Abstract
Background
Current algorithms and device morphology templates have been proposed in current Implantable Cardioverter-Defibrillators (ICDs) to minimize inappropriate therapies (ITS), but this has not been completely successful.
Aim
Assess the impact of a deliberate strategy of using an atrial lead implant with standardized parameters; based on all current ICD discriminators and technologies, on the burden of ITS.
Method
A retrospective single-centre analysis of 250 patients with either dual chamber (DR) ICDs or biventricular ICDs (CRTDs) over a (41.9 ± 27.3) month period was performed. The incidence of ITS on all ICD and CRTD patients was chronicled after the implementation of standardized programming.
Results
39 events of anti-tachycardial pacing (ATP) and/or shocks were identified in 20 patients (8% incidence rate among patients). The total number of individual therapies was 120, of which 34% were inappropriate ATP, and 36% were inappropriate shocks. 11 patients of the 250 patients received ITS (4.4%). Of the 20 patients, four had ICDs for primary prevention and 16 for a secondary prevention. All the episodes in the primary indication group were inappropriate, while seven patients (43%) of the secondary indication group experienced inappropriate therapies.
Conclusions
The burden of ITS in the population of patients receiving ICDs was 4.4% in the presence of atrial leads. The proposed rationalized programming criteria seems an effective strategy to minimize the burden of inappropriate therapies and will require further validation.
Keywords: Implantable cardioverter-defibrillator (ICDs), Inappropriate therapies, Standardized programming
1. Introduction
Inappropriate therapies (ITS) from implantable cardioverter-defibrillators (ICD) lead to significant morbidity either from the painful delivery of shocks or from the pro-arrhythmic potential [1]. Prior studies suggest that 15–28% of anti-tachycardia therapies may be inappropriate [2]. Measures to reduce inappropriate shocks, including an empiric ablative strategy, have been shown to reduce morbidity [3].
It seems intuitive that device specialists should also refine ICD programming to minimize ITS, as this is the least invasive option. Inappropriate therapies occur more frequently in patients having supraventricular arrhythmias, particularly atrial fibrillation, or in younger patients who achieve higher sinus tachycardia rates and may represent up to 12.1% of ITS [4]. We contend that the use of dual chamber devices improves the algorithmic differentiation of atrial from ventricular arrhythmias, but does not completely resolve problem [5].
In this study, we evaluated the burden of ITS affecting a heterogeneous population of recipients of ICDs, with dual chamber (DR) ICD or biventricular ICD (CRTD) defibrillators with current recommended programming standards.
2. Methods
This is a retrospective single centre analysis. We examined the overall device therapies (defined as ATP and/or high voltage (HV) shock) in a cohort of 250 patients implanted with either DR ICDs or CRTDs. Indications for the implants were both primary and secondary, and had a follow up period of 41.9 ± 27.3 months. Follow up was conducted at two months in the first visit, then six-monthly intervals afterwards in our devices clinic. Physicians were to follow current guidelines for pharmacologic therapy if required.
2.1. End point
Inappropriate ICD therapy was defined as all device therapy delivered (by ATP or HV) for sinus tachycardia, atrial fibrillation, atrial flutter, or regular supraventricular tachycardia. Evaluate the impact of a standardized programming regimen on patients.
2.2. Rhythm discrimination
All events associated with therapy were classified as appropriate or inappropriate. Inappropriate therapy was classified as any therapy delivered in a rhythm that was not a true ventricular arrhythmia.
The supraventricular tachycardia was classified as atrial tachycardia (AT) if the atrial near field electromyograms (EGMs) measured as regular. If the variability of the atrial cycle length was more than 50 ms with a rapid atrial rate (<200 ms) recorded, it was diagnosed as atrial fibrillation (AF). A paroxysmal supraventricular tachycardia (either AVNRT/AVRT) or AT was inferred depending on the response to ATP (VAV or VAAV response respectively) [6].
All recorded events with stored EGMs were reviewed independently by two electrophysiologists and by a third reviewer in the case of disagreement. The empiric programming strategy was considered in all devices to minimize inappropriate therapies illustrated in (Table 2-A, Table 2-BB).
Table 2-A.
Empiric programming parameters for devices in primary prevention purpose.
| Medtronic | Abbot “SJM” | Biotronik | Boston Scientific | Sorin | |
|---|---|---|---|---|---|
| VF (VF + FVT) CL in bpm | 200 | 250 | 250 | 220 | 200–240 |
| NID | 30 | 30 | 12/16 | 2.5 s delay | 6 cycles |
| Therapy ATP | ATP during charge | ATP during charge | ATP: Burst 1 | ATP: Burst 1 | ATP: Burst 1 |
| HV | Shock x 6 | Shock x 6 | Shock x 6 | Shock x 6 | Shock x 6 |
| VT2 (FVT via VF) CL in bpm | 250 | 200 | 200 | 200 | 200 |
| NID | 30 | 30 | 28 (RD-14) | 12 s delay | 6 cycles |
| Therapy ATP | ATP: Burst 1 | ATP: Burst 1 | ATP: Burst 1 | ATP: Burst 1 | ATP: Burst 1 |
| HV HV | Shock x 5 | Shock x 4 | Shock x 5 | Shock x 5 | Shock x 5 |
| VT1 CL in bpm | 171 | 171 | 171 | 170 | 170 |
| NID | 28 | 30 | 30 (RD-16) | 5 s delay | 12 cycles |
| Therapy ATP | ATP: Burst 1 + Burst 2 | ATP: Burst 1 + Burst 2 | ATP: Burst 1 + Burst 2 | ATP: Burst 1 + Burst 2 | ATP: Burst 1 + Burst 2 |
| HV | Shock x 4 | Shock x 3 | Shock x 4 | Shock x 4 | Shock x 4 |
| Monitor CL in bpm | 150 | 150 | |||
| NID | 32 | 12 cycles | |||
| Therapy | None | None |
In Biotronik devices, 1 ramp sequence (3 × ATPs) is applied in VT zone.
Burst ATP (each sequences 3 × ATPs): 8 intervals, R-S1 = 88–84%, adaptive to CL (Upper rate ATP cut off 260 b/min (SJM).
Ramp ATP: 8 intervals, R-S1 = 91 90%, 10 ms decrement.
Time out: OFF (Boston Scientific).
Smart mode: OFF (Medtronic).
Progressive therapy: ON (Medtronic >2 Active zone).
ATP Optimization: ON (Biotronik).
Table 2-B.
Empiric programming parameters for devices in Secondary prevention purpose.
| Medtronic | Abbot “SJM” | Biotronik | Boston Scientific | Sorin | |
|---|---|---|---|---|---|
| VF (VF + FVT) CL in bpm | 200 | 250 | 250 | 220 | 200–240 |
| NID | 30 | 30 | 12/16 | 2.5 s delay | 6 cycles |
| Therapy ATP |
ATP during charge | ATP during charge | ATP: Burst 1 | ATP: Burst 1 | ATP: Burst 1 |
| HV | Shock x 6 | Shock x 6 | Shock x 6 | Shock x 6 | Shock x 6 |
| VT2 (FVT via VF) CL in bpm | 250 | 200 | 200 | 200 | 200 |
| NID | 30 | 30 | 28 (RD-14) | 12 s delay | 6 cycles |
| Therapy ATP |
ATP: Burst 1 | ATP: Burst 1 | ATP: Burst 1 | ATP: Burst 1 | ATP: Burst 1 |
| HV HV | Shock x 5 | Shock x 4 | Shock x 5 | Shock x 5 | Shock x 5 |
| VT1 CL in bpm | 171/VTCL-20 | 171/VTCL-20 | 171/VTCL-20 | 170/VTCL-20 | 170/VTCL-20 |
| NID | 28 | 30 | 30 (RD-16) | 5 s delay | 12 cycles |
| Therapy ATP |
ATP: Burst 1 + Burst 2 | ATP: Burst 1 + Burst 2 | ATP: Burst 1 + Burst 2 | ATP: Burst 1 + Burst 2 | ATP: Burst 1 + Burst 2 |
| HV | Shock x 4 | Shock x 3 | Shock x 4 | Shock x 4 | Shock x 4 |
| Monitor CL in bpm | 150/VTCL-30 | 150/VTCL-30 | |||
| NID | 32 | 12 cycles | |||
| Therapy | None | None |
In Biotronik devices, 1 ramp sequence is applied in VT zone.
Burst ATP (each sequences x 3 ATPs): 8 intervals, R-S1 = 88–84%, 20 ms decrement (Upper rate ATP Cut off 260 b/min (St Jude).
Ramp ATP: 8 intervals, R-S1 = 91 90%, 10 ms decrement.
Time out: OFF (Boston Scientific).
Smart mode: OFF (Medtronic).
Progressive therapy: ON (Medtronic).
ATP Optimization: ON (Biotronik).
VT CL in ms is programmed at rate of 171 bpm or presenting VT CL – 20 ms whichever is higher and lower than 200 bpm (300 ms).
2.3. Exclusion criteria
Patients with ITS due to non-arrhythmic episodes e.g. over sensing or detection of non-physiological “noise” were excluded from the analysis. This study was therefore streamlined to evaluate the use of ICD programming to discriminate supraventricular tachycardia (SVT) from ventricular arrhythmias. We excluded patients without atrial leads from the analysis. The atrial lead has been successfully demonstrated to lower the inappropriate shock rate of dual/triple-chamber ICD group when compared to the single-chamber ICD group according to PainFree SST trial [7].
2.4. Proposed device programming
In Table 2-A, Table 2-BB, we proposed our centre and institutional board approved programming based on reviewing all the guidelines on large prospective trials for ICD programming. A proven optimal programming approach would adopt a simple therapy prescription, reduce inadvertent programming errors and reduce shock related morbidity, thereby improving therapy outcomes. The available sources for programming as per manufacturer were PROVE trial for St Jude Medical (SJM - now Abbott) [8]; PAINFREE II for Medtronic [7]; MADIT- RIT trial for Boston Scientific devices [9]; and general consensus of the American Heart Association (AHA) recommendations [10], [11]. All these proposed programming parameters discussed and adjusted in conjunction with manufacturers continuous collaborations.
2.5. Statistical analysis
The quantitative variables with normal distribution are presented as a mean and SD. The other variables (qualitative) are represented as a percentage. Statview version 5.0 for Windows (SAS Institute Inc. Cary, NC, USA) was used for statistical analysis.
3. Results
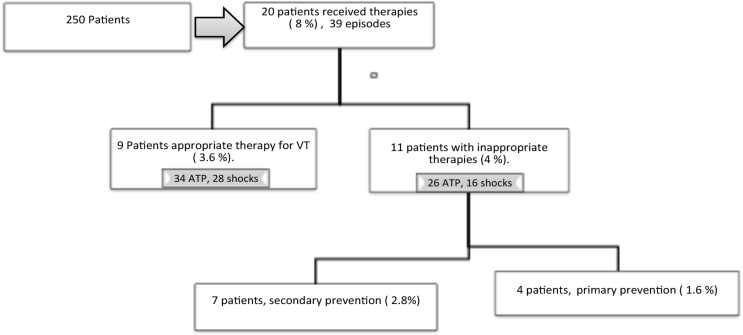
In total, we implanted 250 devices of them 165 were DR ICDs and 85 were CRTDs. Cohort classification according to device indication and all therapies are shown in Fig. 1.
Fig. 1.
This depicts study population and device therapy delivered over the follow up period in the whole study population. The proportion of appropriate and inappropriate therapies and distribution of ITS according to the cause of the underlying cardiomyopathy.
We identified 39 events (giving a total of 120 therapies) in 20 patients. Inappropriate therapies “ITS” were identified in 11 patients. We presented the characteristics and baseline demographics of ITS group in Table 1. We focused our study and data analysis on the identified 20 patients who received device therapies.
Table 1.
Characteristics of patients receiving inappropriate therapies.
| Baseline demographics | |
| Sex (Female) | 4, 20% |
| Age (mean years± SD) | 73.1 ± 7.1 |
| Hypertension (n, %) | 15, 75% |
| DM (n, %) | 6, 30% |
| LVEF (mean ± SD) | 31.1 ± 13.3 |
| Follow up (months mean ± SD) | 41.9 ± 27.3 |
| SVT history (overlap of SVTs is observed) | 12, 60% |
| a. AF/AT history (n, %) | 5, 25% |
| b. A Flutter (n, %) | 5, 25% |
| c. Reentrant Tachycardia (n, %) | 2, 10% |
| Underlying heart disease | |
| Ischemic (n, %) | 13, 65% |
| DCM (non-ischemic) (n, %) | 6, 30% |
| Medications | |
| Beta Blockers (n, %) | 17, 85% |
| Amiodarone (n, %) | 8, 40% |
| Anticoagulant (n, %) | 11, 55% |
| Ca2+ antagonists (n, %) | 1, 5% |
| Devices | |
| CRTD (n, %) | 10, 50% |
| ICD (n, %) | 10, 50% |
| Indication | |
| Primary prevention (n, %) | 4, 20% |
| Secondary prevention (n, %) | 16, 80% |
According to indication of device implantation in our cohort of those 20 patients; we had four devices implanted for a primary indication (n = 2 DR ICD and n = 2 CRTD), while the remaining 16 patients had ICDs implanted for a secondary indication (n = 8 DR ICD and n = 8 CRTD). See Table 1 & Fig. 1
The event rate of the patients that received therapies in regards to the whole cohort was 8% (20/250 patients) over a 41-month period, with an average of 0.95 events per month (39 events/41 months) for the whole cohort.
However, Sub analysis of the 20 patients that received therapies identified that 100% (n = 4/4) patients in the primary prevention group had ITS for a SVT. In secondary prevention group (n = 16), 44% (n = 7/16) of patients had ITS (Fig. 2), whilst the remaining 56% (n = 916) had appropriate therapies for ventricular tachycardia (VT). The overall incidence of ITS delivered in both primary and secondary prevention group was 4.4% (11/250 patients) in comparison to the whole study cohort.
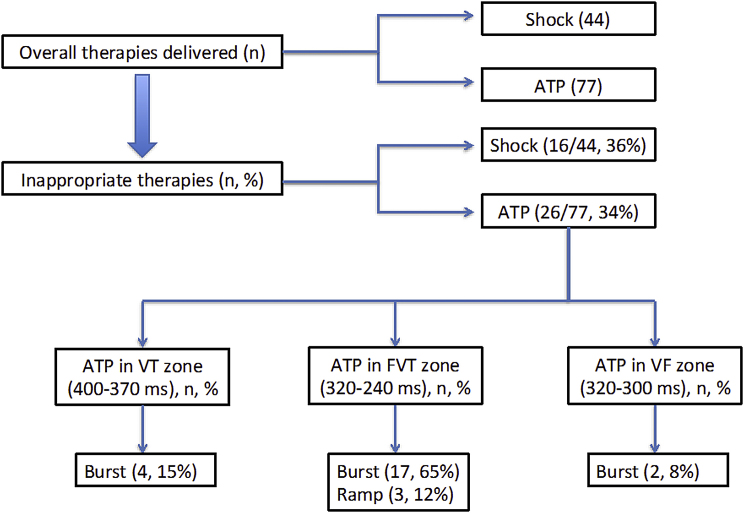
Fig. 2.
Analysis of delivered therapies and SVT episodes eliciting inappropriate therapies. VT = ventricular tachycardia zone, FVT = Fast ventricular tachycardia zone.
According to the type of therapies delivered, were 120 in total; we found 76 sequences of ATP and 44 HV shocks (High volt shocks). Out of these, inappropriate therapies included 26 ATP sequences (34.2%) and 16 (36.4%) HV shocks (Fig. 2)
4. Discussion
Implantable defibrillators have improved survival in patients at risk of sudden cardiac arrhythmia [6], [12]. They have also impacted on the quality of life for patients with heart failure [13]. Despite advances in ICD development and advanced programming, ITS have not been fully overcome. Sinus tachycardia in younger individuals is especially challenging due to the sinus tachycardia rates approaching the tachycardia detection intervals, as are supraventricular arrhythmias, such as AF [14]. The delivery of inappropriate shocks has been linked to decreased survival and increased morbidity, and measures to reduce this phenomenon are imperative [15]. The presence of an atrial lead has demonstrated success in PainFree SST trial with lower inappropriate shock rates of the dual/triple-chamber ICD group when compared to single-chamber ICD group [7]. This accounts for better discrimination of SVT and therefore a lower incidence of ITS.
In our heterogeneous cohort (both primary and secondary) we proposed programming parameters that coalesce the most common programming trials tailored as device manufacturer specifications to minimize ITS.
Our study population entailed a cross section of heterogeneous patients with a predominance of patients with secondary indications for ICD implantation (80%). The remaining 20% had a primary indication. Only four patients implanted with a primary indication for an ICD received inappropriate therapies with EGM analysis revealing underlying AF or AT. Keruz et al. showed a higher incidence of ITS in patients having an ICD for a primary indication (range between 9 and 15%), however in our study its 1.8% [16].
16 patients in the secondary prevention received therapies, of which only 2.8% were ITS (7/250), characterized by inappropriate classification of AF/AT with one suspected SVT. This ITS ratio in our cohort is comparable to previous studies as we considered dual chamber devices in all our new implants [4], [7], [14], [17], [18].
ATP was the initial therapy in all episodes whether they were classified at VT or SVT. A long detection interval was programmed as per the PainFREE Rx II study protocol [7], [19] and ADVANCE III trial, proving a long detection interval resulted in a lower rate of inappropriate HV shocks [20]. This would allow for at least one burst of ATP even in the ventricular fibrillation (VF) zone to terminate fast VT during a capacitor charge to obviate the need for a shock if the ATP was effective. ATP programming was shown to be a highly effective, safe and painless method of terminating VT in the EMPIRIC and PROVE trial [8].
The categorization of ITS was therefore based on delivery of ATP and/or HV shocks in this study to increase the detection rate of episodes. The delivery of ATP is not innocuous as rapid ventricular pacing may well be pro-arrhythmic, particularly in this cohort due to the likely presence of an underlying macro re-entrant substrate [21] The mean ATP CL in our cohort was 365 ± 31 ms.
Shock morbidity has previously been quantified by determining the following [16]:
-
•
Proportion of true VT/VF episodes that are shocked
-
•
Proportion of true SVT episodes that are shocked
-
•
Time to first shock (VT/VF or SVT)
-
•
Time to first VT/VF shock
-
•
Time to first SVT shock
Ultimately there were 44 shocks delivered with 36% (n = 16/44) of these delivered for an SVT. It is noteworthy that six sequences of ATP were successful in terminating the SVT.
Programming measures that may reduce the risk of an ITS include [10], [11], [22].
-
1.
Programming a higher time delay interval (TDI) in patients with AF/AT or in the case of younger patients receiving ICDs where a higher intrinsic sinus rate may be anticipated.
-
2.
Avoid programming ONSET and STABILITY discriminators as these tend to require fewer detection intervals to evaluate a rhythm, therefore dominating over other more sensitive algorithms, thus increasing the chance of ITS.
-
3.
Programming a slower VT detection interval with advanced discriminators so even if an inappropriate shock is delivered it is synchronized to an R wave and therefore less chance of ventricular arrhythmia induction.
-
4.
If a VT zone is considered then advanced discriminators should be applied to overlap into the VF zone when permitted. This allows rhythm discriminators to be applied in the VF zone as well, rather than relying purely on rate.
Therefore, there may be a place for patient-tailored device programming to minimize the risk of ITS in those at risk of this phenomenon when indicated. Good programming choices are essential for patient acceptance of ICD therapy. We do not discount the advantage that for the remaining population receiving ICDs, there is merit in an empiric-programming regimen [23]. We propose a new standardized parameter programming that would favor ATP over HV shock therapy and increase the threshold of the device to deliver ITS. These programming parameters are illustrated in Table 2-A, Table 2-Ba and 2b
4.1. Limitations
The small population size studied makes it difficult to make recommendations on intervention. However, the low incidence of ITS in this cohort validates the effectiveness of the programming measures we adopted in these patients. Our cohort included only a few patients with Boston Scientific devices and the programming recommendation were derived from MADIT III trial and the manufacturer's scientific recommendations.
This study only included the devices with atrial leads, however comparison with single chamber devices may be interesting. PainFree SST trial also favors atrial lead implant for rhythm discrimination [7]. Finally, whilst over sensing of noise on the atrial lead was excluded from this trial to focus on SVTs as the main culprit for ITS, it would be interesting to see if by programing these parameters reduces ITS by allowing corresponding algorithms activation prior to therapy initiation.
5. Conclusion
The burden of ITS in the population of patients receiving ICDs was 4.4% in the presence of atrial leads. The proposed rationalized programming criteria seems an effective strategy to minimize the burden of inappropriate therapies and will require further validation.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Rinaldi C.A., Simon R.D., Baszko A., Bostock J., Elliot D., Bucknall C.A. A 17 year experience of inappropriate shock therapy in patients with implantable cardioverter-defibrillators: are we getting any better? Heart. 2004;90(3):330–331. doi: 10.1136/hrt.2003.026195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weber M., Bocker D., Bansch D., Brunn J., Castrucci M., Gradaus R. Efficacy and safety of the initial use of stability and onset criteria in implantable cardioverter defibrillators. J Cardiovasc Electrophysiol. 1999;10(2):145–153. doi: 10.1111/j.1540-8167.1999.tb00655.x. [DOI] [PubMed] [Google Scholar]
- 3.Reddy V.Y., Reynolds M.R., Neuzil P., Richardson A.W., Taborsky M., Jongnarangsin K. Prophylactic catheter ablation for the prevention of defibrillator therapy. N. Engl J Med. 2007;357(26):2657–2665. doi: 10.1056/NEJMoa065457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jodko L., Kornacewicz-Jach Z., Kazmierczak J., Rzeuski R., Zielonka J., Kaliszczak R. Inappropriate cardioverter-defibrillator discharge continues to be a major problem in clinical practice. Cardiol J. 2009;16(5):432–439. [PubMed] [Google Scholar]
- 5.Dorian P., Philippon F., Thibault B., Kimber S., Sterns L., Greene M. Randomized controlled study of detection enhancements versus rate-only detection to prevent inappropriate therapy in a dual-chamber implantable cardioverter-defibrillator. Heart Rhythm Official J Heart Rhythm Soc. 2004;1(5):540–547. doi: 10.1016/j.hrthm.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Michael K.A., Enriquez A., Baranchuk A., Haley C., Caldwell J., Simpson C.S. Failed anti-tachycardia pacing can be used to differentiate atrial arrhythmias from ventricular tachycardia in implantable cardioverter-defibrillators. Eur Eur pacing, Arrhythm cardiac Electrophysiol J Work Groups Cardiac Pacing, Arrhythm cardiac Cell Electrophysiol Eur Soc Cardiol. 2015;17(1):78–83. doi: 10.1093/europace/euu169. [DOI] [PubMed] [Google Scholar]
- 7.Auricchio A., Schloss E.J., Kurita T., Meijer A., Gerritse B., Zweibel S. Low inappropriate shock rates in patients with single- and dual/triple-chamber implantable cardioverter-defibrillators using a novel suite of detection algorithms: PainFree SST trial primary results. Heart rhythm Official J Heart Rhythm Soc. 2015;12(5):926–936. doi: 10.1016/j.hrthm.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Saeed M., Neason C.G., Razavi M., Chandiramani S., Alonso J., Natarajan S. Programming antitachycardia pacing for primary prevention in patients with implantable cardioverter defibrillators: results from the PROVE trial. J Cardiovasc Electrophysiol. 2010;21(12):1349–1354. doi: 10.1111/j.1540-8167.2010.01825.x. [DOI] [PubMed] [Google Scholar]
- 9.Moss A.J., Schuger C., Beck C.A., Brown M.W., Cannom D.S., Daubert J.P. Reduction in inappropriate therapy and mortality through ICD programming. N. Engl J Med. 2012;367(24):2275–2283. doi: 10.1056/NEJMoa1211107. [DOI] [PubMed] [Google Scholar]
- 10.Wilkoff B.L., Fauchier L., Stiles M.K., Morillo C.A., Al-Khatib S.M., Almendral J. 2015 HRS/EHRA/APHRS/SOLAECE expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing. Eur Eur pacing, Arrhythm Cardiac Electrophysiol J Work Groups Cardiac Pacing, Arrhythm cardiac Cell Electrophysiol Eur Soc Cardiol. 2016;18(2):159–183. [Google Scholar]
- 11.Wilkoff B.L., Fauchier L., Stiles M.K., Morillo C.A., Al-Khatib S.M., Almendral J. 2015 HRS/EHRA/APHRS/SOLAECE expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing. Heart rhythm Official J Heart Rhythm Soc. 2016;13(2):e50–86. doi: 10.1016/j.hrthm.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 12.Birnie D.H., Sambell C., Johansen H., Williams K., Lemery R., Green M.S. Use of implantable cardioverter defibrillators in Canadian and US survivors of out-of-hospital cardiac arrest. CMAJ. 2007;177(1):41–46. doi: 10.1503/cmaj.060730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saksena S., Nagarakanti R. The future of implantable defibrillator and cardiac resynchronization therapy trials. J interventional cardiac Electrophysiol Int J Arrhythm Pacing. 2008;23(1):29–39. doi: 10.1007/s10840-008-9302-6. [DOI] [PubMed] [Google Scholar]
- 14.Yang J.H., Byeon K., Yim H.R., Park J.W., Park S.J., Huh J. Predictors and clinical impact of inappropriate implantable cardioverter-defibrillator shocks in Korean patients. J Korean Med Sci. 2012;27(6):619–624. doi: 10.3346/jkms.2012.27.6.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poole J.E., Johnson G.W., Hellkamp A.S., Anderson J., Callans D.J., Raitt M.H. Prognostic importance of defibrillator shocks in patients with heart failure. N. Engl J Med. 2008;359(10):1009–1017. doi: 10.1056/NEJMoa071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kreuz J., Balta O., Liliegren N., Mellert F., Esmailzadeh B., Nickenig G. Incidence and characteristics of appropriate and inappropriate therapies in recipients of ICD implanted for primary prevention of sudden cardiac death. Pacing and clinical electrophysiology. PACE. 2007;30(1):S125–S127. doi: 10.1111/j.1540-8159.2007.00621.x. [DOI] [PubMed] [Google Scholar]
- 17.Diemberger I., Martignani C., Biffi M., Frabetti L., Valzania C., Cooke R.M. Arrhythmia discrimination by physician and defibrillator: importance of atrial channel. Int J Cardiol. 2012;154(2):134–140. doi: 10.1016/j.ijcard.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Kuhlkamp V., Wilkoff B.L., Brown A.B., Volosin K.J., Hugl B.J., Stafford W. Experience with a dual chamber implantable defibrillator. Pacing Clin Electrophysiol PACE. 2002;25(7):1041–1048. doi: 10.1046/j.1460-9592.2002.01041.x. [DOI] [PubMed] [Google Scholar]
- 19.Sweeney M.O., Wathen M.S., Volosin K., Abdalla I., DeGroot P.J., Otterness M.F. Appropriate and inappropriate ventricular therapies, quality of life, and mortality among primary and secondary prevention implantable cardioverter defibrillator patients: results from the Pacing Fast VT REduces Shock ThErapies (PainFREE Rx II) trial. Circulation. 2005;111(22):2898–2905. doi: 10.1161/CIRCULATIONAHA.104.526673. [DOI] [PubMed] [Google Scholar]
- 20.Gasparini M., Proclemer A., Klersy C., Kloppe A., Lunati M., Ferrer J.B. Effect of long-detection interval vs standard-detection interval for implantable cardioverter-defibrillators on antitachycardia pacing and shock delivery: the ADVANCE III randomized clinical trial. Jama. 2013;309(18):1903–1911. doi: 10.1001/jama.2013.4598. [DOI] [PubMed] [Google Scholar]
- 21.Sweeney M.O., Ruetz L.L., Belk P., Mullen T.J., Johnson J.W., Sheldon T. Bradycardia pacing-induced short-long-short sequences at the onset of ventricular tachyarrhythmias: a possible mechanism of proarrhythmia? J Am Coll Cardiol. 2007;50(7):614–622. doi: 10.1016/j.jacc.2007.02.077. [DOI] [PubMed] [Google Scholar]
- 22.Wilkoff B.L. 2015 HRS/EHRA/APHRS/SOLAECE expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing. Eur Eur Pacing, Arrhythm Cardiac Electrophysiol J Work Groups Cardiac Pacing, Arrhythm Cardiac Cell Electrophysiol Eur Soc Cardiol. 2017;19(4):580. [Google Scholar]
- 23.Morgan J.M., Sterns L.D., Hanson J.L., Ousdigian K.T., Otterness M.F., Wilkoff B.L. A trial design for evaluation of empiric programming of implantable cardioverter defibrillators to improve patient management. Curr Control Trials Cardiovasc Med. 2004;5(1):12. doi: 10.1186/1468-6708-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]