Abstract
Although late gadolinium enhancement on cardiac magnetic resonance imaging remains the reference standard for scar assessment, it does not provide quantitative information about the extent of pathophysiological changes within the scar tissue. T1 mapping and extracellular volume (ECV) mapping are steadily becoming diagnostic and prognostically useful tests for in vivo myocardial histology, influencing clinical decision-making. Quantitative native T1 maps (acquired without a contrast agent) represent the longitudinal relaxation time within the myocardium and changes with myocardial extracellular water (edema, focal, or diffuse fibrosis), fat, iron, and amyloid protein content. Post-contrast ECV maps estimate the size of the extracellular space and have sensitivity in the identification of interstitial disease. Both pre- and post-contrast T1 mapping are emerging as comprehensive tools for the assessment of numerous conditions including ischemic scarring that occurs post myocardial infarction (MI). This review outlines the current evidence and potential future role of T1 mapping in MI. We conclude by highlighting some of the remaining challenges such as quality control, standardization of image acquisition for clinical practice, and automated methods for quantifying infarct size, area at risk, and myocardial salvage post MI.
Keywords: magnetic resonance imaging, gadolinium, extracellular space, myocardial infarction, edema
Introduction
Myocardial infarction (MI) remains one of the leading causes of death worldwide. New serological biomarkers, such as high sensitive troponins, have radically improved the diagnosis of MI, but even then, the diagnosis of MI can be still challenging. Cardiac magnetic resonance imaging (MRI) generates exquisite tissue contrast to characterize the myocardium, and late gadolinium enhancement (LGE) is the current reference standard for non-invasive assessment of the presence of a myocardial scar (1). Additionally, cardiac MRI provides a robust means of structural and functional assessment of regional myocardial motion and thickening, rest and stress perfusion, and myocardial edema following an acute ischemic event. Post reperfused ST-elevation MI (STEMI), early and LGE also show other independent poor prognostic biomarkers such as microvascular obstruction (MVO) and intra-myocardial hemorrhage (IMH) (2, 3).
Standard cardiac MRI sequences and protocols for MI imaging T2-weighted imaging for myocardial edema
Myocardial edema develops before ischemic necrosis or even troponin release (4), and it can persist for up to 1 month even when electrocardiogram changes and myocardial dysfunction resolves (5). Early studies have demonstrated that edema-sensitive T2-weighted (T2W) imaging significantly improved the diagnostic power of cardiac MRI for MI by recognizing post-ischemia edema (6). Moreover, in the same study, Cury et al. (6) demonstrated that T2W imaging accurately identified all cases of acute MI, often before cardiac biomarkers were elevated. T2W cardiac MRI can also be used to determine the area at risk (AAR) in reperfused and non-reperfused infarction (Fig. 1). When combined with contrast-enhanced imaging, the salvaged area and thus the success of early coronary revascularization can be quantified. Strong evidence for the prognostic value of myocardial salvage has facilitated its use as a primary endpoint in clinical trials.
Figure 1.
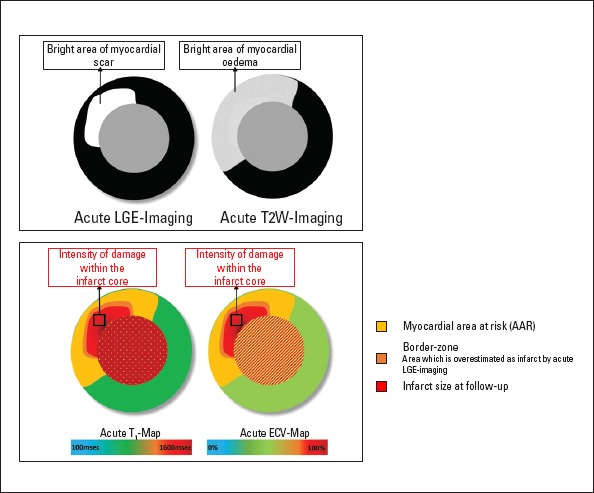
Illustration demonstrating different cardiac magnetic resonance imaging (MRI) techniques for infarct assessment. Note that only T1 map and extracellular volume (ECV) map inform about the extent of damage within the infarction
LGE imaging for myocardial scar
The most validated cardiac MRI technique for myocardial scar assessment remains LGE imaging. LGE imaging employs an inversion recovery T1-weighted (T1W) gradient echo acquisition approximately 10 min after the intravenous administration of the gadolinium contrast. With appropriate settings, normal myocardium appears black or nulled, whereas non-viable regions appear bright or hyper-enhanced. Gadolinium contrast distribution volume and tissue concentration are low in normal myocardium. With acute necrosis (e.g., acute MI and myocarditis), there is membrane rupture, which allows gadolinium contrast to diffuse into the myocytes. This results in increased gadolinium concentration, shortened T1 relaxation, and hyper-enhancement. In the chronic MI setting, a scar replaces the necrotic tissue, and the interstitial myocardial space is expanded. This again leads to increased gadolinium concentration and hyper-enhancement.
Post MI, infarct size (IS) is a marker of the extent of damage secondary to myocardial necrosis. It has been proposed as an imaging biomarker of clinical outcomes and as a measure of potential efficacy of new treatment approaches. IS and infarct shape on LGE imaging has been validated against histopathology (7). Additionally, IS (quantified as % of the left ventricular (LV) volume/mass) is an independent predictor of prognosis post MI (8). Depending on the extent of ischemia, the infarct can be limited to the sub-endocardium (with limited loss of viability) or be transmural in larger infarcts. Infarct transmurality assessment on LGE also predicts functional recovery post reperfusion therapy and has become an important clinical tool in routine cardiac MRI practice (9).
IS and transmurality on LGE imaging can be quantified using manual contours (10-12) or by semi-automated image thresholding techniques, which include enhancing myocardium exceeding a pre-defined signal intensity (SI) threshold, typically 2-6 standard deviations above that of remote (non-infarcted) myocardium. Currently, the most validated and commonly used is the full-width at half maximum (FWHM) thresholding method (13), defining infarct as myocardium with an SI greater than 50% of the peak SI in the infarct core.
Limitations of T2W and LGE imaging
Conventional T2W images are acquired using the short-tau inversion recovery (STIR) sequence (14). Limitations of this technique include signal drop-out, bright signals adjacent to the sub-endocardium due to slow-flow blood, image quality impairment in tachyarrhythmias, and long breath-holds (15, 16). Moreover, there is need for a “normal” reference region of interest (ROI), either in remote myocardium, or in adjacent skeletal muscle for conditions with diffuse myocardial edema. The use of an ROI outside the myocardium typically requires using the body-coil to minimize signal inhomogeneity, which gives a lower signal-noise-ratio. In systemic conditions like viral myocarditis, the reference skeletal muscle may also be inflamed, leading to false-negative results. In a pre-clinical study, semi-automated, FWHM thresholding demonstrated promising results to quantify AARs (17). However, more recent evidence has debated this in humans (18, 19), and currently, there is no consensus on the use semi-automated thresholding method on T2W imaging for the quantification of myocardial edema.
Similarly, for LGE imaging, it is still debated if semi-automated segmentation techniques provide the best reproducibility between different users to quantify the IS (18, 20). Semi-automated thresholding method is dependent on several factors, including 1) windowing by the operator to decide where the ROI is placed, 2) the variation of SI within the ROI, and 3) the size of the ROI can also affect the signal intensity variations (21). The cumulative effect of these factors can significantly influence quantification and intra-/inter-observer variation of the measurements. Moreover, recent literature suggests that IS on LGE imaging has limited value in acute MI (22) and compared with histopathology, it overestimates the actual IS (23, 24). Also, SI changes seen with infarction do not necessarily represent extracellular matrix (25, 26).
Hence, there has been a drive to move toward more quantitative methods for accurate and precise, in vivo assessment of histopathological changes in the myocardium.
Principles of T1 mapping
T1 mapping measures the longitudinal relaxation time of tissue, which is determined by how rapidly proton spins re-equilibrate their longitudinal magnetization after being excited by a radiofrequency pulse (27). T1 mapping refers to pixel-wise measurement of absolute T1 relaxation times on a quantitative map. T1 mapping circumvents some of the issues of the influence of windowing and nulling (as in LGE) and allows direct, pixel-wise T1 quantification. T1 mapping has the potential to detect diffuse myocardial structural alterations not assessable by other non-invasive methods and may be more sensitive than LGE in detecting diffuse myocardial fibrosis (28). The two most important biological determinants of an increase in native T1 are edema (increase of tissue water in e.g., acute infarction of inflammation) and increase of collagen associated with rise in interstitial space [e.g., fibrosis of infarction (scar) or cardiomyopathy and in amyloid deposition]. Native T1 mapping is feasible even in patients with severe renal impairment in whom gadolinium-based contrast agents are contraindicated.
Native T1 values are a composite signal of intracellular volume and ECV with the potential of pseudo-normalization of abnormal values (e.g., low native T1 values of the Anderson-Fabry disease cancelled out by the presence of infarction/fibrosis). Native T1 values are influenced by the scanner’s static magnetic field strength, with higher native T1 values at 3T than at 1.5T. The accuracy and precision of measured T1 values depends on the pulse sequence used. The most commonly used methods in clinical practice are Modified Look Locker Inversion recovery (MOLLI) and short MOLLI (shMOLLI) as well as Saturation Recovery Single Shot Acquisition (SASHA). Of these methods, SASHA appears to be more accurate but less reproducible than MOLLI and shMOLLI, which tend to underestimate the absolute T1 value (29). Another disadvantage of MOLLI sequences is that they interleave images from three inversion recoveries, which results in a long breath-hold and some heart rate dependency (29). ShMOLLI sequences have been developed to reduce heart rate dependency and breath-hold and may be more clinically applicable for patients who experience breathlessness (30).
T1 is also sensitive to the choice of readout flip angle used in inversion recovery sequences and the cardiac phase (diastole versus systole). Normal native T1 values are thus specific to the imaging set up, and changes to the acquisition method may result in changes to T1 values. Additionally, there can be regional variation of T1 values across normal myocardium and hence placement of ROI used to assess T1 requires consistency (31). Placement of ROI also needs to avoid partial volume effects at the blood–myocardium boundary, where myocardium values may be artificially increased by the inclusion of blood pool in the pixels of interest. However, high intra-observer, inter-observer, and inter-study reproducibility can be achieved with consistent methodology (31).
Contrast-enhanced T1 mapping is mostly used for calculating the ECV fraction in combination with native T1 mapping. Standard gadolinium-based contrast agents are distributed throughout the extracellular space and shorten T1 relaxation times of the myocardium proportional to the local concentration of gadolinium. Areas of fibrosis and scar will therefore exhibit shorter T1 relaxation times than healthy tissue after contrast administration. The hematocrit represents the cellular fraction of blood. Estimation of ECV (interstitial and extracellular matrix) requires the measurement of myocardial and blood T1 before and after the administration of contrast agents as well as the patient’s hematocrit value (27). The ECV formula is as follows:
ECV = (1 − hematocrit) × (1/post-contrast T1 myo − 1/native T1 myo)/(1/post-contrast T1 blood − 1/native T1 blood)
ECV is a marker of myocardial tissue remodeling and provides a physiologically intuitive unit of measurement with normal ECV values of 25.3±3.5% in normal myocardium (27).
In future, the requirement for an additional blood test to derive hematocrit could be avoided using the so-called synthetic ECV formulas (32, 33). Synthetic ECV is calculated by estimating the hematocrit from native T1 values of blood pool within the LV. Currently, this has been validated on 1.5T Siemens systems (32) and 1.5T/3T Philips systems (33). The equations for computing synthetic ECV are listed in Table 1.
Table 1.
Synthetic hematocrit formulas for different magnetic resonance systems to derive synthetic extracellular volume
| Vendor | Field strength | Synthetic hematocrit formula | Ref. |
|---|---|---|---|
| Siemens | 1.5 Tesla | Synthetic Hct MOLLI=(866.0·[1/T1blood])−0.1232 | (32) |
| Siemens | 1.5 Tesla | Synthetic Hct ShMOLLI=(727.1·[1/T1blood])−0.0675 | (32) |
| Philips | 1.5 Tesla | Synthetic Hct MOLLI=(922.6·[1/T1blood])–0.1668 | (33) |
| Philips | 3 Tesla | Synthetic Hct MOLLI=(869.7·[1/T1blood])–0.071 | (33) |
Hct - hematocrit; MOLLI - Modified Look Locker Inversion recovery; shMOLLI - short Modified Look Locker Inversion recovery; Ref - references
ECV can either be calculated for myocardial ROIs or visualized on ECV maps. As ECV is derived from the ratio of T1 signal values and is expressed in percentage (%), it is less influenced by the different field strengths, vendors, and acquisition techniques versus native or post-contrast T1 (34). This makes ECV more robust for imaging biomarker for clinical applications across all vendors. ECV measures also exhibit better agreement with histological measures of the collagen volume fraction than with isolated post-contrast T1 (35). However, it should be noted that low ECV values occur in thrombus, MVO, IMH, and fat/lipomatous metaplasia (Fig. 2).
Figure 2.
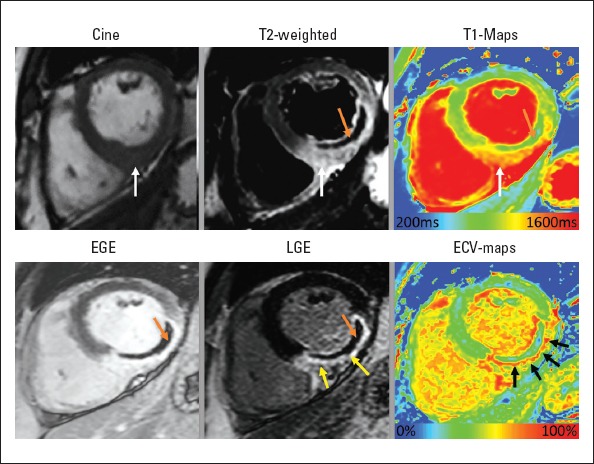
In vivo histopathology assessment by multi-parametric cardiac magnetic resonance imaging (MRI) of a patient who presented with acute ST-elevation myocardial infarction. On cine images, inferio-lateral segments demonstrate increased thickness secondary to myocardial edema. T2-weighted imaging (white arrow) and T1 maps (white arrow) confirm the presence of myocardial edema. A hypointense core is seen on T2-weighted imaging, which is consistent with the diagnosis of intra-myocardial hemorrhage (orange arrow). This is also present in EGE imaging and LGE imaging confirming the presence of MVO. LGE imaging confirms large infero-lateral MI and MVO. ECV maps demonstrate >60% ECV in the scar tissue, suggesting severe tissue damage (black arrows). Also, note that IMH/MVO within the infarct results in pseudo-normalization of native T1 and ECV
In patients with acute or chronic MI, T1 mapping and ECV provide complementary information to standard LGE imaging, both diagnostically and prognostically (Table 2).
Table 2.
Comprehensive assessment by multi-parametric cardiac magnetic resonance imaging in patients presenting with myocardial infarction
| Regional and global LV functional assessment |
|---|
| Volumetric cine imaging |
| Myocardial edema assessment |
| T2-weighted imaging |
| T1 or T2 maps |
| ECV (>33%) |
| Microvascular obstruction and LV thrombus assessment |
| Early gadolinium enhancement imaging |
| Intra-myocardial hemorrhage assessment |
| T2-weighted imaging (hypointense core in the infarcted area) |
| T2 maps or T2-star maps |
| Infarct size/viability assessment to predict functional recovery and prognosis |
| LGE imaging |
| Native T1 maps |
| ECV maps (Acute MI infarct size: ECV >46%) |
| Extent of damage within the infarct to predict functional recovery and prognosis |
| Native T1 maps and/or ECV maps (>50% ECV is associated with poor functional recovery in respective segments) |
| Myocardial salvage index |
| [AAR (% of LV on T2-weighted imaging) − IS (% of LV, on LGE)]/AAR (% of LV on T2-weighted imaging) |
| or |
| [AAR (% of LV with ECV >33%) − IS (% of LV with ECV >46%)]/AAR (% of LV with ECV >33%) |
Acute MI
Differentiation between acute and chronic MI can be of clinical relevance because both disorders require a different diagnostic work-up, including conventional coronary angiography and medical therapy. Myocardial edema develops within minutes of coronary occlusion secondary to ischemia and resolves over weeks or sometimes months after MI. Native T1 reliably detects segmental abnormalities caused by acute MI with high sensitivity and specificity (36). Also, T1 mapping detects myocardial edema in both STEMI and non-STEMI patients and is at least as sensitive as T2-STIR, in particular in patients with smaller IS (36). A recent study demonstrated that native T1 maps perform better than T2W imaging in differentiating acute from chronic MI (area under the curve 0.97 versus 0.86, p<0.01) (37). Furthermore, T1 values progress from normal myocardium to that of maximal injury and can be used for defining the peri-infarct zone/AAR in combination with LGE imaging (5). Native T1 values in acute MI are high, and ECV values are among the highest of all cardiac disease (56±1.4) (38), most likely due to the disruption of cardiomyocyte membrane integrity and subsequent expansion of the distribution volume of extracellular contrast agents.
Infarct core: Intensity of damage within the infarct is prognostically relevant
Native T1 in the infarct core predicts 6-month mortality in STEMI patients even after adjustment for LV ejection fraction (39). Similarly, a study investigating the ability of acute infarct ECV and acute transmural extent of LGE to predict convalescent function demonstrated that ECV had higher accuracy than LGE extent in predicting improved wall motion (area under the curve was 0.77 versus 0.66; p=0.02). In the same study, an infarct ECV of <50% had 81% sensitivity and 65% specificity for the prediction of improvement in segmental function at follow-up and on the contrary, LGE transmural extent ≤0.5 had 61% sensitivity and 71% specificity.
Remote myocardium: adverse tissue level remodeling in normal myocardium
T1 mapping and ECV have also been used to predict remote myocardial remodeling after STEMI. In a study by Carrick et al. (40), native remote myocardium T1 predicted LV adverse remodeling in patients with acute MI. In a more recent work by the same group, acute remote zone ECV and changes in ECV (between acute and follow-up visit) were associated with MI severity (41). However, the authors concluded that changes in acute/follow-up ECV in remote zone were small and had limited use as a clinical biomarker of remodeling. More recently, we demonstrated that segmental ECV expansion of normal myocardium is associated with worsening contractile function (42).
Quantification of AAR and IS
Another recent study in a STEMI population demonstrated that acute ECV maps could be used to quantify AAR and IS on both 1.5T and 3T systems (43). The ECV threshold for AAR was 33% and 46% for IS. ECV maps demonstrated better agreement with final IS than with acute IS on LGE [ECV maps: bias, 1.9; 95% confidence interval (CI), 0.4-3.4 versus LGE imaging: bias, 10; 95% CI, 7.7–12.4]. More recently, native T1 maps have also been used to distinguish between reversible (injured myocardial cells with edema) and irreversible (necrosed myocardial cells leading to permanent scar) injury post STEMI (44). T1 cut-off values for edematous versus necrotic myocardium were identified as 1251 ms and 1400 ms on a 3T MR system. Additionally, a native T1 threshold of 1400 ms for IS volume demonstrated a good agreement with 6-month LGE IS (r=0.99). T1-derived infarct extent strongly correlated with the log area under the curve troponin (r=0.80) and 6-month ejection fraction (r=−0.73). Quantification thresholds for IS and AAR are lacking for native T1 mapping on 1.5T MR systems.
Being able to accurately quantify AAR and MI size using a pixel-wise quantitative map would be appealing and could be potentially easily implemented in clinical practice with the wider availability of in-line, automated map generation from the scanner, to improve workflow. However, more validation work is needed across vendor platforms and field strengths before reliable quantification of AAR, IS and myocardial salvage index can be made from quantitative native T1 or ECV maps.
Challenges of T1 mapping in acute MI
Undertaking comprehensive cardiac MRI in acute infarct patients can be challenging because of multiple breath-holds required, patients having to lie flat in the scanner, and relatively long acquisition times. The majority of studies included in this review were conducted in small selected patient groups and in expert centers, and it remains unclear if they can be translated to clinical routine. However, free breathing cardiac T1 mapping sequences, which may improve clinical feasibility, are being developed (45). MVO in the infarct core (no-reflow phenomenon) results in a pseudo-normalization of T1 values in this area and if not factored in, can result in the underestimation of the extent of injury (36). Additionally, T1 can even be decreased in the case of IMH, which is due to the accumulation of methemoglobin (T1 shortening effect). Most of the above discussed studies were conducted within 4–5 days of the index presentation. However, it is well recognized that acute MI size is dynamic and acutely reduces in size within the first week. Hence, clinical cardiac MRI protocols aimed to characterize acute MI need to be standardized to take into account the dynamic biological changes post-acute myocardial ischaemic injury. More recently, a pre-clinical and in vivo study demonstrated that post-MI myocardial edema follows a bimodal pattern, which affects cardiac MRI estimates of AAR (46). According to the study findings, a time window between day 4 and 7 post-MI seems a good compromise solution for standardization. It is also note-worthy that multi-parametric assessment can offer mechanistic insights into infarct-associated complications and differential diagnosis (47, 48).
Chronic MI
In chronic MI, the necrotic and edematous infarct tissue of an acute infarct is replaced by a smaller area of increased extracellular collagen (fibrous scar). Native T1 values are therefore lower and less extensive in chronic MI than in acute MI (49). The ECV of chronically infarcted myocardium has been shown to be markedly elevated (51±8%) compared with that of normal myocardium but slightly lower than in acutely infarcted myocardium. T1 mapping is also able to illustrate areas of lipomatous metaplasia in chronic MI, the presence of which alters the electrical properties of the myocardium and might play a role in post-MI arrhythmogenesis. Fat has very low T1 values (230-350 ms at 1.5 T), and the fatty replacement area within the infarct core therefore displays noticeable T1 decrease. It is worth noting that in chronic MI, the remote myocardial native T1 is independently associated with LV systolic dysfunction (50) again affirming the fact that post-MI LV remodeling is more global and also results in diffuse fibrosis in the remote myocardium.
Cardiac MRI MI protocol
As previously discussed in this review, cardiac MRI protocol for MI should be able to perform differential diagnosis, differentiate between acute and chronic MI, aid in the quantification of AAR, IS, and subsequent viability, inform about the intensity of damage within the infarct zone, and finally appropriately define and explain any post-infarct complications (Fig. 3). We proposed a multi-parametric cardiac MRI MI protocol, which is detailed in Figure 4. This cardiac MRI MI protocol will facilitate a comprehensive evaluation and has been tested in several studies (2, 3, 42, 43).
Figure 3.
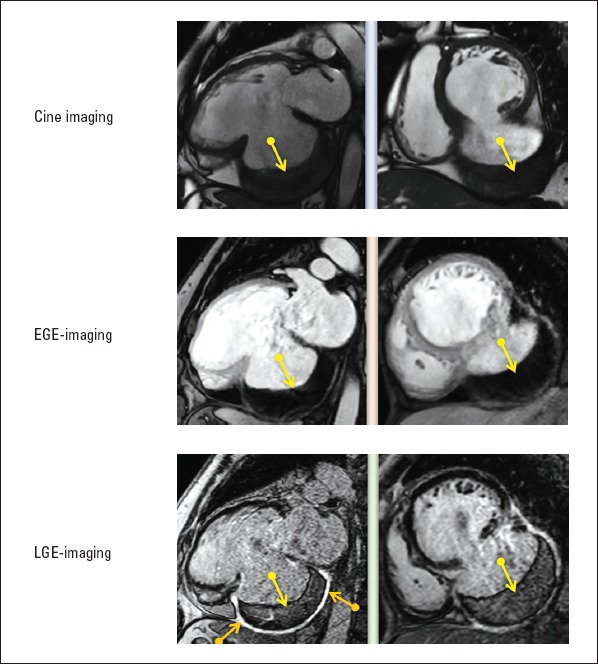
Post-acute myocardial infarct complication: inferior wall rupture and pseudoaneurysm formation (orange arrows demonstrate the uptake of hyper-enhancement during LGE imaging) with a large immobile mural thrombus within the pseudoaneurysm cavity (yellow arrows)
Figure 4.
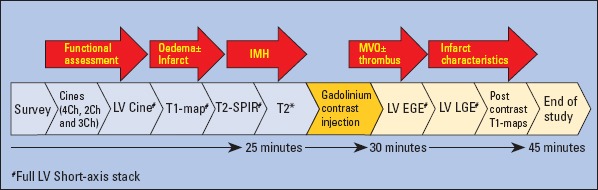
Cardiac MRI MI protocol
Conclusion
Although more pre-clinical validation and human clinical studies need to be conducted, it is evident that native T1 and ECV mapping can add to the assessment of acute and chronic MI patients and may have advantages over conventional cardiac MRI methods. The prospect of a rapid native T1 mapping protocol for patients who cannot tolerate a longer cardiac MRI scan or in those with contraindications to contrast usage is promising. Pixel-wise, T1 and ECV maps not only allow IS and AAR assessment but also inform about the extent of damage within the scar, which is associated with both functional recovery and prognosis. In both acute and chronic MI, native T1 maps and ECV maps are paving the road for a more automated, user-independent, advanced analysis, which will eventually inform future clinical studies and trials testing the potential efficacy of new therapeutic approaches.
Footnotes
Conflict of interest: None declared.
Peer-review: Internally peer-reviewed.
Authorship contributions: Concept – P.G., S.P.; Design – L.C.S., A.J.S., J.M.W.; Supervision – J.M.W., S.P.; Materials – P.G., L.C.S., A.J.S.; Data collection &/or processing – P.G., L.C.S., A.J.S.; Analysis &/or interpretation – P.G., L.C.S., A.J.S., J.M.W., S.P.; Literature search – P.G., L.C.S., A.J.S., J.M.W., S.P.; Writing – P.G., L.C.S.; Critical review – A.J.S., J.M.W., S.P.
References
- 1.Garg P, Underwood SR, Senior R, Greenwood JP, Plein S. Noninvasive cardiac imaging in suspected acute coronary syndrome. Nat Rev Cardiol. 2016;13:266–75. doi: 10.1038/nrcardio.2016.18. [DOI] [PubMed] [Google Scholar]
- 2.Garg P, Kidambi A, Foley JRJ, Musa TA, Ripley DP, Swoboda PP, et al. Ventricular longitudinal function is associated with microvascular obstruction and intramyocardial haemorrhage. Open Heart. 2016;3:e000337. doi: 10.1136/openhrt-2015-000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garg P, Kidambi A, Swoboda PP, Foley JRJ, Musa TA, Ripley DP, et al. The role of left ventricular deformation in the assessment of microvascular obstruction and intramyocardial haemorrhage. Int J Cardiovasc Imaging. 2017;33:361–70. doi: 10.1007/s10554-016-1006-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdel-Aty H, Cocker M, Meek C, Tyberg JV, Friedrich MG. Edema as a very early marker for acute myocardial ischemia:a cardiovascular magnetic resonance study. J Am Coll Cardiol. 2009;53:1194–201. doi: 10.1016/j.jacc.2008.10.065. [DOI] [PubMed] [Google Scholar]
- 5.h-Ici DO, Jeuthe S, Al-Wakeel N, Berger F, Kuehne T, Kozerke S, et al. T1 mapping in ischaemic heart disease. Eur Heart J Cardiovasc Imaging. 2014;15:597–602. doi: 10.1093/ehjci/jeu024. [DOI] [PubMed] [Google Scholar]
- 6.Cury RC, Shash K, Nagurney JT, Rosito G, Shapiro MD, Nomura CH, et al. Cardiac magnetic resonance with T2-weighted imaging improves detection of patients with acute coronary syndrome in the emergency department. Circulation. 2008;118:837–44. doi: 10.1161/CIRCULATIONAHA.107.740597. [DOI] [PubMed] [Google Scholar]
- 7.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 8.Husser O, Monmeneu JV, Bonanad C, Gomez C, Chaustre F, Nunez J, et al. Head-to-head comparison of 1 week versus 6 months CMR-derived infarct size for prediction of late events after STEMI. Int J Cardiovasc Imaging. 2013;29:1499–509. doi: 10.1007/s10554-013-0239-1. [DOI] [PubMed] [Google Scholar]
- 9.Kim RJ, Wu E, Rafael A, Chen E-L, Parker MA, Simonetti O, et al. The Use of Contrast-Enhanced Magnetic Resonance Imaging to Identify Reversible Myocardial Dysfunction. N Engl J Med. 2000;343:1445–53. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 10.Hombach V, Grebe O, Merkle N, Waldenmaier S, Höher M, Kochs M, et al. Sequelae of acute myocardial infarction regarding cardiac structure and function and their prognostic significance as assessed by magnetic resonance imaging. Eur Heart J. 2005;26:549–57. doi: 10.1093/eurheartj/ehi147. [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim T, Hackl T, Nekolla SG, Breuer M, Feldmair M, Schömig A, et al. Acute Myocardial Infarction:Serial Cardiac MR Imaging Shows a Decrease in Delayed Enhancement of the Myocardium during the 1st Week after Reperfusion. Radiology. 2010;254:88–97. doi: 10.1148/radiol.09090660. [DOI] [PubMed] [Google Scholar]
- 12.Ørn S, Manhenke C, Anand IS, Squire I, Nagel E, Edvardsen T, et al. Effect of Left Ventricular Scar Size, Location, and Transmurality on Left Ventricular Remodeling With Healed Myocardial Infarction. Am J Cardiol. 2007;99:1109–14. doi: 10.1016/j.amjcard.2006.11.059. [DOI] [PubMed] [Google Scholar]
- 13.Larose E, Rodés-Cabau J, Pibarot P, Rinfret S, Proulx G, Nguyen CM, et al. Predicting late myocardial recovery and outcomes in the early hours of ST-segment elevation myocardial infarction traditional measures compared with microvascular obstruction, salvaged myocardium, and necrosis characteristics by cardiovascular magnetic resonance. J Am Coll Cardiol. 2010;55:2459–69. doi: 10.1016/j.jacc.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 14.Simonetti OP, Finn JP, White RD, Laub G, Henry DA. “Black blood” T2-weighted inversion-recovery MR imaging of the heart. Radiology. 1996;199:49–57. doi: 10.1148/radiology.199.1.8633172. [DOI] [PubMed] [Google Scholar]
- 15.Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, et al. Cardiovascular magnetic resonance in myocarditis:A JACC White Paper. J Am Coll Cardiol. 2009;53:1475–87. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kellman P, Aletras AH, Mancini C, McVeigh ER, Arai AE. T2-prepared SSFP improves diagnostic confidence in edema imaging in acute myocardial infarction compared to turbo spin echo. Magn Reson Med. 2007;57:891–7. doi: 10.1002/mrm.21215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aletras AH, Tilak GS, Natanzon A, Hsu L-Y, Gonzalez FM, Hoyt RF, Jr, et al. Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging:histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation. 2006;113:1865–70. doi: 10.1161/CIRCULATIONAHA.105.576025. [DOI] [PubMed] [Google Scholar]
- 18.McAlindon E, Pufulete M, Lawton C, Angelini GD, Bucciarelli-Ducci C. Quantification of infarct size and myocardium at risk:evaluation of different techniques and its implications. Eur Heart J Cardiovasc Imaging. 2015;16:738–46. doi: 10.1093/ehjci/jev001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan JN, Nazir SA, Horsfield MA, Singh A, Kanagala P, Greenwood JP, et al. Comparison of semi-automated methods to quantify infarct size and area at risk by cardiovascular magnetic resonance imaging at 1.5T and 3.0T field strengths. BMC Res Notes. 2015;8:52. doi: 10.1186/s13104-015-1007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bulluck H, Rosmini S, Abdel-Gadir A, Bhuva AN, Treibel TA, Fontana M, et al. Impact of microvascular obstruction on semiautomated techniques for quantifying acute and chronic myocardial infarction by cardiovascular magnetic resonance. Open Heart. 2016;3:e000535. doi: 10.1136/openhrt-2016-000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwong RY, Farzaneh-Far A. Measuring myocardial scar by CMR. JACC Cardiovasc Imaging. 2011;4:157–60. doi: 10.1016/j.jcmg.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 22.El Aidi H, Adams A, Moons KGM, Den Ruijter HM, Mali WPTM, Doevendans PA, et al. Cardiac magnetic resonance imaging findings and the risk of cardiovascular events in patients with recent myocardial infarction or suspected or known coronary artery disease:a systematic review of prognostic studies. J Am Coll Cardiol. 2014;63:1031–45. doi: 10.1016/j.jacc.2013.11.048. [DOI] [PubMed] [Google Scholar]
- 23.Hammer-Hansen S, Bandettini WP, Hsu L-Y, Leung SW, Shanbhag S, Mancini C, et al. Mechanisms for overestimating acute myocardial infarct size with gadolinium-enhanced cardiovascular magnetic resonance imaging in humans:a quantitative and kinetic study. Eur Heart J Cardiovasc Imaging. 2016;17:76–84. doi: 10.1093/ehjci/jev123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jablonowski R, Engblom H, Kanski M, Nordlund D, Koul S, van der Pals J, et al. Contrast-Enhanced CMR Overestimates Early Myocardial Infarct Size:Mechanistic Insights Using ECV Measurements on Day 1 and Day 7. JACC Cardiovasc Imaging. 2015;8:1379–89. doi: 10.1016/j.jcmg.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Garg P, Kidambi A, Ripley P, Dobson PPs, Musa TA, Erhayiem B, et al. 137 Extra-Cellular Volume Mapping and Late Gadolinium Enhancement in Acute Myocardial Infarction. Heart. 2015;101(Suppl 4):A79. [Google Scholar]
- 26.Garg P, Kidambi A, Ripley DP, Dobson LE, Swoboda PP, Musa TA, et al. Is signal intensity of late gadolinium enhancement a substitute for extracellular volume mapping in acute myocardial infarction? J Cardiovasc Magn Reson. 2015;17(Suppl 1):156. [Google Scholar]
- 27.Haaf P, Garg P, Messroghli DR, Broadbent DA, Greenwood JP, Plein S. Cardiac T1 Mapping and Extracellular Volume (ECV) in clinical practice:a comprehensive review. J Cardiovasc Magn Reson. 2016;18:89. doi: 10.1186/s12968-016-0308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roller FC, Wiedenroth C, Breithecker A, Liebetrau C, Mayer E, Schneider C, et al. Native T1 mapping and extracellular volume fraction measurement for assessment of right ventricular insertion point and septal fibrosis in chronic thromboembolic pulmonary hypertension. Eur Radiol. 2017;27:1980–91. doi: 10.1007/s00330-016-4585-y. [DOI] [PubMed] [Google Scholar]
- 29.Kellman P, Hansen MS. T1-mapping in the heart:accuracy and precision. J Cardiovasc Magn Reson. 2014;16:2. doi: 10.1186/1532-429X-16-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piechnik SK, Ferreira VM, Dall'Armellina E, Cochlin LE, Greiser A, Neubauer S, et al. Shortened Modified Look-Locker Inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J Cardiovasc Magn Reson. 2010;12:69. doi: 10.1186/1532-429X-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dabir D, Child N, Kalra A, Rogers T, Gebker R, Jabbour A, et al. Reference values for healthy human myocardium using a T1 mapping methodology:results from the International T1 Multicenter cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2014;16:69. doi: 10.1186/s12968-014-0069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Treibel TA, Fontana M, Maestrini V, Castelletti S, Rosmini S, Simpson J, et al. Automatic Measurement of the Myocardial Interstitium:Synthetic Extracellular Volume Quantification Without Hematocrit Sampling. JACC Cardiovasc Imaging. 2016;9:54–63. doi: 10.1016/j.jcmg.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Fent GJ, Garg P, Foley JRJ, Swoboda PP, Dobson LE, Erhayiem B, et al. Synthetic Myocardial Extracellular Volume Fraction. JACC Cardiovasc Imaging. 2017;10:1402–4. doi: 10.1016/j.jcmg.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M, et al. Society for Cardiovascular Magnetic Resonance Imaging;Cardiovascular Magnetic Resonance Working Group of the European Society of Cardiology. Myocardial T1 mapping and extracellular volume quantification:a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson. 2013;15:92. doi: 10.1186/1532-429X-15-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sibley CT, Noureldin RA, Gai N, Nacif MS, Liu S, Turkbey EB, et al. T1 Mapping in Cardiomyopathy at Cardiac MR:Comparison with Endomyocardial Biopsy. Radiology. 2012;265:724–32. doi: 10.1148/radiol.12112721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dall'Armellina E, Piechnik SK, Ferreira VM, Si Q Le, Robson MD, Francis JM, et al. Cardiovascular magnetic resonance by non contrast T1-mapping allows assessment of severity of injury in acute myocardial infarction. J Cardiovasc Magn Reson. 2012;14:15. doi: 10.1186/1532-429X-14-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tahir E, Sinn M, Bohnen S, Avanesov M, Säring D, Stehning C, et al. Acute versus Chronic Myocardial Infarction:Diagnostic Accuracy of Quantitative Native T1 and T2 Mapping versus Assessment of Edema on Standard T2-weighted Cardiovascular MR Images for Differentiation. Radiology. 2017;285:83–91. doi: 10.1148/radiol.2017162338. [DOI] [PubMed] [Google Scholar]
- 38.Kidambi A, Motwani M, Uddin A, Ripley DP, McDiarmid AK, Swoboda PP, et al. Myocardial Extracellular Volume Estimation by CMR Predicts Functional Recovery Following Acute MI. JACC Cardiovasc Imaging. 2017;10:989–99. doi: 10.1016/j.jcmg.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carrick D, Haig C, Rauhalammi S, Ahmed N, Mordi I, McEntegart M, et al. Prognostic significance of infarct core pathology revealed by quantitative non-contrast in comparison with contrast cardiac magnetic resonance imaging in reperfused ST-elevation myocardial infarction survivors. Eur Heart J. 2016;37:1044–59. doi: 10.1093/eurheartj/ehv372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carrick D, Haig C, Rauhalammi S, Ahmed N, Mordi I, McEntegart M, et al. Pathophysiology of LV Remodeling in Survivors of STEMI:Inflammation, Remote Myocardium, and Prognosis. JACC Cardiovasc Imaging. 2015;8:779–89. doi: 10.1016/j.jcmg.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carberry J, Carrick D, Haig C, Rauhalammi SM, Ahmed N, Mordi I, et al. Remote Zone Extracellular Volume and Left Ventricular Remodeling in Survivors of ST-Elevation Myocardial Infarction. Hypertension. 2016;68:385–91. doi: 10.1161/HYPERTENSIONAHA.116.07222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garg P, Broadbent DA, Swoboda PP, Foley JRJ, Fent GJ, Musa TA, et al. Extra-cellular expansion in the normal, non-infarcted myocardium is associated with worsening of regional myocardial function after acute myocardial infarction. J Cardiovasc Magn Reson. 2017;19:73. doi: 10.1186/s12968-017-0384-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garg P, Broadbent DA, Swoboda PP, Foley JRJ, Fent GJ, Musa TA, et al. Acute Infarct Extracellular Volume Mapping to Quantify Myocardial Area at Risk and Chronic Infarct Size on Cardiovascular Magnetic Resonance Imaging. Circ Cardiovasc Imaging. 2017;10:e006182. doi: 10.1161/CIRCIMAGING.117.006182. [DOI] [PubMed] [Google Scholar]
- 44.Liu D, Borlotti A, Viliani D, Jerosch-Herold M, Alkhalil M, De Maria GL, et al. CMR Native T1 Mapping Allows Differentiation of Reversible Versus Irreversible Myocardial Damage in ST-Segment–Elevation Myocardial Infarction. Circ Cardiovasc Imaging. 2017;10:e005986. doi: 10.1161/CIRCIMAGING.116.005986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chow K, Yang Y, Shaw P, Kramer CM, Salerno M. Robust free-breathing SASHA T1 mapping with high-contrast image registration. J Cardiovasc Magn Reson. 2016;18:47. doi: 10.1186/s12968-016-0267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fernández-Jiménez R, Barreiro-Pérez M, Martin-García A, Sánchez-González J, Agüero J, Galán-Arriola C, et al. Dynamic Edematous Response of the Human Heart to Myocardial Infarction:Implications for Assessing Myocardial Area at Risk and Salvage. Circulation. 2017;136:1288–1300. doi: 10.1161/CIRCULATIONAHA.116.025582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garg P, Abdel-Rahman SED, Greenwood JP, Plein S. Free-Wall Rupture Post-Reperfused Acute Myocardial Infarction:Insights From Multimodality Cardiovascular Imaging. Circulation. 2015;132:e245–7. doi: 10.1161/CIRCULATIONAHA.115.018932. [DOI] [PubMed] [Google Scholar]
- 48.Garg P, Greenwood JP, Plein S. Multiparametric relaxometry by cardiac magnetic resonance imaging in Takotsubo cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2015;16:1174. doi: 10.1093/ehjci/jev167. [DOI] [PubMed] [Google Scholar]
- 49.Kali A, Cokic I, Tang RLQ, Yang HJ, Sharif B, Marbán E, et al. Determination of location, size, and transmurality of chronic myocardial infarction without exogenous contrast media by using cardiac magnetic resonance imaging at 3 T. Circ Cardiovasc Imaging. 2014;7:471–81. doi: 10.1161/CIRCIMAGING.113.001541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakamori S, Alakbarli J, Bellm S, Motiwala SR, Addae G, Manning WJ, et al. Native T 1 value in the remote myocardium is independently associated with left ventricular dysfunction in patients with prior myocardial infarction. J Magn Reson Imaging. 2017;46:1073–81. doi: 10.1002/jmri.25652. [DOI] [PMC free article] [PubMed] [Google Scholar]


