Abstract
Diverse animal species primarily rely on sense (left–right) and egocentric distance (proximal–distal) when navigating the environment. Recent neuroimaging studies with human adults show that this information is represented in 2 scene-selective cortical regions—the occipital place area (OPA) and retrosplenial complex (RSC)—but not in a third scene-selective region—the parahippocampal place area (PPA). What geometric properties, then, does the PPA represent, and what is its role in scene processing? Here we hypothesize that the PPA represents relative length and angle, the geometric properties classically associated with object recognition, but only in the context of large extended surfaces that compose the layout of a scene. Using functional magnetic resonance imaging adaptation, we found that the PPA is indeed sensitive to relative length and angle changes in pictures of scenes, but not pictures of objects that reliably elicited responses to the same geometric changes in object-selective cortical regions. Moreover, we found that the OPA is also sensitive to such changes, while the RSC is tolerant to such changes. Thus, the geometric information typically associated with object recognition is also used during some aspects of scene processing. These findings provide evidence that scene-selective cortex differentially represents the geometric properties guiding navigation versus scene categorization.
Keywords: object recognition, occipital place area, parahippocampal place area, retrosplenial complex, scene recognition
Our abilities to navigate the environment and to recognize the objects in it are essential to our survival. Over the past 2 decades, an abundance of research has elucidated the geometric information guiding navigation and object recognition in animals from insects to mammals, and in humans from infants to adults. Humans and many other animals navigate by encoding the “sense” (left–right) relations and egocentric distances (proximal–distal) of large extended surfaces in the environment, but not the relative lengths of these surfaces or the angles at which they meet (O’Keefe and Burgess 1996; Lee et al. 2012, 2013; see Cheng and Newcombe 2005, and Spelke and Lee 2012 for reviews; but see Yousif and Lourenco 2017). By contrast, humans and many other animals recognize and categorize objects by the relative lengths and angles that define their 2D or 3D structure, rather than by their positions relative to the observer (Dehaene et al. 2006; Izard and Spelke 2009). Further, recent functional magnetic resonance imaging (fMRI) studies with human adults have shown that 2 scene-selective regions—the occipital place area (OPA) (Dilks et al. 2013) and the retrosplenial complex (RSC) (Maguire 2001)—are sensitive to the sense and egocentric distance properties guiding navigation (Dilks et al. 2011; Persichetti and Dilks 2016). Surprisingly, however, a third scene-selective region—the parahippocampal place area (PPA) (Epstein and Kanwisher 1998)—is not sensitive to either sense or egocentric distance, challenging its role in navigation, which has been suggested by prior work (Ghaem et al. 1997; Janzen and van Turennout 2004; Cheng and Newcombe 2005; Rosenbaum et al. 2004; Rauchs et al. 2008; Spelke et al. 2010). What geometric information, then, does the PPA represent, and what role might it play in scene processing? We hypothesize that the PPA represents the shape-defining properties of relative length and angle in a scene, and in doing so, might play a role in scene categorization (e.g., recognizing a place as a kitchen or a beach).
Prior studies have demonstrated that the PPA represents the “shape” of a scene, often referred to as its “spatial layout.” For example, the PPA discriminates between open and closed spatial layouts (e.g., a desert vs. a forest; Kravitz et al. 2011; Park et al. 2011) and responds more strongly to intact spatial layouts (e.g., empty apartment rooms) than to fragmented ones in which the walls, floors, and ceilings have been fractured and rearranged (Epstein and Kanwisher 1998; Kamps et al. 2016a). Nevertheless, these are gross definitions of a scene’s shape, and whether sensitivity to shape in the PPA also includes the simple relative lengths and angles in a layout remains unknown. A behavioral study may provide some clues: Walther and Shen (2014) found that participants’ ability to categorize scenes was significantly impaired when contour junctions (i.e., angle), and to a lesser extent, contour lengths were removed, and they conjectured that perhaps activity in the PPA represents such information. Here we directly test whether the PPA, as well as the other scene-selective regions, the OPA and the RSC, represent the shape of a scene by the relative lengths and angles that define its extended surfaces. In addition, we test how these 3 regions represent such information by measuring their sensitivity to isolated changes in relative length or angle.
To test whether and how the PPA and the other scene-selective regions represent relative length and angle, we used an event-related fMRI adaptation paradigm (Grill-Spector and Malach 2001) in human adults. Specifically, participants viewed 2 successively presented pictures of the same scene, of completely different scenes, or of 2 scenes that differed by a relative length and/or angle change. If regions of scene-selective cortex (PPA, OPA, and RSC) are sensitive to the relative length and angle properties of scenes, then the pictures presenting such shape changes will be treated as different, and fMRI adaptation will not occur. If, in contrast, those regions are tolerant to changes to the relative length and angle properties of scenes, then pictures with those changes may be treated as the same, and fMRI adaptation will occur.
Materials and Methods
Participants
A total of 25 healthy adults (ages 20–36; 11 females) participated in this experiment. All participants gave informed consent and had normal or corrected-to-normal vision. One participant was excluded due to excessive motion; we thus report the results from the remaining 24 participants.
Design
First, we used a region of interest (ROI) approach, in which we localized scene-selective cortical regions (localizer runs). Then, we used an independent set of experimental runs to investigate the responses of these regions to pairs of pictures of scenes that were identical, entirely different, or differed by the relative lengths of surfaces and/or the angles at which surfaces met. Object stimuli were also included to test whether any sensitivity to relative length and angle in scene-selective regions was specific to pictures of scenes or generalized to pictures of objects. Finally, we investigated whether object-selective regions were sensitive to relative length and angle changes in these same pictures of scenes and objects.
For the localizer runs, ROIs were identified using a standard method (described previously in Epstein and Kanwisher 1998). Each participant completed 2 localizer runs. In a blocked design, participants saw pictures of faces, objects, scenes, and scrambled objects. Each run was 336 s long and consisted of 4 blocks per stimulus category. The order of the stimulus category blocks in each run was palindromic (e.g., faces, objects, scenes, scrambled objects, scrambled objects, scenes, objects, and faces) and was pseudorandomized across runs. Each block contained 20 pictures from the same category for a total of 16 s blocks. Each picture was presented for 300 ms, followed by a 500 ms interstimulus interval (ISI). We also included five 16 s fixation blocks: 1 at the beginning; 3 in the middle interleaved between each palindrome; and 1 at the end of each run. Participants performed a one-back task, responding with a button press every time the same picture was presented twice in a row.
For the experimental runs, participants completed 10 runs each with 100 experimental trials (50 scene trials and 50 object trials, interleaved), and an average of 44 fixation trials, which were used as a baseline condition. Each run was 390 s long. On each fixation trial, a white cross (subtending 0.5° of visual angle) was displayed on a gray background. On each experimental trial, a picture of either a scene or an object was presented for 300 ms, followed by a 400 ms ISI, followed by another picture in the same stimulus category for 300 ms. After the presentation of the second picture, there was a jittered interval ranging from 1 to 6 s (3 s on average). Each pair of pictures consisted of one of the following: (1) the same picture presented twice (Same condition); (2) two completely different pictures from the same category (Different condition); (3) a picture followed by that same picture but with a change in both its relative length and angle properties (Length and Angle condition [L/A condition]); (4) a picture followed by that same picture but with a relative length change only (Length condition); or (5) a picture followed by that same picture but with an angle change only (Angle condition) (Fig. 1). In total, each participant viewed 100 trials of each condition (Same, Different, L/A, Length, and Angle) for each picture type. The trial sequence was generated by the Free-Surfer optseq2 function, optimized for the most accurate estimations of the hemodynamic response (Burock et al. 1998; Dale et al. 1999).
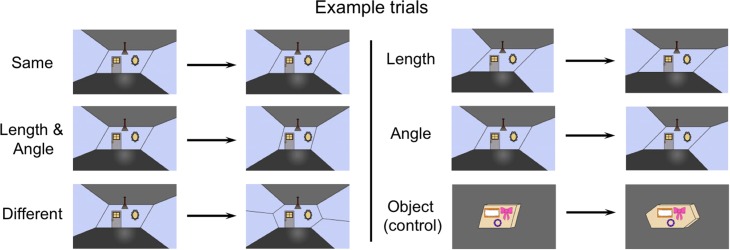
Figure 1.
Example scene stimuli from each condition (i.e., Same, Length and Angle changes, Different, Length changes only, Angle changes only). The object stimuli (bottom right corner) were presented in the same shape-change conditions as the scene stimuli (pictured here is the Different condition). The scene and object stimuli never appeared together in the same trial.
All stimuli subtended 9° × 7° of visual angle, and stimuli in the same category were the same color (Fig. 1). Participants were instructed to keep their eyes fixated on the white cross that appeared in the center of the screen between each picture. After pictures appeared at the central fixation, they moved 1° of visual angle to either the left or right. Participants performed an orthogonal task, indicating by a button press whether the pictures within a pair were moving in the same or opposite directions. This motion task was chosen to eliminate possible early retinotopic confounds and to further disrupt the potential perception of navigating through the scenes.
Stimuli
All scene and object pictures were created from a set of 6 parallelograms. Two of these parallelograms had a side-length ratio of 1:1.33, 3 had a side-length ratio of 1:1.66, and 1 had side-length ratio of 1:1.99. Three parallelograms (one at each side-length ratio) had supplementary angles of 135° and 45°, 2 (at 1:1.33 and 1:1.66 side-length ratios) had supplementary angles of 105° and 75°, and 1 (at the 1:1.66 side-length ratio) had supplementary angles of 120° and 60°. This set of parallelograms afforded 3 groups of 3 pictures: one group that differed in both types of shape properties (L/A condition); one group that differed in relative length only (Length condition); and one group that differed in angle only (Angle condition). These 3 sets of stimuli allowed us to evaluate responses to combined changes to relative length and angle as well as to specific geometric shape properties (i.e., relative length only or angle only). Specifically, we first examined whether each ROI was sensitive to combined changes to relative length and angle, and, if so, then asked how relative length and angle might contribute separately to this sensitivity to the combined changes.
One set of scene pictures was formed by posing each parallelogram as the back wall of a 2D projection of a 3D space, where the corners of the parallelogram were extended forward, in perspective, towards the picture plane. The other set of scene pictures was created using the same method, but the back-wall parallelogram was altered by adding a triangle on the left and right sides to form a hexagon. Both parallelogram and hexagon pictures of scenes were used for the Same, L/A, Length, and Angle conditions. The Different condition consisted of trials in which a parallelogram scene was either preceded or followed by a hexagon scene, with these scenes additionally always differing in relative length and angle. Object pictures used the same parallelogram and hexagon templates, but perspectival depth was projected backwards in the picture plane, to form freestanding “boxes” in an otherwise empty picture. The scene pictures were designed to look like nondescript indoor rooms that contained a door, a mirror, and a hanging light. The object pictures were designed to resemble mail packages that contained 3 stickers. Importantly, the distance between the front edge of the picture and the back wall of the room, and the distance between the front edge of the picture and the package, were held constant so that the rooms and objects never changed in their egocentric distance relative to the viewer (Fig. 1).
For each condition (except the Same condition), there was a small change in the area of the primary shape defining the scene or object between the pair of pictures in each trial. However, independent samples t-tests comparing the area changes between every combination of picture pairs within each condition revealed no significant differences in area changes across the conditions (all P > 0.14). This result suggests that any neural differences across condition were not due to area changes, but rather due to relative length and/or angle changes.
fMRI Scanning
Scanning was completed on a 3T Siemens Trio scanner at the Facility for Education and Research in Neuroscience (FERN) at Emory University (Atlanta, GA). Functional images were acquired using a 32-channel head matrix coil and a gradient echo single-shot echo planar imaging sequence. In total, 16 image slices were acquired for both the localizer scans (repetition time = 2 s), and the experimental scans (repetition time = 1 s). These slices were oriented approximately between perpendicular and parallel to the calcarine sulcus, covering the occipital and temporal lobes and the lower portion of the parietal lobe. For all scans: echo time = 30 ms; voxel size = 3.1 mm × 3.1 mm × 4.0 mm, with a 0.4 mm interslice gap. For each participant, whole-brain, high-resolution T1 weighted anatomical images were acquired for registration of the functional images.
Data Analysis
fMRI data analysis was conducted using the FSL software (Smith et al. 2004) and custom MATLAB code. Before statistical analysis, data from the experimental runs were skull-stripped (Smith 2002), registered to participants’ T1 weighted anatomical image, motion corrected using FSL’s MCFLIRT tool (Jenkinson et al. 2002), and spatially smoothed with a 6 mm kernel. We then conducted analyses on the resultant preprocessed time courses. Following the methods of previous studies (Grill-Spector et al. 1999; Kourtzi and Kanwisher 2001; Dilks et al. 2011; Persichetti and Dilks 2016), the experimental trials were separated by condition for each picture type. Next, for each condition and each picture type, we selected a time-series window from the trial onset to 12 s after onset, and then calculated a mean response for each participant. Finally, the mean response to each condition was compared with a fixation baseline, thus computing percent signal change. The fixation baseline for each participant was identified by averaging the 1 s fixation that preceded the onset of each experimental trial (we deliberately chose this 1 s window to avoid overlapping the previous trial when the jittered intertrial interval was equal to 1 s). Localizer data, but not experimental data, were detrended and then fit with a general linear model that contained covariates that were convolved with a double-gamma function to approximate the hemodynamic response function. After this preprocessing, the scene-selective regions—PPA, OPA, and RSC—were bilaterally defined in each participant as those regions that responded more strongly to scenes than to objects (P < 10−4, uncorrected; as in Epstein and Kanwisher 1998). Each scene-selective ROI was identified in at least one hemisphere for all 24 participants. Object-selective regions—the lateral occipital sulcus (LO) and the posterior fusiform sulcus (pFs)—were bilaterally defined in all participants for LO, and in 20 of the 24 participants for the pFs, as those regions that responded more strongly to objects than to scrambled objects (P < 10−4, uncorrected) (Grill-Spector et al. 1998). As a control region, we also anatomically defined an early visual cortex (EVC) ROI bilaterally in each participant that corresponded to the entirety of V1 (fovea and periphery) using the Jülich histological atlas in FSL (Amunts et al. 2000). For each ROI of each participant, the mean time courses of responses for the experimental conditions (Same, Different, L/A, Length, and Angle) for each picture type were extracted across voxels.
Next, the response time courses were averaged across all conditions (Different, Same, L/A, Length, and Angle) for only the scene stimuli, all scene-selective ROIs, and all participants to identify an average response across “peak” time points. A peak response window was then defined by the whole-second interval in which responses were significantly greater than zero (i.e., the fixation baseline), resulting in a window from 5 to 9 s (all Ps < 0.05). The same method was used to find the peak time points for only the object stimuli in object-selective ROIs, resulting in a window from 6 to 10 s (all Ps < 0.05). Finally, for each participant, we extracted the average activation within each time window for each scene- and object-selective ROI for each picture type (scenes or objects) and each condition (Different, Same, L/A, Length, and Angle). In each ROI and for each picture type, a 2 (Hemisphere: Left, Right) × 5 (Condition: Different, Same, L/A, Length, Angle) repeated-measures ANOVA was performed, and none revealed a significant Hemisphere × Condition interaction (all Ps > 0.10). Thus, data from each hemisphere were collapsed for all further analyses.
Results
Scene-Selective Cortex
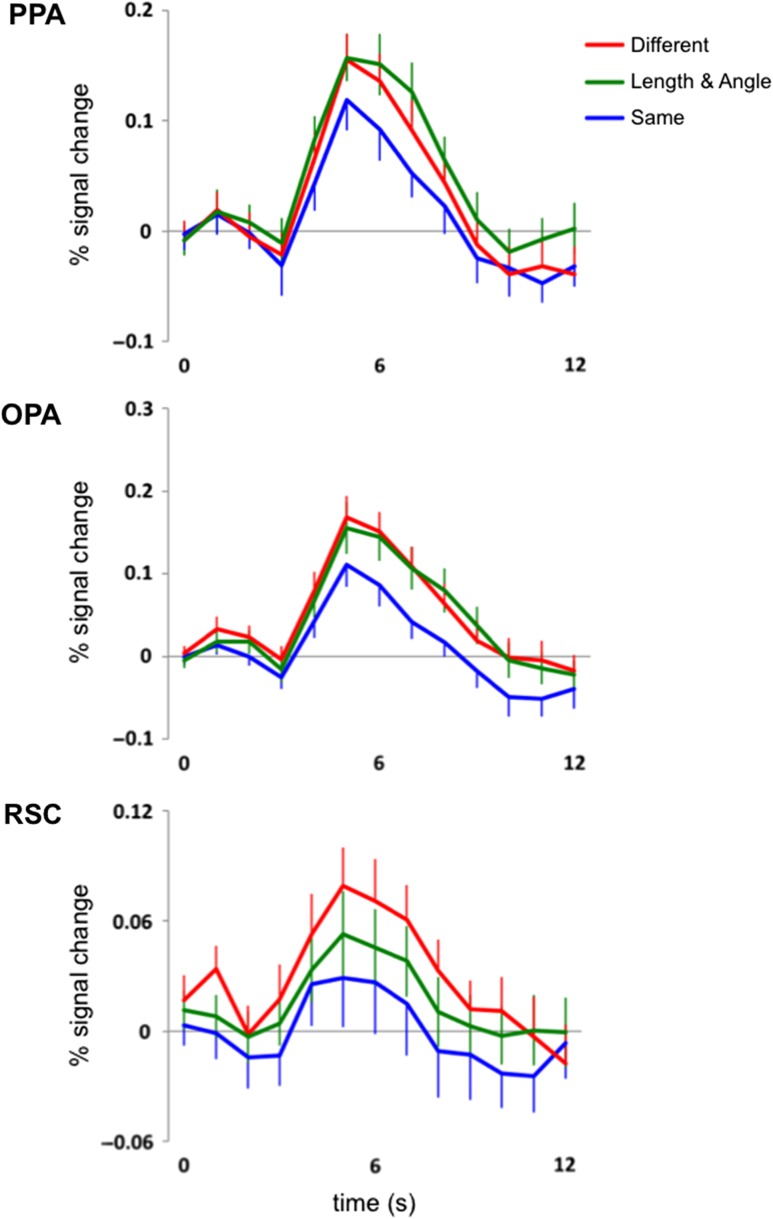
Our first main analysis investigated whether combined changes to relative length and angle (i.e., L/A changes) in pictures of scenes are represented in each scene-selective ROI. As predicted, we found that the PPA was sensitive to L/A changes in pictures of scenes (Fig. 2). In the PPA, a 3-level (Condition: Different, L/A, Same) repeated-measures ANOVA on the average response between 5 s and 9 s revealed a significant main effect of Condition (F2,46 = 5.82, P < 0.01, ηP2 = 0.20), with a greater response to the Different and L/A conditions compared with the Same condition (main effect contrasts, both Ps < 0.05, both Cohen’s ds > 0.54). There was no significant difference between the Different and L/A conditions (main effect contrast, P = 0.20, Cohen’s d = 0.31). These results demonstrate the expected fMRI adaptation (i.e., Different > Same) as well as the predicted sensitivity to combined changes to relative length and angle in pictures of scenes (i.e., L/A > Same) in the PPA.
Figure 2.
Hemodynamic time courses (percent signal change) of 3 scene-selective regions of cortex, the PPA, the OPA, and the RSC to 2 completely different pictures of scenes (red line labeled “Different”), a scene picture followed by that same picture but with a change to both its relative length and angle properties (green line labeled “Length & Angle”), and the same picture of a scene presented twice (blue line labeled “Same”). Note that there is sensitivity to relative length and angle changes in both the PPA and the OPA, while the RSC is tolerant to such changes.
We also found that the OPA was sensitive to L/A changes in pictures of scenes (Fig. 2). A 3-level (Condition: Different, L/A, Same) repeated-measures ANOVA revealed a significant main effect of Condition (F2,46 = 6.55, P < 0.005, ηP2 = 0.22), with a greater response to the Different and L/A conditions compared with the Same condition (main effect contrasts, both Ps < 0.005, both Cohen’s ds > 0.77). There was no significant difference between the Different and L/A conditions (main effect contrast, P = 0.85, Cohen’s d = 0.05). These results demonstrate the expected fMRI adaptation effect (i.e., Different > Same) as well as a sensitivity to combined changes to relative length and angle in pictures of scenes (i.e., L/A > Same) in the OPA.
Unlike the OPA and PPA, the RSC was tolerant to L/A changes in pictures of scenes (Fig. 2). A 3-level (Condition: Different, L/A, Same) repeated-measures ANOVA revealed a significant main effect of Condition (F2,46 = 3.26, P < 0.05, ηP2 = 0.12), with a significantly greater response to the Different condition compared with the Same condition (main effect contrast, P < 0.05, Cohen’s d = 0.70), a marginal difference between the Different and L/A conditions (main effect contrast, P = 0.10, Cohen’s d = 0.51), and no significant difference between the L/A and Same conditions (main effect contrast, P = 0.38, Cohen’s d = 0.28). These results demonstrate the expected fMRI adaptation effect (i.e., Different > Same) in the RSC, but no clear sensitivity to L/A information in pictures of scenes (i.e., L/A ≈ Same). Although the marginal difference between the L/A and Different conditions suggests that the RSC is not totally insensitive to L/A changes in pictures of scenes, it nonetheless shows a tolerance to these changes—i.e., no significant difference between the L/A and Same conditions—in contrast to the PPA and OPA.
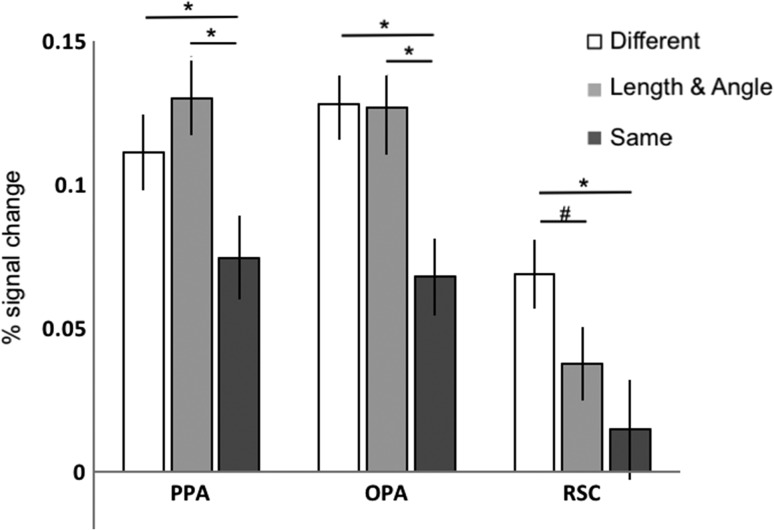
The above analyses suggest that the 3 scene-selective cortical regions respond to L/A changes in pictures of scenes differently. To directly test this suggestion, we compared responses across the 3 ROIs (Fig. 3). A 3 (ROI: PPA, OPA, RSC) × 3 (Condition: Different, L/A, Same) repeated-measures ANOVA revealed a significant ROI × Condition interaction (F4,92 = 2.68, P < 0.05, ηP2 = 0.10). There was no significant difference in response between the Same and Different conditions across any of the ROIs, indicating that all 3 scene-selective regions showed the expected adaptation effect to a similar degree (interaction contrasts, all Ps > 0.15, all ηP2s < 0.09). In contrast, comparison of the L/A and Same conditions revealed a greater difference between these conditions in the PPA relative to the RSC (interaction contrast, P < 0.05, ηP2 = 0.18), a marginal difference between these conditions in the OPA relative to the RSC (interaction contrast, P = 0.10, ηP2 = 0.11), and no significant difference between these conditions in the PPA relative to the OPA (interaction contrast, P = 0.97, ηP2 < 0.001). Taken together, these results demonstrate that the PPA and OPA represent combined changes to relative length and angle in scenes differently from the RSC, with the PPA and OPA sensitive to such changes and the RSC tolerant to such changes.
Figure 3.
The average response (percent signal change) from 5 to 9 s in the Different (white), Length and Angle (light gray bars), and Same (dark gray bars) conditions in each ROI, demonstrating that the PPA and the OPA are sensitive to changes in relative length and angle, while the RSC is tolerant to such changes. (*the difference is significant at P < 0.05, #the difference is marginally significant at P = 0.10.)
Are these responses to L/A changes in the PPA and OPA (and in the RSC, since it is tolerant, not totally insensitive to such changes) specific to pictures of scenes, or might these ROIs respond to any L/A changes regardless of whether they are presented in the context of scenes? To address this question, we measured the response to L/A changes in pictures of objects in each ROI. A 3-level (Condition: Different, L/A, Same) repeated-measures ANOVA on the average response to pictures of objects from 5 to 9 s revealed no significant main effect of condition in any of the scene-selective ROIs (all Ps > 0.15, all ηP2s < 0.08). Thus, none of the scene-selective ROIs even showed the expected adaptation effect (i.e., Different > Same) to object stimuli, revealing a qualitatively different pattern of activation to pictures of objects versus pictures of scenes. These results suggest that the sensitivity to combined changes to relative length and angle in the PPA and the OPA is specific to pictures of scenes.
Might the sensitivity to L/A changes in pictures of scenes in the PPA and OPA be due to a feed-forward effect from earlier visual areas, rather than to a sensitivity intrinsic to each area? We do not think this could be the case because participants were asked to fixate while the stimuli were moving across the fovea, thus disrupting early retinotopic representations. Nevertheless, we directly addressed this question by asking whether L/A changes in our stimuli were represented in an anatomically defined EVC ROI. A 3-level (Condition: Different, L/A, Same) repeated-measures ANOVA on the average response to pictures of scenes from 5 to 9 s revealed that the EVC did not even show the expected fMRI adaptation effect (i.e., Different > Same; F2,46 = 0.47, P = 0.63, ηP2 = 0.02), thus revealing a qualitatively different pattern of activation in the EVC than in the scene-selective cortex. These results suggest that the sensitivity to combined changes to relative length and angle in scenes is not due to adaptation in the early visual areas for either the PPA or the OPA.
Our second main analysis investigated how relative length and angle might contribute separately to the response to the combined changes to relative length and angle across scene-selective cortex, observed in our first analysis. Despite its tolerance to combined changes to relative length and angle compared with, the PPA and OPA, we included the RSC in this analysis because the RSC might nevertheless still be sensitive to changes in relative length alone or to angle alone when other geometric properties are equated. Since we are interested in how changes to relative length only and angle only combine to explain the response to the changes in both relative length and angle (i.e., L/A changes), the crucial comparison is between the isolated changes (i.e., Length or Angle), the combined change (i.e., L/A), and the Same condition. (We still included the Different condition in our analysis for completeness, even though it is irrelevant to how the responses to the isolated changes combine to equal the response to the combined change.) Thus, if relative length alone is driving sensitivity to L/A changes, then the response to the Length changes will look like the L/A condition and will differ from the Angle and Same conditions. By contrast, if angle alone is driving sensitivity to L/A changes, then the response to the Angle changes will resemble the L/A condition and differ from the Length and Same conditions. Other patterns (e.g., Length and Angle somewhere in between both the L/A and Same conditions) would suggest that both geometric properties underlie cortical responses to combined changes in relative length and angle.
We found evidence that both types of information in combination underlie sensitivity to L/A changes in the PPA: A 5-level (Condition: L/A, Length, Angle, Same, Different) repeated-measures ANOVA revealed a significant main effect of Condition (F4,92 = 2.74, P < 0.05, ηP2 = 0.11), with a greater response to both the L/A and Different conditions compared with the Same condition (main effect contrasts, both Ps < 0.05, Cohen’s ds > 0.54), consistent with the PPA’s sensitivity to L/A changes, as reported in our first analysis. Next, the response to the Length condition was marginally greater than the Same condition (main effect contrast, P = 0.06, Cohen’s d = 0.42), and not significantly different from either the L/A or Different conditions (main effect contrasts, both P > 0.20, Cohen’s ds < 0.38), while the response to the Angle condition was not significantly different from the Same condition (main effect contrast, P = 0.22, Cohen’s d = 0.36), marginally different from the L/A condition (main effect contrast, P = 0.06, Cohen’s d = 0.48), and not significantly different from the Different condition (main effect contrast, P = 0.49, Cohen’s d = 0.17). These results could suggest that relative length alone, but not angle alone, drives responses to L/A changes in the PPA. Crucially, however, there was no difference between the Length and Angle conditions (main effect contrast, P = 0.81, Cohen’s d = 0.07): If relative length or angle alone were driving the response to combined length and angle, the response to the isolated changes to relative length and angle would differ from each other. Thus, taken together, these results reveal that the response to the Length and Angle conditions were somewhere in between the L/A and Same conditions, suggesting that both relative length and angle modulate the activity of the PPA.
Similar findings emerged from the analyses of the OPA. A 5-level (Condition: L/A, Length, Angle, Same, Different) repeated-measures ANOVA revealed a significant main effect of Condition (F4,92 = 3.79, P < 0.01, ηP2 = 0.14), with a greater response to both the L/A and Different conditions compared with the Same condition (main effect contrasts, both P < 0.01, Cohen’s ds < 0.96), consistent with the OPA’s sensitivity to L/A changes, as reported in our first analysis. Next, the response to the Length condition was marginally greater than the Same condition (main effect contrast, P = 0.06, Cohen’s d = 0.53), and not significantly different from the L/A or Different conditions (main effect contrasts, both P > 0.19, Cohen’s ds < 0.26), while the response to the Angle condition was marginally different from both the Same condition (main effect contrast, P = 0.10, Cohen’s d = 0.35) and the L/A condition (main effect contrast, P = 0.07, Cohen’s d = 0.47), and significantly different from the Different condition (main effect contrast, P < 0.05, Cohen’s d = 0.61). Finally, there was no difference between the Length and Angle conditions (main effect contrast, P = 0.53, Cohen’s d = 0.21). Taken together, these results suggest that the OPA, like the PPA, responds to both relative length and angle.
By contrast, we did not find clear evidence for sensitivity to any shape changes (L/A, Length, or Angle) in the RSC. A 5-level (Condition: L/A, Length, Angle, Same, Different) repeated-measures ANOVA revealed a marginal main effect of Condition (F4,92 = 2.27, P = 0.07, ηP2 = 0.09). Note, however, that the marginal main effect of condition here is due to the inclusion of the Different condition—which we noted above is not necessary for this analysis, but is included for completeness. When we remove the Different condition from the ANOVA, we find no significant main effect of condition (F3,69 = 1.05, P = 0.38, ηP2 = 0.04). These results demonstrate that while we find the expected adaptation in RSC (i.e., Different > Same), none of the other conditions differed from each other, consistent with the RSC’s tolerance to L/A changes, as reported in our first analysis.
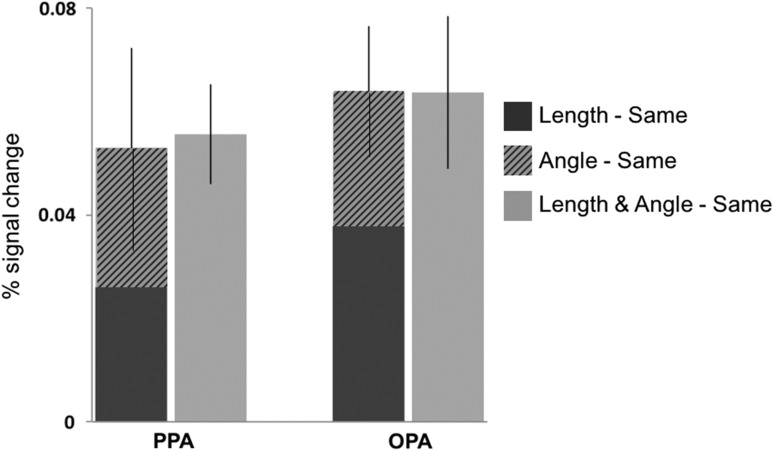
Given that neither length alone nor angle alone accounts for the observed responses to the changes in both length and angle in either the PPA or OPA, we conducted a further analysis probing how relative length and angle might combine to explain the response to shape changes in scenes in these regions. Since we did not find any evidence of sensitivity to any shape changes in the RSC, we did not include this region in this analysis. We first normalized each shape-change condition by subtracting the activation from the Same condition (i.e., Length–Same, Angle–Same, and L/A–Same) in each ROI of each participant, and then we examined how responses to changes in each geometric property separately contributed to the overall response to changes in both properties together (Fig. 4). Using paired samples t-tests, we found that the sum of the activation to the Length–Same and Angle–Same conditions was not significantly different from the L/A–Same condition in either the PPA, (t(23) = 0.13, P = 0.90, Cohen’s d = 0.02) or the OPA (t(23) = 0.21, P = 0.83, Cohen’s d = 0.05), and that the Length–Same and Angle–Same conditions were not significantly different from each other in either region (PPA: t(23) = 0.05, P = 0.96, Cohen’s d = 0.01; OPA: t(23) = 0.64, P = 0.53, Cohen’s d = 0.16). These findings suggest that an equal and additive effect of relative length and angle may underlie sensitivity to shape changes involving both properties in the PPA and OPA. Future studies could more specifically test for combinatorial effects of these geometric changes in the scene-selective ROIs by parametrically varying the magnitude of change in each shape property.
Figure 4.
The stacked responses to Length–Same (dark gray bar) and Angle–Same (striped bar) compared with the (Length and Angle)–Same (light gray bar) condition in the PPA and OPA. We found a roughly equal and additive effect of relative length and angle changes on responses in these regions.
Object-Selective Cortex
Our analyses of scene-selective ROIs demonstrated that the geometric information typically associated with object recognition (i.e., relative length and angle) is represented in some regions of scene-selective cortex when these properties are presented in the context of scenes. Given that relative length and angle are classically associated with object recognition, does object-selective cortex represent these properties in our stimuli? We tested whether and how object-selective ROIs (LO and pFs) represent relative length and angle in pictures of objects and scenes.
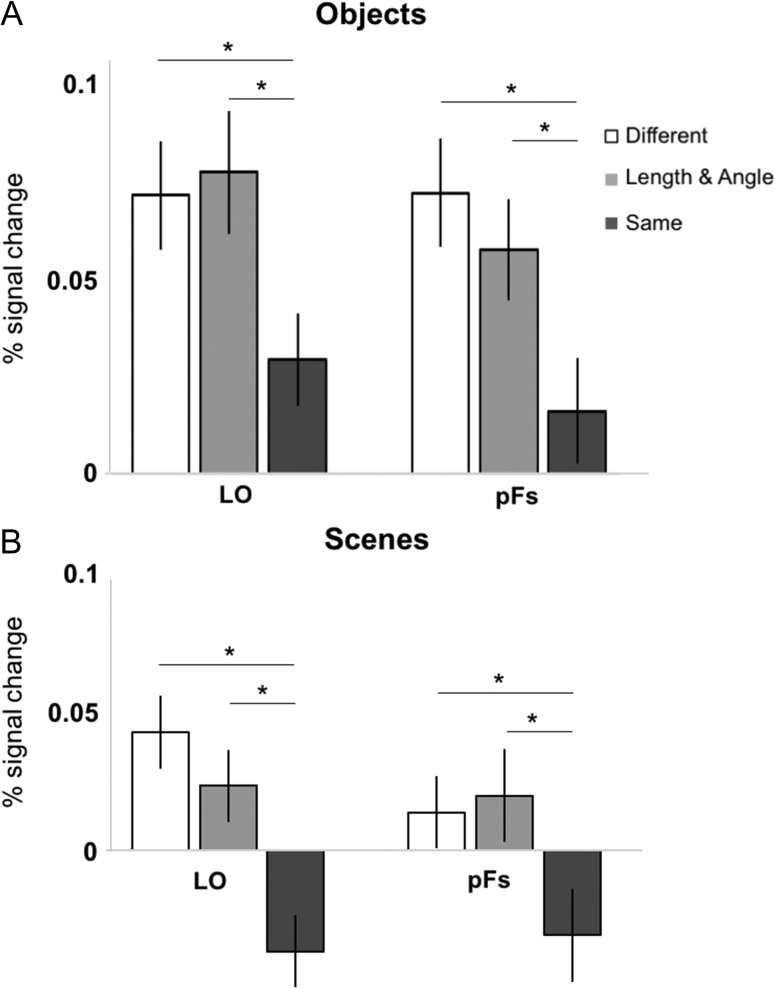
First, we investigated whether the LO and pFs are sensitive to combined changes to relative length and angle in pictures of objects (i.e., their preferred category) (Fig. 5A). As predicted, A 3-level (Condition: Different, L/A, Same) repeated-measures ANOVA on the average response between 6 and 10 s revealed a significant main effect of Condition in both ROIs (LO: F2,46 = 4.07, P < 0.05, ηP2 = 0.15, pFs: F2,38 = 4.97, P < 0.01, ηP2 = 0.21), with a greater response to the Different and L/A conditions compared with the Same condition in both ROIs (main effect contrasts, all Ps < 0.05, all Cohen’s ds > 0.65). There was no significant difference between the Different and L/A conditions in either ROI (main effect contrasts, both Ps > 0.45, both Cohen’s ds < 0.25). These results demonstrate the expected fMRI adaptation effect (i.e., Different > Same) as well as a sensitivity to combined changes to relative length and angle in pictures of objects (i.e., L/A > Same) in both LO and pFs, consistent with a wealth of evidence that object recognition depends in part on integrated representations of length and angle. A 2 (ROI: LO, pFs) × 3 (Condition: Different, L/A, Same) repeated-measures ANOVA revealed no significant ROI × Condition interaction (F2,38 = 1.04, P = 0.37, ηP2 = 0.05), thus confirming that the LO and pFs represent the combined changes to length and angle relations in objects in a similar way.
Figure 5.
(A) The average response (percent signal change) to pictures of objects from 6 to 10 s in the Different (white), Length and Angle (light gray bars), and Same (dark gray bars) conditions in each ROI, demonstrating that the LO and pFs are sensitive to changes in relative length and angle in pictures of objects. (B) The average response (percent signal change) to pictures of scenes from 6 to 10 s in the Different (white), Length and Angle (light gray bars), and Same (dark gray bars) conditions in each ROI, demonstrating that the LO and pFs are sensitive to changes in relative length and angle in pictures of scenes. (*the difference is significant at P < 0.05.)
Next, we asked if the responses to L/A changes in the LO and pFs are specific to pictures of objects, or if these ROIs respond to L/A changes in pictures of scenes as well. Interestingly, a 3-level (Condition: Different, L/A, Same) repeated-measures ANOVA revealed a significant main effect of Condition in both ROIs (LO: F2,46 = 5.82, P < 0.01, ηP2 = 0.20, pFs: F2,38 = 3.17, P = 0.05, ηP2 = 0.14), with a greater response to the Different and L/A conditions compared with the Same condition in both ROIs (main effect contrasts, all Ps < 0.05, all Cohen’s ds > 0.70) (Fig. 5B). There was no significant difference between the Different and L/A conditions in either ROI (main effect contrasts, both Ps > 0.45, both Cohen’s ds < 0.30). These results demonstrate the expected fMRI adaptation effect (i.e., Different > Same) as well as a sensitivity to combined changes to relative length and angle in pictures of scenes (i.e., L/A > Same) in LO and pFs. A 2 (ROI: LO, pFs) × 3 (Condition: Different, L/A, Same) repeated-measures ANOVA revealed no significant ROI × Condition interaction (F2,38 = 1.91, P = 0.18, ηP2 = 0.09). LO and pFs therefore appear to be sensitive to relative length and angle changes in pictures of scenes as well as in pictures of objects.
Though the above analysis suggests that these object-selective regions respond similarly to objects and scenes, a 2 (ROI: LO, pFs) × 2 (Picture Type: Object, Scene) × 3 (Condition: Different, L/A, Same) repeated-measures ANOVA revealed a significant main effect of condition in both ROIs (F1,19 = 18.19, P < 0.001, all ηP2 = 0.49), demonstrating that the object condition elicits a significantly greater response compared with the scene condition in LO and pFs, consistent with object-selectivity in both ROIs. Further, we found no significant Picture Type × Condition interaction (F2,36 = 0.50, P = 0.61, all ηP2 = 0.03), demonstrating that the object-selective ROIs represent changes to relative length and angle similarly regardless of whether those changes occur in the context of objects or scenes. Therefore, we collapsed across objects and scenes in each ROI for further analysis.
Since both the LO and pFs were sensitive to combined changes to relative length and angle in pictures of objects and scenes, our second analysis investigated how relative length and angle might contribute separately to the responses to changes in both properties across object-selective cortex, as observed in our first analysis. We found evidence that both types of information in combination underlie sensitivity to L/A changes in LO: A 5-level (Condition: L/A, Length, Angle, Same, Different) repeated-measures ANOVA revealed a significant main effect of Condition (F4,92 = 7.54, P < 0.001, ηP2 = 0.25), with a greater response to both the L/A and Different conditions compared with the Same condition (main effect contrasts, both Ps < 0.001, Cohen’s ds > 1.33), consistent with LO’s sensitivity to L/A changes, as reported in our first analysis. Next, the response to the Length condition was not significantly different from the Same condition (main effect contrast, P = 0.24, Cohen’s d = 0.36) but was significantly different from the L/A and Different conditions (main effect contrasts, both Ps < 0.05, Cohen’s ds > 0.74). The response to the Angle condition was significantly different from the Same condition (main effect contrast, P < 0.01, Cohen’s d = 0.96), marginally different from the Different condition (main effect contrast, P = 0.07, Cohen’s d = 0.62), and not significantly different from the L/A condition (main effect contrast, P = 0.21, Cohen’s d = 0.40). Finally, there was no difference between the Length and Angle conditions (main effect contrast, P = 0.19, Cohen’s d = 0.42). Taken together, these results reveal that the responses to the Length and Angle conditions were somewhere in between the L/A and Same conditions, suggesting that combined representations of relative length and angle underlie LO’s sensitivity to shape in objects and scenes.
We also found evidence that both geometric properties in combination underlie sensitivity to L/A changes in the pFs. A 5-level (Condition: L/A, Length, Angle, Same, Different) repeated-measures ANOVA revealed a significant main effect of Condition (F4,76 = 4.34, P < 0.01, ηP2 = 0.19), with a greater response to both the L/A and Different conditions compared with the Same condition (main effect contrasts, both Ps < 0.01, Cohen’s ds > 1.00), consistent with the pFs’s sensitivity to L/A changes, as reported in our first analysis. Next, the response to the Length condition was marginally different from both the Same and Different conditions (Same: main effect contrast, P = 0.07, Cohen’s d = 0.60, Different: main effect contrast, P = 0.08, Cohen’s d = 0.64) and significantly different from the L/A condition (main effect contrast, P < 0.05, Cohen’s d = 0.60). The response to the Angle condition was significantly different from the Same condition (main effect contrast, P < 0.05, Cohen’s d = 0.68) but not significantly different from either the L/A or Different conditions (main effect contrasts, both Ps > 0.23, Cohen’s ds < 0.42). Crucially, however, there was no difference between the Length and Angle conditions (main effect contrast, P = 0.64, Cohen’s d = 0.14). Taken together, these results reveal that the response to the Length and Angle conditions lie somewhere between the L/A and Same conditions, suggesting an combined response to relative length and angle in the pFs.
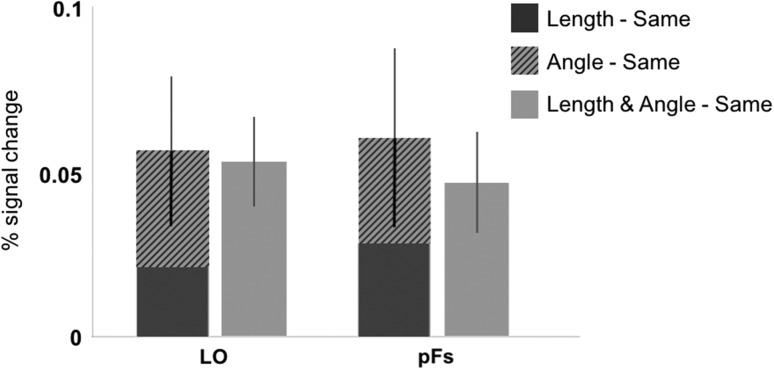
Given that neither length alone nor angle alone drives the sensitivity to the combined length and angle information in the LO and pFS, we conducted a further analysis probing how these properties might combine to explain the response to shape changes in these regions (as conducted above with scene-selective cortex) (Fig. 6). Using paired samples t-tests, we first found that the Length–Same and Angle–Same conditions were not significantly different from each other in either region (LO: t(23) = 1.34, P = 0.19, Cohen’s d = 0.31; pFs: t(19) = 0.48, P = 0.64, Cohen’s d = 0.09). We also found that the sum of the activation to the Length–Same plus Angle–Same condition was not significantly different from the L/A–Same condition in either LO or pFs (LO: t(23) = 0.21, P = 0.84, Cohen’s d = 0.04, pFs: t(19) = 0.79, P = 0.44, Cohen’s d = 0.14). These findings suggest that equal and additive effects of relative length and angle may underlie sensitivity to the combined changes to both properties in the LO and pFS.
Figure 6.
The stacked responses collapsed across object and scene conditions to Length–Same (dark gray bar) and Angle–Same (striped bar) compared with the (Length and Angle)–Same (light gray bar) condition in LO and in pFs. We found a roughly equal and additive effect of relative length and angle changes on responses in LO and pFs.
Discussion
The current study asked whether and how scene-selective cortical regions, especially the PPA, represent shape information about the spatial layout of scenes, specifically relative length and angle. The results demonstrate differential sensitivity to relative length and angle across 3 scene-selective regions. Using an fMRI adaptation paradigm, we found that 2 scene-selective regions, the PPA and the OPA, were sensitive to combined changes to relative length and angle in the layout of scenes, while the RSC, another scene-selective region, was tolerant to such changes. These results are specific to pictures of scenes, not to pictures of objects, and cannot be explained by a feed-forward effect from earlier visual areas. Thus, these findings demonstrate that the geometric properties typically associated with object recognition and categorization are also used during some aspects of scene processing. Moreover, these findings, coupled with the findings that the OPA and the RSC represent the sense and egocentric distance relations used for navigation while the PPA does not (Dilks et al. 2011; Persichetti and Dilks 2016), suggest that the 3 scene-selective ROIs each respond to different geometric properties of scenes. Specifically, the PPA represents relations of length and angle, the RSC represents relations of sense and egocentric distance, and the OPA represents all of this information.
Our finding that the PPA is sensitive to relative length and angle changes in scenes dovetails with previous findings that the PPA represents the shape of a scene in terms of the openness or the continuity of its spatial layout (Epstein and Kanwisher 1998; Kravitz et al. 2011; Park et al. 2011; Kamps et al. 2016a). The present findings, however, extend this work by specifying that the shape sensitivity in the PPA can be explained by a response to basic length and angle relations among the extended surfaces in scenes. The suggested additive effect of relative length and angle changes to scenes, moreover, indicates that the sensitivity to combined changes to relative length and angle in the PPA depends on both properties roughly equally, perhaps by a change in the aspect ratio of a scene, as relative length and angle changes both give rise to such a shape change in planar geometry (Euclid 300 B.C.E./1990). Finally, our results also lend support to the conjecture made by Walther and Shen (2014), based on behavioral data, that the PPA may represent contour junctions and contour lengths in a scene, which are both used for scene categorization. Thus, the main role of the PPA in scene processing may not be for navigation through a scene, but rather for scene categorization (e.g., recognizing a place as a kitchen or beach), consistent with the classic evidence that shape analysis is central to object recognition and categorization (Landau et al. 1992, 1998; Dehaene et al. 2006; Izard and Spelke 2009).
We also found that the OPA is sensitive to relative length and angle in pictures of scenes. Here too the effect of each geometric property appeared to be equal and additive. Nevertheless, we believe that detection of relative length and angle in the OPA may subserve a different role than that of the PPA in scene processing. Given that the PPA is not sensitive to either sense or egocentric distance (Dilks et al. 2011; Persichetti and Dilks 2016), both of which are crucial for navigation (e.g., see Cheng and Newcombe 2005 and Spelke and Lee 2012 for reviews), while the OPA is sensitive to such information (Dilks et al. 2011; Persichetti and Dilks 2016), we propose that the OPA may represent egocentric spatial information about local scene elements, such as the distance and direction of boundaries and obstacles relative to the viewer (Julian et al. 2016), as well as the size and shape (e.g., relative lengths and angles) of boundaries and obstacles in the service of active, visually guided navigation through the local environment (Kamps et al. 2016a; Kamps et al. 2016b; Bonner and Epstein, 2017). During such visually guided navigation, navigators must represent and update their location relative to the boundaries and obstacles in the immediate surroundings, ostensibly requiring sensitivity to all of the types of geometric information shown to be represented in the OPA, including sense, egocentric distance, relative length, and angle.
The RSC was the only scene-selective region found to be tolerant to relative length and angle changes in scenes. While the RSC is sensitive to the intact versus fragmented 3D structure of a scene (Kamps et al. 2016a) and to the presence of rectilinear rather than curvilinear figures (Nasr and Tootell 2012), responses to the basic shape properties of a scene may not be directly relevant to the putative function of the RSC. Specifically, several studies have shown that the RSC integrates representations of the local environment with representations of the broader environment, encodes the locations of stable landmarks in the environment (Auger et al. 2012, 2015; Troiani et al. 2012), and tracks the location and heading direction of a navigator (Epstein et al. 2007; Baumann and Mattingley 2010; Vass and Epstein 2013; Marchette et al. 2014). In doing so, the RSC may underlie memory-guided navigation, situating the immediate surroundings in a broader context and tracking the navigator’s position relative to the stable, enduring environment. Such a function need not directly represent the basic shape properties of the local spatial layout that were tested here.
Finally, we examined whether and how object-selective regions of cortex represent shape in the context of both objects and scenes. We found that both the LO and pFs are sensitive to relative length and angle not only in the context of objects, but also in the context of scenes, suggesting that the lateral occipital complex may support a secondary pathway for scene processing, as has been proposed in prior work (MacEvoy and Epstein 2011; Epstein 2014). We also found that both the LO and pFs may represent relative length and angle in an additive fashion.
In conclusion, we have shown that the PPA is sensitive to the geometric properties classically associated with object recognition and categorization, but only in the context of scenes. Moreover, we have shown that the OPA is also sensitive to such changes, while the RSC is tolerant to such changes. These findings, coupled with the fact that the PPA is not sensitive to the geometric properties that guide navigation (i.e., sense and egocentric distance), while the OPA is sensitive to those properties, suggest that PPA may be involved in scene categorization and the OPA may be involved in visually guided navigation.
Notes
We would like to thank the Facility for Education and Research in Neuroscience (FERN) Imaging Center in the Department of Psychology, Emory University, Atlanta, GA. Conflict of Interest: None declared.
Funding
Emory College, Emory University (to D.D.D.), National Eye Institute (grant T32 EY007092 to A.S.P.), the Harvard University Mind, Brain, and Behavior Initiative (M.R.D. and E.S.S.), the Center for Brains, Minds and Machines (NSF STC award CCF-1231216), and the National Science Foundation Graduate Research Fellowship (DGE-1144152 to M.R.D.; DGE-1444932 to A.S.P.).
References
- Amunts K, Malikovic A, Mohlberg H, Schormann T, Zilles K. 2000. Brodmann’s areas 17 and 18 brought into stereotaxic space-where and how variable? NeuroImage. 11:66–84. [DOI] [PubMed] [Google Scholar]
- Auger SD, Mullally SL, Maguire EA. 2012. Retrosplenial cortex codes for permanent landmarks. PLoS One. 7:e43620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger SD, Zeidman P, Maguire EA. 2015. A central role for the retrosplenial cortex in de novo environmental learning. eLife. 4:e09031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann O, Mattingley JB. 2010. Medial parietal cortex encodes perceived heading direction in humans. J Neurosci Off J Soc Neurosci. 30:12897–12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner MF, Epstein RA. 2017. Coding of navigational affordances in the human visual system. Proc Natl Acad Sci. 114(18):4793–4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burock MA, Buckner RL, Woldorff MG, Rosen BR, Dale AM. 1998. Randomized event-related experimental designs allow for extremely rapid presentation rates using functional MRI. Neuroreport. 9:3737–3739. [DOI] [PubMed] [Google Scholar]
- Cheng K, Newcombe NS. 2005. Is there a geometric module for spatial orientation? Squaring theory and evidence. Psychon Bull Rev. 12:1–23. [DOI] [PubMed] [Google Scholar]
- Dale AM, Greve DN, Burock MA. 1999. Stimulus sequences for event-related fMRI. Hum Brain Mapp. 8:109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Izard V, Pica P, Spelke E. 2006. Core knowledge of geometry in an Amazonian indigene group. Science. 311:381–384. [DOI] [PubMed] [Google Scholar]
- Dilks DD, Julian JB, Kubilius J, Spelke ES, Kanwisher N. 2011. Mirror-image sensitivity and invariance in object and scene processing pathways. J Neurosci. 31:11305–11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilks DD, Julian JB, Paunov AM, Kanwisher N. 2013. The occipital place area is causally and selectively involved in scene perception. J Neurosci. 33:1331–1336a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein RA. 2014. Neural systems for visual scene recognition In: Bar M, Kveraga K, editors. Scene vision: making sense of what we see. Cambridge (MA): MIT Press. [Google Scholar]
- Epstein RA, Higgins JS, Jablonski K, Feiler AM. 2007. Visual scene processing in familiar and unfamiliar environments. J Neurophysiol. 97:3670–3683. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. 1998. A cortical representation of the local visual environment. Nature. 392:598–601. [DOI] [PubMed] [Google Scholar]
- Euclid 1990. Great books of the Western World: The thirteen books of Euclid’s Elements. The works of Archimedes, including the method. Introduction to arithmetic by Nicomachus (2 ed.). Chicago: Encyclopedia Britannica. (Original work written 300 B.C.E.). [Google Scholar]
- Ghaem O, Mellet E, Crivello F, Tzourio N, Mazoyer B, Berthoz A, Denis M. 1997. Mental navigation along memorized routes activates the hippocampus, precuneus, and insula. Neuroreport. 8:739–744. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Edelman S, Avidan G, Itzchak Y, Malach R. 1999. Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron. 24:187–203. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Hendler T, Edelman S, Itzchak Y, Malach R. 1998. A sequence of object-processing stages revealed by fMRI in the human occipital lobe. Hum Brain Mapp. 6:316–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Malach R. 2001. fMR-adaptation: a tool for studying the functional properties of human cortical neurons. Acta Psychol (Amst). 107:293–321. [DOI] [PubMed] [Google Scholar]
- Izard V, Spelke ES. 2009. Development of sensitivity to geometry in visual forms. Hum Evol. 23:213–248. [PMC free article] [PubMed] [Google Scholar]
- Janzen G, van Turennout M. 2004. Selective neural representation of objects relevant for navigation. Nat Neurosci. 7:673–677. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 17:825–841. [DOI] [PubMed] [Google Scholar]
- Julian JB, Ryan J, Hamilton RH, Epstein RA. 2016. The occipital place area is causally involved in representing environmental boundaries during navigation. Curr Biol. 26:1104–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps FS, Julian JB, Kubilius J, Kanwisher N, Dilks DD. 2016. a. The occipital place area represents the local elements of scenes. NeuroImage. 132:417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps FS, Lall V, Dilks DD. 2016. b. The occipital place area represents first-person perspective motion information through scenes. Cortex J Devoted Study Nerv Syst Behav. 83:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtzi Z, Kanwisher N. 2001. Representation of perceived object shape by the human lateral occipital complex. Science. 293:1506–1509. [DOI] [PubMed] [Google Scholar]
- Kravitz DJ, Peng CS, Baker CI. 2011. Real-world scene representations in high-level visual cortex: it’s the spaces more than the places. J Neurosci. 31:7322–7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau B, Smith LB, Jones S. 1992. Syntactic context and the shape bias in children’s and adults’ lexical learning. J Mem Lang. 31:807–825. [Google Scholar]
- Landau B, Smith L, Jones S. 1998. Object shape, object function, and object name. J Mem Lang. 38:1–27. [Google Scholar]
- Lee SA, Vallortigara G, Flore M, Spelke ES, Sovrano VA. 2013. Navigation by environmental geometry: the use of zebrafish as a model. J Exp Biol. 216:3693–3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Kravitz DJ, Baker CI. 2012. Disentangling visual imagery and perception of real-world objects. Neuroimage. 59:4064–4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacEvoy SP, Epstein RA. 2011. Constructing scenes from objects in human occipitotemporal cortex. Nat Neurosci. 14:1323–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA. 2001. The retrosplenial contribution to human navigation: a review of lesion and neuroimaging findings. Scand J Psychol. 42:225–238. [DOI] [PubMed] [Google Scholar]
- Marchette SA, Vass LK, Ryan J, Epstein RA. 2014. Anchoring the neural compass: coding of local spatial reference frames in human medial parietal lobe. Nat Neurosci. 17:1598–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasr S, Tootell RBH. 2012. A cardinal orientation bias in scene-selective visual cortex. J Neurosci. 32:14921–14926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, Burgess N. 1996. Geometric determinants of the place fields of hippocampal neurons. Nature. 381:425–428. [DOI] [PubMed] [Google Scholar]
- Park S, Brady TF, Greene MR, Oliva A. 2011. Disentangling scene content from spatial boundary: complementary roles for the parahippocampal place area and lateral occipital complex in representing real-world scenes. J Neurosci. 31:1333–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persichetti AS, Dilks DD. 2016. Perceived egocentric distance sensitivity and invariance across scene-selective cortex. Cortex. 77:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauchs G, Orban P, Balteau E, Schmidt C, Degueldre C, Luxen A, Maquet P, Peigneux P. 2008. Partially segregated neural networks for spatial and contextual memory in virtual navigation. Hippocampus. 18:503–518. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Ziegler M, Winocur G, Grady CL, Moscovitch M. 2004. “I have often walked down this street before”: fMRI studies on the hippocampus and other structures during mental navigation of an old environment. Hippocampus. 14:826–835. [DOI] [PubMed] [Google Scholar]
- Smith SM. 2002. Fast robust automated brain extraction. Hum Brain Mapp. 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. 2004. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 23(Suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- Spelke ES, Lee SA. 2012. Core systems of geometry in animal minds. Philos Trans R Soc Lond B Biol Sci. 367:2784–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spelke E, Lee SA, Izard V. 2010. Beyond core knowledge: natural geometry. Cogn Sci. 34(5):863–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troiani V, Stigliani A, Smith ME, Epstein RA. 2012. Multiple object properties drive scene-selective regions. Cereb Cortex. 24:883–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vass LK, Epstein RA. 2013. Abstract representations of location and facing direction in the human brain. J Neurosci. 33:6133–6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousif SR, Lourenco SF. 2017. Are all geometric cues created equal? Children's use of distance and length for reorientation. Cogn Dev. 43:159–169. [Google Scholar]
- Walther DB, Shen D. 2014. Nonaccidental properties underlie human categorization of complex natural scenes. Psychol Sci. 25:851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]