Key Points
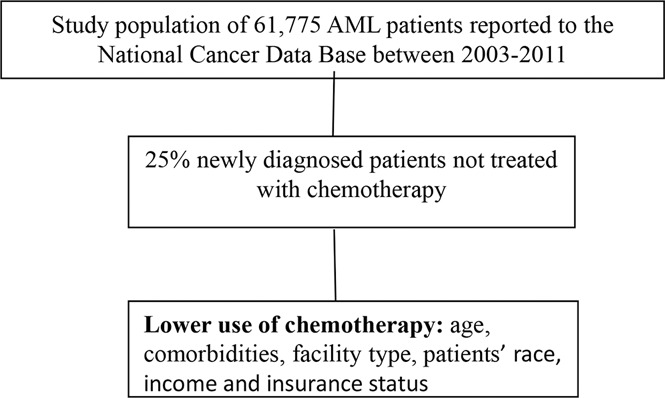
An analysis of 61 775 adults with AML diagnosed between 2003 and 2011 demonstrated that 25% did not receive any chemotherapy.
Factors such as facility type, patients’ race, income, and insurance status were associated with the rates of use of chemotherapy.
Abstract
The use of chemotherapy in patients with acute myeloid leukemia (AML) is associated with survival benefits and alleviation of symptoms related to AML. Prior studies have demonstrated a lower receipt of chemotherapy with increasing age and comorbidities. We hypothesized that socioeconomic and health system factors also determine the use of chemotherapy. We included 61 775 adults with AML diagnosed between 2003 and 2011 from the National Cancer Database, and performed a multivariable logistic regression model to determine the association between receipt of chemotherapy and several factors. A total of 15 608 patients (25.3%) did not receive chemotherapy. In a multivariable analysis, the likelihood of getting chemotherapy declined with increasing age and comorbidities and among patients with therapy-related and intermediate-/high-risk AML. Other factors associated with a lower likelihood of receiving chemotherapy included receipt of care in nonacademic centers, African American race, lower income status, uninsured or Medicare insurance status, and female sex. Compared with the previous studies, our study is novel because it provides data from a large, unselected cohort of patients diagnosed in the United States in recent years, and simultaneously examines the effect of various biological, socioeconomic, and health system factors. The results of our study raise a possibility of leukemia care disparity based on socioeconomic and health system factors. Better understanding of ways such factors may influence receipt of chemotherapy may allow an increase in the use of chemotherapy.
Visual Abstract
Introduction
The use of the appropriate intensity of chemotherapy in patients with acute myeloid leukemia (AML) can result in alleviation of symptoms related to AML, improvement in quality of life, and prolongation of survival despite the potential risks and toxicities of chemotherapy.1,2 These benefits are observed even among older patients or those with comorbidities,3,4 who can be treated with low-intensity chemotherapy, such as hypomethylating agent or low-dose cytarabine, when appropriate. Without chemotherapy, many patients succumb to AML within a few months.5 For these reasons, the National Comprehensive Cancer Network guidelines in AML6 and the European LeukemiaNet expert panel guidelines7 recommend initial use of chemotherapy in patients with AML.
Prior studies have demonstrated that many patients, particularly those with older age, do not receive chemotherapy for the management of AML.8-11 In our prior study, we have also observed that about a quarter of patients with newly diagnosed AML did not receive chemotherapy.12 Although avoiding chemotherapy in critically ill patients or those who prefer not to undergo chemotherapy is appropriate, and some patients may die of AML before receiving chemotherapy, disparity may exist in the use of chemotherapy among other patients. Prior studies have demonstrated a lower receipt of chemotherapy with increasing age of patients and increasing comorbidities.8-11,13 Previously, we have demonstrated that the likelihood of receiving chemotherapy was higher among patients treated in academic as compared with nonacademic centers.12 Extending on our initial observations, we have performed a study using the large National Cancer Database (NCDB) specifically to determine the use of upfront chemotherapy in AML in more recent years and to determine several factors associated with the receipt of chemotherapy. We hypothesized that additional health system and socioeconomic factors such as facility type, income, and insurance status also determine the rates of use of chemotherapy.
Methods
Data source and patient selection
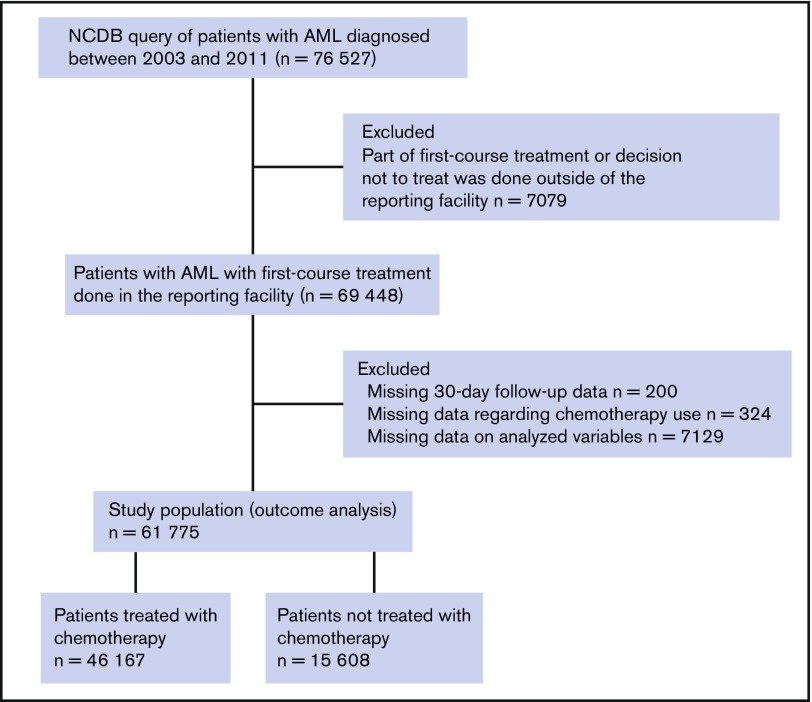
The NCDB, maintained by the Commission on Cancer of the American College of Surgeons and the American Cancer Society, captures approximately 70% of new diagnoses of cancer in the United States. A standard operating policy is used at more than 1,500 accredited cancer programs to capture the data and monitor quality control.14 The NCDB provided deidentified patient-level data of adult patients with a new diagnosis of AML during the years 2003 to 2011. International Classification of Diseases for Oncology, Version 3, codes 9840-9861, 9865-9874, and 9891-9931 were used to capture the patients. The NCDB provided records of 76 527 patients with newly diagnosed AML. The cohort selection is described in the CONSORT diagram (Figure 1). We excluded patients who received part of a first course of treatment or who made the decision not to treat outside of the reporting facility, as well as those with missing data.
Figure 1.
CONSORT diagram for cohort selection.
Study end points and variables analyzed
The primary objective of this study was to determine the factors associated with the receipt of initial chemotherapy for patients with AML. Initial chemotherapy or first course of therapy, defined as first-line therapy used before disease progression or recurrence,15 and use of single or multiagent chemotherapy, are captured by NCDB; however, NCDB does not record specific chemotherapeutic agents.
Variables analyzed included receipt of chemotherapy, sex, age, race, education, income, facility type, hospital volume, distance traveled for therapy, urban/rural location of facility type, insurance, Charlson comorbidity score, subtypes of AML, and time from diagnosis to treatment initiation (Table 1). AML subtypes were divided into acute promyelocytic leukemia, core-binding factor AML, therapy-related AML, and other intermediate-/high-risk AML. NCDB specifically captures the diagnosis of acute promyelocytic leukemia with t(15;17), and core binding factor AML with inv(16)/t(16;16) or t(8;21), using unique International Classification of Diseases for Oncology, Version 3, codes and specific genetic abnormalities. Therapy-related AML is also a specific entity in the NCDB; however, intermediate-risk AML cannot always be differentiated from high-risk AML in this database. For this reason, intermediate-risk AML and high-risk AML were combined for analysis in this study. Income and education are not of the patient but, rather, area-based metrics derived from Zip code data. Further details on classification of other variables can be obtained from our prior publication.12 The institutional review board at the University of Nebraska Medical Center determined that the retrospective study did not require IRB approval because of the use of deidentified data.
Table 1.
Demographic characteristics of the study population based on the receipt of chemotherapy
| Variable | Received chemotherapy, n (%) (N = 46 167) | Did not receive chemotherapy, n (%) (N = 15 608) | P |
|---|---|---|---|
| Age, y | <.0001 | ||
| 18-40 | 6 858 (14.8) | 418 (2.7) | |
| 41-59 | 14 280 (30.9) | 1 232 (7.9) | |
| 60-70 | 12 194 (26.4) | 2 314 (14.8) | |
| 71-80 | 9 480 (20.5) | 5 210 (33.4) | |
| ≥81 | 3 355 (7.3) | 6 434 (41.2) | |
| Male sex | 24 865 (53.9) | 8 125 (52.1) | <.0001 |
| Race | <.0001 | ||
| White | 39 719 (86.0) | 13 802 (88.4) | |
| African American | 4 018 (8.7) | 1 211 (7.8) | |
| Other | 2 430 (5.3) | 595 (3.8) | |
| No high school diploma, %* | .04 | ||
| ≥29 | 7 608 (16.5) | 2 559 (16.4) | |
| 20-28.9 | 10 559 (22.9) | 3 642 (23.3) | |
| 14-19.9 | 11 170 (24.2) | 3 896 (25.0) | |
| <14 | 16 830 (36.4) | 5 511 (35.3) | |
| Median household income* | <.0001 | ||
| <$30 000 | 5 991 (13.0) | 2 205 (14.1) | |
| $30 000-$34 999 | 8 552 (18.5) | 3 004 (19.2) | |
| $35 000-$45 999 | 12 874 (27.9) | 4 583 (29.4) | |
| ≥$46 000 | 18 750 (40.6) | 5 816 (37.3) | |
| Insurance status | <.0001 | ||
| Not insured | 1 867 (4.0) | 376 (2.4) | |
| Private insurance/managed care | 20 790 (45.0) | 2 679 (17.2) | |
| Medicaid | 4 049 (8.8) | 478 (3.1) | |
| Medicare | 18 915 (41.0) | 11 978 (76.7) | |
| Other government | 546 (1.2) | 97 (0.6) | |
| Charlson comorbidity score | <.0001 | ||
| 0 | 34 466 (74.7) | 9 752 (62.5) | |
| 1 | 8 623 (18.7) | 3 729 (23.9) | |
| ≥2 | 3 078 (6.7) | 2 127 (13.6) | |
| Distance traveled, miles | <.0001 | ||
| 0-4.9 | 10 040 (21.7) | 5 769 (36.7) | |
| 5-11.9 | 11 401 (24.7) | 4 313 (27.6) | |
| 12-34.7 | 12 275 (26.6) | 3 294 (21.1) | |
| ≥34.8 | 12 451 (27.0) | 2 232 (14.3) | |
| AML subtypes | <.0001 | ||
| Acute promyelocytic leukemia | 4 507 (9.8) | 712 (4.6) | |
| Core binding factor AML | 1 535 (3.3) | 243 (1.6) | |
| Therapy-related AML | 701 (1.5) | 200 (1.3) | |
| Other intermediate/high risk | 39 424 (85.4) | 14 453 (92.6) | |
| Receipt of care in academic centers | 25 890 (56.1) | 4 583 (29.4) | <.0001 |
| Annual hospital volume, median (quartiles) [range] | 15 (7-35) [1-122] | 7 (4-14) [1-122] | <.0001 |
Based on Zip code.
Statistical analysis
PC SAS version 9.4 was used for all summaries and analyses. χ2 tests were used to compare the demographic and baseline variables for patients who did vs did not receive chemotherapy. Frequency and percentages are presented. The variables hospital volume and time from diagnosis to initiation of therapy were summarized using the median, quartiles, and range. Those demographic and baseline variables that had statistically significant P values from the univariable analysis were included in a multivariable logistic regression model, using the glimmix procedure. The model accounted for clustering of facility locations and used backward selection to keep terms in the model that were statistically significant. The 2-way interactions, age by insurance status and age by Charlson comorbidity score, were tested and kept in the model because they were statistically significant. One-month mortality was analyzed using a χ2. Kaplan-Meier curves were compared to examine overall survival.
Results
The study population included 61 775 patients. The majority (97.7%) received care in a facility situated in an urban location. A total of 46 167 patients (74.7%) received chemotherapy within a median of 4 days of diagnosis (interquartile range, 2-10 days; range, 0-1098 days), and 15 608 patients (25.3%) did not receive chemotherapy. Patients received a multiagent (74.9%, n = 34 576), single-agent (21.8%, n = 10 086), or unspecified (3.3%, n = 1505) chemotherapy regimen. Reasons for not receiving chemotherapy were cited as unspecified reason (72.8%, n = 11 358), the presence of contraindication such as comorbidities or advanced age (11.4%, n = 1786), patient’s or family’s refusal (11.3%, n = 1763), death before therapy (3.8%, n = 598), and recommended but not administered for unspecified reason (0.7%, n = 103). The 1-month mortality was 11.8% and 53.6% among patients who did vs did not receive chemotherapy. Patients who did not receive chemotherapy had a poorer 5-year overall survival (2.6%) compared with patients treated with chemotherapy (25.8%; Figure 2).
In a univariable analysis, increasing age (particularly 60 years and older) and Charlson comorbidity score correlated with a lower probability of receiving chemotherapy (Table 1). The diagnosis of therapy-related AML or intermediate-/high-risk AML compared with core binding factor AML and acute promyelocytic leukemia, was associated with a lower likelihood of receiving chemotherapy. Female sex, white race, lower educational status, lower income, uninsured status, and receipt of care in nonacademic centers and low hospital volume were associated with a lower use of chemotherapy. Patients who traveled a longer distance more frequently received chemotherapy.
In a multivariable analysis, 2-way interactions, age by insurance status, and age by Charlson comorbidity score were statistically significant (Table 2; supplemental Tables 1 and 2). The likelihood of receiving chemotherapy declined with increasing age, with higher Charlson comorbidity score, and with uninsured or Medicare insurance status. Chemotherapy was used less often for therapy-related AML and intermediate-/high-risk AML. Other factors associated with lower likelihood of receiving chemotherapy included lower hospital volume, female sex, African American race, and lower income status. Patients treated in academic centers had a 1.5-fold higher likelihood of receiving chemotherapy. Patients who traveled a longer distance to receive therapy were also more likely to receive chemotherapy.
Table 2.
Multivariable logistic regression model predicting receipt of chemotherapy
| Variable | Odds ratio (95% confidence interval) | Overall P value |
|---|---|---|
| Sex | .001 | |
| Male vs female | 1.07 (1.03-1.12) | |
| Race | 0009 | |
| African American vs other | 0.84 (0.73-0.96) | |
| African American vs white | 0.85 (0.78-0.93) | |
| Other vs white | 1.02 (0.91-1.14) | |
| Median household income | <.0001 | |
| <$30 000 vs $30 000-$34 999 | 0.90 (0.83-0.97) | |
| <$30 000 vs $35 000-$45 999 | 0.90 (0.84-0.98) | |
| <$30 000 vs ≥ $46 000 | 0.80 (0.74-0.87) | |
| $30 000-$34 999 vs $35 000-$45 999 | 1.00 (0.94-1.07) | |
| $30 000-$34 999 vs ≥ $46 000 | 0.90 (0.83-0.96) | |
| $35 000-$45 999 vs ≥ $46 000 | 0.89 (0.84-0.94) | |
| Distance traveled, miles | <.0001 | |
| 5-11.9 vs 12-34.7 | 0.92 (0.86-0.98) | |
| 5-11.9 vs ≥ 34.8 | 0.82 (0.76-0.89) | |
| 5-11.9 vs 0-4.9 | 1.16 (1.09-1.23) | |
| 12-34.7 vs ≥ 34.8 | 0.89 (0.83-0.96) | |
| 12-34.7 vs 0-4.9 | 1.26 (1.18-1.34) | |
| ≥34.8 vs 0-4.9 | 1.41 (1.31-1.52) | |
| AML subtypes | <.0001 | |
| Core binding factor AML vs therapy-related AML | 2.32 (1.82-2.96) | |
| Core binding factor AML vs other intermediate/high risk | 1.43 (1.22-1.68) | |
| Core binding factor AML vs acute promyelocytic leukemia | 1.26 (1.05-1.52) | |
| Therapy-related AML vs other intermediate/high risk | 0.62 (0.51-0.74) | |
| Therapy-related AML vs acute promyelocytic leukemia | 0.54 (0.44-0.67) | |
| Other intermediate/high risk vs acute promyelocytic leukemia | 0.88 (0.80-0.97) | |
| Annual hospital volume (as a continuous variable)* | 1.02 (1.02-1.03) | <.0001 |
| Facility type | <.0001 | |
| Academic vs nonacademic | 1.51 (1.36-1.67) | |
| Age | NP | <.0001 |
| Insurance status | NP | <.0001 |
| Charlson comorbidity score | NP | <.0001 |
| Age × insurance status | Supplemental Table 1 | <.0001 |
| Age × Charlson comorbidity score | Supplemental Table 2 | <.0001 |
NP, not provided because of interaction.
For every single increase in number of patients treated annually in a hospital, odds of receiving chemotherapy increase by a factor of 1.02.
Discussion
Our large study demonstrated that approximately a quarter of patients with newly diagnosed AML do not receive any chemotherapy despite survival benefits associated with the use of chemotherapy.1-4 The majority of patients (74.9%) received multiagent chemotherapy; however, NCDB does not record specific chemotherapeutic agents. Although the use of specific chemotherapy influences the remission rate and survival, the prognosis is more profoundly affected by whether patients receive chemotherapy in the first place. Although chemotherapy was not used in some patients because of refusal, early death, or contraindication, no specific reason was recorded in the database for three-quarters of the patients.
The receipt of chemotherapy was lower with increasing age and comorbidities, and among patients with therapy-related AML and intermediate-/high-risk AML, these factors are associated with lower anticipated benefits or higher risks for complications with the use of chemotherapy. In addition, socioeconomic factors such as lower income status, uninsured or Medicare insurance status, African American race, and to some extent, female sex were associated with a lower use of chemotherapy. Although the univariable analysis demonstrated a lower use of chemotherapy in whites, African American race was associated with a lower use of chemotherapy in the multivariable analysis that adjusted for other variables. Patients treated at academic centers were more likely to receive chemotherapy. We also noted a higher probability of receiving chemotherapy among patients who traveled a greater distance to receive care.
Prior studies have also demonstrated a lower receipt of chemotherapy with increasing age and greater burden of comorbidities8-11,13 (Table 3). Two of the prior studies8,9 were performed only on Medicare patients aged 65 years or older; hence, these 2 studies do not inform factors associated with the receipt of chemotherapy in younger patients. The third study used the California cancer registry to analyze the racial differences in the use of chemotherapy, did not adjust for potential confounders such as socioeconomic factors, and demonstrated a lower rate of use of chemotherapy in African Americans.10 This study had suggested that future studies should explore the role of socioeconomic factors and treatment facility location on leukemia care disparity. The study from Denmark13 highlighted that the utilization of intensive chemotherapy was lower among older patients, particularly those with lower education and lower income levels. The country has universal healthcare; hence, the study only focused on understanding the effect of education and income levels. Further, the emphasis of this study was on use of intensive chemotherapy, and details are lacking on patients who did not receive therapy. Compared with the previous studies, our study is novel because it provides data from a large unselected cohort of patients diagnosed in the United States in recent years and simultaneously examines the effect of several biological, socioeconomic, and health system factors. The effect of health system factors was not analyzed by the aforementioned studies. We have demonstrated that the likelihood of undergoing chemotherapy was significantly higher in patients treated at academic centers. Most patients in our study were treated in an urban location, which did not influence the probability of receiving chemotherapy. Patients who traveled a longer distance to receive therapy were also more likely to receive chemotherapy. Although we do not fully understand the reason, the longer travel distance may indicate a greater performance status or receipt of initial care at academic centers.
Table 3.
Key factors associated with a lower utilization of initial chemotherapy for acute myeloid leukemia
| Study | Patients not receiving chemotherapy | Older age | Comorbidities | Race | Lower education | Lower income | Insurance status |
|---|---|---|---|---|---|---|---|
| Present (US) (2003-2012) | 25% | Yes | Yes | Lower in African American | No | Yes | Yes |
| SEER-Medicare (2000-2007)*,8 | 61% among patients ≥ 65 y | Yes | Yes | No | Yes | Yes | Medicare patients only |
| SEER-Medicare (1997-2007)9 | 57% among patients ≥ 65 y | Yes | Yes | Lower in African American | Medicare patients only | ||
| California Cancer Registry (1998-2008)10 | 25%-39% depending on race | Yes | Yes | Lower in African American | |||
| Denmark (2000-2014)13 | † | Yes† | Yes† | Yes† | Universal healthcare system | ||
| Netherlands (2007-2012)11 | 5-66%‡ | Yes |
Our study also demonstrated a lower use of chemotherapy in patients treated at non-academic centers, hospital with low volume of AML patients, and patients with therapy-related, intermediate or high-risk acute myeloid leukemia, and a higher use in patients who traveled greater distance to receive care.
This study also demonstrated lower rates of receipt of chemotherapy in patients from rural areas and those with prior diagnosis of myelodysplastic syndrome.
47% of patients received palliative intent therapy; however, whether chemotherapy or only supportive care therapy is not specified. The receipt of intensive chemotherapy was lower among older patients and those with lower education and lower income levels (mainly among older patients).
Depending on the age: 5% in patients aged 18-40 years and 66% in patients aged >70 years. The rates of chemotherapy use were higher for patients with acute promyelocytic leukemia and those treated in 2007-2012 compared with prior years.
The possibility of healthcare disparity based on socioeconomic factors is concerning and needs further research to understand various mechanisms and ways these factors can influence the receipt of chemotherapy. Insurance status and income levels may limit access to chemotherapy, supportive care, intensive monitoring, or other resources (eg, transportation) necessary to obtain treatment, or in some situations, patients may prioritize other basic needs over getting chemotherapy. Racial background of patients may affect patients’ understanding of the disease process or the risk-benefit analysis of receiving chemotherapy. In addition, physicians or other healthcare providers may base their recommendations on the racial or socioeconomic background of patients. Educational status was associated with the use of chemotherapy in univariable, but not in multivariable, analysis. This discrepancy from the results of other studies may be related to the fact that educational status is derived from Zip code data in the NCDB. This is a limitation of the database. Also, we could not ascertain molecular features of AML and performance status of the patients at the time of diagnosis; the latter could influence the decision to receive chemotherapy. The Commission on Cancer-approved facilities that report cases of AML to the NCDB are more frequently located in urban locations, which explains the finding that the majority of patients in this study received treatment in urban locations.
In conclusion, a quarter of patients with newly diagnosed AML do not receive chemotherapy in the United States. Whereas the lower receipt of chemotherapy is expected among older patients, patients with significant comorbidities, and those with high-risk AML, racial, insurance and income status and facility type also influenced the likelihood of receiving chemotherapy. These findings raise a possibility of leukemia care disparity based on socioeconomic and health system factors. Early diagnosis of AML, multidisciplinary management of leukemia emergencies and comorbidities, and strengthening the partnership between academic leukemia experts and community oncologists are some of the potential ways to increase the use of chemotherapy. Addressing the effect of racial, income, and insurance status may be more complex and requires better understanding of ways such factors may influence receipt of chemotherapy. Some of the potential strategies to improve the receipt of chemotherapy in African American patients may include frequent discussions about the risk-benefit analysis of chemotherapy, taking into consideration the educational and cultural background of the patients, increasing diversity in the healthcare workforce, and enhancing cultural competence among members of a healthcare team.16 Chemotherapy use should be maximized, especially as we enter the era of more effective and better tolerated novel and molecularly targeted therapies.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The data used in the study are derived from a deidentified National Cancer Database file.
The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology used or the conclusions drawn from these data by the investigators.
Authorship
Contribution: V.R.B. developed the concept of the study; V.R.B. and V.S. designed the study and were involved with data analysis; all authors contributed to the interpretation of the results; the initial manuscript was drafted by V.R.B. and V.S.; all authors reviewed and critically revised the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: V.R.B. reports serving as a consultant for Pfizer. J.O.A. reports serving as a consultant for Conatus-IDMC, Samus Therapeutics, and Ascentage, and is on the board of directors for Tesaro bio, Inc. The remaining authors declare no competing financial interests.
Correspondence: Vijaya Raj Bhatt, University of Nebraska Medical Center, Department of Internal Medicine, Division of Hematology-Oncology, 986840 Nebraska Medical Center, Omaha, NE 68198-6840; e-mail: vijaya.bhatt@unmc.edu.
References
- 1.Bhatt VR, Gundabolu K, Koll T, Maness LJ. Initial therapy for acute myeloid leukemia in older patients: principles of care. Leuk Lymphoma. 2018;59(1):29-41. [DOI] [PubMed] [Google Scholar]
- 2.Estey EH. How to manage high-risk acute myeloid leukemia. Leukemia. 2012;26(5):861-869. [DOI] [PubMed] [Google Scholar]
- 3.Sorror ML, Storer BE, Fathi AT, et al. . Development and validation of a novel acute myeloid leukemia-composite model to estimate risks of mortality. JAMA Oncol. 2017;3(12):1675-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Østgård LS, Nørgaard JM, Sengeløv H, et al. . Comorbidity and performance status in acute myeloid leukemia patients: a nation-wide population-based cohort study. Leukemia. 2015;29(3):548-555. [DOI] [PubMed] [Google Scholar]
- 5.Freireich EJ, Wiernik PH, Steensma DP. The leukemias: a half-century of discovery. J Clin Oncol. 2014;32(31):3463-3469. [DOI] [PubMed] [Google Scholar]
- 6.O’Donnell MR, Tallman MS, Abboud CN, et al. . Acute myeloid leukemia, version 3.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15(7):926-957. [DOI] [PubMed] [Google Scholar]
- 7.Döhner H, Estey E, Grimwade D, et al. . Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica. 2012;97(12):1916-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyers J, Yu Y, Kaye JA, Davis KL. Medicare fee-for-service enrollees with primary acute myeloid leukemia: an analysis of treatment patterns, survival, and healthcare resource utilization and costs. Appl Health Econ Health Policy. 2013;11(3):275-286. [DOI] [PubMed] [Google Scholar]
- 10.Patel MI, Ma Y, Mitchell B, Rhoads KF. How do differences in treatment impact racial and ethnic disparities in acute myeloid leukemia? Cancer Epidemiol Biomarkers Prev. 2015;24(2):344-349. [DOI] [PubMed] [Google Scholar]
- 11.Dinmohamed AG, Visser O, van Norden Y, et al. . Treatment, trial participation and survival in adult acute myeloid leukemia: a population-based study in the Netherlands, 1989-2012. Leukemia. 2016;30(1):24-31. [DOI] [PubMed] [Google Scholar]
- 12.Bhatt VR, Shostrom V, Giri S, et al. . Early mortality and overall survival of acute myeloid leukemia based on facility type. Am J Hematol. 2017;92(8):764-771. [DOI] [PubMed] [Google Scholar]
- 13.Østgård LSG, Nørgaard M, Medeiros BC, et al. . Effects of education and income on treatment and outcome in patients with acute myeloid leukemia in a tax-supported health care system: a national population-based cohort study. J Clin Oncol. 2017;35(32):3678-3687. [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Data Base. About the National Cancer Database. Available at: https://www.facs.org/quality-programs/cancer/ncdb/about. Accessed 12 October 2016.
- 15.National Cancer Data Base. Data Dictionary PUF 2014. Treatment. Available at: http://ncdbpuf.facs.org/node/408. Accessed 23 October 2016.
- 16.Bhatt VR, Chen B, Lee SJ. Use of hematopoietic cell transplantation in younger patients with acute myeloid leukemia: A National Cancer Database Study [published online ahead of print 5 February 2018]. Bone Marrow Transplant. doi:10.1038/s41409-018-0105-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.