Abstract
The axonal guidance proteins semaphorin (Sema)4D and Sema3A play important roles in communication between osteoclasts and osteoblasts. As stimulation of adenosine A2A receptors (A2AR) regulates both osteoclast and osteoblast function, we asked whether A2AR regulates both osteoclast and osteoblast expression of Semas. In vivo bone formation and Sema3A/PlexinA1/Neuropilin-1, Sema4D/PlexinB1 protein expression were studied in a murine model of wear particle-induced osteolysis. Osteoclast/osteoblast differentiation were studied in vitro as the number of tartrate-resistant acid phosphatase+/Alizarin Red+ cells after challenge with CGS21680 (A2AR agonist, 1 µM) or ZM241385 (A2AR antagonist, 1 µM), with or without Sema4D or Sema3A (10 ng/ml). Sema3A/PlexinA1/Neuropilin-1, Sema4D/PlexinB1, and receptor activator of NF-κB ligand/osteoprotegerin (RANKL/OPG) expression was studied by RT-PCR and Western blot. β-Catenin activation and cytoskeleton changes were studied by fluorescence microscopy and Western blot. In mice with wear particles implanted over the calvaria, CGS21680 treatment increased bone formation in vivo, reduced Sema4D, and increased Sema3A expression compared with mice with wear particle-induced osteolysis treated with vehicle alone. During osteoclast differentiation, CGS21680 abrogated RANKL-induced Sema4D mRNA expression (1.3 ± 0.3- vs. 2.5 ± 0.1-fold change, P < 0.001, n = 4). PlexinA1, but not Neuropilin-1, mRNA was enhanced by CGS21680 treatment. CGS21680 enhanced Sema3A mRNA expression during osteoblast differentiation (8.7 ± 0.2-fold increase, P < 0.001, n = 4); PlexinB1 mRNA was increased 2-fold during osteoblast differentiation and was not altered by CGS21680. Similar changes were observed at the protein level. CGS21680 decreased RANKL, increased OPG, and increased total/nuclear β-catenin expression in osteoblasts. Sema4D increased Ras homolog gene family, member A phosphorylation and focal adhesion kinase activation in osteoclast precursors, and CGS21680 abrogated these effects. In summary, A2AR activation diminishes secretion of Sema4D by osteoclasts, inhibits Sema4D-mediated osteoclast activation, and enhances secretion of Sema3A by osteoblasts, increasing osteoblast differentiation and diminishing inflammatory osteolysis.—Mediero, A., Wilder, T., Shah, L., Cronstein, B. N. Adenosine A2A receptor (A2AR) stimulation modulates expression of semaphorins 4D and 3A, regulators of bone homeostasis.
Keywords: Sema4D, Sema3A, cell communication, RhoA, β-catenin
Communication between osteoclasts and osteoblasts plays a critical role in bone homeostasis (1, 2). Among the signals sent between osteoclasts and osteoblasts are members of the family of axonal guidance proteins, including neurotrophins, semaphorins (Semas), eph/ephorins, and netrins and their receptors, which mediate regulation of osteoblasts by osteoclasts and vice versa (3–5). Semas include both secreted and membrane-associated molecules that were first discovered as regulators of the axonal growth cone. Subsequent to their discovery as axonal guidance proteins, they have now been shown to participate in regulation in a number of different functions. Plexins are the receptors for membrane-associated Semas, and Neuropilins are required coreceptors for class III Semas (6–8). Sema4D is expressed and secreted by osteoclasts, and in contrast, Sema3A is produced by osteoblasts (9), and these molecules clearly play an important role in the regulation of bone homeostasis.
Sema4D was first identified as a regulator of immune response when it was shown to mediate cell–cell interactions in the immune synapse, as well as regulation of the immune response (10). Interestingly, the Sema4D homodimer has strong similarities with the αVβ3 integrin heterodimer, which is the most abundant integrin receptor expressed in bone-resorbing osteoclasts (11). Sema4D is secreted by osteoclasts in the presence of receptor activator of NF-κB ligand (RANKL) and binds to its receptor PlexinB1 on osteoblasts to inhibit osteoblast differentiation and function by activating Ras homolog gene family, member A (RhoA)-Rho kinase (ROCK), which inhibits IGF-1 signaling (12–14), thereby inhibiting osteoblast differentiation induced by the RhoA pathway (12, 15). In addition, it can downregulate TGF-β1 to inhibit the function of osteoblasts (16). Mice lacking Sema4D and Plexin1 have high bone mass as a result of increased bone formation (12, 17). The silencing of Sema4D expression in osteoclasts produces an increase in alkaline phosphatase (ALP) activity and other differentiation markers in osteoblasts in vivo (18).
Sema3A, originally identified as a diffusible axonal chemorepellent that modulates axon guidance and growth (19), is produced by osteoblasts and binds to PlexinA1/Neuropilin-1 to inhibit both RANKL-induced osteoclast differentiation by inhibition of immunoreceptor tyrosine-based activation motif and RhoA signaling (14, 20) and to stimulate osteoblast differentiation and function (12, 20–22). By binding to Neuropilin-1, Sema3A activates canonical Wnt/β-catenin signaling on mesenchymal precursors to stimulate osteoblast differentiation and inhibit adipocyte formation (14). In murine models of cortical bone defects, treatment with Sema3A inhibits bone resorption and increases bone formation (20). It has been observed that Sema3A is critical for bone and cartilage development (21) and osteointegration (23–25). Moreover, Sema3A participates in the invasion of the bone by blood vessels and nerve fibers, and this molecule and its receptors can be found in hypertrophic chondrocytes in ossification centers (21).
Adenosine, a nucleoside released at sites of injury and hypoxia, modulates cell function via specific cell-surface receptors [adenosine A1, A2A, A2B, A3 receptors (A1R, A2AR, A2BR, A3R)] (1). Prior studies show that A2AR activation suppresses osteoclast formation in vitro via PKA-dependent inhibition of NF-κB nuclear translocation and reduces wear particle-induced inflammation (26–29). Activation of A2AR increases osteoprotegerin (OPG) levels (a decoy receptor for RANKL) and decreases levels of RANKL, M-CSF, IL-1β, and TNF-α (29, 30). In a murine model of wear particle-induced bone resorption, activation of the A2AR reduced particle-induced bone pitting and porosity and increased cortical bone and bone volume compared with vehicle-treated mice, concomitant with reduced levels of bone resorption markers (RANK, RANKL, cathepsin K, CD163, and osteopontin) after A2AR activation (26). Moreover, A2AR activation increased secretion of the potent anti-inflammatory cytokine IL-10 with reduction of proinflammatory cytokines IL-1β and TNF-α (26). Activation of A2AR plays a critical role in promoting the proliferation of mouse bone marrow-derived fibroblast-like mesenchymal stem cells, and A2AR is upregulated in later osteoblast differentiation stages (31, 32). We have demonstrated a direct effect of A2AR activation on bone regeneration in vivo following either direct activation of the receptor or by enhancing extracellular adenosine levels by blockade of equilibrative nucleoside transporter 1 (27, 30).
Therefore, we hypothesized that A2ARs regulate the production of signals between osteoclasts and osteoblasts that regulate bone formation in vivo and therefore, sought to determine the effect of A2AR stimulation on production of Semas involved in the intercellular communication involved in bone homeostasis. Here, we report that A2AR stimulation increases SEMA3A production by osteoblasts and reduces SEMA4D production by osteoclasts.
MATERIALS AND METHODS
Reagents
Recombinant mouse M-CSF, RANKL, and Sema3A and Sema4D were from R&D Systems (Minneapolis, MN, USA). α-minimum essential medium (α-MEM), fetal bovine serum, penicillin/streptomycin, Alexa Fluor 555, sodium acetate, glacial acetic acid, Naphthol AS-MX phosphate disodium salt, Fast Red Violet LB, sodium tartrate, Alizarin Red, Toluidine blue, RIPA buffer, protease inhibitor cocktail, phosphatase inhibitor cocktail, dexamethasone, β-glycerophosphate, and L-ascorbic acid were from Thermo Fisher Scientific (Waltham, MA, USA). Phosphorylated RhoA (pRhoA), RhoA, focal adhesion kinase (FAK), cdc42, goat anti-rabbit-horseradish peroxidase (HRP), and goat anti-mouse-HRP were from Santa Cruz Biotechnology (Dallas, TX, USA). Sodium tartrate was from Thermo Fisher Scientific. Primary antibodies against Sema4D, Sema3A, PlexinB1, PlexinA1, Neuropilin-1, and FAK were from Abcam (Cambridge, United Kingdom). β-Catenin antibodies were from Cell Signaling Technology (Danvers, MA, USA). Goat anti-rabbit IRDye 800CW and goat anti-mouse IRDye 680 RD were from Li-Cor Biosciences (Lincoln, NE, USA). CGS21680 and ZM241385 were from Tocris Bioscience (Bristol, United Kingdom).
Osteoclast differentiation
Bone marrow cells (BMCs) were isolated from 6 to 8-wk-old female C57BL/6 mice, as previously described (27). In brief, the marrow cavity was flushed out with α-MEM from aseptically removed femora and tibiae, and marrow was incubated overnight in α-MEM containing 10% fetal bovine serum and 1% penicillin/streptomycin to obtain a single-cell suspension. Two hundred thousand nonadherent cells were collected and seeded in α-MEM with 30 ng/ml M-CSF (R&D Systems) for 2 d. At d 3 (d 0 of differentiation), 30 ng/ml RANKL (R&D Systems) was added to cultures in the presence/absence of CGS21680 (A2AR agonist) 1 µM and ZM241385 (A2AR antagonist) 1 µM, together with recombinant Sema4D or Sema3A 10 ng/ml each, alone or in the presence of their correspondence antibodies (0.5 µg/ml; n = 6). Medium and reagents were replaced every 3rd d. After incubation for 7 d, cells were stained for tartrate-resistant acid phosphatase (TRAP) for osteoclast quantification, as previously described (26, 27). The number of TRAP-positive multinucleated cells containing ≥3 nuclei/cell was scored (28).
To assay resorption activity, 200,000 nonadherent cells were collected and seeded on dentin slides (Immunodiagnostic Systems, Scottsdale, AZ, USA) and were treated in the same conditions as described for TRAP staining. After 7 d of differentiation, resorption was assayed by staining the dentin slides with 1% Toluidine blue in 0.5% sodium tetraborate solution, following the manufacturer’s recommendations. The pits developed blue to purple color.
Morphologic characterization of cultured osteoclasts
Osteoclasts were generated from BMCs, as previously described. Seven thousand five hundred cells per milliliter were plated on fibronectin-coated glass coverslips under the same conditions as described above. After 7 d in culture, cells were fixed with 4% paraformaldehyde in PBS, blocked with PBS 1% bovine serum albumin (BSA) and 0.1% Triton X-100 for 30 min, stained fluorescently with Alexa Fluor 555 phalloidin (Thermo Fisher Scientific) for 30 min, and counterstained with DAPI (Fluoroshield with DAPI mounting medium; Sigma-Aldrich, St. Louis, MO, USA), as previously described (33). To evaluate osteoclast morphology, 400 osteoclasts were examined in each sample using confocal microscopy (Leica SP5 Confocal System; Leica Microsystems, Buffalo Grove, IL, USA).
Osteogenesis assay
Osteogenesis assays were performed as previously described (1). BMCs were isolated by flushing out the bone marrow cavity from 6 to 8-wk-old wild-type female mice. BMCs were cultured for 3 d, nonadherent cells were discarded, and adherent cells were cultured until confluent. Stromal cells were washed and reseeded in culture dishes at 1 × 105 cell/cm2 density with osteogenic medium (α-MEM containing 1 µM dexamethasone, 50 µg/ml ascorbic acid, and 10 mM β-glycerophosphate) in the presence/absence of CGS21680 1 µM and ZM241385 1 µM, together with recombinant Sema4D or Sema3A 10 ng/ml each alone or in the presence of their correspondence antibodies (0.5 µg/ml; n = 6). Ten days after culture beginning, cells were fixed in 4% paraformaldehyde and stained for 45 min with 2% Alizarin Red.
Western blot
Primary murine BMCs were treated with CGS21680 1 µM and ZM241385 1 µM in the presence/absence of recombinant Sema4D or Sema3A 10 ng/ml for 15 min or 24 h (n = 5 each). Cells were lysed with RIPA buffer, and the supernatant contents were extracted with cold ethanol–1% SDS and sonicated. Cytoplasmic and nuclear fraction protein extraction was performed using NE-PER Nuclear and Cytoplasmic Extraction Reagents Kit. Protein concentration was determined by BCA. Four to 10 mg protein was subjected to 7.5 or 10% SDS-PAGE and transferred to a nitrocellulose membrane. Nonspecific binding was blocked with Tris-buffered saline/Tween-20 0.05–5% skimmed milk. Membranes were incubated overnight (4°C) with primary antibodies against Sema4D, Sema3A, PlexinB1, PlexinA1, Neuropilin-1, pRhoA, RhoA, FAK, Rac1, cdc42, β-catenin total, β-catenin nonphosphorylated, pβ-catenin (Ser522), and actin (1:1000 each). Membranes were incubated with goat anti-rabbit IRDye 800CW 1:10,000 and goat anti-mouse IRDye 680 RD 1:10,000 (Li-Cor Biosciences) in the dark. Proteins were visualized by Li-Cor Biosciences Odyssey equipment, which detects near-infrared fluorescence. As each secondary antibody emits a signal in a different spectrum, reprobing with actin (to check that all lanes were loaded with the same amount of protein) was performed simultaneously with primary antibody incubation. Coomassie blue was used as a loading marker for the supernatant proteins. Intensities of the respective band were quantitated by densitometric analysis using Image Studio 2.0.38 software (Li-Cor Biosciences). Variations in band intensity were expressed as percentage of unstimulated controls to minimize disparities among different experiments.
Real-time quantitative RT-PCR
To validate the effect of A2AR in Sema4D and Sema3A expression and activity, primary osteoclast and osteoblast precursors were challenged with CGS21680 1 µM and ZM241385 1 µM for 7 and 10 d, respectively, and mRNA expression of Sema4D/PlexinB1, Sema3A/PlexinA1/Neuropilin-1 was analyzed (n = 4). In other experiments, cells were cultured with CGS21680 1 µM and ZM241385 1 µM in the presence of recombinant Sema4D or Sema3A 10 ng/ml, and Cathepsin K, nuclear factor of activated T cells c1 (NFATc1), osteopontin, RANKL, and OPG were analyzed. Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Germantown, MD, USA), following the manufacturer’s protocol, including sample homogenization with QiaShredder columns (Qiagen) and the on-column DNA digestion step. Total RNA was retrotranscribed using the MuLV Reverse Transcriptase PCR Kit (Applied Biosystems, Foster City, CA, USA) at 2.5 U/μl, including in the same reaction, the following reagents: RNase inhibitor 1 U/μl (Applied Biosystems), random hexamers 2.5 U/μl (Applied Biosystems), MgCl2 5 mM (Applied Biosystems), PCR Buffer II one time (Applied Biosystems), and deoxynucleotides 1 mM (Applied Biosystems). Relative quantification of gene expression was performed using real-time RT-PCR on a Stratagene Mx3005P (Agilent Technologies, La Jolla, CA, USA) with Brilliant SYBR Green Kit QPCR Master Mix (Stratagene; Agilent Technologies), according to the manufacturer’s protocol. The following primers were used in real-time PCR amplification: Cathepsin K forward, 5′-GCTGAACTCAGGACCTCTGG-3′ and reverse, 5′-GAAAAGGGAGGCATGAATGA-3′; NFATc1 forward, 5′-TCATCCTGTCCAACACCAAA-3′ and reverse, 5′-TCACCCTGGTGTTCTTCCTC-3′; osteopontin forward: 5′-TCTGATGAGACCGTCACTGC-3′ and reverse, 5′-TCTCCTGGCTCTCTTTGGAA-3′; RANKL forward, 5′-AGCCGAGACTACGGCAAGTA-3′ and reverse, 5′-GCGCTCGAAAGTACAGGAAC-3′; OPG forward, 5′-CTGCCTGGGAAGAAGATCAG-3′ and reverse, 5′-TTGTGAAGCTGTGCAGGAAC-3′; osteocalcin forward, 5′-AATCCCCTTGGCTTCTGACT-3′ and reverse, 5′-AGCCCTCTGCAGGTCATAGA-3′, Runt-related transcription factor 2 (Runx2) forward, 5′-CCCAGCCACCTTTACCTACA-3′ and reverse, 5′-TATGGAGTGCTGCTGGT-3′; osteonectin forward, 5′-TGGGAGAATTTGAGGACGGTG-3′ and reverse, 5′-GAGTCGAAGGTCTTGTTGTCAT-3′; Sema4D forward, 5′-TCTTTGCTGACGTGATCCAG-3′ and reverse, 5′-CAGATCAGCCTGGCCTTTAG-3′; PlexinB1 forward, 5′-TGGGTCATGTGCAGTACGAT-3′ and reverse, 5′-CACTGCTCTCCAGGTTCTCC-3′; Sema3A forward, 5′-CCTCCCAAAACCTCAAACAA-3′ and reverse, 5′-TGATCTCTGTCAAGCGTTGG-3′; PlexinA1 forward, 5′-CTTCTGGACTGGGCTCTGAC-3′ and reverse, 5′-TAGAGGGTGGCTCTGAGCAT-3′; Neuropilin-1 forward, 5′-GGAGCTACTGGGCTGTGAAG-3′ and reverse, 5′-ACCGTATGTCGGGAACTCTG-3′; and glyceraldehyde 3-phosphate dehydrogenase forward, 5′-CTACACTGAGGACCAGGTTGTCT-3′ and reverse, 5′-GGTCTGGGATGGAAATTGTG-3′. The Pfaffl method (34) was used for relative quantification.
Immunocytochemistry
Osteoblast precursors were plated in chamber slides and treated with CGS21680 1 µM, alone or in the presence of ZM241385 1 µM. After 24 h in culture, cells were fixed with 4% paraformaldehyde in PBS, blocked with PBS 3% BSA and 0.1% Triton X-100 for 30 min, and incubated overnight with anti-total β-catenin, anti-active β-catenin, and anti-pSer522 β-catenin antibodies. Secondary anti-rabbit FITC was incubated for 1 h in the dark, and slides were mounted with Fluoroshield with DAPI mounting medium (Sigma-Aldrich).
Surgical procedure
All protocols followed internationally recognized guidelines and were approved by the New York University School of Medicine Institutional Animal Care and Use Committee. Osteolysis was induced by implantation of ultrahigh molecular weight polyethylene (UHMWPE) particles over the mouse calvaria, prepared as previously described (26). In brief, 6- to 8-wk-old male C57BL/6 mice were anesthetized by intraperitoneal injection of 100 mg/kg ketamine and 10 mg/kg xylazine, and a 1 cm midline sagittal incision was made over the calvaria anterior to the line connecting both ears, as previously described (26). Five mice received no particles (sham-surgery group, untreated mice), and the incision was closed without any further intervention. The remaining animals (n = 10) received 3 mg dried UHMWPE particles. Of these animals, 5 mice received 20 µl of 0.9% saline at the site of surgery, and the rest received 20 µl CGS21680 µM, beginning immediately before surgery and continuing every day until euthanasia (2 wk later). Water and food were given ad libitum until euthanasia. Animals were euthanized after 14 d in a CO2 chamber, and the calvaria were removed, fixed, and prepared for histologic staining.
In vivo bone formation quantification
One week after surgery, 2 nM/150 µl XenoLight RediJect Bone Probe 680 conjugate (Caliper, Newton, MA, USA) was intravenously injected, and in combination with IVIS imaging system (Caliper), skeletal changes were in vivo detected and measured. Fluorescence images were taken with 68 nm excitation peak and 698 nm emission peak. Images were analyzed following the manufacturer’s instructions. Total flux (photons/second) were normalized and expressed as percentage of control to avoid intrinsic changes among animals.
Histological studies
Calvarias were fixed in 4% paraformaldehyde for 48 h, followed by decalcification in 10% EDTA for 4 wk and paraffin embedding (n = 5/treatment). Sections (5 µm) were cut, and hematoxylin and eosin staining was performed.
Immunohistochemistry analysis for Sema4D, PlexinB1, Sema3A, PlexinA1, Neuropilin-1, CD68, and ALP were carried out, as previously described (26). In brief, deparaffinized and hydrated sections were incubated with Proteinase K solution (20 μg/ml in Tris/EDTA buffer, pH 8.0) for 15 min in a water bath at 37°C for antigen retrieval. After blocking of nonspecific binding with PBS 3% BSA and 0.1% Triton X-100 for 1 h, primary antibodies anti-Sema4D, PlexinB1, Sema3A, PlexinA1, Neuropilin-1, anti-CD68, and anti-ALP 1:200 were incubated overnight at 4°C in a humidifying chamber. Secondary antibodies [goat anti-rabbit-FITC (1:200), goat anti-mouse-FITC (1:200), or goat anti-rabbit tetramethylrhodamine (1:200)] were incubated for 1 h in the dark. Slides were mounted with Fluoroshield with DAPI mounting medium (Sigma-Aldrich). For some studies, secondary goat-anti-mouse-HRP or goat anti-rabbit-HRP antibodies 1:200 were used, and sections were developed with Fast 3′3′-Diaminobenzidine (Sigma-Aldrich), counterstained with hematoxylin (Sigma-Aldrich), and slides were mounted using Permount mounting medium (Thermo Fisher Scientific). Images were observed under a fluorescence microscope (Nikon, Tokyo, Japan), equipped with Nis Elements F3.0 SP7 or Act1 software, and under a Leica microscope, equipped with SlidePath Digital Image Hub v.3.0 software.
Statistical analysis
Statistical significance for differences among groups was determined by use of 1-way ANOVA and Bonferroni post hoc test or Student’s t test, as appropriate. All statistics were calculated using GraphPad software (La Jolla, CA, USA).
RESULTS
Activation of A2AR increases new bone formation and changes SEMA4D and SEMA3A expression at sites of wear particle-induced osteolysis
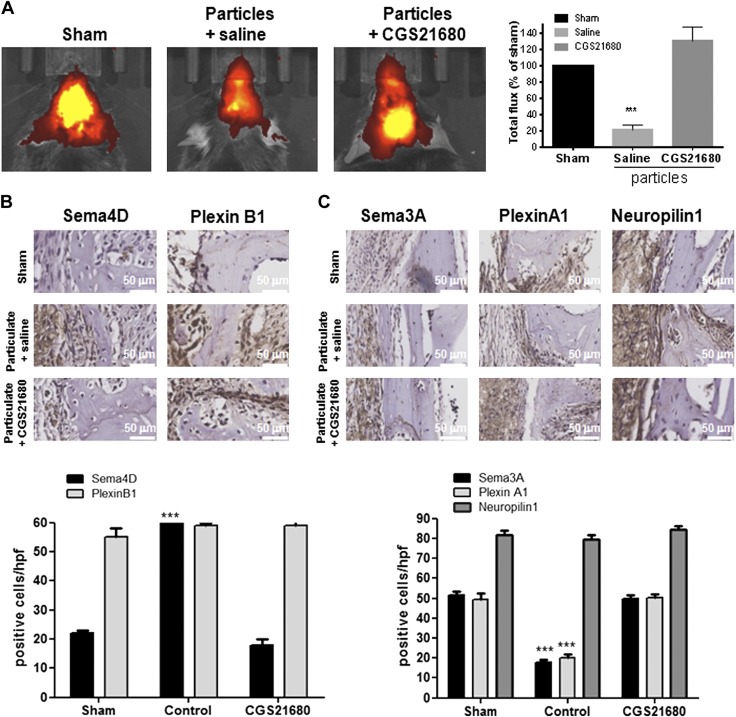
We have previously reported that activation of A2AR reduces wear particle-induced bone pitting and loss and diminishes inflammation and the number of osteoclasts present in bone in a dose-dependent fashion (26). As activation of A2AR by specific agonists or by increasing endogenous adenosine levels stimulates new bone formation, as well as bone morphogenetic protein 2, and represents a novel approach to stimulating bone regeneration (30), we determined whether wear particles had any effect on new bone formation and if activation of A2AR affected bone formation. XenoLight RediJect Bone Probe 680 conjugate targets hydroxyapatite, a biomarker for osteoblast activity, and allows in vivo detection and measurement of skeletal changes (35). Fluorescence analysis of calvariae (depicted as red for low intensity of fluorescence, low volume of new bone formation and yellow for high intensity, high volume of new bone formation) revealed that wear particle exposure decreased bone formation by as much as 79 ± 6% when compared with sham-surgery mice (P < 0.001), and treatment with the selective A2AR agonist CGS21680 restored bone formation (30 ± 18% increase compared with sham group; Fig. 1A).
Figure 1.
Activation of A2AR increases new bone formation at sites of wear particle-induced osteolysis by changes in axon guidance molecule expression. Mice were administered UHMWPE particles and either treated with saline or the A2AR agonist CGS21680. Control mice (sham) were not exposed to particles (n = 5). Mice were injected daily, and after 2 wk, animals were euthanized. A) XenoLight RediJect Bone Probe 680 conjugate was injected intravenously, and the fluorescence image was captured 1 wk after surgery. Total flux in photons per second was normalized and expressed as a percentage of control to avoid intrinsic changes among animals. Red indicates low-signal intensity and low rates of new bone formation, whereas yellow indicates high-signal intensity and high rates of new bone formation. B) Representative images for Sema4D and PlexinB1 immunohistochemistry and quantification of the number of positive cells/hpf. Cells were counted in 5 different images for each of 5 mice. Data are means ± sem. C) Representative images for Sema3A, PlexinA1, and Neuropilin-1 immunohistochemistry and quantification of the number of positive cells/hpf. Cells were counted in 5 different images for each of 5 mice. Data are expressed as means ± sem (n = 5/group). ***P < 0.001 compared with control (ANOVA). Original magnification, ×40. Original scale bars, 50 µm.
The A2AR-mediated change in new bone formation in wear particle-induced osteolysis suggested that adenosine and its receptors affect signals required for bone homeostasis. To test this hypothesis, we analyzed the expression of Sema4D and Sema3A and their receptors in this murine model of inflammatory osteolysis. As shown in Fig. 1B, there is a small number of Sema4D-positive cells in sham-surgery mice, but this number increased in the presence of wear particles [60 ± 1 cells/high-power field (hpf) vs. 22 ± 2 cells/hpf for sham group, P < 0.001], a change completely reversed in mice treated with CGS21680 1 µM (18 ± 3 cells/hpf vs. 22 ± 2 cells/hpf for sham group). There was no change in the number of Plexin B1-positive cells in any of the experimental groups (59 ± 1 cells/hpf for particles, 59 ± 2 for CGS21680 vs. 55 ± 4 cells/hpf for sham group; Fig. 1B). We also found that wear particle exposure diminishes the number of cells expressing Sema3A (19 ± 1 cells/hpf for particles vs. 51 ± 2 cells/hpf for sham group, P < 0.001) and its receptor PlexinA1 when compared with sham-surgery mice (21 ± 1 cells/hpf for particles vs. 48 ± 3 cells/hpf for sham group, P < 0.001), but no changes were observed for Neuropilin-1-positive cells (50 ± 2 cells/hpf for particles vs. 51 ± 2 cells/hpf for sham group). Treatment with CGS21680 1 µM also reversed the effects of wear particle exposure on the number of cells expressing Sema3A (47 ± 1 cells/hpf for particles vs. 51 ± 2 cells/hpf for sham group) and PlexinA1 (48 ± 2 cells/hpf for particles vs. 48 ± 3 cells/hpf for sham group; Fig. 1C).
To corroborate which cells were positive for both Sema4D and Sema3A, double-labeling was performed (Supplemental Fig. 1). We did not find colocalization of Sema4D and Sema3A in the calvaria of mice with implanted wear particles, whether treated with CGS21680 or ZM241385, but we found colocalization between Sema4D and CD68 (macrophages) and between Sema3A and ALP (osteoblast; Supplemental Fig. 1).
Osteoclast Sema4D and osteoblast Sema3A expression/secretion are modified by A2AR activation
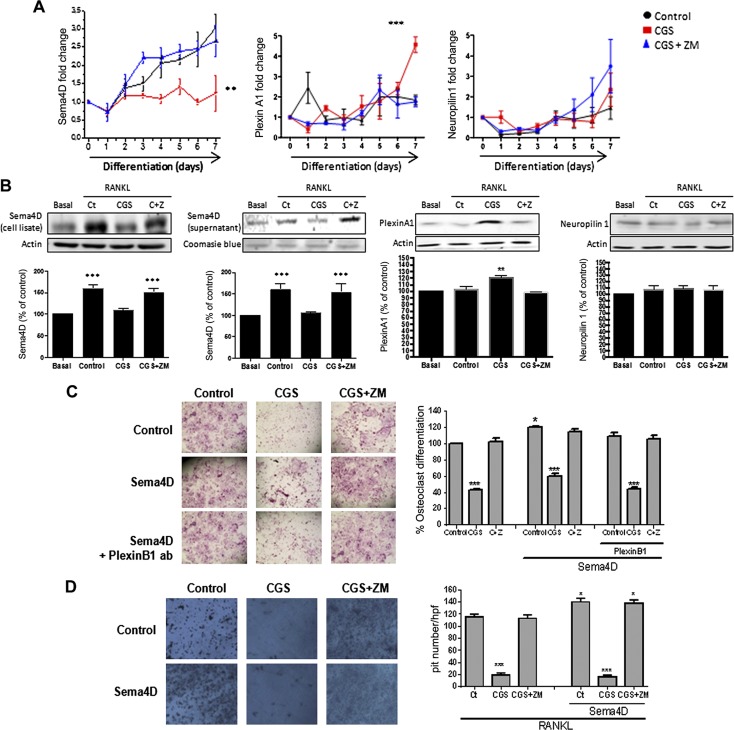
The results described above suggested that there might be a crosstalk between A2AR activation and expression of Sema4D and Sema3A, critical regulators of bone homeostasis. To test this hypothesis, expression of Sema4D/PlexinB1 and Sema3A/PlexinA1/Neuropilin-1 was analyzed both at the mRNA and protein level during osteoclast and osteoblast differentiation in the presence/absence of CGS21680 and ZM241385, a selective A2AR antagonist (Figs. 2 and 3). Sema4D mRNA expression increased during osteoclast differentiation in primary hematopoietic precursors in the presence of 30 ng/ml RANKL alone, but this increase was blocked by treatment of cells with CGS21680 alone (1 µM; Fig. 2A), a change completely reversed by the A2AR antagonist ZM241385 1 µM (up to 2.5-fold change, P < 0.01). The presence of 30 ng/ml RANKL alone or in the presence of ZM241385 1 µM and increased PlexinA1 mRNA expression and treatment with CGS21680 1 µM enhanced this expression (4.5 ± 0.4-fold increase compared with 1.75 ± 0.2 for RANKL, P < 0.001). Neuropilin-1 mRNA was increased during osteoclast differentiation, but no changes were observed in cells treated with CGS21680 or ZM241385 treatments (2.1 ± 1-fold increase for CGS21680 and 3.3 ± 0.6 for ZM241385 compared with 1.5 ± 0.5 for RANKL; Fig. 2A). Similar results were found at the protein level. As with mRNA, Sema4D protein expression and secretion were increased 24 h after RANKL exposure during osteoclast differentiation, and A2AR stimulation by CGS21680 (1 µM) blocked this increase, an effect completely reversed by ZM241385 (1 µM, 60 ± 4% increased vs. basal, P < 0.001; Fig. 2B). PlexinA1 expression was not increased 24 h after RANKL stimulation, but its expression was enhanced in the presence of CGS21680 (6 ± 3% increased for control and 20 ± 3% increased for CGS21680 vs. basal). ZM241385 treatment blocked the CGS21680-mediated increase. Neuropilin-1 expression was not changed by any treatment (Fig. 2B).
Figure 2.
Osteoclast molecules and receptor expression/secretion are modified by A2AR activation. A) Fold change in Sema4D, PlexinA1, and Neuropilin-1 mRNA during osteoclast differentiation in murine BMCs in the presence of CGS21680 (CGS) alone or in combination with ZM241385 (ZM). B) Protein expression for Sema4D, PlexinA1, and Neuropilin-1 after challenge in the presence of CGS21680 alone (CGS) or in combination with ZM241385 (C+Z). C) Wild-type mice osteoclast primary culture cells were fixed and stained for TRAP after being cultured for 7 d in the presence of recombinant Sema4D, alone or combined with PlexinB1 antibody, and in combination with CGS21680 ± ZM241385. TRAP-positive cells containing 3 or more nuclei were counted as osteoclasts. D) Toluidine blue staining to assay osteoclast activity. Nonadherent cells were seeded in dentin slides and treated for 7 d in the presence of recombinant Sema4D, alone or combined with CGS21680 ± ZM241385. Data are expressed as means ± sem. ***P < 0.001, **P < 0.01, *P > 0.05 vs. nonstimulated control (Ct; ANOVA).
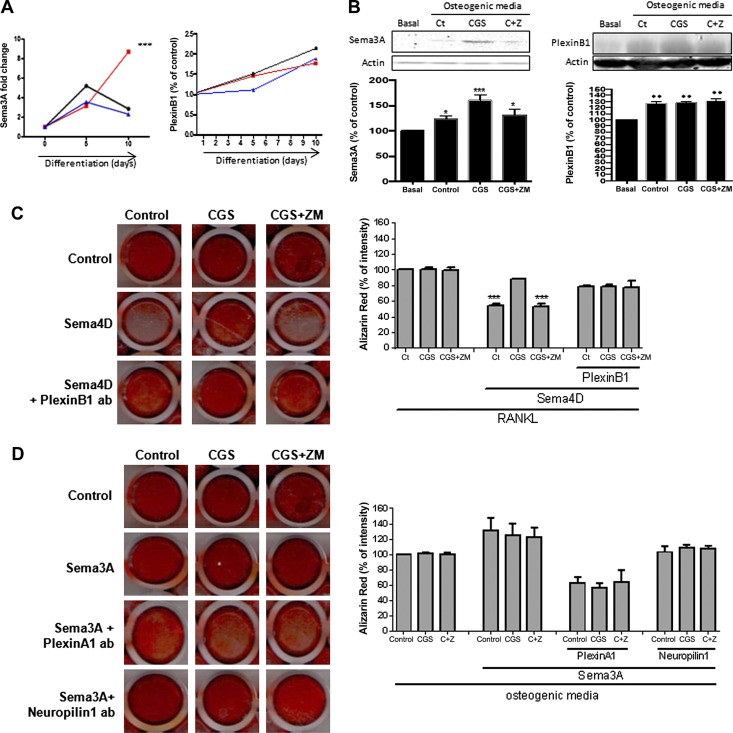
Figure 3.
Osteoblast molecules and receptor expression/secretion are modified by A2AR activation. A) Fold change in Sema3A and PlexinB mRNA during osteoblast differentiation in murine BMCs in the presence of CGS21680, alone or in combination with ZM241385. B) Protein expression for Sema3A and Plexin B1 after challenge in the presence of CGS21680, alone or in combination with ZM241385. C) Wild-type mice osteoblast primary culture cells were fixed and stained for Alizarin Red after being cultured for 14 d in the presence of recombinant Sema4D, alone or combined with PlexinB1 antibody (ab) and in combination with CGS21680 ± ZM241385. D) Wild-type mice osteoblast primary culture cells were fixed and stained for Alizarin Red after being cultured for 14 d in the presence of recombinant Sema3A, alone or combined with PlexinA1 or Neuropilin-1 antibodies, alone or combined with PlexinB1 antibody, and in combination with CGS21680 ± ZM241385. Data are expressed as means ± sem. ***P < 0.001, **P < 0.01, *P > 0.05 vs. nonstimulated control (ANOVA).
Sema3A mRNA increases during osteoblast differentiation (3.5 ± 0.5-fold increased), and the presence of CGS21680 1 µM enhanced this increase (8.7 ± 0.2-fold increase, P < 0.001; Fig. 3A), whereas pretreatment with ZM241385 did not affect Sema3A expression when compared with osteogenic medium alone (Fig. 3A). PlexinB1 mRNA expression was increased during osteoblast differentiation (up to 2-fold change, P < 0.1), and neither activation nor inhibition of the A2AR affected the change in PlexinB1 expression (Fig. 3A). Similar results were observed at the protein level. Sema3A expression was increased 24 h after osteogenic medium stimulation, and it was enhanced in the presence of CGS21680 (23 ± 4% increased for control and 61 ± 7% increased for CGS21680 vs. basal, P < 0.1 and P < 0.001, respectively). ZM241385 completely reversed the A2AR-mediated increase in expression (26 ± 5% increased for ZM241385 vs. basal, P < 0.1; Fig. 3B). PlexinB1 expression in osteoblast-derived cells was increased 24 h after osteogenic medium incubation, and neither CGS21680 nor ZM241385 changed the expression (Fig. 3B).
A2AR activation reverses the effect of Sema4D on osteoclast and osteoblast differentiation
To determine whether A2AR-mediated inhibition of Sema4D expression affected osteoclast and osteoblast differentiation and function, we examined osteoclast and osteoblast differentiation and function in the presence of both Sema4D and CGS21680. As previously described, CGS21680 decreases the number of TRAP-positive cells when compared with RANKL alone, and this change was reversed by ZM241385 (Fig. 2C). Treatment with Sema4D (10 ng/ml) increases osteoclast differentiation (21 ± 1% increase vs. basal, P < 0.05), and CGS21680 reversed this effect (40 ± 3% decreased vs. basal, P < 0.001); treatment with ZM241385 exerted similar results on osteoclast differentiation as Sema4D alone (Fig. 2C). When osteoclast precursors were cultured in the presence of 0.5 µg/ml PlexinB1 antibody, no change in osteoclast differentiation was observed when compared with Sema4D alone; treatment with CGS21680 reversed the Sema4D-mediated increase in osteoclast differentiation, and ZM241385 did not affect osteoclast differentiation when compared with PlexinB1 antibody treatment alone (Fig. 3C). Similar results were observed for osteoclast function. Morphometric measurement of Toluidine blue-stained dentin clearly demonstrated a marked reduction in pit formation when osteoclasts were differentiated in the presence of CGS21680 alone and or in combination with Sema4D (Fig. 2D), an effect that was reversed in the presence of ZM241385.
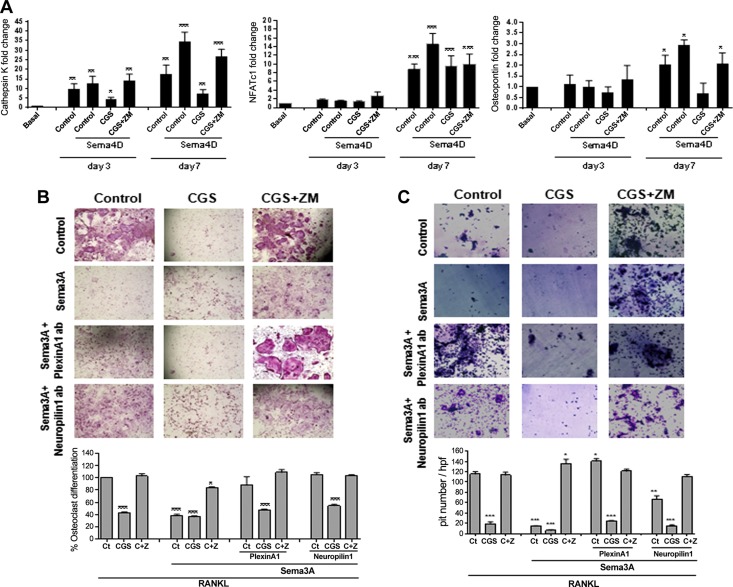
To explore the crosstalk further between Sema4D and A2AR in osteoclast differentiation, we analyzed the expression of key markers for osteoclast differentiation. As previously described, treatment with 30 ng/ml RANKL, alone or in the presence of ZM241385, increased the expression of mRNA for Cathepsin K (by up to 17 ± 4-fold change on d 7, P < 0.01), an effect that was abrogated by CGS21680 (1 µM) (27). When cells were treated in the presence of Sema4A 10 ng/ml, mRNA expression for Cathepsin K was enhanced (to 34 ± 5-fold change on d 7, P < 0.001), an increase that was also abrogated by A2AR stimulation with CGS21680 but not when A2ARs were blocked by ZM241385 (Fig. 4A). The same effect was found for NFATc1 mRNA expression: RANKL increased NFATc1 mRNA expression by 9 ± 1-fold on d 7 of differentiation (P < 0.001; Fig. 4A), and this upregulation was enhanced in the presence of Sema4D (to 15 ± 2-fold increase on d 7, P < 0.001) and partially abrogated by CGS21680, alone or in combination with ZM241385 (Fig. 4A). Finally, mRNA expression for osteopontin was also increased in the RANKL-stimulated cells (by up to 2 ± 0.4-fold on d 7, P < 0.05), and Sema4D treatment enhanced this increase (to 3 ± 0.2-fold change on d 7, P < 0.05), all of which were abrogated by A2AR stimulation with CGS21680 but not in the presence of ZM241385 (Fig. 4A, C).
Figure 4.
A2AR activation reverses the effect of Sema4D and enhances the effect of Sema3A on osteoclast. A) Fold changes in cathepsin K, NFATc1, and osteopontin mRNA during the 7 d osteoclast differentiation process in the presence of recombinant Sema4D, alone or combined with combination with CGS21680 ± ZM241385. B) Wild-type mice osteoclast primary culture cells were fixed and stained for TRAP after being cultured for 7 d in the presence of recombinant Sema3A, alone or combined with PlexinA1 or Neuropilin-1 antibodies and in combination with CGS21680 ± ZM241385. TRAP-positive cells containing 3 or more nuclei were counted as osteoclasts. C) Toluidine blue staining to assay osteoclast activity. Nonadherent cells were seeded in dentin slides and treated for 7 d in the presence of recombinant Sema3A, alone or combined with PlexinA1 or Neuropilin-1 antibodies, alone or combined with a combination with CGS21680 ± ZM241385. All data are expressed as means ± sem of 4 independent cultures. ***P < 0.001, **P < 0.01, *P > 0.05 related to control (ANOVA).
When osteoblast differentiation was analyzed (Fig. 3C), we found that no changes in alizarin staining were observed in the presence of CGS21680, alone or in the presence of ZM241385, but the presence of CGS21680 reversed the inhibitory effect of Sema4D on osteoblast differentiation. When PlexinB1 antibody was added to the culture, no changes in osteoblast differentiation were observed either with CGS21380 or ZM241385 (Fig. 3C)
A2AR activation enhances the effect of Sema3A on osteoclast and osteoblast differentiation
Sema3A inhibits osteoclast differentiation (20), as does A2AR activation (27). Therefore, we determined whether Sema3A enhanced the effect of A2AR activation on osteoclast differentiation. The A2AR agonist CGS21680 decreases osteoclast differentiation, an effect completely reversed by the A2AR antagonist ZM241385 (Fig. 4B). When osteoclast precursors were cultured in the presence of Sema3A, osteoclast differentiation was inhibited (61 ± 2% decreased vs. basal, P < 0.001), an effect not significantly enhanced by CGS21680 (64 ± 1% decreased vs. basal; Fig. 4B). Moreover, when Sema3A was present, ZM241385 did not completely reverse the effect of CGS21680 (16 ± 1% decrease vs. basal, P < 0.05). Treatment with either anti-PlexinA1 and anti-Neuropilin-1 antibodies reversed the effects of Sema3A on osteoclast differentiation, but neither antibody affected the capacity of CGS21680 nor ZM241385 to diminish or increase, respectively, osteoclast differentiation (Fig. 4B). Morphometric measurement of Toluidine blue-stained dentin clearly demonstrated a marked reduction in pit formation when osteoclasts were differentiated in the presence of Sema3A 10 ng/ml, combined with CGS21680 1 µM (Fig. 4C), and ZM241385 reversed this effect. The presence of the PlexinA1 antibody showed an increase in osteoclast differentiation when compared with Sema3A alone, which was reversed in the presence of CGS21680 but not ZM241385 (Fig. 4C). Finally, the presence of the Neuropilin-1 antibody, in combination with Sema3A, diminished pit formation (P < 0.05), a reduction that was greater in the presence of CGS21680 and reversed by ZM241385 (Fig. 4C).
When osteoblast differentiation was analyzed (Fig. 3D), we found that no changes in Alizarin Red staining were observed in the presence of CGS21680, alone or in the presence of ZM241385 1 µM, but treatment with Sema3A (10 ng/ml) enhanced Alizarin Red intensity, an effect reversed by PlexinA1 antibody pretreatment but not with Neuropilin-1 antibody pretreatment (Fig. 3D).
A2AR activation alters osteoclast cytoskeletal assembly
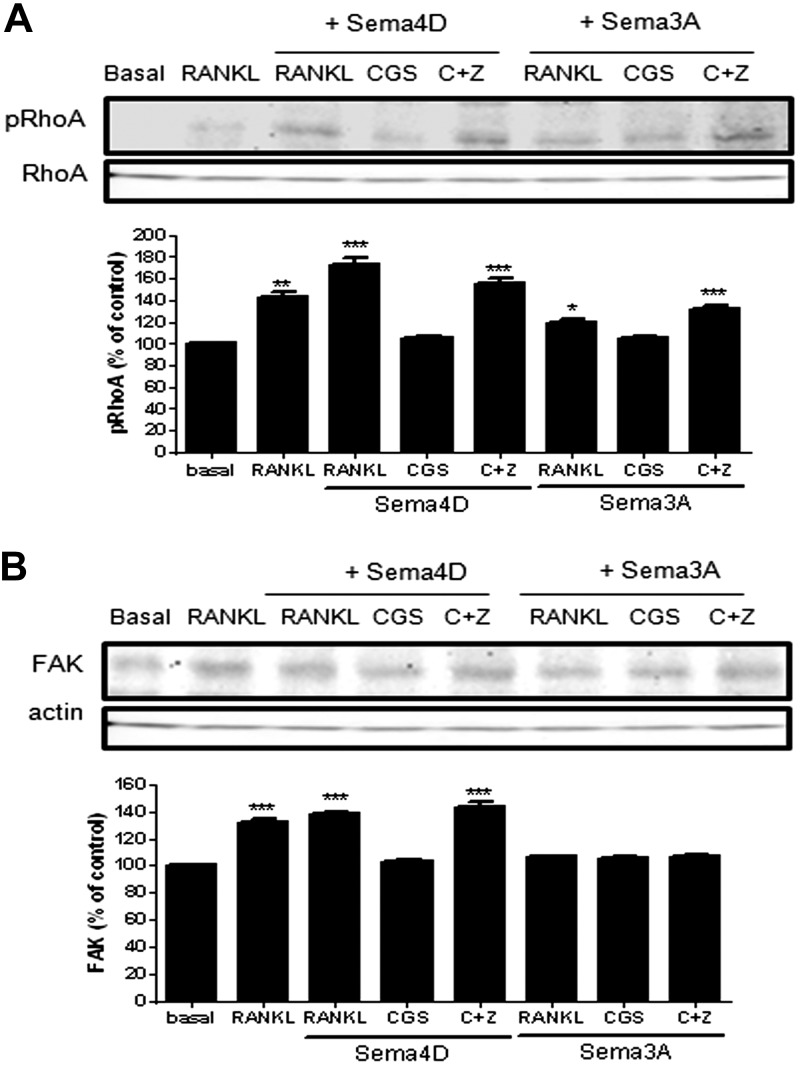
As Sema4D and Sema3A regulate cytoskeletal assembly in both osteoclasts and osteoblasts (17), we determined whether A2AR stimulation affected the cytoskeletal changes induced by Sema4D and Sema3A. Sema4D increases pRhoA in osteoclast precursors stimulated by RANKL, an effect completely reversed by treatment with CGS21680 (73 ± 6% increased for Sema4D and 5 ± 2% for CGS21680 vs. basal, P < 0.001, P = ns, respectively, n = 4; Fig. 5A). Sema3A stimulated a significantly (P < 0.001) smaller increase in pRhoA when compared with Sema4D (19 ± 3% increase for Sema3A vs. basal, P < 0.05, n = 4). Stimulation of A2AR completely blocked pRhoA, induced by both Sema4D and Sema3A, and blockade of A2AR reversed this effect. Interestingly, treatment with ZM241385 increased pRhoA (33 ± 3% increased vs. basal, P < 0.001, n = 4; Fig. 5A). When we studied FAK activation, we observed that in the presence of RANKL, Sema4D increased FAK in osteoclasts, but A2AR stimulation blocked this increase (39 ± 2% increase for Sema4D and 3 ± 1% for CGS21680 vs. basal, P < 0.001, P = ns, respectively, n = 4; Fig. 5B). In contrast, Sema3A did not increase FAK activation (6 ± 1% increased for Sema3A vs. basal, P = ns, n = 4), and neither activation nor blockade of A2AR altered this effect (5 ± 1% increased for CGS21680 and 7 ± 2% for CGS21680 + ZM241385 vs. basal, P = ns, n = 4; Fig. 5B).
Figure 5.
A2AR activation alters osteoclast cytoskeleton. A) pRhoA was analyzed 15 min after stimulation with CGS21680 ± ZM241385 in combination with Sema4D or Sema3A by Western blot of lysates. B) FAK expression was analyzed 15 min after stimulation with CGS21680 ± ZM241385 in combination with Sema4D or Sema3A by Western blot of lysates. To normalize for protein loading, the membranes were reprobed with RhoA or actin, respectively, and results normalized appropriately. Data are expressed as the means of 4 independent experiments. ***P < 0.001, **P < 0.01, *P > 0.05 vs. nonstimulated control.
To prove cytoskeletal changes, phalloidin staining was performed (Supplemental Fig. 2). As previously described (27), treatment with CGS21680 increased least-differentiated osteoclasts that had been this reversed when cells were treated with ZM241385. When cells were treated in the presence of 10 ng/ml Sema4D, we observed that treatment with CGS21680 was able to reverse osteoclast maturation induced by Sema4D, and this was reversed in the presence of ZM241385. When cells were treated with 10 ng/ml Sema3A, we observed that either Sema3A alone or in combination with CGS21680 inhibits osteoclast maturation, and ZM241385 was able to increase the number of maturing osteoclasts.
A2AR activation triggers nuclear translocation of β-catenin in osteoblasts
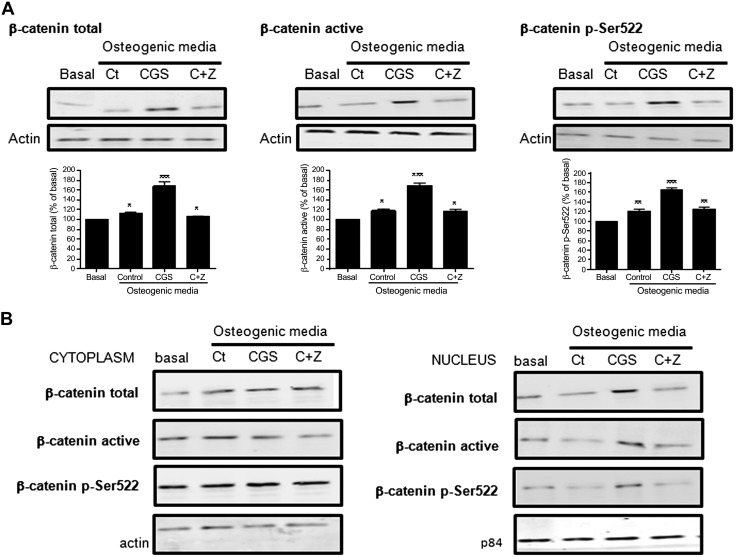
It is clear that Sema3A stimulates osteoblast differentiation and inhibits adipocyte differentiation via the canonical Wnt/β-catenin signaling pathway (20). Likewise, in fibroblasts, A2AR stimulates activation of the Wnt/β-catenin signaling pathway (36). As shown in Fig. 4C, when PlexinA1 is blocked by mAb, both the effect on osteoblast differentiation by Sema3A and CGS21680 was diminished. Therefore, to determine whether activation of A2AR and Sema3A interacted in stimulating the β-catenin signaling pathway, primary osteoblast precursors were incubated with the A2AR agonist and antagonist, or their combination for 24 h and β-catenin signal were analyzed. As shown in Fig. 6A, both canonical and noncanonical β-catenin pathways are activated in the presence of osteogenic medium (control), and this increase was enhanced in the presence of CGS21680 (68 ± 8% increase for total β-catenin, 69 ± 4% increase for active β-catenin, and 64 ± 4% increased for pSer522 β-catenin vs. basal, P < 0.001, n = 4), an effect completely abrogated by ZM241385 (Fig. 6A). To determine whether the crosstalk between A2AR activation and β-catenin activation is internal or via production of a Wnt ligand, we studied β-catenin nuclear translocation in the presence of CGS21680, alone or in the presence of ZM241385, in primary osteoblast precursors. When we analyzed total β-catenin, we observed no changes in the cytoplasm in the presence of CGS21680 when compared with osteogenic medium alone (Fig. 6B), but the amount of total β-catenin in the nucleus is markedly increased (55 ± 3% increased for CGS21680 vs. 14 ± 2% increased for control, P < 0.001, n = 4), and this increase was blocked in the presence of ZM241385. In the case of active β-catenin, we observed that treatment with CGS21680 increased active β-catenin in the nucleus when compared with osteogenic medium (46 ± 3% increased for CGS21680 vs. 10 ± 2% increased for control, P < 0.001, n = 4), and this effect was reversed by ZM241385 (Fig. 6B). Similar results were obtained for noncanonical pSer522 β-catenin nuclear translocation (Fig. 6B).
Figure 6.
A2AR activation triggers β-catenin in osteoblast. A) β-Catenin total, active, and pSer522 activation was analyzed 24 h after stimulation with CGS21680 ± ZM241385 by Western blot of osteoblast lysates. B) β-Catenin total, active, and pSer522 activation in cytoplasm and nucleus was analyzed 24 h after stimulation with CGS21680 ± ZM241385. Data are expressed as the means of 4 independent experiments. ***P < 0.001, **P < 0.01; *P > 0.05 vs. nonstimulated control.
To confirm Western blot results, immunofluorescence for the 3 β-catenin isoforms was carried out (Supplemental Fig. 3). As observed, total β-catenin, active β-catenin, and pSer522 β-catenin nuclear translocation were increased when cells were treated with CGS21680, and this nuclear translocation was reversed when ZM241385 was present in the culture.
A2AR activation modifies RANKL/OPG secretion from osteoblasts
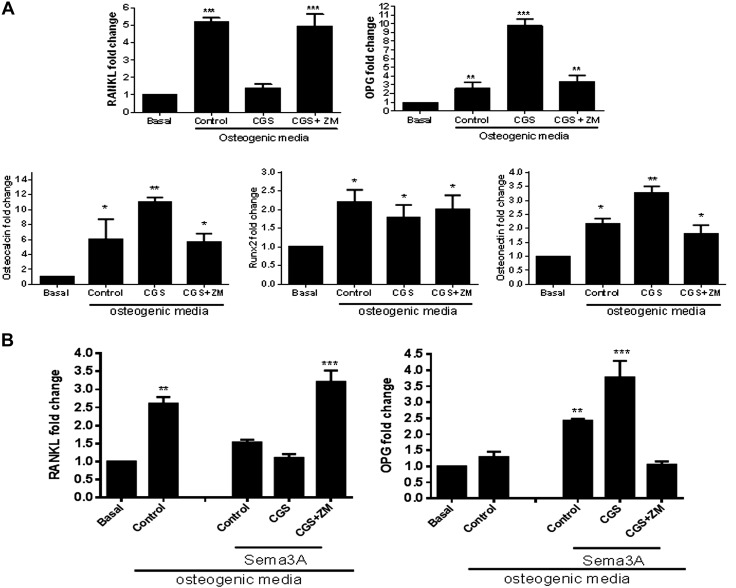
Culture of osteoblast precursors in osteogenic medium for 24 h increased RANKL mRNA expression 5-fold compared with basal expression (P < 0.001, n = 4), and CGS21680 completely inhibited this increase (0.39 ± 0.2-fold change, P = ns vs. control), an effect that was reversed by ZM241385 (Fig. 7A). In contrast, CGS21680 markedly enhanced OPG mRNA expression (up to 10-fold, P < 0.001, n = 4), and ZM241385 completely blocked the CGS21680-enhanced increase in OPG (Fig. 7A). When we analyzed mRNA expression for several osteoblast markers, we observed that osteocalcin mRNA expression was increased for 24 h in the presence of osteogenic medium, and treatment with CGS21680 enhanced osteocalcin mRNA expression (up to 11-fold, P < 0.01, n = 4), which was reduced to control levels in the presence of ZM241385 (Fig. 7A). Runx2 was increased in the presence of osteogenic medium, but no significant changes were observed when cells were treated with CGS21680 or ZM241385 (Fig. 7A). The effect of A2AR stimulation on osteonectin was similar to the effect on osteocalcin, CGS21680 markedly enhanced osteonectin mRNA expression (up to 3-fold, P < 0.01, n = 4), and ZM241385 completely blocks the CGS21680-enhanced increased (Fig. 7A).
Figure 7.
A2AR activation modifies RANKL/OPG secretion from osteoblast. A) RANKL, OPG, osteocalcin, Runx2, and osteonectin mRNA fold change in murine osteoblast precursors after 24 h challenge with osteogenic medium in the presence of CGS21680, alone or with ZM241385, compared with control (n = 4). B) RANKL and OPG mRNA fold change in murine osteoblast precursors after 24 h challenge with osteogenic medium in the presence of CGS21680, alone or with ZM241385, and in combination with Sema3A compared with control (n = 4). ***P < 0.001, **P < 0.01, *P > 0.05 (ANOVA).
To determine whether the interaction between Sema3A and A2AR is related to Wnt signaling, RANKL and OPG mRNA expression was studied in the presence of the A2AR agonist and antagonist, alone or in the presence of Sema3A. As shown in Fig. 7B, Sema3A inhibits RANKL expression and increases OPG expression (1.1 ± 0.1-fold RANKL change vs. 2.6 ± 0.2 for control and 2.4 ± 0.1-fold OPG change vs. 1.3 ± 0.1 for control, P < 0.5, respectively, n = 4), and treatment with CGS21680 enhanced the decrease in RANKL and increased OPG expression (1.5 ± 0.1-fold RANKL change vs. 2.6 ± 0.2 for control and 3.8 ± 0.5-fold OPG change vs. 1.3 ± 0.1 for control, P < 0.001 respectively, n = 4). These effects were reversed in the presence of A2AR blockade (Fig. 7B). These results indicate that both Sema3A and A2AR activate Wnt signaling in a complementary fashion.
DISCUSSION
Bone remodeling involves multiple cell types; osteoclasts resorb bone, and osteoblasts generate new bone. Osteocytes within the bone matrix regulate bone remodeling as well, as do the bone lining cells covering the bone surface and the capillary blood supply (2, 37). Signaling and crosstalk among the cells involved is important in controlling this process. In this sense, coupling factors that coordinate the temporal activation and function of these cells are being studied to identify new, potential therapeutic targets (2). Among these factors, axonal guidance molecules and neurotrophins play an important role, as bone is innervated by sympathetic and sensory nerves in mammals (38, 39). Togari and colleagues (5) demonstrated the expression of mRNA for axonal guidance proteins, including Semas, netrins, and neurotrophins, in human osteoblast cell lines, osteosarcoma cell lines, and osteoclasts, and it has been previously demonstrated that Semas and ephrins play important roles in regulating bone cell differentiation and activity (3, 12, 15, 20, 40). More recently, we have demonstrated that netrin-1 and its receptor unc5b play an important role in maintaining bone homeostasis as well (4).
In the presence of RANKL, Sema4D expression is rapidly induced in osteoclast precursors (12, 13). Sema4D, secreted by osteoclasts, binds to its receptor Plexin-B1 on osteoblasts, resulting in the activation and autophosphorylation of ErbB2 and RhoA-ROCK activation and the inhibition of insulin receptor substrate 1, thereby diminishing osteoblast differentiation induced by RhoA activation (12, 15). Mice lacking Sema4D had higher bone mass as a result of augmented bone formation, without any abnormality in bone resorption, suggesting that osteoclastic expression of Sema4D inhibits osteoblastic bone formation and mediates osteoclast–osteoblast communication (17). In confirmation, with the use of polymeric nanoparticles/a small interfering RNA approach, it was demonstrated that interference with the expression of Sema4D in osteoclasts led to significantly higher levels of ALP activity, mineralization, and bone differentiation markers in osteoblasts, both in control and ovariectomized mice (18). Sema4D has been recently associated with postmenopausal osteoporosis, representing a novel, predictive indicator of postmenopausal osteoporosis (41), and it has been shown that the targeting of Sema4D in ovariectomized animals leads to increased bone loss (42). Sema4D also contributes to the pathogenesis of rheumatoid arthritis by inducing TNF-α/a disintegrin and metalloproteinase with thrombospondin motif 4 (22) and to skeletal metastasis in breast cancer (43).
In contrast, Sema3A, produced by osteoblasts among other cells, binds to its receptor, Neuropilin-1, both to inhibit RANKL-induced osteoclast differentiation and to stimulate osteoblast differentiation and function (20, 44). Sema3A signaling seems to precede or coincide at the temporal and spatial level with the invasion of bone by blood vessels and nerve fibers, and deletion of the Sema3A gene induces abnormal bone and cartilage development (21). It has been observed that Sema3A is critical for osteointegration in diabetes (23), diminishes differentiation of mesenchymal stem cells to adipocytes, promotes differentiation to an osteogenic phenotype, and may play a role in bone repair (45). In ovariectomized rats, Sema3A, suspended in matrigel or chitosan film, improves titanium implant osseointegration and fixation (24, 25). Surprisingly, it has also been observed that Sema3A, Neuropilin-1, and PlexinA1 may be involved in peri-prosthetic osteolysis (46) as Sema3A, Neuropilin-1, and PlexinA1 mRNA levels in bone increased in the presence of polyethylene particles.
As we have previously reported, A2AR activation promotes bone formation by 2 mechanisms. On 1 hand, activation of the A2AR diminishes osteoclast differentiation and function both in vitro and in vivo (27, 30, 47, 48). On the other hand, A2AR stimulation does not alter osteoblast differentiation in vitro directly, although it clearly regulates osteoblast expression of proteins involved in bone formation, and stimulation of A2AR by specific agonists or by increasing endogenous adenosine levels stimulates new bone formation, as well as bone morphogenetic protein 2 (30, 47). A2AR stimulation diminishes the number of RANKL-expressing cells present at the site of wear particle-induced osteolysis (26), and here, we show that A2AR stimulation promotes osteoblast expression of key markers for bone formation, such as osteocalcin and osteonectin (26). These results suggest that adenosine and A2AR are involved in the crosstalk between osteoclasts and osteoblasts. Therefore, we asked whether A2AR activation modulates bone homeostasis by regulating Sema4D and Sema3A expression, at least in part. Our results demonstrate that A2AR activation diminishes secretion of Sema4D by osteoclasts and enhances secretion of Sema3A by osteoblasts, leading to an increase in osteoblast differentiation and function and in combination with the suppressive effects of A2AR on osteoclast differentiation and function, diminishes bone osteolysis (Fig. 8). Moreover, we have demonstrated that stimulation of A2AR blocked pRhoA and FAK activation mediated by Sema4D in osteoclasts, and this is potentiated when Sema3A is present in the medium. In osteoclasts, RhoA, Rac, Cdc42, RhoU, and also ADP-ribosylation factor 6 regulate podosome assembly and their organization into the sealing zone. Therefore, the combinatory effect of A2AR and Sema3A blocks the regulation of cytoskeletal organization and polarization of osteoclasts and avoids osteoclast differentiation, leading to fewer fully differentiated osteoclasts, as we have previously demonstrated for A2AR activation (27). These findings further demonstrate that adenosine is a mediator in maintaining bone homeostasis both under control and inflammatory conditions.
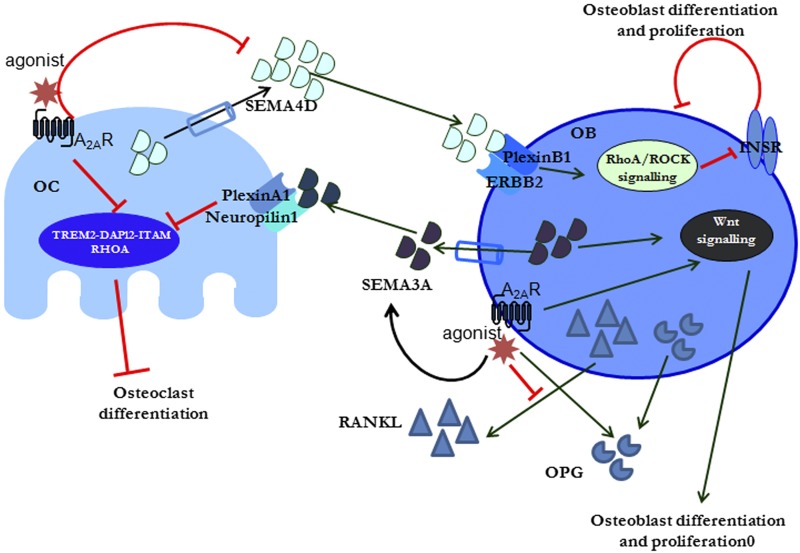
Figure 8.
Proposed crosstalk pathway between A2AR signaling and Semas. Activation of A2AR in osteoclast (OC) precursors directly inhibits osteoclast differentiation and blocks Sema4D secretion. In osteoblast (OB) precursors, A2AR activations promotes Sema3A activation and secretion; alters cytoskeleton signaling, RANKL, and OPG secretion; and promotes Wnt signaling to promote osteoblast differentiation and inhibit osteoclast differentiation. ERBB2, receptor tyrosine-protein kinase erbB-2; INSR, insulin receptor; ITAM, immunoreceptor tyrosine-based activation motif; TREM2, triggering receptor expressed on myeloid cells 2.
In previous studies, we have demonstrated that signaling at A2AR is mediated, in part, through crosstalk with WNT/β-catenin signaling in human fibroblasts (36), and here, we report that A2AR stimulation regulates osteoblast function with similar signaling crosstalk. We have demonstrated that both canonical and noncanonical WNT/β-catenin signaling pathways are involved during A2AR activation in osteoblasts. Indeed, it is likely that many of the effects of A2AR stimulation on new bone formation, as seen in vivo in these studies, are mediated through the WNT/β-catenin signaling pathway. In fibroblasts, the A2AR most likely mediates its effects on WNT signaling via activation of exchange protein directly activated by cAMP 1/2 (EPAC1/2) and downstream activation of p38MAPK and PKB (AKT) (36, 49, 50). The precise signaling interactions and the downstream signaling elements involved in EPAC1/2 signaling for osteoclast differentiation are not completely understood, although they are likely to involve regulation of cytoskeletal assembly, the effects for which EPAC1/2 signaling is best understood (48).
In summary, the targeting of A2ARs might constitute a novel, therapeutic approach for bone remodeling, as it regulates bone homeostasis by directly regulating the cells involved in bone formation and remodeling and indirectly by monitoring the expression and response to intercellular signals important for maintaining bone homeostasis.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by the U.S. National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases (Grants AR68593 and AR56672), the New York University–Health and Hospitals Corporation Clinical and Translational Science Institute (Grant U54TR001364), and a New York University Caregiver Intervention Center Support Grant (9NIH/NCI 5 P30CA16087-310). A.M. is funded by Instituto de Salud Carlos III through the Miguel Servet Program (CP15/00053), and cofunded by Fondo Europeo de Desarrollo Regional (FEDER) with a research grant from the Spanish Instituto de Salud Carlos III (PI16/00991). A.M. and B.N.C. have filed a patent on use of A2AR agonists to prevent prosthesis loosening (pending), and a separate patent on use of A2AR agonists and agents that increase adenosine levels to promote bone formation/regeneration. B.N.C. is a consultant for Bristol-Myers Squibb, AstraZeneca, Novartis, and CanFite Biopharmaceuticals and has stock in CanFite Biopharmaceuticals. The remaining authors declare no conflicts of interest.
Glossary
- α-MEM
α-minimum essential medium
- ALP
alkaline phosphatase
- AR
adenosine receptor
- BMC
bone marrow cell
- BSA
bovine serum albumin
- EPAC1/2
exchange protein directly activated by cAMP 1/2
- FAK
focal adhesion kinase
- hpf
high-power field
- HRP
horseradish peroxidase
- NFATc1
nuclear factor of activated T cells c1
- OPG
osteoprotegerin
- RANKL
receptor activator of NF-κB ligand
- RhoA
Ras homolog gene family, member A
- ROCK
Ras homolog gene family kinase
- Runx2
Runt-related transcription factor 2
- Sema
semaphorin
- TRAP
tartrate-resistant acid phosphatase
- UHMWPE
ultrahigh molecular weight polyethylene
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
B. N. Cronstein and A. Mediero designed the experiments, analyzed and interpreted results, and revised the manuscript; A. Mediero was the primary person responsible for carrying out all experimental procedures and writing the manuscript; T. Wilder assisted with surgery and animal treatments and revised the manuscript; and L. Shah helped to design and execute these experiments and edited the manuscript.
REFERENCES
- 1.He W., Mazumder A., Wilder T., Cronstein B. N. (2013) Adenosine regulates bone metabolism via A1, A2A, and A2B receptors in bone marrow cells from normal humans and patients with multiple myeloma. FASEB J. 27, 3446–3454 https://doi.org/10.1096/fj.13-231233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kular J., Tickner J., Chim S. M., Xu J. (2012) An overview of the regulation of bone remodelling at the cellular level. Clin. Biochem. 45, 863–873 https://doi.org/10.1016/j.clinbiochem.2012.03.021 [DOI] [PubMed] [Google Scholar]
- 3.Edwards C. M., Mundy G. R. (2008) Eph receptors and ephrin signaling pathways: a role in bone homeostasis. Int. J. Med. Sci. 5, 263–272 https://doi.org/10.7150/ijms.5.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mediero A., Ramkhelawon B., Perez-Aso M., Moore K. J., Cronstein B. N. (2015) Netrin-1 is a critical autocrine/paracrine factor for osteoclast differentiation. J. Bone Miner. Res. 30, 837–854 https://doi.org/10.1002/jbmr.2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Togari A., Mogi M., Arai M., Yamamoto S., Koshihara Y. (2000) Expression of mRNA for axon guidance molecules, such as semaphorin-III, netrins and neurotrophins, in human osteoblasts and osteoclasts. Brain Res. 878, 204–209 https://doi.org/10.1016/S0006-8993(00)02700-1 [DOI] [PubMed] [Google Scholar]
- 6.Takahashi T., Fournier A., Nakamura F., Wang L. H., Murakami Y., Kalb R. G., Fujisawa H., Strittmatter S. M. (1999) Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell 99, 59–69 https://doi.org/10.1016/S0092-8674(00)80062-8 [DOI] [PubMed] [Google Scholar]
- 7.Tamagnone L., Artigiani S., Chen H., He Z., Ming G. I., Song H., Chedotal A., Winberg M. L., Goodman C. S., Poo M., Tessier-Lavigne M., Comoglio P. M. (1999) Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell 99, 71–80 https://doi.org/10.1016/S0092-8674(00)80063-X [DOI] [PubMed] [Google Scholar]
- 8.Winberg M. L., Noordermeer J. N., Tamagnone L., Comoglio P. M., Spriggs M. K., Tessier-Lavigne M., Goodman C. S. (1998) Plexin A is a neuronal semaphorin receptor that controls axon guidance. Cell 95, 903–916 https://doi.org/10.1016/S0092-8674(00)81715-8 [DOI] [PubMed] [Google Scholar]
- 9.Sims N. A., Martin T. J. (2014) Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. Bonekey Rep. 3, 481 https://doi.org/10.1038/bonekey.2013.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kikutani H., Suzuki K., Kumanogoh A. (2007) Immune semaphorins: increasing members and their diverse roles. Adv. Immunol. 93, 121–143 https://doi.org/10.1016/S0065-2776(06)93003-X [DOI] [PubMed] [Google Scholar]
- 11.Love C. A., Harlos K., Mavaddat N., Davis S. J., Stuart D. I., Jones E. Y., Esnouf R. M. (2003) The ligand-binding face of the semaphorins revealed by the high-resolution crystal structure of SEMA4D. Nat. Struct. Biol. 10, 843–848 https://doi.org/10.1038/nsb977 [DOI] [PubMed] [Google Scholar]
- 12.Negishi-Koga T., Shinohara M., Komatsu N., Bito H., Kodama T., Friedel R. H., Takayanagi H. (2011) Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat. Med. 17, 1473–1480 https://doi.org/10.1038/nm.2489 [DOI] [PubMed] [Google Scholar]
- 13.Dacquin R., Domenget C., Kumanogoh A., Kikutani H., Jurdic P., Machuca-Gayet I. (2011) Control of bone resorption by semaphorin 4D is dependent on ovarian function. PLoS One 6, e26627 https://doi.org/10.1371/journal.pone.0026627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyce B. F. (2013) Advances in the regulation of osteoclasts and osteoclast functions. J. Dent. Res. 92, 860–867 https://doi.org/10.1177/0022034513500306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang S., Kumanogoh A. (2013) Semaphorins in bone development, homeostasis, and disease. Semin. Cell Dev. Biol. 24, 163–171 https://doi.org/10.1016/j.semcdb.2012.09.008 [DOI] [PubMed] [Google Scholar]
- 16.Nogi T., Yasui N., Mihara E., Matsunaga Y., Noda M., Yamashita N., Toyofuku T., Uchiyama S., Goshima Y., Kumanogoh A., Takagi J. (2010) Structural basis for semaphorin signalling through the plexin receptor. Nature 467, 1123–1127 https://doi.org/10.1038/nature09473 [DOI] [PubMed] [Google Scholar]
- 17.Negishi-Koga T., Takayanagi H. (2012) Bone cell communication factors and semaphorins. Bonekey Rep. 1, 183 https://doi.org/10.1038/bonekey.2012.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y., Wei L., Miron R. J., Shi B., Bian Z. (2015) Anabolic bone formation via a site-specific bone-targeting delivery system by interfering with semaphorin 4D expression. J. Bone Miner. Res. 30, 286–296 https://doi.org/10.1002/jbmr.2322 [DOI] [PubMed] [Google Scholar]
- 19.Kruger R. P., Aurandt J., Guan K. L. (2005) Semaphorins command cells to move. Nat. Rev. Mol. Cell Biol. 6, 789–800 https://doi.org/10.1038/nrm1740 [DOI] [PubMed] [Google Scholar]
- 20.Hayashi M., Nakashima T., Taniguchi M., Kodama T., Kumanogoh A., Takayanagi H. (2012) Osteoprotection by semaphorin 3A. Nature 485, 69–74 https://doi.org/10.1038/nature11000 [DOI] [PubMed] [Google Scholar]
- 21.Gomez C., Burt-Pichat B., Mallein-Gerin F., Merle B., Delmas P. D., Skerry T. M., Vico L., Malaval L., Chenu C. (2005) Expression of semaphorin-3A and its receptors in endochondral ossification: potential role in skeletal development and innervation. Dev. Dyn. 234, 393–403 https://doi.org/10.1002/dvdy.20512 [DOI] [PubMed] [Google Scholar]
- 22.Yoshida Y., Ogata A., Kang S., Ebina K., Shi K., Nojima S., Kimura T., Ito D., Morimoto K., Nishide M., Hosokawa T., Hirano T., Shima Y., Narazaki M., Tsuboi H., Saeki Y., Tomita T., Tanaka T., Kumanogoh A. (2015) Semaphorin 4D contributes to rheumatoid arthritis by inducing inflammatory cytokine production: pathogenic and therapeutic implications. Arthritis Rheumatol. 67, 1481–1490 https://doi.org/10.1002/art.39086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang K., Song W., Wang L., Xu X., Tan N., Zhang S., Wei H., Song Y. (2016) Semaphorin 3A-modified adipose-derived stem cell sheet may improve osseointegration in a type 2 diabetes mellitus rat model. Mol. Med. Rep. 14, 2449–2456 https://doi.org/10.3892/mmr.2016.5568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y., He D., Liu B., Hu J. (2017) SEMA3A suspended in matrigel improves titanium implant fixation in ovariectomized rats. J. Biomed. Mater. Res. B Appl. Biomater. 105, 2060–2065 [DOI] [PubMed] [Google Scholar]
- 25.Fang K., Song W., Wang L., Jia S., Wei H., Ren S., Xu X., Song Y. (2014) Immobilization of chitosan film containing semaphorin 3A onto a microarc oxidized titanium implant surface via silane reaction to improve MG63 osteogenic differentiation. Int. J. Nanomedicine 9, 4649–4657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mediero A., Frenkel S. R., Wilder T., He W., Mazumder A., Cronstein B. N. (2012) Adenosine A2A receptor activation prevents wear particle-induced osteolysis. Sci. Transl. Med. 4, 135ra65 https://doi.org/10.1126/scitranslmed.3003393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mediero A., Kara F. M., Wilder T., Cronstein B. N. (2012) Adenosine A(2A) receptor ligation inhibits osteoclast formation. Am. J. Pathol. 180, 775–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yasuda H., Shima N., Nakagawa N., Yamaguchi K., Kinosaki M., Goto M., Mochizuki S. I., Tsuda E., Morinaga T., Udagawa N., Takahashi N., Suda T., Higashio K. (1999) A novel molecular mechanism modulating osteoclast differentiation and function. Bone 25, 109–113 https://doi.org/10.1016/S8756-3282(99)00121-0 [DOI] [PubMed] [Google Scholar]
- 29.Mediero A., Perez-Aso M., Cronstein B. N. (2013) Activation of adenosine A(2A) receptor reduces osteoclast formation via PKA- and ERK1/2-mediated suppression of NFκB nuclear translocation. Br. J. Pharmacol. 169, 1372–1388 https://doi.org/10.1111/bph.12227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mediero A., Wilder T., Perez-Aso M., Cronstein B. N. (2015) Direct or indirect stimulation of adenosine A2A receptors enhances bone regeneration as well as bone morphogenetic protein-2. FASEB J. 29, 1577–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katebi M., Soleimani M., Cronstein B. N. (2009) Adenosine A2A receptors play an active role in mouse bone marrow-derived mesenchymal stem cell development. J. Leukoc. Biol. 85, 438–444 https://doi.org/10.1189/jlb.0908520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gharibi B., Abraham A. A., Ham J., Evans B. A. (2011) Adenosine receptor subtype expression and activation influence the differentiation of mesenchymal stem cells to osteoblasts and adipocytes. J. Bone Miner. Res. 26, 2112–2124 https://doi.org/10.1002/jbmr.424 [DOI] [PubMed] [Google Scholar]
- 33.Chitu V., Pixley F. J., Macaluso F., Larson D. R., Condeelis J., Yeung Y. G., Stanley E. R. (2005) The PCH family member MAYP/PSTPIP2 directly regulates F-actin bundling and enhances filopodia formation and motility in macrophages. Mol. Biol. Cell 16, 2947–2959 https://doi.org/10.1091/mbc.E04-10-0914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfaffl M. W. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45 https://doi.org/10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harmatys K. M., Cole E. L., Smith B. D. (2013) In vivo imaging of bone using a deep-red fluorescent molecular probe bearing multiple iminodiacetate groups. Mol. Pharm. 10, 4263–4271 https://doi.org/10.1021/mp400357v [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaikh G., Zhang J., Perez-Aso M., Mediero A., Cronstein B. (2016) Adenosine A2A receptor promotes collagen type III synthesis via β-catenin activation in human dermal fibroblasts. Br. J. Pharmacol. 173, 3279–3291 https://doi.org/10.1111/bph.13615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sims N. A., Walsh N. C. (2012) Intercellular cross-talk among bone cells: new factors and pathways. Curr. Osteoporos. Rep. 10, 109–117 https://doi.org/10.1007/s11914-012-0096-1 [DOI] [PubMed] [Google Scholar]
- 38.Bjurholm A. (1991) Neuroendocrine peptides in bone. Int. Orthop. 15, 325–329 https://doi.org/10.1007/BF00186871 [DOI] [PubMed] [Google Scholar]
- 39.Hohmann E. L., Elde R. P., Rysavy J. A., Einzig S., Gebhard R. L. (1986) Innervation of periosteum and bone by sympathetic vasoactive intestinal peptide-containing nerve fibers. Science 232, 868–871 https://doi.org/10.1126/science.3518059 [DOI] [PubMed] [Google Scholar]
- 40.Fukuda T., Takeda S., Xu R., Ochi H., Sunamura S., Sato T., Shibata S., Yoshida Y., Gu Z., Kimura A., Ma C., Xu C., Bando W., Fujita K., Shinomiya K., Hirai T., Asou Y., Enomoto M., Okano H., Okawa A., Itoh H. (2013) Sema3A regulates bone-mass accrual through sensory innervations [Erratum in Nature 2013, 500, 612]. Nature 497, 490–493 https://doi.org/10.1038/nature12115 [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y., Feng E., Xu Y., Wang W., Zhang T., Xiao L., Chen R., Lin Y., Chen D., Lin L., Chen K., Lin Y. (2015) Serum Sema4D levels are associated with lumbar spine bone mineral density and bone turnover markers in patients with postmenopausal osteoporosis. Int. J. Clin. Exp. Med. 8, 16352–16357 [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y., Wei L., Miron R. J., Zhang Q., Bian Z. (2014) Prevention of alveolar bone loss in an osteoporotic animal model via interference of semaphorin 4d. J. Dent. Res. 93, 1095–1100 https://doi.org/10.1177/0022034514552676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang Y. H., Buhamrah A., Schneider A., Lin Y. L., Zhou H., Bugshan A., Basile J. R. (2016) Semaphorin 4D promotes skeletal metastasis in breast cancer. PLoS One 11, e0150151 https://doi.org/10.1371/journal.pone.0150151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu R. (2014) Semaphorin 3A: a new player in bone remodeling. Cell Adh. Migr. 8, 5–10 https://doi.org/10.4161/cam.27752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu X., Tan N., Zhou Y., Zhou X., Chen H., Wei H., Chen J., Xu X., Zhang S., Yang G., Song Y. (2016) Semaphorin 3A shifts adipose mesenchymal stem cells towards osteogenic phenotype and promotes bone regeneration in vivo. Stem Cells Int. 2016, 2545214 https://doi.org/10.1155/2016/2545214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saad S., Dharmapatni A. A. S. S. K., Crotti T. N., Cantley M. D., Algate K., Findlay D. M., Atkins G. J., Haynes D. R. (2016) Semaphorin-3a, neuropilin-1 and plexin-A1 in prosthetic-particle induced bone loss. Acta Biomater. 30, 311–318 https://doi.org/10.1016/j.actbio.2015.11.025 [DOI] [PubMed] [Google Scholar]
- 47.Mediero A., Cronstein B. N. (2013) Adenosine and bone metabolism. Trends Endocrinol. Metab. 24, 290–300 https://doi.org/10.1016/j.tem.2013.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mediero A., Perez-Aso M., Cronstein B. N. (2014) Activation of EPAC1/2 is essential for osteoclast formation by modulating NFκB nuclear translocation and actin cytoskeleton rearrangements. FASEB J. 28, 4901–4913 https://doi.org/10.1096/fj.14-255703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shaikh G., Cronstein B. (2016) Signaling pathways involving adenosine A2A and A2B receptors in wound healing and fibrosis. Purinergic Signal. 12, 191–197 https://doi.org/10.1007/s11302-016-9498-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perez-Aso M., Mediero A., Cronstein B. N. (2013) Adenosine A2A receptor (A2AR) is a fine-tune regulator of the collagen1:collagen3 balance. Purinergic Signal. 9, 573–583 https://doi.org/10.1007/s11302-013-9368-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.