Key Points
TL for age shortens over time in patients with the TBD DC, irrespective of treatment with androgens.
Prospective long-term research is needed to understand the extra-hematopoietic effects of androgens for management of TBDs.
Abstract
Dyskeratosis congenita (DC) is an inherited bone marrow failure syndrome and the prototypic telomere biology disorder (TBD). Leukocyte telomere length (TL) less than the first percentile for age, measured by flow cytometry with in situ hybridization (flow FISH), is diagnostic of DC. Androgens are a therapeutic option for DC/TBD-associated bone marrow failure (BMF). One report has shown an apparent increase in TL in patients while on treatment with the attenuated androgen danazol. The aim of this study was to compare TL over time in 10 androgen-treated and 16 untreated patients with DC. All subjects were enrolled in institutional review board–approved longitudinal cohort studies of inherited BMF. TL in 6-panel leukocyte subsets was measured by flow FISH. Generalized estimating equations (GEE) methodology was used to compare TL changes over time between groups. Unadjusted analyses showed annual median total lymphocyte TL attrition of −62 base pairs/year (bp/y) in androgen-treated patients with DC compared with −76 bp/y in untreated DC patients (P = .71). Longitudinal analysis using a GEE model, adjusted for age at sample collection, showed no statistically significant difference in TL change over time between treated and untreated patients (P = .24). The results were similar for each individual leukocyte subset evaluated. In summary, our data show the expected age-associated longitudinal telomere shortening in patients with DC, irrespective of androgen therapy. Caution is warranted when recommending androgen therapy for non-BMF manifestations of DC or TBDs until the biological mechanisms are better understood.
Visual Abstract
Introduction
Dyskeratosis congenita (DC) is an inherited bone marrow failure syndrome (IBMFS) and the prototypic telomere biology disorder (TBD). Telomeres, nucleotide repeats and a protein complex at chromosome ends, are essential for genomic stability and shorten with each cell division. Although the classic mucocutaneous triad of dysplastic nails, oral leukoplakia, and reticular skin pigmentation is diagnostic of DC, a wide range of medical problems result in a spectrum of clinical manifestations now recognized as the TBDs. These medical problems include bone marrow failure (BMF), pulmonary fibrosis, liver disease, squamous cell carcinomas of the head and neck, leukemia, and myelodysplastic syndrome, as well as other manifestations.1,2 Telomere length (TL) less than the first percentile for age measured in leukocyte subsets by flow cytometry with in situ hybridization (flow FISH) is diagnostic of DC.3,4 DC and the clinically related TBDs are caused by X-linked recessive, autosomal dominant, or autosomal recessive inheritance of pathogenic germline variants in key telomere biology genes (DKC1, TERC, TERT, TINF2, NOP10, NHP2, CTC1, WRAP53, RTEL1, ACD, STN1, NAF1, or PARN).5-7
Approximately 50% of patients with DC develop clinically significant BMF by 40 years of age.2,8 Hematopoietic cell transplantation (HCT) is a curative option for DC-related BMF; however, some patients may be medically unable or not ready to undergo HCT. We previously showed that up to 70% of patients with DC and severe BMF attain independence from transfusions for anemia or thrombocytopenia with androgen therapy.9 The biological mechanisms by which androgens effectively treat BMF are not understood. It has been proposed that androgens may directly increase the production of erythropoietin or may act on the erythropoietin receptor to elicit a hematological response.10,11 A limited number of studies on human cell lines and mouse models of aplastic anemia suggest that androgens may increase telomerase expression and, in turn, increase TL.12,13 A recent observational study of the attenuated androgen, danazol, was recently completed in patients with TBDs with telomere elongation as the primary end point and TL measured by quantitative polymerase chain reaction (qPCR) of blood-derived DNA.14 The data in that study suggested lengthening of telomeres while patients were taking danazol, with accelerated telomere shortening occurring after treatment cessation.14 This observation conflicts with our prior report showing that leukocyte TL (measured by flow FISH) continued to decline in DC patients on androgens for BMF.9 The primary aim of the current study was to evaluate the long-term effect of androgens on TL in patients with DC. Here, we compared the rate of TL change in patients with DC receiving androgens with those who have never been treated with androgens.
Methods
Patient characteristics
This retrospective observational study included patients with DC enrolled in the National Cancer Institute’s (NCI) institutional review board–approved inherited bone marrow failure syndrome study (NCI 02-C-0052, NCT00027274, www.marrowfailure.cancer.gov)2 Baylor College of Medicine institutional review board–approved Genetic and Biologic Determinants of Bone Marrow Failure study (H-17698) in accordance with the Declaration of Helsinki. Patients were classified as having DC if they had a germline mutation in 1 of the known DC genes, or if they had at least 2 features of the classic diagnostic triad and other clinical findings consistent with DC, such as hematologic or neoplastic complications.1,15 All participants had a minimum of 2 flow FISH leukocyte TL measurements performed at an interval of at least 1 year. Medical records were reviewed for androgen treatment details and response to therapy. Androgens for the treatment of BMF were started and managed by the primary local hematologist, often in consultation with the study team. All treated participants were taking androgens at the most recent TL measurement.
Telomere length
TL in all samples was measured at a single laboratory, which is also Clinical Laboratory Improvement Amendments certified for clinical testing (Repeat Diagnostics, Inc, Vancouver, BC, Canada). Six panel leukocyte subsets TL (granulocytes, total lymphocytes, CD45+ lymphocytes, CD45− lymphocytes, CD20+ lymphocytes, and CD57+ lymphocytes) was measured by flow FISH performed on fresh blood samples as previously described.16
Statistical analysis
We compared the annual TL change in androgen-treated and untreated DC patients using the Mann-Whitney U test. To account for the correlations between serially collected samples, we used the generalized estimating equations (GEE) to perform longitudinal TL change analysis; models were adjusted for age at sample collections. All analyses were 2 sided, and P < .05 was considered statistically significant. Excel (Microsoft, 2007 release) and SPSS Statistics, version 23 (IBM Corp, Armonk, NY) were used for all analyses.
Results
Participant characteristics and response to therapy
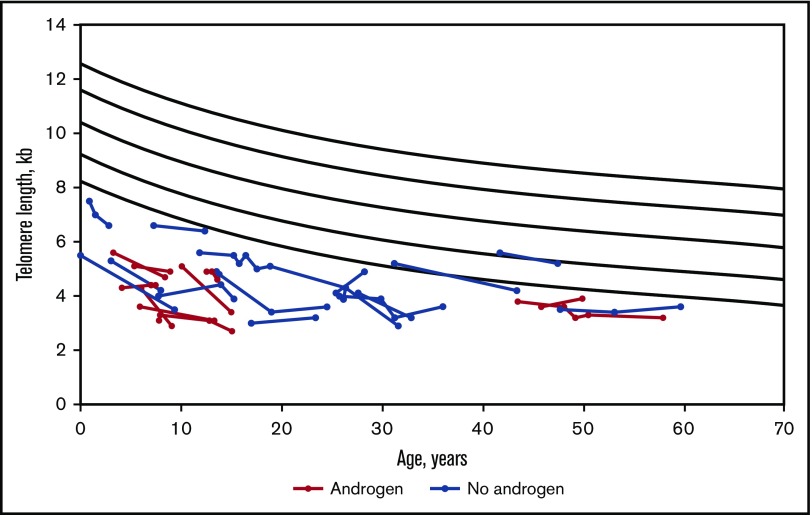
The study included 26 patients with DC who had TL testing by flow FISH at study enrollment and subsequent serial samples (Table 1; Figure 1). Sixteen patients never received androgens. The 10 patients treated with androgens were treated with oxymetholone (n = 4), danazol (n = 5), or halotestin (n = 1). The indications, doses, and adverse effects (if any) of androgens are detailed in Table 2. Patients on androgens were followed for a median of 5 years (range 1-15 years) after baseline TL measurement and were treated with androgens for a median of 3 years (range 0.5-15 years). Untreated patients were followed for a median of 6 years (range 2-15 years) (Table 1). Baseline TL was below the first percentile for age in all study participants (Figure 1).
Table 1.
Demographic and genetic details of participants
| DC patients treated with androgens (n = 10) | DC patients never treated with androgens (n = 16) | |
|---|---|---|
| Feature* | ||
| Median age at baseline TL measurement (range), y | 7 (3-46) | 21 (0-48) |
| Median age at most recent TL measurement (range), y | 13.5 (3-48) | 27 (3-60) |
| Median follow-up time (range), y | 5 (1-15) | 6 (2-15) |
| Male:female | 9:1 | 3:1 |
| Gene (inheritance) (%) | ||
| DKC1 (XLR) | 3 (30) | 1 (6) |
| TERT (AD) | 0 (0) | 3 (19) |
| TERC (AD) | 1 (10) | 2 (12) |
| TINF2 (AD) | 2 (20) | 4 (25) |
| RTEL1(AD/AR†) | 2 (20) | 1 (6) |
| PARN (AR) | 1 (10) | 3 (19) |
| Unknown | 1 (10) | 2 (12) |
AD, autosomal dominant; AR, autosomal recessive; DKC1, dyskerin; PARN, poly(A)-specific ribonuclease; RTEL1, regulator of telomere elongation helicase 1; TERC, telomerase RNA component; TERT, telomerase reverse transcriptase; TINF2, TRF1-interacting nuclear factor 2; XLR, X-linked recessive.
P values of age at baseline TL, age at most recent TL, length of follow-up, and sex were all >.05.
RTEL1 is AD inheritance in 1 treated and 1 untreated patient, and AR in 1 treated patient.
Figure 1.
Longitudinal flow FISH lymphocyte TL in DC patients, comparing androgen-treated and untreated participants. Total lymphocyte TL is shown in kilobases (kb). The curvilinear black lines denote the range of normal TLs, with the bottom line indicating first percentile for age and the top line indicating 99th percentile for age.
Table 2.
Clinical details for patients with DC treated with androgens
| Patient | Gene | Indication for androgens | Androgen used | Dose | Hematological response* | Side effects | Outcome/last follow-up |
|---|---|---|---|---|---|---|---|
| NCI-6-1 | TERC | SAA | Halotestin | 10 mg/d (0.14 mg/kg/d) | Yes | Virilization | Alive, on androgen, bone marrow stable, progressive pulmonary fibrosis |
| NCI-106-1 | DKC1 | SAA | Oxymetholone | 0.8 mg/kg/d, then 2 mg/kg/d | Yes | Splenic peliosis and rupture when oxymetholone and granulocyte colony-stimulating factor coadministered; oxymetholone stopped and restarted at higher dose after recovery due to drop in blood counts; advanced bone age | Died age 19, post-HCT |
| NCI 164-2 | RTEL1 | Thrombocytopenia | Oxymetholone | 0.5 mg/kg/d | Initial response, lost after 3 y | None known | Died age 14 of pulmonary AVM complications, post-HCT |
| NCI 165-1 | PARN | Transfusion-dependent anemia | Oxymetholone | 0.75 mg/kg/d | Initial response, lost after 5 y when developed MDS | None known | Died age 14, post-HCT |
| NCI 180-1 | RTEL1 | SAA | Oxymetholone | 1 mg/kg/d | Yes | Hypercholesterolemia with low HDL, high LDL requiring treatment with statin; advanced bone age | Alive, post-HCT |
| NCI 288-1 | DKC1 | Short telomeres, moderate thrombocytopenia | Danazol | 800 mg/d × 2 y, 400 mg/d thereafter | No | Hypercholesterolemia with low HDL, high LDL | Died age 54 of SCC tongue |
| NCI 474-1 | Unknown | Thrombocytopenia | Danazol | 4 mg/kg/d | Yes | None known | Alive, on androgen |
| Baylor 1 | TINF2 | Thrombocytopenia | Danazol | 1.5-5 mg/kg/d | Yes | Premature puberty, lipid abnormalities with low HDL | Alive, danazol discontinued due to side effects |
| Baylor 2 | TINF2 | Thrombocytopenia | Danazol | 1.6-2.7 mg/kg/d | Yes | None known | Alive, on androgen |
| Baylor 3 | DKC1 | Thrombocytopenia | Danazol | 5 mg/kg/d | Yes | None known | Alive, on androgen |
AVM, arteriovenous malformation; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; MDS, myelodysplastic syndrome; SAA, severe aplastic anemia; SCC, squamous cell carcinoma.
Hematological response defined as increase in hemoglobin by 2 g/dL, platelet count >30 × 109/L, ANC >1.5 × 109/L, or stabilization of progressively declining blood counts.
Response to therapy was defined as either red blood cell and platelet transfusion independence, or stabilization of previously declining blood counts. Of the 10 treated patients, 7 showed a consistent red blood cell and/or platelet response to androgen therapy. One patient lost his response to androgen therapy after 5 years of consistent response, developing progressive pancytopenia and myelodysplastic changes in the bone marrow requiring a therapeutic HCT.17 His last TL reported was prior to cessation of androgen therapy. Another patient lost hematologic response after 3 years of therapy. One patient concurrently treated with oxymetholone and granulocyte colony-stimulating factor developed splenic peliosis requiring cessation of the oxymetholone for recovery, and resulting in a drop in blood counts.18 Oxymetholone was subsequently restarted successfully in this patient with transfusion independence.
One adult male with a DKC1 mutation, who was started on danazol for short telomeres and moderate thrombocytopenia at 52 years of age, developed lipid abnormalities with elevated LDL and decreased HDL cholesterol while on danazol. His longitudinal TL appeared to elongate between the 2 TL measurements performed, and danazol was continued, although he did not have a hematologic response. Unfortunately, he developed an aggressive squamous cell carcinoma of the tongue at age 53 years, underwent extensive surgical resection and radiation therapy, and died due to complications of his malignancy, 6 months after diagnosis.
TL change over time
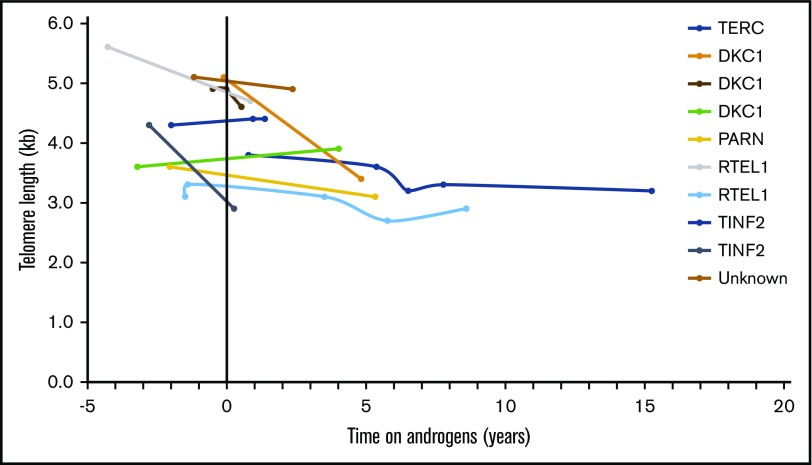
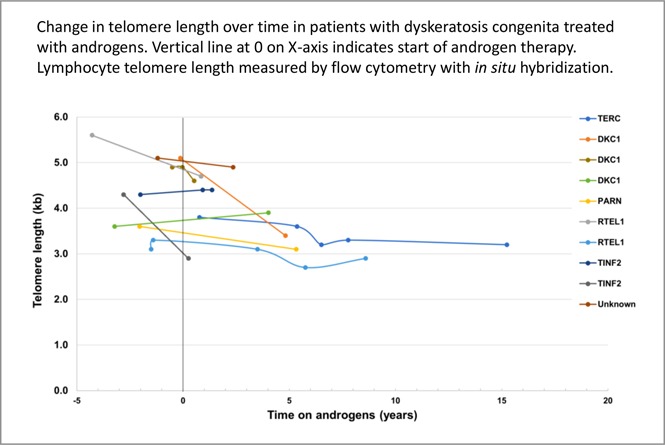
Telomeres shortened over time in both treated and untreated DC patient groups. As shown in Figure 2, individual lymphocyte TL shortened over time in all treated patients, except for 3 individuals in whom TL was stable or minimally longer. Unadjusted analysis showed that there was no statistically significant difference in median annual total lymphocyte TL change between treated (median −62 base pairs/year [bp/y]) and untreated patients (median −76 bp/y, P = .71) (Figure 1; Table 3). Similar loss of TL per year in treated and untreated patients was also observed in 4 lymphocytes subsets, CD45+, CD20+, CD57+, and NK cells and in granulocytes (Table 3). Changes in granulocyte TL, the least specific diagnostic subset,3 were inconsistent in androgen-treated patients showing a median loss of 27 bp/y but mean gain of 9 bp/y. Untreated patients had a median granulocyte TL loss of 89 bp/y and mean gain of 33 bp/y (P = .66) (Table 3).
Figure 2.
Lymphocyte TL change in DC patients treated with androgens, in relation to commencement of androgen therapy. Time 0 (vertical black line) indicates start of androgen therapy. The y-axis shows TL in kilobases, at each measurement point over time on androgen therapy (time in years depicted in x-axis).
Table 3.
Median annual TL change in androgen-treated and untreated participants by leukocyte subset
| Leukocyte subset | Total granulocytes | Total lymphocytes | CD45+ lymphocytes | CD45− lymphocyte | CD20+ lymphocytes | CD57+ lymphocyte |
|---|---|---|---|---|---|---|
| Median annual TL change in bp (range in bp) | ||||||
| Androgen-treated | −27 (−678 to +954) | −62 (−343 to +41) | −69 (−581 to +55) | −89 (−384 to +89) | −69 (−387 to +296) | −118 (−1118 to −13) |
| Untreated | −89 (−263 to +1346) | −76 (−465 to +282) | −87 (−413 to + 55) | −52 (−51 to +130) | −79 (−413 to +27) | −62 (−404 to +279) |
| P | .66 | .71 | .96 | .96 | .79 | .32 |
Longitudinal analysis using GEEs, adjusting for age at sample collection and accounting the correlations between multiple measures, showed TL shortening in both groups (mean annual rate of TL attrition = −25 bp/y in treated and −44 bp/y in untreated patients). This difference was not statistically significant (P = .24). Sensitivity analysis performed with the linear random effects mixed model yielded similar results.
To understand the suggested possible lower rate of telomere attrition in treated vs untreated, we repeated the longitudinal TL analysis, restricting the treatment group to patients with >2 time points of TL measured (range 3-5 TL measurements) compared with TL change in the untreated patients. The results showed that the change of TL over time was not statistically significantly different between the 2 groups (P = .08).
Discussion
This longitudinal study measured DC patient TL in leukocyte subsets by flow FISH and found no statistically significant difference in the long-term TL change between androgen-treated DC patients compared with untreated patients. This finding was irrespective of the leukocyte subset analyzed or type of androgen and suggests that the hematologic response to androgens for BMF may not be related to telomere biology effects.
There are several explanations as to why our data do not agree with the prior study reporting TL elongation in 11 of 12 patients treated with danazol for 24 months.14 It is possible that our patient population may have had shorter telomeres and more complex medical problems than those in the danazol study (Table 2). Our study also looked at long-term effects of androgens on TL with androgen treatment of a median of 3 years (range 0.5-15 years), with longer-term follow-up on hematologic response and adverse effects of treatment. Finally, observed differences may also be attributed to different methods of TL measurement in both studies. Here, we used the highly accurate flow FISH assay as compared with qPCR assay in the danazol study. It has been reported that TL by qPCR is highly dependent on DNA extraction methods and other preanalytic factors.19,20 Notably, qPCR has been shown to be less accurate for diagnosing DC than flow FISH.21,22 We also compared the longitudinal TL change in patients treated with androgens to the untreated patients. Although this is not a randomized trial, it offers a comparator group with the same underlying disease biology and similar study referral patterns.
Previously published studies, based upon cross-sectional data,4,16 have demonstrated an “oscillating” nature of intraindividual TL changes and suggested that TL elongation over a short period of time may represent measurement variation or error due to nonlinear TL changes as opposed to the general trend of attrition over longer periods of follow-up.23-25 We observed intra-individual TL variation over time in the study DC patients, but the overall trend with longer follow-up was for shortening in both androgen-treated and untreated patients (Figure 1).
The biological mechanism by which androgens improve blood cell counts in patients with BMF or why they would alter telomere attrition in other populations is not understood. A previously proposed hypothesis is that increased expression of telomerase may be mediated by an estrogen-sensitive element in the telomerase promoter.12,26 However, it should be noted that danazol is not aromatized to estradiol, a conversion that would be required if the mechanism of action was via the estrogen response element in the telomerase promoter.27 A cross-sectional study of 980 men found a positive correlation with between blood testosterone metabolite levels and TL, independent of age, and that aromatase gene polymorphisms were correlated with lower serum estradiol and shorter TL.28 Conversely, a longitudinal interventional study in 40 prostate cancer patients and 25 radiotherapy-matched controls showed that severe and prolonged androgen deprivation therapy did not cause accelerated shortening of telomeres.29 These studies may provide insight into further research on the biologic effects of androgens on sex hormone metabolism and associations with TL change over time.
Of the 5 patients in our study who were treated with danazol, 2 (with DKC1 and TINF2) showed minimal elongation of TL, 1 showed stabilization of TL, and 2 showed shortening of TL. However, the patients treated with danazol had shorter follow-up time on androgens (median 1.4 years, range 0.5-4 years) than the treated patients as a group (median 3 years on androgens, range 0.5-15 years). It is important to note that the patient with DKC1 with a small degree of telomere lengthening developed aggressive tongue cancer leading to death while on danazol. Patients with DC are at very high risk of head and neck squamous cell carcinoma, and this patient may illustrate the natural progression of DC30; however, this case also illustrates the importance of vigilance and long-term follow-up for potential late effects. Extensive longer-term trials and research are required to understand whether there is a differential effect on TL based on specific androgen used, by length of treatment, and importantly, the potential implications of TL effect of androgens (if any) on disease phenotype.
The strengths of this study include long-term longitudinal follow-up of TL of up to 15 years of androgen-treated and untreated patients with DC and the use of flow FISH, the most sensitive and specific clinical TL measurement method. A limitation of our study is that it was not a randomized trial, and androgen therapy was managed by the local physician and not directly by the study team. Hence, TL was not always measured at commencement of androgens. There is also heterogeneity among the treated and untreated patients with respect to age, genotype, and clinical manifestations. However, a strength of this study is that it reflects data from the real-world use of androgens for DC-related BMF over a long period of time. As is often the challenge in rare diseases, this study is limited by sample size. The ideal solution would be to perform a long-term prospective randomized trial within an international research consortium to clearly delineate the effects, if any, of androgen therapy on TL and disease phenotype.
In conclusion, our data show that patients with DC continue to have telomere shortening over time, regardless of treatment with androgens. There could be a small difference in the rate of decline between androgen-treated and untreated patients, but the sample size is too small to make definitive conclusions. Androgens continue to be an effective therapeutic option for BMF associated with DC/TBD. However, their use for management of other medical complications requires further understanding of the biological and clinical effects of androgens on telomeres.
Acknowledgments
The authors thank the patients, their families, and the referring clinicians for their valuable contributions to this study. Lisa Leathwood and Maureen Risch of Westat, Inc provided excellent study support.
This work was supported by the intramural research program of the National Institutes of Health, National Cancer Institute.
Authorship
Contribution: P.P.K. and S.A.S. conceptualized and designed the study; P.P.K. and S.M.G. provided the study analyses; P.P.K., B.P.A., N.G., A.A.B., and S.A.S. collected data; P.P.K., S.M.G., and S.A.S. interpreted the results; and P.P.K., A.A.B., S.M.G., N.G., B.P.A., and S.A.S. wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sharon A. Savage, Clinical Genetics Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, 9609 Medical Center Dr, Room 6E456, Bethesda, MD 20892; e-mail: savagesh@mail.nih.gov.
References
- 1.Ballew BJ, Savage SA. Updates on the biology and management of dyskeratosis congenita and related telomere biology disorders. Expert Rev Hematol. 2013;6(3):327-337. [DOI] [PubMed] [Google Scholar]
- 2.Alter BP, Giri N, Savage SA, et al. Malignancies and survival patterns in the National Cancer Institute inherited bone marrow failure syndromes cohort study. Br J Haematol. 2010;150(2):179-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alter BP, Baerlocher GM, Savage SA, et al. Very short telomere length by flow fluorescence in situ hybridization identifies patients with dyskeratosis congenita. Blood. 2007;110(5):1439-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alter BP, Rosenberg PS, Giri N, Baerlocher GM, Lansdorp PM, Savage SA. Telomere length is associated with disease severity and declines with age in dyskeratosis congenita. Haematologica. 2012;97(3):353-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertuch AA. The molecular genetics of the telomere biology disorders. RNA Biol. 2016;13(8):696-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanley SE, Gable DL, Wagner CL, et al. Loss-of-function mutations in the RNA biogenesis factor NAF1 predispose to pulmonary fibrosis-emphysema. Sci Transl Med. 2016;8(351):351ra107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simon AJ, Lev A, Zhang Y, et al. Mutations in STN1 cause Coats plus syndrome and are associated with genomic and telomere defects. J Exp Med. 2016;213(8):1429-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vulliamy T, Dokal I. Dyskeratosis congenita. Semin Hematol. 2006;43(3):157-166. [DOI] [PubMed] [Google Scholar]
- 9.Khincha PP, Wentzensen IM, Giri N, Alter BP, Savage SA. Response to androgen therapy in patients with dyskeratosis congenita. Br J Haematol. 2014;165(3):349-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shahidi NT. A review of the chemistry, biological action, and clinical applications of anabolic-androgenic steroids. Clin Ther. 2001;23(9):1355-1390. [DOI] [PubMed] [Google Scholar]
- 11.Maggio M, Snyder PJ, Ceda GP, et al. Is the haematopoietic effect of testosterone mediated by erythropoietin? The results of a clinical trial in older men. Andrology. 2013;1(1):24-28. [DOI] [PubMed] [Google Scholar]
- 12.Calado RT, Yewdell WT, Wilkerson KL, et al. Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood. 2009;114(11):2236-2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bär C, Huber N, Beier F, Blasco MA. Therapeutic effect of androgen therapy in a mouse model of aplastic anemia produced by short telomeres. Haematologica. 2015;100(10):1267-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Townsley DM, Dumitriu B, Liu D, et al. Danazol treatment for telomere diseases. N Engl J Med. 2016;374(20):1922-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savage SA, Bertuch AA. The genetics and clinical manifestations of telomere biology disorders. Genet Med. 2010;12(12):753-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baerlocher GM, Vulto I, de Jong G, Lansdorp PM. Flow cytometry and FISH to measure the average length of telomeres (flow FISH). Nat Protoc. 2006;1(5):2365-2376. [DOI] [PubMed] [Google Scholar]
- 17.Burris AM, Ballew BJ, Kentosh JB, et al. Hoyeraal-Hreidarsson syndrome due to PARN mutations: fourteen years of follow-up. Pediatr Neurol. 2016;56:62-68.e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giri N, Pitel PA, Green D, Alter BP. Splenic peliosis and rupture in patients with dyskeratosis congenita on androgens and granulocyte colony-stimulating factor. Br J Haematol. 2007;138(6):815-817. [DOI] [PubMed] [Google Scholar]
- 19.Dagnall CL, Hicks B, Teshome K, et al. Effect of pre-analytic variables on the reproducibility of qPCR relative telomere length measurement. PLoS One. 2017;12(9):e0184098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunningham JM, Johnson RA, Litzelman K, et al. Telomere length varies by DNA extraction method: implications for epidemiologic research. Cancer Epidemiol Biomarkers Prev. 2013;22(11):2047-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gadalla SM, Khincha PP, Katki HA, et al. The limitations of qPCR telomere length measurement in diagnosing dyskeratosis congenita. Mol Genet Genomic Med. 2016;4(4):475-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutierrez-Rodrigues F, Santana-Lemos BA, Scheucher PS, Alves-Paiva RM, Calado RT. Direct comparison of flow-FISH and qPCR as diagnostic tests for telomere length measurement in humans. PLoS One. 2014;9(11):e113747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benetos A, Kark JD, Susser E, et al. Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell. 2013;12(4):615-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verhoeven JE, Lin J, Révész D, Wolkowitz OM, Penninx BW. Unresolved issues in longitudinal telomere length research: response to Susser et al. Am J Psychiatry. 2016;173(11):1147-1149. [DOI] [PubMed] [Google Scholar]
- 25.Chen W, Kimura M, Kim S, et al. Longitudinal versus cross-sectional evaluations of leukocyte telomere length dynamics: age-dependent telomere shortening is the rule. J Gerontol A Biol Sci Med Sci. 2011;66(3):312-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nanni S, Narducci M, Della Pietra L, et al. Signaling through estrogen receptors modulates telomerase activity in human prostate cancer. J Clin Invest. 2002;110(2):219-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grossmann M. Danazol treatment for telomere diseases. N Engl J Med. 2016;375(11):1095-1096. [DOI] [PubMed] [Google Scholar]
- 28.Yeap BB, Knuiman MW, Divitini ML, et al. Epidemiological and Mendelian randomization studies of dihydrotestosterone and estradiol and leukocyte telomere length in men. J Clin Endocrinol Metab. 2016;101(3):1299-1306. [DOI] [PubMed] [Google Scholar]
- 29.Cheung AS, Yeap BB, Hoermann R, Hui J, Beilby JP, Grossmann M. Effects of androgen deprivation therapy on telomere length. Clin Endocrinol (Oxf). 2017;87(4):381-385. [DOI] [PubMed] [Google Scholar]
- 30.Alter BP, Giri N, Savage SA, Rosenberg PS. Cancer in the National Cancer Institute inherited bone marrow failure syndrome cohort after fifteen years of follow-up. Haematologica. 2018;103(1):30-39. [DOI] [PMC free article] [PubMed] [Google Scholar]