Abstract
β2-Adrenergic receptors (β2ARs) desensitize during continuous agonist activation, which manifests clinically as tachyphylaxis. β-Agonist desensitization of β2ARs in human airway smooth muscle (HASM) cells is recognized in the treatment of asthma and may be related to poor outcomes. Rapid events in desensitization include receptor phosphorylation and internalization, but mechanisms responsible for the decrease in receptor protein after prolonged agonist exposure (down-regulation) are ill defined. The microRNA (miRNA) let-7f regulates β2AR expression by translational repression. In cultured HASM cells from nonasthmatic and asthmatic lungs, 18 h of β-agonist exposure increased let-7f by 2–3-fold, concomitant with a ∼90% decrease in β2ARs. Inhibition of let-7f attenuated this down-regulation response by ∼50%. The let-7f increase was found to be cAMP/PKA–dependent. The mechanism of the let-7f increase was found by chromatin immunoprecipitation to be from activated cAMP response element–binding protein (CREB) binding to the let-7f promoter, thereby increasing let-7f expression. Knockdown of CREB attenuated agonist-promoted β2AR down-regulation by ∼50%. Thus, β2AR down-regulation occurs as a result of not only internalized receptor degradation but also a novel cAMP/PKA/CREB-mediated increase in let-7f, which causes enhanced repression of the β2AR gene, adrenoreceptor β2 (ADRB2) translation and represents ∼50% of the net loss of receptors observed after prolonged agonist exposure. This mechanism is apparent in asthmatic HASM cells, indicating relevance in a disease model.—Kim, D., Cho, S., Woo, J. A., Liggett, S. B. A CREB-mediated increase in miRNA let-7f during prolonged β-agonist exposure: a novel mechanism of β2-adrenergic receptor down-regulation in airway smooth muscle.
Keywords: G protein, tachyphylaxis, asthma, microRNA
Like many other 7 transmembrane-spanning GPCRs, the β2-adrenergic receptor (β2AR) undergoes agonist-promoted desensitization (1, 2). Desensitization is defined as the loss of receptor function during continuous agonist activation, and represents a homeostatic mechanism by which cells and organs regulate receptor function within the context of multiple signals. Receptor desensitization is considered integral to normal physiologic conditions, and can be adaptive or maladaptive in disease states (3). GPCR desensitization is also the basis for reduced effectiveness of therapeutic agonists administered on a chronic basis, clinically recognized as tolerance or tachyphylaxis. Early events in agonist-promoted desensitization of β2ARs include phosphorylation of the agonist-occupied receptor by GPCR kinases (GRKs). This establishes a substrate for receptor binding of β-arrestins, which partially disrupt the coupling of the receptor to the G protein (4). For β2ARs and many other GPCRs, β-arrestins also mediate receptor internalization from the cell surface to the interior of the cell. At this juncture, the internalized receptor either proceeds toward a degradation pathway, or can be recycled to the cell surface if an agonist is no longer present (5). Owing to their adapter functions, β-arrestins also initiate other signaling events, as reviewed in Lefkowitz et al. (6). These early processes are followed by a gradual decrease in β2AR expression (regardless of cellular localization) when agonist exposure is on the order of several hours, a process termed down-regulation. This decrease in expression can be substantial, resulting in a ≥90% loss of cell surface expression. For many cell types, down-regulation represents the main mechanism of desensitized cellular responses during long-term agonist exposure.
β2ARs are expressed in airway smooth muscle cells to relax the muscle, leading to bronchodilation and increased airflow. Agonists to β2ARs, often termed “β-agonists,” are the primary therapy for acute reversal of airway narrowing in asthma and chronic obstructive pulmonary disease (COPD). These drugs are also utilized in chronic conditions to maintain airway patency and prevent exacerbations. Repetitive or prolonged β-agonist treatment has been shown to result in loss of bronchodilator sensitivity to acute β-agonists (7, 8), a decrease in the bronchoprotective effect of β-agonists to inhaled bronchoconstrictors (9, 10), tachyphylaxis (8, 11–14), and adverse effects including increased exacerbations and death (15–18). Thus, there continues to be interest in the molecular basis of long-term agonist-promoted down-regulation of β2ARs in human airway smooth muscle (HASM) cells. As detailed in the Discussion, there is ample evidence suggesting additional mechanisms in play during down-regulation other than degradation of β-arrestin–mediated internalized receptors. This evidence suggests that down-regulation is not simply the result of a continuum from the short-term events to this long-term outcome. We have recently shown that expression of the gene encoding the β2AR, adrenoreceptor β2 (ADRB2), is significantly regulated by the small, noncoding microRNA (miRNA) let-7f, which binds to ADRB2 3’ untranslated regions and represses translation (19). In model transfected cell systems and in cells that endogenously express β2ARs, the expression levels of let-7f are inversely proportional to the expression of β2ARs, consistent with the role of miRNAs in repressing translation. In the current work, the potential for chronic β-agonists to alter let-7f expression in HASM cells was examined and observed, and studies were carried out to determine the mechanism by which this occurs. This novel let-7f–mediated mechanism appears to be responsible for ∼50% of the total β2AR down-regulation found with chronic β-agonist exposure, and accounts for the majority of the previously unexplained down-regulation observed in HASM cells.
MATERIALS AND METHODS
Cell culture
The HASM cells, derived from donor lungs of asthmatic and nonasthmatic patients, have been previously described (20, 21). We used cells between passages 5 and 7, which were cultured in DMEM supplemented with 10% fetal bovine serum in an environment composed of 95% air and 5% CO2 at 37°C. The immortalized HASM cell line denoted D9 hTERT was developed as described (22), and was grown in the same manner. For the treatment with isoproterenol (ISO), cells between 80 and 90% confluency were placed in fresh medium with either 30 µM ISO, 100 µM isobutylmethylxanthine (to inhibit phosphodiesterase), and 100 µM ascorbic acid (to inhibit oxidation of ISO), or ascorbic acid alone, and incubated for 18 h. Cells were then washed 3 times with room temperature PBS and processed for protein or for RNA. In a similar manner, cells were treated with 100 µM dibutyryl-cAMP or 10 µM forskolin. In some experiments, pretreatment with 10 µM N-(2-[p-bromocinnamylamino]ethyl)-5-isoquinoline-sulfonamide (H89) occurred for 1 h prior to the aforementioned treatments.
Transfections
HASM cells in 6-well plates were transfected using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA) as described in detail elsewhere (22, 23). Cells were in a final volume of 1.0 ml and the indicated constructs along with 10 µl of Lipofectamine 2000 were added to initiate the transfection. The constructs and final concentrations were: cAMP receptor element–binding protein [CREB (in pcDNA3, 4 μg/ml)], siCREB (40 nM of siGenome SmartPool; Dharmacon, Lafayette, CO, USA), and to inhibit let-7f, a competitive inhibitor (Dharmacon) that binds to let-7f (24, 25) at a final concentration of 40 nM (24, 25). The cells were studied 48 h after transfection. If cells were also treated with a drug, the agent was added at the indicated concentration at the 48-h point for an additional 18 h.
Western blots and quantitative RT-PCR
SDS-PAGE was performed over 7 h using 12% polyacrylamide gels on whole-cell lysates solubilized in RIPA buffer (∼10 µg), which were then transferred to PVDF membranes over 16 h, as previously described (22, 23). The titer (v/v) and antibodies were: SC-569 (β2AR, 1: 500; Santa Cruz Biotechnology, Dallas, TX, USA), 9197 (CREB, 1:1000; Cell Signaling Technology, Danvers, MA, USA), A1978 (actin, 1:2000; Sigma-Aldrich, St. Louis, MO, USA). Secondary antibody for chemoluminescence (Thermo Fisher Scientific) was at a titer of 1:10,000. ChemiDoc (Bio-Rad, Hercules, CA, USA) was used to detect bands, which were quantitated using ImageJ (National Institutes of Health, Bethesda, MD, USA). RT-PCR was performed using a LightCycler 96 system (Roche, Basel, Switzerland) and TaqMan polymerase (Applied Biosystems, Foster City, CA, USA) as previously described (26, 27). The RT reactions utilized Oligo d(T)16 for ADRB2 and glyceraldehyde-3 phosphate dehydrogenase (control), and the primers included with the TaqMan MicroRNA assay (Applied Biosystems) for the reaction with let-7f and U6 snRNA (control). The following assay IDs (Thermo Fisher Scientific) were used for PCR: ADRB2 (Hs00240532_sl), glyceraldehyde-3 phosphate dehydrogenase (Hs02786624_gl), let-7f (000382), and U6 snRNA (001973). Quantitation of transcripts was made by the 2−ΔCt method (28, 29).
Chromatin immunoprecipitation
Adhered HASM cells were treated with 1% formaldehyde in media for 10 min at 25°C, followed by the addition of 125 mM glycine to quench the fixation. After washing 3 times with PBS, cells were lysed and sheared by sonication. After brief centrifugation, pellets were diluted in 0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 167 mM NaCl, 16.7 mM Tris, (pH 8.0) buffer and precleared by incubation with sheared salmon sperm DNA and A/G agarose beads for 1 h at 4°C and then centrifuged at 2000 rpm for 4 min at 4°C. An aliquot of the supernatant was collected to represent the input. The remaining supernatants were subjected to immunoprecipitation (12 h at 4°C) using A/G agarose beads and an anti-CREB antibody (1:100; Cell Signaling Technology) or normal rabbit IgG as a control (Thermo Fisher Scientific). Subsequently, the agarose beads were sequentially washed with a low salt, high salt, and LiCl-based buffers as described in Qiu et al. (30), and then eluted with a 1% SDS, 0.1 M NaHCO2 buffer. Crosslinking, RNase A treatment, and DNA purification were performed as previously described (30). PCR to identify predicted CREB binding sites in pri-let-7f was carried out using GoTaq (Promega, Madison, WI, USA) and the following primers: 5′-TGTGTGTTTTGCACACCAGTT-3′ (forward) and 5′-AGACAATTCAACTGGGAATCG-3′ (reverse) (site 1), 5′-GCTACCTCCTAAATATGAAGTCTGT-3′ (forward) and 5′-TGAAGGCAGAGTCCAAAATCT (reverse) (site 2), 5′-TCGTTG TATGTTAGTGCATTTGGA-3′ (forward) and 5′-CAGAAAAACATGACAGCCTCTAA-3′ (reverse) (site 3), and 5′-ACACCCACCACTGGGAGATAA-3′ (forward) and 5′-ACTGACTTTCTATCAGACCGCC-3′ (reverse) (site 4).
Radioligand binding
Confluent cells in 10 cm dishes were washed 3 times with cold PBS followed by disruption with a cell scraper in buffer consisting of 5 mM Tris and 2 mM EDTA at 4°C (pH 7.4). Crude membranes were collected by centrifugation at 30,000 g for 15 min at 4°C, and resuspended in assay buffer consisting of 75 mM Tris, 12 mM MgCl2, and 5 mM EDTA at 25°C (pH 7.4). Saturation radioligand binding was performed using ∼20 µg membranes and the β2AR radioligand [125I]-cyanopindolol in the absence (total binding) and presence (nonspecific binding) of 10 µM alprenolol as described in Mialet Perez et al. (31). After incubation for 1.5 h at 25°C, bound radioligands were separated from free radioligands by rapid dilution and vacuum filtration with buffer containing 15 ml of 5 mM Tris and 2 mM EDTA at 25°C (pH 7.4) over Whatman GF/C filters (GE Healthcare Bio-Sciences, Piscataway, NJ, USA) using a Brandel cell harvester (Brandel, Gaithersberg, MD, USA). Specific binding was divided by the added cell membrane protein and β2AR expression stated as femtomoles per milligram.
Informatics and statistical analysis
Potential CREB binding sites in pri-miRNA let-7f (GenBank NR_029483; National Center for Biotechnology Information, Bethesda, MD, USA: https://www.ncbi.nlm.nih.gov/genbank/) were determined using the JASPAR database (http://jaspar.genereg.net) (32). The sequence surrounding pri-miRNA let-7f was taken from human chromosome 9q22.2-31.1 sequence deposited as GenBank AL158152 v.18. Queries were made for CREB binding sites 1750 bp upstream of the initiator ATG. The consensus CREB binding sequence was considered TGACGTCA and the profile score threshold set at 80%. Statistical comparisons of biochemical studies were made using GraphPad Prism 6.0 (La Jolla, CA, USA) using 2-tailed, paired Student’s t tests with a value of P < 0.05 considered significant. Data are shown as means ± se.
RESULTS
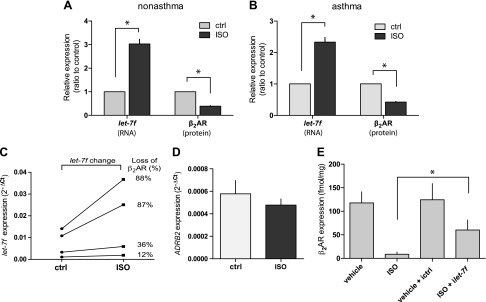
It has previously been shown that HASM cells from asthmatic lungs maintained in culture retain signaling characteristics consistent with a bronchospastic phenotype (33). Thus, we aimed to establish the relevance of let-7f to β2AR expression and regulation not only in cells of physiologic relevance, but also to confirm that results found in HASM cells obtained from normal lungs were recapitulated in those from asthmatic lungs. Initial efforts were focused on whether let-7f in HASM cells is regulated by long-term β-agonist exposure, and the consequences of such regulation on β2AR expression. As shown in Fig. 1A, B 18 h of exposure to the β-agonist ISO caused a ∼3-fold increase in let-7f miRNA expression in both asthmatic and nonasthmatic HASM cells. This was also accompanied by a decrease in β2AR expression in both groups of cells. In addition, cells that displayed the most β2AR down-regulation had the greatest change in let-7f, while those with the least change in β2AR expression had the smallest change in let-7f after ISO exposure, consistent with a potential causal relationship (Fig. 1C). We also utilized the immortalized D9 HASM cells in certain studies because these are more readily transfected compared with the primary lines. These cells showed the same β2AR and let-7f responses to ISO as the primary HASM cells (data not shown). In D9 HASM cells, ADRB2 mRNA was not found to be changed due to ISO exposure (Fig. 1D). Furthermore, inhibition of let-7f markedly attenuated (but did not fully abrogate) β2AR down-regulation by ISO, further indicating a causal relationship between the change in let-7f and β2AR down-regulation by agonist exposure (Fig. 1E).
Figure 1.
Agonist regulation of β2AR and let-7f in HASM cells. A, B) HASM cells from nonasthmatic or asthmatic lung donors were maintained in culture and treated with 100 µM ascorbic acid (control) or ascorbic acid with 30 µM ISO for 18 h at 37°C. RNA and protein were derived from these cells and let-7f miRNA and β2AR protein expression were determined as described in Materials and Methods (n = 4 experiments each from a different donor). C) The 2 greatest and 2 least changes in β2AR expression found in experiments from panels A and B are indicated with the changes in let-7f levels. D) ADRB2 mRNA is not altered in HASM cells by 18 h ISO treatment (n = 4 experiments from the D9 line). E) Knockdown of let-7f attenuates ISO-promoted down-regulation of β2AR in HASM cells (n = 6 experiments) *P < 0.01.
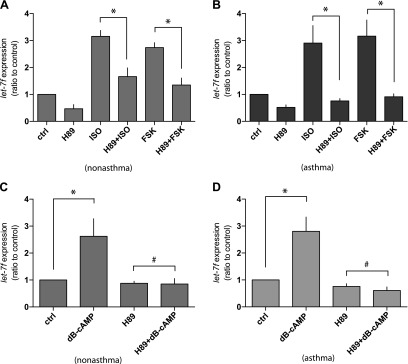
Having established a link between let-7f increases from β-agonist exposure and agonist-promoted down-regulation of β2AR, studies were undertaken to ascertain the mechanism by which let-7f is increased by agonists. We considered that the increase in let-7f might be dependent on activation of PKA, which is promoted by β2AR stimulation of intracellular cAMP. To address this, cells were treated with the diterpene forskolin, thus activating adenylyl cyclase and increasing cAMP in a receptor-independent fashion. The dose of forskolin was equieffective in stimulating cAMP as ISO (data not shown). To separate a PKA-dependent mechanism from a non-PKA event evoked by cAMP, the PKA inhibitor H89 was also utilized. As shown in Fig. 2A, B, forskolin increased let-7f to a similar extent as ISO in nonasthmatic and asthmatic HASM cells. Both responses were inhibited by H89. Additional studies were also performed using the cell-permeable cAMP analog dibutyryl-cAMP, to further confirm the dependency on cAMP. The expression of let-7f increased after an 18 h treatment with dibutyryl-cAMP, which was blocked by H89 (Fig. 2C, D).
Figure 2.
cAMP/PKA-dependent agonist regulation of let-7f in HASM cells. A, B) Cultured HASM cells derived from asthmatic (A) and nonasthmatic (B) donor lungs were treated with 10 µM H89, 30 µM ISO, H89 + ISO, 10 µM forskolin, or H89 + forskolin for 18 h at 37°C and let-7f miRNA expression was determined by quantitative RT-PCR. The expression of let-7f miRNA was increased by ISO and forskolin, which was blocked by the PKA inhibitor H89 (n = 4 experiments, each from a different donor). C, D) The same cells in panels A and B were treated with 100 µM cell-permeable cAMP analog dibutyryl cAMP (dB-cAMP) and let-7f miRNA expression was determined by quantitative RT-PCR. dB-cAMP increased let-7f expression, which was blocked by H89. (n = 4 experiments, each from a different donor) *P < 0.01, #P > 0.05.
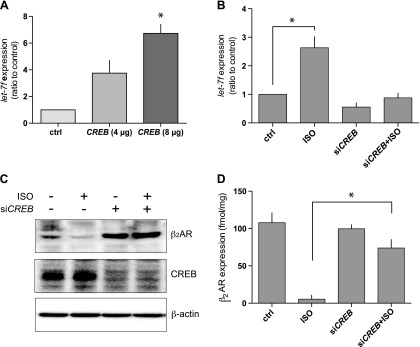
These results indicated that a PKA-mediated event promotes the increase in let-7f observed with chronic β-agonist exposure. It was hypothesized that the mechanism of this response could be due to activation of CREB, which then binds to a putative binding site in the promoter of let-7f. Initial studies of CREB overexpression in HASM cells (in the absence of an agonist) were performed, which showed an increase of let-7f in a gene-dose–dependent fashion (Fig. 3A). A moderate degree of native CREB expression was found in HASM cells, so the CREB was silenced using siRNA, and then the primary phenotype of β-agonist–induced increase in let-7f expression was examined. As shown in Fig. 3B, siCREB reduced the baseline expression of let-7f. More importantly, 18 h ISO exposure in siCREB-transfected cells caused no increase in let-7f, in contrast to nontransfected control cells studied in parallel (Fig. 3B).
Figure 3.
CREB modulates let-7f expression and long-term agonist-promoted down-regulation of β2AR in HASM cells. A) Transfection of CREB increases let-7f expression in HASM cells (n = 3). B) Knockdown of CREB by siCREB ablates agonist-promoted increases in let-7f (n = 4). C) Knockdown of CREB impairs agonist-promoted down-regulation of β2AR. A representative experiment (1 of 4) shows the silencing of CREB expression by siCREB, and the lack of ISO-promoted down-regulation under these conditions. D) Knockdown of CREB attenuates ISO-promoted down-regulation of β2AR (n = 5). All experiments performed with ISO used a concentration of 30 µM with an exposure period of 18 h at 37°C. *P < 0.01.
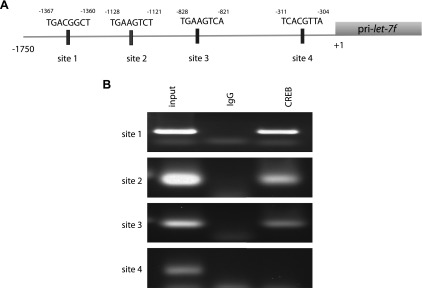
Taken together, the data indicated a PKA-mediated, CREB-based regulation of let-7f expression. Using the program JASPAR, 4 potential CREB sites in the promoter of the let-7f pri-miRNA sequence, as found in human chromosome 9q 22.2–31.1 sequence (GenBank) were found. (The pri-miRNA is initially transcribed and subsequently cleaved to the 22 bp let-7f miRNA). These potential CREB sites are arbitrarily defined as sites 1, 2, 3, and 4 (Fig. 4A). The binding scores for the 4 sites were 6.51, 6.36, 7.86, and 6.9, respectively. PCR primers were established to amplify each potential site after chromatin immunoprecipitation (see Materials and Methods). Sheared DNA protein complexes from HASM cells were immunoprecipitated with a CREB antibody, and the subsequent DNA was subjected to site-specific PCR. As shown in Fig. 4B, PCRs for predicted sites 1, 2, and 3 in the let-7f promoter were amplified, which indicates binding of these regions to CREB. Site 4 was not amplified, indicating that CREB did not bind to this region. When IgG was utilized instead of the CREB antibody, no specific PCR products were detected. PCR with the input DNA (prior to immunoprecipitation) revealed products of the expected molecular size, indicating the validity of the primers.
Figure 4.
Localization of CREB binding sites in the let-7f pri-miRNA promoter. A) Potential CREB binding sites in the 5′-upstream region of pri-let-7f as identified by JASPER. B) Chromatin immunoprecipitation results using a CREB antibody and pri-let-7f primers for PCR (see Materials and Methods). Sites 1, 2, and 3 were identified as CREB binding sites based on the presence of a PCR product. Results are representative of 4 experiments performed. IgG was utilized as a negative control in the immunoprecipitation step. The input refers to cell extracts prior to immunoprecipitation and the primers were validated by the presence of PCR products.
We considered that PKA activation evoked by β-agonists causes CREB phosphorylation, leading to its binding to the CREs in the promoter of let-7f. This binding increases let-7f and leads to translational repression of ADRB2, and a subsequent decrease in β2AR protein expression. This was tested by knocking down CREB in HASM cells in the absence and presence of long-term ISO exposure, and measuring β2AR expression. A representative experiment wherein β2AR expression was ascertained by Western blot is shown in Fig. 3C. The top panel shows the down-regulation of β2ARs by agonists in control cells and that this effect is not observed when CREB is silenced. Figure 3D shows the results of multiple experiments in which β2AR expression was quantitatively determined through radioligand binding. Under control conditions, ISO treatment resulted in a significant decrease in β2AR expression (>90%). With CREB knockdown, down-regulation by ISO was only ∼20% (Fig. 3D), indicating that this CREB–let-7f mechanism is a significant component of agonist-promoted down-regulation in HASM cells. The magnitude of this effect on β2AR down-regulation observed in the CREB knockdown experiments is remarkably similar to that observed when let-7f is ablated (compare Figs. 3D and 1D), consistent with the serial nature of the pathway identified.
DISCUSSION
The mechanisms at play in the regulation of β2AR function during continuous agonist exposure have been relatively well-established for short-term exposure, but there are multiple gaps in our understanding of events responsible for long-term agonist regulation. There has been a general assumption that the early events dictate or are a prerequisite for down-regulation, which has led to the belief that the early and late events represent a simple continuum. Applying this linear paradigm suggests that the dominant mechanism in long-term regulation is agonist-promoted phosphorylation by GRKs, which promote β-arrestin binding (early events) leading to ongoing receptor internalization and ultimate degradation of internalized receptors (late events), resulting in a net reduction in β2AR expression (down-regulation).
Several lines of evidence, however, do not support this as the singular mechanism. For example, studies have shown that, with impaired short-term desensitization, mutated β2ARs lacking all potential serine or threonine GRK phosphorylation sites nevertheless display long-term down-regulation similar to that of wild-type receptors (34). In other studies, mutations of the β2AR that alter the agonist-bound “active” state (equivalent to the GRK-phosphorylated and β-arrestin–bound state) have no effect on agonist-mediated internalization (35). Similarly, others have observed that mutants with wild-type agonist-promoted phosphorylation and internalization exhibit impaired long-term down-regulation, again showing a separation between these processes and suggesting additional mechanisms (35). Furthermore, some agonists that cause similar degrees of GRK-mediated phosphorylation of wild-type β2ARs display markedly different degrees of down-regulation (36). Of particular interest is the observation that β2AR down-regulation can be evoked to nearly the same extent as that induced by β-agonists through incubating cells with dibutyryl-cAMP and forskolin, pointing to cAMP-dependent events that lead to decreased receptor expression (37).
The intronless human ADRB2 gene does not have a CRE in the 5′-region including the promoter, so feedback at this level due to a cAMP/PKA mechanism is not expected. It was postulated, however, that a CREB-dependent mechanism evoked by receptor activation, cAMP generation, and PKA activation might down-regulate another gene whose product participates in β2AR down-regulation. We have previously shown that there is a 100% match between the seed region for the miRNA let-7f and a 3′UTR sequence of the ADRB2 (19). Using a reporter system with human embryonic kidney 293 cells, transfected wild-type let-7f inhibited translation of the reporter linked to wild-type ADRB2 3′UTR. In contrast, mutation of the ADRB2 sequence within the predicted binding site, or mutation of let-7f in the seed region, resulted in no change in reporter expression (19). These results prompted studies of endogenous expression of β2ARs in H292 cells (an epithelial carcinoma cell line). As has been previously established (38, 39), the guide miRNA strand is assembled into the miRNA-induced silencing complex (miRISC) while the passenger strand is degraded. A core component of miRISC is Ago2, a member of the Argonaute endonucleases, which binds to the guide miRNA strand and silences translation of the targeted mRNA. Overexpression of let-7f was found to increase in ADRB2 mRNA immunoprecipated with Ago2, thus confirming that let-7f binds to ADRB2 and functional interaction with the silencing mechanism occurs (19). Furthermore, there was a correlation between β2AR protein expression and let-7f expression. So, these studies revealed that let-7f regulates β2AR expression, but its potential role in β2AR down-regulation by agonist in HASM cells, or the mechanism of such a potential effect, was not defined.
In the current work, it is shown in HASM cells that let-7f expression increases with chronic β-agonist exposure, and, greater increases in let-7f are associated with greater degrees of down-regulation. Furthermore, agonist-promoted down-regulation was attenuated in HASM cells by ∼50% when let-7f was silenced, indicating a nontrivial role of this mechanism in the down-regulation process. Multiple approaches showed that the agonist-promoted increase in let-7f was cAMP and PKA dependent, indicating that let-7f was the intermediate component in a negative feedback loop. Informatic analysis revealed 4 potential CRE sequences in the 5′promoter of let-7f, and chromatin immunoprecipitation studies in HASM cells showed that 3 of these sites bind CREB. When CREB was silenced, agonist-promoted down-regulation was attenuated by ∼50%, an effect with a magnitude similar to that observed when let-7f was silenced.
Thus, in HASM cells a mechanism has been delineated that accounts for ∼50% of the magnitude of the long-term agonist-promoted down-regulation response (summarized in Fig. 5). This mechanism is due to a cAMP-dependent transcriptional regulation of let-7f by CREB, which represses translation of β2AR. The other components of down-regulation include the degradation of internalized receptors (40), and potentially other mechanisms. The HASM cell type is of particular interest because chronic β-agonists, acting on β2ARs on smooth muscle cells of the airway, are utilized for the treatment of airflow obstruction in asthma and COPD. Long-term treatment with β-agonists is not uncommon, and is utilized for maintenance or preventative treatment in chronic asthma and COPD. And, as indicated in the Introduction, there are multiple adverse effects with chronic β-agonist exposure. Many of these, such as a decrease in bronchodilation, a decrease in sensitivity to acute β-agonists, and loss of the bronchoprotective effects of acute β-agonists against inhaled constrictive agents, are consistent with depressed airway smooth muscle β2AR function evoked by prolonged β2AR activation. Furthermore, the corollary to these physiologic measurements may be adverse clinical outcomes, such as increased exacerbations and hospitalizations.
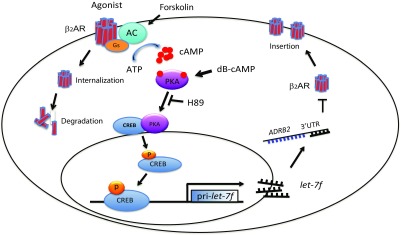
Figure 5.
Summary of the mechanisms responsible for β2AR down-regulation by CREB-mediated/let-7f processes in HASM cells. HASM cells express β2AR at a stable level when receptor production and degradation are at an equilibrium. Upon agonist activation of β2AR, intracellular cAMP levels increase due to receptor-Gs coupling. cAMP activates PKA, which in turn activates the CREB transcription factor. The transcription factor subsequently binds to the promoter of pri-let-7f, increasing expression of let-7f. Then let-7f binds to the 3′UTR of ADRB2 and represses translation, contributing to agonist-promoted long-term down-regulation of β2AR. Another mechanism of down-regulation that is apparent in HASM cells is the degradation of β-arrestin–mediated internalized receptors.
This let-7f-mediated down-regulation of β2ARs occurs regardless of how cAMP levels are increased, as indicated by the fact that β2AR activation, a cAMP analog, and activation of adenylyl cyclase with forskolin, all evoke the response. Thus, any potential new bronchodilator that acts via binding to a Gs-coupled receptor (41), would ultimately lead to β2AR down-regulation. This event would be deleterious for asthma therapy, as β-agonist responsiveness would be reduced, leaving less “β-agonist reserve” for acute treatment of severe bronchospasm. Bronchodilators acting via completely different mechanisms, such as agonists for bitter taste receptors, which relax HASM cells via a non-cAMP–dependent mechanism, have been proposed for add-on (or replacement) therapy to β-agonists, in part because of this lack of crosstalk (42). Interestingly, the up-regulation of let-7f by β-agonists may be cell-type dependent. We previously observed a small decrease in let-7f from whole lung homogenates from mice chronically treated with β-agonists (19). However, the lung consists of ∼40 cell types, of which many express β2ARs. Indeed, in whole lung homogenates the great majority of β2ARs come from type II cells of the alveoli (43). The physiologic role of β2ARs in these cells is not well established, and they are not utilized as therapeutic targets, so while this response is intriguing, its relevance is unclear. Nevertheless, these results suggest the potential for cell type specificity in the let-7f mediated regulation of β2AR down-regulation. In addition, the relative contribution of cAMP-dependent vs. agonist-dependent down-regulation of β2ARs appears to differ based on cell type or experimental conditions. For example, stably transfected human embryonic kidney 293 cells greatly overexpressing β2ARs have been reported to have a relatively small degree of dibutyryl cAMP-mediated down-regulation of β2ARs compared with that of ISO (40). This may be due to cell type characteristics, the expression vector, or the marked overexpression. In contrast, a cAMP-mediated pathway predominates in Chinese hamster fibroblast 1102 cells transfected to express β2ARs (37). In the current study, we have exclusively utilized HASM cells that endogenously express β2AR, so artifacts from overexpression are avoided. Regardless, the characteristics of the down-regulation phenotype in these cells is relevant, since β2ARs in HASM cells have a well-recognized physiologic role and are targets for β-agonists in the treatment of obstructive lung disease.
In summary, a previously undescribed mechanism that is responsible for ∼50% of the agonist-promoted down-regulation of β2ARs has been identified (Fig. 5). This mechanism is instigated by a β2AR-mediated increase in intracellular cAMP, which activates PKA, and PKA in turn activates CREB. CREB then binds to several CREs in the promoter of the miRNA let-7f. This causes increased cellular expression of let-7f, which then binds to the 3′UTR of ADRB2 and represses ADRB2 translation, leading to a progressive loss of β2AR protein expression. This pathway was established in the pharmacologically relevant HASM cell, where β2ARs act as targets for β-agonist treatment to relieve airway obstruction in asthma. These events were also observed in HASM cells derived from donor lungs of asthmatics, indicating that this regulatory pathway is intact in a pathophysiologic model. Given that the adverse effects of prolonged β-agonist treatment can be attributed to β2AR down-regulation, adjunct therapy aimed at blocking this pathway may prove clinically useful.
ACKNOWLEDGMENTS
The authors thank Ashley Goss, Maria Castano, and Tara Rosin for technical assistance, and Lisa Steward for manuscript preparation and editing (all from the University of South Florida). This work was funded by U.S. National Institutes of Health, National Heart, Lung, and Blood Institute Grants HL045967 and HL114471. The authors declare no conflicts of interest.
Glossary
- ADRB2
adrenoceptor β2
- β2AR
β2-adrenergic receptor
- COPD
chronic obstructive pulmonary disease
- CREB
cAMP response element–binding protein
- GRK
GPCR kinase
- H89
N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinoline-sulfonamide
- HASM
human airway smooth muscle
- ISO
isoproterenol
- miRNA
microRNA
AUTHOR CONTRIBUTIONS
D. Kim, J. A. Woo, and S. B. Liggett designed and performed the experiments, analyzed and interpreted the data, and wrote the paper; and S. Cho performed experiments.
REFERENCES
- 1.Liggett S. B. (1991) Desensitization of the β-adrenergic receptor: distinct molecular determinants of phosphorylation by specific kinases. Pharmacol. Res. 24(Suppl 1), 29–41 [DOI] [PubMed] [Google Scholar]
- 2.Kohout T. A., Lefkowitz R. J. (2003) Regulation of G protein–coupled receptor kinases and arrestins during receptor desensitization. Mol. Pharmacol. 63, 9–18 [DOI] [PubMed] [Google Scholar]
- 3.Lohse M. J., Engelhardt S., Eschenhagen T. (2003) What is the role of β-adrenergic signaling in heart failure? Circ. Res. 93, 896–906 [DOI] [PubMed] [Google Scholar]
- 4.Liggett S. B. (2011) Phosphorylation barcoding as a mechanism of directing GPCR signaling. Sci. Signal. 4, pe36 [DOI] [PubMed] [Google Scholar]
- 5.Hanyaloglu A. C., von Zastrow M. (2008) Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu. Rev. Pharmacol. Toxicol. 48, 537–568 [DOI] [PubMed] [Google Scholar]
- 6.Lefkowitz R. J. (2013) Arrestins come of age: a personal historical perspective. In Progress in Molecular Biology and Translational Science, Vol. 118 (Ed. Luttrell L. M.), pp. 3–18, Academic Press, New York: [DOI] [PubMed] [Google Scholar]
- 7.Lipworth B. J. (1997) Airway subsensitivity with long-acting β2-agonists. Is there cause for concern? Drug Saf. 16, 295–308 [DOI] [PubMed] [Google Scholar]
- 8.Newnham D. M., Grove A., McDevitt D. G., Lipworth B. J. (1995) Subsensitivity of bronchodilator and systemic beta 2 adrenoceptor responses after regular twice daily treatment with eformoterol dry powder in asthmatic patients. Thorax 50, 497–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalra S., Swystun V. A., Bhagat R., Cockcroft D. W. (1996) Inhaled corticosteroids do not prevent the development of tolerance to the bronchoprotective effect of salmeterol. Chest 109, 953–956 [DOI] [PubMed] [Google Scholar]
- 10.Harvey J. E., Tattersfield A. E. (1982) Airway response to salbutamol: effect of regular salbutamol inhalations in normal, atopic, and asthmatic subjects. Thorax 37, 280–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhagat R., Kalra S., Swystun V. A., Cockcroft D. W. (1995) Rapid onset of tolerance to the bronchoprotective effect of salmeterol. Chest 108, 1235–1239 [DOI] [PubMed] [Google Scholar]
- 12.Grove A., Lipworth B. J. (1995) Bronchodilator subsensitivity to salbutamol after twice daily salmeterol in asthmatic patients. Lancet 346, 201–206 [DOI] [PubMed] [Google Scholar]
- 13.Booth H., Bish R., Walters J., Whitehead F., Walters E. H. (1996) Salmeterol tachyphylaxis in steroid treated asthmatic subjects. Thorax 51, 1100–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Israel E., Chinchilli V. M., Ford J. G., Boushey H. A., Cherniack R., Craig T. J., Deykin A., Fagan J. K., Fahy J. V., Fish J., Kraft M., Kunselman S. J., Lazarus S. C., Lemanske R. F., Jr., Liggett S. B., Martin R. J., Mitra N., Peters S. P., Silverman E., Sorkness C. A., Szefler S. J., Wechsler M. E., Weiss S. T., Drazen J. M.; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network . (2004) Use of regularly scheduled albuterol treatment in asthma: genotype-stratified, randomised, placebo-controlled cross-over trial. Lancet 364, 1505–1512 [DOI] [PubMed] [Google Scholar]
- 15.Salpeter S. R., Buckley N. S., Ormiston T. M., Salpeter E. E. (2006) Meta-analysis: effect of long-acting β-agonists on severe asthma exacerbations and asthma-related deaths. Ann. Intern. Med. 144, 904–912 [DOI] [PubMed] [Google Scholar]
- 16.Sears M. R. (1998) Role of β2-agonists in asthma fatalities. In Fatal Asthma (Sheffer A. L., ed.), pp. 457–481, Vol. 115, Marcel Dekker, New York [Google Scholar]
- 17.Nelson H. S., Weiss S. T., Bleecker E. R., Yancey S. W., Dorinsky P. M.; SMART Study Group . (2006) The salmeterol multicenter asthma research trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest 129, 15–26 [DOI] [PubMed] [Google Scholar]
- 18.Grainger J., Woodman K., Pearce N., Crane J., Burgess C., Keane A., Beasley R. (1991) Prescribed fenoterol and death from asthma in New Zealand, 1981–7: a further case-control study. Thorax 46, 105–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang W. C. H., Juan A. H., Panebra A., Liggett S. B. (2011) MicroRNA let-7 establishes expression of β2-adrenergic receptors and dynamically down-regulates agonist-promoted down-regulation. Proc. Natl. Acad. Sci. USA 108, 6246–6251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deshpande D. A., Wang W. C. H., McIlmoyle E. L., Robinett K. S., Schillinger R. M., An S. S., Sham J. S. K., Liggett S. B. (2010) Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat. Med. 16, 1299–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aisenberg W. H., Huang J., Zhu W., Rajkumar P., Cruz R., Santhanam L., Natarajan N., Yong H. M., De Santiago B., Oh J. J., Yoon A.-R., Panettieri R. A., Homann O., Sullivan J. K., Liggett S. B., Pluznick J. L., An S. S. (2016) Defining an olfactory receptor function in airway smooth muscle cells. Sci. Rep. 6, 38231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim D., Woo J. A., Geffken E., An S. S., Liggett S. B. (2017) Coupling of airway smooth muscle bitter taste receptors to intracellular signaling and relaxation is via Gαi1,2,3. Am. J. Respir. Cell Mol. Biol. 56, 762–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim D., Pauer S. H., Yong H. M., An S. S., Liggett S. B. (2016) β2-adrenergic receptors chaperone trapped bitter taste receptor 14 to the cell surface as a heterodimer and exert unidirectional desensitization of taste receptor function. J. Biol. Chem. 291, 17616–17628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xue H., Gao X., Xu S., Zhang J., Guo X., Yan S., Li T., Guo X., Liu Q., Li G. (2016) MicroRNA-Let-7f reduces the vasculogenic mimicry of human glioma cells by regulating periostin-dependent migration. Oncol. Rep. 35, 1771–1777 [DOI] [PubMed] [Google Scholar]
- 25.Barnes N. A., Stephenson S., Cocco M., Tooze R. M., Doody G. M. (2012) BLIMP-1 and STAT3 counterregulate microRNA-21 during plasma cell differentiation. J. Immunol. 189, 253–260 [DOI] [PubMed] [Google Scholar]
- 26.Panebra A., Wang W. C., Malone M. M., Pitter D. R. G., Weiss S. T., Hawkins G. A., Liggett S. B. (2010) Common ADRB2 haplotypes derived from 26 polymorphic sites direct β2-adrenergic receptor expression and regulation phenotypes. PLoS One 5, e11819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panebra A., Schwarb M. R., Swift S. M., Weiss S. T., Bleecker E. R., Hawkins G. A., Liggett S. B. (2008) Variable-length poly-C tract polymorphisms of the β2-adrenergic receptor 3′-UTR alter expression and agonist regulation. Am. J. Physiol. Lung Cell. Mol. Physiol. 294, L190–L195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmittgen T. D., Livak K. J. (2008) Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3, 1101–1108 [DOI] [PubMed] [Google Scholar]
- 29.Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 30.Qiu Z., Dyer K. D., Xie Z., Rådinger M., Rosenberg H. F. (2009) GATA transcription factors regulate the expression of the human eosinophil-derived neurotoxin (RNase 2) gene. J. Biol. Chem. 284, 13099–13109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mialet Perez J., Rathz D. A., Petrashevskaya N. N., Hahn H. S., Wagoner L. E., Schwartz A., Dorn G. W., II, Liggett S. B. (2003) β1-adrenergic receptor polymorphisms confer differential function and predisposition to heart failure. Nat. Med. 9, 1300–1305 [DOI] [PubMed] [Google Scholar]
- 32.Sandelin A., Alkema W., Engström P., Wasserman W. W., Lenhard B. (2004) JASPAR: an open-access database for eukaryotic transcription factor binding profiles. Nucleic Acids Res. 32(Suppl 1), D91–D94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.An S. S., Mitzner W., Tang W.-Y., Ahn K., Yoon A.-R., Huang J., Kilic O., Yong H. M., Fahey J. W., Kumar S., Biswal S., Holgate S. T., Panettieri R. A., Jr., Solway J., Liggett S. B. (2016) An inflammation-independent contraction mechanophenotype of airway smooth muscle in asthma. J. Allergy Clin. Immunol. 138, 294–297.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liggett S. B., Bouvier M., Hausdorff W. P., O’Dowd B., Caron M. G., Lefkowitz R. J. (1989) Altered patterns of agonist-stimulated cAMP accumulation in cells expressing mutant beta 2–adrenergic receptors lacking phosphorylation sites. Mol. Pharmacol. 36, 641–646 [PubMed] [Google Scholar]
- 35.Campbell P. T., Hnatowich M., O’Dowd B. F., Caron M. G., Lefkowitz R. J., Hausdorff W. P. (1991) Mutations of the human beta 2–adrenergic receptor that impair coupling to Gs interfere with receptor down-regulation but not sequestration. Mol. Pharmacol. 39, 192–198 [PubMed] [Google Scholar]
- 36.Swift S. M., Schwarb M. R., Mihlbachler K. A., Liggett S. B. (2007) Pleiotropic β-agonist–promoted receptor conformations and signals independent of intrinsic activity. Am. J. Respir. Cell Mol. Biol. 36, 236–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bouvier M., Collins S., O’Dowd B. F., Campbell P. T., de Blasi A., Kobilka B. K., MacGregor C., Irons G. P., Caron M. G., Lefkowitz R. J. (1989) Two distinct pathways for cAMP-mediated down-regulation of the β2-adrenergic receptor: phosphorylation of the receptor and regulation of its mRNA level. J. Biol. Chem. 264, 16786–16792 [PubMed] [Google Scholar]
- 38.Han J., Lee Y., Yeom K.-H., Kim Y.-K., Jin H., Kim V. N. (2004) The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 18, 3016–3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chendrimada T. P., Gregory R. I., Kumaraswamy E., Norman J., Cooch N., Nishikura K., Shiekhattar R. (2005) TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature 436, 740–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore R. H., Tuffaha A., Millman E. E., Dai W., Hall H. S., Dickey B. F., Knoll B. J. (1999) Agonist-induced sorting of human beta2-adrenergic receptors to lysosomes during downregulation. J. Cell Sci. 112, 329–338 [DOI] [PubMed] [Google Scholar]
- 41.Mizuta K., Zhang Y., Xu D., Mizuta F., D’Ovidio F., Masaki E., Emala C. W. (2013) The dopamine D1 receptor is expressed and facilitates relaxation in airway smooth muscle. Respir. Res. 14, 89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liggett S. B. (2013) Bitter taste receptors on airway smooth muscle as targets for novel bronchodilators. Expert Opin. Ther. Targets 17, 721–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGraw D. W., Fukuda N., James P. F., Forbes S. L., Woo A. L., Lingrel J. B., Witte D. P., Matthay M. A., Liggett S. B. (2001) Targeted transgenic expression of β2-adrenergic receptors to type II cells increases alveolar fluid clearance. Am. J. Physiol. Lung Cell. Mol. Physiol. 281, L895–L903 [DOI] [PubMed] [Google Scholar]