Abstract
Acid-sensing ion channels (ASICs) are the major proton receptor in the brain and a key mediator of acidosis-induced neuronal injuries in disease. Most of published data on ASIC function came from studies performed in mice, and relatively little is known about potential differences between human and mouse ASICs (hASIC and mASIC, respectively). This information is critical for us to better interpret the functional importance of ASICs in human disease. Here, we examined the expression of ASICs in acutely resected human cortical tissue. Compared with mouse cortex, human cortical tissue showed a similar ratio of ASIC1a:ASIC2a expression, had reduced ASIC2b level, and exhibited a higher membrane:total ratio of ASIC1a. We further investigated the mechanism for higher surface trafficking of hASIC1a in heterologous cells. A single amino acid at position 285 was critical for increased N-glycosylation and surface expression of hASIC1a. Consistent with the changes in trafficking and current, cells expressing hASIC1a or mASIC1a S285P mutant had a higher acid-activated calcium increase and exhibited worsened acidotoxicity. These data suggest that ASICs are likely to have a larger impact on acidosis-induced neuronal injuries in humans than mice, and this effect is, at least in part, a result of more efficient trafficking of hASIC1a.—Xu, Y., Jiang, Y.-Q., Li, C., He, M., Rusyniak, W. G., Annamdevula, N., Ochoa, J., Leavesley, S. J., Xu, J., Rich, T. C., Lin, M. T., Zha, X.-M. Human ASIC1a mediates stronger acid-induced responses as compared with mouse ASIC1a.
Keywords: acidosis, trafficking, neuronal injury, calcium
In recent years, acid-induced signaling has attracted more and more attention in neuroscience research. Synaptic transmission reduces pH at the synaptic cleft, and this acidification contributes to postsynaptic currents and synaptic plasticity (1–4). Moreover, acidosis is common in neurologic diseases and is a major cause of neuronal injury in diseases, such as ischemia (5, 6). These observations indicate that proton-mediated signaling is important for both physiologic and pathophysiological responses. One key neuronal proton receptor in the brain is the acid-sensing ion channels (ASICs), a family of proton-gated cation channels (7). The ASIC subunits expressed throughout mouse brain are ASIC1a, -2a, and -2b (8). Electrophysiological studies further showed that ASIC1a and ASIC2 subunits are the main contributor to acid-activated currents in the vast majority of brain neurons (5). ASIC1a is the key determinant of the acid-activated current, whereas ASIC2 plays important modulator roles. Most ASIC channels conduct primarily Na+, whereas ASIC1a homomeric channels conduct both Na+ and to a less extent, Ca2+ (7). The deletion of the ASIC1a gene reduced long-term potentiation and altered the density of dendritic spines (1, 3, 4, 9). ASIC1a null mice have altered behavioral outcome in multiple fear- and anxiety-related tests (10–12). In addition, in acidotic conditions in vitro, as well as in rodent models of ischemia, multiple sclerosis, and traumatic brain injury, the deletion of ASIC1a or ASIC2 has a protective effect (13–17). These data demonstrate that ASICs, especially ASIC1a, are important mediators of acid signaling in the brain and a promising therapeutic target to alleviate acidosis-related neuronal injuries.
Whereas the findings on ASICs, especially ASIC1a, are inspiring, the studies mostly were performed in rodents. To explore the function and regulation of ASICs in humans, it is important to define better the expression of ASICs in the human brain, as well as mechanisms regulating human ASIC (hASIC) trafficking and function. Although mouse ASIC1a (mASIC1a) and hASIC1a share high homology (98% amino acid identity), previous studies have implicated several differences between hASICs and mASICs. Acutely cultured human neurons had acid-activated currents that suggest an increased contribution from ASIC1a (18). In our previous study, when transfected into Chinese hamster ovary (CHO) cells or Xenopus oocytes, hASIC1a generated a higher acid-activated current than mASIC1a (19). In addition, the expression of hASIC1a increased spine density in hippocampal slices, whereas the expression of mASIC1a had little effect (9, 19). These results indicate that despite the high homology, there exist key differences in the biogenesis and/or function of hASIC and mASICs.
Here, we determined the relative expression of ASIC1a and ASIC2 in human tissue. We found that human cortical tissues had a higher level of ASIC1a in the membrane fraction compared with mouse cortical tissues. We further used heterologous cells to determine the mechanism that underlies differential surface expression of hASIC1a and mASIC1a and examined their differential effects on acid-activated current, acid-induced increase of intracellular calcium, and acid-induced injury.
MATERIALS AND METHODS
Human brain tissue
The protocols for obtaining and using the human tissues were reviewed and approved by the Institutional Review Board and Institutional Biosafety Committee at the University of South Alabama. Cortical human tissues were obtained with patient consent. Human tissues used in this study were acutely resected from 10 patients (7 females and 3 males; age range from 17 to 64, with an average age of 41.1 ± 5.9) who underwent surgical treatment of intractable epilepsy. The resection of a small piece of temporal lobe tissue is necessary to gain access to the epilepsy loci, which typically were located to hippocampus (also see Fig. 1A). These cortical tissues were normally considered surgical waste (20). Resected tissues were stored at −80°C until biochemical analysis. For the experiments on isolation of membrane preparation, some tissues underwent a period at approximately −20°C, as a result of an unexpected failure of a −80°C freezer. However, these tissues yielded similar results on ASIC expression compared with those that were frozen at −80°C all the time, and the results were combined in our analysis (see Fig. 1).
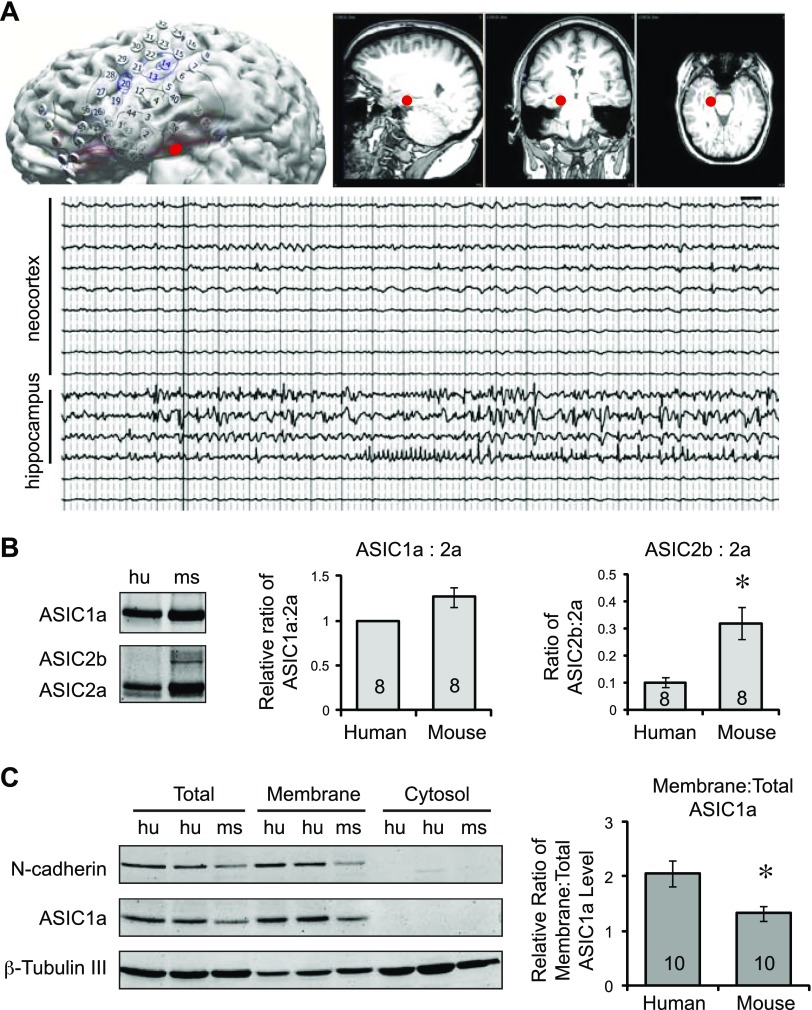
Figure 1.
Expression of ASIC1 and -2 in human cortical tissue. A) A typical set of images and traces showing the identification of epileptic foci in 1 patient. Diagram illustrates the layout of the recording electrodes (top left), whereas the MRIs on the right illustrate the location of the epileptic foci (indicated by a red dot) in hippocampus. EEG traces from neocortex and hippocampus: hippocampus exhibited epileptic patterns of activities, which were absent in cortical traces (bottom). Note that the cortical samples were used in this study for comparing ASIC expression between human and mouse brain. B) Representative Western blot images and quantification showing the expression of ASIC1a, -2a, and -2b in human (hu) and mouse (ms) cortical tissues (left). Acutely resected human cortical tissues were obtained, as described in Materials and Methods. Human and mouse cortical tissues were blotted for ASIC1 and ASIC2, which recognize both ASIC2a and -2b. Antibody specificity was verified using the corresponding ASIC knockouts (14, 21). Human and mouse tissues were not statistically different in the relative ratio of ASIC1a:ASIC2a (P > 0.05, either by Student’s t test or Wilcoxon signed rank test) (middle). The relative ASIC2b:ASIC2a ratio was significantly lower in human tissue. P = 0.006 (Student’s t test) (right). C) Representative Western blot images and quantification showing ASIC1a levels in membrane (left). Membrane fractions from human and mouse cortical tissues were prepared, as described in Materials and Methods. Total lysate, membrane fraction, and cytosol fraction were blotted for ASIC1a, N-cadherin, and β-tubulin III. The latter 2 proteins served as controls for the quality of the preparation. Right: quantification of relative membrane:total ASIC1a ratios. Asterisks (B, C) indicate significant differences between mouse and human. Numbers in bars indicate number of repeats.
Mice
Wild-type C57BL/6 mice were maintained as breeding colonies at the University of South Alabama. Animal care met National Institutes of Health (NIH; Bethesda, MD, USA) standards, and all procedures were approved by the University of South Alabama Animal Care and Use Committee. Mice (both males and females), from 4 wk to 9 mo of age, were used. We used this age range because the relative expression ratio of ASIC1a:ASIC2a:ASIC2b in mouse brain remained constant between 3 wk and 8 mo of age (21).
Constructs and reagents
Lymphocyte-specific protein tyrosine kinase-green fluorescent protein (Lck-GFP) was kindly provided by Dr. Steven Green. N-Terminal hemagglutinin (HA)-tagged wild-type mASIC1a has been described earlier (22). HA-tagged hASIC1a was generated by PCR-mediated subcloning. Mouse ASIC1a S285P, C70H, C70H/S285P, and human P285S mutants were generated with a Quickchange Mutagenesis Kit (Stratagene, La Jolla, CA, USA), following the manufacturer’s instructions. All constructs were verified by sequencing. ASIC antibodies used the following: a rabbit anti-ASIC1 antibody (14) and a goat anti-ASIC1 (SC-13905; Santa Cruz, Biotechnology, Dallas, TX, USA) and rabbit anti-ASIC2 (21). Antibody specificity was verified by the lack of signals in corresponding ASIC knockout mice (14, 21). Other antibodies used were the following: mouse anti-N-cadherin (MA1-159; Thermo Fisher Scientific, Waltham, MA, USA); Alexa 488-, 568-, 680-, and 800-conjugated secondary antibodies (Thermo Fisher Scientific and Li-Cor Biosciences, Lincoln, NE, USA); and Dylight 488-, 680-, and 800-conjugated secondary antibodies (Thermo Fisher Scientific). Other reagents used include the following: endoglycosidase H (Endo H) and peptide-N-glycosidase F (PNGase F; New England Biolabs, Ipswich, MA, USA); trypsin (Sigma-Aldrich, St. Louis, MO, USA); Sulfo-NHS-Biotin, Sulfo-NHS-LC-Biotin, and NeutrAvidin Beads (Pierce, Rockford, IL, USA); Psalmotoxin 1 (PcTx-1; Abcam, Cambridge, MA, USA); culture medium and serum [GE Healthcare Bio-Sciences (Pittsburgh, PA, USA) or Thermo Fisher Scientific); Lipofectamine 2000 (Thermo Fisher Scientific); and Fugene 6 (Promega, Madison, WI, USA).
Electroencephalogram in patients
Electrode placement in epilepsy patients was performed as previously described (23). Electroencephalography (EEG) was recorded with a sampling rate of 500 Hz using the Neuvo system (Compumedics, Abbotsford, VIC, Australia). Raw data were acquired at 10 kHz, and a software second-order Infinite Impulse Response Butterworth low-pass filter was applied at 40% of the sampling frequency.
Brain tissue membrane preparation
For mouse tissue, a small piece (∼2–3 mm cube) of cortical tissue at the temporal lobe region was used. For human cortical tissue, a similar-sized gray-matter tissue was used. Preparation of membrane fraction was achieved using a Mem-PER Plus Membrane Protein Extraction Kit (89842; Thermo Fisher Scientific), following the manufacturer’s instructions. In brief, dissected cortical tissues were placed in 2-ml microcentrifuge tubes, rinsed once by the wash solution, transferred to the tissue grinder, and homogenized in 2 ml permeabilization solution. The suspension was transferred into a new tube, incubated for 10 min at 4°C with gentle rotation; 0.5 ml suspension was transferred to a new tube and saved as the total fraction. The remaining 1.5 ml suspension was centrifuged at 14,000 g for 15 min at 4°C. The supernatant was saved as the cytosol fraction. The pellet was resuspended with 1 ml solubilization buffer, incubated for 30 min at 4°C with gentle rotation, and centrifuged at 14,000 g for 15 min at 4°C. The supernatant was collected as the membrane fraction.
CHO cell culture and transfection
CHO-K1 cells were purchased from American Type Culture Collection (Manassas, VA, USA). CHO cell culture and transfection were performed similar to what was described in our previous studies (19, 24). In brief, CHO cells were grown in F-12K, supplemented with 10% fetal bovine serum in a humidified 5% CO2 incubator. For biochemistry experiments, cells were plated onto 35 or 60 mm dishes at a density of ∼4 × 104 cells/cm2. For immunofluorescence experiments, cells were plated onto collagen-coated glass coverslips. CHO cells were transfected using Lipofectamine 2000 or Fugene 6, following the manufacturer’s instructions. The amount of DNA transfected was 1–2 µg/35 mm dish or 2–5 µg/60 mm dish.
Immunofluorescence and confocal microscopy
Confocal images were captured using a laser-scanning microscope (A1R; Nikon, Tokyo, Japan), similar to procedures previously described (19, 21). In brief, illumination was provided by an argon (458, 488, and 514 nm lines) and a 561 diode laser. For ASIC1a immunofluorescence, green and red channels were imaged sequentially to eliminate bleedthrough. Alexa 488 and GFP were imaged with 488 nm excitation and a 525/50 emission filter. Alexa 568 and 555 were imaged with 561 nm excitation and a 595/50 emission filter. Images were captured with a ×60/1.2 PL Apochromatic (APO) water lens, at a z step of 0.4 μm. Each captured image was an average of 4 scans in a single plane.
Deglycosylation
Cell lysate was deglycosylated with PNGase F or Endo H, similar to what was described earlier (19, 25). In brief, the samples were denatured at 95°C for 10 min and cooled to room temperature. For PNGase F digestion, Nonidet P-40 was added to a final concentration of 1%. The reaction mixture was incubated overnight with 0.1–0.3 μl of PNGase F or Endo H per sample at 37°C.
Surface biotinylation, NeutrAvidin pulldown, and Western blot
Surface biotinylation and NeutrAvidin pulldown were performed as previously described (14, 21). In brief, cells were washed 3 times with ice-cold PBS+/+, followed by 30 min incubation at 4°C in 1.5 ml PBS+/+ containing 0.5 mg/ml sulfo-NHS-biotin or sulfo-NHS-LC-biotin. Cells were washed once with cold PBS+/+, and the reaction was quenched by 100 mM glycine in PBS+/+. Cells were lysed in 300 μl NeutrAvidin lysis buffer (PBS, 1% Triton, 0.5% SDS, 0.5 mg/ml N-ethylmorpholine, with protease inhibitors). For precipitation of surface proteins, cell lysates were sonicated briefly and centrifuged at full speed with a desktop centrifuge for 10 min at 4°C. Forty microliters NeutrAvidin agarose beads was added to 200 μl cell lysate (∼600 μg proteins), and the precipitation was carried out overnight at 4°C with gentle rotation, followed by 3 washes with PBS containing 1% Triton.
Surface fraction was eluted with 80 μl 2× sample buffer containing 10% 2-ME. Total lysate was mixed with an equal volume of sample buffer. Equal volume of surface and total fraction was loaded per lane so the loading into the surface fraction is ∼5 times that of total. The samples were separated by 8 or 10% SDS-PAGE and transferred to nitrocellulose membranes. Blotting was performed, according to instructions of the Odyssey Imaging System (Li-Cor Biosciences). Membranes were blocked in blocking buffer (0.1% casein in 0.2 × PBS, pH 7.4) for 1 h. Primary antibodies were diluted with blocking buffer containing 0.1% Tween-20 and incubated at 4°C overnight or at room temperature for 2 h. Secondary antibodies were diluted in blocking buffer containing 0.1% Tween-20 and 0.01% SDS and incubated at room temperature for 1 h. Antibody dilutions were the following: rabbit anti-ASIC1a 1:1000; goat anti-ASIC1 1:1000; rabbit anti-ASIC2 1:1000; mAb anti-HA 1:1000–2000; mAb anti-tubulin 1:30,000; and anti-β-tubulin III 1:10,000–30,000. Secondary antibodies were used at 1:10,000–16,000 dilutions. Blots were imaged using an Odyssey Infrared Imaging System, according to the manufacturer’s instructions. Densitometry of imaged bands was performed as previously described (14, 21).
Electrophysiology
Whole-cell patch-clamp recordings were performed, as previously described (19). In brief, patch electrodes were constructed from borosilicated glass (BF150-86-10; Sutter Instrument, Novato, CA, USA) on a horizontal micropipette puller (P-97; Sutter Instrument). The resistance of the patch electrode ranged from 3 to 5 MΩ when filled with intracellular solution. Whole-cell currents were elicited by a drop in pH from 7.4 to 6.8/6.0 at a holding potential of −60 mV and recorded using Axopatch 200B amplifiers (Axon CNS; Molecular Devices, Foster City, CA, USA). Data were filtered at 2 kHz and digitized at 5 Hz using Digidata 1322A unit (Axon CNS; Molecular Devices). The online acquisition was done using pCLAMP software (v.10.2; Axon CNS; Molecular Devices). Standard extracellular fluid (ECF) contained (mm) the following: 140 NaCl, 5.4 KCl, 2.0 CaCl2, 1.0 MgCl2, 20 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), and 10 glucose (pH 7.4; 320∼330 mOsm). For pH 6.0 solution, 2-(N-morpholino)ethanesulfonic acid (MES) was used instead of HEPES for more reliable pH buffering. The pipette solution contained (mm) the following: 140 K-gluconate, 10 HEPES, 11 EGTA, 2 tetraethylammonium, 1 CaCl2, 2 MgCl2, and 4 K2ATP (pH 7.2∼7.3; 290∼300 mOsm). In general, ASIC channels were triggered by a drop in pH, from 7.4 to 6.0, every 2 min, to allow a complete recovery of the channel from desensitization. pH-buffered ECF was exchanged through gravity-fed pipes, similar to what has been used in previous studies (26, 27). The perfusion rate was ∼1 ml/min. To achieve fast exchange of extracellular pH around the recorded cell, a Perfusion Pencil (with a 250 µm tip; Automate Scientific, Berkeley, CA, USA) was mounted on a micromanipulator with the tip positioned directly above the recorded cell. The tip was ∼200 µm away from the cell (estimated based on the pore size and the distance of the tip from the cell), and the flow of the solution was directed toward the cell.
During each experiment, a voltage step of –10 mV from the holding potential was applied periodically to monitor the cell capacitance and the access resistance. Recordings in which either the access resistance or the capacitance changed by >10% during the experiment were excluded from data analysis. To determine the time constant of the desensitizing portion of the ASIC currents, pH 6.0-activated currents were fitted by a single, standard exponential equation using Clampfit10.2 (Axon CNS; Molecular Devices).
Calcium imaging and spectral FRET analysis
CHO-K1 cells were plated onto collagen-coated coverglass-bottom dishes at a density of 1 × 104 cells/cm2, transfected with cameleon YC3.60 (28), alone or together with hASIC1a, mASIC1a, or mASIC1a S285P. The ratio of cameleon:ASIC was 1:3. Two days after transfection, cells were used for calcium imaging. The culture medium was changed to ECF containing the following (mM): 140 NaCl, 5.4 KCl, 20 glucose, 2 CaCl2, and 1 MgCl2. Osmolarity of ECF was 320–335 mOsm. pH 7.4 was buffered with 5 mM HEPES. Transfected cells were imaged with a Nikon A1R inverted confocal microscope. Cameleon was excited with a 405 nm laser line, and the spectrum between 460.75 and 560.75 nm was captured. Cells were imaged for 45 s at pH 7.4 for baseline, followed by pH 6.5 for 2–3 min. pH 6.5 in bath was achieved by adding 0.5 ml pH 6.3 ECF (buffered with 20 mM MES) to the dish, which contained 1 ml pH 7.4 ECF.
Confocal images were exported as 16-bit unscaled Tagged Image File Format files. A spectral library, consisting of pure spectra of cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP), was obtained using cells expressing either cerulean or citrine alone. Spectral images from calcium (cameleon) imaging were unmixed into separate CFP and YFP channels, based on the spectral library of CFP or YFP and using a custom-written script in MATLAB (29, 30). The unmixed CFP and YFP were used to calculate the fluorescence resonance energy transfer (FRET) ratio. The fluorescence intensity of selected cells was quantified in ImageJ (NIH) and exported to Excel for further analysis.
Cell survival
CHO-K1 cells were plated in a 96-well dish at a density of 3000 cells/well. At the second day after plating, cells were transfected with Lck-GFP, alone or together with hASIC1a, mASIC1a, and S285P. For cotransfection, the ratio of GFP:ASIC was 1:3. Two days after the transfection, cell injury was analyzed using a similar protocol, as described in one previous study (15). Cells were rinsed twice with ECF, buffered at pH 7.4 (with 20 mM HEPES) or pH 6.0 (with 20 mM MES) and then incubated in pH 7.4 or pH 6.0 ECF for 2 h. Following the 2-h incubation, the cells were rinsed once with pH 7.4 ECF and changed to regular culture medium for 24 h. Fluorescence images were taken at the end of the 24-h incubation. For each well, 5 fields were randomly captured using an Olympus IX70 inverted fluorescence microscope with Spot Imaging software. For each condition, the total number of GFP-positive cells from all 5 fields was counted. The reduction in GFP+ cells in the pH 6-treated condition (compared with those in pH 7.4) indicates cell injury. The percentage of injury was calculated as the following: cells (n) in pH 7.4 − cells (n) in pH 6/cells (n) in pH 7.4.
For examining the effect of PcTx-1 on ASIC1-induced cell injury, cells were plated in 96-well plates and transfected as above for 24 h. Cells were pretreated with vehicle or PcTx-1 (100 ng/ml) for 2 h, followed by pH treatment for 2 h in pH 7.4, 6.0, or 6.0 + PcTx-1. Cells were then incubated in regular culture medium for 24 h, followed by imaging using a Nikon Eclipse Ti fluorescence microscope with NIS-Elements software. For each well, 5 fields were randomly captured. Quantification of cell injury was performed as described above.
Statistical analysis
For paired comparisons, we used a 2-tailed Student’s t test or Wilcoxon signed-rank test. For multiple comparisons, we used ANOVA, followed by Tukey’s honest significant difference post hoc correction. Data were reported as means ± sem. for the number of samples or number of repeats, as indicated.
RESULTS
Human brain tissue exhibits higher levels of ASIC1a in the membrane fraction
To analyze ASIC expression in the human brain, we obtained human cortical tissue that was acutely resected from patients with intractable epilepsy. The epileptic foci in these patients were verified by EEG recordings. In Fig. 1A, the upper left diagram illustrates a typical layout of the recording electrodes, whereas the MRIs illustrate the location of the epileptic loci (as indicated by a red dot) from 1 patient. A representative set of EEG recordings are presented in Fig. 1A (lower panel). Traces from hippocampus exhibited typical epileptic patterns of activities. In contrast, the neocortex did not exhibit abnormal activities, even when the hippocampus was seizing. In this study, to alleviate the concern that epileptic activities alter channel trafficking, we used these cortical tissues for studying ASIC expression in the human brain. Of note, the resection of a small piece of cortical tissue, typically from the anterior temporal lobe location, is a necessary surgical routine to gain access to the epileptic foci in the hippocampus. To determine the expression of ASIC1 and ASIC2 proteins, we blotted the human cortical tissue with ASIC1 and ASIC2 antibodies and compared that with the mouse cortex. Human and mouse tissues showed comparable ratios of ASIC1a:ASIC2a (Fig. 1B). However, there was little ASIC2b protein in the human tissue. Next, we prepared the membrane fraction of human and mouse cortical tissue and analyzed the relative levels of ASIC1a in the membrane to that of total lysate. To control for the quality of the preparation, we blotted for N-cadherin (a membrane protein) and β-tubulin III (a cytosolic protein). N-Cadherin and β-tubulin III were preferentially segregated in the membrane and cytosol fractions, respectively (Fig. 1C). We quantified the relative level of ASIC1a in membrane and total fractions. Human tissue exhibited higher membrane:total ratio of ASIC1a compared with mouse.
hASIC1a shows more efficient surface expression than mASIC1a in heterologous cells
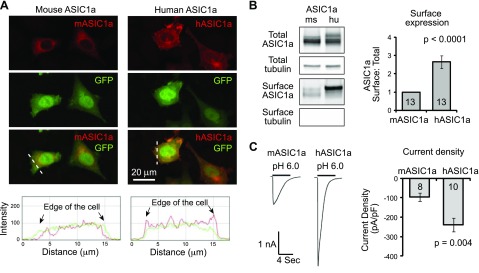
The result from membrane preparation suggests that hASIC1a traffics more efficiently than mASIC1a. However, the membrane preparation included all membranes—both intracellular and extracellular. To assess directly whether surface trafficking of hASIC1a is higher, we transfected CHO-K1 cells with mASIC1a and hASIC1a, together with enhanced GFP (eGFP) to identify transfected cells. We performed immunostaining using an anti-ASIC1 antibody and visualized ASIC1a localization with confocal microscopy. hASIC1a exhibited higher staining at the cell periphery (Fig. 2A). To determine quantitatively surface expression of the 2 homologs, we performed surface biotinylation, pulled down the surface fraction with NeutrAvidin beads, and analyzed surface and total fractions using Western blot. hASIC1a showed higher surface:total ratio than mASIC1a (Fig. 2B). To determine whether these changes in localization result in changes in acid-activated currents, we performed patch-clamp analysis. pH 6-activated current density in cells expressing hASIC1a was ∼2-fold higher than in cells expressing mASIC1a (Fig. 2C).
Figure 2.
hASIC1a shows higher surface expression than mASIC1a. A) Representative confocal images showing distribution of mASIC1a and hASIC1a in CHO cells (top). CHO cells were transfected with mASIC1a or hASIC1a (red), together with eGFP. ASIC1a localization was visualized by immunofluorescence using an anti-ASIC1a antibody. Plots show the line profile of ASIC1a (red) and eGFP (green) along the dashed lines, as indicated (bottom). Note that hASIC1a exhibited a higher signal at the edge of the cell. B) Representative Western blot images showing surface and total levels of ASIC1a (left). CHO cells expressing mASIC1a and hASIC1a were surface biotinylated, followed by pulldown of surface fraction with NeutrAvidin beads (see Materials and Methods). Total and surface fractions were blotted with ASIC1a and tubulin at the same time. Quantification of surface:total ratio of ASIC1a (right). Numbers in bars indicate the number of repeats. C) Representative traces and quantification of pH 6-activated current density of CHO cells expressing mASIC1a or hASIC1a. Numbers in bars indicate the number of cells.
Efficient N-glycosylation of hASIC1a leads to a higher surface expression than mASIC1a
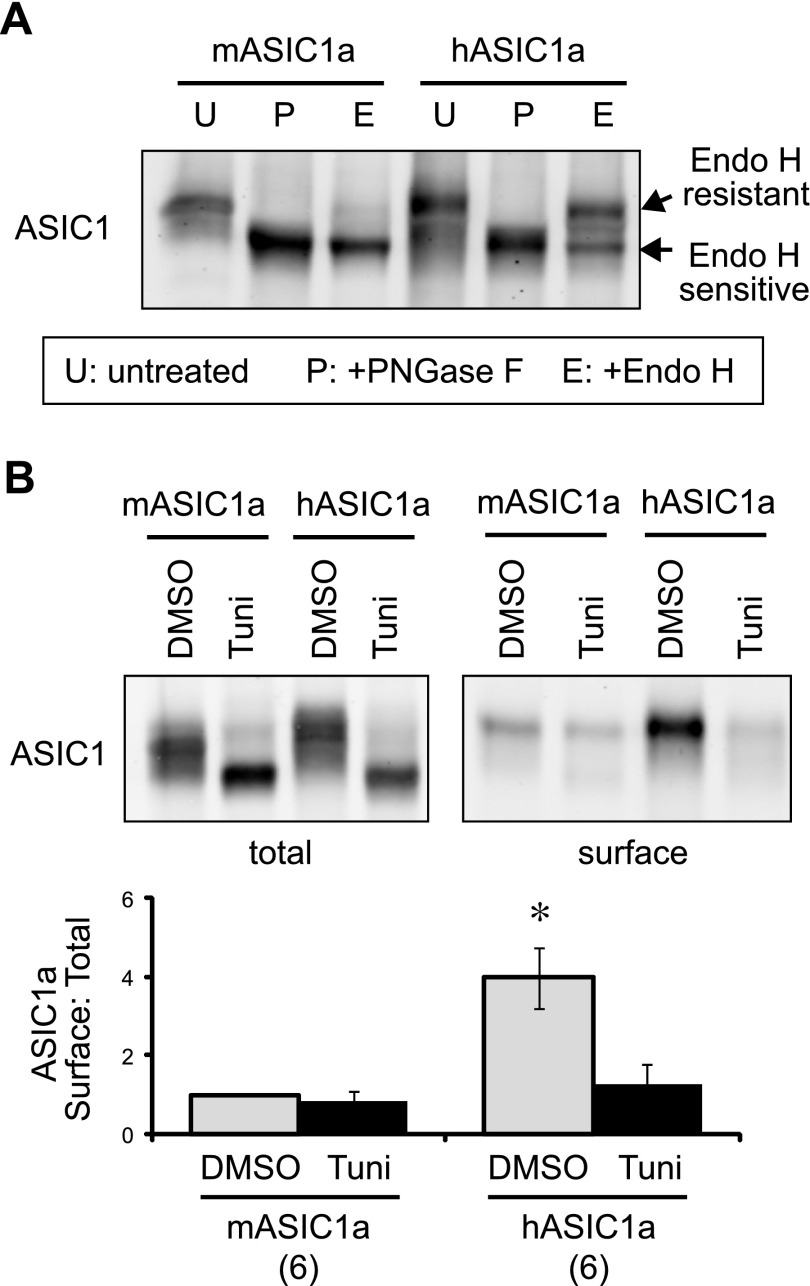
While performing this experiment, we noticed a difference in migration patterns of mASIC1a and hASIC1a. The human homolog had an increased proportion of slower migration bands on the gel (Fig. 2B). In our previous studies, we found that the difference in migration of mASIC1a was largely a result of differential N-glycosylation (14, 19, 24). Therefore, we analyzed N-glycosylation of the 2 homologs. We treated cell lysates with 2 endoglycosidases; Endo H and PNGase F. Endo H can remove unmodified (immature) N-linked core glycans, whereas PNGase F cleaves all N-linked glycans (31, 32). hASIC1a showed a significantly higher Endo H-resistant (slow-migrating) fraction as compared with mASIC1a (Fig. 3A). These data are consistent with our previous finding showing that efficient N-glycosylation of mASIC1a facilitates its surface expression (19).
Figure 3.
Efficient N-glycosylation is responsible for higher surface trafficking of hASIC1a. A) CHO cells were transfected with hASIC1a or mASIC1a, lysed, and either untreated (U) or treated with PNGase F (P) or Endo H (E), as described in Materials and Methods. The migration patterns of ASIC1a were analyzed by Western blot. Note that PNGase F treatment removed all N-linked glycans, and the protein migrated faster on the gel. Endo H-treated samples migrated as 2 populations, and the slower-migrating population represents the population that carries matured (or processed) N-glycans, which are resistant to Endo H treatment. B) ASIC1a-expressing cells were treated with DMSO (vehicle control) or 0.5 µg/ml tunicamycin (Tuni) overnight. Surface biotinylation and analysis were performed as in Fig. 2B. Tunicamycin treatment reduced surface expression of hASIC1a and mASIC1a to a comparable level. Asterisk indicates that the vehicle-treated hASIC1a group is significantly different from all other groups. P < 0.01 (ANOVA).
To test directly whether the increased surface trafficking of hASIC1a was a result of more efficient N-glycosylation, we treated CHO cells with tunicamycin, which inhibits the addition of N-linked glycans in the endoplasmic reticulum (33). Similar to our previous study (19), tunicamycin treatment converted most ASIC1a to the fast-migrating species, a result expected with the inhibition of glycosylation (Fig. 3B). Tunicamycin treatment significantly (P < 0.01, Tukey’s honest significant difference test) reduced surface:total ratio of hASIC1a, and there was no significant differences in surface:total ratio between tunicamycin-treated hASIC1a and mASIC1a.
aa 285 is responsible for differential N-glycosylation and surface expression of hASIC1a and mASIC1a
hASIC1a and mASIC1a share high homology, with 98% amino acid identity (see complete amino acid alignment in Supplemental Fig. 1). Our results here indicate that the few amino acid differences are indeed critical for their differential biogenesis process. In our previous study, we found that the interaction between 2 regions—1 at approximately aa 287 and 1 at approximately aa 71—is important for efficient glycosylation, trafficking, and channel function of mASIC1a (24). This result is consistent with others studying biophysical properties of this channel (34, 35). Interestingly, mouse and human differ at 2 aa in these regions: aa 70, which is cysteine in mouse and histidine in human, and 285, which is serine in mouse and proline in human. Given the previous findings, we hypothesized that these 2 aa are responsible for differential N-glycosylation and surface expression between the 2 homologs. To test this hypothesis, we generated mASIC1a C70H and S285P mutants, which substitute the amino acids at the corresponding positions to those in hASIC1a. The mASIC1a S285P mutant exhibited similar N-glycosylation levels as hASIC1a, whereas the mASIC1a C70H mutant showed slightly higher glycosylation (Fig. 4A). We also generated a mASIC1a C70H/S285P double mutant. The double mutant showed similar levels of N-glycosylation compared with mASIC1a S285P.
Figure 4.
aa 285 is the key residue determining differential biogenesis of hASIC1a and mASIC1a. A) Representative Western blot and quantification showing the effect of amino acid substitutions at positions 285 and 70 on N-glycosylation of ASIC1a. Note that the alignment of hASIC1a and mASIC1a was shown in Supplemental Fig. 1. Wild-type mASIC1a, hASIC1a, and the mASIC1a S285P, C70H, C70H/S285P mutants were transfected into CHO-K1 cells. Surface biotinylation and Endo H treatment were performed, as described in Materials and Methods and previous figures. Note that mASIC1a S285P exhibited a similar glycosylation rate as hASIC1a. B) The effect of amino acid substitutions on surface trafficking of mASIC1a. The quantification was shown in 2 separate graphs, as the controls were different in the 2 sets of analysis data presented. Asterisk indicates significant differences from wild-type mASIC1a (ANOVA). C) The effect of P285S mutant of hASIC1a on surface trafficking. Wild-type hASIC1a had significantly higher (ANOVA) surface expression than mASIC1a or hASIC1a P285S.
Next, we examined their surface expression. The mASIC1a S285P, but not the mASIC1a C70H, mutation significantly increased its surface expression (Fig. 4B). The substitution of both amino acids did not lead to a further increase in surface expression. We further generated the reverse mutant in hASIC1a-substituting Pro285 in hASIC1a with serine. hASIC1a P285S had significantly reduced surface expression as compared with wild-type hASIC1a (Fig. 4C).
The substitution of aa 285 is sufficient for changing acid-activated current and acid-induced calcium increase
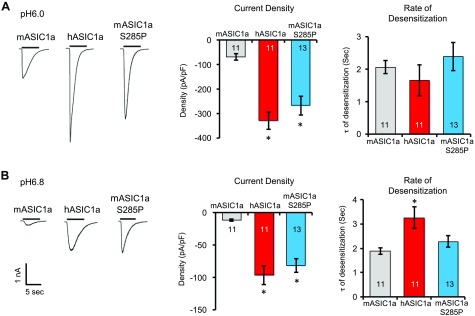
The biochemical data suggest that the difference at aa 285 is responsible for the increased acid-activated currents in cells expressing hASIC1a. To determine whether this is the case, we analyzed acid-activated currents in CHO cells. At both pH 6 and pH 6.8, cells expressing hASIC1a possessed larger acid-activated currents (Fig. 5). The mASIC1a S285P mutant exhibited a similar-sized current compared with hASIC1a. At pH 6, the rate of desensitization was not different among mASIC1a, hASIC1a, or the S285P mutant. However, at pH 6.8, hASIC1a exhibited a slower rate of desensitization (Fig. 5B, right).
Figure 5.
hASIC1a exhibits a higher acid-activated current. CHO cells were transfected with mASIC1a, hASIC1a, or mASIC1a S285P, together with eGFP, to identify transfected cells. A, B) Acid-activated currents were evoked by rapid perfusion with pH 6 (A) or 6.8 (B) solutions. Typical traces of acid-activated currents (left). Quantification of current density (middle). Quantification of desensitization rate (right). Numbers in bars denote total number of cells. Asterisk indicates difference from mASIC1a (ANOVA).
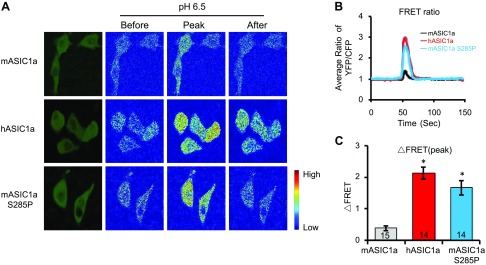
The data on acid-activated currents indicate that cells expressing hASIC1a will result in a larger calcium rise in response to acidic stimulation. To visualize acid-induced calcium responses, we transfected CHO-K1 cells with a ratiometric calcium reporter, cameleon YC3.60 (28), either alone or together with the above ASIC1a constructs. Cells expressing cameleon alone did not generate noticeable changes in FRET in response to pH 6.5 (data not shown). Cells expressing mASIC1a had an ∼39% increase in the FRET ratio (Fig. 6). In contrast, cells expressing hASIC1a or mASIC1a S285P led to a significantly larger acid-induced increase in FRET.
Figure 6.
hASIC1a and mASIC1a S285P elicit a larger acid-activated calcium increase. CHO cells were transfected with various ASIC1 constructs, as indicated together with cameleon YC3.60, a ratiometric calcium reporter. FRET imaging and spectral unmixing were performed, as described in Materials and Methods. A) Representative images showing the fluorescent images of transfected cells (leftmost) and ratiometric images (right 2 sets) before, at peak, and after stimulation with a pH 6.5 solution. For ratio images, red and blue indicate high and low FRET, respectively. B) Average time-dependent changes in FRET (YFP/CFP ratio) of cells expressing mASIC1a, hASIC1a, and mASIC1a S285P. C) Quantification of peak change in FRET (ΔFRET) of cells expressing the 3 different constructs. Bar numbers indicate total number of cells analyzed (B, C). Asterisk indicates differences from mASIC1a (ANOVA). Numbers in bars indicate number of repeats.
Cells expressing hASIC1a exhibit worsened acidosis-induced injury
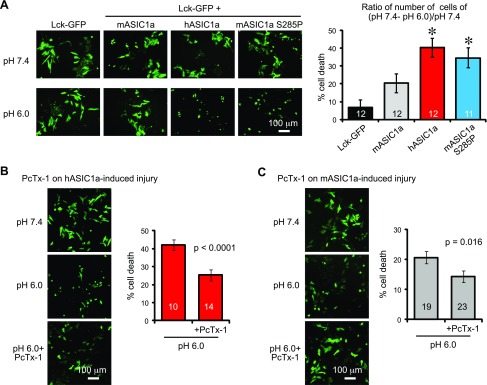
Previous studies have shown that acid-induced calcium overload is a main contributor to acidosis-induced injuries (15, 17). Therefore, we asked whether mASIC1a and hASIC1a exhibit differential capacity in inducing acidosis-induced injuries. We transfected CHO-K1 cells with Lck-GFP, either alone or together with mASIC1a, hASIC1a, or mASIC1a S285P. We allowed the cells to express the constructs for 2 d, treated the cells with solutions buffered at pH 7.4 or pH 6.0 for 2 h, and counted the number of remaining GFP-positive cells, 24 h later. In Lck-GFP-transfected cells, the number of GFP+ cells was comparable in pH 7.4- and pH 6-treated conditions (Fig. 7A), and the reduction in GFP+ cells was minimal (6.4 ± 4.6%). Cells expressing mASIC1a exhibited ∼20% cell death in the pH 6 condition. As expected, cells expressing either hASIC1a or mASIC1a S285P showed a higher percentage of cell death: 38.6 and 35.6% for hASIC1a and the S285P mutant, respectively. To determine whether pH 6-induced injury was a result of ASIC1a activation, we inhibited ASIC1a with the PcTx-1 peptide, a specific inhibitor of ASIC1a (36). PcTx-1 significantly inhibited pH 6-induced cell death in cells expressing either hASIC1a or mASIC1a (Fig. 7B, C).
Figure 7.
Cells expressing hASIC1a exhibit increased acidotoxicity. A) Left: representative images showing the effect of acidotoxicity in cells expressing Lck-GFP alone or together with mASIC1a, hASIC1a, or mASIC1a S285P. CHO cells were transfected with the above constructs for 2 d and treated with pH 7.4 or 6.0 for 2 h. Approximately 24 h after the treatment, GFP+ cells were visualized and counted. Bar graph shows quantification of cell death after 2 h pH 6 treatment (right). Cell death was calculated by subtracting the number of cells in pH 6 from that in pH 7.4. Asterisk indicates difference from the vector control (Lck-GFP). The hASIC1a group was also significantly higher than the mASIC1a group. P < 0.05 (ANOVA). Numbers in bars indicate number of repeats from 9 separate experiments. B, C) Representative images and quantification showing the effect of PcTx-1 on acidosis-induced injury in cells expressing hASIC1a (B) or mASIC1a (C). PcTx-1 was used at 100 ng/ml. Numbers in bars denote number of repeats from 4 (hASIC1a) or 9 (mASIC1a) separate experiments. Significance value resulted from 1-tailed Student’s t test.
DISCUSSION
Our data revealed several findings regarding hASICs. We reported a differential expression of ASIC1 and ASIC2 in human and mouse cortical tissues. Compared with mouse cortex, human cortical tissue expressed similar ratios of ASIC1a:ASIC2a proteins but had reduced levels of ASIC2b. Our biochemical analysis further showed that hASIC1a trafficked more efficiently to the cell membrane. In addition, heterologous cells expressing hASIC1a had larger acid-activated currents and acid-induced calcium rise and exhibited worsened acidosis-induced injuries. Whereas we do not have direct evidence for acidosis-induced signaling in human neurons, our data implicate that compared with mouse brain tissue, acid likely will have a more prominent impact on human brain tissue.
ASIC1a and ASIC2 are 2 major ASIC subunits expressed in the mouse brain. Other ASIC subunits, e.g., ASIC3 and -4, are also present in mouse brain. However, the expression of ASIC3 and -4 is restricted to specific nuclei and/or cell types (37–39). In dissociated human neurons, acid-activated currents also suggest the main contribution from ASIC1a and ASIC2a (18). For this reason, we focused our analysis on ASIC1a and ASIC2 and found that human cortical tissue expressed ASIC1a and ASIC2a but had reduced ASIC2b. Qualitatively, this finding is consistent with the previous electrophysiological recordings. A similar ratio of ASIC1a:ASIC2a subunits in human and mouse tissues suggests that acid-activated currents in human neurons will be similar to the currents recorded from mouse neurons and have a mixed contribution from ASIC1a homomers and -1a/2a heteromers (27, 40–43). However, an earlier study showed that acid-activated currents recorded from cultured human neurons suggested a predominant contribution from ASIC1a homomers, whereas the ASIC1a/-2a heteromers played a relatively minor role (18). The reason for this discrepancy is unclear. We speculate that the differences in samples may provide an explanation. In our study, we analyzed acutely resected normal cortical tissues from patients with intractable epilepsy (the epileptic foci were mostly in hippocampus). In contrast, Li et al. (18) studied neurons in culture, and the cultures were prepared from tissues resected from patients with brain tumors. Besides the difference in samples, our biochemical data analyzed overall protein expression in the tissue. In contrast, the recorded acid-activated currents were from individual neurons. It is conceivable that the relative ASIC subunit ratios in different types of neurons (e.g., excitatory vs. inhibitory neurons) are different from the overall expression levels in the tissue. One important caution on these data is that the human and mouse tissues were very different in nature. The human tissues were resected from patients with intractable epilepsy, whereas the mice were nonepileptic. Although the epileptic loci resided in hippocampus (Fig. 1A), we cannot rule out the possibility that the potential spreading of activities to the cortex alters the expression and/or trafficking of ASICs. Another consideration is that the patients were typically older, with the age range from 17 to 61 yr old and an average age of 41 yr. Nevertheless, these data together indicate that ASIC1a and -2a are the main ASIC subunits expressed in human neurons. In addition, our in vivo biochemical data on membrane vs. total ASIC1a were consistent with our in vitro trafficking result.
Given the high degree of homology—98% aa identity—between hASIC1a and mASIC1a, it is somewhat surprising to observe a large (>3-fold) difference in trafficking between the 2 homologs. Our data show that a single amino acid at position 285 is critical for this difference. What makes this position so critical? In previous studies, the interaction between the first transmembrane domain 1 (TM1) and the β turn that links to the thumb region is critical for ASIC channel gating (34, 35). We have shown that mutations in these regions reduced N-glycosylation and trafficking of ASIC1a (24). These data suggest that this interaction between the turn and TM1, probably through modulating glycosylation and folding of the extracellular domain, regulates ASIC1a trafficking and channel function. We speculate that the substitution at aa 285 (serine in mASIC1a and proline in hASIC1a), which is located at the β turn region at the thumb, achieves its effect through modulation of the interaction between the TM1 and the thumb region. Regardless of the exact mechanism, these data imply that pharmacological targeting of extracellular regions of ASIC can provide an efficient approach to alter the biogenesis and/or channel properties of these channels and consequently, ASIC-mediated responses in neurons.
Previous studies have discovered important roles of acid signaling and ASICs in rodents. ASICs regulate neuron excitability and mediate acidosis-related neuronal injury in ischemia, multiple sclerosis, and traumatic brain injury (13–16, 44–48). ASIC knockout animals also reduce fear and anxiety and exhibit less depressive behavior (6). Our results here showed that ASIC1a levels in the membrane fraction of human cortical tissues were higher than that of the mouse brain. Although the membrane preparation here includes both cell membrane and intracellular membranes, the result suggests that the surface ASIC1a level is likely higher in human neurons. As support of this speculation, hASIC1a exhibited higher surface trafficking in heterologous cells. Cells expressing hASIC1a also showed larger acid-activated calcium rise and worsened acidosis-induced cell injury. These data suggest that the biologic role of the acid/proton-ASIC pathway is conserved from mouse to human. Besides calcium overload-induced injuries, recent studies have shown that ASIC1a can elicit a channel activity-independent necroptosis pathway and can drive changes in α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors following ischemia (49, 50). Regardless of the relative contribution of these novel mechanisms in human neurons, the increased trafficking of hASIC1a suggests that extracellular protons or acidosis are likely to elicit a larger impact in humans compared with mice.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Lan Jing, Yufan Zhou, Nan Jiang, and Junjun Wu (all from the University of South Alabama) for assistance on pilot experiments. Y.X. was supported, in part, by a fellowship from South Medical University. Y.-Q.J. was supported by a fellowship from The Third Hospital of Hebei Medical University. C.L. was supported, in part, by a scholarship from the China Scholarship Council. N.A. was supported, in part, by a fellowship from the American Heart Association (AHA; 16PRE27130004). The Nikon A1R confocal microscope, FRET imaging, and analysis were funded by U.S. National Institutes of Health (NIH)/American Recovery and Reinvestment Act equipment grants (S10RR027535 and S10OD020149). This work was supported, in part, by the National Natural Science Foundation of China (Grant 81773698 to J.X.) and NIH/National Institute of Neurological Disorders and Stroke Grants R01NS102495 and R21NS093522, and AHA Grant 13SDG13970009 (to X.-M.Z.). The authors declare no conflicts of interest.
Glossary
- ASIC
acid-sensing ion channel
- CFP
cyan fluorescent protein
- CHO
Chinese hamster ovary
- ECF
extracellular fluid
- EEG
electroencephalography
- eGFP
enhanced green fluorescent protein
- Endo H
endoglycosidase H
- FRET
fluorescence resonance energy transfer
- GFP
green fluorescent protein
- HA
hemagglutinin
- hASIC
human ASIC
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- Lck
lymphocyte-specific protein tyrosine kinase
- mASIC
mouse ASIC
- MES
2-(N-morpholino)ethanesulfonic acid
- NHS
N-hydroxysuccinimide
- PcTx-1
psalmotoxin 1
- PNGase F
peptide-N-glycosidase F
- TM1
transmembrane domain 1
- YFP
yellow fluorescent protein
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
Y. Xu designed and performed studies on human tissue analysis, calcium imaging, patch clamp, cell survival, and immunolocalization and analyzed the results; Y.-Q. Jiang designed and performed surface trafficking and glycosylation in CHO cells and analyzed the results; C. Li performed analysis of human tissues and analyzed the results; W. G. Rusyniak provided human cortical tissue; J. Ochoa performed EEG recording and analysis; M. T. Lin provided assistance on electrophysiology studies; M. He, N. Annamdevula, S. J. Leavesley, and T. C. Rich provided study materials and/or technical support; J. Xu provided important discussions; and X.-M. Zha conceived of the overall hypothesis and design, analyzed the data, and wrote the manuscript; all authors reviewed the manuscript.
REFERENCES
- 1.Du J., Reznikov L. R., Price M. P., Zha X. M., Lu Y., Moninger T. O., Wemmie J. A., Welsh M. J. (2014) Protons are a neurotransmitter that regulates synaptic plasticity in the lateral amygdala. Proc. Natl. Acad. Sci. USA 111, 8961–8966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.González-Inchauspe C., Urbano F. J., Di Guilmi M. N., Uchitel O. D. (2017) Acid-sensing ion channels activated by evoked released protons modulate synaptic transmission at the mouse calyx of held synapse. J. Neurosci. 37, 2589–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu M. G., Li H. S., Li W. G., Wu Y. J., Deng S. N., Huang C., Maximyuk O., Sukach V., Krishtal O., Zhu M. X., Xu T. L. (2016) Acid-sensing ion channel 1a contributes to hippocampal LTP inducibility through multiple mechanisms. Sci. Rep. 6, 23350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiang P. H., Chien T. C., Chen C. C., Yanagawa Y., Lien C. C. (2015) ASIC-dependent LTP at multiple glutamatergic synapses in amygdala network is required for fear memory. Sci. Rep. 5, 10143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang Y., Jiang N., Li J., Ji Y. H., Xiong Z. G., Zha X. M. (2015) Two aspects of ASIC function: synaptic plasticity and neuronal injury. Neuropharmacology 94, 42–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wemmie J. A., Taugher R. J., Kreple C. J. (2013) Acid-sensing ion channels in pain and disease. Nat. Rev. Neurosci. 14, 461–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gründer S., Pusch M. (2015) Biophysical properties of acid-sensing ion channels (ASICs). Neuropharmacology 94, 9–18 [DOI] [PubMed] [Google Scholar]
- 8.Zha X. M. (2013) Acid-sensing ion channels: trafficking and synaptic function. Mol. Brain 6, 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zha X. M., Wemmie J. A., Green S. H., Welsh M. J. (2006) Acid-sensing ion channel 1a is a postsynaptic proton receptor that affects the density of dendritic spines. Proc. Natl. Acad. Sci. USA 103, 16556–16561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziemann A. E., Allen J. E., Dahdaleh N. S., Drebot I. I., Coryell M. W., Wunsch A. M., Lynch C. M., Faraci F. M., Howard M. A., III, Welsh M. J., Wemmie J. A. (2009) The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell 139, 1012–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coryell M. W., Ziemann A. E., Westmoreland P. J., Haenfler J. M., Kurjakovic Z., Zha X. M., Price M., Schnizler M. K., Wemmie J. A. (2007) Targeting ASIC1a reduces innate fear and alters neuronal activity in the fear circuit. Biol. Psychiatry 62, 1140–1148 [DOI] [PubMed] [Google Scholar]
- 12.Pidoplichko V. I., Aroniadou-Anderjaska V., Prager E. M., Figueiredo T. H., Almeida-Suhett C. P., Miller S. L., Braga M. F. (2014) ASIC1a activation enhances inhibition in the basolateral amygdala and reduces anxiety. J. Neurosci. 34, 3130–3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friese M. A., Craner M. J., Etzensperger R., Vergo S., Wemmie J. A., Welsh M. J., Vincent A., Fugger L. (2007) Acid-sensing ion channel-1 contributes to axonal degeneration in autoimmune inflammation of the central nervous system. Nat. Med. 13, 1483–1489 [DOI] [PubMed] [Google Scholar]
- 14.Jiang N., Wu J., Leng T., Yang T., Zhou Y., Jiang Q., Wang B., Hu Y., Ji Y. H., Simon R. P., Chu X. P., Xiong Z. G., Zha X. M. (2017) Region specific contribution of ASIC2 to acidosis-and ischemia-induced neuronal injury. J. Cereb. Blood Flow Metab. 37, 528–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiong Z. G., Zhu X. M., Chu X. P., Minami M., Hey J., Wei W. L., MacDonald J. F., Wemmie J. A., Price M. P., Welsh M. J., Simon R. P. (2004) Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell 118, 687–698 [DOI] [PubMed] [Google Scholar]
- 16.Yin T., Lindley T. E., Albert G. W., Ahmed R., Schmeiser P. B., Grady M. S., Howard M. A., Welsh M. J. (2013) Loss of acid sensing ion channel-1a and bicarbonate administration attenuate the severity of traumatic brain injury. PLoS One 8, e72379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yermolaieva O., Leonard A. S., Schnizler M. K., Abboud F. M., Welsh M. J. (2004) Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc. Natl. Acad. Sci. USA 101, 6752–6757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li M., Inoue K., Branigan D., Kratzer E., Hansen J. C., Chen J. W., Simon R. P., Xiong Z. G. (2010) Acid-sensing ion channels in acidosis-induced injury of human brain neurons. J. Cereb. Blood Flow Metab. 30, 1247–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jing L., Chu X. P., Jiang Y. Q., Collier D. M., Wang B., Jiang Q., Snyder P. M., Zha X. M. (2012) N-glycosylation of acid-sensing ion channel 1a regulates its trafficking and acidosis-induced spine remodeling. J. Neurosci. 32, 4080–4091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frenkel C., Duch D. S., Urban B. W. (1993) Effects of i.v. anaesthetics on human brain sodium channels. Br. J. Anaesth. 71, 15–24 [DOI] [PubMed] [Google Scholar]
- 21.Wu J., Xu Y., Jiang Y. Q., Xu J., Hu Y., Zha X. M. (2016) ASIC subunit ratio and differential surface trafficking in the brain. Mol. Brain 9, 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zha X. M., Wang R., Collier D. M., Snyder P. M., Wemmie J. A., Welsh M. J. (2009) Oxidant regulated inter-subunit disulfide bond formation between ASIC1a subunits. Proc. Natl. Acad. Sci. USA 106, 3573–3578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ochoa J. G., Rusyniak W. G. (2016) Description of Ictal HFO mapping in patients with both temporal and extratemporal seizure focus. Neurol. Res. Int. 2016, 5380907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jing L., Jiang Y. Q., Jiang Q., Wang B., Chu X. P., Zha X. M. (2011) Interaction between the first transmembrane domain and the thumb of acid-sensing ion channel 1a is critical for its N-glycosylation and trafficking. PLoS One 6, e26909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu J., Leng T., Jing L., Jiang N., Chen D., Hu Y., Xiong Z. G., Zha X. M. (2016) Two di-leucine motifs regulate trafficking and function of mouse ASIC2a. Mol. Brain 9, 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Askwith C. C., Wemmie J. A., Price M. P., Rokhlina T., Welsh M. J. (2004) Acid-sensing ion channel 2 (ASIC2) modulates ASIC1 H+-activated currents in hippocampal neurons. J. Biol. Chem. 279, 18296–18305 [DOI] [PubMed] [Google Scholar]
- 27.Sherwood T. W., Askwith C. C. (2009) Dynorphin opioid peptides enhance acid-sensing ion channel 1a activity and acidosis-induced neuronal death. J. Neurosci. 29, 14371–14380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagai T., Yamada S., Tominaga T., Ichikawa M., Miyawaki A. (2004) Expanded dynamic range of fluorescent indicators for Ca(2+) by circularly permuted yellow fluorescent proteins. Proc. Natl. Acad. Sci. USA 101, 10554–10559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leavesley S. J., Britain A. L., Cichon L. K., Nikolaev V. O., Rich T. C. (2013) Assessing FRET using spectral techniques. Cytometry A 83, 898–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Annamdevula N. S., Sweat B., Favreau P., Lindsey A. S., Alvarez D. F., Rich T. C., Leavesley S. J. (2013) An approach for characterizing and comparing hyperspectral microscopy systems. Sensors (Basel) 13, 9267–9293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helenius A., Aebi M. (2004) Roles of N-linked glycans in the endoplasmic reticulum. Annu. Rev. Biochem. 73, 1019–1049 [DOI] [PubMed] [Google Scholar]
- 32.Rotin D., Kanelis V., Schild L. (2001) Trafficking and cell surface stability of ENaC. Am. J. Physiol. Renal Physiol. 281, F391–F399 [DOI] [PubMed] [Google Scholar]
- 33.Prescher J. A., Bertozzi C. R. (2006) Chemical technologies for probing glycans. Cell 126, 851–854 [DOI] [PubMed] [Google Scholar]
- 34.Yang H., Yu Y., Li W. G., Yu F., Cao H., Xu T. L., Jiang H. (2009) Inherent dynamics of the acid-sensing ion channel 1 correlates with the gating mechanism. PLoS Biol. 7, e1000151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li T., Yang Y., Canessa C. M. (2009) Interaction of the aromatics Tyr-72/Trp-288 in the interface of the extracellular and transmembrane domains is essential for proton gating of acid-sensing ion channels. J. Biol. Chem. 284, 4689–4694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Escoubas P., De Weille J. R., Lecoq A., Diochot S., Waldmann R., Champigny G., Moinier D., Ménez A., Lazdunski M. (2000) Isolation of a tarantula toxin specific for a class of proton-gated Na+ channels. J. Biol. Chem. 275, 25116–25121 [DOI] [PubMed] [Google Scholar]
- 37.Lin S. H., Chien Y. C., Chiang W. W., Liu Y. Z., Lien C. C., Chen C. C. (2015) Genetic mapping of ASIC4 and contrasting phenotype to ASIC1a in modulating innate fear and anxiety. Eur. J. Neurosci. 41, 1553–1568 [DOI] [PubMed] [Google Scholar]
- 38.Meng Q. Y., Wang W., Chen X. N., Xu T. L., Zhou J. N. (2009) Distribution of acid-sensing ion channel 3 in the rat hypothalamus. Neuroscience 159, 1126–1134 [DOI] [PubMed] [Google Scholar]
- 39.Babinski K., Lê K. T., Séguéla P. (1999) Molecular cloning and regional distribution of a human proton receptor subunit with biphasic functional properties. J. Neurochem. 72, 51–57 [DOI] [PubMed] [Google Scholar]
- 40.Chen X., Whissell P., Orser B. A., MacDonald J. F. (2011) Functional modifications of acid-sensing ion channels by ligand-gated chloride channels. PLoS One 6, e21970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chu X. P., Close N., Saugstad J. A., Xiong Z. G. (2006) ASIC1a-specific modulation of acid-sensing ion channels in mouse cortical neurons by redox reagents. J. Neurosci. 26, 5329–5339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li M., Kratzer E., Inoue K., Simon R. P., Xiong Z. G. (2010) Developmental change in the electrophysiological and pharmacological properties of acid-sensing ion channels in CNS neurons. J. Physiol. 588, 3883–3900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baron A., Waldmann R., Lazdunski M. (2002) ASIC-like, proton-activated currents in rat hippocampal neurons. J. Physiol. 539, 485–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao J., Duan B., Wang D. G., Deng X. H., Zhang G. Y., Xu L., Xu T. L. (2005) Coupling between NMDA receptor and acid-sensing ion channel contributes to ischemic neuronal death. Neuron 48, 635–646 [DOI] [PubMed] [Google Scholar]
- 45.Pignataro G., Simon R. P., Xiong Z. G. (2007) Prolonged activation of ASIC1a and the time window for neuroprotection in cerebral ischaemia. Brain 130, 151–158 [DOI] [PubMed] [Google Scholar]
- 46.Chen X., Orser B. A., MacDonald J. F. (2010) Design and screening of ASIC inhibitors based on aromatic diamidines for combating neurological disorders. Eur. J. Pharmacol. 648, 15–23 [DOI] [PubMed] [Google Scholar]
- 47.Petroff E. Y., Price M. P., Snitsarev V., Gong H., Korovkina V., Abboud F. M., Welsh M. J. (2008) Acid-sensing ion channels interact with and inhibit BK K+ channels. Proc. Natl. Acad. Sci. USA 105, 3140–3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ziemann A. E., Schnizler M. K., Albert G. W., Severson M. A., Howard M. A., III, Welsh M. J., Wemmie J. A. (2008) Seizure termination by acidosis depends on ASIC1a. Nat. Neurosci. 11, 816–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quintana P., Soto D., Poirot O., Zonouzi M., Kellenberger S., Muller D., Chrast R., Cull-Candy S. G. (2015) Acid-sensing ion channel 1a drives AMPA receptor plasticity following ischaemia and acidosis in hippocampal CA1 neurons. J. Physiol. 593, 4373–4386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y. Z., Wang J. J., Huang Y., Liu F., Zeng W. Z., Li Y., Xiong Z. G., Zhu M. X., Xu T. L. (2015) Tissue acidosis induces neuronal necroptosis via ASIC1a channel independent of its ionic conduction. eLife 4, e05682 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.