Key Points
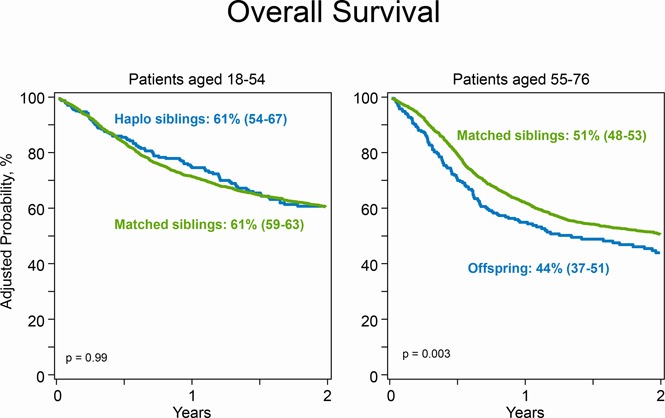
Patient age, 18 to 54 years: comparable survival after transplants from an HLA-matched sibling and a haploidentical sibling.
Patient age, 55 to 76 years: better survival after transplants from an HLA-matched sibling compared with offspring.
Abstract
We sought to identify whether posttransplantation cyclophosphamide (PT-Cy) reduces or eliminates the detrimental impact of HLA mismatching on outcomes of HLA-haploidentical related donor transplantation for acute leukemia. Data from 2143 donor-recipient pairs (n = 218 haploidentical sibling; n = 218 offspring; n = 1707 HLA-matched sibling) with acute myeloid or lymphoblastic leukemia were studied. All received a calcineurin inhibitor for graft-versus-host disease (GVHD) prophylaxis while high-dose PT-Cy was also given to recipients of haploidentical transplant. Patient age correlated with donor-recipient relationship: haploidentical siblings donated to patients aged 18 to 54 years whereas offspring donated to patients aged 55 to 76 years. Therefore, transplant outcomes were examined separately in the 2 patient age groups. In patients aged 18 to 54 years, there were no significant differences in outcomes except chronic GVHD, which was lower after haploidentical sibling compared to HLA-matched sibling transplant (hazard ratio [HR], 0.63; P < .001). In patients aged 55 to 76 years, despite lower chronic GVHD (HR, 0.42; P < .001), graft failure (14% vs 6%; P = .003), nonrelapse mortality (HR, 1.48; P = .02), and overall mortality (HR, 1.32; P = .003) were higher after transplant from offspring compared with an HLA-matched sibling. These data demonstrate a superior outcome in older recipients when using an HLA-matched sibling instead of offspring, although there were differences in transplant platforms (GVHD prophylaxis and graft type) between the 2 groups. Validation of these findings requires a prospective randomized trial wherein the transplant platforms can be closely matched.
Visual Abstract
Introduction
For patients with high-risk, relapsed, or refractory acute leukemia, allogeneic hematopoietic stem cell transplantation sometimes represents the only treatment option with curative potential. The degree of HLA matching between the donor and recipient has been the paramount consideration for donor selection as transplantation from an HLA-matched donor has historically been associated with superior outcomes as compared with transplants from HLA-mismatched donors.1 Recent studies have confirmed the importance of HLA matching at the allele level for unrelated donor transplantation for hematologic malignancy.2,3 Studies on selection of unrelated adult donors have shown that, in addition to donor-recipient HLA-match, the age of the donor impacts survival; for every 10-year increment in donor age there is a 5.5% increase in the hazard ratio (HR) for mortality.4 The advent of high-dose posttransplantation cyclophosphamide (PT-Cy) for graft-versus-host disease (GVHD) prophylaxis has broadened transplantation from donors by reducing the toxicity of transplantation from an HLA-mismatched related donor, especially an HLA-haploidentical related donor.5,6 There are also reports of comparable overall survival after transplantation from an unrelated donor or an HLA-matched sibling compared with transplantation from a haploidentical related donor with the PT-Cy approach for acute leukemia and lymphoma, but the number of haploidentical transplantations was modest in comparison with the other donor sources in those reports implying those reports were unlikely to adequately powered to detect differences between donor groups.7-9 With the increasing use of T-cell replete haploidentical transplantation with PT-Cy for hematologic malignancy in adults, our group recently reported that patient and disease characteristics are more important than either the age of the donor or donor-recipient relationship with regard to survival and GVHD.10
As potential adult transplant candidates may have HLA-mismatched sibling(s) or offspring who are younger than HLA-matched sibling(s), the question we sought to address in the era of PT-Cy for haploidentical transplantation was whether donor age affects transplant outcomes more than histocompatibility. This becomes increasingly more relevant as reduced-intensity conditioning regimens have enabled the transplantation of older patients, most of whom have offspring. Donor selection for those patients often involves choosing between an HLA-matched or -haploidentical sibling of similar age (usually within the same decade), or an offspring, typically 2 to 3 decades younger than the patient.
Methods
Patients
Data on transplantations were obtained from the Center for International Blood and Marrow Transplant and the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplant. Transplant centers report consecutive transplantations at their center with longitudinal follow-up until death or lost to follow-up. Eligible patients were aged 18 years and older with acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL). Patients received unmanipulated bone marrow or peripheral blood from haploidentical sibling or offspring (defined as ≥2 HLA mismatches between donor and recipient) or an HLA-matched sibling. Transplants were performed between 2008 and 2015. Patients provided informed consent for research. The institutional review board of the National Marrow Donor Program approved this study.
End points
The primary end points were leukemia-free survival and overall survival. For leukemia-free survival, death from any cause or relapse were considered events. For overall survival, death from any cause was considered an event. All surviving patients were censored at last follow-up. Secondary end points were acute and chronic GVHD, graft failure, relapse, and nonrelapse mortality. Acute grade II-IV and chronic GVHD were assigned based on reports from transplant centers using standard criteria.11,12 Primary and secondary graft failure were considered as a single outcome. Primary graft failure was defined as failure to achieve an absolute neutrophil count of ≥0.5 × 109/L for 3 consecutive days or <5% donor chimerism (peripheral blood CD3+ or bone marrow).13 Secondary graft failure was defined as initial donor engraftment followed by graft loss, evidenced by a persistent decline in the absolute neutrophil count (<0.5 × 109/L), loss of donor chimerism, or second transplant in patients with documented clinical remission.13 Relapse was defined as disease recurrence (morphologic, cytogenetic, or molecular) or progression. Nonrelapse mortality was defined as death in remission.
Statistical methods
Patient age was correlated with donor-recipient relationship (r = 0.66; P < .001). Exploratory analysis confirmed differences in overall mortality risks between patients aged 18 to 54 years and those aged 55 to 77 years (HR, 1.39; 95% confidence interval [CI], 1.28-1.51; P < .0001). Most patients aged 18 to 54 years received grafts from their haploidentical sibling (218 of 299; 72%; transplants from offspring [n = 81] were excluded) and those aged 55 to 76 years, from their offspring (218 of 279; 78%; n = 61 transplants from haploidentical siblings were excluded).
Recipients of transplants from a haploidentical sibling and offspring (cases) were matched on age, disease, and disease risk index to recipients of transplants from an HLA-matched sibling (controls). Matching was an iterative process, whereby each case was matched to a pool of possible controls on disease and disease risk index, and the control with the smallest age difference was selected. This was done for each case, after which the process started over to add additional controls. The matching process was done until each case had 4 controls, or no further controls could be matched. Ninety-two percent of cases (N = 402) were matched to 4 controls, 7% of cases (N = 31) were matched to 3 controls and the remaining 1% of cases (N = 3) to 2 controls.
Separate multivariate marginal Cox regression models were built to compare the effect of donor type for the 2 patient age groups.14 In patients aged 18 to 54 years, outcomes after haploidentical sibling were compared with that after transplantation from an HLA-matched sibling and in patients aged 55 to 74 years, transplantation from haploidentical offspring to HLA-matched sibling.14 The variable for donor type was held in all steps of model building regardless of level of significance. Other variables tested include patient and donor sex, graft type, conditioning regimen intensity, and transplant period. The incidences of acute and chronic GVHD, nonrelapse mortality, and relapse were calculated using cumulative incidence estimator,15 and the probabilities of leukemia-free and overall survival were calculated from the final Cox model with adjustment for other factors that were associated with these outcomes. All variables tested met the assumptions for proportionality and there were no first-order interactions between the variables for donor type and other variable in the final model. Variables that attained P ≤ .05 were held in the final multivariate model. The cumulative incidences of graft failure at 1 year was calculated using the cumulative incidence estimator to accommodate competing risk.15 All P values are 2-sided and analyses were done using SAS version 9.4 (Cary, NC).
Results
Patient, disease, and transplant characteristics
The characteristics of recipients of haploidentical transplants (cases) matched on age, disease, and disease risk index to recipients of transplants from HLA-matched sibling (controls) are shown in Tables 1 and 2. The median differences in age between cases and controls were 0.08 years (range, 0-9.9) for patients aged 18 to 54 years and 0.05 years (range, 0-9.5) for patients aged 55 to 76 years. For patients aged 18 to 54 years, the median age of haploidentical sibling donors was 39 years compared with 40 years for HLA-matched sibling donors. For patients aged 55 to 76 years, the age of donors differed in that the median age of offspring donors was 34 years compared with 61 years for HLA-matched sibling donors. Although recipients of both donor types were equally likely to receive reduced-intensity conditioning, bone marrow was the predominant graft from haploidentical donors of all donor relationships compared with peripheral blood from HLA-matched siblings. Recipients of haploidentical transplants received a uniform GVHD prophylaxis: PT-Cy with tacrolimus or cyclosporine and mycophenolate. Recipients of transplants from an HLA-matched sibling received tacrolimus or cyclosporine with mycophenolate or methotrexate. As most haploidentical transplants occurred after 2011, all outcomes were censored at 2 years to accommodate differences in follow-up between recipients of haploidentical transplants and transplants from an HLA-matched sibling.
Table 1.
Characteristics for patients 18 to 54 years old
| Characteristics | Haplo sibling, N = 218 | Matched sibling, N = 843 | P |
|---|---|---|---|
| Recipient age, y | |||
| Median (range) | 41 (18-55) | 42 (18-55) | .36 |
| 18-39, n (%) | 102 (47) | 360 (43) | |
| 40-54, n (%) | 116 (53) | 483 (57) | |
| Recipient sex, n (%) | .07 | ||
| Male | 129 (59) | 441 (52) | |
| Female | 89 (41) | 402 (48) | |
| Donor age, y | .14 | ||
| Median (range) | 39 (15-69) | 40 (8-68) | |
| Disease, n (%) | .75 | ||
| AML | 146 (67) | 574 (68) | |
| ALL | 72 (33) | 269 (32) | |
| Disease status, n (%) | .07 | ||
| CR1 | 119 (55) | 533 (63) | |
| ≥CR2 | 48 (22) | 149 (18) | |
| Relapse/primary induction failure | 51 (23) | 161 (19) | |
| Disease risk index, n (%) | .98 | ||
| Low | 8 (3) | 32 (3) | |
| Intermediate | 135 (62) | 534 (63) | |
| High | 71 (33) | 261 (31) | |
| Not reported | 4 (2) | 16 (2) | |
| Graft type, n (%) | <.001 | ||
| Bone marrow | 125 (57) | 99 (12) | |
| Peripheral blood | 93 (43) | 744 (88) | |
| Conditioning intensity, n (%) | <.001 | ||
| Myeloablative | 131 (60) | 704 (84) | |
| Reduced intensity | 87 (40) | 139 (16) | |
| GVHD prophylaxis, n (%) | NA | ||
| CNI + MMF + PT-Cy | 218 (100) | — | |
| CNI + MMF | — | 167 (20) | |
| CNI + MTX | — | 542 (64) | |
| CNI alone | — | 134 (16) | |
| Transplant period, n (%) | <.001 | ||
| 2008-2011 | 52 (24) | 546 (65) | |
| 2012-2015 | 166 (76) | 297 (35) |
CNI, calcineurin inhibitor; CR, complete remission; Haplo, haploidentical; MMF, mycophenolate mofetil; MTX, methotrexate; NA, not applicable.
Table 2.
Characteristics for patients 55 to 76 years old
| Characteristics | Offspring, N = 218 | Matched sibling, N = 864 | P |
|---|---|---|---|
| Recipient age, y | |||
| Median (range) | 63 (55-76) | 63 (55-76) | .40 |
| 55-64, n (%) | 127 (58) | 536 (62) | |
| 65-76, n (%) | 91 (42) | 328 (38) | |
| Recipient sex, n (%) | .80 | ||
| Male | 129 (59) | 803 (58) | |
| Female | 89 (41) | 361 (42) | |
| Donor age, y | <.001 | ||
| Median (range) | 34 (20-55) | 61 (28-105) | |
| Disease, n (%) | .77 | ||
| AML | 192 (88) | 767 (89) | |
| ALL | 26 (12) | 97 (11) | |
| Disease status, n (%) | .05 | ||
| CR1 | 129 (59) | 587 (68) | |
| ≥CR2 | 37 (17) | 115 (13) | |
| Relapse/primary induction failure | 52 (24) | 162 (19) | |
| Disease risk index, n (%) | .99 | ||
| Low | 6 (3) | 24 (3) | |
| Intermediate | 135 (62) | 539 (63) | |
| High | 73 (33) | 285 (31) | |
| Not reported | 4 (2) | 16 (2) | |
| Graft type, n (%) | <.001 | ||
| Bone marrow | 153 (70) | 49 (6) | |
| Peripheral blood | 65 (30) | 815 (94) | |
| Conditioning intensity, n (%) | .87 | ||
| Myeloablative | 60 (28) | 233 (27) | |
| Reduced intensity | 158 (72) | 631 (73) | |
| GVHD prophylaxis, n (%) | NA | ||
| CNI + MMF + PT-Cy | 218 (100) | — | |
| CNI + MMF | — | 324 (38) | |
| CNI + MTX | — | 385 (45) | |
| CNI alone | — | 155 (18) | |
| Transplant period, n (%) | <.001 | ||
| 2008-2011 | 40 (18) | 434 (50) | |
| 2012-2015 | 178 (82) | 430 (50) |
Outcomes in patients aged 18 to 54 years: transplants from a haploidentical sibling vs an HLA-matched sibling
In this cohort, the 2-year incidence of graft failure did not differ significantly between recipients of transplants from a haploidentical sibling or an HLA-matched sibling donor, 6% (95% CI, 3-10) and 6% (95% CI, 5-7), respectively (P = .88). The degree of HLA matching did not have an effect on acute GVHD, as there were no differences in risks for grade II-IV and III-IV acute GVHD after transplantation of grafts from either donor type (Table 3). However, chronic GVHD risks were lower after haploidentical sibling compared with transplant from an HLA-matched sibling (Table 3; Figure 1A). Among patients who developed chronic GVHD, its severity differed by donor type; extensive chronic GVHD was reported in 55% of transplants from a haploidentical sibling compared with 75% of transplants from an HLA-matched sibling (P < .001). There were no differences in nonrelapse mortality, relapse, leukemia-free survival and overall survival (Table 3; Figures 2A and 3A). The predominant causes of death after transplants from a haploidentical sibling or an HLA-matched sibling were recurrent disease (52% vs 43%), GVHD (24% vs 40%), infection (10% vs 8%), and organ failure (7% vs 3%), respectively. To summarize, in patients aged 18 to 54 years, chronic GVHD was lower after transplant from a haploidentical sibling compared with an HLA-matched sibling, but all other outcomes studied were equivalent.
Table 3.
Results of multivariate analysis
| Haploidentical vs HLA-matched sibling | Offspring vs HLA-matched sibling | |||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | Probability, % | HR (95% CI) | P | Probability, % | |
| Acute GVHD II-IV at day 100 | 1.03 (0.78-1.35) | .86 | 32 vs 26 | 0.65 (0.44-0.97) | .034 | 16 vs 23 |
| Acute GVHD III-IV at day 100 | 1.05 (0.64-1.73) | .84 | 12 vs 10 | 0.48 (0.25-0.92) | .028 | 5 vs 9 |
| Chronic GVHD at 2 y | 0.63 (0.48-0.82) | <.001 | 42 vs 51 | 0.42 (0.30-0.58) | <.001 | 28 vs 46 |
| Nonrelapse mortality at 2 y | 1.14 (0.77-1.69) | .52 | 15 vs 13 | 1.48 (1.05-2.09) | .025 | 21 vs 17 |
| Relapse at 2 y | 1.01 (0.80-1.26) | .97 | 37 vs 37 | 1.17 (0.95-1.45) | .14 | 45 vs 41 |
| Leukemia-free survival at 2 y | 1.04 (0.85-1.26) | .70 | 48 vs 50 | 1.27 (1.06-1.51) | .008 | 34 vs 42 |
| Overall survival at 2 y | 1.00 (0.81-1.24) | .99 | 61 vs 61 | 1.32 (1.10-1.59) | .003 | 44 vs 51 |
Bold values represent statistical significance.
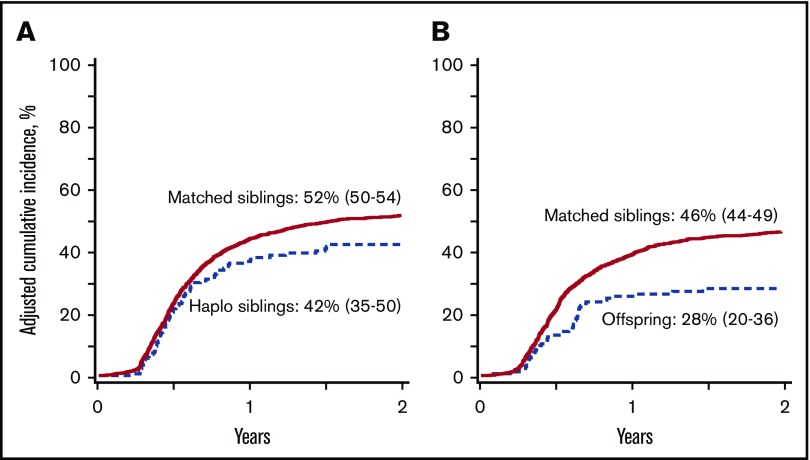
Figure 1.
Chronic GVHD. (A) The 2-year cumulative incidence of chronic GVHD after transplantation of grafts from haploidentical sibling (42%; 95% CI, 35-50) and HLA-matched sibling (52%; 95% CI, 50-54); P < .001. (B) The 2-year cumulative incidence of chronic GVHD after transplantation of grafts from offspring (28%; 95% CI, 20-36) and HLA-matched sibling (46%; 95% CI, 44-49); P < .001.
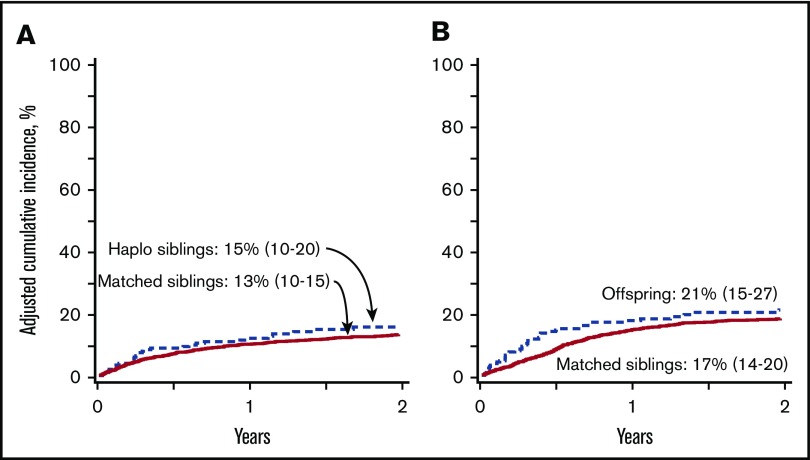
Figure 2.
Nonrelapse mortality. (A) The 2-year cumulative incidence of nonrelapse mortality after transplantation of grafts from haploidentical sibling (15%; 95% CI, 10-20) and HLA-matched sibling (13%; 95% CI, 10-15); P = .52. (B) The 2-year cumulative incidence of nonrelapse mortality after transplantation of grafts from offspring (21%; 95% CI, 15-27) and HLA-matched sibling (17%; 95% CI, 14-20); P = .025.
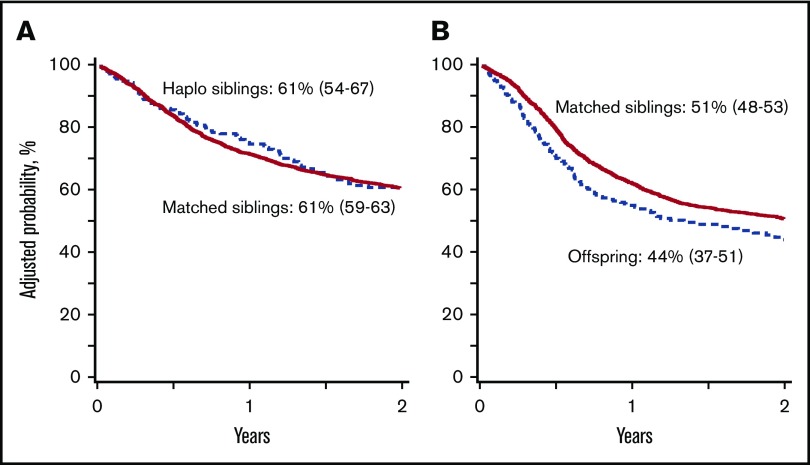
Figure 3.
Overall survival. (A) The 2-year probability of overall survival after transplantation of grafts from haploidentical sibling (61%; 95% CI, 54-67) and HLA-matched sibling (61%; 95% CI, 59-63); P = .99. (B) The 2-year probability of overall survival after transplantation of grafts from offspring (44%; 95% CI, 37-51) and HLA-matched sibling (51%; 95% CI, 48-53); P = .003.
Outcomes in patients aged 55 to 76 years: transplant from a haploidentical sibling vs an HLA-matched sibling
The 2-year graft failure rates were higher after transplant from haploidentical offspring compared with an HLA-matched sibling, 14% (95% CI, 9-18) and 6% (95% CI, 5-8), respectively (P = .003). Among recipients of transplantation from offspring, graft failure did not differ by paternal and maternal recipients (12% vs 16%, respectively). Risks for grade II-IV and III-IV acute and chronic GVHD were lower after transplantation of grafts from haploidentical sibling compared with HLA-matched sibling (Table 3; Figure 1B). There were no differences in severity of chronic GVHD by donor type; 53% of offspring and 61% of HLA-matched sibling transplant recipients reported extensive chronic GVHD (P = .67). Despite lower rates of acute and chronic GVHD, nonrelapse mortality risks were higher after transplantation of grafts from offspring compared with HLA-matched siblings (Table 3; Figure 2B). There were no differences in relapse risks by donor type (Table 3). Leukemia-free survival and overall survival were lower after transplant from haploidentical offspring compared with an HLA-matched sibling (Table 3; Figure 3B). The causes of death did not differ by donor type; predominant causes of death after transplants from offspring or an HLA-matched sibling include recurrent disease (52% vs 45%), GVHD (21% vs 37%), and infection (14% vs 9%), respectively. To summarize, in patients aged 55 to 74 years, acute and chronic GVHD were lower after transplantation of grafts from offspring but higher nonrelapse mortality resulted in lower leukemia-free and overall survival compared with HLA-matched siblings.
Discussion
In this report, we studied the effect of donor-recipient relationship and indirectly, donor age on transplant outcomes in adults with acute leukemia in the setting of related donor transplants. All mismatched related donors (siblings or offspring) were mismatched to their recipients at ≥2 HLA loci. Furthermore, donor-recipient relationship segregated by age in that patients younger than 55 years mostly received grafts from their HLA-matched or haploidentical sibling and those older than 55 years, mostly from their offspring or HLA-matched sibling. The preferential utilization of haploidentical offspring over a haploidentical sibling when a suitable offspring was available raises the possibility that clinicians may be extrapolating the superior outcomes of transplants from younger unrelated donors to the setting of transplantation from a haploidentical related donor.4 Given this skewing in the selection of haploidentical donors, we are unable to study the effects of transplant from an offspring donor in patients aged 18 to 54 years and transplant from a haploidentical sibling donor in patients aged 55 to 74 years. Although the ages of patients and their sibling donors were within the same decade, the offspring were about 3 decades younger than their parents. As patient age is an important predictor of transplant outcomes, we ensured the median difference in age between recipients of transplants from a haploidentical sibling or an HLA-matched sibling was 0.08 years (patient age group 18-54 years) and for recipients of transplants from haploidentical offspring or an HLA-matched sibling, 0.05 years (patient age group 55-74 years).
Our analyses support transplantation of grafts from a haploidentical sibling using the PT-Cy platform for GVHD prophylaxis or an HLA-matched sibling using a calcineurin inhibitor with methotrexate or mycophenolate are acceptable options for patients with acute leukemia aged 18 to 54 years. On the other hand, for patients aged 55 to 74 years with acute leukemia our analyses support transplantation of grafts from an HLA-matched sibling instead of a haploidentical offspring using the PT-CY platform for GVHD prophylaxis. The incorporation of PT-Cy to haploidentical transplantation regimens has eliminated the detrimental impact of HLA mismatching on acute and chronic GVHD.5 Yet, the higher graft failure and mortality demonstrated after transplantation from a haploidentical offspring compared with an HLA-matched sibling imply PT-Cy does not fully overcome the HLA barrier. We hypothesize that in the setting of transplantation from an offspring there may be immune mediated effects of noninherited maternal and paternal antigens contributing to higher mortality.16 A thorough examination of immune mediated effects of noninherited maternal and paternal antigens or the effect of HLA mismatching is beyond the scope of the current study. Even in the setting of transplantation from an adult unrelated donor in which survival is better with younger donors, the age of the donor per se does not overcome the adverse effect of HLA disparity on survival.4
In the current analyses, we matched recipients of haploidentical transplantations to recipients of transplantations from an HLA-matched sibling on age, disease, and disease risk index. We used disease risk index, a composite for disease status at transplantation and cytogenetic risk as a marker for disease severity as others have validated the effectiveness of disease risk index to adjust for disease type and disease severity in the setting of haploidentical transplants and transplantation from an HLA-matched sibling.17,18 In contrast to other reports that have shown a reciprocal relationship between relapse and chronic GVHD we did not observe differences in relapse by donor type despite lower chronic GVHD after haploidentical transplants.19,20 The GVHD prophylaxis strategies differed between donor types and a better comparison would have been one in which recipients of transplantation from an HLA-matched sibling also received PT-Cy in addition to calcineurin inhibitor. Such a comparison is not possible now as relatively few transplants from HLA-matched siblings use PT-Cy with a calcineurin inhibitor for GVHD prophylaxis.
We compared transplant outcomes after transplantation from mismatched and matched related donors using data reported to 2 transplant registries and subject to biases in regards to patient and donor selection for transplantation. We ensured comparability between the treatment arms (ie, donor types) by matching patients in each of the donor groups on patient age, disease and disease risk index17,18 and adjusted for other factors such as performance score, comorbidity index,21 transplant conditioning regimen intensity22-24 and transplant period. Yet there were differences between the donors groups that could not be adjusted for. Bone marrow was used as the graft source more often among recipients of HLA-haploidentical transplants as compared with recipients of transplants from HLA-matched sibling,25 which may account in part for the lower incidence of GVHD in the former group. Further, all haploidentical transplant recipients received PT-Cy for GVHD prophylaxis, which may also account for the lower incidence of GVHD in that group. A more appropriate comparison is one in which both donor types use the same graft and GVHD prophylaxis regimen. Such a comparison is not possible now as transplantation strategies generally follow the accepted practices for matched and mismatched related donors.
Despite these limitations the data support the hypothesis that transplantation from a haploidentical sibling results in comparable leukemia-free and overall survival to that after transplantation from an HLA-matched sibling in patients aged 18 to 54 years. Therefore, in clinical practice a haploidentical sibling may be considered instead of an HLA-matched unrelated donor when a HLA-matched sibling is not available. In regards to older patients (≥55 years) their offspring served as haploidentical donors and the data showed higher nonrelapse mortality and lower overall survival compared with transplantation from an HLA-matched sibling. Therefore, in clinical practice, an HLA-matched sibling regardless of their age is better suited to serve as the donor. Randomized clinical trials offer the highest quality data for modifying clinical practice and provide unbiased allocation to the treatment arms being studied. Yet it is challenging to conduct a randomized trial as less than a third of patients have a suitable HLA-matched sibling and that would incur a lengthy accrual period. A prospective trial in which patients are assigned to donor type according to the availability of an HLA-matched sibling would accrue faster but is not entirely free from bias. In summary, the PT-Cy platform has eliminated the detrimental impact of HLA mismatching for adults with acute leukemia transplants when the mismatched relative is a sibling. Yet, when the relative is an offspring, transplantation from an HLA-matched sibling offers better survival. These results may differ if the transplant platforms are similar.
Acknowledgments
The Center for International Blood and Marrow Transplant Research was supported primarily by Public Health Service Grant/Cooperative Agreement 5U24-CA076518 from the National Institutes of Health, National Cancer Institute, National Heart, Lung, and Blood Institute, and National Institute of Allergy and Infectious Diseases; 5U10HL069294 from the National Heart, Lung, and Blood Institute and the National Cancer Institute; contract HHSH250201200016C with the Health Resources and Services Administration/Department of Health and Human Services; and grants N00014-15-1-0848 and N00014-16-1-2020 from the Office of Naval Research.
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration, or any other agency of the US government.
Authorship
Contribution: T.M.R., E.J.F., M.-J.Z., A.S.M., M.L., A.N., and M.E. designed the study; M.L., A.S.M., and D.A.K. prepared the study file for analyses; M.-J.Z. and A.S.M. analyzed the data; T.M.R., M.-J.Z., A.S.M., D.A.K., E.J.F., M.L., A.N., and M.E. summarized and interpreted the findings; T.M.R. and E.J.F. drafted the manuscript; M.-J.Z., A.S.M., M.L., D.A.K., D.B., A.B., J.-H.B., F.C., S.O.C., S.M.D., M.M., S.R.M., N.M., I.K.M., V.R., R.R., G.S., I.Y.-A., R.J.S., M.E., and A.N. critically reviewed and edited the manuscript; and all authors approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mary Eapen, Center for International Blood and Marrow Transplant, Department of Medicine, Medical College of Wisconsin, 8701 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: meapen@mcw.edu.
References
- 1.Szydlo R, Goldman JM, Klein JP, et al. . Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. J Clin Oncol. 1997;15(5):1767-1777. [DOI] [PubMed] [Google Scholar]
- 2.Lee SJ, Klein J, Haagenson M, et al. . High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110(13):4576-4583. [DOI] [PubMed] [Google Scholar]
- 3.Pidala J, Lee SJ, Ahn KW, et al. . Nonpermissive HLA-DPB1 mismatch increases mortality after myeloablative unrelated allogeneic hematopoietic cell transplantation. Blood. 2014;124(16):2596-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kollman C, Spellman SR, Zhang M-J, et al. . The effect of donor characteristics on survival after unrelated donor transplantation for hematologic malignancy. Blood. 2016;127(2):260-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kasamon YL, Luznik L, Leffell MS, et al. . Nonmyeloablative HLA-haploidentical bone marrow transplantation with high-dose posttransplantation cyclophosphamide: effect of HLA disparity on outcome. Biol Blood Marrow Transplant. 2010;16(4):482-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasamon YL, Bolaños-Meade J, Prince GT, et al. . Outcomes of nonmyeloablative HLA-haploidentical blood or marrow transplantation with high-dose post-transplantation cyclophosphamide in older adults. J Clin Oncol. 2015;33(28):3152-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciurea SO, Zhang M-J, Bacigalupo AA, et al. . Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126(8):1033-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh N, Karmali R, Rocha V, et al. . Reduced-intensity transplantation for lymphomas using haploidentical related donors versus HLA-matched sibling donors: a Center for International Blood and Marrow Transplant Research analysis. J Clin Oncol. 2016;34(26):3141-3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanate AS, Mussetti A, Kharfan-Dabaja MA, et al. . Reduced-intensity transplantation for lymphomas using haploidentical related donors vs HLA-matched unrelated donors. Blood. 2016;127(7):938-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCurdy SR, Zhang MJ, St Martin A, et al. . Effect of donor characteristics on haploidentical transplantation with posttransplantation cyclophosphamide. Blood Adv. 2018;2(3):299-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Przepiorka D, Weisdorf D, Martin P, et al. . 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825-828. [PubMed] [Google Scholar]
- 12.Shulman HM, Sullivan KM, Weiden PL, et al. . Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204-217. [DOI] [PubMed] [Google Scholar]
- 13.Olsson R, Remberger M, Schaffer M, et al. . Graft failure in the modern era of allogeneic hematopoietic SCT [published correction appears in Bone Marrow Transplant. 2013;48(4):616]. Bone Marrow Transplant. 2013;48(4):537-543. [DOI] [PubMed] [Google Scholar]
- 14.Cox DR. Regression models and life-tables. J R Stat Soc Series B Stat Methodol. 1972;34:187-220. [Google Scholar]
- 15.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16(8):901-910. [DOI] [PubMed] [Google Scholar]
- 16.van Rood JJ, Loberiza FR Jr, Zhang M-J, et al. . Effect of tolerance to noninherited maternal antigens on the occurrence of graft-versus-host disease after bone marrow transplantation from a parent or an HLA-haploidentical sibling. Blood. 2002;99(5):1572-1577. [DOI] [PubMed] [Google Scholar]
- 17.Armand P, Kim HT, Logan BR, et al. . Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664-3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCurdy SR, Kanakry JA, Showel MM, et al. . Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood. 2015;125(19):3024-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nordlander A, Mattsson J, Ringdén O, et al. . Graft-versus-host disease is associated with a lower relapse incidence after hematopoietic stem cell transplantation in patients with acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2004;10(3):195-203. [DOI] [PubMed] [Google Scholar]
- 20.Signori A, Crocchiolo R, Oneto R, et al. . Chronic GVHD is associated with lower relapse risk irrespective of stem cell source among patients receiving transplantation from unrelated donors. Bone Marrow Transplant. 2012;47(11):1474-1478. [DOI] [PubMed] [Google Scholar]
- 21.Sorror ML, Maris MB, Storb R, et al. . Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott BL, Pasquini MC, Logan BR, et al. . Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol. 2017;35(11):1154-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marks DI, Wang T, Pérez WS, et al. . The outcome of full-intensity and reduced-intensity conditioning matched sibling or unrelated donor transplantation in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia in first and second complete remission. Blood. 2010;116(3):366-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bachanova V, Marks DI, Zhang MJ, et al. . Ph+ ALL patients in first complete remission have similar survival after reduced intensity and myeloablative allogeneic transplantation: impact of tyrosine kinase inhibitor and minimal residual disease. Leukemia. 2014;28(3):658-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bashey A, Zhang M-J, McCurdy SR, et al. . Mobilized peripheral blood stem cells versus unstimulated bone marrow as a graft source for T-cell replete haploidentical donor transplantation using post-transplant cyclophosphamide. J Clin Oncol. 2017;35(26):3002-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]