Abstract
Neurogenesis in the healthy adult murine brain is based on proliferation and integration of stem/progenitor cells and is thought to be restricted to 2 neurogenic niches: the subventricular zone and the dentate gyrus. Intriguingly, cells expressing the immature neuronal marker doublecortin (DCX) and the polysialylated-neural cell adhesion molecule reside in layer II of the piriform cortex. Apparently, these cells progressively disappear along the course of ageing, while their fate and function remain unclear. Using DCX-CreERT2/Flox-EGFP transgenic mice, we demonstrate that these immature neurons located in the murine piriform cortex do not vanish in the course of aging, but progressively resume their maturation into glutamatergic (TBR1+, CaMKII+) neurons. We provide evidence for a putative functional integration of these newly differentiated neurons as indicated by the increase in perisomatic puncta expressing synaptic markers, the development of complex apical dendrites decorated with numerous spines and the appearance of an axonal initial segment. Since immature neurons found in layer II of the piriform cortex are generated prenatally and devoid of proliferative capacity in the postnatal cortex, the gradual maturation and integration of these cells outside of the canonical neurogenic niches implies that they represent a valuable, but nonrenewable reservoir for cortical plasticity.
Keywords: doublecortin, integration, maturation, neuroplasticity, piriform cortex, PSA-NCAM
Introduction
Inducible cortical neurogenesis outside the canonical neurogenic niches of the adult rodent brain can be detected in lesions, such as stroke, and mostly depends on the migration of neuroblasts from the neurogenic regions towards the lesion site (Arvidsson et al. 2002; Jin et al. 2003). However, some studies suggested the presence of immature and/or adult-born neurons outside of the canonical neurogenic regions even under physiological conditions (Dayer et al. 2005; Takemura 2005; Gould 2007; Luzzati et al. 2009; Shapiro et al. 2009). The piriform cortex is one of these structures where immature neurons have been reported, although no local proliferative neuroblasts have been detected under physiological conditions (Gómez-Climent et al. 2008; Luzzati et al. 2009; Klempin et al. 2011; Yang et al. 2015; Rubio et al. 2016). Whether these brain regions in which immature neurons have been detected support the events that define adult neurogenesis, that is, proliferation, differentiation, maturation, and network-integration, is currently under debate (Bonfanti and Peretto 2011; Bonfanti and Nácher 2012; Feliciano and Bordey 2013; Ernst et al. 2014; Wang et al. 2014; Feliciano et al. 2015).
In adult mice and rats, immature neurons are mainly restricted to layer II of the paleocortex. However, a more extensive distribution of immature neurons residing in this cortical layer, including the neocortex, has been reported in mammals with larger cerebral cortices, for example, in guinea pigs, rabbits, cats, nonhuman-primates, and humans, (Ní Dhúill et al. 1999; Bonfanti 2006; Gómez-Climent et al. 2008; Luzzati et al. 2009; Zhang, Cai, et al. 2009; Varea et al. 2011; De Nevi et al. 2013; He et al. 2014; Patzke et al. 2014; Rubio et al. 2016), reviewed in (König et al. 2016).
The majority of the immature neurons detected in layer II of the piriform cortex of mice, rats, guinea pigs, cats, and most likely in all mammals, appears to have been generated prenatally (Gómez-Climent et al. 2008; Varea et al. 2011; Bonfanti and Nácher 2012; Yang et al. 2015; König et al. 2016; Rubio et al. 2016). Nevertheless, some studies have suggested that immature neurons of the piriform cortex can also originate from progenitors generated in the SVZ of young mammals that migrate towards the piriform cortex and integrate locally (Bernier et al. 2002; Pekcec et al. 2006; Shapiro et al. 2007, 2009). Other investigations reported that a local cell population expressing neural/glial antigen 2 (NG2) with low level of doublecortin (DCX) (Tamura et al. 2007; Rivers et al. 2008; Guo et al. 2010) or progenitors residing in layer I (Xiong et al. 2010) are the origin of the immature neurons detected in the piriform cortex layer II. However, these findings could not be reproduced by other studies (Nácher et al. 2002; Gómez-Climent et al. 2008; Luzzati et al. 2009; Varea et al. 2011), which suggests that, at best, these sources of immature neurons might only contribute at a very low level or have a transient existence (Pekcec et al. 2006; Shapiro et al. 2007; 2009).
Intriguingly, 2 subpopulations of immature neurons with distinct cell morphologies have been detected in layer II of the adult piriform cortex (Gómez-Climent et al. 2008; Rubio et al. 2016). The first population represents so-called “tangled cells”, which are endowed with a small diameter (~9 μm) and a few short intricate dendrites. These small cells strongly express DCX, the polysialylated neural cell adhesion molecule (PSA-NCAM) and the neuron-specific class III beta-tubulin (TuJ-1), which are classical markers found in immature neurons (Seki and Arai 1991; Bonfanti et al. 1992; Nàcher et al. 2001; Nácher et al. 2002; Brown et al. 2003; Couillard-Despres et al. 2005; Gómez-Climent et al. 2008; Luzzati et al. 2009). In contrast, tangled cells do not express markers of stem cells (nestin), mature neurons (e.g., NeuN, CaMKII, GAD67), oligodendrocytes (RIP), or astrocytes (GFAP) (Gómez-Climent et al. 2008; Rubio et al. 2016). Tangled cells appear to be inactive according to the lack of expression of functionality markers like c-Fos or the activity-regulated cytoskeleton-associated protein (Arc) (Gómez-Climent et al. 2008; Carceller et al. 2016) and due to the ensheathment of their soma by astrocytic end feet (Gómez-Climent et al. 2008).
The second group of immature neuronal cells residing in layer II of the piriform cortex displays a larger soma than tangled cells, that is, a diameter of ~15 μm, and a rather elaborated dendritic tree decorated with some dendritic spines. These larger immature neurons express DCX and also PSA-NCAM, albeit at a lower intensity than observed in tangled cells. Furthermore, the occasional low expression of NeuN could be detected as well (Gómez-Climent et al. 2008; Rubio et al. 2016). These larger cells have the typical morphology of semilunar–pyramidal transitional neurons, a common population of excitatory neurons found in the piriform cortex layer II. These cells will be thereafter referred to as “complex cells.”
Complex cells with morphology between those of tangled cells and semilunar–pyramidal transitional neurons are found in layer II as well. Since tangled cells appear more immature than semilunar–pyramidal transitional neurons, it has been hypothesized that the former progressively differentiate into semilunar–pyramidal neurons. Considering that both subpopulations of immature neurons express the transcription factor Tbr1, which is exclusively expressed by pallial-derived excitatory neurons (Gómez-Climent et al. 2008; Luzzati et al. 2009; Varea et al. 2011; Rubio et al. 2016), we hypothesized that tangled cells could generate immature complex cells, which turn into glutamatergic pyramidal neurons upon maturation.
In rats (Gómez-Climent et al. 2008; Varea et al. 2009), dogs (De Nevi et al. 2013), guinea pigs (Xiong et al. 2008), and nonhuman primates (Cai et al. 2009; Zhang, Cai, et al. 2009), the immature cortical neurons gradually vanished with age. Two hypotheses have been formulated to explain the disappearance of the immature neurons in the cortical layer II (Abrous et al. 1997; Varea et al. 2009; Gómez-Climent et al. 2010). On the one hand, the immature neurons may simply die due to their lack of functional integration. However, no evidence of augmented cell death has been observed in structures bearing the immature neuron populations (Bonfanti and Nácher 2012). On the other hand, the immature neurons in layer II may remain in a dormant or standby stage until they resume their delayed final maturation step and become functionally integrated neurons. The latter has been supported by recent immunohistochemical (Gómez-Climent et al. 2008; Luzzati et al. 2009; Rubio et al. 2016) and electrophysiological analyses (Klempin et al. 2011) of cells expressing immature neuronal markers in adult cortical layer II.
Until now, the lack of an adequate fate-mapping tool precluded the demonstration that immature neurons residing in the adult piriform cortex can differentiate and incorporate as principal neurons. To this end, we made use of an inducible transgenic mouse model in which DCX-expressing cells can be permanently labeled following tamoxifen administration and monitored with a green fluorescent protein (GFP) reporter (short DCX-CreERT2/Flox-EGFP mouse) (Zhang et al. 2010). With this approach, we have been able to show that immature neurons residing in layer II of the mouse adult piriform cortex remain alive in the course of ageing, developing the morphological requirements for synaptic input and action potential output (i.e., synapses and axonal initial segments). Taken together, our observations suggest the gradual integration of the immature neurons of the piriform cortex into the surrounding network as local principal neurons.
Material and Methods
Animals
Transgenic DCX-CreERT2/Flox-EGFP mice were used to label and follow the fate of neuronal precursor cells (Zhang et al. 2010). All experiments were performed in accordance with the guidelines of the “Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes” and were approved by the national animal care authorities.
Induction of the GFP Reporter Gene in DCX-Expressing Cells
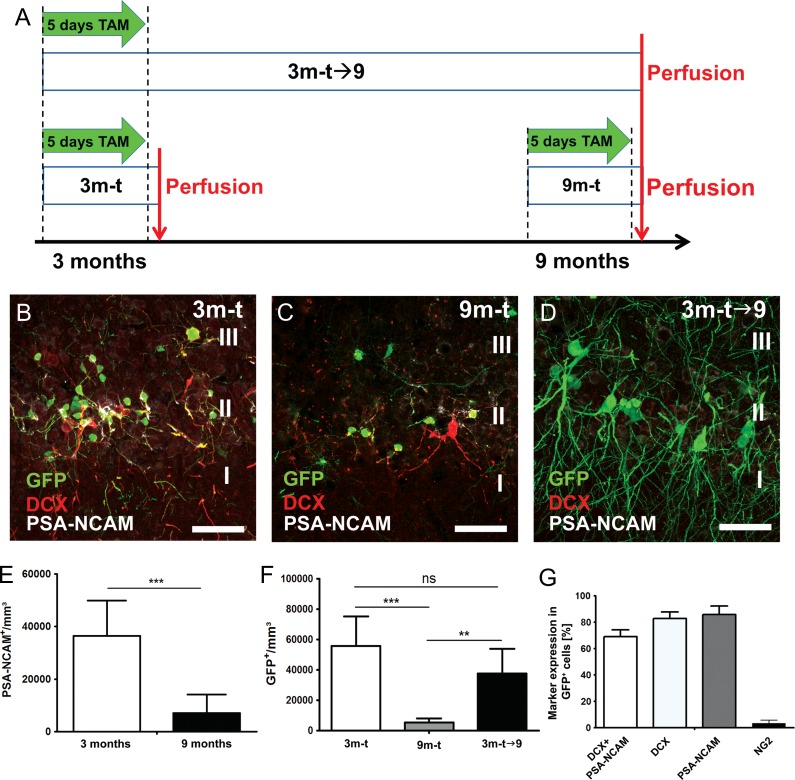
Mice were divided into 3 experimental groups and tamoxifen (100 mg/kg of bodyweight dissolved in corn oil, Sigma-Aldrich) was administered orally by gavage once daily for 5 consecutive days (days 1–5) (Fig. 1A). To study the fate and distribution of GFP+ cells over time, mice of group 1 received tamoxifen at the age of 3 months and were sacrificed on day 8 following the first tamoxifen administration (3m-t, n = 7). Mice from group 2 received tamoxifen at 9 months of age and were sacrificed on day 8 after the first administration (9m-t, n = 5). Finally, mice of group 3 received tamoxifen at 3 months of age and were sacrificed 6 months later at the age of 9 months (3m-t→9, n = 8).
Figure 1.
Overview of the experimental groups and detection of immature neurons in the piriform cortex. (A) DCX-CreERT2/Flox-EGFP transgenic mice were split into 3 experimental groups and administered daily with tamoxifen for 5 consecutive days. First and second group received tamoxifen with 3 months (3m-t) or 9 months (9m-t) of age and were sacrificed on day 8 following administration. The third group (3m-t→9) received tamoxifen with 3 months of age and was perfused with 9 months of age. Numerous DCX / PSA-NCAM-expressing cells (red) could be detected in layer II of the piriform cortex in 3-month-old mice (B) and to lesser extent at 9 months of age (C and D). The majority of cells expressing GFP (green) coexpressed PSA-NCAM (white) or DCX (red) in mice sacrificed shortly after tamoxifen administration (B and C). Expression of immature markers in GFP+ was seldom detected in the 3m-t→9 group (D). Density of cells expressing PSA-NCAM (E) and GFP (F) in the layer II of the piriform cortex. (G) Coexpression of markers for immature neurons and NG2 in GFP+ cells of the 3m-t group., Scale bars = 50 μm.
To scrutinize whether tangled cells in the piriform cortex proliferate in the adult brain or remain postmitotic, mice (n = 5) were injected intraperitoneally with BrdU (50 mg/kg bodyweight) at the age of 3 months once daily for 5 consecutive days. Simultaneously, mice also received standard oral application of tamoxifen (100 mg/kg bodyweight). Mice were sacrificed on day 8 following the first BrdU administration and the brains were further processed for immunohistochemistry. In a second group, pregnant mice received BrdU (50 mg/kg bodyweight) to label the brain of developing fetuses at embryonic age E14 and E15 (estimated by plug-check of the mother). The progeny (n = 5) of these pregnant mice received the standard oral application of tamoxifen (100 mg/kg bodyweight) as for the 3m-t group and sacrificed on day 8. Moreover, possible leakage of the system resulting in the activation of the EGFP reporter expression in the absence of tamoxifen was addressed in 2-year-old DCX-CreERT2/Flox-EGFP naive mice (n = 2) which where compared with 2-year-old transgenic mice treated with tamoxifen (100 mg/kg bodyweight daily for 5 consecutive days) at the age of 3 months (n = 2).
Immunohistochemistry and Image Analysis
For immunohistochemistry, mice were transcardially perfused with 0.9% NaCl for 5 min followed by 0.1 M phosphate buffered 4% paraformaldehyde pH 7.4 for 10 min. Brains were dissected and postfixed in the same paraformaldehyde solution overnight at 4 °C and then transferred in 0.1 M phosphate buffered 30% sucrose solution pH 7.4 at 4 °C for at least 48 h. Brains were cut in 40 μm sagittal sections using a sliding microtome (Leica) on dry ice and sections were stored at −20 °C until further processing in cryoprotectant (25% v/v glycerol, 0.05 M sodium phosphate buffer pH 7.4, 25% v/v ethylene glycol).
Following antigen-retrieval (citrate buffer pH 6.0 [Sigma-Aldrich], 10 min at 100 °C), fluorescent immunohistological analyses were performed as previously described (Couillard-Despres et al. 2005; Rubio et al. 2016). Antibodies: rat anti-BrdU (Bio-Rad AbD Serotec) 1:500; mouse anti-CaMKII (Abcam) 1:500; goat anti-ChAT (Novus Biologicals) 1:100; rabbit anti-DCX (Cell Signaling Technology) 1:300; mouse anti-GAD67 (Millipore) 1:500; guinea pig anti-GFAP (Progen) 1:500; chicken anti-GFP (Invitrogen) 1:500; guinea pig anti-NeuN (Millipore) 1:500; rabbit anti-NG2 (Millipore) 1:200; mouse anti-PSA-NCAM (Millipore) 1:1000; rabbit anti- βIV-spectrin (selfmade) (Schlüter et al. 2017) 1:500; goat anti-Sox2 (Santa Cruz Biotechnology) 1:1000; mouse anti-synaptophysin (Sigma Aldrich) 1:500; rabbit anti-Tbr1 (Abcam) 1:500; rabbit anti-VGAT (Synaptic Systems) 1:500. Fluorescence images were acquired using a LSM 710 confocal microscope and ZEN 2011 Black Software (Carl Zeiss) and a TSC SPE confocal microscope (Leica). Z-stacks were acquired over the whole thickness of the section and co-localization was confirmed by the analysis of successive optical slices. For image-analysis, ImageJ Software 1.46r (National Institutes of Health) and FIJI based on ImageJ 1.50a (Schindelin et al. 2012) were used.
The analysis of the marker profile and fate of GFP+ cells involved 50 GFP+ cells in layer II of the piriform cortex per mouse and staining and were analyzed for double- and triple-labeling with cell type-specific antibodies.
The leakiness of the system in the absence of tamoxifen was evaluated based on the density of GFP-expressing cells in 2-year-old naive transgenic mice, compared with the density observed in mice induced at 3 months of age and perfused at 2 years. The density of GFP-expressing cells in the layer II of the piriform cortex was assessed in 10 randomly chosen fields of view per mice acquired with a ×20 objective (Z-stack step size = 1 μm).
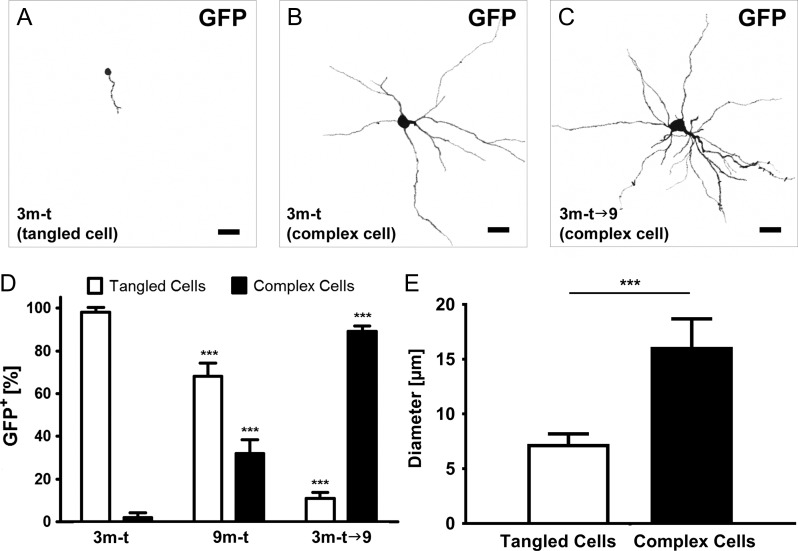
Cell morphometric analysis was performed using confocal microscopy with a ×63 oil immersion objective and z-series of optical sections (0.2 μm step size). The soma diameter of GFP-expressing cells was measured in 10 randomly chosen cells of each type from 5 mice per group. The confocal stacks were then processed using FIJI to render 3D reconstructions (Fig. 2A–C).
Figure 2.
Maturation of immature neurons in the layer II of the piriform cortex. According to a 3D reconstruction, the majority of GFP+ cells in group 3m-t exhibited a tangled cell morphology (A), whereas a smaller population could be classified as semilunar–pyramidal transitional neuron (B). When cells were given 6 months to mature (group 3m-t→9), cells morphologically increased in complexity (C). Scale bars for A–C: 15 μm. (D) In group 3m-t, the majority of GFP+ cells could be morphologically classified as tangled cells and only a small fraction showed a more complex morphology. The tangled cell population decreased significantly in group 9m-t and 3m-t→9, while cells gained a more complex morphology. (E) Mean diameter of the soma of tangled cells in the 3m-t group in comparison to cells with a complex morphology of the 3m-t→9.
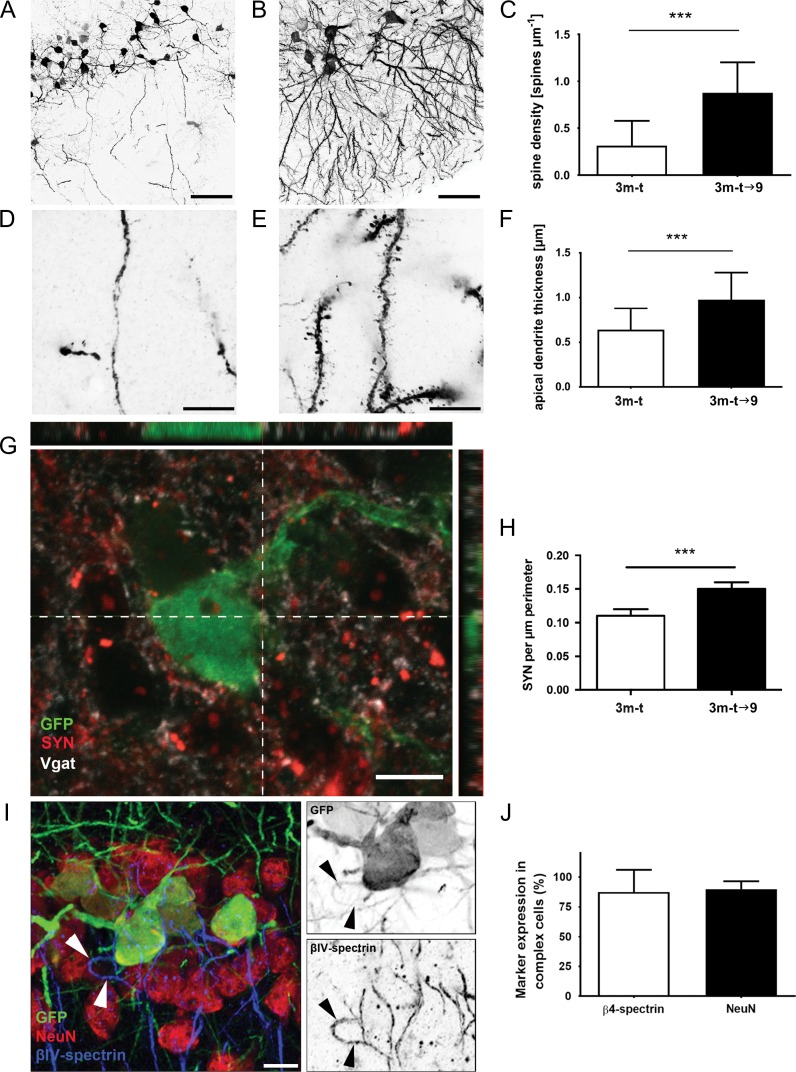
Spine density on the apical dendrite of GFP-expressing neurons and the density of immunoreactive puncta in their perisomatic region were analyzed as described previously (Guirado et al. 2014). For the analysis of the spine density, a ×63 oil immersion objective with an additional ×3.5 digital zoom and a 0.8 μm Z-step size was used. Five randomly chosen apical dendrites of GFP-positive neurons within the piriform cortex layer II of 5 mice per group were analyzed (n = 25). Selected dendrites from GFP-positive neurons had to meet the following criteria: measure at least 150 μm from the soma and not be intersected by another dendrite along their trajectory. Apical dendrite thickness was also measured at a distance of 120 μm from the cell soma.
For the analyses of perisomatic puncta containing synaptophysin (SYN) and vesicular GABA transporter (VGAT) apposed to GFP+ neurons, micrographs were acquired using a ×63 oil objective. From each animal, 5 GFP+ expressing cells from piriform cortex layer II were randomly selected. Z-series of optical sections covering all 3D extensions of the somata were acquired using sequential scanning mode (Z-step size, 0.5 μm). Stacks were processed with Fiji software. The profile of every soma was delimited manually and puncta placed within a range of 0.5 μm from the edge of this profile were analyzed. SYN and VGAT containing puncta were defined as having an area larger than 0.15 μm2 and smaller than 2.5 μm2. Puncta linear density values were determined and expressed as number of puncta per micron of soma perimeter.
Micrographs of the axonal initial segment were acquired using a ×63 oil objective (Z-series step size 1 μm). Analysis of NeuN and βIV-spectrin coexpression was performed in at least 100 GFP-expressing cells per transgenic mouse.
GraphPad Prism 5 (GraphPad Software Inc.) with 2-tailed one-way ANOVA and Bonferroni post hoc test or a 2-tailed unpaired t-test was used for statistical analyses. The Mann Whitney test was used to analyze the proportion of complex cells carrying an axon initial segment. Graphs show mean values with standard deviation as error bars. Significance: P > 0.05 ns, P < 0.05*, P < 0.01**, P < 0.001***.
Results
DCX-CreERT2/Flox-EGFP transgenic mice were divided into 3 experimental groups and either received tamoxifen at 3 or 9 months of age and were perfused one week later (3m-t and 9m-t), or received tamoxifen at 3 months of age and were perfused 6 months later at the age of 9 months (3m-t→9) (see Material and Methods and Fig. 1A). We confirmed that the density of immature neurons expressing PSA-NCAM in the piriform cortex layer II of these mice (Fig. 1B–D, Supplementary Fig. S1) decreased in the course of aging as previously reported (Varea et al. 2009). Indeed, the density of PSA-NCAM+ cells decreased significantly from 36 443 ± 13 468 cells/mm3 in the 3-month-old transgenic mice to 7049 ± 7036 cells/mm3 in the 9-month-old mice (two-tailed t-test, P = 0.0002***) (Fig. 1E). The density of GFP+ cells in layer II of the piriform cortex was thereafter determined in the 3 experimental groups (Fig. 1B–D, Supplementary Fig. S1). Similar to the PSA-NCAM+ cell population, the density of GFP+ cells decreased significantly in 9-month-old mice when compared with 3-month-old (3m-t: 55 829 ± 19 444 cells/mm3 vs. 9m-t: 5405 ± 2516 cells/mm3, one-way ANOVA, P = 0.0002***) (Fig. 1F).
The overwhelming majority of GFP+ cells expressed markers of immature neurons when animals were sacrificed 1 week after tamoxifen administration, both at 3 months (PSA-NCAM+ 85.7 ± 2.7%, DCX+ 82.7 ± 2.0%, DCX+/PSA-NCAM+ 69.0 ± 2.1%) and at 9 months (PSA-NCAM+ 63.1 ± 8.9%, DCX+ 77.8 ± 3.3%, DCX+/PSA-NCAM+ 58.1 ± 8.6%) (Fig. 1B,C,G). These results indicated that shortly after tamoxifen induction at 3 months or 9 months of age, most GFP+ cells in the piriform cortex layer II of our transgenic mice were immature neurons. BrdU was also injected in 2 different groups of transgenic mice, either at embryonic age E14–15 or at the age of 3 months, to label proliferating cells. Tamoxifen induction took place at 3 months of age in both groups. Overlap between BrdU and GFP-expression could be detected in the piriform cortex layer II only in mice that were labeled with BrdU at E14–15, but not in mice that received BrdU at the age of 3 months, thus confirming the postmitotic nature of the tangled cells in the adult brain (Supplementary Fig. S2). In contrast, numerous GFP-expressing cells had integrated BrdU in the dentate gyrus, a proliferative neurogenic niche of the adult mouse brain (Supplementary Fig. S2).
The number of GFP+ cells coexpressing markers of immature neurons decreased dramatically when the animals were sacrificed 6 months after tamoxifen administration (3m-t→9) (PSA-NCAM+ 4.7 ± 1.7%, P < 0.0001***; DCX+ 6.0 ± 1.4%, P < 0.0001***; DCX+/PSA-NCAM+ 3.25 ± 1%, P < 0.0001***) (Fig. 1D, Supplementary Fig. S1). In contrast, a nonsignificant reduction of the density of GFP-expressing cells was detected between the mice that were treated with tamoxifen at 3 months of age and sacrificed after one week and the mice that were sacrificed 6 months later (3m-t: 55 829 ± 19 444 cells/mm3 vs. 3m-t→9:37 562 ± 16 173 cells/mm3) (Fig. 1F). While it cannot be excluded that some tangled cells died along ageing, the large number of surviving cells expressing GFP in old mice and the prominent decline in expression of immature markers indicate that most of the GFP+ cells, which were expressing DCX and/or PSA-NCAM in the piriform cortex at 3 months of age, had survived for 6 months and by then were no longer immature.
The distribution of GFP signal within expressing cells allows for a detailed morphological analysis (Fig. 2A–C). Mice of the 3m-t group exhibited a high proportion of small GFP+ cells in layer II of the piriform cortex (98.0 ± 2.3% of GFP+ cells; mean soma diameter 7.3 ± 0.9 μm), displaying a very low number of dendrites, and were therefore classified as tangled cells (Fig. 2A,D,E). Very few larger and morphologically complex cells could be detected (2.0 ± 2.3% of GFP+ cells; mean soma diameter 14.6 ± 2.1 μm), which were classified as semilunar–pyramidal transitional neurons or intermediate cell types (Fig. 2B). By contrast, in the 9m-t group, the proportion of tangled cells significantly decreased to 68.0 ± 6.3% of GFP+ cells (P < 0.0001***), whereas the percentage of GFP+ complex cells increased to 32.0 ± 6.3% of all GFP+ cells (P < 0.0001***) (Fig. 2D). Six months following tamoxifen administration (3m-t→9 group), GFP-expressing cells were densely intermingled and most of them exhibited a complex morphology (tangled cells 11.0 ± 2.8% vs. complex cells 89.0 ± 2.8%) (Fig. 2D). In comparison to the tangled cells of the 3m-t group, complex cells of the 3m-t→9 group had remarkably larger cell soma (P < 0.001) with average diameter equal to 16.1 ± 2.5 μm (Fig. 2E, Supplementary Table S1). These data indicate a progressive development from small tangled cells to complex cells resembling semilunar–pyramidal transitional neurons over time.
To further monitor this progressive maturation, we measured and compared the thickness of the principal apical dendrite of GFP+ cells at a distance of 120 μm from the soma (Fig. 3A–C). Since tangled cells only have few stunted nonspinous dendrites, we compared the few complex GFP+ cells in the 3m-t group with the complex GFP+ cells found in the 3m-t→9 group. The apical dendrite thickness increased significantly in the 3m-t→9 group and reached 0.98 ± 0.30 μm as compared with 0.64 ± 0.24 μm in the 3m-t group (two-tailed t-test, P < 0.0001***). This observation regarding apical dendrite thickness further indicates a maturation from the GFP+ tangled cell morphology into GFP+ semilunar–pyramidal transitional neurons with increasing complexity over the follow-up period of 6 months.
Figure 3.
Morphological maturation of synapses, dendrites and axons over time. (A–C) Maturation of dendrites from GFP+ cells projecting in the layer I. (A) Few thin and sparse dendrites were detected in the GFP+ cells from the 3m-t group. (B) dense distribution of broader dendrites could be detected in the 3m-t→9 group. (C) The apical dendrite thickness increased significantly from group 3m-t to group 3m-t→9. Dendrites of cells in 3m-t mice were almost devoid of synaptic spine structures (D), while numerous spines were decorating the dendrites of group 3m-t→9 mice (E). (F) Comparison of dendritic spine densities between the 3m-t and 3m-t→9 groups suggesting functional integration of complex cells. Scale bars = 50 μm (A, B) and 10 μm (D, E). To measure the synaptic innervation of GFP+ cells in layer II of the piriform cortex, a linear density of synaptophysin (SYN) containing puncta apposed to the cell soma of GFP+ cells was calculated. (G) GFP+ complex cell (green) surrounded by SYN (red) and VGAT (white) immunoreactive puncta. Scale bar = 10 μm. (H) The linear density of SYN significantly increased between GFP+ cells found in group 3m-t compared with GFP+ cells measured in group 3m-t→9. (I, J) In 2-year-old transgenic mice that received tamoxifen at 3 months of age, the large majority of GFP+ complex cells were endowed with a βIV-spectrin-positive AIS and expressed the mature neuronal marker NeuN. Scale bar = 20 μm.
In order to functionally integrate into the existing network, immature neurons must be able to receive inputs via their dendritic tree and further propagate the information in form of action potentials. Therefore, we analyzed the density of spines decorating a 40 μm dendritic segment starting from a distance of 100 μm from the cell body (Fig. 3D–F). The spine density detected on GFP-labeled dendrites increased markedly and significantly during the 6 months separating the 3m-t group (0.31 ± 0.27 spines/μm, n = 25) from the 3m-t→9 group (0.88 ± 0.32 spines/μm, n = 25; 2-tailed t-test, P < 0.0001***) Structural integration of immature neurons into the surrounding neuronal network was further examined using the linear density of synaptophysin (SYN) containing puncta, a marker of active synapses, apposed to the soma of GFP+ cells. This parameter has been described as a surrogate marker of synaptic integration (Calhoun et al. 1996). The density of SYN+-puncta on GFP+ cells increased significantly over time (3m-t: 0.11 ± 0.01 puncta/μm vs. 3m-t→9:0.15 ± 0.01 puncta/μm; one-way ANOVA, P = 0.012*), demonstrating that neurons of the surrounding network increased their connectivity with the maturing GFP+ neurons (Fig. 3G,H). Similar results were observed when analyzing the density of VGAT immunoreactive puncta (3m-t: 0.1 ± 0.01 puncta/μm vs. 3m-t→9:0.14 ± 0.01 puncta/μm; one-way ANOVA, P < 0.0001***).
Similarly, we investigated the appearance of an axonal initial segment (AIS), a specialized axonal microdomain usually localized at the emergence of the axon (Leterrier 2016). It fulfills 2 main functions: 1) it is the initiation site of action potentials, with a 40-fold higher density of voltage-gated sodium channels compared with the soma (Kole and Stuart 2012) and 2) it has been shown to be a major player for the regulation of cellular excitability (Wefelmeyer et al. 2016). A main molecular component of the AIS is the master scaffolding protein AnkyrinG (AnkG), which in turn recruits other proteins enriched at the AIS (Jones and Svitkina 2016). Voltage-gated sodium channels, voltage-gated potassium channels, as well as scaffolding proteins such as βIV-spectrin and neurofascins cluster at the AIS (Yoshimura and Rasband 2014). Although the presence of an AIS based on the detection of βIV-spectrin could not be detected in tangled cells, the vast majority of immature neurons with a complex morphology displayed a clear AIS. In transgenic mice treated with tamoxifen at 3 months of age and analyzed at 2 years, AIS could be detected in 87 ± 18% of GFP-expressing cells (Fig. 3I,J). At this time point, 90% ± 6% of the GFP+ complex cells expressed NeuN, thereby substantiating further the advanced structural and functional maturation of the labeled cells.
The possibility that induction of GFP expression occurs stochastically in the absence of tamoxifen was investigated. Such a leakiness of the system could lead to the accumulation of GFP-expressing mature neurons over time. We therefore quantified the number of GFP+ neurons in layer II of the piriform cortex of 2-year-old transgenic DCX-CreERT2/Flox-EGFP mice, which either received tamoxifen at the age of 3 months or remained untreated. Without the application of tamoxifen, we detected 1310 ± 1388 GFP+ cells/mm3 in the piriform cortex of the naive 2-year-old transgenic mice, whereas 44 590 ± 11 309 GFP+ cells/mm3 could still be detected in the 2-year-old transgenic mice induced with tamoxifen at the age of 3 months, corresponding to a leakiness of less than 3% over 2 years (Supplementary Fig. S3). Hence, we concluded that the leakiness of our transgenic DCX-CreERT2/Flox-EGFP model did not interfere with the fate analysis of the tangled cells.
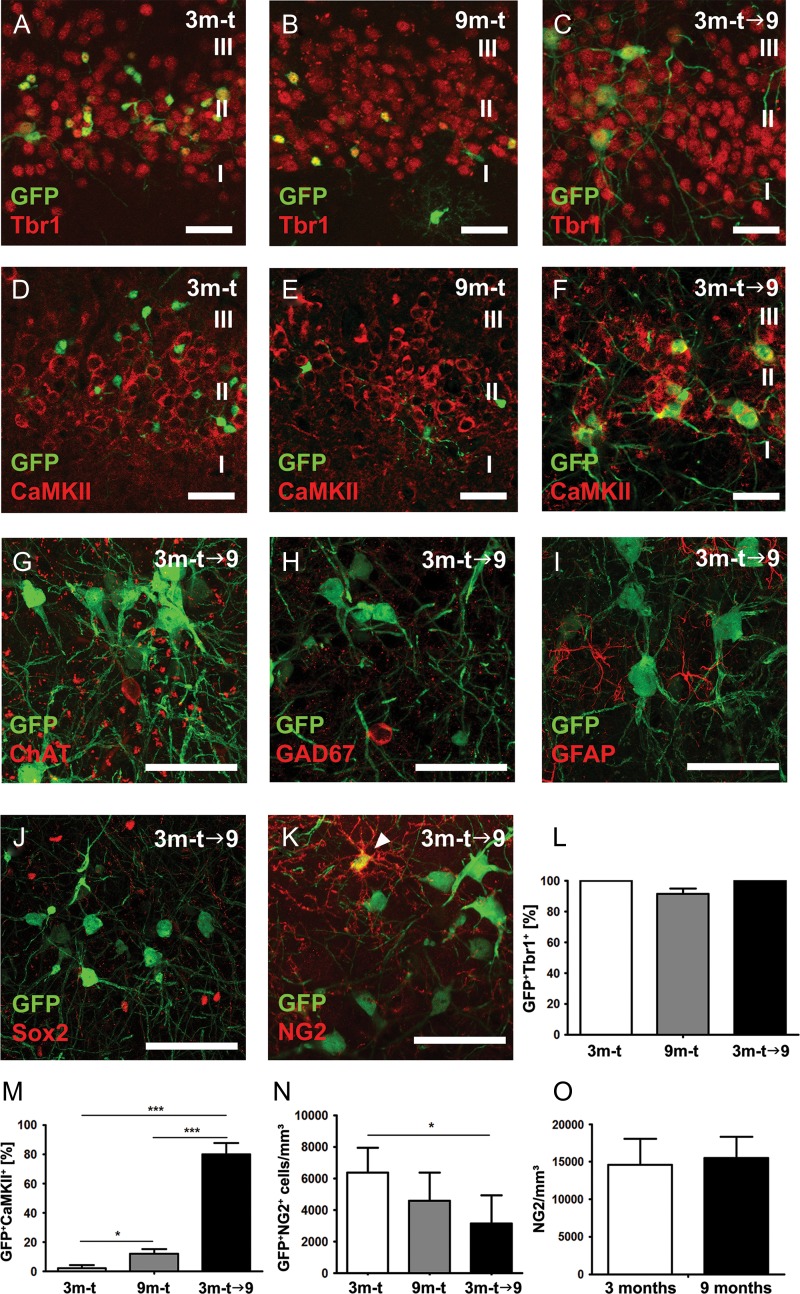
We subsequently analyzed the expression of a collection of mature neuronal and glial markers in GFP+ cells in layer II of the piriform cortex of our 3 experimental groups, with a particular attention to the fate of GFP+ cells 6 months after tamoxifen administration (3m-t→9). Three types of excitatory neurons were examined, namely dopaminergic neurons (expressing tyrosine hydroxylase, TH), cholinergic neurons (expressing choline acetyltransferase, ChAT) and glutamatergic principal neurons (expressing the transcription factor T-Box brain 1 (Tbr1) or Ca2+/calmodulin-dependent protein kinase II (CaMKII)). Tbr1 was coexpressed in virtually all GFP+ cells (3m-t: 100%; 9m-t: 91.5 ± 3.4%; 3m-t→9:100%) (Fig. 4A–C,L, Supplementary Fig. S4). The proportion of GFP+CaMKII+ coexpressing cells increased significantly between the 3m-t group (2.0 ± 2.3% of GFP+ cells) and 9m-t group (12 ± 2.3% of GFP+ cells; one-way ANOVA, P < 0.05*) and this increase was even higher in GFP+ cells that were given 6 months to mature (3m-t→9: 80.0 ± 7.7%; one-way ANOVA, P < 0.0001***) (Fig. 4D–F,M, Supplementary Fig. S4). No GFP+ cells were found to coexpress TH (data not shown) or ChAT (Fig. 4G). A few cells (6 out of 1000 GFP+ cells) were found to express GAD67, a marker for GABAergic neurons. These GFP+GAD67+ cells were found exclusively in the 9m-t group, while this scarce cell population was not detected in the other experimental groups (Fig. 4H, Supplementary Fig. S5). The detection of glial fibrillary acidic protein (GFAP) was used to identify astrocytes, in addition to the transcription factor SRY-box 2 (Sox2), which is expressed in astrocytes as well as in neural stem cells. No GFP+ cell was found to coexpress GFAP and/or Sox2 in any of the 3 experimental groups (Fig. 4I,J). This indicates that the vast majority of former immature GFP+ neurons became mature glutamatergic neurons within the 6 months after induction of the GFP reporter gene.
Figure 4.
Fate of GFP+ cells. (A–C) Virtually all GFP-expressing cells in layer II of the piriform cortex coexpressed Tbr1, a transcription factor expressed in glutamatergic neurons. (D–F) Expression of CaMKII, a marker of mature glutamatergic neurons could be detected in GFP+ cells of all 3 experimental groups at various levels. (G) GFP+ cells did not coexpress the cholinergic neuronal marker ChAT. (H) GFP+ cells only rarely expressed the GABAergic marker GAD67 (see also Supplementary Fig 5). (I–J) No coexpression with astrocyte and stem cell markers GFAP and Sox2 was detected. (K) A small subpopulation of GFP-expressing cells with polydendrocytic morphology expressed NG2. (M) The coexpression of CaMKII in GFP+ cells increased significantly between the 3m-t group and 9m-t group and increased to even higher extent in GFP+ cells that were given 6 months to mature. (N) The density of GFP+NG2+ cells did not significantly differ between groups 3m-t and 9m-t, however, if GFP-labeled cells were analyzed 6 months post tamoxifen administration (3m-t→9), their density was significantly decreased compared with 3m-t. (O) The density of NG2-expressing cells in layer II of the piriform cortex remained stable comparing 3- and 9-month-old mice. Scale bars = 50 μm.
A small population of GFP+ cells in our study presented a morphology resembling that of polydendrocytes and expressed NG2 (Fig. 4K, Supplementary Fig. S4). Previous studies have also reported a subpopulation of PSA-NCAM/DCX+ cells expressing NG2 in layer II of the piriform cortex of both mice and rats (Gómez-Climent et al. 2008; Rubio et al. 2016). Therefore, we further analyzed the NG2+ population by measuring its density in layer II of the piriform cortex in all 3 experimental groups. Our comparison did not reveal any statistical difference in the NG2+ cell densities of 3 and 9-month-old mice (3m-t: 14 622 ± 3432 NG2+ cells/mm3; 9m-t: 15 510 ± 2845 NG2+ cells/mm3; 2-tailed t-test, P = 0.6677) (Fig. 4O). However, the GFP+NG2+ cells density was slightly decreased following a survival of 6 months post tamoxifen administration, that is, 3m-t versus 3m-t→9 (3m-t: 7387 ± 844; 9m-t: 4861 ± 521; 2-tailed t-test P = 0.0142*), thus, suggesting a slow turnover of this cell population (Fig. 4N).
Discussion
In this study, we detected and mapped the fate of immature neurons in the murine piriform cortex layer II, by the use of the transgenic DCX-CreERT2/Flox-EGFP mouse. We demonstrate that immature neurons in the piriform cortex do not die in the course of aging, but structurally integrate as newly matured neurons, as hypothesized by previous studies (Gómez-Climent et al. 2008; Varea et al. 2011; Yang et al. 2015; Rubio et al. 2016). This conclusion was supported by the pattern of marker expression together with the morphological features of GFP+ cells after tamoxifen induction, including the sprouting of synapses and axons. Further evidence against the elimination of immature neurons in the piriform cortex via cell death comes from the lack of elevated TUNEL activity (Xiong et al. 2008) or the absence of pyknotic nuclei (Gómez-Climent et al. 2010). GFP+ cells in layer II of the piriform cortex were found to be postmitotic. Hence, application of BrdU during adulthood did not label any neurons of the piriform cortex. However, in line with previous studies (Gómez-Climent et al. 2008; Luzzati et al. 2009; Zhang, Cai, et al. 2009; Rubio et al. 2016), we could detect abundant incorporation of BrdU at E14–15, which remained detectable in the immature neurons of the piriform cortex at 3 months of age. Under physiological conditions, newly generated cells in the rodent adult piriform cortex thus appear to be marginal and might originate from cells migrating out of the SVZ (Shapiro et al. 2007).
Virtually all GFP+ immature neurons in the piriform cortex expressed the Tbr1 transcription factor, suggesting a determined glutamatergic phenotype and fate. This observation substantiated previous reports indicating that immature neurons in the piriform cortex express Tbr1 (Gómez-Climent et al. 2008; Luzzati et al. 2009; Rubio et al. 2016), while they were very rarely found to express the LIM/homeobox protein Lhx6 or distal-less DLL, arguing therefore for a pallial origin, rather than subpallial structures in which most cortical interneurons originate (Luzzati et al. 2009). In contrast to Tbr1, CaMKII, a Ca2+/calmodulin-dependent protein kinase, is expressed only in mature principal neurons. We observed that coexpression of GFP with CaMKII, which was marginal shortly after application of tamoxifen in DCX-CreERT2/Flox-EGFP, irrespective of the age of mice (3m-t and 9m-t groups), impressively increased to approximately 80% following a maturation period of 6 months. This marker profile suggests the progressive maturation of tangled cells into mature glutamatergic neurons.
To explore the integration of these “newly matured” glutamatergic neurons within the neuronal network, we analyzed morphological correlates of functionality, namely, dendritic spines and axon initial segments (AIS). Upon 6 months of maturation, the apical dendrites of GFP+ complex cells in layer I displayed an increase in their thickness and in the linear density of spines, suggesting an increase in synaptic input.
Similarly, we detected the appearance of an AIS upon maturation. Since AIS are necessary and sufficient structures to define any cell as a neuron able to generate action potentials, an effective strategy for tracing the transition between tangled cells and neurons is to evaluate the maturation of an AIS according to the presence of AIS-specific scaffolding proteins (Gutzmann et al. 2014; Yoshimura and Rasband 2014; Schlüter et al. 2017). Although tangled cells were devoid of AIS, based on the detection of βIV-spectrin, this key feature of neuronal identity was present in the vast majority of GFP-expressing cells that underwent 6 months of maturation. Taken together, we observed that the GFP-expressing cells, which corresponded to tangled cells shortly after tamoxifen application, adopted a morphology consistent with the functional semilunar–pyramidal transitional neurons or pyramidal neurons upon 6 months of maturation.
Moreover, the surrounding neuronal network increasingly contacted the GFP-expressing immature neurons upon maturation. The analysis of SYN+ and VGAT+ perisomatic puncta, which correspond to presynaptic structures that could be used as a surrogate marker of synaptic integration (Calhoun et al. 1996), has revealed that, regardless of the age of the mice analyzed, the densities of these puncta on tangled cells were very low. In contrast, in the 3m-t→9 group, the GFP+ neurons which acquired a complex morphology at this time point had significantly higher density of these SYN+ and VGAT+ presynaptic structures, arguing for an increased synaptic connectivity with the surrounding network. These observations are in accordance with a study performed in the piriform cortex by Klempin and colleagues (2011), who reported that cells with a low DCX expression and a complex morphology, reminiscent of immature semilunar–pyramidal transitional neurons, exhibited an electrophysiological profile consistent with neurons undergoing maturation (Klempin et al. 2011).
Studies investigating various cortical structures in different mammalian species suggested that immature cortical neurons located in layer II can mature into GABAergic interneurons (Xiong et al. 2008; Cai et al. 2009; Zhang, Zhang, et al. 2009; König et al. 2016). This possibility that some GFP+ neurons belong to an interneuronal population was examined using the marker GAD67 expressed in inhibitory neurons (Young and Sun 2009). Only a few GFP+/GAD67+ cells could be detected in the 9m-t group. Due to the very low abundance of this cell population, we can neither exclude that they are the result of a labeling artifact, nor that they remained undetected in the 2 other experimental groups. On the other hand, we could clearly exclude that GFP+ cells in the piriform cortex undergo cholinergic or dopaminergic maturation.
Intriguingly, we found low frequency of coexpression of the proteoglycan NG2 in GFP+ cells. Previous studies in rats and mice have also indicated that a small subpopulation of NG2-expressing cells in the piriform cortex layer II coexpressed markers usually found in immature neurons (Gómez-Climent et al. 2008; Rubio et al. 2016). NG2+ cells have been generally considered oligodendrocyte precursors, but many of them remain in the adult CNS as polydendrocytes, a specific glial cell type displaying a distinct morphology capable of lineage plasticity (Nishiyama et al. 2009; Huang et al. 2014). However, in contrast to the PSA-NCAM+ and DCX+ cell populations progressively disappearing, the density of NG2+ cells in layer II remained stable during aging. The very low number of GFP+ cells expressing NG2 and the absence of significant cell proliferation, rules out this cell population as potential source for the numerous glutamatergic neurons observed in the 3m-t→9. Similarly, the absence of coexpression of GFP, shortly after tamoxifen application or following 6 months of maturation, with GFAP or Sox2 ruled out the association with an astrocytic or neural stem cell phenotype.
The mechanisms enabling postmitotic tangled cells to survive in a dormant state during adulthood still have to be deciphered. Similarly, the nature of the signals triggering completion of their neuronal maturation processes and the functional relevance of such immature cells in the piriform cortex remain largely unknown. Although the maturation of the immature neurons in the piriform cortex does not seem to play a crucial role in olfactory memory, an activity-dependent control of maturation has been suggested by the observation that the number of PSA-NCAM/DCX-expressing cells dramatically decreased following bulbectomy, whereas the expression of mature markers increased (Gómez-Climent et al. 2011). The continuous maturation of glutamatergic neurons under physiological conditions offers a precious opportunity for neuronal plasticity in piriform cortex layer II. Analyses of the classical neurogenic niches demonstrated that the integration of immature neurons not only adds new synapses in the neuronal circuitry, but also showed that the activity of the surrounding neuronal network is profoundly influenced by immature neurons due to their distinct electrophysiological properties (Couillard-Despres et al. 2006; Ge et al. 2007).
In summary, we demonstrated that in layer II of the murine adult piriform cortex, a population of postmitotic immature neurons is continuously providing newly matured glutamatergic principal neurons, which structurally integrate in the surrounding neuronal network according to the increase in synaptic contacts and the appearance of an AIS. These findings suggest that new functional neurons could mature and integrate within in the adult central nervous system outside of the classical neurogenic niches. It is tempting to speculate that these cortical immature neurons, which are more widely distributed in mammals with gyrencephalic brains, such as humans (reviewed in König et al. 2016), progressively integrate into the neuronal network throughout adulthood and thereby provide an unsuspected capacity of network plasticity. Such matter will be of crucial relevance for the neurobiology of human aging, because the maturation and integration of postmitotic immature neurons will also result in a progressive depletion of this resource. In light of the reported scarce proliferative neurogenesis in the adult human brain (Sanai et al. 2011; Wang et al. 2011; Bergmann et al. 2012; Obernier et al. 2018), the existence of latent nonproliferative neuronal precursor populations in several brain areas might constitute an unsuspected resource for the plasticity of neuronal networks in higher mammals (König et al. 2016).
Supplementary Material
Notes
Conflict of Interest: None declared.
Supplementary Material
Supplementary material is available at Cerebral Cortex online.
Funding
The authors are grateful for the technical support of Dominika Jakubecova and Pasquale Romanelli. Spanish Ministry of Economy and Competitiveness SAF2015-68436-R, Generalitat Valenciana Prometeo Excellence Program PROMETEO2013/069 and the Fundación Alicia Koplowitz to J.N. M.B. has an Atracció de Talent predoctoral fellowship from the Universitat de València. This work was supported by the Austrian Science Fund (FWF) Special Research Program (SFB) F44010 and F4413-B23 “Cell Signaling in Chronic CNS Disorders,” The Propter Homines foundation (Liechtenstein), the State Government of Salzburg (Austria), the Deutsche Forschungsgemeinschaft (SFB 1134/A03), and the research support funds from the Paracelsus Medical University PMU-FFF (R-14/04/063-KÖN; R-16/04/084-BEN).
References
- Abrous DN, Montaron MF, Petry KG, Rougon G, Darnaudéry M, Le Moal M, Mayo W. 1997. Decrease in highly polysialylated neuronal cell adhesion molecules and in spatial learning during ageing are not correlated. Brain Res. 744:285–292. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. 2002. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 8:963–970. [DOI] [PubMed] [Google Scholar]
- Bergmann O, Liebl J, Bernard S, Alkass K, Yeung MSY, Steier P, Kutschera W, Johnson L, Landén M, Druid H, et al. . 2012. The age of olfactory bulb neurons in humans. Neuron. 74:634–639. [DOI] [PubMed] [Google Scholar]
- Bernier PJ, Bedard A, Vinet J, Levesque M, Parent A. 2002. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc Natl Acad Sci USA. 99:11464–11469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfanti L. 2006. PSA-NCAM in mammalian structural plasticity and neurogenesis. Prog Neurobiol. 80:129–164. [DOI] [PubMed] [Google Scholar]
- Bonfanti L, Nácher J. 2012. New scenarios for neuronal structural plasticity in non-neurogenic brain parenchyma: the case of cortical layer II immature neurons. Prog Neurobiol. 98:1–15. [DOI] [PubMed] [Google Scholar]
- Bonfanti L, Olive S, Poulain DA, Theodosis DT. 1992. Mapping of the distribution of polysialylated neural cell adhesion molecule throughout the central nervous system of the adult rat: an immunohistochemical study. Neuroscience. 49:419–436. [DOI] [PubMed] [Google Scholar]
- Bonfanti L, Peretto P. 2011. Adult neurogenesis in mammals—a theme with many variations. Eur J Neurosci. 34:930–950. [DOI] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. 2003. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 467:1–10. [DOI] [PubMed] [Google Scholar]
- Cai Y, Xiong K, Chu Y, Luo D-W, Luo X-G, Yuan X-Y, Struble RG, Clough RW, Spencer DD, Williamson A, et al. . 2009. Doublecortin expression in adult cat and primate cerebral cortex relates to immature neurons that develop into GABAergic subgroups. Exp Neurol. 216:342–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun ME, Jucker M, Martin LJ, Thinakaran G, Price DL, Mouton PR. 1996. Comparative evaluation of synaptophysin-based methods for quantification of synapses. J Neurocytol. 25:821–828. [DOI] [PubMed] [Google Scholar]
- Carceller H, Rovira-Esteban L, Nácher J, Castrén E, Guirado R. 2016. Neurochemical phenotype of reelin immunoreactive cells in the piriform cortex layer II. Front Cell Neurosci. 10:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couillard-Despres S, Winner B, Karl C, Lindemann G, Schmid P, Aigner R, Laemke J, Bogdahn U, Winkler J, Bischofberger J, et al. . 2006. Targeted transgene expression in neuronal precursors: watching young neurons in the old brain. Eur J Neurosci. 24:1535–1545. [DOI] [PubMed] [Google Scholar]
- Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn H-G, Aigner L. 2005. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 21:1–14. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Cleaver KM, Abouantoun T, Cameron HA. 2005. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J Cell Biol. 168:415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nevi E, Marco-Salazar P, Fondevila D, Blasco E, Pérez L, Pumarola M. 2013. Immunohistochemical study of doublecortin and nucleostemin in canine brain. Eur J Histochem. 57:e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst A, Alkass K, Bernard S, Salehpour M, Perl S, Tisdale J, Possnert G, Druid H, Frisén J. 2014. Neurogenesis in the striatum of the adult human brain. Cell. 156:1072–1083. [DOI] [PubMed] [Google Scholar]
- Feliciano DM, Bordey A. 2013. Newborn cortical neurons: only for neonates? Trends Neurosci. 36:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliciano DM, Bordey A, Bonfanti L. 2015. Noncanonical sites of adult neurogenesis in the mammalian brain. Cold Spring Harbor Perspect Biol. 7:a018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Yang C-H, Hsu K-S, Ming G-L, Song H. 2007. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 54:559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E. 2007. How widespread is adult neurogenesis in mammals? Nat Rev Neurosci. 8:481–488. [DOI] [PubMed] [Google Scholar]
- Guirado R, Perez-Rando M, Sanchez-Matarredona D, Castillo-Gómez E, Liberia T, Rovira-Esteban L, Varea E, Crespo C, Blasco-Ibáñez JM, Nácher J. 2014. The dendritic spines of interneurons are dynamic structures influenced by PSA-NCAM expression. Cereb Cortex. 24:3014–3024. [DOI] [PubMed] [Google Scholar]
- Guo F, Maeda Y, Ma J, Xu J, Horiuchi M, Miers L, Vaccarino F, Pleasure D. 2010. Pyramidal neurons are generated from oligodendroglial progenitor cells in adult piriform cortex. J Neurosci. 30:12036–12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutzmann A, Ergül N, Grossmann R, Schultz C, Wahle P, Engelhardt M. 2014. A period of structural plasticity at the axon initial segment in developing visual cortex. Front Neuroanat. 8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Climent MA, Castillo-Gómez E, Varea E, Guirado R, Blasco-Ibáñez JM, Crespo C, Martínez-Guijarro FJ, Nàcher J. 2008. A population of prenatally generated cells in the rat paleocortex maintains an immature neuronal phenotype into adulthood. Cereb Cortex. 18:2229–2240. [DOI] [PubMed] [Google Scholar]
- Gómez-Climent MA, Guirado R, Varea E, Nàcher J. 2010. “Arrested development.” Immature, but not recently generated, neurons in the adult brain. Arch Ital Biol. 148:159–172. [PubMed] [Google Scholar]
- Gómez-Climent MA, Hernández-González S, Shionoya K, Belles M, Alonso-Llosa G, Datiche F, Nàcher J. 2011. Olfactory bulbectomy, but not odor conditioned aversion, induces the differentiation of immature neurons in the adult rat piriform cortex. Neuroscience. 181:18–27. [DOI] [PubMed] [Google Scholar]
- He X, Zhang X-M, Wu J, Fu J, Mou L, Lu D-H, Cai Y, Luo X-G, Pan A, Yan X-X. 2014. Olfactory experience modulates immature neuron development in postnatal and adult guinea pig piriform cortex. Neuroscience. 259:101–112. [DOI] [PubMed] [Google Scholar]
- Huang W, Zhao N, Bai X, Karram K, Trotter J, Goebbels S, Scheller A, Kirchhoff F. 2014. Novel NG2-CreERT2 knock-in mice demonstrate heterogeneous differentiation potential of NG2 glia during development. Glia. 62:896–913. [DOI] [PubMed] [Google Scholar]
- Jin K, Sun Y, Xie L, Peel A, Mao XO, Batteur S, Greenberg DA. 2003. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol Cell Neurosci. 24:171–189. [DOI] [PubMed] [Google Scholar]
- Jones SL, Svitkina TM. 2016. Axon initial segment cytoskeleton: architecture, development, and role in neuron polarity. Neural Plast. 2016:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempin F, Kronenberg G, Cheung G, Kettenmann H, Kempermann G. 2011. Properties of doublecortin-(DCX)-expressing cells in the piriform cortex compared to the neurogenic dentate gyrus of adult mice. PLoS One. 6:e25760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole MHP, Stuart GJ. 2012. Signal processing in the axon initial segment. Neuron. 73:235–247. [DOI] [PubMed] [Google Scholar]
- König R, Benedetti B, Rotheneichner P, O’ Sullivan A, Kreutzer C, Belles M, Nácher J, Weiger TM, Aigner L, Couillard-Despres S. 2016. Distribution and fate of DCX/PSA-NCAM expressing cells in the adult mammalian cortex: a local reservoir for adult cortical neuroplasticity? Front Biol. 11:193–213. [Google Scholar]
- Leterrier C. 2016. The axon initial segment, 50 years later: a nexus for neuronal organization and function. Curr Top Membr. 77:185–233. [DOI] [PubMed] [Google Scholar]
- Luzzati F, Bonfanti L, Fasolo A, Peretto P. 2009. DCX and PSA-NCAM expression identifies a population of neurons preferentially distributed in associative areas of different pallial derivatives and vertebrate species. Cereb Cortex. 19:1028–1041. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Komitova M, Suzuki R, Zhu X. 2009. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 10:9–22. [DOI] [PubMed] [Google Scholar]
- Nàcher J, Crespo C, McEwen BS. 2001. Doublecortin expression in the adult rat telencephalon. Eur J Neurosci. 14:629–644. [DOI] [PubMed] [Google Scholar]
- Nácher J, Alonso-Llosa G, Rosell D, McEwen B. 2002. PSA-NCAM expression in the piriform cortex of the adult rat. Modulation by NMDA receptor antagonist administration. Brain Res. 927:111–121. [DOI] [PubMed] [Google Scholar]
- Ní Dhúill CM, Fox GB, Pittock SJ, O’Connell AW, Murphy KJ, Regan CM. 1999. Polysialylated neural cell adhesion molecule expression in the dentate gyrus of the human hippocampal formation from infancy to old age. J Neurosci Res. 55:99–106. [DOI] [PubMed] [Google Scholar]
- Obernier K, Cebrián-Silla A, Thomson M, Parraguez JI, Anderson R, Guinto C, Rodriguez JR, Garcia-Verdugo JM, Alvarez-Buylla A. 2018. Adult neurogenesis is sustained by symmetric self-renewal and differentiation. Stem Cell. 22:221–234.e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzke N, LeRoy A, Ngubane NW, Bennett NC, Medger K, Gravett N, Kaswera-Kyamakya C, Gilissen E, Chawana R, Manger PR. 2014. The distribution of doublecortin-immunopositive cells in the brains of four afrotherian mammals: the Hottentot golden mole (Amblysomus hottentotus), the rock hyrax (Procavia capensis), the eastern rock sengi (Elephantulus myurus) and the four-toed sengi (Petrodromus tetradactylus). Brain Behav Evol. 84:227–241. [DOI] [PubMed] [Google Scholar]
- Pekcec A, Löscher W, Potschka H. 2006. Neurogenesims in the adult rat piriform cortex. NeuroReport. 17:571–574. [DOI] [PubMed] [Google Scholar]
- Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. 2008. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 11:1392–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio A, Belles M, Belenguer G, Vidueira S, Fariñas I, Nàcher J. 2016. Characterization and isolation of immature neurons of the adult mouse piriform cortex. Dev Neurobiol. 76:748–763. [DOI] [PubMed] [Google Scholar]
- Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z, Tsai H-H, Wong M, Gupta N, Berger MS, Huang E, Garcia-Verdugo JM, et al. . 2011. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 478:382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. . 2012. Fiji – an Open-Source platform for biological image analysis. Nat Methods. 9:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlüter A, Del Turco D, Deller T, Gutzmann A, Schultz C, Engelhardt M. 2017. Structural plasticity of synaptopodin in the axon initial segment during visual cortex development. Cereb Cortex. 27:4662–4675. [DOI] [PubMed] [Google Scholar]
- Seki T, Arai Y. 1991. Expression of highly polysialylated NCAM in the neocortex and piriform cortex of the developing and the adult rat. Anat Embryol. 184:395–401. [DOI] [PubMed] [Google Scholar]
- Shapiro LA, Ng KL, Kinyamu R, Whitaker-Azmitia P, Geisert EE, Blurton-Jones M, Zhou Q-Y, Ribak CE. 2007. Origin, migration and fate of newly generated neurons in the adult rodent piriform cortex. Brain Struct Funct. 212:133–148. [DOI] [PubMed] [Google Scholar]
- Shapiro LA, Ng K, Zhou Q-Y, Ribak CE. 2009. Subventricular zone-derived, newly generated neurons populate several olfactory and limbic forebrain regions. Epilepsy Behav. 14(Suppl 1):74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura NU. 2005. Evidence for neurogenesis within the white matter beneath the temporal neocortex of the adult rat brain. Neuroscience. 134:121–132. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Kataoka Y, Cui Y, Takamori Y, Watanabe Y, Yamada H. 2007. Multi-directional differentiation of doublecortin- and NG2-immunopositive progenitor cells in the adult rat neocortex in vivo. Eur J Neurosci. 25:3489–3498. [DOI] [PubMed] [Google Scholar]
- Varea E, Belles M, Vidueira S, Blasco-Ibáñez JM, Crespo C, Pastor AM, Nácher J. 2011. PSA-NCAM is expressed in immature, but not recently generated, neurons in the adult cat cerebral cortex layer II. Front Neurosci. 5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varea E, Castillo-Gómez E, Gómez-Climent MA, Guirado R, Blasco-Ibáñez JM, Crespo C, Martínez-Guijarro FJ, Nácher J. 2009. Differential evolution of PSA-NCAM expression during aging of the rat telencephalon. Neurobiol Aging. 30:808–818. [DOI] [PubMed] [Google Scholar]
- Wang C, Liu F, Liu Y-Y, Zhao C-H, You Y, Wang L, Zhang J, Wei B, Ma T, Zhang Q, et al. . 2011. Identification and characterization of neuroblasts in the subventricular zone and rostral migratory stream of the adult human brain. Cell Res. 21:1534–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, You Y, Qi D, Zhou X, Wang L, Wei S, Zhang Z, Huang W, Liu Z, Liu F, et al. . 2014. Human and monkey striatal interneurons are derived from the medial ganglionic eminence but not from the adult subventricular zone. J Neuroscii. 34:10906–10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefelmeyer W, Puhl CJ, Burrone J. 2016. Homeostatic plasticity of subcellular neuronal structures: from inputs to outputs. Trends Neurosci. 39:656–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong K, Cai Y, Zhang X-M, Huang J-F, Liu Z-Y, Fu G-M, Feng J-C, Clough RW, Patrylo PR, Luo X-G, et al. . 2010. Layer I as a putative neurogenic niche in young adult guinea pig cerebrum. Mol Cell Neurosci. 45:180–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong K, Luo D-W, Patrylo PR, Luo X-G, Struble RG, Clough RW, Yan X-X. 2008. Doublecortin-expressing cells are present in layer II across the adult guinea pig cerebral cortex: partial colocalization with mature interneuron markers. Exp Neurol. 211:271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Xie M-X, Li J-M, Hu X, Patrylo PR, Luo X-G, Cai Y, Li Z, Yan X-X. 2015. Prenatal genesis of layer II doublecortin expressing neurons in neonatal and young adult guinea pig cerebral cortex. Front Neuroanat. 9:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T, Rasband MN. 2014. Axon initial segments: diverse and dynamic neuronal compartments. Curr Opin Neurobiol. 27:96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A, Sun Q-Q. 2009. GABAergic inhibitory interneurons in the posterior piriform cortex of the GAD67-GFP mouse. Cereb Cortex. 19:3011–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X-M, Cai Y, Chu Y, Chen E-Y, Feng J-C, Luo X-G, Xiong K, Struble RG, Clough RW, Patrylo PR, et al. . 2009. Doublecortin-expressing cells persist in the associative cerebral cortex and amygdala in aged nonhuman primates. Front Neuroanat. 3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L-M, Zhang Y-Z, Liu Y-Q, Gong Z-H, Zhao Y-M, Li Y-F. 2009. CTN-986, a compound extracted from cottonseeds, increases cell proliferation in hippocampus in vivo and in cultured neural progenitor cells in vitro. Eur J Pharmacol. 607:110–113. [DOI] [PubMed] [Google Scholar]
- Zhang J, Giesert F, Kloos K, Weisenhorn DMV, Aigner L, Wurst W, Couillard-Despres S. 2010. A powerful transgenic tool for fate mapping and functional analysis of newly generated neurons. BMC Neurosci. 11:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.