Abstract
Objective:
Resistin, a cysteine-rich peptide, is associated with atherosclerosis and diabetes. Resistin levels increase corresponding to coronary artery disease (CAD) and heart failure severity. Since resistin level tends to elevate with symptomatic heart failure, it is expected to be associated with left ventricular end-diastolic pressure (LVEDP). However, there is no relevant literature on the relationship between resistin levels and LVEDP. We aimed to evaluate the association between resistin levels and LVEDP, severity of CAD, carotid intima-media thickness (CIMT), and echocardiographic diastolic dysfunction parameters.
Methods:
For this study, 128 euvolemic patients with creatinine clearance >50 mg/dL and without acute coronary syndrome, who had typical chest pain or were stress test positive, were enrolled. Resistin level was measured by Enzyme-linked immunosorbent assays (ELISA) method. Severe CAD is defined as ≥50% stenosis in one of the major coronary arteries. LVEDP was measured during left heart catheterization.
Results:
After coronary angiography, 60 patients (46.9%) had severe CAD. The mean LVEDPs were similar for patients with and without severe CAD (p=0.480). The resistin levels did not differ between the groups (p=0.154). The resistin levels did not correlate with LVEDP (r=−0.045, p=0.627), ejection fraction (EF; r=0.110, p=0.228), the Gensini score (r=−0.091, p=0.328), and CIMT (r=0.082, p=0.457). No significant correlation was found between the echocardiographic diastolic dysfunction parameters and resistin levels.
Conclusion:
There was no significant correlation between resistin level and LVEDP, CAD severity, echocardiographic diastolic dysfunction parameters, and CIMT. Further studies are warranted to determine the efficacy of resistin in clinical use.
Keywords: resistin, left ventricular end-diastolic pressure, coronary artery disease, carotid intima-media thickness, diastolic dysfunction
Introduction
Cardiovascular diseases are the primary cause of mortality and morbidity worldwide (1). In parallel with the increase in the number of cardiovascular diseases, the developments in new invasive and noninvasive techniques that evaluate the severity of cardiovascular diseases still continue. Left ventricular end-diastolic pressure (LVEDP) measurement during left heart catheterization is an invasive method used as an indicator of left ventricular function. Several clinical disturbances that can affect left ventricular performance may cause elevated LVEDPs. However, since this technique requires arterial puncture and left heart catheterization, noninvasive alternative methods that can provide the information about LVEDP are needed.
Resistin is a cysteine-rich polypeptide and a member of a family known as resistin-like molecules. It was first discovered in rodents for its role in insulin resistance and obesity (2). Rodent resistin is mostly secreted from adipose tissue (3). In contrast to rodents, human resistin is mostly expressed from inflammatory cells (4,5). Human resistin is 59% similar to rodent insulin in terms of amino acid level. Several studies have indicated resistin’s relationship with health issues such as insulin resistance, obesity, nonalcoholic liver diseases, rheumatological diseases, malignancies, asthma, inflammatory bowel diseases, and chronic kidney diseases in humans (2, 6-10). There is also a relationship between the elevated levels of resistin and inflammatory processes and atherosclerosis in humans (11,12). Resistin levels also increase as the symptoms of heart failure worsen (13). Some studies show that the high levels of resistin predict the development of heart failure (14,15). Higher resistin levels are measured in acute coronary syndrome patients (16-18). Other studies on coronary angiography found resistin levels to be increased according to the number of coronary arteries with ≥50% stenosis (19,20). In contrast, other publications show no relationship between resistin levels and coronary artery diseases (CAD) (21-24).
Some studies suggest that resistin levels increase as heart failure symptoms worsen and that hypervolemia in these patients is symptomatic (13). Since LVEDP increases with hypervolemia, we hypothesized that resistin level would be higher as LVEDP increases. In our present study, we examined whether the serum levels of resistin are associated with LVEDP and whether we can noninvasively predict LVEDP. We also addressed the relationship between resistin levels and the severity of CAD, CIMT, and the echocardiographic parameters of diastolic heart failure.
Methods
Study participants
The study was reviewed and approved by the Ethics Committee of Başkent University, and informed consent was obtained from each subject. Serum resistin levels were measured in 128 subjects who were referred to the Invasive Laboratory for coronary angiography between February 2014 and September 2014. Coronary angiography was performed in 63 (49.6%) patients due to typical angina pectoris symptoms, 48 (37.8%) patients due to positive stress tests, and 17 (12.6%) patients due to other reasons, including ischemia on ECG and left ventricular systolic dysfunction on echocardiography. Hypervolemic patients, tachycardic patients (heart rate >100 bpm), patients with acute coronary syndrome, serious valve regurgitation, stenosis, previous diagnosis of asthma, chronic kidney diseases, chronic inflammatory diseases, or malignancy, obese patients, and diabetic patients were excluded from this study. All study participants belonged to class 1 according to New York Heart Association classification.
Evaluated parameters
Blood samples for serum resistin levels were obtained after the admission of the patients to the Invasive Laboratory and just before the coronary angiography procedure. The samples were then centrifuged at 3,500×g or 15 min, and the serum obtained was stored at −20°C until analysis. The serum resistin concentration was measured using enzyme-linked immunosorbent assay (ELISA; eBioscience, Vienna, Austria).
Clinical data were obtained from patient interviews and hospital medical records. Hypertensive patients were defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or as those using antihypertensive medication for the study purpose according to the 2013 guidelines of the European Society of Hypertension and the European Society of Cardiology task force for the management of arterial hypertension. Hyperlipidemia was defined LDL >130 mg/dL, triglyceride > 200 mg/dL, and HDL <50 mg/dL for females and <40 mg/dL for males. Coronary angiography was performed using a modified Seldinger technique through a radial or femoral approach. LVEDP was measured before coronary angiography and before the injection of contrast material. Severe CAD was defined as ≥50% stenosis in a minimum of one of the major coronary arteries. The severity of CAD was evaluated by the Gensini score. A two-dimensional echocardiography was performed after hospitalization and before the CAG procedure. Ejection fraction (EF), the ratio of peak velocity flow in early diastole to the peak velocity flow in the late diastole (E/A), the ratio of E to the early diastolic mitral annular velocity (E/e’), mitral deceleration time (MDT), left atrium volume index (LAVi), mitral propagation time (Vp), E/Vp, and isovolemic relaxation time (IVRT) were also examined. CIMT was measured after the echocardiography with a 4.5–12.0 MHz linear transducer.
Statistical methods
Data are presented as mean ± standard deviation and as proportions for categorical variables. The t-test and chi-square test was used for the comparisons of continuous and categorical variables, respectively. The data distribution for normality was tested by the Shapiro-Wilk test, and the homogeneity of group variances were tested by the Levene test. For the parameters that were not normally distributed, the Mann-Whitney U-test was used. ANOVA model was used for comparisons across >2 groups. The Spearman correlation test was used for correlation analysis. P<0.05 was considered to be statistically significant. The data were analyzed using SPSS 20.0 (IBM SPSS Ver. 20.0; IBM Corp, Armonk NY, USA).
Results
A total of 445 patients who applied to the Invasive Laboratory for coronary angiography between February 2014 and September 2014 were evaluated in this study. Among these patients, 317 of them were excluded because they did not meet the inclusion criteria. For this study, 128 patients were finally enrolled. The mean age of the study population was 59±12.5 years; 66.4% were male and 33.6% were female. The baseline characteristics of the study population are shown in Table 1.
Table 1.
Baseline characteristics of the study population
| Age, years | 59.0±12.5 |
|---|---|
| Gender, Male/Female | 85/43 |
| Hypertension, % | 65.5 |
| Hyperlipidemia, % | 54.3 |
| Stroke, % | 1.6 |
| Peripheral vascular disease, % | 1.6 |
| Smoking, % | 35.6 |
| Family history of CAD, % | 59.8 |
| Ejection fraction, % | 52.6±9.7 |
| LVEDP, mm Hg | 18.6±7.5 |
| GFR, mg/dL | 58.6±7.8 |
| Blood examination | |
| FBS mg/dL | 97.6±9.9 |
| Creatinine, mg/dL | 0.9±0.2 |
| HDL-C, mg/dL | 46.7±18.4 |
| LDL-C, mg/dL | 128.7±56.0 |
| TG, mg/dL | 152.4±87.8 |
| Hb, g/dL | 14.5±1.5 |
| WBC count | 7800±1900 |
| Platelet count | 255400±73200 |
| Resistin, pg/mL | 2845.1±1588.9 |
CAD - coronary artery disease; GFR - glomerular filtration rate; FBS - fasting blood sugar; HDL-C - high-density lipoprotein cholesterol; Hb - hemoglobin; LDL-C - low-density lipoprotein cholesterol; LVEDP - left ventricular end-diastolic pressure; TG - triglyceride; WBC - white blood cell
After CAG, 60 patients (46.9%) had severe CAD and were classified as the severe CAD group, whereas 68 patients (53.1%) did not have severe CAD and were classified as the control group.
The proportion of male population was significantly higher in the CAD group than that in the control group. Additionally, the mean age and serum creatinine levels were significantly higher in the CAD group than those in the control group. EF was significantly higher in the control patients when compared with that in the CAD group (p=0.003). However, there was no difference in the incidences of hypertension, dyslipidemia, stroke, peripheral vascular disease, smoking, and family history of CAD. The mean fasting blood sugar, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglyceride levels were also similar between the groups. LVEDP results were also similar (Table 2).
Table 2.
Study group characteristics and laboratory parameters
| CAD group | Control group | P-value | |
|---|---|---|---|
| (n=60) | (n=68) | ||
| Age, years | 61.5±12.6 | 56.7±12.0 | 0.033 |
| Male, % | 48 (80.0) | 37 (54.4) | 0.002 |
| HT, % | 42 (71.2) | 41 (60.3) | 0.198 |
| HL, % | 35 (59.3) | 34 (50.0) | 0.293 |
| Stroke, % | 2 (3.4) | 0 (0) | 0.126 |
| PVD, % | 2 (3.4) | 0 (0) | 0.126 |
| Smoking, % | 17 (31.5) | 24 (38.7) | 0.417 |
| Family history of CAD, % | 26 (60.5) | 35 (59.3) | 0.907 |
| Ejection fraction, % | 49.8±10.9 | 55.1±8.0 | 0.003 |
| LVEDP, mm Hg | 19.1±7.9 | 18.1±7.2 | 0.480 |
| FBS, mg/dL | 99.7±10.4 | 96.0±9.3 | 0.061 |
| Creatinine, mg/dL | 0.9±0.2 | 0.8±0.1 | 0.006 |
| HDL-C, mg/dL | 43.6±16.6 | 49.3±19.8 | 0.228 |
| LDL-C, mg/dL | 127.9±63.8 | 129.5±48.6 | 0.880 |
| TG, mg/dL | 151.7±75.6 | 153.1±99.2 | 0.936 |
| Resistin, pg/mL | 2626.2±1513.3 | 3031.9±1638.6 | 0.154 |
CAD - coronary artery disease; FBS - fasting blood sugar; HDL-C - high-density lipoprotein cholesterol; HL -hyperlipidemia; HT - hypertension; LDL-C - low-density lipoprotein cholesterol; LVEDP - left ventricular end-diastolic pressure; PVD - peripheral vascular disease; TG - triglyceride
When the drug use of the study groups was examined, a statistically significant difference was found in the use of acetylsalicylic acid (p=0.006), clopidogrel (p=0.005), angiotensin converting enzyme inhibitor or angiotensin receptor blocker (p=0.004), beta blocker (p=0.032), calcium channel blocker (p=0.004), nitrate (p=0.027), and statin (p=0.001) between the groups (Table 3).
Table 3.
Drug use in study groups
| Drugs | Control group | CAD group | P value |
|---|---|---|---|
| (n=68) | (n=60) | ||
| Acetylsalicylic acid n (%) | 22 (32.4%) | 33 (56.9%) | 0.006 |
| Clopidogrel n (%) | 3 (4.4%) | 12 (20.7%) | 0.005 |
| ACEI/ARB n (%) | 25 (36.8%) | 37 (62.7%) | 0.004 |
| Beta blocker n (%) | 24 (35.3%) | 32 (54.2%) | 0.032 |
| Calcium channel blocker n (%) | 4 (5.9%) | 14 (23.7%) | 0.004 |
| Alpha blocker n (%) | 0 (0%) | 1 (1.7%) | 0.285 |
| Trimetazidine n (%) | 3 (4.4%) | 5 (8.5%) | 0.347 |
| Nitrates n (%) | 2 (2.9%) | 8 (13.6%) | 0.027 |
| Spironolactone n (%) | 2 (3.0%) | 1 (1.7%) | 0.635 |
| Statin n (%) | 13 (19.1%) | 28 (47.5%) | 0.001 |
| Furosemide n (%) | 1 (1.5%) | 4 (6.8%) | 0.125 |
ACEI - angiotensin converting enzyme inhibitor; ARB - angiotensin receptor blocker; CAD - coronary artery disease
The serum resistin levels were 2626.2±1513.3 pg/mL in the CAD group and 3031.9±1638.6 pg/mL in the control group, and the difference was not statistically significant (p=0.154). The mean LVEDPs were also similar for the patients with and without severe CAD (p=0.480; Fig. 1).
Figure 1.
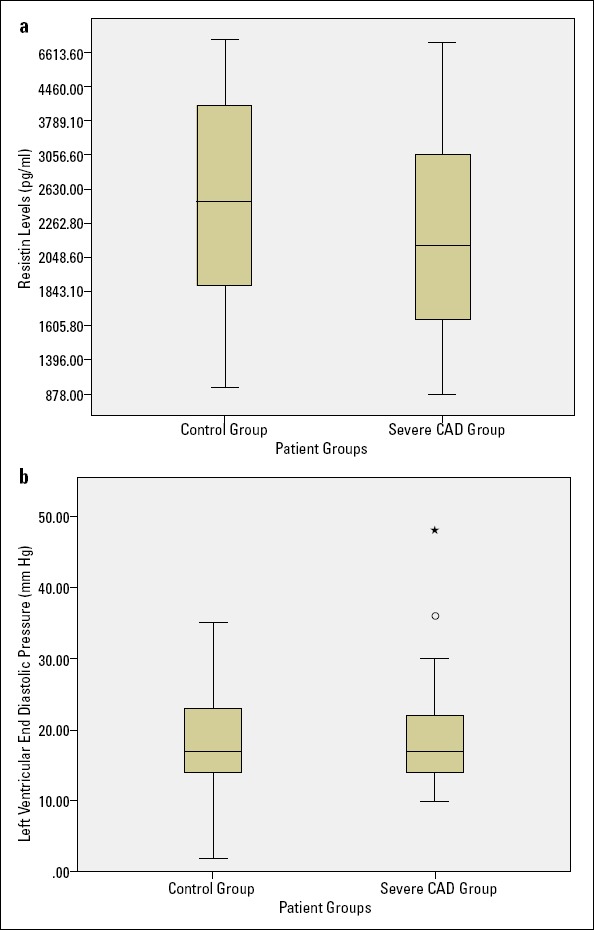
(a) Box-plot graph of patients groups and resistin levels. (b) Box-plot graph of patient groups and left ventricular end-diastolic pressure
CAD - coronary artery disease
The resistin levels were also evaluated between the patients with normal coronary arteries (n=22) and the patients with minimal CAD (n=46) or severe CAD (n=60). There was also no significant difference in the resistin levels among these three groups (Fig. 2).
Figure 2.
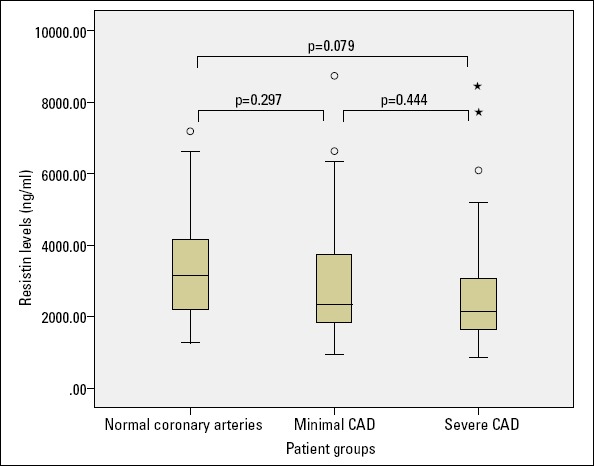
Relationship between the study groups and resistin levels
CAD - coronary artery disease
A comparison of the resistin levels of reduced EF (EF <50%) patients (n=30) and preserved EF (EF ≥50%) patients (n=98) also showed no significant difference (p=0.531). In the subgroup of patients with preserved EF (n=98), no significant difference was found in the resistin levels of the patients with severe CAD or those of the control group (p=0.257). LVEDP was 18.9±8.0 in the preserved EF group and 18.1±6.2 in the reduced EF group, and the results were statistically similar between the groups (p=0.649).
The patients with diastolic dysfunction were further classified as grade 1 (n=53), grade 2 (n=19), and grade 3 (n=2) groups. The serum resistin levels were similar among all diastolic dysfunction groups (p=0.383). A pairwise comparison of the resistin levels of these three groups did not show any significant difference between the resistin levels as shown in Figure 3.
Figure 3.
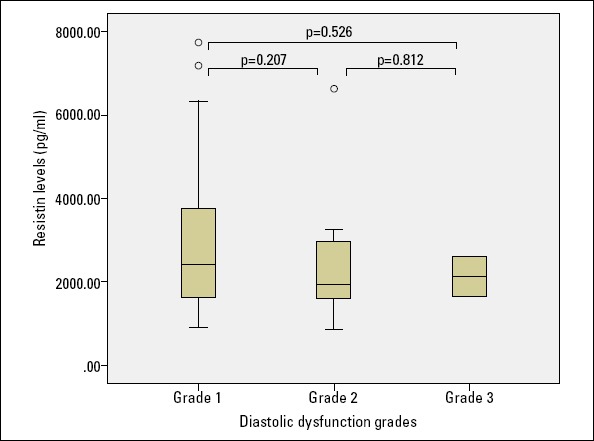
Relationship between the diastolic dysfunction groups and resistin levels
In the complete study population, the resistin levels were not significantly correlated to LVEDP (r=−0.045, p=0.627). Furthermore, there was no correlation between the resistin level and age (r=−0.056, p=0.535), EF (r=0.110, p=0.228), and the Gensini score (r=−0.091, p=0.328).
The resistin levels were compared among the subgroups of drug use, including acetylsalicylic acid, clopidogrel, angiotensin converting enzyme inhibitor or angiotensin receptor blocker, beta blocker, calcium channel blocker, nitrate, and statins. There were no significant differences among these subgroups (p>0.05 for all comparisons).
The Spearman correlation analysis was applied to the echocardiographic diastolic dysfunction parameters and resistin levels, and no relationship was found between the resistin levels and E/e’ (r=−0.072, p=0.468), e’ (r=0.037, p=0.738), E/A (r=0.018, p=0.847), MDT (r=0.022, p=0.828), LAVi (r=−0.087, p=0.432), Vp (r= .102, p=0.391), E/Vp (r=−171, p=0.158), and IVRT (r=−0.017, p=0.866).
The carotid intima-media thickness was measured in 86 patients, and no relationship was found between the resistin level and CIMT (r=0.082, p=0.457). There were statistically significant correlations between the resistin level and CIMT in hypertensive patients (n=55) and the patients with severe CAD (n=41; r=0.282, p=0.041 and r=0.485, p=0.002, respectively).
There was a statistically significant difference between the severe CAD and control groups with respect to CIMT (0.99±0.34 mm for patients with severe CAD and 0.7±0.19 mm for those without severe CAD; p=0.000).
Discussion
Our study showed that resistin has no relationship with LVEDP, CAD severity, CIMT, and the echocardiographic diastolic dysfunction parameters.
Resistin is a peptide implicated in cardiovascular diseases in many studies (25). LVEDP is a parameter that can be measured by left heart catheterization, indicating pressure and volume load conditions. It can also provide valuable data on the relationship of systolic performance of the left ventricle and diastolic pressure volume (26). This study aimed to determine whether LVEDP, an invasively measured parameter, could be predicted by resistin level, a biomarker that has previously been shown to increase during hypervolemia or symptomatic heart failure. We excluded the patients with infection, malignancy, diabetes, and acute coronary syndrome because resistin is also shown to be an inflammatory mediator (9, 11, 19). The relationship between resistin and heart failure has been investigated in many previous studies (13,27). Takeishi et al. (13) compared the resistin levels in symptomatic heart failure patients and control group, which showed that resistin levels increased along with NYHA class. Baldasseroni et al. (27) compared the resistin levels of asymptomatic preserved EF, asymptomatic reduced LVEF, and symptomatic reduced LVEF patients. Only in the symptomatic reduced LVEF patients, the levels were higher. The resistin levels of the asymptomatic reduced LVEF and preserved LVEF patients were statistically similar. This finding suggest that resistin levels only increase in symptomatic, hypervolemic patients and that EF has no effect on resistin levels. Our results suggest no statistically significant relationship in terms of the resistin levels between the reduced LVEF and preserved LVEF groups. As mentioned earlier, the hypervolemic and symptomatic patients were excluded in our study, so this finding is consistent with the study of Baldasseroni et al. (27). In our study, no significant correlation between the resistin levels and LVEDP was observed. The exclusion criteria for this study include all causes that increase LVEDP, except for heart failure. Our goal in this study was to understand LVEDP using only resistin, thus avoiding an invasive examination. As explained before, previous studies found increased resistin levels in hypervolemic patients, so we expected to find increased levels of resistin along with higher LVEDP values. Contrary to our prediction, there was no significant relationship between LVEDP and resistin levels.
EF was higher in the control group compared with that in the severe CAD patients. However, in these patients, the LVEDP results were statistically similar. Left ventricular end-diastolic pressure is expected to be higher when left ventricular volume load is increased; hence, during hypervolemic conditions and heart failure, LVEDP is expected to be higher. However, in our study, although the severe CAD patients had lower LVEF, the LVEDP values were statistically similar. This may be caused by the fact that our patients were all normovolemic and that the number of reduced EF patients were lower.
There are some studies that show positive correlation between resistin levels (16–20) and the severity of CAD and few other studies that report that there is no correlation between them (21–24). In our study, there was no statistically significant relationship between resistin and CAD. When the articles with statistically significant results were examined, it was found that the resistin levels mostly increased in the cases of acute coronary syndromes and that there was no significant increase in the stable angina pectoris cases (16, 18, 20, 22). In some studies, while there was a relationship between resistin and CAD at the beginning, when the inflammatory parameters such as CRP included in the study, it was found that the relationship was originated from inflammation rather than CAD; after adjustment, the association was no longer significant (23,28). The strong relationship between CRP and resistin has been determined repeatedly in many CAD related studies (19–21). In our study, inflammatory conditions such as acute coronary syndrome, infection, chronic inflammatory diseases, and malignancy were excluded. Given this situation, it is not surprising that there is no significant relationship between resistin and CAD.
In our study, a significant relationship between the resistin levels and CIMT was found in hypertensive patients. Shin et al. (29) also reviewed CIMT and resistin levels in 307 hypertensive patients, and a similar relationship was found. Several studies demonstrated a relationship among atherosclerosis, vascular inflammation, and resistin (11,12). Further, resistin expression is increased in human carotid artery samples (30). This increased release in carotid arteries may be the cause of the relationship between CIMT and resistin levels in our study, or this finding may be coincidental. To clarify why resistin levels are not affected by CAD severity but are affected by CIMT, more detailed studies including coroner and carotid arteries should be designed.
Study limitations
A limitation to our work is that the size of the reduced EF patient was low. Additionally, it would be better to simultaneously monitor resistin and CRP levels; however, since we excluded all the inflammatory diseases and CRP increased situations, we believe that this is not a major limitation to our study.
Conclusion
In conclusion, resistin seems to be an inflammatory biomarker with no direct relationship with LVEDP, CAD, systolic, and diastolic dysfunction. Only in hypertensive patients, there is a borderline relationship between resistin and CIMT, which may be caused by atherosclerotic processes.
Acknowledgments:
I would like to thank Hugo Tapia and Gamze Çamdere for their assistance in the language translation process of the article.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – Ö.T.Y., A.Y.; Design – Ö.T.Y., A.Y.; Supervision – Ö.T.Y., A.Y., L.E.S.; Fundings – Ö.T.Y., A.Y., S.H.H., H.K., E.Ö., K.O., U.A.B., A.A., H.M.; Materials – Ö.T.Y., A.Y., S.H.H., H.K., E.Ö., K.O., U.A.B., A.A., H.M.; Data collection &/or processing – Ö.T.Y., A.Y., L.E.S., S.H.H., H.K., E.Ö., K.O., U.A.B., A.A., H.M.; Analysis &/or interpretation – Ö.T.Y., A.Y., L.E.S.; Literature search – Ö.T.Y., A.Y.; Writing – Ö.T.Y., A.Y.; Critical review – A.Y.
References
- 1.Guilbert JJ. The world health report 2002 - reducing risks, promoting healthy life. Educ Health (Abingdon) 2003;16:230. doi: 10.1080/1357628031000116808. [DOI] [PubMed] [Google Scholar]
- 2.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–12. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 3.Kim KH, Lee K, Moon YS, Sul HS. A cysteine-rich adipose tissue-specific secretory factor inhibits adipocyte differentiation. J Biol Chem. 2001;276:11252–6. doi: 10.1074/jbc.C100028200. [DOI] [PubMed] [Google Scholar]
- 4.Fain JN, Cheema PS, Bahouth SW, Lloyd Hiler M. Resistin release by human adipose tissue explants in primary culture. Biochem Biophys Res Commun. 2003;300:674–8. doi: 10.1016/s0006-291x(02)02864-4. [DOI] [PubMed] [Google Scholar]
- 5.Patel L, Buckels AC, Kinghorn IJ, Murdock PR, Holbrook JD, Plumpton C, et al. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun. 2003;300:472–6. doi: 10.1016/s0006-291x(02)02841-3. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh S, Singh AK, Aruna B, Mukhopadhyay S, Ehtesham NZ. The genomic organization of mouse resistin reveals major differences from the human resistin:functional implications. Gene. 2003;305:27–34. doi: 10.1016/s0378-1119(02)01213-1. [DOI] [PubMed] [Google Scholar]
- 7.Gerber M, Boettner A, Siedel B, Lammert A, Bar J, Schuster E, et al. Serum resistin levels of obese and lean children and adolescents:biochemical analysis and clinical relevance. J Clin Endocrinol Metab. 2005;90:4503–9. doi: 10.1210/jc.2005-0437. [DOI] [PubMed] [Google Scholar]
- 8.Degawa-Yamauchi M, Bovenkerk JE, Juliar BE, Watson W, Kerr K, Jones R, et al. Serum resistin (FIZZ) protein is increased in obese humans. J Clin Endocrinol Metab. 2003;88:5452–5. doi: 10.1210/jc.2002-021808. [DOI] [PubMed] [Google Scholar]
- 9.Filkova M, Haluzik M, Gay S, Senolt L. The role of resistin as a regulator of inflammation:implications for various human pathologies. Clin Immunol. 2009;133:157–70. doi: 10.1016/j.clim.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Gnacinska M, Malgorzewicz S, Stolek M, Lysiak-Szydlowska W, Sworczak K. Role of adipokines in complications related to obesity:a review. Adv Med Sci. 2009;54:150–7. doi: 10.2478/v10039-009-0035-2. [DOI] [PubMed] [Google Scholar]
- 11.Reilly MP, Lehrke M, Wolfe ML, Rohatgi A, Lazar MA, Rader DJ. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation. 2005;111:932–9. doi: 10.1161/01.CIR.0000155620.10387.43. [DOI] [PubMed] [Google Scholar]
- 12.Liberale L, Bonaventura A, Vecchie A, Casula M, Dallegri F, Montecucco F, et al. The Role of Adipocytokines in Coronary Atherosclerosis. Curr Atheroscler Rep. 2017;19:21. doi: 10.1007/s11883-017-0657-y. [DOI] [PubMed] [Google Scholar]
- 13.Takeishi Y, Niizeki T, Arimoto T, Nozaki N, Hirono O, Nitobe J, et al. Serum resistin is associated with high risk in patients with congestive heart failure-a novel link between metabolic signals and heart failure. Circ J. 2007;71:460–4. doi: 10.1253/circj.71.460. [DOI] [PubMed] [Google Scholar]
- 14.Frankel DS, Vasan RS, D'Agostino RB, Sr, Benjamin EJ, Levy D, Wang TJ, et al. Resistin, adiponectin, and the risk of heart failure the Framingham offspring study. J Am Coll Cardiol. 2009;53:754–62. doi: 10.1016/j.jacc.2008.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butler J, Kalogeropoulos A, Georgiopolou V, de Rekeneire N, Rodondi N, Smith AL, et al. Serum resistin concentrations and risk of new onset heart failure in older persons:the Health, Aging, and Body Composition (Health ABC) study. Arterioscler Thromb Vasc Biol. 2009;29:1144–9. doi: 10.1161/ATVBAHA.109.186783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu WL, Qiao SB, Hou Q, Yuan JS. Plasma resistin is increased in patients with unstable angina. Chin Med J (Engl) 2007;120:871–5. [PubMed] [Google Scholar]
- 17.Lubos E, Messow CM, Schnabel R, Rupprecht HJ, Espinola-Klein C, Bickel C, et al. Resistin, acute coronary syndrome and prognosis results from atherogene study. Atherosclerosis. 2007;193:121–8. doi: 10.1016/j.atherosclerosis.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 18.Joksic J, Sopic M, Spasojevic-Kalimanovska V, Kalimanovska-Ostric D, Andjelkovic K, Jelic-Ivanovic Z. Circulating resistin protein and mRNA concentrations and clinical severity of coronary artery disease. Biochem Med (Zagreb) 2015;25:242–51. doi: 10.11613/BM.2015.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohmori R, Momiyama Y, Kato R, Taniguchi H, Ogura M, Ayaori M, et al. Associations between serum resistin levels and insulin resistance, inflammation, and coronary artery disease. J Am Coll Cardiol. 2005;46:379–80. doi: 10.1016/j.jacc.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Chen DY, Cao J, He ZY, Zhu BP, Long M. High serum resistin level may be an indicator of the severity of coronary disease in acute coronary syndrome. Chin Med Sci J. 2009;24:161–6. doi: 10.1016/s1001-9294(09)60082-1. [DOI] [PubMed] [Google Scholar]
- 21.Yaturu S, Daberry RP, Rains J, Jain S. Resistin and adiponectin levels in subjects with coronary artery disease and type 2 diabetes. Cytokine. 2006;34:219–23. doi: 10.1016/j.cyto.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Hoefle G, Saely CH, Risch L, Koch L, Schmid F, Rein P, et al. Relationship between the adipose-tissue hormone resistin and coronary artery disease. Clin Chim Acta. 2007;386:1–6. doi: 10.1016/j.cca.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Pilz S, Weihrauch G, Seelhorst U, Wellnitz B, Winkelmann BR, Boehm BO, et al. Implications of resistin plasma levels in subjects undergoing coronary angiography. Clin Endocrinol (Oxf) 2007;66:380–6. doi: 10.1111/j.1365-2265.2007.02743.x. [DOI] [PubMed] [Google Scholar]
- 24.Montazerifar F, Bolouri A, Paghalea RS, Mahani MK, Karajibani M. Obesity, Serum Resistin and Leptin Levels Linked to Coronary Artery Disease. Arg Bras Cardiol. 2016;107:348–53. doi: 10.5935/abc.20160134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee EL, Kim HS. Human resistin in cardiovascular disease. J Smooth Muscle Res. 2012;48:27–35. doi: 10.1540/jsmr.48.27. [DOI] [PubMed] [Google Scholar]
- 26.Borlaug BA, Kass DA. Invasive Hemodynamic Assesment in Heart Failure. Heart Fail Clin. 2009;5:217–28. doi: 10.1016/j.hfc.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baldasseroni S, Mannucci E, Di Serio C, Orso F, Bartoli N, Mossello E, et al. Resistin level in coronary artery disease and heart failure:the central role of kidney function. J Cardiovasc Med (Hagerstown) 2013;14:150–7. doi: 10.2459/JCM.0b013e32834eec93. [DOI] [PubMed] [Google Scholar]
- 28.Pischon T, Bamberger CM, Kratzsch J, Zyriax BC, Algenstaedt P, Boeing H, et al. Association of plasma resistin levels with coronary heart disease in women. Obes Res. 2005;13:1764–71. doi: 10.1038/oby.2005.215. [DOI] [PubMed] [Google Scholar]
- 29.Shin HJ, Park S, Yoon SJ, Choi DS, Cho DK, Kim JS, et al. Association between serum resistin and carotid intima media thickness in hypertension patients. Int J Cardiol. 2008;125:79–84. doi: 10.1016/j.ijcard.2007.02.028. [DOI] [PubMed] [Google Scholar]
- 30.O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]


