Abstract
The transmembrane protein, ADAM10 (a disintegrin and metalloprotease 10), has key physiologic functions—for example, during embryonic development and in the brain. During transit through the secretory pathway, immature ADAM10 (proADAM10) is converted into its proteolytically active, mature form (mADAM10). Increasing or decreasing the abundance and/or activity of mADAM10 is considered to be a therapeutic approach for the treatment of such diseases as Alzheimer’s disease and cancer. Yet biochemical detection and characterization of mADAM10 has been difficult. In contrast, proADAM10 is readily detected—for example, in immunoblots—which suggests that mADAM10 is only a fraction of total cellular ADAM10. Here, we demonstrate that mADAM10, but not proADAM10, unexpectedly undergoes rapid, time-dependent degradation upon biochemical cell lysis in different cell lines and in primary neurons, which prevents the detection of the majority of mADAM10 in immunoblots. This degradation required the catalytic activity of ADAM10, was efficiently prevented by adding active site inhibitors to the lysis buffer, and did not affect proADAM10, which suggests that ADAM10 degradation occurred in an intramolecular and autoproteolytic manner. Inhibition of postlysis autoproteolysis demonstrated efficient cellular ADAM10 maturation with higher levels of mADAM10 than proADAM10. Moreover, a cycloheximide chase experiment revealed that mADAM10 is a long-lived protein with a half-life of approximately 12 h. In summary, our study demonstrates that mADAM10 autoproteolysis must be blocked to allow for the proper detection of mADAM10, which is essential for the correct interpretation of biochemical and cellular studies of ADAM10.—Brummer, T., Pigoni, M., Rossello, A., Wang, H., Noy, P. J., Tomlinson, M. G., Blobel, C. P., Lichtenthaler, S. F. The metalloprotease ADAM10 (a disintegrin and metalloprotease 10) undergoes rapid, postlysis autocatalytic degradation.
Keywords: ADAM17, GI254023X, Alzheimer’s, tetraspanin15, NrCAM
ADAM10 (a disintegrin and metalloprotease 10), has fundamental functions in multicellular organisms, both in health and disease (1–3). ADAM10 is essential for Notch and growth factor signaling (4, 5) during embryonic development, as well as for cell adhesion, migration, and differentiation (6–8) in various organs, including synapse formation and the maintenance or formation of the vasculature (9–13). Dysregulation of ADAM10 is linked to several diseases, which makes ADAM10 an interesting drug target. For example, activation or increased activity of ADAM10 is intended as a therapeutic strategy for the treatment of Alzheimer’s and prion disease, whereas inhibition of ADAM10 is considered to be beneficial for the treatment of allergic reactions and different tumors, such as glioblastoma and pancreatic cancer (14–23).
ADAM10 is a ubiquitously expressed, type I membrane protein and acts as a sheddase—that is, a protease that cleaves off the ectodomain, also referred to as shedding, from its presumably more than 100 different membrane protein substrates, most of which are also single-span membrane proteins (10, 24). For most substrates, how ADAM10 cleavage alters their function remains to be determined, but for a few substrates, the functional consequences are understood in great detail. For example, ligand-induced ADAM10 cleavage of the Notch receptor is required for Notch signaling (25–27), whereas cleavage of the neural glial–related cell adhesion molecule (NrCAM) is essential for correct axon targeting in the brain (10), cleavage of transmembrane activator and CAML interactor is linked to autoimmunity (28), and ADAM10 cleavage of the amyloid precursor protein (APP) prevents the generation of the pathogenic amyloid-β peptide in Alzheimer’s disease (17, 19, 29–31).
ADAM10 belongs to the larger family of ADAM proteases, of which approximately half are proteolytically active. This includes ADAM17, also known as TNF-α–converting enzyme, which, similar to ADAM10, sheds diverse membrane proteins (32). ADAM10 consists of several functional domains. The ectodomain starts with an N-terminal signal peptide, then a prodomain, a metalloprotease domain, a disintegrin domain, and a cysteine-rich domain, followed by a single transmembrane and a cytoplasmic domain (24). After signal peptide cleavage in the endoplasmic reticulum, the proteolytically inactive, immature ADAM (proADAM10) transits via the secretory pathway, where it is cleaved by furin or homologous proteases and converted to the proteolytically active, mature ADAM10 (mADAM10), which cleaves its substrates at or close to the cell surface. A recently reported crystal structure shows that the mature ectodomain adopts a compact, arrowhead-like structure in which the metalloprotease domain is enveloped by the disintegrin and cysteine-rich domains, the latter of which partially blocks the active site (33).
In cell lines, primary cells, and in vivo tissue material, ADAM10 is primarily detected as proADAM10, with only little mADAM10 present (10, 29, 34–37), which suggests that ADAM10 maturation is a tightly controlled process that yields relatively low amounts of the mature, proteolytically active form. In fact, ADAM10 contains an endoplasmic reticulum–retention motif that seems to block the efficient maturation and cell surface localization of ADAM10 (38). Furthermore, association of the cytoplasmic tail of ADAM10 with the synapse-associated protein, SAP97, increases the cell surface levels of ADAM10 in neurons (39). In addition, 6 different members of the tetraspanin family of membrane proteins associate with ADAM10 and contribute to ADAM10 maturation in a cell type–dependent manner (40–43). Whereas these studies suggest that ADAM10 maturation is tightly controlled and seems to limit the levels of mADAM10, other studies have detected equal or even larger amounts of mADAM10 compared with proADAM10 (9, 10, 31, 44). These different studies raise the possibility that the detection of mADAM10 could be highly variable, which would make it difficult to study the maturation and cellular mechanisms of this essential protease.
Here, we demonstrate that mADAM10 undergoes an autocatalytic, most likely intramolecular cleavage event within minutes after cell lysis that interferes with its detection in Western blot analysis. We demonstrate that this autoproteolysis depends on the proteolytic activity of ADAM10 itself and that adding specific ADAM10 inhibitors to the lysis buffer is sufficient to prevent the degradation of mADAM10, which allows for reproducible and efficient detection of mADAM10. Our data also reveal that the relative levels of cellular mADAM10 are greater than those of proADAM10. Finally, we show that mADAM10 has a long half-life of approximately 12 h. Taken together, our results demonstrate that, in contrast to previous assumptions, ADAM10 undergoes efficient maturation, which yields a relatively stable and long-lived mature and active form.
MATERIALS AND METHODS
Materials
The following antibodies were used in this study: ADAM10 C-terminal (1:1000; Abcam, Cambridge, United Kingdom), ADAM10 N-terminal (1:1000; R&D Systems, Minneapolis, MN, USA), ADAM17 C-terminal (1:1000) (45), β-actin (MilliporeSigma, St. Louis, MO, USA; 1:1000), NrCAM (Abcam; 1:1000), APP C-terminal antibody 2C11 (1:10) (46, 47), anti-FLAG (1:000; MilliporeSigma), and horseradish peroxidase–coupled anti-mouse and anti-rabbit secondary antibodies (1:10,000; Dako, Glostrup, Denmark). The following reagents were used: GI254023X (final concentration, 5 µM; MilliporeSigma), broad spectrum metalloprotease inhibitor TAPI-1 (final concentration, 50 µM; MilliporeSigma), 1,10-phenanthroline (final concentration, 100 mM), ADAM10 inhibitors MN8 and LT4 (final concentration, 5 µM) (48, 49), cycloheximide (final concentration, 100 µg/ml; MilliporeSigma), and decanoyl-RVKR-chloromethylketone (dec-CMK; final concentration, 50 µM; Bachem, Bubendorf, Switzerland).
Plasmids
Peak12/hADAM10-sh7/9RNAi—wild-type (wt) ADAM10 with 2 noncoding short hairpin RNA resistance mutation sites—was used as a template for the generation of the E384A mutant ADAM10. The point mutation was introduced with the following primers: E384A A4006C: (reverse) 5′-TGTCCAACTGCGTGAGCAAAAGTAATGTG-3′, (forward) 5′-TTGCTCACGCAGTTGGACATAACTTTGGATCC-3′. The pEF6A FLAG-human tetraspanin15 construct was generated as previously described (40). pcDNA 3.1 Zeo(+)-CD5 signalpeptide-ADAM10 Δcyto (aa 1–693) and pcDNA 3.1 Zeo(+)-CD5 signalpeptide-ADAM10 ecto (aa 1–672) were ordered form ProteoGenix (Portland, OR, USA). Human ADAM10 sequences were used.
Mouse strains and cell lines
The mouse strain wt C57BL/6NCrl (Charles River Laboratories, Wilmington, MA, USA) was used in this study. HEK293 and SH-SY5Y cells were purchased from American Type Culture Collection (Manassas, VA, USA). HEK293 cells were kept in DMEM that was supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. SH-SY5Y cells were kept in DMEM-F12 that was supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, and 1% nonessential amino acids (29).
ADAM10-deficient HEK293T cells—HEK293 cells that express the large T antigen of simian virus 40—were generated by using CRISPR/Cas9. To do this, short-guide RNA sequences were designed to target exon 2 of human ADAM10 (5′-CACCGCGTCTAGATTTCCATGCGCA-3′ and 5′-AAACTGGGCATGGAAATCTAGACGC-3′) by using the Wellcome Trust Sanger Institute Genome Editing Tool (50). Primers that encode this sequence were annealed and cloned into BbsI-digested pSpCas9 (BB)-2A-Puro (PX459), kindly provided by Feng Zhang (51). The resulting ADAM10 CRISPR/Cas9 construct was transfected into HEK293T cells by using a previously described protocol, and transfected cells were selected with 2.5 µg/ml puromycin (Thermo Fisher Scientific, Waltham, MA, USA) (42). Single-cell clones were generated by limiting dilution and knockout of ADAM10 was confirmed by flow cytometry and Western blot analysis.
Isolation of primary neurons
Neurons from wild-type mice were prepared at embryonic day (E)15 and 16 and cultured as previously described (47). Neurons were kept in neurobasal medium that was supplemented with l-glutamine (0.5 mM), 1% penicillin/streptomycin, and B27. At 5 d in vitro, cells were washed with PBS and medium was replaced with fresh neurobasal medium that was supplemented with l-glutamine (0.5 mM), 1% penicillin/streptomycin, B27, and GI254023X (5 µM), TAPI-1 (50 µM), or DMSO. After 48 h of incubation, supernatants were collected and cells were lysed. HEK293 and SH-SY5Y cells were cultured as previously described.
Transfection
HEK293 cells (106/well) were seeded in a 6-well format and transfected in solution in OptiMem (Thermo Fisher Scientific) with the respective vectors (600 ng) using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s protocols, then incubated for 48 h.
Cell lysate and supernatant preparation
Supernatants from HEK293 cells, SH-SY5Y cells, and neurons were collected. Supernatants were then centrifuged at 13,000 rpm and mixed with 4× Laemmli buffer. For HEK293 cells that were transfected with pcDNA 3.1 Zeo(+)-CD5- ADAM10 ecto (for purification of the soluble ADAM10 ectodomain secreted into the conditioned medium), cells were kept in serum-free medium, and the conditioned medium were pulled down by ConA-Sepharose beads. Beads were then washed twice with ice-cold PBS and finally resuspended in 1× nonreducing sample buffer. In contrast to supernatants, cells were washed twice with PBS and lysed in STET lysis buffer (50 mM Tris, pH 7,5, 150 mM NaCl, 2 mM EDTA, 1% Triton -100) that contained a protease inhibitor cocktail (1:500; Sigma-Aldrich) and GI254023X (5 µM), MN8 (5 µM), LT4 (5 µM), TAPI-1 (50 µM), or phenanthroline (10 mM). Samples were centrifuged at 13,000 rpm and 4°C, supernatants were transferred to fresh tubes, and protein concentration was measured by bicinchoninic acid assay (Uptima Interchim, Montluçon Cedex, France). Fifteen to twenty micrograms of total protein was used for SDS-PAGE separation.
Time course experiments
HEK293 cells (106/well) were seeded in a 6-well format and grown nearly to confluency. Cells were then washed 2 times with PBS and lysed with lysis buffer that contained GI254023X (5 µM) and protease inhibitor cocktail or protease inhibitor alone. GI254023X was added to the lysates—without GI254023X—after certain timepoints (0, 2, 5, 10, 30, or 60 min). Cells were kept on ice during the lysis procedure.
Membrane preparation
HEK293 cells were resuspended and scraped in 1× PBS (137 mM sodium chloride, 2.7 mM potassium chloride, 10 mM disodium phosphate, 2 mM monopotassium phosphate, pH 7.4) and centrifuged at 1300 g at 4°C for 5 min. Pellets were resuspended in hypotonic buffer (10 mM Tris, pH 7.4, 1 mM EDTA, 1 mM EGTA) that contained protease inhibitor cocktail (1:500; MilliporeSigma), mixed, and incubated for 10 min on ice. Samples were then pushed 15 times through a 23-gauge syringe and centrifuged at 3800 g at 4°C for 5 min. Pellets were discarded and supernatants were centrifuged at 21,000 g at 4°C for 1 h (29). Supernatants were then removed and membrane pellets were resuspended in 1× reducing Laemmli buffer (2% SDS, 10% glycerol, 0.00625% bromophenol blue, 2% 2-ME, 31.25 mM Tris, pH 6.8) or STET (50 mM Tris, pH 7.5, 150 mM NaCl, 2 mM EDTA) buffer with or without detergent (1% Triton X-100) with or without GI254023X (5 µM). Samples were incubated for 30 min on ice, then Laemmli buffer was added to the STET samples. Samples were boiled at 95°C for 5 min and separated by SDS-PAGE.
Cycloheximide chase experiment
HEK293 cells (3 × 106) were seeded on 6-cm dishes and grown nearly to confluency. Cells were then treated with cycloheximide (100 µg/ml) and lysed at certain time points—0, 2, 6, 12, 18, and 24 h—in the presence of GI254023X (5 µM).
Western blot analysis
Samples were boiled at 95°C for 5 min in Laemmli buffer (8% SDS, 40% glycerol, 0.025% bromophenol blue, 10% 2-ME, 125 mM Tris, pH 6.8) and separated on 8% SDS-PAGE gels. Proteins were transferred to PVDF membranes (EMD Millipore, Billerica, MA, USA) and blocked with 5% milk for 1 h at room temperature. Membranes were incubated for 1–2 h at room temperature or overnight at 4°C with primary antibody solutions. Membranes were then incubated with the secondary antibody for 45 min at room temperature. Membranes were developed with ECL prime (GE Healthcare, Waukesha, WI, USA). For the detection of ADAM10 with the N-terminal antibody or ADAM17, lysates—30 µg of total protein—were pulled down with ConA-Sepharose (30 µl) beads, rotating overnight at 4°C. Beads were then washed 3 times with ice-cold 1× PBS and resuspended in 1× nonreducing Laemmli buffer (2% SDS, 10% glycerol, 0.00625% bromophenol blue, 31.25 mM Tris, pH 6.8) or 1× reducing Laemmli buffer. Please note that the N-terminal shed fragment of NrCAM that was detected in supernatants and cell lysate has the same apparent MW, which can be explained as follows. During maturation in the secretory pathway, NrCAM undergoes proteolytic cleavage by furin (52, 53) within its ectodomain at a site that is farther from the membrane and N-terminal to the ADAM10 cleavage site. ADAM10-mediated processing results in the shedding of the ectodomain, which remains a heterodimer of the large N-terminal fragment that ends at the furin site, and a shorter C-terminal fragment that spans from the furin cleavage site to the ADAM10 cleavage site. Upon SDS-PAGE, both fragments are separated. As the NrCAM antibody binds to the NrCAM N terminus, only the N-terminal fragment of the mature NrCAM heterodimer is detected, which has the same length as that in the membrane-anchored and soluble shed form of NrCAM.
ADAM10 fluorescence resonance energy transfer assay
The ADAM10 fluorescence resonance energy transfer assay was purchased from AnaSpec (Fremont, CA, USA) and performed according to the manufacturer’s instructions. Relative fluorescence units were measured every 5 min for different inhibitor concentrations at Ex/Em 490/520 nm. Rates (fluorescence per 5 min) were calculated by applying a linear regression model for every sample (Prism v.7; GraphPad Software, La Jolla, CA, USA) when fluorescence intensities were still in the linear range (between 10 and 40 min). Ki values were calculated by plotting inhibitor concentrations against the rates, then inserting the values into Bieth’s equation (54) using Prism:
 |
E is the enzyme concentration (4 × 109 M; calculated with an MW of 50 kDa for ADAM10), X is the inhibitor concentration (molar), Y is the rate, and K is the Ki value.
Statistical analysis
All tests were performed on an exploratory 2-sided 5% significance level. P values for Western blots were calculated with relative log2 transformed values using 2-sided Student’s t test with a Welch’s correction and post hoc Bonferroni correction for multiple hypothesis testing (Prism v.7).
RESULTS
Specific ADAM10 inhibition prevents postlysis degradation of mature ADAM10
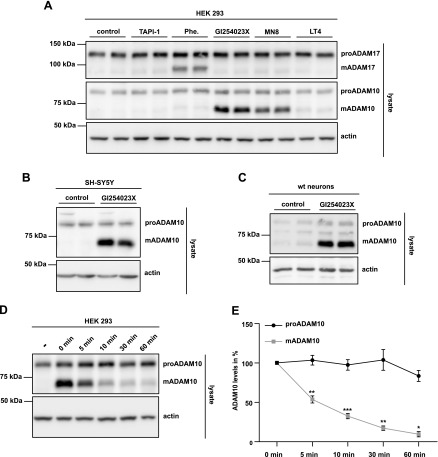
In an initial experiment that was aimed at studying the role of ADAM10 in the shedding of substrates from neuronal cultures, we treated primary murine neurons with the broad-spectrum metalloprotease inhibitor, TAPI-1, or the ADAM10-selective inhibitor, GI254023X (55, 56). In agreement with a previous publication (10), both inhibitors efficiently blocked the shedding of the endogenous neuronal cell adhesion membrane protein, NrCAM, which is a known ADAM10 substrate (10). Upon ADAM10 inhibition, the levels of the soluble NrCAM ectodomain were strongly decreased (Fig. 1A), whereas the amounts of full-length NrCAM were mildly increased, which is in line with its reduced shedding. Surprisingly, the ADAM10-preferring inhibitor, GI254023X, strongly increased the levels of endogenous mADAM10 that could be detected in the cell lysate by Western blot analysis, whereas only little mADAM10 was detected under control or TAPI-1–treated conditions (Fig. 1A and quantification in Fig. 1B). In contrast to mADAM10, levels of proADAM10 remained unchanged (Fig. 1A, B). This suggests that GI254023X either blocks ADAM10 degradation or increases ADAM10 production and/or maturation.
Figure 1.
The ADAM10-preferring inhibitor, GI254023X, increases mature ADAM10 levels. After 5 d in vitro, primary murine neurons (E16.5) were treated with TAPI-1 (50 µM), GI254023X (5 µM), or DMSO (control) for 48 h, then supernatants were collected and cells were lysed. A) Shown are representative Western blots. For the detection of ADAM10, an antibody to its C terminus was used. B) Densitometric quantification of proADAM10 and mADAM10 levels normalized to control levels. Two-sided Student’s t test with Welch’s correction and Bonferroni’s correction for multiple hypothesis testing (n = 6–8). ****P < 0.0001.
To address the underlying molecular mechanism, we assessed the possibility that mADAM10—similar to another ADAM family member, ADAM17 (45)—may undergo an autocatalytic proteolytic cleavage during cell lysis, which prevents the efficient detection of mADAM10. To test this hypothesis, cultured HEK293 cells were lysed with lysis buffer that contained either the broad-spectrum metalloprotease inhibitor, TAPI-1, the Zn2+ chelator, 1,10-phenanthroline, or one of the ADAM10-preferring inhibitors, GI254023X, MN8, or LT4 (48, 49). Of importance—and in contrast to the experiment in Fig. 1—in this experiment, inhibitors were only added to the lysis buffer and were not present during the culture period of HEK293 cells. Inhibitors were added to the lysis buffer in concentrations commonly used for the treatment of viable cells, when inhibition of protein shedding is studied, such as for NrCAM in Fig. 1. If mADAM10 does undergo autoproteolytic degradation upon cell lysis, GI254023X, and potentially additional inhibitors, but not TAPI-1, should block this degradation and lead to enhanced mADAM10 levels that are similar to the increase in mADAM10 observed in Fig. 1. In fact, increased mADAM10 levels were observed for the 2 ADAM10-preferring inhibitors, GI254023X and MN8, whereas TAPI-1, 1,10-phenanthroline, and LT4 had little or no effect on mADAM10 levels (Fig. 2A). As a control, 1,10-phenanthroline blocked the autoproteolysis of the ADAM10-related ADAM17, which is in agreement with a previous study (45), whereas other inhibitors barely affected mADAM17 levels.
Figure 2.
Specific ADAM10 inhibitors block the postlysis degradation of ADAM10. A) HEK293 cells were lysed with lysis buffer that contained GI254023X (5 µM), 1,10-phenanthroline (Phe.; 10 mM), TAPI-1 (50 µM), MN8 (5 µM), LT4 (5 µM), or DMSO as control. For the detection of ADAM17, lysates were subjected to an overnight ConA pull down. B) SH-SY5Y cells were lysed with lysis buffer that contained GI254023X (5 µM) or DMSO as control. C) Primary murine neurons (E16.5) were lysed with lysis buffer that contained GI254023X (5 µM) or DMSO as control. D) HEK293 cells were lysed with lysis buffer that contained GI254023X (5 µM) or DMSO as control. Cells were kept on ice for 60 min. After the indicated time points (0, 5, 10, 30, or 60 min), GI254023X (5 µM) was added to lysates, which previously did not contain GI254023X, to stop the reaction. Shown are representative Western blots. For the detection of ADAM10, C-terminal antibody was used. E) Quantification of mean proADAM10 and mADAM10 levels (n = 6) relative to those at the starting time point. Shown are means ± sem. Relative log2 transformed values using 2-sided Student’s t test with Welch’s correction and post hoc Bonferroni’s correction. *P < 0.05, **P < 0.01, ***P < 0.001.
We next assessed the possibility that the different abilities of the 4 metalloprotease inhibitors to prevent mADAM10 degradation were a result of different inhibitory potencies. To this aim, we estimated the inhibitors’ inhibitory constant, Ki, with an in vitro assay in which a substrate peptide with quenched fluorescence was incubated with recombinant ADAM10. Substrate cleavage was measured as an increase in fluorescence intensity and was observed over time and at different inhibitor concentrations (Supplemental Fig. 1). The 2 inhibitors, GI254023x and MN8, which efficiently blocked mADAM10 degradation in cells, displayed low Ki values that were similar to each other [Ki (GI254023X): 8.109 ± 0.356 nM; Ki (MN8): 4.699 ± 0.371 nM]. In contrast, LT4 and TAPI-1, which failed to block mADAM10 degradation in cells, had much higher estimated Ki values for ADAM10 [Ki (LT4) > 35 nM; Ki (TAPI-1) > 140 nM]. Specifically, TAPI-1 was inefficient in blocking ADAM10 in the in vitro assay at the same concentrations as those used for the other inhibitors.
Inhibition of mADAM10 degradation was not only observed in HEK293 cells, but also in human neuroblastoma SH-SY5Y cells (Fig. 2B) and in primary murine neurons (Fig. 2C). Similar to Fig. 2A, the inhibitor, GI254023X, was added in both experiments only in the lysis buffer. Of importance, in all experiments, increased protein levels were specifically seen for mADAM10, but not for proADAM10. Taken together, these results demonstrate that the addition of specific inhibitors to the cell lysis buffer allows for the stabilization and efficient detection of mADAM10.
To assess the time course of the degradation of mADAM10, HEK293 cells were treated with cell lysis buffer with or without GI254023X and incubated on ice for different time points up to 60 min. GI254023X was added to the lysates at the indicated time points (Fig. 2E). When GI254023X was added immediately to the lysis buffer, it stabilized mADAM10 as in the previous experiments (Fig. 2D); however, when GI254023X was added only 5 min after the addition of the lysis buffer, mADAM10 levels were already reduced by approximately 50% (Fig. 2D and quantification in Fig. 2E). After 30 and 60 min in the absence of GI254023X, mADAM10 levels were nearly as low as in non-inhibitor–treated conditions (Fig. 2D, E). Taken together, this experiment demonstrates that, in the absence of an inhibitor, mADAM10 undergoes rapid degradation upon cell lysis with a half-life of approximately 5 min. Of note, proADAM10 again remained unaffected throughout the entire incubation time, which indicates that, unlike mADAM10, it is not degraded. Likewise, this result suggests that proADAM10 is not converted to mADAM10 during the cell-free incubation period on ice. In contrast, in living cells, proADAM10 is converted to mADAM10 via removal of its N-terminal propeptide by members of the proprotein convertase (PC) protease family, in particular furin and PC7 (57, 58). In fact, HEK293 cells that were treated with the PC inhibitor, dec-CMK, had reduced levels of mADAM10 (Supplemental Fig. 2A), which demonstrated the effectiveness of the inhibitor. In contrast, dec-CMK had no effect on the levels of proADAM10 or mADAM10 in the postlysis incubation period on ice, neither in the presence, nor the absence of GI254023X (Supplemental Fig. 2B). This demonstrates that ADAM10 does not undergo a postlysis PC-dependent maturation process and also shows that PCs do not contribute to mADAM10 degradation.
mADAM10 degradation creates several cleavage products
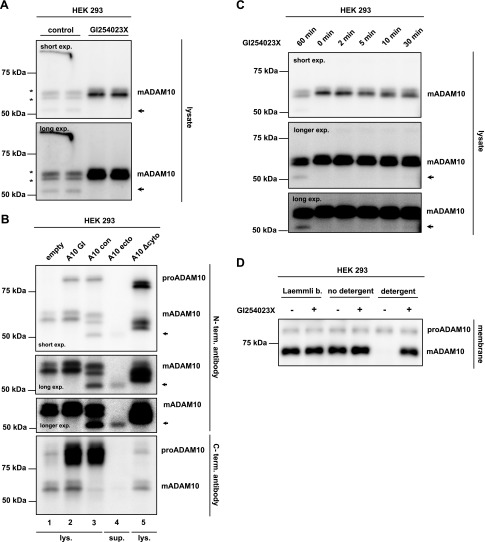
We next tested whether cleavage fragments that resulted from the postlysis degradation of mADAM10 were detectable. To this aim, HEK293 cells that express endogenous ADAM10 were incubated with lysis buffer in the presence or absence of GI254023X. In contrast to the previous experiments that used an antibody to the ADAM10 C terminus, ADAM10 was now detected in immunoblots by using an antibody that binds to the N-terminal ectodomain of ADAM10. This antibody is used under nonreducing electrophoresis conditions and detected endogenous mADAM10, but not proADAM10 (Fig. 3A), presumably because the antibody epitope is not easily accessible in the presence of the ADAM10 prodomain. In contrast to the single band that was detected by the C-terminal antibody used in the figures above, mADAM10 was detected as 2 bands of similar MW when its degradation was blocked with GI254023X (Fig. 3A). Both bands may differ because of unknown post-translational modifications. Of importance, in the absence of GI254023X, 3 changes were observed. First, the intensity of the mADAM10 bands was reduced (Fig. 3A), which is in agreement with the increased mADAM10 degradation observed in the previous figures. Second, a smaller N-terminal fragment of approximately 50 kDa was detected, and its generation was prevented by GI254023X (Fig. 3A). Third, the two closely comigrating mADAM10 bands demonstrated a shift to a mildly lower MW (for a scheme, see Supplemental Fig. 3). To test whether this might be a result of the proteolytic removal of the ADAM10 C terminus, we repeated the experiment of Fig. 3A, but used overexpressed ADAM10 to better visualize the cleavage fragments. Under these overexpression conditions, the N-terminal antibody also detected proADAM10 (Fig. 3B), presumably because of the strong increase in proADAM10 levels compared with the endogenous situation. Of note, in the absence of GI254023X, the 3 bands of mADAM10—the two comigrating bands with the slight MW shift and the 50-kDa fragment—were clearly detected with the N-terminal antibody (Fig. 3B, lane 3; see the upper 3 panels showing 3 different exposure times), but not with an antibody to the C terminus of ADAM10 (Fig. 3B, lane 3, lowest panel). In contrast, proADAM10, which is not subject to degradation, was detected with both antibodies. This experiment demonstrates that all 3 mADAM10 bands detected in the absence of GI254023X have lost their C terminus. Moreover, the 2 closely comigrating bands displayed only a small MW shift in the absence of GI254023X (A10 con) compared with the 2 comigrating bands in the presence of GI254023X (A10 GI; Fig. 3B, lanes 2 and 3). Thus, it is likely that the proteolysis of ADAM10 occurs at a short distance from the cytosolic C terminus.
Figure 3.
mADAM10 degradation creates several fragments, which are precluded by an intact membrane. A) HEK293 cells were lysed with lysis buffer that contained GI254023X (5 µM). Lysates were then subjected to an overnight ConA pull down. ADAM10 was detected under nonreducing conditions with an antibody raised against part of its N-terminal extracellular domain. Arrow indicates the smaller fragment. Asterisks indicate the larger fragments. B) HEK293 cells transfected with wtADAM10 (A10), ADAM10 ecto, ADAM10 Δcyto, or empty vector control. Cells were lysed in the presence of GI254023X (5 µM), with the exception of A10 con. Lysates (30 µl) or supernatants (500 µl) were pulled down by ConA-Sepharose beads. Arrow indicates the smaller fragment. The upper 3 panels are 3 different exposure (exp.) times of the blot with the N-terminal ADAM10 antibody. The lowest panel shows the blot developed with the C-terminal ADAM10 antibody, as indicated. C) HEK293 cells were lysed with lysis buffer that contained GI254023X (5 µM) or DMSO. Cells were kept on ice for 60 min. After the indicated time points (0, 2, 5, 10, 30, or 60 min), GI254023X (5 µM) was added to lysates, which previously did not contain GI254023X, to stop the reaction. Lysates were then subjected to an overnight ConA pull down. Arrow indicates the smaller 50-kDa fragment. Three different exposure times of the blot are shown. D) Membrane pellets were prepared from HEK293 cells. Laemmli buffer (1×; reducing), STET-lysis buffer without Triton X-100, or usual STET-lysis buffer with Triton X-100 was added to pelleted membranes in the presence or absence of GI254023X (5 µM). Samples were then incubated for 15 min on ice, then 1× Laemmli buffer (reducing) was added to the samples not yet containing Laemmli buffer. For the detection of ADAM10 in panel D, C-terminal antibody was used.
To better characterize the size of the smaller 50-kDa fragment, we created 2 truncated ADAM10 mutants that were expressed in HEK293 cells—ADAM10 Δcyto, which lacked the C-terminal part after the transmembrane domain, and ADAM10 ecto, which comprised the whole ADAM10 ectodomain, but not the transmembrane and cytoplasmic domains. As expected, both proteins were detected with the antibody to the N terminus (Fig. 3B, lanes 4 and 5, upper panels), but not the C terminus, of ADAM10 (Fig. 3B, lanes 4 and 5, lowest panel). Of importance, ADAM10 ecto had the same apparent MW as the 50-kDa fragment, whereas the MW of ADAM10 Δcyto was higher (Fig. 3B, lane 3 vs. lanes 4 and 5), which suggests that the 50-kDa fragment comprises most or all of the ADAM10 ectodomain and results from a proteolytic cleavage within the juxtamembrane domain of ADAM10. Of interest, the 2 comigrating bands that displayed a slight MW shift as a result of the removal of their C terminus had a higher MW than did ADAM10 Δcyto. This clearly demonstrates that the C terminus must include part of the cytoplasmic domain and that the cleavage site is located close to the C terminus of ADAM10.
Among the 3 degradation fragments, the intensity of the 50-kDa fragment was lower than the 2 comigrating bands of mADAM10 that lost their C-terminal amino acids. Thus, we assessed whether proteolysis first occurred close to the C terminus and was then followed by proteolysis in the juxtamembrane domain or whether both cleavages occurred independently of each other. To address this question, we carried out a time-course experiment in which lysates of HEK293 cells were incubated on ice for up to 60 min in the absence of GI254023X. After different time points, GI254023X was added to block additional degradation. This experiment was similar to that in Fig. 2D, but fragments were detected by using the N-terminal ADAM10 antibody. After 10 min, the 2 comigrating bands started to display a slight molecular shift to a lower MW that was fully visible after 30 min (Fig. 3C). In contrast, the 50-kDa fragment only became visible after 60 min, which suggests that the primary cleavage site is at the C terminus of ADAM10 and is later followed by cleavage within the juxtamembrane domain.
An intact membrane precludes mADAM10 degradation
The active site of ADAM10 is located within its ectodomain. In contrast, the primary cleavage site is located within its cytoplasmic domain and the secondary cleavage site in the juxtamembrane domain in close proximity to the membrane. Whereas the primary cleavage site is not accessible in living cells as the membrane is intact, the secondary cleavage site may also be difficult to reach because of proximity to the lipid bilayer. This is in line with our findings above that the degradation of mADAM10 occurred when cells were lysed with a buffer that contained the detergent, Triton X-100, which efficiently solubilizes the membrane. As an additional control experiment, we predicted that mADAM10 degradation may be prevented, not when cells are lysed in the presence of a detergent, but when cells are simply ruptured and membrane sheets are prepared. To test this possibility, HEK293 cells were treated with a hypotonic buffer without detergent. Membrane sheets were collected after multiple centrifugation steps and incubated in lysis buffer, but without detergent. Under these conditions, mADAM10 was efficiently detected (Fig. 3D), and the addition of GI254023X did not additionally increase mADAM10 levels. In contrast, when membrane sheets were additionally incubated with the detergent, Triton X-100, mADAM10 was barely detectable, which was similar to experiments in which HEK293 cells were directly lysed in detergent-containing lysis buffer. Of importance, in this case, the addition of GI254023X efficiently prevented mADAM10 degradation. Of interest, when membrane sheets were incubated on ice in denaturing Laemmli buffer that contained 1% SDS and the reducing agent, 2-ME, degradation of mADAM10 was prevented. This suggests that, under these harsh conditions with SDS and 2-ME, ADAM10 or potentially other relevant proteases are inactive and no longer able to degrade mADAM10. Again, proADAM10 remained unaffected from any treatment. Taken together, these experiments demonstrate that degradation of membrane-bound mADAM10 is prevented as long as it resides within intact membrane sheets.
Degradation of ADAM10m requires catalytically active ADAM10
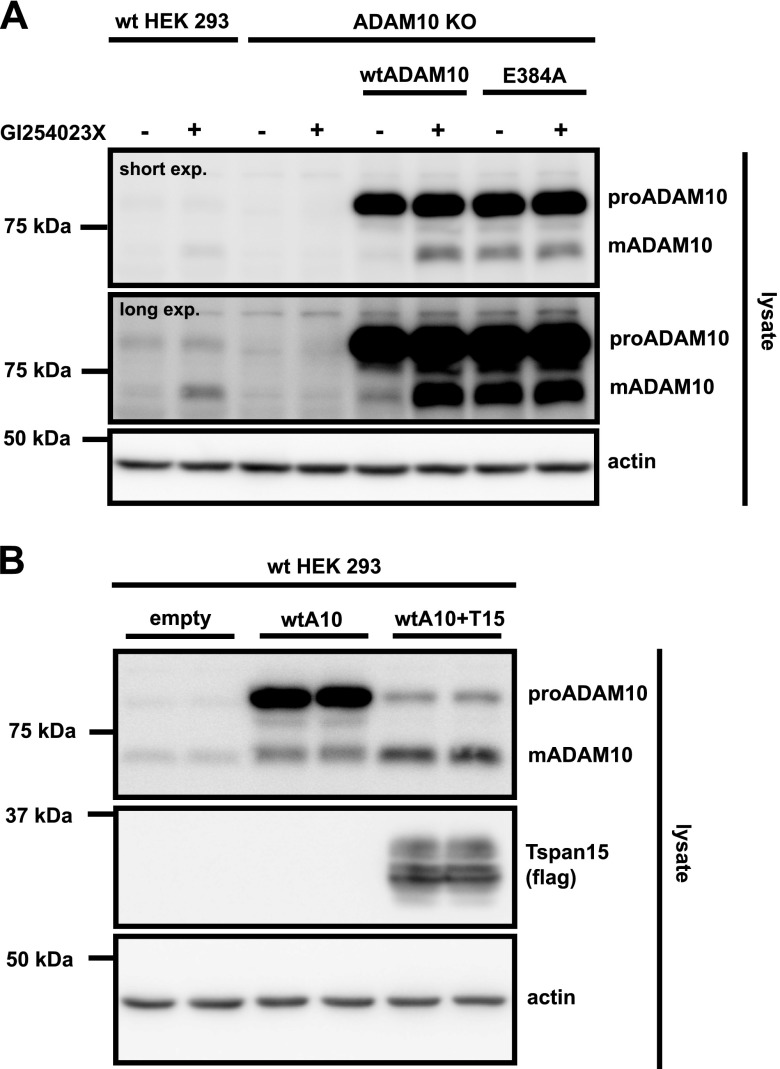
Experiments thus far suggest that mADAM10—once it is extracted from the membrane—cleaves itself in an autocatalytic, proteolytic, and intramolecular manner close to its juxtamembrane domain. If this model is accurate, the degradation of mADAM10 should require catalytic ADAM10 activity, and should be abolished in the active site mutant ADAM10 E384A, which is catalytically inactive (59). To assess this model and exclude any interference by endogenous ADAM10, ADAM10-deficient [ADAM10 knockout (KO)] HEK293 cells were transfected with empty plasmid or with the plasmid that encodes either wtADAM10 or inactive ADAM10 E384A. Cells were lysed in detergent-containing lysis buffer with or without GI254023X. As expected, compared with wtHEK293 cells, no endogenous ADAM10 was detected in ADAM10 KO HEK293 cells (Fig. 4A). Transfected wtADAM10 or ADAM10 E384A was clearly detected in ADAM10 KO HEK293 cells. mADAM10 was barely detected for wtADAM10, but was strongly enriched in the presence of GI254023X. This demonstrates that transfected mADAM10 also undergoes rapid degradation, which is similar to endogenous mADAM10. In contrast, the mature form of ADAM10 E384A was readily detected. Its levels were similar to wt mADAM10 under GI254023X-treated conditions and were not additionally increased in the presence of GI254023X. Of importance, proADAM10 levels were unchanged between wtADAM10 and ADAM10 E384A or in the presence of GI254023X. This demonstrates that the catalytic activity of ADAM10 is required for mADAM10 degradation and is in line with the proposed model in which mADAM10 degradation occurs in an intramolecular cleavage reaction.
Figure 4.
mADAM10 degradation requires catalytically active mADAM10. A) wtHEK293 cells and ADAM10 KO HEK293 cells were transfected with ADAM10, inactive hADAM10 mutant (E384A), or the empty vector. After 48 h of incubation, cells were lysed with lysis buffer that contained GI254023X (5 µM) or solvent. B) HEK293 cells were transfected with hADAM10, hADAM10 and tetraspanin15 (T15), or the empty vector. Cells were lysed in the presence of GI254023X (5 µM). Shown are representative Western blots (n = 3–4). For the detection of ADAM10, C-terminal antibody was used.
Of interest, for transfected ADAM10, we observed relatively less mADAM10 than proADAM10, whereas for endogenous ADAM10, there was more mADAM10 than proADAM10, at least when autoproteolysis was blocked. It is known that overexpressed ADAM10 is partially retained in early compartments of the secretory pathway, presumably because there are not enough cellular protein binding partners to promote its transport from the endoplasmic reticulum to later compartments in the secretory pathway (38, 39, 60). In fact, and in agreement with previous studies (40), co-overexpression of tetraspanin15 increased the maturation of transfected wtADAM10 in HEK293 cells (Fig. 4B).
Determination of the half-life of mADAM10
We next determined the half-life of ADAM10, which thus far had not been possible, as mADAM10 was rapidly degraded during cell lysis. We blocked protein translation and, thus, the synthesis of new ADAM10 in HEK293 cells with cycloheximide, and observed ADAM10 over a chase period of up to 24 h. Cell lysates were prepared in the presence of GI254023X to block postlysis mADAM10 degradation. As a control, we blotted for mature and immature APP in the cell lysate. APP is a known ADAM10 substrate and was found to have a half-life of <2 h [30–60 min (61); Fig. 5A]. In contrast, proADAM10 had a half-life of approximately 6 h, much longer than APP. mADAM10 seemed to have an even longer half-life than proADAM10, with 50% of mADAM10 remaining after 24 h; however, it should be noted that proADAM10 at time point 0 h will eventually mature and generate mADAM10 during the chase period. Thus, total mADAM10 levels at time points of 2–24 h truly represent the sum of the newly formed mADAM10—matured from proADAM10 at time point 0 h—and mADAM10 that remained from time point 0 h and was not yet degraded. Thus, to roughly estimate the half-life of mADAM10, we assumed that proADAM10 only underwent maturation to mADAM10 without autodegradation, and subtracted the reduction in proADAM10 levels compared with time point 0 h from total mADAM10 at every time point. In this curve, the half-life of mADAM10 was approximately 12 h (Fig. 5B). Taken together, mADAM10 is a long-lived protein with a half-life that is much longer than one of its substrates, APP.
Figure 5.
Determination of the half-life of mADAM10. A) HEK293 cells were treated with 100 µg/ml cycloheximide for indicated times (0, 2, 6, 12, 18, or 24 h). Cells were lysed by using STET-lysis buffer that contained GI254023X (5 µM). B) Quantification of mean proADAM10 and mADAM10 levels. mADAM10 corrected: Subtractions of proADAM10 at time points of 2–24 h from proADAM10 at time point 0 h. These values were then subtracted from mADAM10 values at every time point. Shown are representative Western blots (n = 3–4). For the detection of ADAM10, C-terminal antibody was used.
DISCUSSION
The metalloprotease ADAM10 has key functions during development and adulthood, both in healthy and pathologic conditions (1, 4, 11, 24, 31). Despite helpful insights into the function of ADAM10 over the past years, mechanistic and biochemical studies were limited as a result of the difficulty of detecting mADAM10 and the resulting assumption that little mADAM10 is present in cells because of inefficient maturation. Our study demonstrates that the opposite is true, and that endogenous ADAM10 matures efficiently such that more mADAM10 than proADAM10 is present in different cell lines and primary neurons. Mechanistically, we found that ADAM10 undergoes rapid autoproteolysis within a few minutes of lysis of the cellular membrane with a nonionic detergent. Addition of a specific ADAM10 inhibitor prevented this autoproteolysis and, thus, allowed for the efficient detection of mADAM10 and identification of mADAM10 as a long-lived protein. These findings will be instrumental for future mechanistic and functional ADAM10 studies, such as for the identification of ADAM10-interacting proteins or understanding the factors involved in the production of mature ADAM10, as well as for the structure determination of full-length ADAM10 and potential complexes with other accessory membrane proteins, such as tetraspanins.
Our study suggests a model in which mADAM10 cleaves off its own C terminus upon membrane removal in an autocatalytic, intramolecular, proteolytic reaction (Fig. 6). This model is supported by several findings. First, degradation of mADAM10 required the catalytic activity of ADAM10, which was demonstrated with the catalytically inactive mADAM10 E384A mutant that was not subject to autodegradation. Likewise, pharmacologic inhibition by using the ADAM10-preferring inhibitors, GI254023X and MN8 (48, 55, 56), blocked mADAM10 degradation. Second, proADAM10 was not degraded under either endogenous or overexpressed conditions. If the degradation reaction happened in an intermolecular cleavage reaction, one mADAM10 molecule should be able to cleave not only another mADAM10, but also a proADAM10 molecule; however, this was not the case. Third, degradation occurred only after the lysis of the membrane and was prevented when membrane sheets were left intact, which is consistent with an intramolecular reaction. In contrast, an intermolecular reaction could take place in the presence of the membrane when one ADAM10 molecule in one membrane sheet cleaves off the C terminus of another ADAM10 molecule in a neighboring membrane sheet.
Figure 6.
Scheme of the autocatalytic degradation of mADAM10 after membrane disruption. After mADAM10 is released from the membrane during cell lysis, potential cleavage sites—within the juxtamembrane and intracellular domains (indicated in red)—become accessible to the protease. This leads to an autocatalytic, presumably intramolecular cleavage, which creates at least 2 mADAM10 fragments of different sizes that comprise either the ectodomain (smaller fragment) or the ectodomain plus the transmembrane and part of the cytoplasmic domain. Generation of the smaller fragment occurs via an initial cleavage at the C-terminal end (1), followed by a secondary cleavage (2) in the juxtamembrane domain. The vertical dashed lines indicate the binding sites of the antibodies used in this study (black: anti–N-terminal antibody to the ADAM10 ectodomain; red: anti–C-terminal antibody to the cytoplasmic tail of ADAM10; domains not drawn to scale to emphasize the transmembrane and juxtamembrane domains).
The time-course experiment demonstrated that mADAM10 cleaves itself at least twice, first close to the C terminus and again within the juxtamembrane domain [Fig. 6, labeled as (1) and (2)]. Whereas the first cleavage is assumed to happen in an intramolecular reaction as discussed above, the second cleavage, which led to the generation of the 50-kDa fragment, may alternatively occur in an intermolecular reaction. This possibility is in line with the recently determined crystal structure of the recombinant mADAM10 ectodomain (33), which corresponds to our 50-kDa fragment. Of interest, in the crystal structure, mADAM10 was observed as a tetramer, where the C-terminal amino acids of one mADAM10 molecule were bound in the active site of a neighboring mADAM10 molecule, which suggests that an intermolecular cleavage at this mADAM10 site, in principle, may be possible. This second cleavage not only generates the 50-kDa ectodomain fragment, but should also generate a second, small C-terminal fragment that comprises the amino acids between the first and second cleavage sites (Fig. 6). This fragment was not detectable because the first cleavage removes the epitope for the C-terminal ADAM10 antibody.
The autocatalytic cleavage of ADAM10, as reported in our study, was only observed upon the lysis of the cell membrane, but not in living cells in which the membrane is intact. The first cleavage in the cytoplasmic C terminus of ADAM10 is readily explained, as the membrane forms a barrier that prevents the catalytic domain of ADAM10 from accessing its own C terminus; however, the second, smaller cleavage fragment—produced by processing in the extracellular juxtamembrane domain—was also only observed upon cell lysis. This may seem to be surprising given that ADAM9 and ADAM15 can shed ADAM10 (62), and that the recent crystal structure demonstrated a compact folding of the ADAM10 ectodomain, with proximity of the ADAM10 active site to its own juxtamembrane domain (33). This structure provides evidence that the active site of ADAM10 is positioned close to the membrane surface, which is in agreement with ADAM10 functioning as a sheddase that cleaves different membrane protein substrates within their juxtamembrane domains, as well as with our finding that ADAM10 can also cleave within its own juxtamembrane domain. This occurred only when the membrane was removed and sterically may not be possible as long as the membrane is intact. In an alternative mechanistic scenario, juxtamembrane cleavage may not be possible in living cells because the first, autocatalytic cleavage at the C terminus may be a requirement for the second cleavage in the juxtamembrane domain, and, thus, would not take place in living cells in which the first cleavage is prevented by the intact membrane. This mechanistic scenario is in agreement with our time-course experiment, which suggests that the juxtamembrane cleavage is preceded by the C-terminal cleavage. C-terminal cleavage may induce a structural alteration in full-length ADAM10, which allows the ADAM10 active site to access its own juxtamembrane domain. In this context, it is also worth noting that ADAM10 forms complexes with different tetraspanins, which are multipass transmembrane proteins, presumably binding to the transmembrane and juxtamembrane domains of ADAM10 (42). Complex formation between ADAM10 and tetraspanins in living cells may prevent autocatalytic cleavage in the ADAM10 juxtamembrane domain, but could also be disrupted by lysis in detergents.
The autocatalytic degradation of ADAM10 has important implications for the purification of ADAM10—for example, for in vitro assays and the study of ADAM10 regulation, but also for the determination of the X-ray crystallographic or cryo-electron microscopy structure of full-length ADAM10. For example, commercially available, recombinant ADAM10 ectodomain—ending in amino acid Glu672 right before the beginning of the transmembrane domain—is sold with a C-terminal His tag from R&D Systems. The data sheet indicates that most of the recombinant protein lacks the C-terminal His tag, and we did not detect it at all (data not shown). It is likely that autoproteolysis within the juxtamembrane domain described in our study provides the mechanistic explanation for the loss of the His tag. A similar observation was made in a recent study that determined the crystal structure of the recombinant human ADAM10 ectodomain—ending in amino acid 654, followed by a single glycine and a His tag (33). Although the His tag was used for ADAM10 purification, it was no longer visible in the crystal structure, which suggests that it was autocatalytically cleaved off. In support of this, a stretch of amino acids, including the C-terminal Gly655, were found to be bound in the active site of ADAM10 in the crystal structure, suggesting that this was the cleavage site. Thus, our study implies that the purification of the whole, nontruncated ADAM10 ectodomain, as well as the full-length ADAM10, including the cytoplasmic tail, is only achievable when the protease is purified in the presence of ADAM10 inhibitors, such as GI254023X. This will be essential for determining the structure of full-length ADAM10, either alone or in complex with one or several of its interactions partners, which are crucial regulators of ADAM10 activity. Examples are the postsynaptic density protein, SAP97, and tetraspanins that control ADAM10 trafficking and activity (38–40, 42, 43, 60, 63), for example, in the molecular processes that underlie Alzheimer’s disease (64). Another example is the membrane protease, γ-secretase, which seems to form a multiprotease complex with ADAM10 (65).
Of interest, not all metalloprotease inhibitors blocked mADAM10 autoproteolysis in a similar manner, although they are all hydroxamate-based inhibitors. Whereas TAPI-1 blocked cellular ADAM10 activity, including the cleavage of the ADAM10 substrate, NrCAM, in our study, TAPI-1 did not block autoproteolysis of mADAM10 when added to the lysis buffer. A similar result was obtained for LT4. In contrast, GI254023X and MN8 efficiently blocked mADAM10 autoproteolysis. This difference between inhibitors is likely a result of the Ki value, which was in a similarly low nanomolar range for GI254023X and MN8, but was much higher for TAPI-1 and LT4. Thus, whereas there may be slight differences between the inhibitory profiles of GI254023X and MN8, we propose that both remain tightly bound to mADAM10 so that no autoproteolysis can occur within the lysis period on ice. Conversely, we assume that the inhibitors with higher Ki values do not bind mADAM10 as tightly, which leaves mADAM10 temporarily noninhibited. As an intramolecular reaction is kinetically favored over an intermolecular reaction, and thus, more difficult to prevent by using a competitive inhibitor, weaker competitive inhibitors may not suffice to prevent intramolecular autoproteolysis, even though they can prevent the intermolecular processing of such substrates as NrCAM. As a result of this model, and in agreement with our results, both kinds of inhibitors block substrate cleavage, but differ with regard to blocking the autoproteolysis of ADAM10.
Blocking the autoproteolysis of ADAM10 allowed for the determination of the approximate half-lives of mADAM10 and proADAM10 in HEK293 cells by using a cycloheximide chase experiment, demonstrating that mADAM10 is a rather long-lived protein that exceeds the half-lives of such substrates as APP. Whereas the half-life of mADAM10 was approximately 12 h, the half-life of proADAM10 was around 6 h and total ADAM10 had a half-life of 18 h. Given that ADAM10 can bind to different tetraspanins (40, 41, 66), it is possible that the half-life of mADAM10 may be different on the basis of its binding partner.
ADAM10 is part of a larger family of ADAM proteases that includes ADAM17, for which autoproteolysis has also been described (45). In our study, we have confirmed the autoproteolysis of ADAM17 and demonstrated that the inhibitors that block ADAM17 and ADAM10 autoproteolysis are different. Thus, for the detection of both proteases in the same cell lysates, we strongly recommend adding the required inhibitors to the lysis buffer, in particular 1,10-phenanthroline for the detection of mADAM17 and GI254023X, or MN8 for mADAM10 detection. It is possible that other membrane-bound proteases, particularly other members of the ADAM family, or membrane-type matrix metalloproteases may undergo a similar postlysis autoproteolysis, which should be taken into consideration and prevented in mechanistic and functional studies of these proteases.
In summary, our study reveals that the inhibition of mADAM10 autoproteolysis is crucial for an accurate biochemical and cellular characterization of ADAM10. As ADAM10 is considered to be an attractive drug target for the treatment of various conditions, including Alzheimer’s disease, cancer, and inflammatory diseases, the development of new experimental treatment strategies relies heavily on the reliability and reproducibility of mADAM10 detection. Here, we provide a quick and easy approach by which to obtain highly reproducible ADAM10 Western blot data, which should help further our understanding of the mature and active forms of this membrane-anchored protease and thus improve ADAM10-based research.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by the following funding agencies: Deutsche Forschungsgemeinschaft (FOR2290), the Centers of Excellence in Neurodegeneration, the Helmholtz-Israel Program and the Breuer foundation Alzheimer Award (to S.F.L.), and the U.S. National Institutes of Health, National Institute of General Medical Sciences (Grant R01-GM64750; to C.P.B.). T.B. was supported by a fellowship from the medical faculty of Technische Universität München (Klinikum Rechts der Isar). P.J.N. was funded by the British Heart Foundation Project (Grant PG/13/92/30587; to M.G.T.). The authors thank Linda Troeberg (University of Oxford, Oxford, England) for help with the calculation of Ki values. The authors declare no conflicts of interest.
Glossary
- ADAM
a disintegrin and metalloprotease
- APP
amyloid precursor protein
- dec-CMK
decanoyl-RVKR-chloromethylketone
- KO
knockout
- NrCAM
neural glial–related cell adhesion molecule
- PC
proprotein convertase
- wt
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
T. Brummer, M. Pigoni, C. P. Blobel, and S. F. Lichtenthaler designed research; T. Brummer analyzed data; T. Brummer performed research; T. Brummer and S. F. Lichtenthaler wrote the paper; and A. Rossello, H. Wang, P. J. Noy, and M. G. Tomlinson contributed new reagents or analytic tools.
REFERENCES
- 1.Saftig P., Lichtenthaler S. F. (2015) The alpha secretase ADAM10: a metalloprotease with multiple functions in the brain. Prog. Neurobiol. 135, 1–20 https://doi.org/10.1016/j.pneurobio.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 2.Wetzel S., Seipold L., Saftig P. (2017) The metalloproteinase ADAM10: a useful therapeutic target? Biochim. Biophys. Acta. 1864, 2071–2081 [DOI] [PubMed] [Google Scholar]
- 3.Jones J. C., Rustagi S., Dempsey P. J. (2016) ADAM proteases and gastrointestinal function. Annu. Rev. Physiol. 78, 243–276 https://doi.org/10.1146/annurev-physiol-021014-071720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber S., Niessen M. T., Prox J., Lüllmann-Rauch R., Schmitz A., Schwanbeck R., Blobel C. P., Jorissen E., de Strooper B., Niessen C. M., Saftig P. (2011) The disintegrin/metalloproteinase Adam10 is essential for epidermal integrity and Notch-mediated signaling. Development 138, 495–505 https://doi.org/10.1242/dev.055210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groot A. J., Habets R., Yahyanejad S., Hodin C. M., Reiss K., Saftig P., Theys J., Vooijs M. (2014) Regulated proteolysis of NOTCH2 and NOTCH3 receptors by ADAM10 and presenilins. Mol. Cell. Biol. 34, 2822–2832 https://doi.org/10.1128/MCB.00206-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reiss K., Maretzky T., Ludwig A., Tousseyn T., de Strooper B., Hartmann D., Saftig P. (2005) ADAM10 cleavage of N-cadherin and regulation of cell-cell adhesion and beta-catenin nuclear signalling. EMBO J. 24, 742–752 https://doi.org/10.1038/sj.emboj.7600548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maretzky T., Schulte M., Ludwig A., Rose-John S., Blobel C., Hartmann D., Altevogt P., Saftig P., Reiss K. (2005) L1 is sequentially processed by two differently activated metalloproteases and presenilin/gamma-secretase and regulates neural cell adhesion, cell migration, and neurite outgrowth. Mol. Cell. Biol. 25, 9040–9053 https://doi.org/10.1128/MCB.25.20.9040-9053.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan X., Lin J., Talabattula V. A., Mußmann C., Yang F., Wree A., Rolfs A., Luo J. (2014) ADAM10 negatively regulates neuronal differentiation during spinal cord development. PLoS One 9, e84617 https://doi.org/10.1371/journal.pone.0084617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prox J., Bernreuther C., Altmeppen H., Grendel J., Glatzel M., D’Hooge R., Stroobants S., Ahmed T., Balschun D., Willem M., Lammich S., Isbrandt D., Schweizer M., Horré K., De Strooper B., Saftig P. (2013) Postnatal disruption of the disintegrin/metalloproteinase ADAM10 in brain causes epileptic seizures, learning deficits, altered spine morphology, and defective synaptic functions. J. Neurosci. 33, 12915–12928, 12928a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuhn P. H., Colombo A. V., Schusser B., Dreymueller D., Wetzel S., Schepers U., Herber J., Ludwig A., Kremmer E., Montag D., Müller U., Schweizer M., Saftig P., Bräse S., Lichtenthaler S. F. (2016) Systematic substrate identification indicates a central role for the metalloprotease ADAM10 in axon targeting and synapse function. Elife 5, e12748 https://doi.org/10.7554/eLife.12748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glomski K., Monette S., Manova K., De Strooper B., Saftig P., Blobel C. P. (2011) Deletion of Adam10 in endothelial cells leads to defects in organ-specific vascular structures. Blood 118, 1163–1174 https://doi.org/10.1182/blood-2011-04-348557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alabi R. O., Glomski K., Haxaire C., Weskamp G., Monette S., Blobel C. P. (2016) ADAM10-dependent signaling through Notch1 and Notch4 controls development of organ-specific vascular beds. Circ. Res. 119, 519–531 https://doi.org/10.1161/CIRCRESAHA.115.307738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Musardo S., Marcello E., Gardoni F., Di Luca M. (2014) ADAM10 in synaptic physiology and pathology. Neurodegener. Dis. 13, 72–74 https://doi.org/10.1159/000354233 [DOI] [PubMed] [Google Scholar]
- 14.Tippmann F., Hundt J., Schneider A., Endres K., Fahrenholz F. (2009) Up-regulation of the alpha-secretase ADAM10 by retinoic acid receptors and acitretin. FASEB J. 23, 1643–1654 https://doi.org/10.1096/fj.08-121392 [DOI] [PubMed] [Google Scholar]
- 15.Altmeppen H. C., Prox J., Krasemann S., Puig B., Kruszewski K., Dohler F., Bernreuther C., Hoxha A., Linsenmeier L., Sikorska B., Liberski P. P., Bartsch U., Saftig P., Glatzel M. (2015) The sheddase ADAM10 is a potent modulator of prion disease. Elife 4, e04260.https://doi.org/10.7554/eLife.04260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weskamp G., Ford J. W., Sturgill J., Martin S., Docherty A. J., Swendeman S., Broadway N., Hartmann D., Saftig P., Umland S., Sehara-Fujisawa A., Black R. A., Ludwig A., Becherer J. D., Conrad D. H., Blobel C. P. (2006) ADAM10 is a principal ‘sheddase’ of the low-affinity immunoglobulin E receptor CD23. Nat. Immunol. 7, 1293–1298 https://doi.org/10.1038/ni1399 [DOI] [PubMed] [Google Scholar]
- 17.Suh J., Choi S. H., Romano D. M., Gannon M. A., Lesinski A. N., Kim D. Y., Tanzi R. E. (2013) ADAM10 missense mutations potentiate β-amyloid accumulation by impairing prodomain chaperone function. Neuron 80, 385–401 https://doi.org/10.1016/j.neuron.2013.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Endres K., Fahrenholz F., Lotz J., Hiemke C., Teipel S., Lieb K., Tüscher O., Fellgiebel A. (2014) Increased CSF APPs-α levels in patients with Alzheimer disease treated with acitretin. Neurology 83, 1930–1935 https://doi.org/10.1212/WNL.0000000000001017 [DOI] [PubMed] [Google Scholar]
- 19.Postina R., Schroeder A., Dewachter I., Bohl J., Schmitt U., Kojro E., Prinzen C., Endres K., Hiemke C., Blessing M., Flamez P., Dequenne A., Godaux E., van Leuven F., Fahrenholz F. (2004) A disintegrin-metalloproteinase prevents amyloid plaque formation and hippocampal defects in an Alzheimer disease mouse model. J. Clin. Invest. 113, 1456–1464 https://doi.org/10.1172/JCI20864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohutek Z. A., diPierro C. G., Redpath G. T., Hussaini I. M. (2009) ADAM-10-mediated N-cadherin cleavage is protein kinase C-alpha dependent and promotes glioblastoma cell migration. J. Neurosci. 29, 4605–4615 https://doi.org/10.1523/JNEUROSCI.5126-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siney E. J., Holden A., Casselden E., Bulstrode H., Thomas G. J., Willaime-Morawek S. (2017) Metalloproteinases ADAM10 and ADAM17 mediate migration and differentiation in glioblastoma sphere-forming cells. Mol. Neurobiol. 54, 3893–3905 https://doi.org/10.1007/s12035-016-0053-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaida M. M., Haag N., Günther F., Tschaharganeh D. F., Schirmacher P., Friess H., Giese N. A., Schmidt J., Wente M. N. (2010) Expression of a disintegrin and metalloprotease 10 in pancreatic carcinoma. Int. J. Mol. Med. 26, 281–288 [DOI] [PubMed] [Google Scholar]
- 23.Woods N., Trevino J., Coppola D., Chellappan S., Yang S., Padmanabhan J. (2015) Fendiline inhibits proliferation and invasion of pancreatic cancer cells by interfering with ADAM10 activation and β-catenin signaling. Oncotarget 6, 35931–35948 https://doi.org/10.18632/oncotarget.5933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reiss K., Saftig P. (2009) The “a disintegrin and metalloprotease” (ADAM) family of sheddases: physiological and cellular functions. Semin. Cell Dev. Biol. 20, 126–137 https://doi.org/10.1016/j.semcdb.2008.11.002 [DOI] [PubMed] [Google Scholar]
- 25.Van Tetering G., van Diest P., Verlaan I., van der Wall E., Kopan R., Vooijs M. (2009) Metalloprotease ADAM10 is required for Notch1 site 2 cleavage. J. Biol. Chem. 284, 31018–31027 https://doi.org/10.1074/jbc.M109.006775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bozkulak E. C., Weinmaster G. (2009) Selective use of ADAM10 and ADAM17 in activation of Notch1 signaling. Mol. Cell. Biol. 29, 5679–5695 https://doi.org/10.1128/MCB.00406-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan D., Rubin G. M. (1997) Kuzbanian controls proteolytic processing of Notch and mediates lateral inhibition during Drosophila and vertebrate neurogenesis. Cell 90, 271–280 https://doi.org/10.1016/S0092-8674(00)80335-9 [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann F. S., Kuhn P. H., Laurent S. A., Hauck S. M., Berer K., Wendlinger S. A., Krumbholz M., Khademi M., Olsson T., Dreyling M., Pfister H. W., Alexander T., Hiepe F., Kümpfel T., Crawford H. C., Wekerle H., Hohlfeld R., Lichtenthaler S. F., Meinl E. (2015) The immunoregulator soluble TACI is released by ADAM10 and reflects B cell activation in autoimmunity. J. Immunol. 194, 542–552 https://doi.org/10.4049/jimmunol.1402070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuhn P. H., Wang H., Dislich B., Colombo A., Zeitschel U., Ellwart J. W., Kremmer E., Rossner S., Lichtenthaler S. F. (2010) ADAM10 is the physiologically relevant, constitutive alpha-secretase of the amyloid precursor protein in primary neurons. EMBO J. 29, 3020–3032 https://doi.org/10.1038/emboj.2010.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taverna M., Straub T., Hampel H., Rujescu D., Lichtenthaler S. F. (2013) A new sandwich immunoassay for detection of the α-secretase cleaved, soluble amyloid-β protein precursor in cerebrospinal fluid and serum. J. Alzheimers Dis. 37, 667–678 [DOI] [PubMed] [Google Scholar]
- 31.Jorissen E., Prox J., Bernreuther C., Weber S., Schwanbeck R., Serneels L., Snellinx A., Craessaerts K., Thathiah A., Tesseur I., Bartsch U., Weskamp G., Blobel C. P., Glatzel M., De Strooper B., Saftig P. (2010) The disintegrin/metalloproteinase ADAM10 is essential for the establishment of the brain cortex. J. Neurosci. 30, 4833–4844 https://doi.org/10.1523/JNEUROSCI.5221-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gooz M. (2010) ADAM-17: the enzyme that does it all. Crit. Rev. Biochem. Mol. Biol. 45, 146–169 https://doi.org/10.3109/10409231003628015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seegar T. C. M., Killingsworth L. B., Saha N., Meyer P. A., Patra D., Zimmerman B., Janes P. W., Rubinstein E., Nikolov D. B., Skiniotis G., Kruse A. C., Blacklow S. C. (2017) Structural basis for regulated proteolysis by the α-secretase ADAM10. Cell 171, 1638.e7–1648.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagano O., Murakami D., Hartmann D., De Strooper B., Saftig P., Iwatsubo T., Nakajima M., Shinohara M., Saya H. (2004) Cell-matrix interaction via CD44 is independently regulated by different metalloproteinases activated in response to extracellular Ca2+ influx and PKC activation. J. Cell Biol. 165, 893–902 https://doi.org/10.1083/jcb.200310024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romi E., Gokhman I., Wong E., Antonovsky N., Ludwig A., Sagi I., Saftig P., Tessier-Lavigne M., Yaron A. (2014) ADAM metalloproteases promote a developmental switch in responsiveness to the axonal repellant Sema3A. Nat. Commun. 5, 4058 https://doi.org/10.1038/ncomms5058 [DOI] [PubMed] [Google Scholar]
- 36.Nagara Y., Hagiyama M., Hatano N., Futai E., Suo S., Takaoka Y., Murakami Y., Ito A., Ishiura S. (2012) Tumor suppressor cell adhesion molecule 1 (CADM1) is cleaved by a disintegrin and metalloprotease 10 (ADAM10) and subsequently cleaved by γ-secretase complex. Biochem. Biophys. Res. Commun. 417, 462–467 https://doi.org/10.1016/j.bbrc.2011.11.140 [DOI] [PubMed] [Google Scholar]
- 37.Zimmermann M., Gardoni F., Marcello E., Colciaghi F., Borroni B., Padovani A., Cattabeni F., Di Luca M. (2004) Acetylcholinesterase inhibitors increase ADAM10 activity by promoting its trafficking in neuroblastoma cell lines. J. Neurochem. 90, 1489–1499 https://doi.org/10.1111/j.1471-4159.2004.02680.x [DOI] [PubMed] [Google Scholar]
- 38.Marcello E., Gardoni F., Di Luca M., Pérez-Otaño I. (2010) An arginine stretch limits ADAM10 exit from the endoplasmic reticulum. J. Biol. Chem. 285, 10376–10384 https://doi.org/10.1074/jbc.M109.055947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marcello E., Gardoni F., Mauceri D., Romorini S., Jeromin A., Epis R., Borroni B., Cattabeni F., Sala C., Padovani A., Di Luca M. (2007) Synapse-associated protein-97 mediates alpha-secretase ADAM10 trafficking and promotes its activity. J. Neurosci. 27, 1682–1691 https://doi.org/10.1523/JNEUROSCI.3439-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haining E. J., Yang J., Bailey R. L., Khan K., Collier R., Tsai S., Watson S. P., Frampton J., Garcia P., Tomlinson M. G. (2012) The TspanC8 subgroup of tetraspanins interacts with a disintegrin and metalloprotease 10 (ADAM10) and regulates its maturation and cell surface expression. J. Biol. Chem. 287, 39753–39765 https://doi.org/10.1074/jbc.M112.416503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jouannet S., Saint-Pol J., Fernandez L., Nguyen V., Charrin S., Boucheix C., Brou C., Milhiet P. E., Rubinstein E. (2016) TspanC8 tetraspanins differentially regulate the cleavage of ADAM10 substrates, Notch activation and ADAM10 membrane compartmentalization. Cell. Mol. Life Sci. 73, 1895–1915 https://doi.org/10.1007/s00018-015-2111-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noy P. J., Yang J., Reyat J. S., Matthews A. L., Charlton A. E., Furmston J., Rogers D. A., Rainger G. E., Tomlinson M. G. (2016) TspanC8 tetraspanins and a disintegrin and metalloprotease 10 (ADAM10) interact via their extracellular regions: evidence for distinct binding mechanisms for different TspanC8 proteins. J. Biol. Chem. 291, 3145–3157 https://doi.org/10.1074/jbc.M115.703058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dornier E., Coumailleau F., Ottavi J. F., Moretti J., Boucheix C., Mauduit P., Schweisguth F., Rubinstein E. (2012) TspanC8 tetraspanins regulate ADAM10/Kuzbanian trafficking and promote Notch activation in flies and mammals. J. Cell Biol. 199, 481–496 https://doi.org/10.1083/jcb.201201133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pasciuto E., Ahmed T., Wahle T., Gardoni F., D’Andrea L., Pacini L., Jacquemont S., Tassone F., Balschun D., Dotti C. G., Callaerts-Vegh Z., D’Hooge R., Müller U. C., Di Luca M., De Strooper B., Bagni C. (2015) Dysregulated ADAM10-mediated processing of APP during a critical time window leads to synaptic deficits in fragile X syndrome. Neuron 87, 382–398 https://doi.org/10.1016/j.neuron.2015.06.032 [DOI] [PubMed] [Google Scholar]
- 45.Schlöndorff J., Becherer J. D., Blobel C. P. (2000) Intracellular maturation and localization of the tumour necrosis factor alpha convertase (TACE). Biochem. J. 347, 131–138 https://doi.org/10.1042/bj3470131 [PMC free article] [PubMed] [Google Scholar]
- 46.Kuhn P. H., Koroniak K., Hogl S., Colombo A., Zeitschel U., Willem M., Volbracht C., Schepers U., Imhof A., Hoffmeister A., Haass C., Roßner S., Bräse S., Lichtenthaler S. F. (2012) Secretome protein enrichment identifies physiological BACE1 protease substrates in neurons. EMBO J. 31, 3157–3168 https://doi.org/10.1038/emboj.2012.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colombo A., Wang H., Kuhn P. H., Page R., Kremmer E., Dempsey P. J., Crawford H. C., Lichtenthaler S. F. (2013) Constitutive α- and β-secretase cleavages of the amyloid precursor protein are partially coupled in neurons, but not in frequently used cell lines. Neurobiol. Dis. 49, 137–147 https://doi.org/10.1016/j.nbd.2012.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zocchi M. R., Camodeca C., Nuti E., Rossello A., Venè R., Tosetti F., Dapino I., Costa D., Musso A., Poggi A. (2015) ADAM10 new selective inhibitors reduce NKG2D ligand release sensitizing Hodgkin lymphoma cells to NKG2D-mediated killing. OncoImmunology 5, e1123367 https://doi.org/10.1080/2162402X.2015.1123367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Camodeca C., Nuti E., Tepshi L., Boero S., Tuccinardi T., Stura E. A., Poggi A., Zocchi M. R., Rossello A. (2016) Discovery of a new selective inhibitor of a disintegrin and metalloprotease 10 (ADAM-10) able to reduce the shedding of NKG2D ligands in Hodgkin’s lymphoma cell models. Eur. J. Med. Chem. 111, 193–201 https://doi.org/10.1016/j.ejmech.2016.01.053 [DOI] [PubMed] [Google Scholar]
- 50.Hodgkins A., Farne A., Perera S., Grego T., Parry-Smith D. J., Skarnes W. C., Iyer V. (2015) WGE: a CRISPR database for genome engineering. Bioinformatics 31, 3078–3080 https://doi.org/10.1093/bioinformatics/btv308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ran F. A., Hsu P. D., Wright J., Agarwala V., Scott D. A., Zhang F. (2013) Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 https://doi.org/10.1038/nprot.2013.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kayyem J. F., Roman J. M., de la Rosa E. J., Schwarz U., Dreyer W. J. (1992) Bravo/Nr-CAM is closely related to the cell adhesion molecules L1 and Ng-CAM and has a similar heterodimer structure. J. Cell Biol. 118, 1259–1270 https://doi.org/10.1083/jcb.118.5.1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Susuki K., Chang K. J., Zollinger D. R., Liu Y., Ogawa Y., Eshed-Eisenbach Y., Dours-Zimmermann M. T., Oses-Prieto J. A., Burlingame A. L., Seidenbecher C. I., Zimmermann D. R., Oohashi T., Peles E., Rasband M. N. (2013) Three mechanisms assemble central nervous system nodes of Ranvier. Neuron 78, 469–482 https://doi.org/10.1016/j.neuron.2013.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bieth J. G. (1995) Theoretical and practical aspects of proteinase inhibition kinetics. Methods Enzymol. 248, 59–84 https://doi.org/10.1016/0076-6879(95)48007-2 [DOI] [PubMed] [Google Scholar]
- 55.Hundhausen C., Misztela D., Berkhout T. A., Broadway N., Saftig P., Reiss K., Hartmann D., Fahrenholz F., Postina R., Matthews V., Kallen K. J., Rose-John S., Ludwig A. (2003) The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell-cell adhesion. Blood 102, 1186–1195 https://doi.org/10.1182/blood-2002-12-3775 [DOI] [PubMed] [Google Scholar]
- 56.Ludwig A., Hundhausen C., Lambert M. H., Broadway N., Andrews R. C., Bickett D. M., Leesnitzer M. A., Becherer J. D. (2005) Metalloproteinase inhibitors for the disintegrin-like metalloproteinases ADAM10 and ADAM17 that differentially block constitutive and phorbol ester-inducible shedding of cell surface molecules. Comb. Chem. High Throughput Screen. 8, 161–171 https://doi.org/10.2174/1386207053258488 [DOI] [PubMed] [Google Scholar]
- 57.Anders A., Gilbert S., Garten W., Postina R., Fahrenholz F. (2001) Regulation of the alpha-secretase ADAM10 by its prodomain and proprotein convertases. FASEB J. 15, 1837–1839 [DOI] [PubMed] [Google Scholar]
- 58.Lopez-Perez E., Zhang Y., Frank S. J., Creemers J., Seidah N., Checler F. (2001) Constitutive alpha-secretase cleavage of the beta-amyloid precursor protein in the furin-deficient LoVo cell line: involvement of the pro-hormone convertase 7 and the disintegrin metalloprotease ADAM10. J. Neurochem. 76, 1532–1539 https://doi.org/10.1046/j.1471-4159.2001.00180.x [DOI] [PubMed] [Google Scholar]
- 59.Lammich S., Kojro E., Postina R., Gilbert S., Pfeiffer R., Jasionowski M., Haass C., Fahrenholz F. (1999) Constitutive and regulated alpha-secretase cleavage of Alzheimer’s amyloid precursor protein by a disintegrin metalloprotease. Proc. Natl. Acad. Sci. USA 96, 3922–3927 https://doi.org/10.1073/pnas.96.7.3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saraceno C., Marcello E., Di Marino D., Borroni B., Claeysen S., Perroy J., Padovani A., Tramontano A., Gardoni F., Di Luca M. (2014) SAP97-mediated ADAM10 trafficking from Golgi outposts depends on PKC phosphorylation. Cell Death Dis. 5, e1547 https://doi.org/10.1038/cddis.2014.492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Storey E., Katz M., Brickman Y., Beyreuther K., Masters C. L. (1999) Amyloid precursor protein of Alzheimer’s disease: evidence for a stable, full-length, trans-membrane pool in primary neuronal cultures. Eur. J. Neurosci. 11, 1779–1788 https://doi.org/10.1046/j.1460-9568.1999.00599.x [DOI] [PubMed] [Google Scholar]
- 62.Tousseyn T., Thathiah A., Jorissen E., Raemaekers T., Konietzko U., Reiss K., Maes E., Snellinx A., Serneels L., Nyabi O., Annaert W., Saftig P., Hartmann D., De Strooper B. (2009) ADAM10, the rate-limiting protease of regulated intramembrane proteolysis of Notch and other proteins, is processed by ADAMS-9, ADAMS-15, and the gamma-secretase. J. Biol. Chem. 284, 11738–11747 https://doi.org/10.1074/jbc.M805894200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matthews A. L., Noy P. J., Reyat J. S., Tomlinson M. G. (2017) Regulation of a disintegrin and metalloproteinase (ADAM) family sheddases ADAM10 and ADAM17: the emerging role of tetraspanins and rhomboids. Platelets 28, 333–341 https://doi.org/10.1080/09537104.2016.1184751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Epis R., Marcello E., Gardoni F., Vastagh C., Malinverno M., Balducci C., Colombo A., Borroni B., Vara H., Dell’Agli M., Cattabeni F., Giustetto M., Borsello T., Forloni G., Padovani A., Di Luca M. (2010) Blocking ADAM10 synaptic trafficking generates a model of sporadic Alzheimer’s disease. Brain 133, 3323–3335 https://doi.org/10.1093/brain/awq217 [DOI] [PubMed] [Google Scholar]
- 65.Chen A. C., Kim S., Shepardson N., Patel S., Hong S., Selkoe D. J. (2015) Physical and functional interaction between the α- and γ-secretases: a new model of regulated intramembrane proteolysis. J. Cell Biol. 211, 1157–1176 https://doi.org/10.1083/jcb.201502001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matthews A. L., Szyroka J., Collier R., Noy P. J., Tomlinson M. G. (2017) Scissor sisters: regulation of ADAM10 by the TspanC8 tetraspanins. Biochem. Soc. Trans. 45, 719–730 https://doi.org/10.1042/BST20160290 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.