Abstract
Genetic variants within the FTO (α-ketoglutarate–dependent dioxygenase) gene have been strongly associated with a modest increase in adiposity as a result of increased food intake. These risk alleles are associated with decreased expression of both FTO and neighboring RPGRIP1L (retinitis pigmentosa GTPase regulator-interacting protein 1 like). RPGRIP1L encodes a protein that is critical to the function of the primary cilium, which conveys extracellular information to the cell. Rpgrip1l+/− mice exhibit increased adiposity, in part, as a result of hyperphagia. Here, we describe the effects of Rpgrip1l in adipocytes that may contribute to the adiposity phenotype observed in these animals and possibly in humans who segregate for FTO risk alleles. Loss of Rpgrip1l in 3T3-L1 preadipocytes increased the number of cells that are capable of differentiating into mature adipocytes. Knockout of Rpgrip1l in mature adipocytes using Adipoq-Cre did not increase adiposity in mice that were fed chow or a high-fat diet. We also did not observe any effects of Rpgrip1l knockdown in mature 3T3-L1 adipocytes. Thus, to the extent that Rpgrip1l affects cell-autonomous adipose tissue function, it may do so as a result of the effects conveyed in preadipocytes in which the primary cilium is functionally important. We propose that decreased RPGRIP1L expression in preadipocytes in humans who segregate for FTO obesity risk alleles may increase the storage capacity of adipose tissue.—Martin Carli, J. F., LeDuc, C. A., Zhang, Y., Stratigopoulos, G., Leibel, R. L. The role of Rpgrip1l, a component of the primary cilium, in adipocyte development and function.
Keywords: Fto, adipogenesis, obesity
The strongest genetic signal associated with increased adiposity in large groups of humans is located within the first intron of FTO (α-ketoglutarate–dependent dioxygenase; 16q12.2). Originally identified by genome-wide association studies in 2007, single nucleotide polymorphisms (SNPs) within a 47-kb region of linkage disequilibrium are associated with an ∼0.36-kg/m2 increase in body mass index (per risk allele), which corresponds to a ∼1.2-kg increase in body weight in adults (1–3). The genetic mechanism(s) that underlie this association may be various; the primary proximal phenotypic effect is increased food intake (4–7). Studies of loss- and gain-of-function manipulations of Fto, per se, on adiposity in mice have reported conflicting results (8–11). Effects of the obesity-associated FTO intronic sequence variants on vicinal genes have implicated IRX3 (iroquois homeobox 3) and IRX5 (iroquois homeobox 5) by mechanisms that include decreased energy expenditure as a result of impaired browning of adipose tissue, which drives thermogenesis as a result of oxidative phosphorylation uncoupling (12, 13).
We have studied RPGRIP1L (retinitis pigmentosa GTPase regulator-interacting protein 1 like), the transcriptional start for which is ∼100 bases 5′ of FTO in the opposite orientation, for the role it plays in hypothalamic control of energy homeostasis. We have previously demonstrated that the risk alleles, rs8050136 and rs1421085, within intron 1 of FTO are binding sites for isoforms of the transcription factor, cut-like homeobox 1 (CUX1), and thus regulate the expression of both FTO and RPGRIP1L (14–16). Rpgrip1l is a component of the transition zone of the primary cilium (17). The primary cilium—functioning as a cellular antenna—plays a key role in coordinating a wide range of signal transduction systems, including those related to body weight homeostasis (18, 19). Mice that are heterozygous for a null Rpgrip1l allele are hyperphagic and obese (16, 20). Rpgrip1l functions to congregate and assemble 2 modules of the transition zone of the primary cilium: the Meckel syndrome module and the nephronophthisis module. The transition zone functions as a ciliary gate that regulates the compartmentalization of ciliary proteins, restricting them to the ciliary axoneme (21, 22).
The increased adiposity that we observed in Rpgrip1l+/− mice was associated with reduced hypothalamic sensitivity to leptin effects on signal transducer and activator of transcription 3 signaling and food intake. We hypothesized that there might be additional primary effects of Rpgrip1l hypomorphism that impact adipogenesis and/or the regulation of adipocyte secretion of molecules, such as leptin. We found that the loss of Rpgrip1l in 3T3-L1 preadipocytes enhanced the differentiation capacity of these cells, in part, by affecting cell survival. In vivo, this effect could contribute to the obesity of Rpgrip1l+/− mice by increasing the compliant capacity of fat depots. We generated adipocyte-specific Rpgrip1l knockout mice to assess this possibility and found that there was no effect on adiposity. We knocked down Rpgrip1l in mature adipocytes and observed that the increased adipogenesis that was found when Rpgrip1l was knocked down before differentiation was lost. Our results are consistent with other models of impairments in ciliary function that exhibit increased adipogenesis (23–25), and support a growing body of literature that implicates the primary cilium early in adipocyte development (26–29).
MATERIALS AND METHODS
Cell culture and gene knockdown
3T3-L1 preadipocytes were purchased from American Type Culture Collection (Manassas, VA, USA) and maintained in culture without achieving confluence. Growth medium consisted of DMEM—with 25 mM glucose, GlutaMax, and sodium pyruvate—that was supplemented with 10% newborn calf serum (both from Thermo Fisher Scientific, Waltham, MA, USA). The standard 3T3-L1 differentiation protocol (30) requires the incubation of cells for 2 d after they have reached confluence before the induction of differentiation. To amplify the magnitude of the increased adipogenesis that was observed in the Rpgrip1l knockdown condition, we limited differentiation by treating cells with differentiation medium that contained DMEM plus insulin (from bovine pancreas, 1 μg/ml), dexamethasone (0.25 μM), and isobutylmethylxanthine (IBMX; 0.5 mM; all from Sigma-Aldrich, St. Louis, MO, USA) that was supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific) at confluence without waiting for 2 d as in the standard protocol (31–33). Two days later, differentiation medium was replaced with maintenance medium (DMEM that contained only 1 μg/ml insulin and 10% FBS). Maintenance medium was replaced every 2–3 d and cells were filled with lipid by d 6–12, at which point we referred to them as mature adipocytes. We observed lot-to-lot variability in the differentiation capacity of 3T3-L1 cells, with a diminished, but still appreciable, increase in adipogenesis after Rpgrip1l knockdown in better-differentiating lots, which approached maximal differentiation in the control knockdown condition. We performed the experiments reported here on a lot with moderate differentiation potential to more accurately interrogate the phenotype.
By using either Lipofectamine 2000 or 3000 (Thermo Fisher Scientific), 3T3-L1 cells were transfected before differentiation with a mix of 3 Stealth small interfering RNAs (siRNAs; Thermo Fisher Scientific) that were targeted to either Rpgrip1l or nontargeted controls. Knockdown in mature 3T3-L1 adipocytes was achieved by electroporation with the same nontargeted control or Rpgrip1l siRNAs by using a Bio-Rad GenePulser II (Bio-Rad, Hercules, CA, USA) (34, 35) in cells that had been differentiated using the standard differentiation protocol. Cells were incubated for 2 d postconfluence before being treated for 2 d with 10% FBS, insulin, dexamethasone, and IBMX. Cells were then switched to and maintained with medium that contained insulin and FBS only. Mature adipocytes were electroporated 10 d after switching to medium that contained insulin and FBS only.
Hedgehog signaling experiments were performed by adding smoothened agonist (SAg; 1 µM) or cyclopamine-KAAD (1 µM; both from Sigma-Aldrich) concurrently with the transfection reagent added to knockdown Rpgrip1l in 3T3-L1 cells before reaching confluence.
Immunocytochemistry
Cilia were visualized by staining with anti-acetylated α-tubulin Ab (Abcam, Cambridge, MA, USA) and DAPI after fixation with 4% paraformaldehyde. Cilia were imaged at ×63 magnification as z stacks by confocal microscopy (LSM710; Zeiss, Jena, Germany) and displayed as z projections by using maximum intensity (Fiji; https://fiji.sc/) (36). We performed cell proliferation analysis by treating confluent cells with differentiation factors—insulin, dexamethasone, and IBMX—overnight. The next day, while cells remained in the proliferative phase, they were pulsed for 6 h with 5-bromo-2′-deoxyuridine (BrdU; Cell Signaling Technology, Danvers, MA, USA). BrdU incorporation was measured by fixation with 10% paraformaldehyde, permeabilization with Triton X-100, acid wash with HCl (all from Sigma-Aldrich), and subsequent staining with BrdU Ab (Cell Signaling Technology). The percentage of BrdU+ cells was quantified by fluorescence microscopy at ×20 magnification (Eclipse, TS100, and DS-Qi2; Nikon, Tokyo, Japan).
RNA isolation and quantification
RNA was isolated by using the RNeasy Lipid Tissue Kit with On-Column DNase treatment (Qiagen, Germantown, MD, USA) and reverse transcribed by using a Transcriptor First Strand cDNA Synthesis Kit (Roche, Basel, Switzerland) using both OligoDT and random hexamer primers. Quantitative PCR was performed on a LightCycler 480 machine using SYBR Green I Master (Roche). Expression was determined by using LightCycler 480 software (Roche) with the second derivative maximum calculation on the basis of a standard curve. Transcript levels were normalized to 36B4 when comparing a single cell type or tissue, or to the mean of Actb, Gapdh, and Ppia when multiple tissues or cell types were compared. Quantitative PCR primers were designed by using Primer 3 (http://bioinfo.ut.ee/primer3/) to span exon–exon junctions and are listed in Supplemental Table 1.
Protein quantification
We performed leptin and adiponectin ELISAs using kits from R&D Systems (Minneapolis, MN, USA). Cell culture supernatants from mature adipocytes were removed 3 d after medium had been changed. Samples in 12-well plates were diluted 1:20 for the leptin ELISA and 1:1000 for the adiponectin ELISA.
Lipid staining
We performed Oil Red O staining on mature adipocytes that were fixed with 4% paraformaldehyde for 10 min. A stock solution of 25 mg Oil Red O (Sigma-Aldrich) in 50 ml isopropanol was freshly mixed with water 3:2 (Oil Red O:H2O). This solution was filtered and cells were stained for 30 min. After PBS wash, cells were imaged and Oil Red O was extracted in 300 µl isopropanol with 4% Igepal CA-630 for 5 min (Sigma-Aldrich). One hundred microliters were taken to measure absorbance at 490 nm (37). Data are reported as raw absorbance. Nile Red staining protocol was adapted from Smyth et al. (38). In brief, mature adipocytes were trypsinized, fixed for 10 min with 4% paraformaldehyde, and stained with Nile Red at a final concentration of 500 ng/µl (Thermo Fisher Scientific). We added equal volumes of 8.0–12.9 µm of counting beads (2.5 × 104 per condition; Spherotech, Lake Forest, IL, USA) before acquisition by flow cytometry using the tandem conjugate of phycoerythrin and Texas Red (PE-TR) channel to measure Nile Red fluorescence.
Mice
Mice were housed and handled according to guidelines established by Columbia University, and protocols were approved by the Columbia University Institutional Animal Care and Use Committee. Room temperature was maintained at 23 ± 1°C with a 12-h light/dark cycle beginning at 7:00 am. Animals had ad libitum access to food and water. Mice were fed either regular chow (22% calories from fat; Purina, St. Louis, MO, USA), or high-fat diet (HFD; 60% of calories from fat; Research Diets, New Brunswick, NJ, USA) as indicated. Mice were weighed weekly and body weight composition was obtained by time domain (TD)-NMR with a Minispec Analyst AD analyzer (Bruker Optics, Billerica, MA, USA).
For this study, we used mice that segregated for a floxed allele of Rpgrip1l (Rpgrip1ltm1c(EUCOMM)Wtsi), referred to here as Rpgrip1lfl/fl (16), that were then crossed to Adipoq-Cre mice [B6; FVB-Tg (Adipoq-cre) 1Evdr/J; The Jackson Laboratory, Bar Harbor, ME, USA] to generate Rpgrip1ltm1d(EUCOMM)Wtsi (Rpgrip1l+/fl:Adipoq-Cre) mice. These were intercrossed to obtain Rpgrip1lfl/fl:Adipoq-Cre, Rpgrip1l+/fl:Adipoq-Cre, and Rpgrip1l+/+:Adipoq-Cre mice that were used for the chow feeding study. Exon 5 of Rpgrip1l was deleted in only the adipose tissue of Rpgrip1lfl/fl:Adipoq-Cre mice. We also generated Rpgrip1lfl/fl:Adipoq-Cre and Rpgrip1lfl/fl littermates by crossing Rpgrip1lfl/fl:Adipoq-CreCre/+ and Rpgrip1lfl/fl mice for the HFD feeding study. Genotyping for the floxed Rpgrip1l allele was performed as previously described (16). Genotyping for the Adipoq-Cre allele was performed by using Cre primers (forward: 5′-GCGGTCTGGCAGTAAAAACTATC-3′, reverse: 5′-GTGAAACAGCATTGCTGTCACTT-3′).
Adipose tissue analyses
Adipocyte size was measured in 19-wk-old male Rpgrip1l+/+ and Rpgrip1l+/− mice that were fed chow until age 18 wk, at which point they were fed HFD for 1 wk (20). Subcutaneous adipose tissue (SCAT) was paraffin embedded, sectioned, and stained with hematoxylin and eosin. One slide per animal was imaged at ×20 magnification, with 5–6 images captured across each section. Adipocyte size was determined by using the Adiposoft plugin for Fiji (39).
Fractionation of adipose tissue was performed by shaking minced adipose tissue for ∼45 min in adipose tissue digestion solution—DMEM with 20 mg/ml bovine serum albumin (Sigma-Aldrich) and 0.14 U/ml Liberase Thermolysin Medium (TM; Roche)—at 37°C. Digested tissue was passed through a cell strainer and separated via centrifugation. The preadipocyte-containing stromal vascular fraction was pelleted, and mature adipocytes were collected from the floating layer.
Data analysis and statistics
Data were analyzed by using GraphPad Prism (v.7; GraphPad Software, La Jolla, CA, USA). Replicates reported for in vitro knockdown experiments are biological replicates, with control and Rpgrip1l knockdown replicates processed in parallel. Unpaired, 2-tailed Student’s t tests were performed for these experiments. Mouse experiments report the number of mice in each genotype compared—8 L in the chow feeding experiment, and 10 L in the HFD experiment. Unpaired, 2-tailed Student’s t tests were also performed for these experiments, with the exception of the correlation of fat mass and circulating leptin concentrations, for which we used Pearson’s correlation test to determine r2 and P values for linear regressions. ANCOVA was used to compare the slopes and intercepts of the 2 groups.
RESULTS
Rpgrip1l knockdown in 3T3-L1 preadipocytes enhances adipogenesis
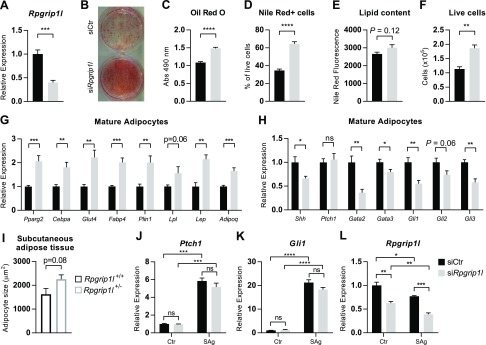
To assess the possible cell-autonomous effects of systemic inactivation of Rpgrip1l on adipose tissue development, we knocked down Rpgrip1l in 3T3-L1 preadipocytes by using siRNA. Knockdown persisted throughout differentiation, and in mature adipocytes (∼12 d after transfection), Rpgrip1l expression was still decreased by 60% (Fig. 1A). Lipid accumulation per well was increased in the Rpgrip1l knockdown condition (Oil Red O; Fig. 1B, C). A partial differentiation protocol was used to limit the time that 3T3-L1 cells spent at confluence before differentiation, which increased the magnitude of the difference between Rpgrip1l and control knockdown. To identify whether this increase in lipid accumulation was a result of increased efficiency of differentiation, the amount of lipid per cell, or both, we performed flow cytometry on Nile Red–stained cells. The observed increase in lipids was attributable mainly to an increase in the percentage of cells that contained lipids (Fig. 1D), although there was a trend toward an increase in the amount of lipid per adipocyte (Fig. 1E; P = 0.12) in Rpgrip1l knockdown cells. In addition, we observed an increase in the total number of live cells after Rpgrip1l knockdown, which suggests that Rpgrip1l limits cell survival and/or proliferation (Fig. 1F). This finding is consistent with a report that has described RPGRIP1L as a tumor suppressor in human hepatocellular carcinoma that acts primarily by affecting cell survival (40). We analyzed cell proliferation by BrdU staining (Supplemental Fig. 1A–D) and did not find an increased percentage of BrdU-stained cells, which indicates that the increase in cell number observed is not a result of the effects on proliferation, but rather cell survival. Rpgrip1l knockdown adipocytes exhibited an increased expression of genes that are involved in glucose uptake (Glut4) and lipid uptake (Fabp4, Lpl) and storage (Plin1), as well as adipokines (Lep, Adipoq). We also observed an increased expression of the developmental regulators of adipogenesis, Pparg2 and Cebpa (Fig. 1G). In previously described male mice that are heterozygous for Rpgrip1l (20), we observed a trend toward an increase in adipocyte size in SCAT of Rpgrip1l+/− mice compared with Rpgrip1l+/+ controls (Fig. 1I and Supplemental Fig. 2A, B). This increase in size is consistent with the trend toward increased lipid filling that was observed in 3T3-L1 by Nile Red staining (Fig. 1E).
Figure 1.
Rpgrip1l knockdown increases adipogenesis in 3T3-L1 adipocytes. A) Rpgrip1l mRNA expression in mature adipocytes after siRNA-induced knockdown before differentiation (siCtr: black bars; siRpgrip1l: light gray bars). B, C) Representative image (B) and quantification (C) of Oil Red O staining of mature adipocytes that were treated with siRNA against Rpgrip1l before differentiation. D, E) Percentage of cells that were positive for Nile Red staining for intracellular lipid acquired by flow cytometry (D) and median fluorescence intensity of Nile Red+ cells (E). F) Total number of live cells that were normalized to the number of flow cytometry counting beads. G) mRNA expression of developmental (Pparg and Cebpa) and functional genes (Glut4, Fabp4, Plin1, Lpl, Adipoq, and Lep) in mature adipocytes when Rpgrip1l was knocked down before differentiation. H) Expression of Shh and target genes of the Hh pathway in mature adipocytes. I) Average adipocyte area determined by hematoxylin and eosin staining of SCAT from 19-wk-old wild-type (open black bars; n = 3) and Rpgrip1l+/− male mice (open gray bars; n = 6) that were fed chow for 18 wk and switched to HFD for 1 wk (20). Five to six images of adipose tissue were analyzed per animal, which yielded ∼300–800 cells/animal. *P < 0.05 (unpaired Student’s t test). J–L) mRNA expression of the Hh target genes, Ptch1 (J) and Gli1 (K), as well as Rpgrip1l (L) expression 24 h after SAg treatment of 3T3-L1 cells added with transfection reagent before confluence. mRNA expression has been normalized to 36B4; n = 3–6/condition. Ns, not significant. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (unpaired Student’s t test).
Rpgrip1l has been previously reported to facilitate the hedgehog (Hh) signaling pathway (17), a known inhibitor of adipogenesis (41–43). We observed a decreased expression of Hh target genes—Gata2, Gata3, Gli1, Gli2, and Gli3—in Rpgrip1l knockdown mature adipocytes, but no change in Ptch1 expression; however, we also observed a decreased expression of Shh, which drives Hh signaling (Fig. 1H). Neither Shh, nor Hh target genes were changed after Rpgrip1l knockdown in either confluent preadipocytes (Supplemental Fig. 1G) or preadipocytes that had been treated with differentiation medium for 2 d (Supplemental Fig. 1H). To directly assess whether Rpgrip1l is required for Hh signaling, we treated preconfluent cells with the Hh activator, SAg, at the time of transfection with Rpgrip1l siRNA, and measured the expression of Hh target genes when these cells reached confluence (∼24 h later). There was no significant impact of Rpgrip1l knockdown on the expression of Ptch1 or Gli1 (Hh mediators) in response to SAg treatment (Fig. 1J, K); however, we did observe an additional decrease in Rpgrip1l expression in response to SAg (Fig. 1L), which suggests that Hh signaling may impact cilia function by regulating the expression of this critical component of the primary cilium’s transition zone (21, 22). We also attempted to inhibit Hh signaling by using cyclopamine-KAAD, but were unable to inhibit Ptch1 or Gli1 expression, which indicates that little endogenous Shh signaling occurs in confluent preadipocytes (Supplemental Fig. 1E, F).
Knockdown of Rpgrip1l is not expected to impair ciliogenesis, per se, as in other cell types the loss of Rpgrip1l may actually increase cilia length (20, 44). We verified that there were no obvious defects in ciliogenesis in the Rpgrip1l knockdown condition by staining for acetylated α-tubulin, a marker of the primary cilium (Supplemental Fig. 1I, J). We found no evidence of an impairment of ciliogenesis, as almost all cells [control siRNA (siCtr): 98%; siRpgrip1l: 97%; P = 0.77] were ciliated after 2 d at confluence.
To examine whether the suppression of Rpgrip1l was sufficient to induce adipogenesis on its own, we knocked down Rpgrip1l expression in preconfluent 3T3-L1 preadipocytes and measured adipogenesis in cells 9 d after they had reached confluence. Rpgrip1l knockdown persisted (−26%; Supplemental Fig. 3A) for this length of time, but we did not observe any evidence of adipogenesis in the siRpgrip1l condition (Supplemental Fig. 3B–E) in the absence of chemically induced differentiation.
Rpgrip1l knockdown does not affect the production or secretion by adipocytes of factors that regulate food intake
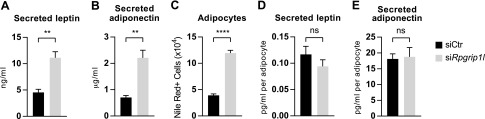
Rpgrip1l hypomorphic mice are obese because, in part, of hyperphagia that results from impaired hypothalamic leptin sensitivity (16, 20). We measured leptin (Fig. 2A) and adiponectin (Fig. 2B) secretion by ELISA in mature 3T3-L1 adipocytes that were transfected with anti-Rpgrip1l siRNA before differentiation. Although we observed increased secretion of both of these hormones in the Rpgrip1l knockdown condition, when normalized to the number of adipocytes in each condition—measured by Nile Red staining detected by flow cytometry (Fig. 2C)—there was no difference in the production of these hormones per cell (Fig. 2D, E).
Figure 2.
Rpgrip1l knockdown does not alter the production of leptin or adiponectin by 3T3-L1 adipocytes. A, B) Secretion of leptin (A) and adiponectin (B) into culture medium by mature adipocytes 3 d after medium change (siCtr: black bars; siRpgrip1l: light gray bars). D, E) Leptin (D) and adiponectin (E) levels in medium that was normalized to the number of adipocytes, measured by Nile Red–stained cells acquired by flow cytometry. C) Adipocyte number was normalized to counting beads; n = 4/condition. Ns, not significant. **P < 0.01, ****P < 0.0001 (unpaired Student’s t test).
Knockout of Rpgrip1l in mature adipocytes does not alter body weight in mice
To determine whether the increased adipogenesis that was observed in Rpgrip1l knockdown adipocytes contributed to the adiposity phenotype that had been previously observed in systemic, congenital Rpgrip1l heterozygotes, we generated adipocyte-specific Rpgrip1l knockout mice (Rpgrip1lfl/fl:Adipoq-Cre) that we compared with either Rpgrip1l+/+:Adipoq-Cre or Rpgrip1lfl/fl controls.
To evaluate the expression of Rpgrip1l and ensure that the decrease was a result of the excision of the Rpgrip1l allele, rather than an off-target effect of the Adipoq-Cre allele, we quantified the transcript levels of Rpgrip1l in perigonadal adipose tissue (PGAT) of male Rpgrip1lfl/fl:Adipoq-Cre, Rpgrip1lfl/+:Adipoq-Cre, and Rpgrip1l+/+:Adipoq-Cre mice. We found that the homozygous and heterozygous Rpgrip1l-floxed animals exhibited dose response–related decreases in Rpgrip1l expression (Supplemental Fig. 4A). In addition, as the aforementioned measurements relied on primers that detected the exon 4–5 junction of Rpgrip1l, which is disrupted by Cre recombinase activity, we verified that levels of the Rpgrip1l transcript downstream of the Cre recombination site were also decreased in Rpgrip1lfl/fl:Adipoq-Cre animals compared with Rpgrip1l+/+:Adipoq-Cre controls (Supplemental Fig. 4B). Adipoq-Cre that was used to generate the loss-of-function allele of Rpgrip1l is highly specific to adipose tissue (45, 46), which is consistent with our observation that, in nonadipose tissues (i.e., muscle, liver, and hypothalamus), there was no difference in Rpgrip1l expression in Rpgrip1lfl/fl:Adipoq-Cre animals compared with Rpgrip1l+/+:Adipoq-Cre controls (Supplemental Fig. 4C).
Rpgrip1l transcript was decreased in both subcutaneous and perigonadal adipose depots of male Rpgrip1lfl/fl:Adipoq-Cre mice [Supplemental Fig. 5A; SCAT: −69%; PGAT: −67%] compared with Rpgrip1lfl/fl controls. In addition, Rpgrip1l expression was decreased or trended toward a decrease in perirenal and mesenteric adipose depots, respectively. Rpgrip1l expression was similarly decreased in female mice (data not shown). Adipose tissue contains diverse cell types, so we separated the stromal vascular and adipocyte fractions in male SCAT and PGAT. We detected Cre transcript only in the adipocyte fraction (Supplemental Fig. 5B), and this fraction had a greater decrease in Rpgrip1l expression (SCAT: −83%; PGAT: −63%) than whole tissue (SCAT: −67%; PGAT: −28%; Supplemental Fig. 5C). There was no change in Rpgrip1l expression in the preadipocyte-containing stromal vascular fraction.
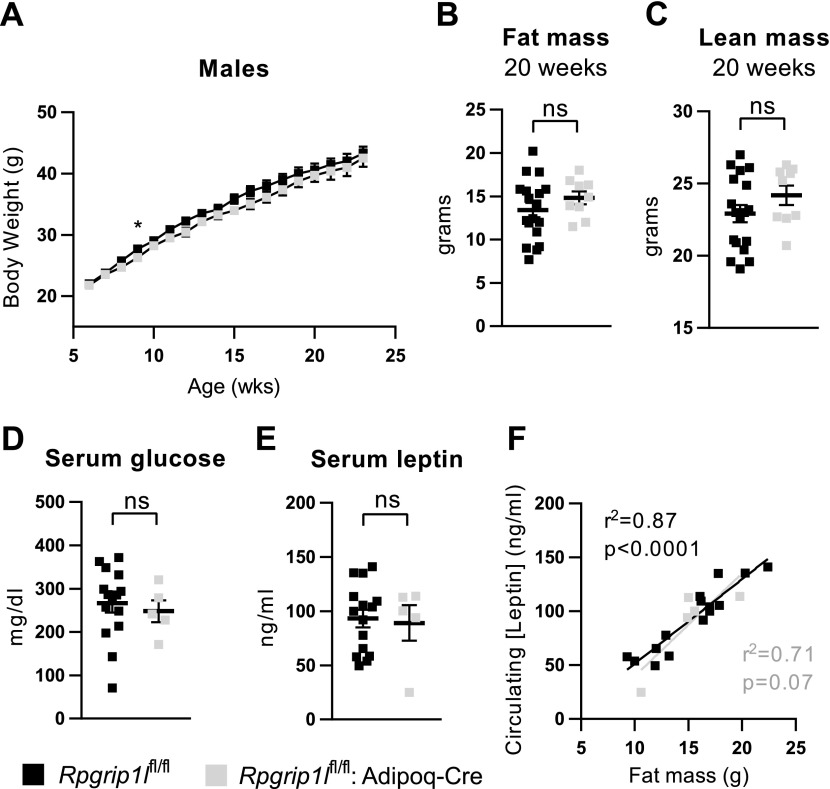
We monitored the body weight of these animals until they reached age 23 wk. During this period, Rpgrip1lfl/fl:Adipoq-Cre and Rpgrip1l+/fl:Adipoq-Cre mice did not exhibit any difference in weight gain compared with Rpgrip1l+/+:Adipoq-Cre littermates (Supplemental Fig. 6A). At age 12 wk, we analyzed body composition and did not detect any differences in either fat (Supplemental Fig. 6B) or lean (Supplemental Fig. 6C) mass. We considered the possibility that Rpgrip1l may not affect body weight unless the animals are overfed. We fed another cohort of mice HFD (60% calories as fat) for 17 wk. We did not observe any differences in body weight (Fig. 3A), body composition (Fig. 3B, C), plasma glucose (Fig. 3D), or leptin concentrations (Fig. 3E) between Rpgrip1lfl/fl:Adipoq-Cre mice and Rpgrip1lfl/fl controls. Regression lines that relate circulating leptin concentration to fat mass were not different (Fig. 3F), which indicates that hypomorphism for Rpgrip1l in adipocytes did not affect the production and/or release of leptin.
Figure 3.
Adipocyte-specific loss of Rpgrip1l function does not affect body weight or fat mass in HFD-fed mice. A) Weekly body weights of male Rpgrip1lfl/fl controls (gray squares; n = 26) and Rpgrip1lfl/fl:Adipoq-Cre mice (black squares; n = 13) that were fed HFD (60% of calories) ad libitum starting at 6 wk. B, C) Fat mass (B) and lean mass (C) were measured by NMR at age 20 wk in a subset of these (Rpgrip1lfl/fl, n = 18; Rpgrip1lfl/fl:Adipoq-Cre n = 9). D, E) Serum glucose (D) and leptin (E) concentrations. F) Correlation of serum leptin concentration with fat mass (Rpgrip1lfl/fl, n = 15; Rpgrip1lfl/fl:Adipoq-Cre, n = 5; Pearson’s correlation test; ANCOVA). Ns, not significant. *P < 0.05 (unpaired Student’s t test except where indicated).
Knockdown of Rpgrip1l in mature 3T3-L1 adipocytes does not alter lipid content
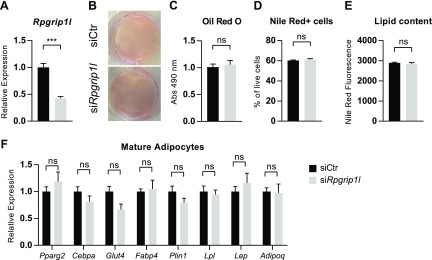
As the Adipoq-Cre driver is not turned on until well after preadipocytes have started to undergo adipogenesis, it is possible that the Rpgrip1lfl/fl:Adipoq-Cre model did not alter Rpgrip1l expression until after its physiologically relevant effects on adipogenesis had occurred. We attempted to model this possibility by knocking down (−54%) Rpgrip1l in fully mature adipocytes at a point at which adiponectin is expressed (Fig. 4A) and observed no difference in lipid content as quantified by Oil Red O (Fig. 4B, C) or Nile Red staining (Fig. 4D, E) in the Rpgrip1l knockdown condition 3 d after knockdown was induced. In addition, we did not find any differences in the mRNA expression of adipocyte developmental—Pparg and Cebpa—and functional genes—Glut4, Fabp4, Plin1, Lpl, Lep, and Adipoq—after Rpgrip1l knockdown in mature adipocytes (Fig. 4F).
Figure 4.
Knockdown of Rpgrip1l in 3T3-L1 mature adipocytes does not affect lipid storage. A) Rpgrip1l mRNA expression 3 d after knockdown in mature 3T3-L1 adipocytes (12 d after differentiation initiation; siCtr: black bars; siRpgrip1l: light gray bars). B, C) Representative image (B) and quantification (C) of Oil Red O staining of mature Rpgrip1l knockdown adipocytes. D, E) Percentage of cells that were positive for Nile Red staining for intracellular lipid acquired by flow cytometry (D) and median fluorescence intensity (E) of Nile Red+ cells. F) mRNA expression of developmental (Pparg and Cebpa) and functional genes (Glut4, Fabp4, Plin1, Lpl, Lep, and Adipoq) in mature adipocytes. Expression is normalized to 36B4; n = 4/condition. Ns, not significant. ***P < 0.001 (unpaired Student’s t test).
DISCUSSION
We have previously demonstrated that RPGRIP1L expression is decreased in induced pluripotent stem cell-derived neurons of humans who segregate for obesity risk alleles in intron 1 of FTO (16). Two of the obesity-associated SNPs—rs8050136 and rs1421085—in this interval are binding sites for the transcriptional regulator, CUX1. Specifically, the risk alleles of these 2 SNPs exhibited decreased in vitro binding of an activating isoform of CUX1 (p110), which decreased RPGRIP1L and FTO minimal promoter activity in N2a murine neuroblastoma cells (14, 16). Rpgrip1l+/− mice are hyperphagic and obese (20). Here, we assessed whether a decrease in Rpgrip1l expression had any direct, cell-autonomous effect on adipocyte development and function that might contribute to the increased adiposity of Rpgrip1l+/− mice.
Knockdown of Rpgrip1l increased adipocyte differentiation and proliferation in 3T3-L1 cells; however, congenital adipocyte-specific loss of Rpgrip1l did not alter body weight or adiposity in mice. The absence of an effect in this circumstance suggests that the function of Rpgrip1l in adipogenesis precedes the expression of Adipoq-Cre recombinase in this model. Consistent with this possibility, knockdown of Rpgrip1l in mature 3T3-L1 adipocytes in vitro does not cause a detectable change in lipid content or expression of adipocyte developmental or functional genes.
The primary cilium is an organelle that is present in almost all mammalian cell types and coordinates cellular development and environmental interactions (47). The role of RPGRIP1L in the assembly of the transition zone of the primary cilium qualifies it to regulate the differentiation of a variety of cell types (21, 22, 48), as the transition zone participates in the compartmentalization of specific signal transduction proteins, such as Hh modulators, as well as platelet-derived growth factor receptor α (Pdgfrα), within the ciliary membrane (49, 50). Mice that are homozygous for a null allele of Rpgrip1l die embryonically with craniofacial and neural tube defects, as well as deranged somatic left–right asymmetry (17). Humans with biallelic RPGRIP1L mutations present with either Joubert syndrome, a developmental disorder with mid-hindbrain malformation and renal and hepatic defects (51), or Meckel syndrome, an autosomal recessive lethal syndrome characterized by CNS malformations that typically include occipital encephalocele (52). Obesity has not been described in patients with RPGRIP1L mutations; however, the systemic consequences are sufficiently severe to mask effects on adiposity. It may be informative to evaluate adiposity in obligate heterozygous parents of these individuals.
A genetically heterogeneous class of disorders, termed ciliopathies, is characterized by mutations in genes that encode more than 40 proteins that contribute to the establishment and/or function of the primary cilium (19). Phenotypes shared among members of this class of disorders include renal and retinal dysfunction as well as developmental malformations, such as polydactyly and defects of the CNS. Some ciliopathies—that is, Bardet-Biedl syndrome (BBS) and Alström syndrome—also present with comorbid severe obesity (18, 19). Hyperphagia, presumably of hypothalamic origin, is a primary contributor to the obesity observed in individuals with ciliopathies (53, 54). We have demonstrated that the congenital absence of Rpgrip1l causes decreased leptin sensitivity as a result of impaired leptin receptor trafficking in relation to the primary cilium (16, 20).
Primary changes in adipose tissue development and/or function have also been implicated in the adiposity observed in individuals with certain ciliopathies (24, 54). Knockdown of the BBS proteins, BBS4, BBS10, and BBS12, increases proliferation and/or adipogenesis in human mesenchymal stem cells and murine 3T3-F442A cells (23–25), which suggests that the disruption of ciliary function—via BBSome activity—conveys primary effects on adipogenesis in addition to CNS-mediated effects on food intake.
Cilia are present on confluent, nondividing human preadipocytes in vitro, and dexamethasone treatment elicits ciliary elongation in the first few days of adipogenesis. Ciliary length then decreases or the cilium disappears entirely as cells become terminally differentiated and filled with lipids (25–27, 29). During this initial period of ciliary elongation, antiadipogenic Hh signaling is inhibited (27) and Igf-1R localizes to the cilium, presumably enhancing the capacity of these cells for differentiation into adipocytes (26, 29). Furthermore, ciliary reabsorption after the initial elongation phase seems to be required for complete adipogenesis (28). The reduction of ciliary proteins, such as Ift88, Kif3a, and Alms1, which are required for ciliary assembly (55–59), impairs adipogenesis (26, 60). Hypomorphic mutations in Ift88 and Kif3a, although embryonically lethal in homozygous mice (56, 57, 61), have also been linked to increased adiposity when induced during adulthood in conditional knockout models (62). However, the effect of Rpgrip1l knockdown on adipogenesis detailed here is consistent with the effects of hypomorphic mutations in BBS10 and BBS12 in human white preadipocytes and mesenchymal stem cells, as well as Bbs4 knockdown in 3T3-F442A preadipocytes, which enhance adipocyte development in several models of adipogenesis (23–25). These differing effects of ciliary proteins on adipogenesis underscore the complicated nature of ciliary function with respect to adipogenesis: ciliary assembly is required to induce adipogenesis, but the maintenance of ciliary function—via BBSome and Rpgrip1l-regulated ciliary gating—and the subsequent reabsorption of the cilium is also critical to restrain excessive adipocyte differentiation. Loss of Rpgrip1l is not anticipated to inhibit cilia formation, and, in fact, loss of Rpgrip1l is associated with increased ciliary length (20, 44). Instead, reduction in Rpgrip1l activity is thought to affect ciliary function by impairing the establishment and regulation of the transition zone and ciliary gate (21, 48). The increased ciliary length itself, as well as the associated inhibition of antiadipogenic pathways or the activation of proadipogenic pathways, may be sufficient to induce adipogenesis, mimicking the lengthening that is observed during early adipogenesis (27, 29).
Timing of ciliary elongation and disassembly may be critical to understanding why we did not observe effects on adiposity in Rpgrip1lfl/fl:Adipoq-Cre animals that were fed either chow or HFD. The adiponectin Cre recombinase in these mice is not expressed in preadipocytes, and is only turned on late in adipogenesis after the reabsorption of the primary cilium (27, 63). Hence, the Adipoq-Cre driver may not have been expressed early enough in adipogenesis to affect ciliary functioning in preadipocytes. Consistent with this possibility, knockdown of Rpgrip1l in fully mature 3T3-L1 adipocytes did not affect lipid content or the expression of adipocyte genes, whereas knockdown of Rpgrip1l in preadipocytes increased the differentiation capacity of 3T3-L1 cells.
As a proposed driver of Hh signaling (17), Rpgrip1l might be expected to limit adipogenesis, as Hh signaling has previously been demonstrated to inhibit adipogenesis in both rodent and human mesenchymal cells (41–43). Hh signaling maintains 3T3-L1 cells in a preadipocyte state, and is decreased as they differentiate into adipocytes (43); however, inhibition of this pathway alone is not sufficient to induce adipogenesis in 3T3-L1 preadipocytes (64). Whereas we observed decreased expression of Hh targets in mature adipocytes after Rpgrip1l knockdown, Shh itself was also decreased. The reduction of Hh target gene expression may be a secondary effect of the increased adipogenesis observed in these cells, as Shh production has been demonstrated to be decreased as adipogenesis proceeds in mouse adipose-derived stromal cells (65); however, there may be a positive feedback loop in mouse adipose-derived stromal cells, as Shh treatment increases Shh expression (65). This complicated regulation of Shh expression and Hh signaling obscures a mechanistic understanding of the role of Rpgrip1l in adipogenesis with respect to this antiadipogenic pathway. In fibroblasts of patients with inactivating mutations in RPGRIP1L, the activating effect of SAg treatment on smoothened localization to the cilium is impaired compared with fibroblasts from healthy controls (48). In postconfluent preadipocytes that were treated for 2 d with the differentiation factors, insulin, dexamethasone, and IBMX, we found no difference in the expression of Hh target genes in the Rpgrip1l knockdown condition. This is approximately the time point during adipogenesis at which Smo localizes to the primary cilium to presumably turn off antiadipogenic Hh signaling (27). Furthermore, Rpgrip1l knockdown did not affect the response of Ptch1 or Gli1 transcription to SAg treatment. However, Hh signaling in response to SAg reduces expression of Rpgrip1l. We are unaware of other instances in which Hh regulates the expression of ciliary proteins. These results expand on previous notions about the ways in which Hh directs cellular activity. In addition to signaling via the smoothened/patched receptors, known to be conveyed on the cilium, Hh signaling also regulates the expression of this critical component of ciliary organization and, presumably, function. The functional consequences of the loss of Rpgrip1l are profound, as knockout mice exhibit embryonic lethality, but additional mechanistic studies are required to understand its activities in the cilium more fully.
Other signaling molecules that are known to regulate adipogenesis, such as PdgfRα, insulin receptor, and Igf1R, localize to the ciliary membrane upon growth arrest and ciliogenesis/ciliary elongation (25, 26, 29, 66–68). There is extensive crosstalk among pathways that are mediated by these molecules (69) and evidence that Rpgrip1l may participate in some of this coordination (70, 71). Recently, an ortholog of Rpgrip1l in Caenorhabditis elegans, Mks5, has been demonstrated to establish a ciliary zone of exclusion that limits phosphatidylinositol 4,5-bisphosphate localization in the ciliary membrane, thereby forming a lipid gate and restricting access of membrane-bound proteins to the ciliary compartment. In addition, Mks5 hypomorphs exhibited dysregulated intraflagellar transport velocity (21). Collectively, these findings support a critical role for Rpgrip1l in adipocyte cell fate commitment, with the potential to affect adipogenesis via multiple avenues.
Are there cell-autonomous contributions by adipocytes to the establishment of body weight, or does CNS regulation of energy intake or energy expenditure entirely drive adiposity? Mouse interstrain adipocyte transplantation experiments (72) and transgenic developmental manipulations of Akt2 (73) and adiponectin (74) implicate adipocyte-autonomous effects on adiposity; however, none of these experiments focused explicitly on the developmental biology of the preadipocyte, per se. For such studies of for example, Rpgrip1l, preadipocyte genes, such as Pparg2, Pdgfra, and Prx1, would be attractive candidates for Cre drivers; however, none of these genes is specific to preadipocytes, which confounds efforts to isolate preadipocyte-autonomous roles in the control of body fat stores (75, 76). In this case, loss of Rpgrip1l in non-preadipocyte cell types in which these Cre drivers are expressed (e.g., neural crest cells and craniofacial mesenchyme) (77, 78) might be expected to confer lethality, as Rpgrip1l is required for neural tube closure and craniofacial development (17).
The brain is the ultimate arbiter of food intake, but adipose tissue storage capacity can affect feedback signals and the facility with which excess calories can be accommodated (79, 80). If the Rpgrip1l knockdown-induced effect on adipogenesis contributes to the whole-body adiposity of Rpgrip1+/− mice, we hypothesized that one way in which adipocytes per se might influence food intake is via the dysregulated production of appetite-regulating factors, such as leptin and/or adiponectin. We observed increased expression of both of these hormones in 3T3-L1 adipocytes after Rpgrip1l knockdown before differentiation, but when normalized to the increased number of adipocytes observed, there was no change in production on a per cell basis. This finding suggests that Rpgrip1l does not regulate adiposity by altering leptin production; however, these results should not be overinterpreted. 3T3-L1 adipocytes express lower levels of leptin than do other adipocyte models and primary adipocytes ex vivo (81). In addition, leptin is known to be differentially expressed in a depot- and sex-specific manner (82) in conditions that were not recapitulated here.
Effects on food intake and adipose tissue differentiation and physiology have been implicated in the very strong genetic signal for adiposity conveyed by non-coding sequences in intron 1 of FTO (1). It is possible that such distinct tissue-related effects are coordinated by single effectors. Earlier we have shown effects of Rpgrip1l hypomorphism on food intake in mice. Here we report a proadipogenic effect of Rpgrip1l hypomorphism in 3T3-L1 cells. Such effects would act coordinately to increase adiposity in genetically susceptible individuals. Increased adipogenesis observed in the early Rpgrip1l knockdown condition in 3T3-L1 cells could implicate Rpgrip1l hypomorphism in adipose tissue—by facilitating preadipocyte production and differentiation—in the increased adiposity of Rpgrip1l+/− mice. A larger depot fat capacity would permit greater fat storage before suppressive consequences on food intake might be invoked (79). The primary cilium - of which RPGRIP1L is a component - conveys many signals which have the potential to regulate adipogenesis, including antiadipogenic Hh signaling (43). Rpgrip1l has been implicated in facilitating Hh signaling (17), an effect which we did not observe in our studies. However, we found increased Rpgrip1l expression after Hh activation, suggesting that ciliary components themselves may be targets of the Hh pathway.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK097399, DK-P30026687-36 (New York Obesity Nutrition Research Center), S10-RR027050 (Columbia Center for Translational Immunology Flow Cytometry Core), and 5P30-DK063608 (Diabetes Education and Research Center). The authors declare no conflicts of interest.
Glossary
- BBS
Bardet-Biedl syndrome
- BrdU
5-bromo-2′-deoxyuridine
- CUX1
cut-like homeobox 1
- FBS
fetal bovine serum
- FTO
α-ketoglutarate–dependent dioxygenase
- HFD
high-fat diet
- Hh
hedgehog
- IBMX
isobutylmethylxanthine
- PGAT
perigonadal adipose tissue
- RPGRIP1L
retinitis pigmentosa GTPase regulator-interacting protein 1 like
- SAg
smoothened agonist
- SCAT
subcutaneous adipose tissue
- siCtr
control small interfering RNA
- siRNA
small interfering RNA
- SNP
single nucleotide polymorphism
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
J. F. Martin Carli and R. L. Leibel conceived of and designed the study and wrote the manuscript; J. F. Martin Carli performed the experiments and analyzed the data; and C. A. LeDuc, Y. Zhang, and G. Stratigopoulos gave technical support and conceptual advice.
REFERENCES
- 1.Frayling T. M., Timpson N. J., Weedon M. N., Zeggini E., Freathy R. M., Lindgren C. M., Perry J. R., Elliott K. S., Lango H., Rayner N. W., Shields B., Harries L. W., Barrett J. C., Ellard S., Groves C. J., Knight B., Patch A. M., Ness A. R., Ebrahim S., Lawlor D. A., Ring S. M., Ben-Shlomo Y., Jarvelin M. R., Sovio U., Bennett A. J., Melzer D., Ferrucci L., Loos R. J., Barroso I., Wareham N. J., Karpe F., Owen K. R., Cardon L. R., Walker M., Hitman G. A., Palmer C. N., Doney A. S., Morris A. D., Smith G. D., Hattersley A. T., McCarthy M. I. (2007) A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316, 889–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scuteri A., Sanna S., Chen W. M., Uda M., Albai G., Strait J., Najjar S., Nagaraja R., Orrú M., Usala G., Dei M., Lai S., Maschio A., Busonero F., Mulas A., Ehret G. B., Fink A. A., Weder A. B., Cooper R. S., Galan P., Chakravarti A., Schlessinger D., Cao A., Lakatta E., Abecasis G. R. (2007) Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 3, e115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dina C., Meyre D., Gallina S., Durand E., Körner A., Jacobson P., Carlsson L. M., Kiess W., Vatin V., Lecoeur C., Delplanque J., Vaillant E., Pattou F., Ruiz J., Weill J., Levy-Marchal C., Horber F., Potoczna N., Hercberg S., Le Stunff C., Bougnères P., Kovacs P., Marre M., Balkau B., Cauchi S., Chèvre J. C., Froguel P. (2007) Variation in FTO contributes to childhood obesity and severe adult obesity. Nat. Genet. 39, 724–726 [DOI] [PubMed] [Google Scholar]
- 4.Berentzen T., Kring S. I., Holst C., Zimmermann E., Jess T., Hansen T., Pedersen O., Toubro S., Astrup A., Sørensen T. I. (2008) Lack of association of fatness-related FTO gene variants with energy expenditure or physical activity. J. Clin. Endocrinol. Metab. 93, 2904–2908 [DOI] [PubMed] [Google Scholar]
- 5.Cecil J. E., Tavendale R., Watt P., Hetherington M. M., Palmer C. N. (2008) An obesity-associated FTO gene variant and increased energy intake in children. N. Engl. J. Med. 359, 2558–2566 [DOI] [PubMed] [Google Scholar]
- 6.Speakman J. R., Rance K. A., Johnstone A. M. (2008) Polymorphisms of the FTO gene are associated with variation in energy intake, but not energy expenditure. Obesity (Silver Spring) 16, 1961–1965 [DOI] [PubMed] [Google Scholar]
- 7.Timpson N. J., Emmett P. M., Frayling T. M., Rogers I., Hattersley A. T., McCarthy M. I., Davey Smith G. (2008) The fat mass- and obesity-associated locus and dietary intake in children. Am. J. Clin. Nutr. 88, 971–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer J., Koch L., Emmerling C., Vierkotten J., Peters T., Brüning J. C., Rüther U. (2009) Inactivation of the Fto gene protects from obesity. Nature 458, 894–898 [DOI] [PubMed] [Google Scholar]
- 9.Church C., Lee S., Bagg E. A., McTaggart J. S., Deacon R., Gerken T., Lee A., Moir L., Mecinović J., Quwailid M. M., Schofield C. J., Ashcroft F. M., Cox R. D. (2009) A mouse model for the metabolic effects of the human fat mass and obesity associated FTO gene. PLoS Genet. 5, e1000599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao X., Shin Y. H., Li M., Wang F., Tong Q., Zhang P. (2010) The fat mass and obesity associated gene FTO functions in the brain to regulate postnatal growth in mice. PLoS One 5, e14005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMurray F., Church C. D., Larder R., Nicholson G., Wells S., Teboul L., Tung Y. C., Rimmington D., Bosch F., Jimenez V., Yeo G. S., O’Rahilly S., Ashcroft F. M., Coll A. P., Cox R. D. (2013) Adult onset global loss of the fto gene alters body composition and metabolism in the mouse. PLoS Genet. 9, e1003166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Claussnitzer M., Dankel S. N., Kim K. H., Quon G., Meuleman W., Haugen C., Glunk V., Sousa I. S., Beaudry J. L., Puviindran V., Abdennur N. A., Liu J., Svensson P. A., Hsu Y. H., Drucker D. J., Mellgren G., Hui C. C., Hauner H., Kellis M. (2015) FTO obesity variant circuitry and adipocyte browning in humans. N. Engl. J. Med. 373, 895–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smemo S., Tena J. J., Kim K. H., Gamazon E. R., Sakabe N. J., Gómez-Marín C., Aneas I., Credidio F. L., Sobreira D. R., Wasserman N. F., Lee J. H., Puviindran V., Tam D., Shen M., Son J. E., Vakili N. A., Sung H. K., Naranjo S., Acemel R. D., Manzanares M., Nagy A., Cox N. J., Hui C. C., Gomez-Skarmeta J. L., Nóbrega M. A. (2014) Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature 507, 371–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stratigopoulos G., LeDuc C. A., Cremona M. L., Chung W. K., Leibel R. L. (2011) Cut-like homeobox 1 (CUX1) regulates expression of the fat mass and obesity-associated and retinitis pigmentosa GTPase regulator-interacting protein-1-like (RPGRIP1L) genes and coordinates leptin receptor signaling. J. Biol. Chem. 286, 2155–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stratigopoulos G., Padilla S. L., LeDuc C. A., Watson E., Hattersley A. T., McCarthy M. I., Zeltser L. M., Chung W. K., Leibel R. L. (2008) Regulation of Fto/Ftm gene expression in mice and humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R1185–R1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stratigopoulos G., Burnett L. C., Rausch R., Gill R., Penn D. B., Skowronski A. A., LeDuc C. A., Lanzano A. J., Zhang P., Storm D. R., Egli D., Leibel R. L. (2016) Hypomorphism of Fto and Rpgrip1l causes obesity in mice. J. Clin. Invest. 126, 1897–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vierkotten J., Dildrop R., Peters T., Wang B., Rüther U. (2007) Ftm is a novel basal body protein of cilia involved in Shh signalling. Development 134, 2569–2577 [DOI] [PubMed] [Google Scholar]
- 18.Vaisse C., Reiter J. F., Berbari N. F. (2017) Cilia and obesity. Cold Spring Harb. Perspect. Biol. 9, a028217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mariman E. C., Vink R. G., Roumans N. J., Bouwman F. G., Stumpel C. T., Aller E. E., van Baak M. A., Wang P. (2016) The cilium: a cellular antenna with an influence on obesity risk. Br. J. Nutr. 116, 576–592 [DOI] [PubMed] [Google Scholar]
- 20.Stratigopoulos G., Martin Carli J. F., O’Day D. R., Wang L., Leduc C. A., Lanzano P., Chung W. K., Rosenbaum M., Egli D., Doherty D. A., Leibel R. L. (2014) Hypomorphism for RPGRIP1L, a ciliary gene vicinal to the FTO locus, causes increased adiposity in mice. Cell Metab. 19, 767–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen V. L., Li C., Bowie R. V., Clarke L., Mohan S., Blacque O. E., Leroux M. R. (2015) Formation of the transition zone by Mks5/Rpgrip1L establishes a ciliary zone of exclusion (CIZE) that compartmentalises ciliary signalling proteins and controls PIP2 ciliary abundance. EMBO J. 34, 2537–2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams C. L., Li C., Kida K., Inglis P. N., Mohan S., Semenec L., Bialas N. J., Stupay R. M., Chen N., Blacque O. E., Yoder B. K., Leroux M. R. (2011) MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J. Cell Biol. 192, 1023–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aksanov O., Green P., Birk R. Z. (2014) BBS4 directly affects proliferation and differentiation of adipocytes. Cell. Mol. Life Sci. 71, 3381–3392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marion V., Mockel A., De Melo C., Obringer C., Claussmann A., Simon A., Messaddeq N., Durand M., Dupuis L., Loeffler J. P., King P., Mutter-Schmidt C., Petrovsky N., Stoetzel C., Dollfus H. (2012) BBS-induced ciliary defect enhances adipogenesis, causing paradoxical higher-insulin sensitivity, glucose usage, and decreased inflammatory response. Cell Metab. 16, 363–377 [DOI] [PubMed] [Google Scholar]
- 25.Marion V., Stoetzel C., Schlicht D., Messaddeq N., Koch M., Flori E., Danse J. M., Mandel J. L., Dollfus H. (2009) Transient ciliogenesis involving Bardet-Biedl syndrome proteins is a fundamental characteristic of adipogenic differentiation. Proc. Natl. Acad. Sci. USA 106, 1820–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu D., Shi S., Wang H., Liao K. (2009) Growth arrest induces primary-cilium formation and sensitizes IGF-1-receptor signaling during differentiation induction of 3T3-L1 preadipocytes. J. Cell Sci. 122, 2760–2768 [DOI] [PubMed] [Google Scholar]
- 27.Forcioli-Conti N., Lacas-Gervais S., Dani C., Peraldi P. (2015) The primary cilium undergoes dynamic size modifications during adipocyte differentiation of human adipose stem cells. Biochem. Biophys. Res. Commun. 458, 117–122 [DOI] [PubMed] [Google Scholar]
- 28.Forcioli-Conti N., Estève D., Bouloumié A., Dani C., Peraldi P. (2016) The size of the primary cilium and acetylated tubulin are modulated during adipocyte differentiation: analysis of HDAC6 functions in these processes. Biochimie 124, 112–123 [DOI] [PubMed] [Google Scholar]
- 29.Dalbay M. T., Thorpe S. D., Connelly J. T., Chapple J. P., Knight M. M. (2015) Adipogenic differentiation of hMSCs is mediated by recruitment of IGF-1r onto the primary cilium associated with cilia elongation. Stem Cells 33, 1952–1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reed B. C., Lane M. D. (1980) Insulin receptor synthesis and turnover in differentiating 3T3-L1 preadipocytes. Proc. Natl. Acad. Sci. USA 77, 285–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green H., Kehinde O. (1975) An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell 5, 19–27 [DOI] [PubMed] [Google Scholar]
- 32.Rubin C. S., Hirsch A., Fung C., Rosen O. M. (1978) Development of hormone receptors and hormonal responsiveness in vitro. Insulin receptors and insulin sensitivity in the preadipocyte and adipocyte forms of 3T3-L1 cells. J. Biol. Chem. 253, 7570–7578 [PubMed] [Google Scholar]
- 33.Russell T. R., Ho R. (1976) Conversion of 3T3 fibroblasts into adipose cells: triggering of differentiation by prostaglandin F2alpha and 1-methyl-3-isobutyl xanthine. Proc. Natl. Acad. Sci. USA 73, 4516–4520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang Z. Y., Zhou Q. L., Coleman K. A., Chouinard M., Boese Q., Czech M. P. (2003) Insulin signaling through Akt/protein kinase B analyzed by small interfering RNA-mediated gene silencing. Proc. Natl. Acad. Sci. USA 100, 7569–7574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okada S., Mori M., Pessin J. E. (2003) Introduction of DNA into 3T3-L1 adipocytes by electroporation. Methods Mol. Med. 83, 93–96 [DOI] [PubMed] [Google Scholar]
- 36.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., Tinevez J. Y., White D. J., Hartenstein V., Eliceiri K., Tomancak P., Cardona A. (2012) Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Church C. D., Berry R., Rodeheffer M. S. (2014) Isolation and study of adipocyte precursors. Methods Enzymol. 537, 31–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smyth M. J., Wharton W. (1992) Differentiation of A31T6 proadipocytes to adipocytes: a flow cytometric analysis. Exp. Cell Res. 199, 29–38 [DOI] [PubMed] [Google Scholar]
- 39.Galarraga M., Campión J., Muñoz-Barrutia A., Boqué N., Moreno H., Martínez J. A., Milagro F., Ortiz-de-Solórzano C. (2012) Adiposoft: automated software for the analysis of white adipose tissue cellularity in histological sections. J. Lipid Res. 53, 2791–2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin Y. W., Yan M. D., Shih Y. L., Hsieh C. B. (2009) The basal body gene, RPGRIP1L, is a candidate tumour suppressor gene in human hepatocellular carcinoma. Eur. J. Cancer 45, 2041–2049 [DOI] [PubMed] [Google Scholar]
- 41.Spinella-Jaegle S., Rawadi G., Kawai S., Gallea S., Faucheu C., Mollat P., Courtois B., Bergaud B., Ramez V., Blanchet A. M., Adelmant G., Baron R., Roman-Roman S. (2001) Sonic hedgehog increases the commitment of pluripotent mesenchymal cells into the osteoblastic lineage and abolishes adipocytic differentiation. J. Cell Sci. 114, 2085–2094 [DOI] [PubMed] [Google Scholar]
- 42.Fontaine C., Cousin W., Plaisant M., Dani C., Peraldi P. (2008) Hedgehog signaling alters adipocyte maturation of human mesenchymal stem cells. Stem Cells 26, 1037–1046 [DOI] [PubMed] [Google Scholar]
- 43.Suh J. M., Gao X., McKay J., McKay R., Salo Z., Graff J. M. (2006) Hedgehog signaling plays a conserved role in inhibiting fat formation. Cell Metab. 3, 25–34 [DOI] [PubMed] [Google Scholar]
- 44.Gerhardt C., Lier J. M., Burmühl S., Struchtrup A., Deutschmann K., Vetter M., Leu T., Reeg S., Grune T., Rüther U. (2015) The transition zone protein Rpgrip1l regulates proteasomal activity at the primary cilium. J. Cell Biol. 210, 115–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eguchi J., Wang X., Yu S., Kershaw E. E., Chiu P. C., Dushay J., Estall J. L., Klein U., Maratos-Flier E., Rosen E. D. (2011) Transcriptional control of adipose lipid handling by IRF4. Cell Metab. 13, 249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee K. Y., Russell S. J., Ussar S., Boucher J., Vernochet C., Mori M. A., Smyth G., Rourk M., Cederquist C., Rosen E. D., Kahn B. B., Kahn C. R. (2013) Lessons on conditional gene targeting in mouse adipose tissue. Diabetes 62, 864–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Satir P., Christensen S. T. (2007) Overview of structure and function of mammalian cilia. Annu. Rev. Physiol. 69, 377–400 [DOI] [PubMed] [Google Scholar]
- 48.Shi X., Garcia G., III, Van De Weghe J. C., McGorty R., Pazour G. J., Doherty D., Huang B., Reiter J. F. (2017) Super-resolution microscopy reveals that disruption of ciliary transition-zone architecture causes Joubert syndrome. Nat. Cell Biol. 19, 1178–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li C., Jensen V. L., Park K., Kennedy J., Garcia-Gonzalo F. R., Romani M., De Mori R., Bruel A. L., Gaillard D., Doray B., Lopez E., Rivière J. B., Faivre L., Thauvin-Robinet C., Reiter J. F., Blacque O. E., Valente E. M., Leroux M. R. (2016) MKS5 and CEP290 dependent assembly pathway of the ciliary transition zone. PLoS Biol. 14, e1002416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goetz S. C., Anderson K. V. (2010) The primary cilium: a signalling centre during vertebrate development. Nat. Rev. Genet. 11, 331–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arts H. H., Doherty D., van Beersum S. E., Parisi M. A., Letteboer S. J., Gorden N. T., Peters T. A., Märker T., Voesenek K., Kartono A., Ozyurek H., Farin F. M., Kroes H. Y., Wolfrum U., Brunner H. G., Cremers F. P., Glass I. A., Knoers N. V., Roepman R. (2007) Mutations in the gene encoding the basal body protein RPGRIP1L, a nephrocystin-4 interactor, cause Joubert syndrome. Nat. Genet. 39, 882–888 [DOI] [PubMed] [Google Scholar]
- 52.Delous M., Baala L., Salomon R., Laclef C., Vierkotten J., Tory K., Golzio C., Lacoste T., Besse L., Ozilou C., Moutkine I., Hellman N. E., Anselme I., Silbermann F., Vesque C., Gerhardt C., Rattenberry E., Wolf M. T., Gubler M. C., Martinovic J., Encha-Razavi F., Boddaert N., Gonzales M., Macher M. A., Nivet H., Champion G., Berthélémé J. P., Niaudet P., McDonald F., Hildebrandt F., Johnson C. A., Vekemans M., Antignac C., Rüther U., Schneider-Maunoury S., Attié-Bitach T., Saunier S. (2007) The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat. Genet. 39, 875–881 [DOI] [PubMed] [Google Scholar]
- 53.Sherafat-Kazemzadeh R., Ivey L., Kahn S. R., Sapp J. C., Hicks M. D., Kim R. C., Krause A. J., Shomaker L. B., Biesecker L. G., Han J. C., Yanovski J. A. (2013) Hyperphagia among patients with Bardet-Biedl syndrome. Pediatr. Obes. 8, e64–e67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oh E. C., Vasanth S., Katsanis N. (2015) Metabolic regulation and energy homeostasis through the primary Cilium. Cell Metab. 21, 21–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pazour G. J., Dickert B. L., Vucica Y., Seeley E. S., Rosenbaum J. L., Witman G. B., Cole D. G. (2000) Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J. Cell Biol. 151, 709–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takeda S., Yonekawa Y., Tanaka Y., Okada Y., Nonaka S., Hirokawa N. (1999) Left-right asymmetry and kinesin superfamily protein KIF3A: new insights in determination of laterality and mesoderm induction by kif3A-/- mice analysis. J. Cell Biol. 145, 825–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marszalek J. R., Ruiz-Lozano P., Roberts E., Chien K. R., Goldstein L. S. (1999) Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc. Natl. Acad. Sci. USA 96, 5043–5048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Graser S., Stierhof Y. D., Lavoie S. B., Gassner O. S., Lamla S., Le Clech M., Nigg E. A. (2007) Cep164, a novel centriole appendage protein required for primary cilium formation. J. Cell Biol. 179, 321–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li G., Vega R., Nelms K., Gekakis N., Goodnow C., McNamara P., Wu H., Hong N. A., Glynne R. (2007) A role for Alström syndrome protein, alms1, in kidney ciliogenesis and cellular quiescence. PLoS Genet. 3, e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang-Doran I., Semple R. K. (2010) Knockdown of the Alström syndrome-associated gene Alms1 in 3T3-L1 preadipocytes impairs adipogenesis but has no effect on cell-autonomous insulin action. Int. J. Obes. 34, 1554–1558 [DOI] [PubMed] [Google Scholar]
- 61.Murcia N. S., Richards W. G., Yoder B. K., Mucenski M. L., Dunlap J. R., Woychik R. P. (2000) The oak ridge polycystic kidney (orpk) disease gene is required for left-right axis determination. Development 127, 2347–2355 [DOI] [PubMed] [Google Scholar]
- 62.Davenport J. R., Watts A. J., Roper V. C., Croyle M. J., van Groen T., Wyss J. M., Nagy T. R., Kesterson R. A., Yoder B. K. (2007) Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr. Biol. 17, 1586–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scherer P. E., Williams S., Fogliano M., Baldini G., Lodish H. F. (1995) A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 270, 26746–26749 [DOI] [PubMed] [Google Scholar]
- 64.Cousin W., Dani C., Peraldi P. (2006) Inhibition of the anti-adipogenic Hedgehog signaling pathway by cyclopamine does not trigger adipocyte differentiation. Biochem. Biophys. Res. Commun. 349, 799–803 [DOI] [PubMed] [Google Scholar]
- 65.James A. W., Leucht P., Levi B., Carre A. L., Xu Y., Helms J. A., Longaker M. T. (2010) Sonic Hedgehog influences the balance of osteogenesis and adipogenesis in mouse adipose-derived stromal cells. Tissue Eng. Part A 16, 2605–2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schneider L., Clement C. A., Teilmann S. C., Pazour G. J., Hoffmann E. K., Satir P., Christensen S. T. (2005) PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr. Biol. 15, 1861–1866 [DOI] [PubMed] [Google Scholar]
- 67.Sun C., Berry W. L., Olson L. E. (2017) PDGFRα controls the balance of stromal and adipogenic cells during adipose tissue organogenesis. Development 144, 83–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gerdes J. M., Christou-Savina S., Xiong Y., Moede T., Moruzzi N., Karlsson-Edlund P., Leibiger B., Leibiger I. B., Östenson C. G., Beales P. L., Berggren P. O. (2014) Ciliary dysfunction impairs beta-cell insulin secretion and promotes development of type 2 diabetes in rodents. Nat. Commun. 5, 5308 [DOI] [PubMed] [Google Scholar]
- 69.Christensen S. T., Morthorst S. K., Mogensen J. B., Pedersen L. B. (2017) Primary cilia and coordination of receptor tyrosine kinase (RTK) and transforming growth factor β (TGF-β) signaling. Cold Spring Harb. Perspect. Biol. 9, a028167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mahuzier A., Gaudé H. M., Grampa V., Anselme I., Silbermann F., Leroux-Berger M., Delacour D., Ezan J., Montcouquiol M., Saunier S., Schneider-Maunoury S., Vesque C. (2012) Dishevelled stabilization by the ciliopathy protein Rpgrip1l is essential for planar cell polarity. J. Cell Biol. 198, 927–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gerhardt C., Lier J. M., Kuschel S., Rüther U. (2013) The ciliary protein Ftm is required for ventricular wall and septal development. PLoS One 8, e57545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liebelt R. A. (1963) Response of adipose tissue in experimental obesity as influenced by genetic, hormonal, and neurogenic factors. Ann. N. Y. Acad. Sci. 110, 723–748 [DOI] [PubMed] [Google Scholar]
- 73.Jeffery E., Church C. D., Holtrup B., Colman L., Rodeheffer M. S. (2015) Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat. Cell Biol. 17, 376–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim J. Y., van de Wall E., Laplante M., Azzara A., Trujillo M. E., Hofmann S. M., Schraw T., Durand J. L., Li H., Li G., Jelicks L. A., Mehler M. F., Hui D. Y., Deshaies Y., Shulman G. I., Schwartz G. J., Scherer P. E. (2007) Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Invest. 117, 2621–2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Berry R., Jeffery E., Rodeheffer M. S. (2014) Weighing in on adipocyte precursors. Cell Metab. 19, 8–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krueger K. C., Costa M. J., Du H., Feldman B. J. (2014) Characterization of Cre recombinase activity for in vivo targeting of adipocyte precursor cells. Stem Cell Reports 3, 1147–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Logan M., Martin J. F., Nagy A., Lobe C., Olson E. N., Tabin C. J. (2002) Expression of Cre recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis 33, 77–80 [DOI] [PubMed] [Google Scholar]
- 78.Soriano P. (1997) The PDGF alpha receptor is required for neural crest cell development and for normal patterning of the somites. Development 124, 2691–2700 [DOI] [PubMed] [Google Scholar]
- 79.Ravussin Y., Leibel R. L., Ferrante A. W., Jr (2014) A missing link in body weight homeostasis: the catabolic signal of the overfed state. Cell Metab. 20, 565–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang Y., Leibel R. L. (2017) Leptin and body weight. In Eating Disorders and Obesity A Comprehensive Handbook, 3rd ed., (Brownell K. D., and Walsh B. T., eds.), Guilford Publications, New York [Google Scholar]
- 81.Slieker L. J., Sloop K. W., Surface P. L. (1998) Differentiation method-dependent expression of leptin in adipocyte cell lines. Biochem. Biophys. Res. Commun. 251, 225–229 [DOI] [PubMed] [Google Scholar]
- 82.Russell C. D., Petersen R. N., Rao S. P., Ricci M. R., Prasad A., Zhang Y., Brolin R. E., Fried S. K. (1998) Leptin expression in adipose tissue from obese humans: depot-specific regulation by insulin and dexamethasone. Am. J. Physiol. 275, E507–E515 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.