Abstract
Evolutionary considerations suggest that the body has been optimized to perform at a high level in the food-deprived state when fatty acids and their ketone metabolites are a major fuel source for muscle cells. Because controlled food deprivation in laboratory animals and intermittent energy restriction in humans is a potent physiologic stimulus for ketosis, we designed a study to determine the impact of intermittent food deprivation during endurance training on performance and to elucidate the underlying cellular and molecular mechanisms. Male mice were randomly assigned to either ad libitum feeding or alternate-day food deprivation (ADF) groups, and half of the mice in each diet group were trained daily on a treadmill for 1 mo. A run to exhaustion endurance test performed at the end of the training period revealed superior performance in the mice maintained on ADF during training compared to mice fed ad libitum during training. Maximal O2 consumption was increased similarly by treadmill training in mice on ADF or ad libitum diets, whereas respiratory exchange ratio was reduced in ADF mice on food-deprivation days and during running. Analyses of gene expression in liver and soleus tissues, and metabolomics analysis of blood suggest that the metabolic switch invoked by ADF and potentiated by exercise strongly modulates molecular pathways involved in mitochondrial biogenesis, metabolism, and cellular plasticity. Our findings demonstrate that ADF engages metabolic and cellular signaling pathways that result in increased metabolic efficiency and endurance capacity.—Marosi, K., Moehl, K., Navas-Enamorado, I., Mitchell, S. J., Zhang, Y., Lehrmann, E., Aon, M. A., Cortassa, S., Becker, K. G., Mattson, M. P. Metabolic and molecular framework for the enhancement of endurance by intermittent food deprivation.
Keywords: exercise, intermittent fasting, muscle, mitochondrial biogenesis, ketone
The usual eating pattern of modern humans, 3 meals plus snacks every day, is abnormal compared to the eating patterns of animals in the wild and our human ancestors before the agricultural revolution (1–3). Competition for limited food sources has been a driving force for evolution, with selection for individuals capable of high levels of physical performance under conditions of intermittent food deprivation (IF). In response to controlled energy restriction for more than 12 to 16 h and/or extended exercise, a major cellular fuel source switches from glucose to fats as liver glycogen stores are depleted, and fatty acids are metabolized to the ketones 3β-hydroxybutyrate (BHB) and acetoacetate (4–6). This switch to fatty acids as a fuel source manifests as a reduction in the respiratory exchange ratio (RER; ratio of CO2 produced to O2 consumed). Intermittent metabolic switching, effected by dietary energy restriction and/or exercise, is associated with health and resistance to a wide range of diseases in humans (5, 7, 8). In laboratory animals, IF protocols, including daily time-restricted feeding and alternate-day food deprivation (ADF), extend life span, and protect against diabetes, cancers, cardiovascular disease, and a range of neurologic disorders in experimental models (8–10). In general, exercise and food deprivation activate adaptive cellular stress response signaling pathways, and pathways that enhance the plasticity and functionality of organ systems (11). For example, both endurance training and IF improve cardiovascular stress adaptation (12, 13) and increase the number synapses in neuronal circuits in the brain (14, 15). Conversely, the sedentary and/or overindulgent lifestyles common in modern societies result in suboptimal physical and mental performance capabilities, impair adaptive cellular responses to stress, and instigate a range of chronic diseases including diabetes, cardiovascular disease, sarcopenia, and a range of neurologic disorders (16, 17).
Adaptive responses of muscle cells to exercise have been intensively studied, resulting in the identification of multiple signaling pathways and subcellular mechanisms involved in enhanced strength and endurance with training. The synaptic stimulation of muscle cells by acetylcholine released from motor neurons results in Ca2+ influx and release from the sarcoplasmic reticulum; Ca2+-activated kinases then mobilize the transcriptional regulators peroxisome proliferator–activated receptor γ coactivator 1α (PGC-1α), nuclear regulatory factor (NRF) 1, pyruvate dehydrogenase kinase 4 (PDK4), and peroxisome proliferator–activated receptor δ (PPAR-δ), which up-regulate genes encoding proteins involved in mitochondrial biogenesis, lipid/ketone metabolism, and mitochondrial uncoupling (18). The large increase in ATP consumption and reactive oxygen species production that occurs in muscle cells during exercise results in an increase in levels of AMP that, together with calcium-dependent kinases, activates AMPK. AMPK phosphorylates proteins that inhibit mammalian target of rapamycin (mTOR) and protein synthesis and that up-regulate autophagy and mitochondrial biogenesis. Several of the major effects of food deprivation on muscle cells are similar to exercise (mitochondrial biogenesis, reduced mTOR activity, and increased autophagy), but they are largely mediated by fatty acid metabolism and PPAR-δ rather than Ca2+ (18). Previous studies that elucidated cellular signaling pathways affected by physiologic bioenergetic challenges focused on either intermittent exercise or food deprivation, but they did not include subjects maintained on food deprivation plus exercise protocols. Recent findings suggest that exercise can prevent loss of muscle mass in humans on low-calorie diets by a mechanism that in part involves up-regulation of leptin receptors and increased fatty acid oxidation in muscle cells (19, 20). Further studies in which the combined effects of dietary energy restriction and exercise on the molecular, cellular, and functional responses of organ systems may lead to novel insight into the mechanisms by which organisms adapt to intermittent metabolic switching from glucose in the fed state to fatty acids and ketones in the food-deprived state.
Historically, endurance athletes consume multiple meals every day and load up on carbohydrates, the notion being that it is important to provide glucose to fuel the muscles (21). However, recent findings argue against a universal recommendation of carbohydrate loading before exercise, as fuel utilization depends on many factors, including timing, intensity, and duration of exercise. One alternative being explored by sports physiologists and endurance athletes is nutritional ketosis, a potential method for attenuating the decrease in physiologic performance associated with the decline in endogenous carbohydrate stores (22). In a study of rats, dietary supplementation with a ketone ester that elevates BHB levels to 1 mM or more resulted in increased performance in treadmill running (23). The same ketone ester increases plasma BHB levels to over 5 mM, significantly reduces RER, and enhances endurance performance in trained cyclists, indicating utilization of the BHB as a fuel source (24). Food deprivation is a potent physiologic stimulus for ketogenesis, but it also engages complex and integrated endocrine signaling, as well as tissue-specific cellular responses (5, 8, 10). If and how such tissue and systemic responses to IF affect the outcomes of endurance training are unknown.
In the present study, we found that ADF during endurance treadmill training enhances performance by a mechanism involving enhanced fatty acid metabolism and up-regulation in soleus muscle cells of molecular pathways involved in mitochondrial biogenesis, mitochondrial stress resistance, and autophagy.
MATERIALS AND METHODS
Animals
Thirty-five male C57 BL/6J mice (The Jackson Laboratory, Bar Harbor, ME, USA) were housed individually with ad libitum access to water. The mice were 6 mo old at the time of initiation of interventions. Animal rooms were maintained at 20 to 22°C with 30 to 70% relative humidity and a 12-h light/dark cycle. Blood samples were collected via eye bleeds, and a maximal O2 consumption (Vo2max) test was performed before the initiation of dietary and exercise interventions. Mice were randomly assigned to 1 of 4 groups: sedentary ad libitum fed control (CTRL; n = 9); sedentary alternate-day food deprivation (ADF; n = 8); ad libitum fed daily treadmill exercise (EX; n = 9); and combined daily treadmill exercise and ADF (EXADF; n = 9). During the first week, 1 mouse in the CTRL group was found dead and was removed from the experiment. All other mice completed all tests in the study, except for the maximum endurance test, in which 1 mouse in the CTRL group and 1 mouse in the EXADF group refused to run on the treadmill. Treadmill training was conducted between 2 and 5 pm daily according to the following schedule: cohort 1 (6 EX mice), 2 to 3 pm; cohort 2 (3 EX and 3 EXADF mice), 3 to 4 pm; and cohort 3 (6 EXADF mice), 4 to 5 pm. For ADF mice, food [standard mouse chow: Teklad Global 18% Protein Rodent Diet (Sterilizable) 2018S; Envigo, Indianapolis, IN, USA] was alternatively provided or removed every day at 5 pm. For EXADF mice, food was provided or removed immediately after treadmill training, at 4 pm for cohort 2 and 5 pm for cohort 3 daily. Body weights were recorded weekly. Average weekly food/energy consumption was calculated (the weight of food provided minus the weight of food remaining uneaten) × kcal/g of food. Blood glucose measurements were made at baseline, then every 2 wk on consecutive feeding and food-deprivation days, before and after exercise, using a FreeStyle Lite Meter (Abbott Laboratories, Chicago, IL, USA). On the fourth week of training, blood ketone levels were measured on consecutive feeding and food-deprivation days before and after exercise with a StatSite M B-HB meter (EKF Diagnostics, Cardiff, United Kingdom). After 8 wk of training, the animals were anesthetized and decapitated. ADF and EXADF mice were killed on a food-deprivation day, and EX mice were killed 18 h after the last exercise session and food was removed 2 h beforehand. All mice were killed between 9 am and noon. Liver and soleus muscle tissues were collected, immediately flash frozen, and stored at −80°C. Trunk blood was collected, centrifuged, flash frozen, and stored at −80°C. All animal procedures were approved by the Animal Care and Use Committee of the National Institutes of Health (NIH) National Institute on Aging Intramural Research Program (Bethesda, MD, USA; Protocol 290-LNS-2019).
VO2max during treadmill exercise
The Vo2max treadmill test was performed twice, once to establish a baseline before initiation of ADF and exercise interventions and once during the fifth week of the interventions. The test was performed (between the hours of 1 and 4 pm) using the Oxymax Comprehensive Lab Animal Monitoring System (CLAMS) equipped with modular treadmills (Columbus Instruments, Columbus, OH, USA). On the acclimation day, the mice walked on the treadmill for 10 min at 10 m/min with the shock stimulus on. The next day the mice acclimated to the stationary treadmill for 10 min. The treadmill speed was then increased progressively from 5 m/min to 70 m/min over a period of 28 min, during which O2 consumption (Vo2) and CO2 consumption (Vco2) were measured. The experimental end point was reached when the animals reached a RER (Vco2/Vo2) of >1.0 or received the shock stimulus 5 times within 30 s. For the second test, mice in the ADF and EXADF groups were tested on a food-deprivation day. Vo2max was determined when oxygen uptake did not increase with increasing treadmill speed and the mouse subsequently failed to maintain effort.
Treadmill training
Training of EX and EXADF groups occurred between 2 and 5 pm daily on an Exer 3/6 treadmill (Columbus Instruments) without a shocker grid. A 2 min warmup was performed before every training session, with the speed increasing every 30 s (7, 10, 12, and 15 m/min). Training sessions were 45 min long. Week 1 training was at 15 m/min with a 10-degree incline. Week 2 training was at 20 m/min with a 10-degree incline. Week 3 training was 15 m/min at a 15-degree incline. Week 4 training was at 20 m/min with a 15-degree incline. Mice continued to train at wk 4 intensity for the next 3 wk on days when not undergoing the Vo2max test (1 d), metabolic assessment by CLAMS (3 d), or running endurance test (1 d).
Indirect calorimetry
Mice were placed in CLAMS metabolic cages 6 wk after initiation of the interventions. Calorimetric data were acquired during a 3 d period, including a 1 d acclimation period during which ADF was continued. Vo2, Vco2, and physical activity levels were measured. These indirect calorimetric data were used to estimate energy expenditure, including RER (25). Mice were removed at 8 am on the third day and returned to their home cages.
Endurance running test
The test was performed during the eighth week of the study between 2 and 5 pm on a Exer 3/6 treadmill without a shocker grid. On d 1, the mice walked on the treadmill for 10 min at 10 m/min with a 10-degree incline. On d 2, the mice acclimated to a stationary treadmill with a 10-degree incline for 10 min. The treadmill speed was then incrementally increased by 5 m/min every 15 min beginning at 10 m/min. After 60 min, the speed remained at 30 m/min. The experiment ended when the mouse fell off the back of the treadmill 3 times within 5 s. The ADF and EXADF mice were tested on a food-deprivation day.
Glucose tolerance test
Seven weeks into the experiment, mice were withheld food overnight, coinciding with food-deprivation days for the ADF and EXADF mice. The next morning, 2 g/kg of 30% glucose solution was injected intraperitoneally, and blood glucose (tail bleeds) was measured using a FreeStyle Lite Meter before the injection, and 15, 30, 60, and 120 min after the injection. Mice were returned to their cages between injections and at the end of the procedure. The test was performed between 11 am and 2 pm.
Metabolomics
Approximately 500 µl of trunk blood was collected in lithium heparin tubes and centrifuged at 12,000 rcf for 15 min at 4°C. Plasma was then collected, flash frozen, and sent to the NIH West Coast Metabolomics Center at the University of California–Davis (Davis, CA, USA) for metabolomics analyses. Analyses were performed using GC-MS methods previously described (26, 27). An Rtx-5Sil GC-MS column (Restek Corp., Bellefonte, PA, USA) was used for retention and separation of primary metabolite classes (amino acids, hydroxyl acids, carbohydrates, sugar acids, sterols, aromatics, nucleosides, amines, and miscellaneous compounds). The mobile phase consisted of helium, with a flow rate of 1 ml/min, an injection volume of 0.5 μl, and injection temperatures of 50°C ramped to 250°C by 12°C/s. Mass spectrometry parameters are used as follows: a Pegasus IV Mass Spectrometer (LECO, St. Joseph, MI, USA) was used with unit mass resolution at 17 spectra/s from 80 to 500 Da at −70 eV ionization energy and 1800 V detector voltage with a 230°C transfer line and a 250°C ion source.
Microarray analysis
Liver RNA was isolated using a RNeasy Mini kit (Qiagen, Germantown, MD, USA). RNA from soleus tissue was isolated using a RNeasy Fibrous Tissue Mini Kit (Qiagen). Total RNA quantity and quality was evaluated using the Bioanalyzer RNA 6000 Chip (Agilent Technologies, Santa Clara, CA, USA). Two hundred nanograms of total RNA was labeled using the Agilent Low-Input QuickAmp Labeling Kit, and was purified and quantified according to the manufacturer’s recommendations. A total of 600 ng Cy3-labeled cRNA was hybridized for 17 h to Agilent SurePrint G3 Mouse Gene Expression 8 × 60 K v.1 oligo microarrays (G4852A). After posthybridization rinses, arrays were scanned using an Agilent SureScan Miroarray Scanner at 3-μm resolution, and hybridization intensity data were extracted from the scanned images using Agilent’s Feature Extraction software. Raw data were subjected to z normalization, as described in Cheadle et al. (28). Principal component analysis (PCA) was performed on the normalized z scores of all of the detectable probes in the samples using DIANE 6.0, a spreadsheet-based microarray analysis program (NIH). Significant genes were selected by the z test <0.05, false discovery rate <0.30, as well as z ratio >1.5 in either direction, and ANOVA P < 0.05.
Mitochondrial DNA relative copy number
DNA from soleus tissue was extracted using a QIAamp DNA Mini kit (Qiagen). Mitochondrial DNA copy number was measured using quantitative PCR (qPCR). PCR analysis was performed using a Bio-Rad Pelthier Thermo Cycler (Bio-Rad, Hercules, CA, USA) and Opticon Monitor 3 software. PCR was performed under the following parameters: 95°C for 5 min, followed by 40 cycles of 95°C for 30 s, then 60°C for 30 s, and 72°C for 1 min, followed by the melting curve analysis (70–90.1°C for 2 s × 100 cycles). The ΔΔCt method was used to determine the normalized changes of MT-ND1 to actin. Supplemental Table S4 lists the primer sequences; 15 µM of primer was used per reaction.
Real-time quantitative PCR
Liver RNA was isolated using a RNeasy Mini Kit (Qiagen). RNA from soleus tissue was isolated using a RNeasy Fibrous Tissue Mini kit. Isolated RNA was reverse transcribed into cDNA using the Invitrogen SuperScript III First-Strand Synthesis System (Thermo Fisher Scientific, Waltham, MA, USA). qPCR analysis was performed as previously described with β2 microglobulin used as the internal control for the soleus and hypoxanthine–guanine phosphoribosyltransferase for the liver. Outliers of ±2 sd were removed from analysis. Supplemental Table S4 lists the primer sequences; 15 µM of primer was used per reaction.
Immunoblot oxidative phosphorylation analysis
Tissues were homogenized in RIPA lysis containing proteinase inhibitor (Roche Life Science, Branford, CT, USA) and phosphatase inhibitor (Sigma-Aldrich, St. Louis, MO, USA) and centrifuged. Protein content of the supernatant was determined using the Pierce BCA Protein Assay Kit (23225; Thermo Fisher Scientific). Thirteen micrograms of protein per lane was lysed in lithium dodecyl sulfate and incubated at 49°C for 10 min, then was run on a 10-lane Invitrogen 4–12% NuPAGE Bis-Tris Gel (NP0321BOX; Thermo Fisher Scientific) before being transferred to a nitrocellulose membrane (Thermo Fisher Scientific). Samples from the 4 groups were distributed equally between gels, with 7 to 8 lanes run per gel. The membrane was blocked with 5% milk for 1 h at room temperature, followed by an overnight incubation at 4°C with the primary antibody and a 1-h incubation at room temperature with the secondary antibody. Blots were probed with MitoProfile Total Oxphos Rodent WB Antibody Cocktail (110413; 1:1000 in Tris-buffered saline with Tween 20) and Sigma-Aldrich actin (A2066; 1:1000 in Tris-buffered saline with Tween 20) and quantified by ImageJ software (NIH), normalized to the actin band of the respective sample.
Statistical analysis
Data are presented as means ± sem. Analyses were performed by GraphPad Prism 5.04 (GraphPad Software, La Jolla CA, USA). The analyses of metabolomics data were implemented in R (R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org/). Comparisons between groups were performed by 1-way ANOVA with Tukey’s post hoc tests as specified. Repeated-measures ANOVA was used to test the effects of interventions on food consumption, body weight, blood glucose levels, and glucose tolerance across the time points. A value of P < 0.05 was considered statistically significant. Global metabolite analysis from metabolomics data was performed using ANCOVA as a function of posttreatment data adjusted for the pretreatment data (wk 0) for each mouse. Adjusted significance values were obtained via the Benjamini-Hochberg procedure to control for multiple testing. The analyses were performed on natural log2-transformed data.
RESULTS
Mice on ADF maintain body weight and exhibit improved glucose tolerance whether or not they exercise daily on a treadmill
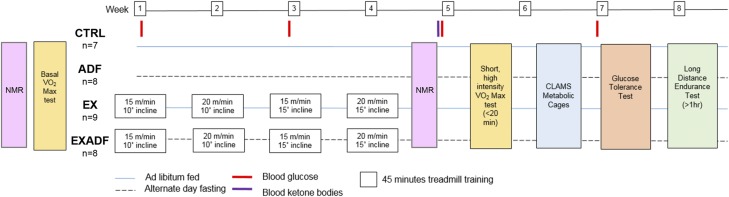
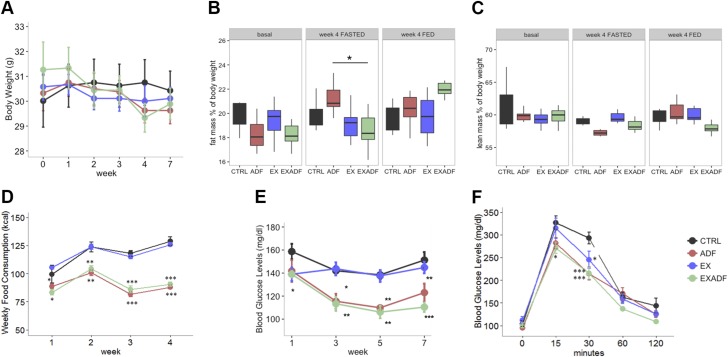
Male C57BL/6J mice that were 6 mo old were randomly assigned to 1 of 4 groups (8–9 mice/group): a sedentary group fed ad libitum (CTRL); a sedentary group on ADF; a group with daily treadmill running exercise fed ad libitum (EX); and a group that ran every day on a treadmill while on ADF (EXADF). The study design, including the timing of experimental interventions and blood and tissue collections, is shown in Fig. 1. There were no significant differences in body weights among the 4 groups at any time point during the study when measurements were made on feeding days (Fig. 2A). NMR body composition analysis during the fourth week revealed the ADF mice had significantly more fat mass than the EXADF group on a food-deprivation day but not a feeding day (Fig. 2B). There were no other significant differences in body fat mass (Fig. 2B) or lean mass (Fig. 2C) among the groups. We previously reported that the same strain and sex of mice used in the present study (C57BL/6) increased their food intake on the feeding days but do not completely compensate, such that they are ∼5 to 10% calorie restricted (29). As shown in Fig. 2D, the overall energy intake of the mice on ADF was ∼10 to 15% lower than mice fed ad libitum during the first week. Mice fed ad libitum increased their food intake during the 1-mo experimental period, whereas ADF mice did not increase their food intake. Exercise did not affect food intake in mice fed ad libitum or ADF (Fig. 2D). Circulating blood glucose levels decreased during the experimental period in mice in the ADF and EXADF groups, while blood glucose levels in the CTRL and EX groups remained unchanged (Fig. 2E). A glucose tolerance test performed on all mice during study wk 4 revealed that mice in the ADF and EXADF groups exhibited significantly lower peak plasma glucose elevation and a more rapid recovery of glucose levels after glucose metabolism compared to mice in the CTRL group (Fig. 2F). The mice in the EX group recovered glucose levels more rapidly than mice in the CTRL group, but less rapidly than mice in the ADF and EXADF groups.
Figure 1.
Experimental design. Mice were randomly assigned to sedentary CTRL, sedentary ADF, daily running (EX), or combined EX and ADF groups.
Figure 2.
Mice on ADF diet, with or without daily treadmill running, have reduced overall calorie intake, retain body mass, and exhibit improved glucose tolerance compared to mice fed ad libitum. A) Body weight. Body weights of mice in ADF, EX, and EXADF groups were not significantly different than mice in CTRL group at any time point. B) Fat mass as percentage of body weight. ADF mice had significantly more fat mass than EXADF group on food-deprivation day but not feeding day. There were no other significant differences in fat mass between groups. C) Lean mass as percentage of body weight. There were no significant differences in lean mass between groups. D) Food consumption during intervention wk 1–4. ADF and EXADF mice consumed fewer calories than CTRL and EX groups during wk 1, 2, 3, and 4. E) Blood glucose levels. Blood glucose levels were significantly lower in mice in ADF and EXADF groups compared to mice in CTRL and EX groups during wk 1–4. F) Results of glucose tolerance test. Blood glucose levels were measured 15, 30, 60, and 120 min after intraperitoneal injection of glucose (2 g/kg of 30% glucose solution). Two-way ANOVA was conducted on influence of ADF and exercise on glucose tolerance over course of 2 h. Mice in ADF and EXADF groups had significantly lower blood glucose levels compared to mice in CTRL group at 15 and 30 min time points. Group × Time interaction effect was not significant: F(12,125) = 1.59, P = 0.1. Values are means ± sem (n = 8 CTRL, 8 ADF, 9 EX, and 9 EXADF mice). *P < 0.05, **P < 0.01, ***P < 0.001.
ADF shifts fuel preference from carbohydrates to fatty acids and enhances endurance in trained mice
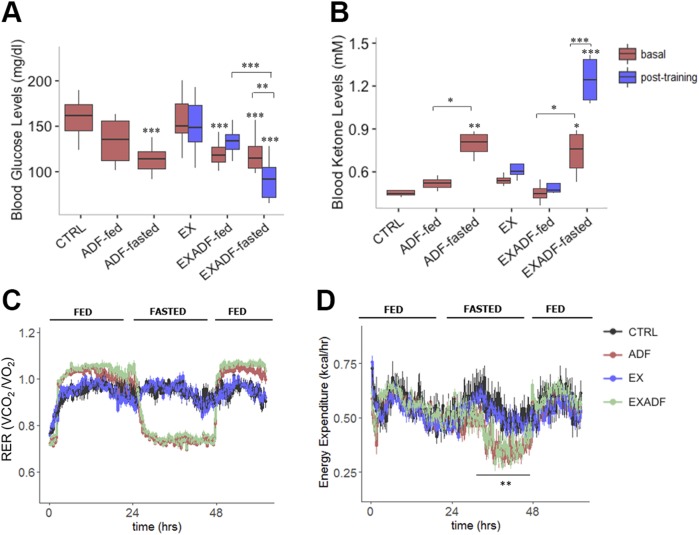
After 4 wk, mice in the ADF and EXADF groups exhibited lower circulating fed glucose levels than mice in the CTRL and EX groups (Fig. 3A). The reduction in plasma glucose levels in mice in the ADF group was particularly pronounced on the food-deprivation days when combined with exercise. Plasma ketone levels were significantly elevated on food-deprivation days in mice in the ADF and EXADF groups, with the highest levels occurring after 45 min of treadmill running on a food-deprivation day (Fig. 3B). These results suggest that ADF results in metabolic flexibility by intermittent switching between ketones and glucose as fuels sources, and by facilitating ketosis during endurance training. To determine the effects of ADF and exercise on energy balance and energy expenditure, we measured oxygen (Vo2) and carbon dioxide (Vco2) exchange rates in metabolic chambers while the mice were maintained in their dietary regimen during the sixth week of training. Over the course of 3 d, EXADF and ADF mice displayed significantly lower RERs (Vco2/Vo2) on food-deprivation days compared to their ad libitum feeding days and compared to mice in the CTRL and EX groups, indicating a more significant contribution of lipids to resting substrate metabolism in the food-deprived state (Fig. 3C) (25, 30). Moreover, on feeding after a food-deprivation day, the RER was elevated above that of mice fed ad libitum, suggesting an enhanced metabolic flexibility that results in a greater contribution of carbohydrates to energy provision in the fed state. Additionally, mice in the EXADF and ADF groups had lower energy expenditure on food-deprivation days compared to feeding days and compared to mice in the CTRL and EX groups (Fig. 3D).
Figure 3.
ADF reduces RER and energy expenditure, and triggers ketogenesis, which is enhanced by exercise. A) Blood glucose levels on feeding and food-deprivation days before and after 45 min training on treadmill during fourth week of training. B). Blood ketone levels on feeding and food-deprivation days before and after 45 min training on treadmill during fourth week of training. C) RER during 60-h time period in mice in CTRL, ADF, EX, and EXADF groups. On food-deprivation days, RER was greatly reduced in mice in ADF and EXADF groups, while on feeding days mice in ADF and EXADF groups exhibited higher RERs compared to mice in CTRL and EX groups. D) Energy expenditure during 60 h time period in mice in CTRL, ADF, EX, and EXADF groups. Mice in ADF and EXADF groups exhibited lower energy expenditure on food-deprivation days compared to feeding days and compared to mice in CTRL and EX groups. V are means ± sem [n = 8 CTRL, n = 8 ADF, n = 9 EX, and n = 9 EXADF mice (A); n = 4 CTRL, n = 4 ADF, n = 4 EX, and n = 4 EXADF mice (B); and n = 7 CTRL, n = 8 ADF, n = 9 EX, and n = 8 EXADF mice (C, D)]. *P < 0.05, **P < 0.01, ***P < 0.001.
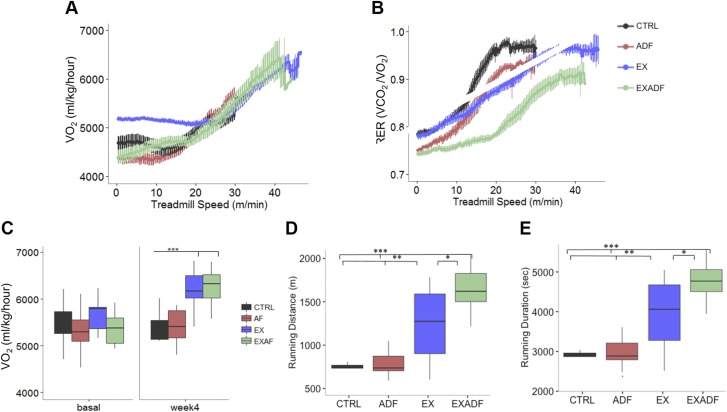
To investigate the impact of ADF on running performance, we tested the mice in a short (<20 min) high-intensity running test during the fifth week of training, and in a maximal endurance test during the eighth week of training. As expected, Vo2 increased progressively in all 4 groups of mice as the treadmill speed increased during the 20-min high-intensity running test (Fig. 4A). During the high-intensity test, the EXADF mice maintained higher levels of fat oxidation (RER <0.8) than their fed counterparts (EX), as did ADF mice vs. CTRL (Fig. 4B) (25, 30). This suggests that ADF enhances the ability to oxidize fatty acids during exercise in the food-deprived state, regardless of fitness level. The EXADF and EX mice achieved similarly higher Vo2max rates compared to untrained mice in the CTRL and ADF groups (Fig. 4C), indicating that ADF had no effect on this well-established physiological adaptation to training. In the maximum endurance test, the trained mice (EX and EXADF groups) ran significantly further and for a longer time period compared to untrained mice in the CTRL and ADF groups (Fig. 4D, E). Notably, mice that were on ADF during the 1 mo of daily treadmill running were able to run significantly further and for a longer time period compared to mice fed ad libitum during the 1-mo training period (Fig. 4D, E).
Figure 4.
ADF switches fuel utilization from carbohydrates to fats during exercise, and enhances endurance running performance without affecting Vo2max. A) VO2 rates during Vo2max exercise test 4 wk after initiation of dietary and exercise interventions. B) RER during Vo2max exercise test 4 wk after initiation of dietary and exercise interventions. RER was significantly lower in EXADF group compared to other groups, indicating high reliance on fat and ketone metabolism during exercise. C) Vo2max rates during exercise before initiation of diet and exercise interventions (basal) and 4 wk after initiation of interventions. D, E) Running endurance test of mice after 7 wk of diet and exercise interventions. Mice in EX and EXADF groups ran significantly further (D) and longer (E) than mice in CTRL and ADF groups. Mice in EXADF group ran significantly further and longer than mice in EX. Values are means ± sem [n = 8 CTRL, n = 8 ADF, n = 9 EX, and n = 9 EXADF mice (A, C); n = 7 CTRL, n = 8 ADF, n = 9 EX, and n = 8 EXADF mice (D, E)]. *P < 0.05, **P < 0.01, ***P < 0.001.
ADF up-regulates pathways involved in fatty acid metabolism, mitochondrial biogenesis, and cellular stress resistance in soleus muscle
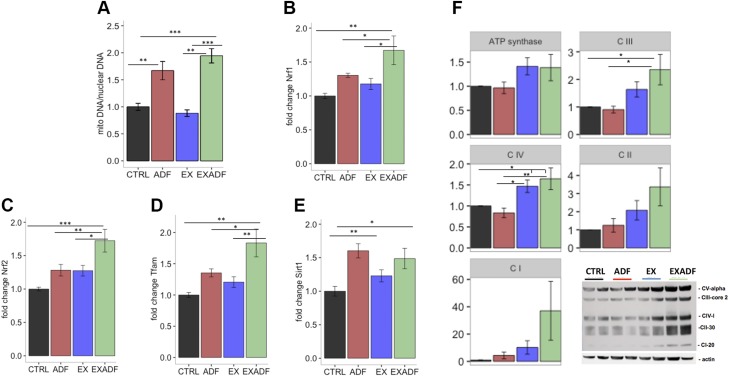
After the endurance test, mice were maintained on their diet and exercise regimens for an additional 3 d, at which time they were humanely killed and blood, soleus muscle, and liver tissues collected. Type I slow-twitch muscle fibers exhibit higher oxidative metabolism and increased mitochondrial density than type II fast-twitch fibers, so we focused on the soleus muscle (predominantly type I fibers), which are known to be critical to endurance running (30, 31). Levels of mitochondrial DNA were significantly increased in muscles of mice in the ADF and EXADF groups compared to the CTRL and EX groups (Fig. 5A). Targeted analyses of mRNAs known to encode proteins involved in cellular stress resistance and mitochondrial biogenesis revealed increased levels of NRF1, NRF2, and mitochondrial transcription factor A (TFAM) mRNAs in mice in the EXADF group compared to the other 3 groups (Fig. 5B–D). Levels of mRNA encoding sirtuin 1 (SIRT1), a protein known to mediate adaptive cellular responses to dietary energy restriction (32), were elevated in muscle of mice in the ADF and EXADF groups compared to CTRL and EX groups (Fig. 5E). Immunoblot for oxidative phosphorylation (oxphos) of the electron transport chain revealed significant increases in complex III in EXADF mice compared to CTRL and ADF mice and complex IV in EXADF mice and EX mice compared to CTRL and ADF mice (Fig. 5F). This increase in oxphos cannot be attributed solely to the increased mitochondrial biogenesis, as there were no increases in the oxphos complexes of ADF mice despite the significant increase in mitochondrial DNA suggested by Fig. 5A.
Figure 5.
Evidence that ADF induces mitochondrial biogenesis in soleus muscle. A) Mitochondrial DNA copy number analyzed by qPCR, normalized to actin, and displayed as relative fold change compared to CTRL group. Mitochondria DNA copy numbers were significantly greater in ADF and EXADF groups compared to CTRL and EX groups. B–E) NRF1, NRF2, TFAM, and SIRT1 mRNA levels were analyzed by qPCR, normalized to actin, and displayed as relative fold change compared to CTRL group. F) Immunoblot and densitometric quantification after actin normalization for oxphos of electron transport chain. Complex III was significantly increased in EXADF mice compared to CTRL and ADF mice, and complex IV was significantly increased in EXADF mice and EX mice compared to CTRL and ADF mice. Values are means ± sem [n = 7 CTRL, n = 8 ADF, n = 9 EX, and n = 8 EXADF mice (A, E); n = 7 CTRL, n = 8 ADF, n = 9 EX, and n = 7 EXADF mice (F)]. *P < 0.05, **P < 0.01, ***P < 0.001.
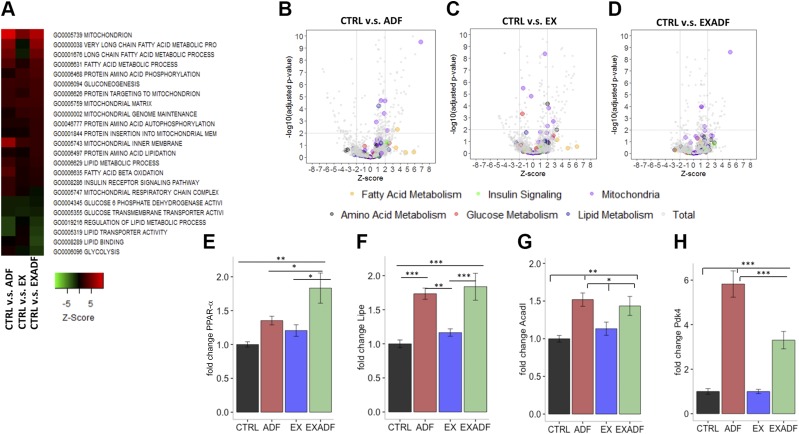
We next performed gene expression microarray analysis of soleus muscle samples from all mice. (The complete gene lists for all microarray analyses in the present study can be accessed using the approved Gene Expression Omnibus [GEO] identifier GSE104079; https://www.ncbi.nlm.nih.gov/geo/). Transcriptome analysis and Gene Ontology (GO) pathway analysis was used to explore the global changes in the gene expression and regulatory pathways in the soleus muscle. Compared to mice in the CTRL group, mice in the ADF and EXADF group exhibited up-regulation of pathways involved in mitochondrial function, and fatty acid and lipid metabolism (Fig. 6A, B, D). Compared to ADF, exercise alone had only modest effects on the expression of genes involved in energy metabolism pathways (Fig. 6A, C). Among all GO process annotations, mitochondria and fatty acid metabolism-related genes were highly enriched in the ADF and EXADF animals, while glycolysis and lipid synthetic pathways were enriched in the down-regulated gene sets in comparison with the CTRL. (Fig. 6B–D). Top significant GO pathways in CTRL-EX, CTRL-ADF, CTRL-EXADF, and EX-EXADF pairwise comparisons are depicted in Supplemental Fig. S1. The autophagy pathway was up-regulated in the muscle of mice in the EX and EXADF groups compared to the CTRL group (Supplemental Fig. S1). Overall, the pathway analyses revealed that exercise alone had a less pronounced impact on the energy metabolism pathways compared to ADF or EXADF.
Figure 6.
ADF affects major metabolic transcriptional pathways in soleus muscle. A) Heat map of significant GO pathways in soleus tissue. ADF and EXADF prominently enhanced expression of genes involved in fatty acid metabolism and mitochondrial activity while down-regulating genes involved in lipid synthesis and glycolysis. B–D) Volcano plots of GO pathways in soleus muscle of ADF, EX, and EXADF animals vs. CTRL. E–H) PPAR-α, hormone-sensitive lipase, ACADL, and PDK4 mRNA levels analyzed by qPCR, normalized to actin, and displayed as relative fold change compared to CTRL group. Values are means ± sem (n = 7 CTRL, n = 8 ADF, n = 9 EX, and n = 8 EXADF mice). *P < 0.05, **P < 0.01, ***P < 0.001.
We further investigated the key regulators of metabolic fuel selection in the soleus muscle by qPCR analysis of mRNAs encoding several major regulatory proteins. Nuclear receptor PPAR-α is fundamentally important in the activation of multiple cellular fatty acid uptake and utilization pathways. EXADF induced PPAR-α expression (Fig. 6E), and markedly increased the expression of the PPAR-α targets hormone-sensitive lipase, acyl–coenzyme A (CoA) dehydrogenase long chain (ACADL), and PDK4 (Fig. 6F–H). PDK4 inhibits the pyruvate dehydrogenase kinase complex and thus reduces the entry of glycolytic products into the tricarboxylic acid (TCA) cycle in the mitochondria (33). These data reveal that ADF enhances fatty acid oxidation and suppresses glucose metabolism in the skeletal muscle, suggesting that these metabolic adaptations contribute to the enhanced running endurance.
Examination of lists of individual genes that were differentially expressed in soleus muscles revealed marked up-regulation of acyl-CoA thioesterases 1 and 2 (Acot1 and Acot2) in soleus muscle in the ADF and EXADF groups, but not in the EX group (Supplemental Table S1). Compared to the CTRL group, mice in the ADF group exhibited up-regulation of genes encoding PDK4, mitochondrial uncoupling protein 3 (UCP3), potassium channel subfamily T, member 2 (KCNT2), and fatty acid 2 hydrolase (FA2H). PDK4 and FA2H promote fatty acid utilization and inhibit glucose utilization (33), UCP3 protects cells against mitochondrial stress (34), and KCNT2 is a mitochondrial potassium channel protein (35). Genes down-regulated by ADF in muscle cells included those involved in regulating cell excitability (HCN3 and GABRG2) and cell proliferation and differentiation (CELF3 and ONECUT1) (Supplemental Table S1). Genes of interest up-regulated by EX included the potassium channel KCNV2 and sphingosine kinase 2 (SPHK2). Compared to mice in the EX alone group, those in the EXADF group exhibited up-regulation of ACOT1, DOC2B (a calcium sensor protein involved in muscle insulin sensitivity) (36), MAPK4, and BCL2L1 (a mitochondria-associated protein that inhibits apoptosis). Genes up-regulated in muscle in the EXADF group compared to the CTRL group included ACOT1, DDIT4 (DNA damage-inducible transcript 4, a protein that inhibits mTORC1), CEBPD (CCAAT-enhancer binding protein δ, a protein associated with muscle strength (37), and CMKLR1 (chemokine-like receptor 1, which is involved in glucose tolerance) (38). Down-regulated in the EXADF group compared to the CTRL group were NALCN, FABP6 (fatty acid binding protein 6), and PON1 (paraoxonase 1).
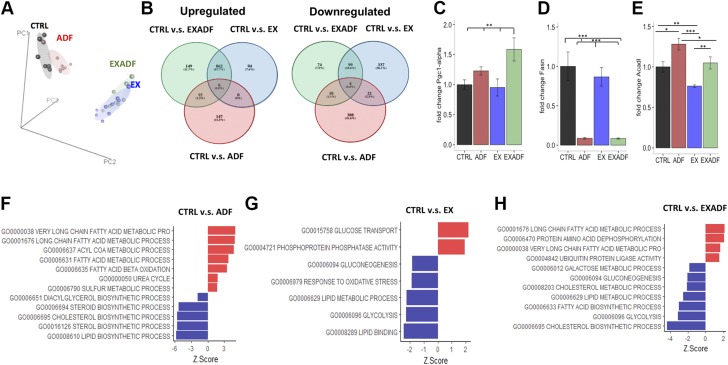
ADF and exercise differentially modify liver transcriptome
The liver plays a central role in the metabolic switch from carbohydrates to ketones as the major cellular fuel during food deprivation and extended exercise. We therefore performed gene expression analyses of liver tissue from mice in all 4 groups. PCA revealed that the liver transcriptomes of mice in the CTRL and ADF groups were similar and distinct from the transcriptomes of mice in the EX and EXADF groups (Fig. 7A). This contrasts with the muscle transcriptome, which was more greatly affected by ADF than exercise (Fig. 6). The number of transcripts that were significantly up-regulated or down-regulated in the ADF, EX, and EXADF groups compared to the CTRL group are shown in Fig. 7B. There were 868 genes that were up-regulated and 103 genes down-regulated in both the EX and EXADF groups. In contrast, only 21 of the same genes were up-regulated and 14 down-regulated in both the ADF and EXADF groups (Fig. 7B). Supplemental Table S2 includes the top 20 significantly up- and down-regulated genes in the 4 intergroup comparisons in the liver. Liver expression of PGC-1α was increased significantly in the EXADF group compared to the CTRL, ADF, and EX groups (Fig. 7C). Fatty acid synthase (FASN) was strongly down-regulated by ADF regardless of whether mice were exercising or sedentary (Fig. 7D). Levels of the mRNA encoding ACADL were significantly increased in response to ADF and decreased in response to EX (Fig. 7E). GO pathway analysis demonstrated strong induction of fatty acid metabolic processes and down-regulation of lipid biosynthetic pathways by ADF and EXADF, and down-regulation of glycolysis in the EX and EXADF groups (Fig. 7F–H and Supplemental Fig. S2). Ubiquitin protein ligase activity was up-regulated in the EXADF group, suggesting an increase in the cellular stress response as well. The autophagy pathway was significantly up-regulated in liver cells in response to ADF (Supplemental Fig. S2).
Figure 7.
Impact of treadmill running and ADF on liver transcriptome. A) PCA of liver gene microarray data reveals that liver transcriptomes of mice in EX and EXADF groups closely overlap and are distinct from transcriptomes of mice in CTRL and ADF groups. B) Venn diagrams of up-regulated and down-regulated gene transcripts present in CTRL-ADF, CTRL-EX, and CTRL-EXADF pairwise comparisons. C–E) PGC-1α, FASN, and ACADL mRNA levels analyzed by qPCR, normalized to actin, and displayed as relative fold change compared to CTRL group. Values are means ± sem (n = 7 CTRL, n = 8 ADF, n = 9 EX, and n = 8 EXADF mice). *P < 0.05, **P < 0.01, ***P < 0.001. F–H) Top metabolic GO pathways exhibiting differences between mice in CTRL groups and mice in ADF, EX, and EXADF groups (ranked by z-score values).
Among the mRNAs most greatly up-regulated in livers of mice in the ADF group compared to the CTRL groups were acetyl-CoA thioesterase 3 (ACOT3); flavin-containing monooxygenase 3 (FMO3), which is involved in detoxification pathways; and cutlike homeobox 2 (CUX2), which encodes a transcription factor involved in growth hormone signaling (39). Genes strongly down-regulated by ADF included those encoding the fatty acid binding protein 5 (FABP5), FASN, and early growth response 1 (ERG1), a transcriptional regulator of cell proliferation and differentiation (Supplemental Table S2). Similar to ADF alone, mice in the EXADF group exhibited up-regulation of FMO3 and CUX2, and down-regulation of FASN and FABP5. Compared to livers in mice in the CTRL group, those in the EX group exhibited highly significant up-regulation of genes encoding calcium/calmodulin dependent kinase 2a (CAMK2A), RNA-binding protein motif 3 (RBM3), which is involved in cellular stress adaptation (40), and membrane metallo-endopeptidase–like 1 (MMEL1) (41). Strongly down-regulated by EX were δ-like noncanonical Notch ligand 2 (DLK2), which is involved in Notch signaling and cell growth regulation, and sulfotransferase 4A1 (SULT4A1) (Supplemental Table S2). Overall, the liver gene expression analyses reveal major effects of ADF on lipid metabolism and cell growth, whereas exercise affects calcium signaling and stress adaptation.
ADF increases levels of plasma branched-chain amino acids, free fatty acids, and ketogenic metabolites
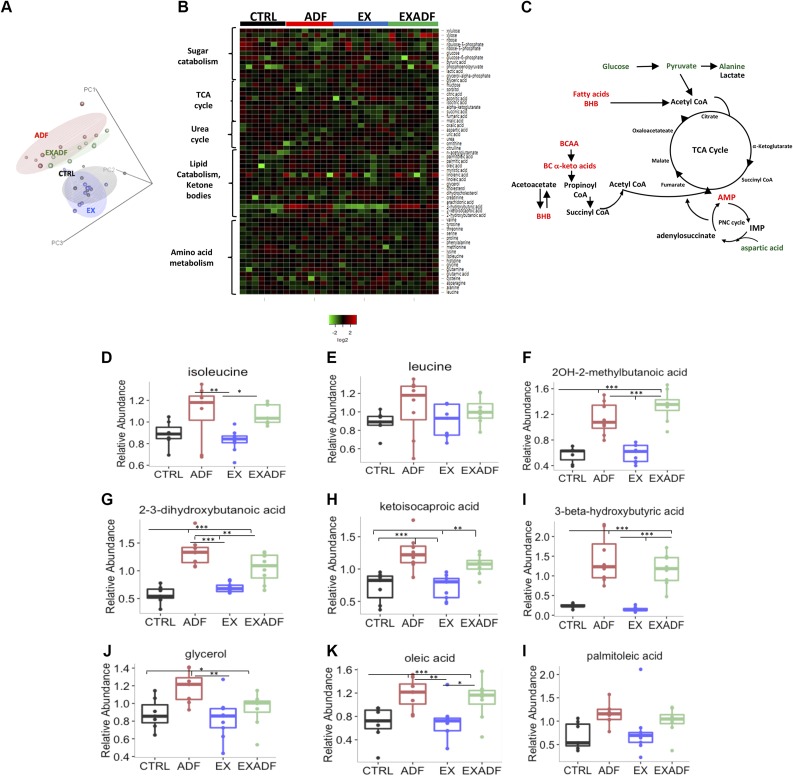
To characterize the metabolic mediators of food deprivation and exercise, we evaluated metabolites in plasma samples of each mouse. The raw data for the metabolomics analysis is included in the Supplemental Data). PCA was performed on 185 identified metabolites and revealed that the metabolomes of mice in the ADF and EXADF groups were similar to each other and distinct from mice in the CTRL and EX groups (Fig. 8A). The heat map in Fig. 8B depicts a subset of metabolites grouped by major metabolic pathways. Levels of branched chain amino acids, free fatty acids, and keto acids were elevated in the plasma of mice in the ADF and EXADF groups compared to the CTRL group, whereas levels of metabolites feeding glycolysis (glucose and pyruvate) were reduced. Notably, metabolites implicated in the urea cycle (urea, citrulline, ornithine, and N-acetylglutamate) were not up-regulated, suggesting that gluconeogenesis fueled by the catabolism of amino acids was not a major source of fuel for the unfed-state mice.
Figure 8.
Impact of treadmill running and ADF on plasma metabolome. A) PCA of plasma metabolomics data reveals that metabolomes of mice in ADF and EXADF groups closely overlap and are distinct from metabolomes of mice in CTRL and EX groups. B) Heat map showing relative levels of major metabolites in each of indicated pathways from each mouse in each group. C) Diagram showing metabolites feeding into TCA cycle that are either elevated (red) or reduced (green) in mice in ADF and EXADF groups compared to CTRL group. BCAA, branched chain amino acids; CoA, coenzyme A; IMP, inositol monophosphate; PNC, purine nucleotide cycle. D, E). Relative concentrations of ketogenic amino acids isoleucine and leucine in plasma of mice in 4 different groups. F–H) Relative concentrations of branched-chain α-keto acids plasma of mice in 4 different groups. I) Relative concentrations of ketone β-hydroxybutyrate in plasma of mice in 4 different groups. J–L) Relative concentrations of fatty acid metabolites glycerol, oleic acid, and palmitoleic acid in plasma of mice in 4 different groups. Values are means ± sem (n = 7 CTRL, n = 8 ADF, n = 9 EX, and n = 8 EXADF mice). *P < 0.05, **P < 0.01, ***P < 0.001.
These data are consistent with a metabolic shift in cellular fuel source from glucose to fatty acids (Fig. 8C). Levels of the ketogenic amino acids isoleucine and leucine were elevated in the plasma of mice in the ADF and EXADF groups compared to the CTRL group, whereas the EX group exhibited no significant effect on the levels of these amino acids (Fig. 8D, E). Levels of many different fatty acid metabolites were elevated in plasma of mice in the ADF and EXADF groups, but not in the EX group. These included 2OH-2-methylbutanoic acid, 2,3-dihydroxybutanoic acid, ketoisocaproic acid, BHB, glycerol, oleic acid, and palmitoleic acid (Fig. 8F–L). Other metabolites significantly increased in response to ADF included intermediates of amino acid catabolism such as N-acetylglycine (Supplemental Table S3). Circulating levels of glucose and glycolytic intermediates were decreased in response to ADF (Supplemental Table S3).
DISCUSSION
The major novel finding in the present study is that the training effect of treadmill running on endurance is enhanced when mice are maintained on an ADF intermittent feeding schedule during training. The enhanced performance afforded by ADF during training was not the result of an effect on Vo2max because Vo2max increased by similar amounts by treadmill training in mice in both the ad libitum and ADF diet groups. Instead, RER during exercise was significantly reduced in mice on ADF compared to the CTRL diet group, indicating that ADF causes a switch in fuel source from carbohydrates to fats. This fuel source switch was confirmed by metabolomic analysis of plasma and by gene expression analyses of soleus muscle and liver tissue samples. Accordingly, levels of circulating fatty acids, ketogenic amino acids, and ketones were elevated in mice in the ADF and EXADF groups, compared to the ad libitum–fed CTRL and EX groups. Pathways involved in fatty acid and ketone metabolism were up-regulated and glycolytic pathways down-regulated in soleus muscle of mice in the ADF and EXADF groups. Additional interrogation of levels of specific mRNAs and proteins in muscle provided evidence that ADF promotes mitochondrial biogenesis (increased mitochondrial/nuclear DNA ratio, and up-regulation of TFAM) and increases cellular resistance to oxidative and metabolic stress (up-regulation of NRF1, NRF2, and SIRT1). Therefore, our findings demonstrate that an eating pattern that includes intermittent metabolic switching can enhance the training effects of a running exercise program by mechanisms involving ketogenesis, mitochondrial biogenesis, and enhanced cellular stress adaptation in muscle cells.
A shift in substrate utilization from glucose toward free fatty acids and ketones is a well-known metabolic adaptation to endurance exercise. In humans, efficient fatty acid utilization contributes to high intrinsic exercise capacity and the health benefits associated with enhanced fitness, while poor metabolic health is associated with high basal use of glucose and impaired fuel switching during the transition from fasting to feeding (42, 43). Improvement in physical performance with training involves increased muscle oxidative capacity (44). We found that mice on ADF during daily treadmill training exhibit superior endurance and a robust switch to fatty acid utilization during exercise compared to mice fed ad libitum during training. Differences in body weight and Vo2max were not responsible for the endurance-enhancing effect of ADF because body weights and Vo2max of mice in the EXADF and EX groups were not different. Instead, mice on ADF utilized fatty acids during exercise, whereas mice fed ad libitum utilized carbohydrates, as indicated by elevated plasma BHB levels and reduced RER during exercise in mice on ADF. Our metabolomics and gene expression analyses indicate that ADF up-regulates fatty acid metabolism and ketogenesis, down-regulates the glycolytic intermediates of the TCA cycle, and stimulates mitochondrial biogenesis and cellular stress resistance in muscle cells. Ketogenesis may play a key role in the enhancement of endurance by ADF because BHB levels were elevated to higher levels in mice in the EXADF group compared to mice in in the ADF or EX groups. Earlier studies in humans suggested that once adapted to the metabolic switch, endurance can be enhanced by consumption of ketogenic diets/fatty acids (45, 46) or ketogenic amino acids (47–49), although other studies did not reveal beneficial effects of ketogenic diets (50, 51). It should also be noted that high-volume exercise may increase glycogen stores. For example, data from a study of sled dogs competing in a 1600-mile race suggest that glycogen stores increase in response to high-volume, moderate-intensity training (52). Interestingly, our RER data (Fig. 2C) show that mice on ADF use mainly fats as a fuel on the food-deprivation days and relatively more carbohydrates on the feeding days compared to mice fed ad libitum. This result is consistent with the possibility that glycogen stores are increased in response to IF.
Although dietary supplementation with a ketone ester consistently elevates plasma BHB levels, the implications of nutritional ketosis for elite physical performance must be further investigated. For example, while ketone ester supplementation improved endurance in rats and in human cyclists who fasted overnight, another study reported elite cyclists given ketone ester after a typical high-carbohydrate meal before a race experienced gastrointestinal distress and decreased endurance (53). However, in contrast to the study showing enhanced endurance with ketone ester supplementation (24), Leckey et al. (53) did not attempt to adapt the subjects to the ketogenic state before the performance test. Cyclists consumed a carbohydrate breakfast and caffeine 2 h before the time trial, and all subjects reported gastrointestinal distress and poorer performance. On the other hand, participants in the Cox et al. study (24) fasted overnight before the test and abstained from caffeine. Studies of ketogenic diets and exercise performance in animals and humans have yielded mixed results. Whereas short-term (several days) ingestion of high-fat diets may impair aerobic exercise capacity, long-term adaptation to such ketogenic diets may not adversely affect endurance capacity (54). Although high-fat diets can be ketogenic, they are typically high in saturated fats and protein and can be proinflammatory (55). In contrast, food deprivation in laboratory animals and voluntary fasting in humans mobilizes fatty acid ketone precursors in a regulated manner and also engages many adaptive signaling pathways that promote cellular stress resistance and resilience (4, 5). With the latter thought in mind, our findings suggest that the impact of intermittent dietary energy restriction on the performance during prolonged high-intensity interval regimens merits investigation in human subjects. Our findings demonstrate that mice fed a standard high-carbohydrate diet but deprived of food every other day exhibit elevated BHB levels and improved running endurance, similar to what may occur with ketone ester supplementation once adapted to the metabolic switching.
Previous studies in rodents and humans have demonstrated beneficial effects of IF on general health and disease resistance. In human subjects, systemic responses to intermittent energy restriction include increased insulin sensitivity, reduction in abdominal fat mass, reductions in markers of oxidative stress and inflammation, and improved lipid profiles (56–63). In rodents, IF reduces tumor incidence and growth (64, 65) prevents and reverses type 2 diabetes (4), and protects the heart and brain against damage in models of myocardial infarction (66) and stroke (67, 68). The signaling pathways and molecular mechanisms by which ADF affects cells has been previously investigated in studies of the brain and heart (4, 5). The mechanisms include activation of trophic factor signaling pathways, autophagy and mitochondrial biogenesis, up-regulation of protein chaperones and antioxidant defenses, and suppression of inflammation (4, 5, 69–71). While the effects of exercise on muscle cells have been intensively studied (7, 72), and several studies have elucidated effects of acute food deprivation on muscle energy metabolism (73–75), molecular adaptations of muscles to IF and interactive effects of IF and exercise on physical performance are largely unknown. One previous study reported that when mice are fed a high-fat diet and deprived of food 15 h every day, they do not become obese, in contrast to mice fed the same diet ad libitum (9). In the latter study, the mice on daily time-restricted feeding performed better on a rotarod test of motor function and ran further on a treadmill compared to the mice fed ad libitum—differences in performance that may be explained by the lower body weight of mice on the time-restricted feeding schedule. In contrast, in the present study, there were no significant differences in body weights of mice in the CTRL, ADF, EX, and EXADF groups, a phenomenon observed previously in C57BL/6 male mice on an ADF regimen (29). This maintenance of body weight precludes an effect of body weight on the enhancement of endurance performance by ADF during 1 mo of treadmill training. ADF also did not significantly affect the endurance performance of untrained mice, indicating that ADF may not be beneficial for endurance in the absence of exercise training.
Our PCA of gene microarray data revealed major changes in the soleus muscle transcriptome in response to ADF, with exercise having less of an overall effect. We found that major transcriptomic responses of muscle to ADF that prominently involve genes encoding proteins involved in fatty acid metabolism, mitochondrial function, and amino acid metabolism. ACOT1, which encodes an acyl-CoA thioesterase, was the most highly up-regulated gene in soleus muscle of mice in both the ADF and EXADF groups. It was recently reported that ACOT1 translocates to the nucleus during dietary energy restriction and regulates PPAR-α in liver cells (76). The latter study also provided evidence that ACOT1 suppresses hepatic oxidative stress and inflammation. If ACOT1 also plays similar roles in muscle cells, it may mediate beneficial effects of ADF on muscle health. Mitochondrial uncoupling proteins play important roles in the adaptation of cells with a high energy demand (muscle cells and neurons) to excitatory and metabolic stress (77). UCP3 is the most abundantly expressed uncoupling protein in skeletal muscle, wherein it may play an important role in reduction of oxidative stress and food deprivation–induced fatty acid oxidation (78). UCP3 expression is also up-regulated by exercise (79), but its role in adaptations to endurance training have not been established. Interestingly, mice overexpressing UCP3 only in skeletal muscles are lean and exhibit reduced plasma glucose and insulin levels despite being hyperphagic (80). Our findings therefore suggest a role for muscle UCP3 in the reductions in fat mass and improvements in glucose metabolism that occur in response to IF. We also found that ADF affects the expression of genes encoding proteins involved in regulating cell excitability, including the potassium channel KCNT2. KCNT2 (also known as SLICK) is widely expressed in excitable cells, wherein it is activated by intracellular Na+, is inhibited by ATP, and contributes to the resting membrane potential (81, 82). Evidence suggests that KCNT2 is also present in the mitochondrial membrane, where it plays a role in adaptation of cells to metabolic stress and the clinically important process of ischemic preconditioning (35). Whether changes in muscle cell excitability occur in response to changes in muscle cell metabolism during ADF, and whether KCNT2 plays a role in the enhancement of endurance by ADF remain to be determined.
Thus far, randomized controlled trials of IF in humans have focused on evaluation of general health indicators and biomarkers of risk for cardiovascular disease and diabetes (8, 83). Inasmuch as many physiologic responses to IF are similar in mice and humans, our findings suggest a potential for IF eating patterns to enhance the training effects of endurance exercise in humans. Historically, endurance athletes consume diets with a high carbohydrate content throughout the day and during events to support their high level of energy expenditure (84, 85). However, such diets preclude metabolic switching, and the associated ketogenesis and activation of signaling pathways that bolster the health and functionality of the excitable cells critical for optimal performance (skeletal and cardiac muscle cells and neurons) (4, 5). Our findings demonstrate a performance-enhancing effect of ADF on endurance running in mice and elucidate the underlying metabolic and molecular mechanisms. IF eating patterns enable loss of fat mass with maintenance of lean mass in laboratory animals and humans (60, 61, 86). Moreover, it was recently reported that daily time-restricted feeding diets (16 h of fasting) enables men performing resistance training to lose body fat while increasing muscle mass and functionality (87, 88). Recent findings suggest that exercise can prevent loss of muscle mass in humans on low-calorie diets by mechanisms involving up-regulation of leptin receptors and increased fatty acid oxidation in muscle cells (19, 20).
The evidence that evolutionary pressures favored individuals capable of maximal physical and mental performance in the food-deprived state (3, 5), as well as the general health-promoting and supposedly antiaging effects of IF documented in laboratory animals (8), suggest the possibility that IF and exercise may synergistically attenuate the inevitable mental and physical age-related decline. It will be of considerable interest to perform randomized controlled trials of IF in human endurance athletes to determine whether intermittent metabolic fuel switching during training affects performance. Finally, it is conceivable that effects of intermittent energy restriction on energy metabolism and exercise performance may differ in rodents and humans, may depend on the type of exercise, and may differentially affect different muscle types. Further studies will be required to address these important issues.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Program of the U.S. National Institutes of Health, National Institute of Aging. The authors declare no conflicts of interest.
Glossary
- ACADL
acyl–coenzyme A dehydrogenase long chain
- ACOT
acetyl–coenzyme A thioesterase
- ADF
alternate-day food deprivation
- BHB
3β-hydroxybutyrate
- CLAMS
Comprehensive Lab Animal Monitoring System
- CoA
coenzyme A
- CTRL
ad libitum fed control
- CUX2
cutlike homeobox 2
- EX
ad libitum fed daily treadmill exercise
- EXADF
daily treadmill exercise and alternate-day food deprivation
- FA2H
fatty acid 2 hydrolase
- FABP5
fatty acid binding protein 5
- FASN
fatty acid synthase
- FMO3
flavin-containing monooxygenase 3
- GO
Gene Ontology
- IF
intermittent food deprivation
- KCNT2
potassium channel subfamily T member 2
- mTOR
mammalian target of rapamycin
- NIH
U.S. National Institutes of Health
- NRF1/2
nuclear regulatory factor 1/2
- oxphos
oxidative phosphorylation
- PCA
principal component analysis
- PDK4
pyruvate dehydrogenase kinase 4
- PGC-1α
peroxisome proliferator–activated receptor γ coactivator 1α
- PPAR-δ
peroxisome proliferator–activated receptor δ
- qPCR
quantitative PCR
- RER
respiratory exchange ratio
- SIRT1
sirtuin 1
- TCA
tricarboxylic acid
- TFAM
mitochondrial transcription factor A
- UCP3
uncoupling protein 3
- Vco2
CO2 consumption
- Vo2
O2 consumtion
- Vo2max
maximal O2 consumption
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
K. Marosi and M. P. Mattson designed the experiments; K. Marosi, K. Moehl, I. Navas-Enamorado, S. J. Mitchell, and E. Lehrmann performed the experiments; K. Marosi, K. Moehl, Y. Zhang, M. A. Aon, and S. Cortassa analyzed the data; and K. Marosi, K. Moehl, K. G. Becker, and M. P. Mattason wrote the article.
REFERENCES
- 1.Hawkes K., O’Connell J. F., Jones N. G. (1991) Hunting income patterns among the Hadza: big game, common goods, foraging goals and the evolution of the human diet. Philos. Trans. R. Soc. Lond. B Biol. Sci. 334, 243–250 [DOI] [PubMed] [Google Scholar]
- 2.Stanford C. B., Wallis J., Matama H., Goodall J. (1994) Patterns of predation by chimpanzees on red colobus monkeys in Gombe National Park, 1982–1991. Am. J. Phys. Anthropol. 94, 213–228 [DOI] [PubMed] [Google Scholar]
- 3.Mattson M. P. (2015) Lifelong brain health is a lifelong challenge: from evolutionary principles to empirical evidence. Ageing Res. Rev. 20, 37–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anton S. D., Moehl K., Donahoo W. T., Marosi K., Mainous A. G., Leeuwenburgh C., Mattson M. P. (2018) Flipping the metabolic switch: understanding and applying health benefits of fasting. Obesity (Silver Spring) 26, 254–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattson M. P., Moehl K., Ghena N., Schmaedick M., Cheng A. (2018) Intermittent metabolic switching, neuroplasticity and brain health. Nat. Rev. Neurosci. 19, 63–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans M., Cogan K. E., Egan B. (2017) Metabolism of ketone bodies during exercise and training: physiological basis for exogenous supplementation. J. Physiol. 595, 2857–2871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neufer P. D., Bamman M. M., Muoio D. M., Bouchard C., Cooper D. M., Goodpaster B. H., Booth F. W., Kohrt W. M., Gerszten R. E., Mattson M. P., Hepple R. T., Kraus W. E., Reid M. B., Bodine S. C., Jakicic J. M., Fleg J. L., Williams J. P., Joseph L., Evans M., Maruvada P., Rodgers M., Roary M., Boyce A. T., Drugan J. K., Koenig J. I., Ingraham R. H., Krotoski D., Garcia-Cazarin M., McGowan J. A., Laughlin M. R. (2015) Understanding the cellular and molecular mechanisms of physical activity-induced health benefits. Cell Metab. 22, 4–11 [DOI] [PubMed] [Google Scholar]
- 8.Mattson MP, Longo VD, Harvie M. (2017) Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 39, 46–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaix A., Zarrinpar A., Miu P., Panda S. (2014) Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 20, 991–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Longo V. D., Mattson M. P. (2014) Fasting: molecular mechanisms and clinical applications. Cell Metab. 19, 181–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattson M. P. (2012) Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 16, 706–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wan R., Camandola S., Mattson M. P. (2003) Intermittent food deprivation improves cardiovascular and neuroendocrine responses to stress in rats. J. Nutr. 133, 1921–1929 [DOI] [PubMed] [Google Scholar]
- 13.Seals D. R., Walker A. E., Pierce G. L., Lesniewski L. A. (2009) Habitual exercise and vascular ageing. J. Physiol. 587, 5541–5549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stranahan A. M., Khalil D., Gould E. (2007) Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus 17, 1017–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stranahan A. M., Lee K., Martin B., Maudsley S., Golden E., Cutler R. G., Mattson M. P. (2009) Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus 19, 951–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Booth F. W., Roberts C. K., Laye M. J. (2012) Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2, 1143–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalinkovich A., Livshits G. (2017) Sarcopenic obesity or obese sarcopenia: a cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 35, 200–221 [DOI] [PubMed] [Google Scholar]
- 18.Nakamura M. T., Yudell B. E., Loor J. J. (2014) Regulation of energy metabolism by long-chain fatty acids. Prog. Lipid Res. 53, 124–144 [DOI] [PubMed] [Google Scholar]
- 19.Perez-Suarez I., Ponce-González J. G., de La Calle-Herrero J., Losa-Reyna J., Martin-Rincon M., Morales-Alamo D., Santana A., Holmberg H. C., Calbet J. A. L. (2017) Severe energy deficit upregulates leptin receptors, leptin signaling, and PTP1B in human skeletal muscle. J. Appl. Physiol. 123, 1276–1287 [DOI] [PubMed] [Google Scholar]
- 20.Calbet J. A. L., Ponce-González J. G., Calle-Herrero J., Perez-Suarez I., Martin-Rincon M., Santana A., Morales-Alamo D., Holmberg H. C. (2017) Exercise preserves lean mass and performance during severe energy deficit: the role of exercise volume and dietary protein content. Front. Physiol. 8, 483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burke L. M., Millet G., Tarnopolsky M. A.; International Association of Athletics Federations . (2007) Nutrition for distance events. J. Sports Sci. 25(Suppl 1), S29–S38; erratum: 2009; 27, 667 [DOI] [PubMed] [Google Scholar]
- 22.Volek J. S., Noakes T., Phinney S. D. (2015) Rethinking fat as a fuel for endurance exercise. Eur. J. Sport Sci. 15, 13–20 [DOI] [PubMed] [Google Scholar]
- 23.Murray A. J., Knight N. S., Cole M. A., Cochlin L. E., Carter E., Tchabanenko K., Pichulik T., Gulston M. K., Atherton H. J., Schroeder M. A., Deacon R. M., Kashiwaya Y., King M. T., Pawlosky R., Rawlins J. N., Tyler D. J., Griffin J. L., Robertson J., Veech R. L., Clarke K. (2016) Novel ketone diet enhances physical and cognitive performance. FASEB J. 30, 4021–4032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox P. J., Kirk T., Ashmore T., Willerton K., Evans R., Smith A., Murray A. J., Stubbs B., West J., McLure S. W., King M. T., Dodd M. S., Holloway C., Neubauer S., Drawer S., Veech R. L., Griffin J. L., Clarke K. (2016) Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab. 24, 256–268 [DOI] [PubMed] [Google Scholar]
- 25.Simonson D. C., DeFronzo R. A. (1990) Indirect calorimetry: methodological and interpretative problems. Am. J. Physiol. 258, E399–E412 [DOI] [PubMed] [Google Scholar]
- 26.Fiehn O., Wohlgemuth G., Scholz M., Kind T., Lee D. Y., Lu Y., Moon S., Nikolau B. (2008) Quality control for plant metabolomics: reporting MSI-compliant studies. Plant J. 53, 691–704 [DOI] [PubMed] [Google Scholar]
- 27.Mitchell S. J., Madrigal-Matute J., Scheibye-Knudsen M., Fang E., Aon M., González-Reyes J. A., Cortassa S., Kaushik S., Gonzalez-Freire M., Patel B., Wahl D., Ali A., Calvo-Rubio M., Burón M. I., Guiterrez V., Ward T. M., Palacios H. H., Cai H., Frederick D. W., Hine C., Broeskamp F., Habering L., Dawson J., Beasley T. M., Wan J., Ikeno Y., Hubbard G., Becker K. G., Zhang Y., Bohr V. A., Longo D. L., Navas P., Ferrucci L., Sinclair D. A., Cohen P., Egan J. M., Mitchell J. R., Baur J. A., Allison D. B., Anson R. M., Villalba J. M., Madeo F., Cuervo A. M., Pearson K. J., Ingram D. K., Bernier M., de Cabo R. (2016) Effects of sex, strain, and energy intake on hallmarks of aging in mice. Cell Metab. 23, 1093–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheadle C., Cho-Chung Y. S., Becker K. G., Vawter M. P. (2003) Application of z-score transformation to Affymetrix data. Appl. Bioinformatics 2, 209–217 [PubMed] [Google Scholar]
- 29.Anson R. M., Guo Z., de Cabo R., Iyun T., Rios M., Hagepanos A., Ingram D. K., Lane M. A., Mattson M. P. (2003) Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc. Natl. Acad. Sci. USA 100, 6216–6220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egan B., Zierath J. R. (2013) Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 17, 162–184 [DOI] [PubMed] [Google Scholar]
- 31.Wernig A., Irintchev A., Weisshaupt P. (1990) Muscle injury, cross-sectional area and fibre type distribution in mouse soleus after intermittent wheel-running. J. Physiol. 428, 639–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boutant M., Cantó C. (2013) SIRT1 metabolic actions: integrating recent advances from mouse models. Mol. Metab. 3, 5–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pilegaard H., Neufer P. D. (2004) Transcriptional regulation of pyruvate dehydrogenase kinase 4 in skeletal muscle during and after exercise. Proc. Nutr. Soc. 63, 221–226 [DOI] [PubMed] [Google Scholar]
- 34.Nabben M., Hoeks J. (2008) Mitochondrial uncoupling protein 3 and its role in cardiac- and skeletal muscle metabolism. Physiol. Behav. 94, 259–269 [DOI] [PubMed] [Google Scholar]
- 35.Smith C. O., Nehrke K., Brookes P. S. (2017) The Slo(w) path to identifying the mitochondrial channels responsible for ischemic protection. Biochem. J. 474, 2067–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramalingam L., Oh E., Yoder S. M., Brozinick J. T., Kalwat M. A., Groffen A. J., Verhage M., Thurmond D. C. (2012) Doc2b is a key effector of insulin secretion and skeletal muscle insulin sensitivity. Diabetes 61, 2424–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harries L. W., Pilling L. C., Hernandez L. D., Bradley-Smith R., Henley W., Singleton A. B., Guralnik J. M., Bandinelli S., Ferrucci L., Melzer D. (2012) CCAAT-enhancer–binding protein-beta expression in vivo is associated with muscle strength. Aging Cell 11, 262–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ernst M. C., Haidl I. D., Zúñiga L. A., Dranse H. J., Rourke J. L., Zabel B. A., Butcher E. C., Sinal C. J. (2012) Disruption of the chemokine-like receptor-1 (CMKLR1) gene is associated with reduced adiposity and glucose intolerance. Endocrinology 153, 672–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conforto T. L., Steinhardt G. F., IV, Waxman D. J. (2015) Cross talk between GH-regulated transcription factors HNF6 and CUX2 in adult mouse liver. Mol. Endocrinol. 29, 1286–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Danno S., Nishiyama H., Higashitsuji H., Yokoi H., Xue J. H., Itoh K., Matsuda T., Fujita J. (1997) Increased transcript level of RBM3, a member of the glycine-rich RNA-binding protein family, in human cells in response to cold stress. Biochem. Biophys. Res. Commun. 236, 804–807 [DOI] [PubMed] [Google Scholar]
- 41.Hirschfield G. M., Liu X., Han Y., Gorlov I. P., Lu Y., Xu C., Lu Y., Chen W., Juran B. D., Coltescu C., Mason A. L., Milkiewicz P., Myers R. P., Odin J. A., Luketic V. A., Speiciene D., Vincent C., Levy C., Gregersen P. K., Zhang J., Heathcote E. J., Lazaridis K. N., Amos C. I., Siminovitch K. A. (2010) Variants at IRF5-TNPO3, 17q12-21 and MMEL1 are associated with primary biliary cirrhosis. Nat. Genet. 42, 655–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Overmyer K. A., Evans C. R., Qi N. R., Minogue C. E., Carson J. J., Chermside-Scabbo C. J., Koch L. G., Britton S. L., Pagliarini D. J., Coon J. J., Burant C. F. (2015) Maximal oxidative capacity during exercise is associated with skeletal muscle fuel selection and dynamic changes in mitochondrial protein acetylation. Cell Metab. 21, 468–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelley D. E., Mandarino L. J. (2000) Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes 49, 677–683 [DOI] [PubMed] [Google Scholar]
- 44.Gibala M. J., Jones A. M. (2013) Physiological and performance adaptations to high-intensity interval training. Nestle Nutr. Inst. Workshop Ser. 76, 51–60 [DOI] [PubMed] [Google Scholar]
- 45.Van Zyl C. G., Lambert E. V., Hawley J. A., Noakes T. D., Dennis S. C. (1996) Effects of medium-chain triglyceride ingestion on fuel metabolism and cycling performance. J. Appl. Physiol. 80, 2217–2225 [DOI] [PubMed] [Google Scholar]
- 46.Lambert E. V., Speechly D. P., Dennis S. C., Noakes T. D. (1994) Enhanced endurance in trained cyclists during moderate intensity exercise following 2 weeks adaptation to a high fat diet. Eur. J. Appl. Physiol. Occup. Physiol. 69, 287–293 [DOI] [PubMed] [Google Scholar]
- 47.Blomstrand E., Eliasson J., Karlsson H. K., Köhnke R. (2006) Branched-chain amino acids activate key enzymes in protein synthesis after physical exercise. J. Nutr. 136(1 Suppl)269S–273S [DOI] [PubMed] [Google Scholar]
- 48.Gualano A. B., Bozza T., Lopes De Campos P., Roschel H., Dos Santos Costa A., Luiz Marquezi M., Benatti F., Herbert Lancha Junior A. (2011) Branched-chain amino acids supplementation enhances exercise capacity and lipid oxidation during endurance exercise after muscle glycogen depletion. J. Sports Med. Phys. Fitness 51, 82–88 [PubMed] [Google Scholar]
- 49.Kephart W. C., Wachs T. D., Mac Thompson R., Brooks Mobley C., Fox C. D., McDonald J. R., Ferguson B. S., Young K. C., Nie B., Martin J. S., Company J. M., Pascoe D. D., Arnold R. D., Moon J. R., Roberts M. D. (2016) Ten weeks of branched-chain amino acid supplementation improves select performance and immunological variables in trained cyclists. Amino Acids 48, 779–789 [DOI] [PubMed] [Google Scholar]
- 50.Nelson A. R., Phillips S. M., Stellingwerff T., Rezzi S., Bruce S. J., Breton I., Thorimbert A., Guy P. A., Clarke J., Broadbent S., Rowlands D. S. (2012) A protein–leucine supplement increases branched-chain amino acid and nitrogen turnover but not performance. Med. Sci. Sports Exerc. 44, 57–68 [DOI] [PubMed] [Google Scholar]
- 51.Burke L. M., Ross M. L., Garvican-Lewis L. A., Welvaert M., Heikura I. A., Forbes S. G., Mirtschin J. G., Cato L. E., Strobel N., Sharma A. P., Hawley J. A. (2017) Low carbohydrate, high fat diet impairs exercise economy and negates the performance benefit from intensified training in elite race walkers. J. Physiol. 595, 2785–2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller B. F., Drake J. C., Peelor F. F., III, Biela L. M., Geor R., Hinchcliff K., Davis M., Hamilton K. L. (2015) Participation in a 1,000-mile race increases the oxidation of carbohydrate in Alaskan sled dogs. J. Appl. Physiol. 118, 1502–1509 [DOI] [PubMed] [Google Scholar]
- 53.Leckey J. J., Ross M. L., Quod M., Hawley J. A., Burke L. M. (2017) Ketone diester ingestion impairs time-trial performance in professional cyclists. Front. Physiol. 8, 806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Conlee R. K., Hammer R. L., Winder W. W., Bracken M. L., Nelson A. G., Barnett D. W. (1990) Glycogen repletion and exercise endurance in rats adapted to a high fat diet. Metabolism 39, 289–294 [DOI] [PubMed] [Google Scholar]
- 55.Lyons CL, Kennedy EB, Roche HM. (2016) Metabolic inflammation-differential modulation by dietary constituents. Nutrients 8, E247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duan W., Guo Z., Jiang H., Ware M., Mattson M. P. (2003) Reversal of behavioral and metabolic abnormalities, and insulin resistance syndrome, by dietary restriction in mice deficient in brain-derived neurotrophic factor. Endocrinology 144, 2446–2453 [DOI] [PubMed] [Google Scholar]
- 57.Duan W., Guo Z., Jiang H., Ware M., Li X. J., Mattson M. P. (2003) Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc. Natl. Acad. Sci. USA 100, 2911–2916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson J. B., Summer W., Cutler R. G., Martin B., Hyun D. H., Dixit V. D., Pearson M., Nassar M., Telljohann R., Maudsley S., Carlson O., John S., Laub D. R., Mattson M. P. (2007) Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic. Biol. Med. 42, 665–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bhutani S., Klempel M. C., Kroeger C. M., Trepanowski J. F., Varady K. A. (2013) Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity (Silver Spring) 21, 1370–1379 [DOI] [PubMed] [Google Scholar]
- 60.Harvie M. N., Pegington M., Mattson M. P., Frystyk J., Dillon B., Evans G., Cuzick J., Jebb S. A., Martin B., Cutler R. G., Son T. G., Maudsley S., Carlson O. D., Egan J. M., Flyvbjerg A., Howell A. (2011) The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int. J. Obes. 35, 714–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harvie M., Wright C., Pegington M., McMullan D., Mitchell E., Martin B., Cutler R. G., Evans G., Whiteside S., Maudsley S., Camandola S., Wang R., Carlson O. D., Egan J. M., Mattson M. P., Howell A. (2013) The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br. J. Nutr. 110, 1534–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hatori M., Vollmers C., Zarrinpar A., DiTacchio L., Bushong E. A., Gill S., Leblanc M., Chaix A., Joens M., Fitzpatrick J. A., Ellisman M. H., Panda S. (2012) Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 15, 848–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Varady K. A., Bhutani S., Klempel M. C., Kroeger C. M., Trepanowski J. F., Haus J. M., Hoddy K. K., Calvo Y. (2013) Alternate day fasting for weight loss in normal weight and overweight subjects: a randomized controlled trial. Nutr. J. 12, 146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berrigan D., Perkins S. N., Haines D. C., Hursting S. D. (2002) Adult-onset calorie restriction and fasting delay spontaneous tumorigenesis in p53-deficient mice. Carcinogenesis 23, 817–822 [DOI] [PubMed] [Google Scholar]
- 65.Mukherjee P., El-Abbadi M. M., Kasperzyk J. L., Ranes M. K., Seyfried T. N. (2002) Dietary restriction reduces angiogenesis and growth in an orthotopic mouse brain tumour model. Br. J. Cancer 86, 1615–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ahmet I., Wan R., Mattson M. P., Lakatta E. G., Talan M. (2005) Cardioprotection by intermittent fasting in rats. Circulation 112, 3115–3121 [DOI] [PubMed] [Google Scholar]
- 67.Yu Z. F., Mattson M. P. (1999) Dietary restriction and 2-deoxyglucose administration reduce focal ischemic brain damage and improve behavioral outcome: evidence for a preconditioning mechanism. J. Neurosci. Res. 57, 830–839 [PubMed] [Google Scholar]
- 68.Arumugam T. V., Phillips T. M., Cheng A., Morrell C. H., Mattson M. P., Wan R. (2010) Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann. Neurol. 67, 41–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mattson M. P., Wan R. (2005) Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J. Nutr. Biochem. 16, 129–137 [DOI] [PubMed] [Google Scholar]
- 70.Camandola S., Mattson M. P. (2017) Brain metabolism in health, aging, and neurodegeneration. EMBO J. 36, 1474–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheng A., Wan R., Yang J. L., Kamimura N., Son T. G., Ouyang X., Luo Y., Okun E., Mattson M. P. (2012) Involvement of PGC-1α in the formation and maintenance of neuronal dendritic spines. Nat. Commun. 3, 1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Godar R. J., Ma X., Liu H., Murphy J. T., Weinheimer C. J., Kovacs A., Crosby S. D., Saftig P., Diwan A. (2015) Repetitive stimulation of autophagy–lysosome machinery by intermittent fasting preconditions the myocardium to ischemia–reperfusion injury. Autophagy 11, 1537–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Powers S. K., Jackson M. J. (2008) Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol. Rev. 88, 1243–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vijayakumar A., Wu Y., Buffin N. J., Li X., Sun H., Gordon R. E., Yakar S., LeRoith D. (2012) Skeletal muscle growth hormone receptor signaling regulates basal, but not fasting-induced, lipid oxidation. PLoS One 7, e44777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wijngaarden M. A., van der Zon G. C., van Dijk K. W., Pijl H., Guigas B. (2013) Effects of prolonged fasting on AMPK signaling, gene expression, and mitochondrial respiratory chain content in skeletal muscle from lean and obese individuals. Am. J. Physiol. Endocrinol. Metab. 304, E1012–E1021 [DOI] [PubMed] [Google Scholar]
- 76.Franklin M. P., Sathyanarayan A., Mashek D. G. (2017) Acyl-CoA thioesterase 1 (ACOT1) regulates PPARα to couple fatty acid flux with oxidative capacity during fasting. Diabetes 66, 2112–2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Geisler J. G., Marosi K., Halpern J., Mattson M. P. (2017) DNP, mitochondrial uncoupling, and neuroprotection: a little dab’ll do ya. Alzheimers Dement. 13, 582–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seifert E. L., Bézaire V., Estey C., Harper M. E. (2008) Essential role for uncoupling protein-3 in mitochondrial adaptation to fasting but not in fatty acid oxidation or fatty acid anion export. J. Biol. Chem. 283, 25124–25131 [DOI] [PubMed] [Google Scholar]
- 79.Zhou M., Lin B. Z., Coughlin S., Vallega G., Pilch P. F. (2000) UCP-3 expression in skeletal muscle: effects of exercise, hypoxia, and AMP-activated protein kinase. Am. J. Physiol. Endocrinol. Metab. 279, E622–E629 [DOI] [PubMed] [Google Scholar]
- 80.Clapham J. C., Arch J. R., Chapman H., Haynes A., Lister C., Moore G. B., Piercy V., Carter S. A., Lehner I., Smith S. A., Beeley L. J., Godden R. J., Herrity N., Skehel M., Changani K. K., Hockings P. D., Reid D. G., Squires S. M., Hatcher J., Trail B., Latcham J., Rastan S., Harper A. J., Cadenas S., Buckingham J. A., Brand M. D., Abuin A. (2000) Mice overexpressing human uncoupling protein-3 in skeletal muscle are hyperphagic and lean. Nature 406, 415–418 [DOI] [PubMed] [Google Scholar]
- 81.Bhattacharjee A., Joiner W. J., Wu M., Yang Y., Sigworth F. J., Kaczmarek L. K. (2003) Slick (Slo2.1), a rapidly-gating sodium-activated potassium channel inhibited by ATP. J. Neurosci. 23, 11681–11691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Santi C. M., Ferreira G., Yang B., Gazula V. R., Butler A., Wei A., Kaczmarek L. K., Salkoff L. (2006) Opposite regulation of slick and slack K+ channels by neuromodulators. J. Neurosci. 26, 5059–5068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mattson M. P., Allison D. B., Fontana L., Harvie M., Longo V. D., Malaisse W. J., Mosley M., Notterpek L., Ravussin E., Scheer F. A., Seyfried T. N., Varady K. A., Panda S. (2014) Meal frequency and timing in health and disease. Proc. Natl. Acad. Sci. USA 111, 16647–16653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tarnopolsky M. A., Atkinson S. A., Phillips S. M., MacDougall J. D. (1995) Carbohydrate loading and metabolism during exercise in men and women. J. Appl. Physiol. 78, 1360–1368 [DOI] [PubMed] [Google Scholar]
- 85.Cermak N. M., van Loon L. J. (2013) The use of carbohydrates during exercise as an ergogenic aid. Sports Med. 43, 1139–1155 [DOI] [PubMed] [Google Scholar]
- 86.Catenacci V. A., Pan Z., Ostendorf D., Brannon S., Gozansky W. S., Mattson M. P., Martin B., MacLean P. S., Melanson E. L., Troy Donahoo W. (2016) A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity (Silver Spring) 24, 1874–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moro T., Tinsley G., Bianco A., Marcolin G., Pacelli Q. F., Battaglia G., Palma A., Gentil P., Neri M., Paoli A. (2016) Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med. 14, 290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tinsley G. M., Forsse J. S., Butler N. K., Paoli A., Bane A. A., La Bounty P. M., Morgan G. B., Grandjean P. W. (2017) Time-restricted feeding in young men performing resistance training: a randomized controlled trial. Eur. J. Sport Sci. 17, 200–207 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.