelination in transgenic mice. Neuron 10, 729–740 [DOI] [PubMed] [Google Scholar]
- 40.D'Ercole A.J., Ye P., and O'Kusky J.R. (2002). Mutant mouse models of insulin-like growth factor actions in the central nervous system. Neuropeptides 36, 209–220 [DOI] [PubMed] [Google Scholar]
- 41.Llorens-Martin M., Torres-Aleman I., and Trejo J.L. (2010). Exercise modulates insulin-like growth factor 1-dependent and -independent effects on adult hippocampal neurogenesis and behaviour. Mol. Cell. Neurosci. 44, 109–117 [DOI] [PubMed] [Google Scholar]
- 42.Trejo J.L., Carro E., and Torres-Aleman I. (2001). Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J. Neurosci. 21, 1628–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trejo J.L., Llorens-Martin M.V., and Torres-Aleman I. (2008). The effects of exercise on spatial learning and anxiety-like behavior are mediated by an IGF-I-dependent mechanism related to hippocampal neurogenesis. Mol. Cell. Neurosci. 37, 402–411 [DOI] [PubMed] [Google Scholar]
- 44.Mir S., Cai W., Carlson S.W., Saatman K.E., and Andres D.A. (2017). IGF-1 mediated neurogenesis involves a novel RIT1/Akt/Sox2 cascade. Sci. Rep. 7, 3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beck K.D., Powell-Braxton L., Widmer H.R., Valverde J., and Hefti F. (1995). Igf1 gene disruption results in reduced brain size, CNS hypomyelination, and loss of hippocampal granule and striatal parvalbumin-containing neurons. Neuron 14, 717–730 [DOI] [PubMed] [Google Scholar]
- 46.Carro E., Trejo J.L., Gomez-Isla T., LeRoith D., and Torres-Aleman I. (2002). Serum insulin-like growth factor I regulates brain amyloid-beta levels. Nat. Med. 8, 1390–1397 [DOI] [PubMed] [Google Scholar]
- 47.Busiguina S., Fernandez A.M., Barrios V., Clark R., Tolbert D.L., Berciano J., and Torres-Aleman I. (2000). Neurodegeneration is associated to changes in serum insulin-like growth factors. Neurobiol. Dis. 7, 657–665 [DOI] [PubMed] [Google Scholar]
- 48.Mustafa A., Lannfelt L., Lilius L., Islam A., Winblad B., and Adem A. (1999). Decreased plasma insulin-like growth factor-I level in familial Alzheimer's disease patients carrying the Swedish APP 670/671 mutation. Dement. Geriatr. Cogn. Disord. 10, 446–451 [DOI] [PubMed] [Google Scholar]
- 49.Markowska A.L., Mooney M., and Sonntag W.E. (1998). Insulin-like growth factor-1 ameliorates age-related behavioral deficits. Neuroscience 87, 559–569 [DOI] [PubMed] [Google Scholar]
- 50.Madathil S.K., Carlson S.W., Brelsfoard J.M., Ye P., D'Ercole A.J., and Saatman K.E. (2013). Astrocyte-specific overexpression of insulin-like growth factor-1 protects hippocampal neurons and reduces behavioral deficits following traumatic brain injury in mice. PLoS One 8, e67204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saatman K.E., Contreras P.C., Smith D.H., Raghupathi R., McDermott K.L., Fernandez S.C., Sanderson K.L., Voddi M., and McIntosh T.K. (1997). Insulin-like growth factor-1 (IGF-1) improves both neurological motor and cognitive outcome following experimental brain injury. Exp. Neurol. 147, 418–427 [DOI] [PubMed] [Google Scholar]
- 52.Rubovitch V., Edut S., Sarfstein R., Werner H., and Pick C.G. (2010). The intricate involvement of the Insulin-like growth factor receptor signaling in mild traumatic brain injury in mice. Neurobiol. Dis. 38, 299–303 [DOI] [PubMed] [Google Scholar]
- 53.Paxinos G., and Watson C. (2009). The Rat Brain in Stereotaxic Coordinates. Compact sixth ed. Elsevier: London [Google Scholar]
- 54.Gluckman P.D., Guan J., Beilharz E.J., Klempt N.D., Klempt M., Miller O., Sirimanne E., Dragunow M., and Williams C.E. (1993). The role of the insulin-like growth factor system in neuronal rescue. Ann. N. Y. Acad. Sci. 692, 138–148 [DOI] [PubMed] [Google Scholar]
- 55.Guan J., Bennet L., George S., Wu D., Waldvogel H.J., Gluckman P.D., Faull R.L., Crosier P.S., and Gunn A.J. (2001). Insulin-like growth factor-1 reduces postischemic white matter injury in fetal sheep. J. Cereb. Blood Flow Metab. 21, 493–502 [DOI] [PubMed] [Google Scholar]
- 56.Zhu Z.F., Wang Q.G., Han B.J., and William C.P. (2010). Neuroprotective effect and cognitive outcome of chronic lithium on traumatic brain injury in mice. Brain Res. Bull. 83, 272–277 [DOI] [PubMed] [Google Scholar]
- 57.Selvamani A., and Sohrabji F. (2010). The neurotoxic effects of estrogen on ischemic stroke in older female rats is associated with age-dependent loss of insulin-like growth factor-1. J. Neurosci. 30, 6852–6861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Escartin C., Boyer F., Bemelmans A.P., Hantraye P., and Brouillet E. (2004). Insulin growth factor-1 protects against excitotoxicity in the rat striatum. Neuroreport 15, 2251–2254 [DOI] [PubMed] [Google Scholar]
- 59.Perez-Martin M., Azcoitia I., Trejo J.L., Sierra A., and Garcia-Segura L.M. (2003). An antagonist of estrogen receptors blocks the induction of adult neurogenesis by insulin-like growth factor-I in the dentate gyrus of adult female rat. Eur. J. Neurosci. 18, 923–930 [DOI] [PubMed] [Google Scholar]
- 60.Pleasant J.M., Carlson S.W., Mao H., Scheff S.W., Yang K.H., and Saatman K.E. (2011). Rate of neurodegeneration in the mouse controlled cortical impact model is influenced by impactor tip shape: implications for mechanistic and therapeutic studies. J. Neurotrauma 28, 2245–2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whalen M.J., Carlos T.M., Dixon C.E., Robichaud P., Clark R.S., Marion D.W., and Kochanek P.M. (2000). Reduced brain edema after traumatic brain injury in mice deficient in P-selectin and intercellular adhesion molecule-1. J. Leuk. Biol. 67, 160–168 [DOI] [PubMed] [Google Scholar]
- 62.Stewart-Wallace A.M. (1939). A biochemical study of cerebral tissue and of the changes in cerebral oedema. Brain 62, 426–438 [Google Scholar]
- 63.Nagaraja T.N., Patel P., Gorski M., Gorevic P.D., Patlak C.S., and Fenstermacher J.D. (2005). In normal rat, intraventricularly administered insulin-like growth factor-1 is rapidly cleared from CSF with limited distribution into brain. Cerebrospinal Fluid Res. 2, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Terpolilli N.A., Kim S.W., Thal S.C., Kuebler W.M., and Plesnila N. (2013). Inhaled nitric oxide reduces secondary brain damage after traumatic brain injury in mice. J. Cereb. Blood Flow Metab. 33, 311–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trabold R., Krieg S., Scholler K., and Plesnila N. (2008). Role of vasopressin V(1a) and V2 receptors for the development of secondary brain damage after traumatic brain injury in mice. J. Neurotrauma 25, 1459–1465 [DOI] [PubMed] [Google Scholar]
- 66.Kenne E., Erlandsson A., Lindbom L., Hillered L., and Clausen F. (2012). Neutrophil depletion reduces edema formation and tissue loss following traumatic brain injury in mice. J. Neuroinflammation 9, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marmarou A., Lu J., Butcher I., McHugh G.S., Mushkudiani N.A., Murray G.D., Steyerberg E.W., and Maas A.I. (2007). IMPACT database of traumatic brain injury: design and description. J. Neurotrauma 24, 239–250 [DOI] [PubMed] [Google Scholar]
- 68.Guan J., Skinner S.J., Beilharz E.J., Hua K.M., Hodgkinson S., Gluckman P.D., and Williams C.E. (1996). The movement of IGF-I into the brain parenchyma after hypoxic-ischaemic injury. Neuroreport 7, 632–636 [DOI] [PubMed] [Google Scholar]
- 69.Guan J., Krishnamurthi R., Waldvogel H.J., Faull R.L., Clark R., and Gluckman P. (2000). N-terminal tripeptide of IGF-1 (GPE) prevents the loss of TH positive neurons after 6-OHDA induced nigral lesion in rats. Brain Res. 859, 286–292 [DOI] [PubMed] [Google Scholar]
- 70.Thorne R.G., Pronk G.J., Padmanabhan V., and Frey W.H., II (2004). Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience 127, 481–496 [DOI] [PubMed] [Google Scholar]
- 71.Song Y., Pimentel C., Walters K., Boller L., Ghiasvand S., Liu J., Staley K.J., and Berdichevsky Y. (2016). Neuroprotective levels of IGF-1 exacerbate epileptogenesis after brain injury. Sci. Rep. 6, 32095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reinhardt R.R. and Bondy C.A. (1994). Insulin-like growth factors cross the blood-brain barrier. Endocrinology 135, 1753–1761 [DOI] [PubMed] [Google Scholar]
- 73.Clemmons D.R., Jones J.I., Busby W.H., and Wright G. (1993). Role of insulin-like growth factor binding proteins in modifying IGF actions. Ann. N. Y. Acad. Sci. 692, 10–21 [DOI] [PubMed] [Google Scholar]
- 74.Contreras P.C., Steffler C., Yu E., Callison K., Stong D., and Vaught J.L. (1995). Systemic administration of rhIGF-I enhanced regeneration after sciatic nerve crush in mice. J. Pharmacol. Exp. Ther. 274, 1443–1449 [PubMed] [Google Scholar]
- 75.Rubovitch V., Shachar A., Werner H., and Pick C.G. (2011). Does IGF-1 administration after a mild traumatic brain injury in mice activate the adaptive arm of ER stress? Neurochem. Int. 58, 443–446 [DOI] [PubMed] [Google Scholar]
- 76.Ennaceur A., and Delacour J. (1988). A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 31, 47–59 [DOI] [PubMed] [Google Scholar]
- 77.Ennaceur A. (2010). One-trial object recognition in rats and mice: methodological and theoretical issues. Behav. Brain Res. 215, 244–254 [DOI] [PubMed] [Google Scholar]
- 78.Suto T., Eisenach J.C., and Hayashida K. (2014). Peripheral nerve injury and gabapentin, but not their combination, impair attentional behavior via direct effects on noradrenergic signaling in the brain. Pain 155, 1935–1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Craig M.M., and Bajic D. (2015). Long-term behavioral effects in a rat model of prolonged postnatal morphine exposure. Behav. Neurosci. 129, 643–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bondy C.A., and Cheng C.M. (2004). Signaling by insulin-like growth factor 1 in brain. Eur. J. Pharmacol. 490, 25–31 [DOI] [PubMed] [Google Scholar]
- 81.Adamo M., Raizada M.K., and LeRoith D. (1989). Insulin and insulin-like growth factor receptors in the nervous system. Mol. Neurobiol. 3, 71–100 [DOI] [PubMed] [Google Scholar]
- 82.Zhang J., Moats-Staats B.M., Ye P., and D'Ercole A.J. (2007). Expression of insulin-like growth factor system genes during the early postnatal neurogenesis in the mouse hippocampus. J. Neurosci. Res. 85, 1618–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fukunaga K., and Kawano T. (2003). Akt is a molecular target for signal transduction therapy in brain ischemic insult. J. Pharmacol. Sci. 92, 317–327 [DOI] [PubMed] [Google Scholar]
- 84.Peruzzi F., Prisco M., Dews M., Salomoni P., Grassilli E., Romano G., Calabretta B., and Baserga R. (1999). Multiple signaling pathways of the insulin-like growth factor 1 receptor in protection from apoptosis. Mol. Cell. Biol. 19, 7203–7215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Noshita N., Lewen A., Sugawara T., and Chan P.H. (2001). Evidence of phosphorylation of Akt and neuronal survival after transient focal cerebral ischemia in mice. J. Cereb. Blood Flow Metab. 21, 1442–1450 [DOI] [PubMed] [Google Scholar]
- 86.Noshita N., Lewen A., Sugawara T., and Chan P.H. (2002). Akt phosphorylation and neuronal survival after traumatic brain injury in mice. Neurobiol. Dis. 9, 294–304 [DOI] [PubMed] [Google Scholar]
- 87.Madathil S.K., Evans H.N., and Saatman K.E. (2010). Temporal and regional changes in IGF-1/IGF-1R signaling in the mouse brain after traumatic brain injury. J. Neurotrauma 27, 95–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang X., Chen Y., Ikonomovic M.D., Nathaniel P.D., Kochanek P.M., Marion D.W., DeKosky S.T., Jenkins L.W., and Clark R.S. (2006). Increased phosphorylation of protein kinase B and related substrates after traumatic brain injury in humans and rats. J. Cereb. Blood Flow Metab. 26, 915–926 [DOI] [PubMed] [Google Scholar]
- 89.Rudick R.A., Zirretta D.K., and Herndon R.M. (1982). Clearance of albumin from mouse subarachnoid space: a measure of CSF bulk flow. J. Neurosci. Methods 6, 253–259 [DOI] [PubMed] [Google Scholar]
- 90.Pardridge W.M. (1991). Peptide Drug Delivery to the Brain. Raven: New York [Google Scholar]
- 91.Hatton J., Kryscio R., Ryan M., Ott L., and Young B. (2006). Systemic metabolic effects of combined insulin-like growth factor-I and growth hormone therapy in patients who have sustained acute traumatic brain injury. J. Neurosurg. 105, 843–852 [DOI] [PubMed] [Google Scholar]
- 92.Yu T.S., Zhang G., Liebl D.J., and Kernie S.G. (2008). Traumatic brain injury-induced hippocampal neurogenesis requires activation of early nestin-expressing progenitors. J. Neurosci. 28, 12901–12912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cai W., Carlson S.W., Brelsfoard J.M., Mannon C.E., Moncman C.L., Saatman K.E., and Andres D.A. (2012). Rit GTPase signaling promotes immature hippocampal neuronal survival. J. Neurosci. 32, 9887–9897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Russell J.W., Windebank A.J., Schenone A., and Feldman E.L. (1998). Insulin-like growth factor-I prevents apoptosis in neurons after nerve growth factor withdrawal. J. Neurobiol. 36, 455–467 [DOI] [PubMed] [Google Scholar]
- 95.Bendall S.C., Stewart M.H., Menendez P., George D., Vijayaragavan K., Werbowetski-Ogilvie T., Ramos-Mejia V., Rouleau A., Yang J., Bosse M., Lajoie G., and Bhatia M. (2007). IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature 448, 1015–1021 [DOI] [PubMed] [Google Scholar]
- 96.Brywe K.G., Mallard C., Gustavsson M., Hedtjarn M., Leverin A.L., Wang X., Blomgren K., Isgaard J., and Hagberg H. (2005). IGF-I neuroprotection in the immature brain after hypoxia-ischemia, involvement of Akt and GSK3beta? Eur. J. Neurosci. 21, 1489–1502 [DOI] [PubMed] [Google Scholar]