Abstract
Resolvins are innate, immune responsive, bioactive mediators generated after myocardial infarction (MI) to resolve inflammation. The MI-induced bidirectional interaction between progressive left ventricle (LV) remodeling and kidney dysfunction is known to advance cardiorenal syndrome (CRS). Whether resolvins limit MI-induced cardiorenal inflammation is unclear. Thus, to define the role of exogenous resolvin D (RvD)-1 in post-MI CRS, we subjected 8- to 12-wk-old male C57BL/6 mice to coronary artery ligation. RvD1 was injected 3 h after MI. MI mice with no treatment served as MI controls (d 1 and 5). Mice with no surgery served as naive controls. In the injected mice, RvD1 promoted neutrophil (CD11b+/Ly6G+) egress from the infarcted LV, compared with the MI control group at d 5, indicative of neutrophil clearance and thereby resolved inflammation. Further, RvD1-injected mice showed higher reparative macrophages (F4/80+/Ly6Clow/CD206+) in the infarcted LV than did MI control mice at d 5 after MI. RvD1 suppressed the miRNA storm at d 1 and limited the MI-induced edematous milieu in a remote area of the LV compared with the MI control at d 5 after MI. Also, RvD1 preserved the nephrin expression that was diffuse in the glomerular membrane at d 5 and 28 in MI controls, indicating renal injury. RvD1 attenuated MI-induced renal inflammation, decreasing neutrophil gelatinase-associated lipocalin and proinflammatory cytokines and chemokines in the kidney compared with the MI control. In summary, RvD1 clears MI-induced inflammation by increasing resolving leukocytes and facilitates renoprotective mechanisms to limit CRS in acute and chronic heart failure.—Halade, G. V., Kain, V., Serhan, C. N. Immune responsive resolvin D1 programs myocardial infarction–induced cardiorenal syndrome in heart failure.
Keywords: leukocytes, inflammation resolution, neutrophil clearance, miRNA storm
An acute primordial inflammatory response to myocardial ischemic injury is evident through activation of damage- and pathogen-associated molecular proteins, but the resolution response has remained ignored (1). Historically, the resolution has been perceived as a passive event, but it has now been established that a leukocyte-mediated endogenous physiologic inflammatory response directs the active inflammation–resolution process (2, 3). Immune responsive bioactive lipid mediators formed in the acute inflammatory cascade are responsible for programming resolution physiology in heart failure (HF) (2, 4). Therefore, in the current report, we describe the resolvin D (RvD)-1 circuit that limits cardiorenal syndrome (CRS) after myocardial infarction (MI) in HF.
MI-induced renal dysfunction and altered synchrony between the heart and kidney are primary contributors to the onset of acute injury leading to progressive HF or kidney failure (5, 6). The cardiorenal network is a unique component of comprehensive systems biology that integrates with renal dysfunction and increases inflammatory markers in HF (6). To mimic translational acute HF (fractional shortening, ∼10%), we used a permanent coronary ligation model instead of reperfusion injury to study the temporal acute and resolving responses after MI. Advanced renal inflammation coincides with myocardium injury and is a critical predictor of cardiorenal pathobiology. Like the pathology of HF stages (7), mild stage I to chronic stage IV, CRS is divided into 1–5 stages from acute to chronic. MI triggers CRS with acute MI-induced hypoxic myocardial damage that coordinates a feed-forward stimulus to activate acute kidney injury markers (KIMs). In this study, we identified the mechanism of RvD1 that limits acute kidney injury in an acute ischemic HF mouse model, conceivably establishing a clinically relevant mouse model of CRS (8).
In response to myocardium injury or infection, resolution of inflammation is an active dynamic process marked by leukocyte infiltration (get-in signal) and exit (get-out signal) in a temporal manner, to coordinate optimal healing (9–11). Inflammation research is usually focused on the recruitment cascade and blockade or the inhibition of leukocytes. However, the clearance or resolution mechanisms remain unclear in acute HF (12). Despite the intervention of vascular reperfusion and use of thrombolytic agents, the collateral damage caused by noncleared leukocytes (↓get-out signal) causes progressive acute-to-chronic inflammation and CRS. Our other reports indicated that an overactive or uncontrolled acute inflammatory response sets the stage for nonresolution after MI inflammation in aging mice (13, 14). Also, we identified that immune responsive RvD1 directly activates the resolving sensor (lipoxin A4 receptor/formyl peptide receptor-2) to promote clearance in the infarcted heart (15). RvD1 is a known ligand for the lipoxin A4 receptor/formyl peptide receptor-2 receptor and GPCR-32 (16, 17). However, the mechanism of RvD1 in acute and chronic cardiorenal signaling remains of interest. In surgically induced acute HF, using a permanent coronary artery ligation model, we defined the role of RvD1 in CRS pathology. We validated that RvD1 limits MI-induced cardiorenal inflammation in a murine model of acute HF. The results suggest that RvD1 accelerates clearance of leukocytes from an infarcted area with activation of the miRNA (miR) circuit and decreases markers of acute kidney injury in HF. In a post-MI setting, the RvD1-mediated cardiorenal resolution is marked by neutrophil clearance, macrophage plasticity toward reparative phenotype, miR regulation in the infarcted heart, and reduced inflammatory markers [neutrophil gelatinase-associated lipocalin (NGAL) and cytokines] in the kidney during HF.
MATERIALS AND METHODS
Animal care and compliance
All animal surgical procedures and treatments were conducted according to the Guide for the Care and Use of Laboratory Animals [National Institutes of Health (NIH), Bethesda, MD, USA], the AVMA Guidelines for the Euthanasia of Animals (2013 edition; American Veterinary Medical Association, Schaumburg, IL, USA), and were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham.
Coronary ligation and RvD1 intervention
C57BL/6 mice (8–12 wk old) were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and were maintained at a constant temperature (19.8–22.2°C). The mice were anesthetized with 2% isoflurane, and the left anterior descending coronary artery was permanently ligated with a prolene 6-0 suture (15, 18, 19). Three hours after MI, RvD1 was injected (3 µg/kg/d; s.c.) and then daily for data collection on post-MI d 1 or 5. The mice were given free access to water and a standard chow diet. To study the MI-induced cardiorenal axis (Fig. 1A; study design) the mice were divided into 3 groups: 1) no surgery (d 0 naive control or no-MI control); 2) MI control; and 3) MI+RvD1. For the chronic HF model, MI-induced mice were monitored until d 28, with and without RvD1 injection daily.
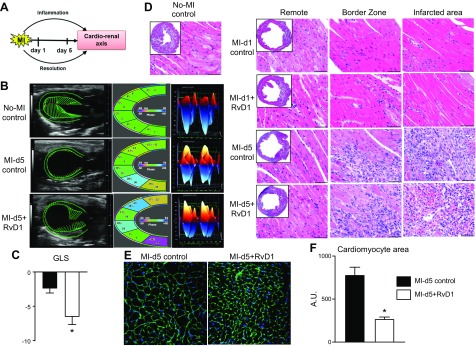
Figure 1.
RvD1 improves cardiac strain and LV performance with marked attenuated remote hypertrophy after MI. A) Study design scheme. B) Speckle tracking–based LV function analyses of echocardiography (left panel; long axis B-mode, middle panel; LV classified regions; right panel; 3D longitudinal strain). C) GLS at d 5 in MI control and RvD1-injected mice after MI. D) H&E images of no-MI, MI control, and MI+RvD1-injected mice LV (border, remote, and infarcted zones) at d 1 and 5 after MI. E) Images of WGA at d 5 after MI from MI control and MI+RvD1-injected mice. Scale bar, 20 μm. F) Quantitation of cardiomyocyte area per section per mouse. Data are means ± sem (n = 5–8 mice/group). *P < 0.05 vs. MI control at d 5 after MI.
Transthoracic echocardiography
Transthoracic echocardiography before and after MI was performed with a Vevo 3100 (VisualSonics, Inc., Toronto, ON, Canada). Mice were maintained under anesthesia with isoflurane and placed on a temperature-controlled platform, maintaining their physiology for normal heart and respiration rates. Ultrasound gel was applied to the chest above the heart area, and imaging was performed with an MX400 transducer (18–38 MHz center transmit; axial resolution, 50 μm; VisualSonics, Inc.), and comprehensive function was analyzed as described elsewhere (20).
Left ventricle and kidney harvest in control and post-MI mice
No-MI control d 0, post-MI RvD1-injected, or MI control d 1 and d 5 mice were anesthetized with isoflurane briefly and then were maintained in a mixture of 2% isoflurane anesthesia and 100% oxygen, and heparin (4 IU/g) was injected. The kidney, lungs, left ventricle (LV), and right ventricle were separated and weighed individually. The LV was divided into apex (infarcted area), midcavity, and base (remote area) under a microscope. The kidneys and LV are collected and weighed. Half of the kidney and the middle section were either fixed in 10% zinc formalin for histology or kept at optimal cutting temperature (OCT; Sakura Finetek, Torrance, CA, USA) for cryosectioning. The remainder of kidney and LV were snap frozen for molecular analysis, as previously described (13, 18).
LV histology
For histologic measurements, transverse LV sections were embedded in paraffin and sectioned. Hematoxylin and eosin (H&E)–stained images were acquired from the sections for all mice. A total of 5–7 images were acquired of the LV infarct area, including the border zone and remote area, with a microscope (BX43) equipped with a camera (DP73) (both from Olympus America, Melville, NY, USA) (13, 18).
LV and kidney immunofluorescence
LV midcavity and kidney cryosections were used for immunofluorescence. For wheat germ agglutinin (WGA) staining, frozen LV sections were fixed with 3% paraformaldehyde and blocked with 10% normal goat serum. For staining, AlexaFluor 488-conjugated WGA (Thermo Fisher Scientific, Waltham, MA, USA) was added to the tissue in a 1:1000 dilution for 1 h. Samples were then washed with PBS and mounted with ProLong Gold Antifade Reagent (Thermo Fisher Scientific). Myocytes were quantified from 5–6 high-power fields per section with ImageJ software (NIH). Cardiomyocyte data from each group were calculated from 30 images from 4–5 mice/group (21). For kidney tissues, a frozen kidney section was fixed in 3% paraformaldehyde, permeabilized with 0.1% Triton, and blocked for 1 h in 10% goat serum. Cells were subsequently incubated with nephrin and NGAL antibody (R&D Systems, Minneapolis, MN, USA) overnight and AlexaFluor 555–labeled anti-mouse antibody (Thermo Fisher Scientific), for 60 min at room temperature. The nucleus was stained with Hoechst (Thermo Fisher Scientific). Stained sections were visualized and photographed with an A1 high-speed laser confocal microscope (Nikon, Melville, NY, USA). The images are representative of 7–8 sections per area from 3–4 mice per group (22).
LV flow cytometry
Single mononuclear cells isolated from LV of no-MI control, MI control (post-MI d 1 and 5), and MI+RvD1 (post-MI d 1 and 5) were analyzed by flow cytometry (FACS) (13). The cell count for LV mononuclear cells was adjusted to ∼1–2 million cells per stain. Isolated cell suspensions were finally suspended in 200 µl of 1:500 Fc block and incubated for 10 min on ice. A cocktail of fluorophore-labeled mAb in 2× concentration was added for 30 min on ice, as appropriate for each experiment. We used CD45-PE-CY7 (BD Biosciences, San Jose, CA, USA), CD11b-APC, F4/80-Percp (Thermo Fisher Scientific), Ly6C-FITC (BD Biosciences), Ly6G-pacific blue (eBioscience, San Diego, CA, USA), and CD206-PE (BD Biosciences) in a cocktail. The cell population was primarily gated with CD45+ markers for hematopoietic cells. Further, the neutrophils were defined as CD11b+/Ly6G+ cells. Activated macrophages were defined as cells dually expressing the CD11b (Mac-1) and F4/80+ surface markers. The monocytes/macrophages were also classified based on CD11b expression as CD11b/F4/80+. The macrophages (F4/80+) were also classified as M1 (classically activated macrophages) based on Ly6chigh expression and M2 (alternatively activated or reparative macrophages) using Ly6Clow and CD206+ surface markers (Supplemental Fig. S1). Cell count was measured on the LSRII Flow cytometer (BD Biosciences), and data were analyzed with FlowJo software (v.7.6.3.) (Ashland, OR, USA) (13).
miR array profiling
miR gene expression profiling was performed with miR PCR arrays in frozen infarcted LV tissue for no-MI control, MI control, and MI+RvD1 groups, per the manufacturer’s instructions (Supplemental Fig. S2). In brief, miR was isolated with the miRNeasy Mini Kit (217004; Qiagen, Germantown, MD, USA), and cDNA synthesis was performed with an miScript II RT Kit (Qiagen). Each sample was loaded on the RT2-PCR plate (mi-Script miRNA PCR array MIMM-113ZE-4; Qiagen) and was run on an ABI 7900HT PCR system (Thermo Fisher Scientific). The results were reported as 2−ΔΔCt values.
Real-time quantitative PCR for infarcted LV and kidney tissue after MI
For quantitative PCR, reverse transcription was performed with 2.5 μg total kidney RNA with the SuperScript Vilo cDNA Synthesis Kit (Thermo Fisher Scientific). Quantitative PCR for the Tnfa, Il6, Il1b, Mrc1, Ifng, Tgfb, and Ym1 genes were performed with TaqMan probes (Thermo Fisher Scientific) on the 7900HT master cycler (Thermo Fisher Scientific). Gene levels were normalized to hypoxanthine phosphoribosyltransferase-1 (Hprt-1 gene) as the housekeeping gene control. The results were reported as 2−ΔΔCt values. All the experiments were performed in duplicate with n = 5–6 per group. For every milligram of infarcted LV tissue, 16 µl of reagent A (1× PBS from Thermo Fisher Scientific, without calcium and with 1× proteinase inhibitor; Roche Diagnostics, Indianapolis, IN, USA) was used. The tissues were homogenized in short (5 s) intervals at up to 100 amps, with a sonic homogenizer until completely homogenous. Homogenates were centrifuged at the maximum speed (14,000 rpm) for 5 min at 4°C. The supernatant was transferred to a fresh tube, snap frozen, and used as fraction A soluble protein. The pellet was washed 3 times with PBS, centrifuged at maximum speed after each wash and then resuspended in 16 µl reagent B (Reagent 4; MilliporeSigma, Billerica, MA, USA) and 1× protease inhibitor) per milligram of original tissue weight, and the pellets were homogenized by the same method as the A fraction. The new homogenous solution was snap frozen and used as fraction B for insoluble protein.
Kidney protein immunoblot analysis
Kidney protein expression was quantified by immunoblot analysis. Each immunoblot was begun with the running of a Criterion XT Bis-Tris 4–12% 18 gel (Bio-Rad, Hercules, CA, USA), in 3-(N-morpholino)propanesulfonic acid (MOPS) buffer (Bio-Rad). The Kaleidoscope Precision Plus Standard (Bio-Rad) was used to determine the molecular mass. Total 10 μg of protein was loaded, run, and transferred to nitrocellulose membrane (Bio-Rad). The total protein stain was acquired with the Pierce Reversible Protein Stain Kit for Nitrocellulose Membranes (Thermo Fisher Scientific). After rinsing with water, the membrane was blocked for 1 h at room temperature with 5% nonfat milk powder (Bio-Rad) dissolved in Triton-PBS and probed with primary antibody (KIM-1, 1:1000; NGAL, 1:1000) overnight at 4°C, followed by probing with a secondary antibody (Bio-Rad). The proteins were detected with the Pierce SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific). Densitometry was performed with ImageJ software (13).
Plasma creatinine
Plasma creatinine levels for the no-MI controls, MI controls, and MI+RvD1 groups were determined with liquid chromatography–tandem mass spectrometry (13).
NGAL ELISA
The NGAL or Lipocalin-2 Mouse Simple Step ELISA Kit (MLCN20; R&D Systems) was used to determine plasma NGAL levels, according to the manufacturer’s instructions.
Urea assay
Plasma urea was measured with the QuantiChrom urea Assay Kit (DIUR-100; BioAssays, Hayward, CA, USA) according to the manufacturer’s instructions.
Hierarchical clustering and gene network analysis of miR arrays
Hierarchical clustering analysis was used to organize the genes based on similarities in their expression profiles. The values of genes were normalized by taking the geometric mean of the genes for statistical significance. A list of putative miR targets was identified with the prediction algorithm DNA Intelligent Analysis (DIANA) microT-CDS (v.5.0) (23). The predicted miR targets were annotated into functional pathways with DIANA-miRPath (v.2.0). The gene-network was generated by using the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING; https//:string-db.org). A volcano plot was generated using R (https://www.r-project.org). The cutoff was a 2-fold change, with values of P < 0.01 indicating significance.
Statistical analysis
Data are expressed as means ± sem. Statistical analyses were performed with Prism 5 (GraphPad, San Diego, CA, USA). ANOVA followed by Newman-Keuls post hoc test was for multiple comparisons of post-MI d 1 and 5 for RvD1-injected and noninjected MI controls compared to the d 0 naive control. All immunoblot densitometry data were normalized to the total protein lane. For 2-group comparison, the unpaired Student’s t test was applied. Values of P < 0.05 were considered statistically significant.
RESULTS
Immune responsive RvD1 improved LV function, strain, and cardiac performance after MI
To determine the global heart functional benefit of RvD1 in acute HF, LV function and strain were measured with echocardiography. The longitudinal, radial, and circumferential strains of RvD1-injected mice were compared with those of MI controls and naive controls. As is obvious, in response to myocardial injury, the fractional shortening decreased to 10–12%, compared with the 35–40% decrease in naive controls. Despite similar infarct areas, RvD1-injected mice improved global longitudinal strain (GLS) after MI (Fig. 1B, C). Immune responsive RvD1 increased strain, which is a dimensionless parameter, and indicated that myocardial tissue had reformed to a matured scar in the resolving phase, compared with MI controls. The RvD1-mediated improvement in global functioning was illustrated by speckle tracking–based strain analysis, as illustrated in Fig. 1B. Multiple parameters, including wall thinning and longitudinal strain, depicted with green speckle tracks, suggest that MI+RvD1–injected mice showed less myocardial deformation in midsystole than did MI control mice at d 5 after MI, whereas the naive control showed a functional heart in no-MI control mice. RvD1-injected mice displayed enhanced longitudinal strain and a less dyssynchronous myocardial pattern than did the MI control mice at d 5 after MI. The longitudinal strain was reduced after MI toward a positive number (Fig. 1B, middle panel), in contrast, RvD1 attenuated longitudinal strain loss at d 5 after MI, maintaining a higher negative number. The left panel in Figure 1B represents a 3D mode of strain analysis; GLS was observed to −2.4 ± 0.6 in MI control mice, indicating LV dysfunction, whereas RvD1-injected mice displayed improved strain, particularly GLS (−4.2 ± 0.7), was indicative of improved LV function in pathologic remodeling after MI (Fig. 1C).
As expected, the LV was dilated in MI controls compared with naive controls. Histologic examination of RvD1-injected mouse LV suggest that the infarcted regions showed a lower myofibrillar loss and dead cells in the border zone and infarcted area after MI. H&E-stained sections revealed hypereosinophilic change, contraction, necrosis in the myocardial infarct sections at d 5 after MI. The morphologic changes were less frequent in the border zone area after MI. RvD1-injected mice showed an attenuated MI-induced edematous milieu in the remote area of the LV that was notable compared with that in the same area in MI control mice at d 5 after MI (Fig. 1D). Further, the MI+RvD1 group had a reduced myocyte area, indicative of a less edematous myocardium, validated with WGA staining (Fig. 1E). Overall, RvD1-injected mice reduced MI-induced edema and improved LV strain and cardiac function after MI.
RvD1 facilitated CD11b+Ly6G+ clearance without affecting the innate immune response after MI
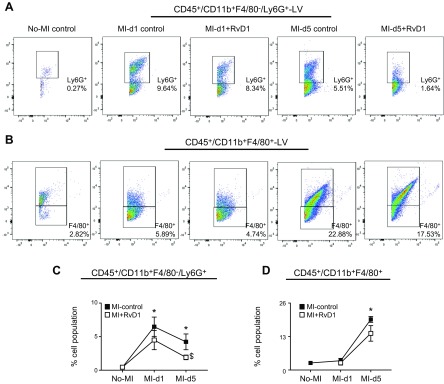
Our previous semiquantitative study has shown (15) that RvD1 coordinates with the spleen after MI to facilitate rapid clearance of neutrophil density after MI. Here, we applied a comprehensive quantitative approach and validated the recruitment (get-in signal) and clearance (get-out signal) of neutrophils (CD45+/F4/80−/CD11b+/Ly6G+) in the LV after MI. We used a detailed flow cytometric approach (Supplemental Fig. S1) to detect CD45+F4/80−/CD11b+Ly6G+ neutrophils that allowed understanding of the temporal kinetics of leukocyte infiltration (get-in signal) and exit (get-out signal) after MI. Results revealed the presence of a minor population of CD45+F4/80−CD11b+Ly6G+ cells (0.27 ± 0.11%) in no-MI control hearts. After MI, there was an immediate rapid infiltration (get-in signal) of CD45+F4/80−CD11b+Ly6G+, peaking within 24 h (post-MI d 1) to 6.5 ± 2% in MI control. The RvD1-injected group displayed steady recruitment in the acute response of CD45+F4/80−CD11b+Ly6G+ neutrophils, similar to the MI control at d 1 (4.5 ± 1.5%) after MI. In cardiac injury, recruited neutrophils are the first invaders at the site of injury, and then monocyte/macrophage phagocytose neutrophils to facilitate the resolution signal (24). Clearance of neutrophils was marked by a lower number of CD45+F4/80−CD11b+Ly6G+ cells at d 5 after MI, which showed that RvD1 expedited the egress of neutrophils (2.0 ± 0.3%; get-out signal) from the infarcted LV in injected mice, compared with MI control (Fig. 2A, C). Now, it is clear that residential macrophages are present in the heart in steady state. The FACS analysis showed that no-MI control LV held nearly 2.7 ± 0.3% of CD45+CD11b+F4/80+ cells, which peaked at d 5 at 19.6 ± 1.1%, indicating the continuous get-in signal. The RvD1-injected group displayed a decrease in CD45+CD11b+F4/80+ cells (14.2 ± 3.0%) at d 5 after MI (Fig. 2B, D). Thus, RvD1 activated a feed-forward loop that supports neutrophil clearance, without affecting the acute recruitment response which is essential for removing cardiomyocyte debris and stable matrix formation after MI.
Figure 2.
RvD1 activated the clearance of neutrophils after MI. A) Representative FACS dot plots showing the LV neutrophil population (CD11b+/Ly6G+) in MI control and MI+RvD1 groups at d 1 and 5 after MI, compared with the no-MI control. B) Representative FACS dot plots showing the LV mononuclear macrophage (CD11b+/F4/80+) population in MI control and MI+RvD1 groups at d 1 and 5 after MI, compared with no-MI controls. C) Data suggest an RvD1-mediated decrease in the Ly6G+ population in the infarcted LV at d 5 after MI. D) Cell population in the LV at d 1 and 5 after MI, compared with d 0 no-MI controls. RvD1-injected mice showed a lower percentage of CD11b+/F4/80+population compared with MI control mice after MI (n = 5 mice/group). Data are means ± sem. *P < 0.05 vs. no-MI, $P < 0.05 MI control vs. MI+RvD1.
RvD1 polarized macrophages toward a reparative phenotype after MI
Large eater macrophages are unique phagocytic effector cells in the innate immune response and are highly responsive to change in the microenvironment and aging (11). Based on the infiltrative expression markers, macrophages are classified as Ly6C+/− and CD206+/− to indicate classic vs. reparative macrophages (Supplemental Fig. S1; see gating strategy). Post-MI cardiac Ly6Clow/CD206+ and Ly6Chigh/CD206+ macrophages were quantified by FACS after MI and RvD1 injection. The Ly6Clow (2.1 ± 0.2%) and Ly6ClowCD206+ (2.1 ± 0.3%) macrophages represent the dominant part of the whole F4/80+ cardiac macrophage population in no-MI control LV (Fig. 3A, B). Cardiac Ly6Chigh macrophages increased at d 1 after MI (1.4 ± 0.7% vs. 0.6 ± 0.1% no-MI) but tended to decrease at d 5 after MI. The number of Ly6Clow macrophages increased at d 5 after MI in the MI control group. The RvD1-injected group displayed a remarkable increase in Ly6Clow (19.6 ± 1.1%) and Ly6ClowCD206+ (18.6 ± 0.7%) macrophages at d 5 after MI (Fig. 3). Thus, the MI+RvD1 group polarized the macrophages with a high proportion of reparative Ly6Clow subpopulations, indicative of an expedited healing response in acute HF.
Figure 3.
RvD1 activated CD206+/Ly6Clow with decrease in CD206+/Ly6Chi in the infarcted LV after MI. A) Representative FACS dot plots identifying the LV F4/80+/Ly6Chigh and F4/80+/Ly6Clow populations in MI+RvD1–injected mice at d 1 and 5, compared with the MI control and no-MI control mice. B) Representative dot plots showing the LV CD206+/Ly6Chigh and CD206+/Ly6Clow populations in MI+RvD1–injected mice at d 1 and 5 compared with no-MI controls. C–F) LV percentage of F4/80+/Ly6Chigh (C), F4/80+/Ly6Clow (D), Ly6Chigh/CD206+ (E), and Ly6Clow/CD206+ (F) populations (n = 5 mice/group). Data are means ± sem. *P < 0.05 vs. no-MI, $P < 0.05 MI control vs. MI+RvD1.
RvD1 governs the miR storm in acute response and accelerates regenerative miRs in the resolving phase after MI
After myocardial injury in MI, like cytokines and chemokines, the miR storm is set to turn on and influence cardiac remodeling. Comprehensive analysis of miR revealed that RvD1 governed the change of expression of numerous miRs in the infarcted LV area after MI. Heat map cluster analysis (Supplemental Fig. S3) showed that MI control and RvD1-injected mice had globally activated miRs after MI. A correlation analysis of the MI-d 1 vs. no-MI control groups indicated generation of the miR storm. The more significant correlations were observed at MI d 5 vs. the no-MI control. RvD1-injected mice displayed a normalized expression, similar to the no-MI control at d 1 after MI (Fig. 4A; open green circle, red arrow), indicating the comprehensive impact of RvD1 on the miR storm. The global change in gene expression was further exemplified with principal component analysis (PCA), to determine changes in MI control and RvD1-injected mice after MI (Fig. 4B). Volcano plot analysis revealed that on post-MI d 1, let-7e-5p miR was significantly down-regulated, whereas miR-342-3p was significantly up-regulated. RvD1-injected mice at d 1 after MI displayed down-regulation of miR-30a-5p, but up-regulation of miR-195a-5p (Supplemental Fig. S2B). A total of 13 miRs (miR-149-5p, miR-99a-5p, miR-486-5p, miR-328-3p, miR-100-5p, miR-499-5p, miR-133a-3p, miR-150-5p, miR-206-3p, miR185-5p, miR-208-3p, miR-320-3p, and miR-181-5p) were significantly up-regulated, whereas 7 miRs (miR-145a-5p, miR-22-3p, miR-30d-5p, miR-378a-3p, miR-30e-5p, miR-125a-5p, and miR-30a-5p) were significantly down-regulated at d 5 after MI (Fig. 4C). RvD1 injection normalized these changes in the gene expression; only miR-1a-3p was significantly up-regulated (8-fold change) and miR-126a-3p, miR-23a-3p, and miR-125b-5p were down-regulated. The string network analysis revealed differentially regulated miRs that are related to extracellular matrix remodeling and leukocyte kinetic gene pathways (Fig. 4D). Overall, RvD1 facilitated miR-directed transcriptional changes in the post-MI healing response.
Figure 4.
RvD1 governs the miR storm in the acute response and accelerates regenerative miRs after MI. A) Correlation graph showing generation of the miR storm after MI. B) PCA of miRs suggesting that RvD1 normalized miR expression at d 5 after MI (n = 4 mice/group). C) Volcano plot for miRs at d 5 after MI in MI control and MI+RvD1 injected group. The x axis is log2 fold-change, and the y axis is log fold change. In each graph, every point represents an individual transcript. The up-regulated (right, red dots) and down-regulated (left, green dots) miRs are shown. D) RvD1 oriented protein–protein interaction network generated through SPRING. The cutoff is 2-fold change (n = 4 mice/group for each time point).
RvD1-mediated leukocyte clearance reduced collateral kidney injury after MI
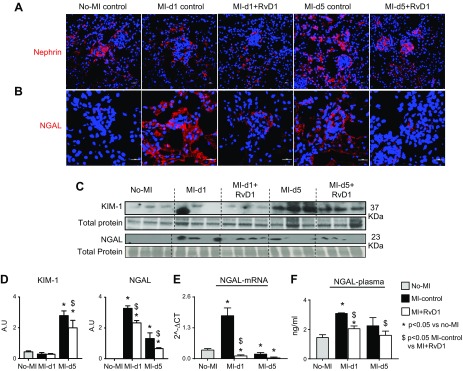
To test whether RvD1 preserves nephrons with a marked reduction in CRS in acute HF, we evaluated the degree of podocyte injury among the experimental groups by immunofluorescence for nephrin. In the no-MI control mice, localization of nephrin (red) was observed along the glomerular capillary wall as a continuous pattern, suggesting localization in podocytes. In the MI control mice, at d 1 after MI, the intensity of nephrin was diminished and was observed as a discontinuous pattern along the glomerular capillary walls and persisted at d 5 after MI, suggesting podocyte injury. The MI+RvD1 group showed restored protein levels, as well as a continuous pattern of nephrin in the glomerulus at d 5 after MI (Fig. 5A). Further, post-MI renal inflammation was confirmed by early up-regulation of NGAL in MI controls. The immunofluorescence analysis of NGAL expression showed that there was an overall and immediate increase of NGAL expression in the MI controls compared with the no-MI naive controls at d 5 after MI. In kidney glomerulus structure, the NGAL expression was higher in the MI controls at d 1 after MI compared to that in the naive controls. There was a decrease in NGAL mRNA expression at d 5 after MI, but the glomerulus consistently displayed higher expression of NGAL suggestive of an inflamed kidney. RvD1-injected mice had limited NGAL expression after MI, specifically in the glomerulus, compared with MI controls (Fig. 5B). RvD1-mediated nephroprotection was further suggested by reduced levels of KIM-1 and NGAL compared with MI controls at d 5 after MI (Fig. 5C, D). The NGAL mRNA, protein expression, and plasma levels displayed an immediate increase (6-fold; P < 0.01 for mRNA) at d 1 in the MI control, whereas RvD1 suppressed NGAL expression after MI, compared with the respective MI control (Fig. 5E, F). Thus, RvD1 attenuated MI-induced collateral kidney injury, thereby limiting CRS syndrome in acute HF.
Figure 5.
RvD1 attenuates cardiorenal inflammation by limiting acute KIM NGAL and retains nephrin expression after MI. A) No-MI control kidney sections showed linear, homogeneous staining along the outer portion of the glomerular basal membrane. The expression of nephrin was diffused and patchy at d 1 after MI, and later, at d 5, in only the outer portion of the glomerular basal membrane. RvD1-injected mice showed diffuse expression at d 1, but at d 5 after MI, the RvD1-injected mice displayed linear homogeneous staining comparable to the no-MI controls. B) Immunofluorescence images showing an immediate increase in NGAL expression at d 1 after MI in the kidney glomerulus and tubular area. RvD1 reduced the NGAL expression after MI in kidney. A total of 3–4 images were taken per section per slide. Magnification, ×40. C) KIM-1 and NGAL protein expression. D) Densitometry analyses of KIM-1 and NGAL normalized to total protein. E) mRNA expression of NGAL normalized to HPRT-1. F) Plasma NGAL levels. Data are means ± sem (n = 3–5/group). *P < 0.05 vs. no-MI, $P < 0.05 MI control vs. MI+RvD1.
RvD1 attenuated renal inflammation in HF
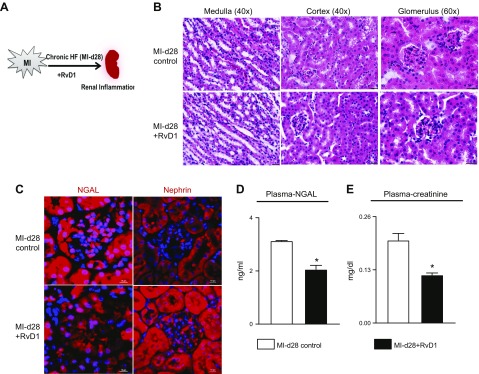
Because RvD1 prevented renal inflammation in acute HF, to replicate the role of RvD1 in chronic HF-induced renal inflammation, the kidney histology and inflammation markers were measured (Fig. 6A). The evaluation of gross kidney morphology by H&E staining showed a distorted medulla and cortex and an impaired glomerulus structure, leaving no space between the basement membrane and mesangial matrix, indicating renal inflammation. RvD1 prevented distortion of renal structure in HF (Fig. 6B). Enhanced NGAL expression (red) is indicative of MI-induced inflamed kidney, as the glomerulus consistently displayed expression of NGAL and diminished nephrin in podocytes, whereas RvD1 attenuated NGAL expression and preserved nephrin expression in podocytes, indicating a decrease in renal inflammation (Fig. 6C). Similar changes were detected in the plasma-NGAL level (Fig. 6D). During advanced HF, the plasma creatinine levels were significantly down-regulated in RvD1-injected mice compared to MI-d 28 control mice (Fig. 6E); however, there was no difference in plasma–urea levels between MI-d 28 and RvD1-treated mice (Supplemental Fig. S8). Thus, RvD1 attenuated MI-induced renal inflammation in advanced HF.
Figure 6.
RvD1 attenuates chronic renal inflammation after MI. A) A chronic HF model. B) H&E-stained kidney of MI control and MI+RvD1 groups at post-MI d 28 displaying structural changes in the cortex, medulla, and glomerulus. C) Immunofluorescence images showing changes in NGAL and nephrin expression at d 28 after MI and the MI+RvD1–injected group in kidney. A total of 3–4 images were taken per section per slide. Magnification, ×40. D) Plasma NGAL level in MI d 28 and MI+RvD1 mice. E) Plasma creatinine level in MI d 28 and MI+RvD1 injected mice. *P < 0.05 vs. MI d 28.
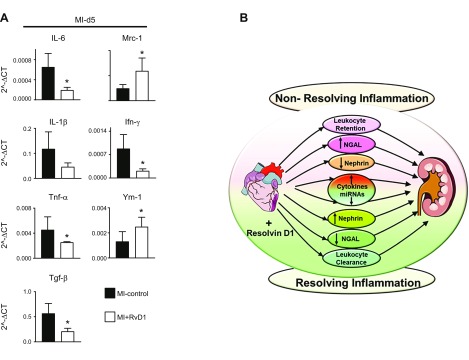
RvD1 control inflammatory cytokines and amplify reparative cytokines after MI
Secreted cytokines are markers of immune cell existence or trafficking at the site of injury, and there is a known amplified leukocyte presence in the circulation after MI. To test whether an amplified leukocyte presence in the circulation contributes to kidney inflammation, we determined the levels of proinflammatory and reparative cytokines after MI. In kidney tissue, Il-6, Il-1β, Tnf-α, and Tgf-β mRNA expression increased significantly at d 5 after MI in MI control mice, indicating localized inflammation in the kidneys, whereas RvD1-injected mice displayed lower levels of those cytokines, indicative of limited kidney inflammation. The expression of the cytokines mannose receptor C-type 1 (Mrc-1) and Ym-1 (chitinase-like 3) was considerably higher in the RvD1-injected mice at d 5 after MI (Fig. 7A). Thus, the MI-induced cytokine outbreak was reduced in RvD1-injected mice, thereby reducing systemic inflammation after MI.
Figure 7.
RvD1 tempered MI-induced renal inflammation in acute HF. A) mRNA expression of proinflammatory (Il6, Il1b, Tnfa, and Tgfb) and proresolving (Mrc1, Ifng, and Ym1) cytokine genes in kidney at d 5 after MI in MI control and MI+RvD1 groups. B) The cardiorenal axis, depicting the role of the RvD1 mechanistic circuit in MI-induced CRS1 pathology (n = 6 mice/group). Data are means ± sem. *P < 0.05 vs. MI control at d 5 after MI.
DISCUSSION
The prime objective of this study was to determine the therapeutic applicability of immune responsive RvD1 to limit MI-induced inflammation in the cardiorenal axis. Our splenocardiac outcome with emphasis on infarct healing (15) and current cardiorenal results indicates that RvD1 improves leukocyte clearance, governs the miR circuit, and limits cardiorenal pathology after MI. We report the systemic mechanisms circuit whereby RvD1 controls the collateral impact of acute myocardial injury of the kidney by resolving inflammation (Fig. 7B). RvD1 increases infarct healing and suppresses renal inflammation with the marked regulation of miRs through direct and indirect effects on the myocardium and kidney. Key findings after RvD1 injection in myocardial healing are as follows: 1) marked leukocyte clearance (get-out signal) without affecting the acute response (get-in signal); 2) reduced MI-induced KIMs such as NGAL; and 3) governed miR circuit (let-7e-5p and miR-342-5p) and amplified reparative cytokines (MRC and YM-1) in kidney after MI. Thus, we report 3 innovative mechanistic actions of RvD1 that are essential for development of a therapeutic modality to amplify proresolution activity in MI-induced acute HF and cardiorenal pathology.
The first innovative action of RvD1 was validated by a quantitative FACS approach that suggested active clearance of neutrophils (CD45/CD11b/Ly6G+) without affecting the primordial innate response or immunosuppression. Clearance of neutrophils is of paramount importance, because, within a few minutes to hours after the onset of myocardium injury, infiltrated neutrophils assemble (get-in signal) in the infarcted cardiac tissue in response to the release of the chemoattractant-like leukotriene B4, oxidative stress, chemokines, cytokines, and activated complement that feed forward a collateral injury program (25, 26). Post-MI magnitude of neutrophilia (neutrophil swarming) is significantly associated with the early progressive development of congestive HF (27). Injury-dependent residual time of neutrophils in the myocardium defines the resolving or nonresolving inflammation in the post-MI setting. Our previous report indicated that obesity superimposed on aging promotes nonresolving inflammation with a marked increase in the cell adhesion molecule-1, proinflammatory mediator 12-hydroperoxyeicosatetraenoic acid, and neutrophils and thereby early cardiac dysfunction (11, 14). Post-MI physiologic inflammation was therapeutically treated using antibodies against cell-adhesion molecules and the inhibition of complement or cytokine (TNF-α or IL-β) activation that perhaps suppress essential acute response; therefore, those anti-inflammatory clinical studies have been largely negative or trigger immunosuppression (25, 28, 29). The cytokine inhibitory approach that triggers the immune suppression is evident in IL-1β blockade by canakinumab, which develops fatal infection in cardiac patients noted in the CANTOS trial (29). Likewise, TNF-α inhibition in the Renewal (Renaissance and Recover) trial confirmed that blockade of inflammation worsens prognosis and HF (28).
The second innovative action of RvD1 is marked with the regulation of miRs, which are abundantly expressed in cardiac developmental physiology, myocardium healing, and HF (30, 31). After MI, not only the cardiomyocytes but nonmyocyte immune cells and fibroblasts play a critical role in remodeling processes, contributing to the regeneration of heart tissue (32). The miRs coordinate regulation of macrophage plasticity and responsiveness to cytokine signals indicated by diversified miRs circuit patterns in RvD1-injected mice after MI. RvD1 limited the MI-induced miR storm at d 1 with significant changes in the expression levels of specific miRs at d 5 after MI. The progression of HF was unexpectedly evidenced by increased levels of multiple miRs. Let-7e-5p is the mechano-miR that is highly regulated in an opposite manner at d 1 after MI (33). Although the DIANA miRPath identified 18 pathways related to skeletal muscle function and extracellular matrix–receptor interaction, let-7e-5p is the key target that is mainly associated with cardiac inflammation and fibrosis. Further up-regulation of miR-342-5p indicates activation of leukocyte response as it facilitates classic macrophage activation through AKT1 and subsequent signaling (34). In the murine peritonitis model, RvD1 regulated miR-21, -146b, -208a, -219, and -466L, with activation of GPCR-32 to clear inflammation is indicative of the comprehensive role of RvD1 in proresolution physiology (35–38).
The third innovative action of RvD1 is the consequence of active neutrophil clearance (get-out signal) after MI. Lipocalin 2 is also widely known as NGAL, which is related to high levels of neutrophils after MI and is lowered in RvD1-injected mouse kidney. This finding is supported by mRNA and protein expression and plasma NGAL data in acute and chronic HF. Thus, immune-responsive immunoresolvents, such as RvD1, are effective in multiple organs and differ from immunosuppression in promoting clearance of neutrophils with marked activation of the resolution program without affecting innate response after MI. Our previous work has suggested that, particularly in aging mice or chronic HF, the NGAL marker correlates with plasma creatinine; however, in the current report, plasma creatinine remains unchanged, perhaps because of age of the young mice (13, 39). To validate reduced injury in RvD1-injected mice, nephrin expression was analyzed. Nephrin is a transmembrane protein of the kidney filtration assembly present on the tips of podocytes and essential in maintaining normal function. Within 24 h after MI, myocardium injury reduced nephrin expression, which was preserved in RvD1-injected mice, indicating attenuated MI-induced impact on the kidney in acute and chronic HF. Nephrin-deficient subjects of a Finnish study shows congenital nephrosis, and nephrin-deficient mice and rats show a defective filtration assembly that indicates the critical role of nephrin, even in the setting of hypertension (40–42). Dynamic changes in NGAL and nephrin are reflected in proinflammatory and anti-inflammatory cytokines, given that the RvD1-injected mice showed reduced levels of Il1b, Tnfa, IL6, and Ifng (proinflammatory), with marked increase of Mrc1 and Ym1 (reparative) cytokines at d 5 after MI in HF suggested active prolonged resolution. Thus, RvD1-mediated active neutrophil clearance in the infarcted heart lowered acute KIMs and preserved the infiltration of assembly structural proteins, indicative of reduced CRS in MI-induced cardiorenal pathology.
Limitations and perspective
In the present study, exogenous RvD1 was tested in young C57BL/6 male 2- to 4-mo-old mice in a model of MI-induced cardiorenal injury. Other strains of male and female mice or female C57BL/6 or obese, hypertensive, diabetic, or aged mice would respond differently to CRS in acute and chronic HF. Therefore, in the clinical setting, the collaboration of the HF specialist with the nephrocardiologist is optimal in delaying progressive acute and chronic HF.
In summary, the results suggest that immune-responsive RvD1 improves the cardiorenal microenvironment to clear inflammation and facilitate resolution in HF (Fig. 7B). The study identifies the mechanistic circuit and cardioprotective role of RvD1 in post-MI cardiorenal resolution physiology.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health (NIH) National Center for Complementary and Integrative Health Grant AT006704; NIH National Heart, Lung and Blood Institute Grant HL132989; The University of Alabama at Birmingham (UAB) Pittman Scholar Award (to G.V.H.); American Heart Association Postdoctoral Fellowship Grant POST31000008 (to V.K.); and NIH National Institute of General Medical Sciences Grants P01-GM095467 and GM038765-32 (to C.N.S.) The authors declare no conflicts of interest.
Glossary
- CRS
cardiorenal syndrome
- FACS
fluorescence-activated cell sorting
- GLS
global longitudinal strain
- H&E
hematoxylin and eosin
- HF
heart failure
- KIM
kidney injury marker
- LV
left ventricle
- MI
myocardial infarction
- Mrc-1
mannose receptor C-type 1
- NGAL
neutrophil gelatinase-associated lipocalin
- RvD1
resolvin D1
- WGA
wheat germ agglutinin
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
G. V. Halade and C. N. Serhan conceived and designed the project; G. V. Halade and V. Kain performed the experiments, interpreted the results, and generated the figures; G. V. Halade supervised the project and interpreted the results; G. V. Halade and C. N. Serhan provided important input to the data analysis; G. V. Halade, V. Kain, and C. N. Serhan wrote the manuscript; and all authors discussed the results and edited and approved the manuscript.
REFERENCES
- 1.Prabhu S. D., Frangogiannis N. G. (2016) The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ. Res. 119, 91–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serhan C. N. (2014) Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckley C. D., Gilroy D. W., Serhan C. N. (2014) Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 40, 315–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kain V., Prabhu S. D., Halade G. V. (2014) Inflammation revisited: inflammation versus resolution of inflammation following myocardial infarction. Basic Res. Cardiol. 109, 444 [DOI] [PubMed] [Google Scholar]
- 5.Fox C. S., Muntner P., Chen A. Y., Alexander K. P., Roe M. T., Wiviott S. D. (2012) Short-term outcomes of acute myocardial infarction in patients with acute kidney injury: a report from the national cardiovascular data registry. Circulation 125, 497–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damman K., Testani J. M. (2015) The kidney in heart failure: an update. Eur. Heart J. 36, 1437–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yancy C. W., Jessup M., Bozkurt B., Butler J., Casey D. E., Jr., Drazner M. H., Fonarow G. C., Geraci S. A., Horwich T., Januzzi J. L., Johnson M. R., Kasper E. K., Levy W. C., Masoudi F. A., McBride P. E., McMurray J. J., Mitchell J. E., Peterson P. N., Riegel B., Sam F., Stevenson L. W., Tang W. H., Tsai E. J., Wilkoff B. L.; American College of Cardiology Foundation ; American Heart Association Task Force on Practice Guidelines . (2013) 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 62, e147–e239 [DOI] [PubMed] [Google Scholar]
- 8.Roy A. K., Mc Gorrian C., Treacy C., Kavanaugh E., Brennan A., Mahon N. G., Murray P. T. (2013) A comparison of traditional and novel definitions (RIFLE, AKIN, and KDIGO) of acute kidney injury for the prediction of outcomes in acute decompensated heart failure. Cardiorenal Med. 3, 26–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nathan C., Ding A. (2010) Nonresolving inflammation. Cell 140, 871–882 [DOI] [PubMed] [Google Scholar]
- 10.Tabas I., Glass C. K. (2013) Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science 339, 166–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tourki B., Halade G. (2017) Leukocyte diversity in resolving and nonresolving mechanisms of cardiac remodeling. FASEB J. 31, 4226–4239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muller W. A. (2015) The regulation of transendothelial migration: new knowledge and new questions. Cardiovasc. Res. 107, 310–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halade G. V., Kain V., Black L. M., Prabhu S. D., Ingle K. A. (2016) Aging dysregulates D- and E-series resolvins to modulate cardiosplenic and cardiorenal network following myocardial infarction. Aging (Albany N.Y.) 8, 2611–2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez E. F., Kabarowski J. H., Ingle K. A., Kain V., Barnes S., Crossman D. K., Lindsey M. L., Halade G. V. (2015) Obesity superimposed on aging magnifies inflammation and delays the resolving response after myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 308, H269–H280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kain V., Ingle K. A., Colas R. A., Dalli J., Prabhu S. D., Serhan C. N., Joshi M., Halade G. V. (2015) Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function. J. Mol. Cell. Cardiol. 84, 24–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krishnamoorthy S., Recchiuti A., Chiang N., Yacoubian S., Lee C. H., Yang R., Petasis N. A., Serhan C. N. (2010) Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc. Natl. Acad. Sci. USA 107, 1660–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norling L. V., Dalli J., Flower R. J., Serhan C. N., Perretti M. (2012) Resolvin D1 limits polymorphonuclear leukocyte recruitment to inflammatory loci: receptor-dependent actions. Arterioscler. Thromb. Vasc. Biol. 32, 1970–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma Y., Halade G. V., Zhang J., Ramirez T. A., Levin D., Voorhees A., Jin Y. F., Han H. C., Manicone A. M., Lindsey M. L. (2013) Matrix metalloproteinase-28 deletion exacerbates cardiac dysfunction and rupture after myocardial infarction in mice by inhibiting M2 macrophage activation. Circ. Res. 112, 675–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halade G. V., Ma Y., Ramirez T. A., Zhang J., Dai Q., Hensler J. G., Lopez E. F., Ghasemi O., Jin Y. F., Lindsey M. L. (2013) Reduced BDNF attenuates inflammation and angiogenesis to improve survival and cardiac function following myocardial infarction in mice. Am. J. Physiol. Heart Circ. Physiol. 305, H1830–H1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bauer M., Cheng S., Jain M., Ngoy S., Theodoropoulos C., Trujillo A., Lin F. C., Liao R. (2011) Echocardiographic speckle-tracking based strain imaging for rapid cardiovascular phenotyping in mice. Circ. Res. 108, 908–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ingle K. A., Kain V., Goel M., Prabhu S. D., Young M. E., Halade G. V. (2015) Cardiomyocyte-specific Bmal1 deletion in mice triggers diastolic dysfunction, extracellular matrix response, and impaired resolution of inflammation. Am. J. Physiol. Heart Circ. Physiol. 309, H1827–H1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kain V., Liu F., Kozlovskaya V., Ingle K. A., Bolisetty S., Agarwal A., Khedkar S., Prabhu S. D., Kharlampieva E., Halade G. V. (2017) Resolution agonist 15-epi-Lipoxin A4 programs early activation of resolving phase in post-myocardial infarction healing. Sci. Rep. 7, 9999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paraskevopoulou M. D., Georgakilas G., Kostoulas N., Vlachos I. S., Vergoulis T., Reczko M., Filippidis C., Dalamagas T., Hatzigeorgiou A.G. (2013) DIANA-microT web server v5.0: service integration into miRNA functional analysis workflows. Nucleic Acids Res. 41, W169–W173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silva M. T. (2011) Macrophage phagocytosis of neutrophils at inflammatory/infectious foci: a cooperative mechanism in the control of infection and infectious inflammation. J. Leukoc. Biol. 89, 675–683 [DOI] [PubMed] [Google Scholar]
- 25.Hausenloy D. J., Yellon D. M. (2013) Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J. Clin. Invest. 123, 92–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serhan C. N. (2017) Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. FASEB J. 31, 1273–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kyne L., Hausdorff J. M., Knight E., Dukas L., Azhar G., Wei J. Y. (2000) Neutrophilia and congestive heart failure after acute myocardial infarction. Am. Heart J. 139, 94–100 [DOI] [PubMed] [Google Scholar]
- 28.Coletta A. P., Clark A. L., Banarjee P., Cleland J. G. (2002) Clinical trials update: RENEWAL (RENAISSANCE and RECOVER) and ATTACH. Eur. J. Heart Fail. 4, 559–561 [DOI] [PubMed] [Google Scholar]
- 29.Ridker P. M., Everett B. M., Thuren T., MacFadyen J. G., Chang W. H., Ballantyne C., Fonseca F., Nicolau J., Koenig W., Anker S. D., Kastelein J. J. P., Cornel J. H., Pais P., Pella D., Genest J., Cifkova R., Lorenzatti A., Forster T., Kobalava Z., Vida-Simiti L., Flather M., Shimokawa H., Ogawa H., Dellborg M., Rossi P. R. F., Troquay R. P. T., Libby P., Glynn R. J.; CANTOS Trial Group . (2017) Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 [DOI] [PubMed] [Google Scholar]
- 30.Chistiakov D. A., Orekhov A. N., Bobryshev Y. V. (2016) Cardiac-specific miRNA in cardiogenesis, heart function, and cardiac pathology (with focus on myocardial infarction). J. Mol. Cell. Cardiol. 94, 107–121 [DOI] [PubMed] [Google Scholar]
- 31.Olson E. N. (2014) MicroRNAs as therapeutic targets and biomarkers of cardiovascular disease. Sci. Transl. Med. 6, 239ps3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehta A., Baltimore D. (2016) MicroRNAs as regulatory elements in immune system logic. Nat. Rev. Immunol. 16, 279–294 [DOI] [PubMed] [Google Scholar]
- 33.Mohamed J. S., Hajira A., Lopez M. A., Boriek A. M. (2015) Genome-wide mechanosensitive microRNA (MechanomiR) screen uncovers dysregulation of their regulatory networks in the mdm mouse model of muscular dystrophy. J. Biol. Chem. 290, 24986–25011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei Y., Nazari-Jahantigh M., Chan L., Zhu M., Heyll K., Corbalán-Campos J., Hartmann P., Thiemann A., Weber C., Schober A. (2013) The microRNA-342-5p fosters inflammatory macrophage activation through an Akt1- and microRNA-155-dependent pathway during atherosclerosis. Circulation 127, 1609–1619 [DOI] [PubMed] [Google Scholar]
- 35.Recchiuti A., Krishnamoorthy S., Fredman G., Chiang N., Serhan C. N. (2011) MicroRNAs in resolution of acute inflammation: identification of novel resolvin D1-miRNA circuits. FASEB J. 25, 544–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krishnamoorthy S., Recchiuti A., Chiang N., Fredman G., Serhan C. N. (2012) Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. Am. J. Pathol. 180, 2018–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y., Dalli J., Chiang N., Baron R. M., Quintana C., Serhan C. N. (2013) Plasticity of leukocytic exudates in resolving acute inflammation is regulated by microRNA and proresolving mediators. Immunity 39, 885–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Recchiuti A., Serhan C. N. (2012) Pro-Resolving Lipid Mediators (SPMs) and their actions in regulating miRNA in novel resolution circuits in inflammation. Front. Immunol. 3, 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halade G. V., Kain V., Ingle K. A. (2018) Heart functional and structural compendium of cardiosplenic and cardiorenal networks in acute and chronic heart failure pathology. Am. J. Physiol. Heart Circ. Physiol. 314, H255–H267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knier B., Cordasic N., Klanke B., Heusinger-Ribeiro J., Daniel C., Veelken R., Hartner A., Hilgers K. F. (2011) Effect of the plasminogen-plasmin system on hypertensive renal and cardiac damage. J. Hypertens. 29, 1602–1612 [DOI] [PubMed] [Google Scholar]
- 41.Maisel A. S., Katz N., Hillege H. L., Shaw A., Zanco P., Bellomo R., Anand I., Anker S. D., Aspromonte N., Bagshaw S. M., Berl T., Bobek I., Cruz D. N., Daliento L., Davenport A., Haapio M., House A. A., Mankad S., McCullough P., Mebazaa A., Palazzuoli A., Ponikowski P., Ronco F., Sheinfeld G., Soni S., Vescovo G., Zamperetti N., Ronco C.; Acute Dialysis Quality Initiative Consensus Group . (2011) Biomarkers in kidney and heart disease. Nephrol. Dial. Transplant. 26, 62–74 [DOI] [PubMed] [Google Scholar]
- 42.Sekulic M., Pichler Sekulic S. (2013) A compendium of urinary biomarkers indicative of glomerular podocytopathy. Pathol. Res. Int. 2013, 782395 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.