Abstract
Resolution of acute inflammation is governed, in part, by lipid mediator class switching from proinflammatory eicosanoids to specialized proresolving mediators, including a recently identified new pathway of mediators, termed maresin conjugates in tissue regeneration (MCTR), which includes MCTR1, MCTR2, and MCTR3. Here, we addressed whether each MCTR can impact the known vascular actions of cysteinyl leukotrienes. Leukotriene D4 (LTD4; 1.5 nmol/mouse) initiated vascular leakage in mouse cremaster vessels, which was reduced (>75%) by MCTR1 and MCTR2 (0.15 nmol each). With isolated Ciona intestinalis (sea squirt) primordial hearts, LTD4 (1–100 nM) induced negative inotropic action and lowered heartbeats 20–30%. Each MCTR (1–100 nM) prevented LTD4-reduced heart rates. With human cysteinyl leukotriene receptor-1 (CysLT1) expressed in CHO cells, each MCTR (10–100 nM) significantly reduced LTD4-initiated signaling. To assess the contribution of CysLT1 in the proresolving actions of MCTR, we carried out human macrophage (MΦ) phagocytosis. Each MCTR (0.1–10 nM) stimulated human MΦ phagocytosis of live Escherichia coli, whereas LTD4 did not stimulate phagocytosis. MCTR-activated phagocytosis was significantly blocked by a pharmacologic receptor antagonist (MK571). With both CHO-CysLT1 and human MΦs, each MCTR competed for specific [3H]-LTD4 binding with apparent lower affinity than LTD4. Thus, each MCTR functionally interacts with human CysLT1 to pharmacologically counter-regulate vascular responses and stimulate physiologic phagocytosis with MΦs.—Chiang, N., Riley, I. R., Dalli, J., Rodriguez, A. R., Spur, B. W., Serhan, C. N. New maresin conjugates in tissue regeneration pathway counters leukotriene D4–stimulated vascular responses.
Keywords: resolution, inflammation, resolvin, omega-3, leukocyte, lipoxygenase
Injury or infection initiates the acute inflammatory response that is vital and normally host protective. The acute inflammatory response, ideally, is self-limited and subsequently leads to complete resolution (1). The self-resolving phase of the process is governed by lipid mediator class switching from the production of proinflammatory prostaglandins (PG) and leukotrienes in the initiation phase to the biosynthesis of specialized proresolving mediators (SPMs). These include eicosapentaenoic acid–derived E-series resolvins (Rvs) and docosahexaenoic acid–derived D-series Rv, protectin, and maresin (MaR) families (1, 2). Structure elucidation of SPMs and their functional characterization indicate that the resolution of inflammation is an active biochemical process with SPMs controlling the key steps by stimulating resolution. We recently identified a new series of evolutionarily conserved chemical signals that are peptide-lipid conjugated mediators in the MaR biosynthesis pathway (3), coined MaR conjugates in tissue regeneration (MCTR).
Biosynthesis of MCTRs is initiated via lipoxygenation of docosahexaenoic acid at the carbon-14 position to form 14S-hydro(peroxy)-4Z,7Z,10Z,12E,16Z,19Z-docosahexaenoic acid, followed by epoxidation to a MaR-epoxide intermediate (i.e., epoxy-MaR, eMaR, 13S,14S-epoxy-4Z,7Z,9E,11E,16Z,19Z-docosahexaenoic acid), which is further converted to MCTR (1). All 3 MCTRs belong to the MaR family, as indicated by the presence of a carbon-14 hydroxyl group. The basic structures of each bioactive MCTR were deduced by determining UV chromophores, fragmentation ions from MS/MS spectra of natural and deuterium-labeled products, and derivatives that were obtained with MCTR trimethyl ester and Raney nickel desulfurization (3). The complete stereochemical structure of MCTR1 proved to be 13R-glutathionyl, 14S-hydroxy-4Z,7Z,9E,11E,13R,14S,16Z,19Z-docosahexaenoic acid, and the MCTR2 structure is 13R-cysteinylglycinyl, 14S-hydroxy-4Z,7Z,9E,11E,13R,14S,16Z,19Z-docosahexaenoic acid. These structures and potent biologic actions were confirmed by the total organic synthesis of each compound (4, 5).
We recently identified the third pathway member, denoted MCTR3, and established its function and complete stereochemistry as 13R-cysteinyl, 14S-hydroxy-4Z,7Z,9E,11E,13R,14S,16Z,19Z-docosahexaenoic acid (4). MCTRs are present in self-limited infectious murine exudates, regenerating planaria, human tissues, and organs, including breast milk, macrophages (MΦs), serum, lymph nodes, and plasma (3, 6). MCTRs enhance host responses to contain infections, stimulate tissue regeneration, and promote the restoration of function in model organisms (3–5). These results demonstrate new chemical signals and pathways that stimulate the resolution of inflammation and evoke the acceleration of tissue regeneration.
Cysteinyl leukotrienes (CysLTs), such as leukotriene C4 (LTC4) and leukotriene D4 (LTD4), are biosynthesized from arachidonic acid and are potent mediators that increase vascular permeability. They are the principal components of the slow-reacting substance of anaphylaxis (7–9). Their vascular-directed actions include a negative inotropic action on heart tissues (10, 11). Given that specific SPMs counter-regulate LTB4 actions (1, 12), we questioned whether the new MCTRs interact with CysLT vascular actions in model organisms. Results of the current study demonstrate that MCTRs functionally antagonize LTD4-stimulated cardiovascular responses and directly interact with recombinant human CysLT receptor-1 (CysLT1), which indicates pharmacologic interactions that are potentially physiologically relevant.
MATERIALS AND METHODS
Materials
LTD4 and MK571, a CysLT1 antagonist, were purchased from Cayman Chemicals (Ann Arbor, MI, USA). Each MCTR was prepared by total organic synthesis as previously described (5), and these physical properties matched with endogenous MCTR as in Dalli et al. (3, 4). The integrity of each MCTR was assessed by using liquid chromatography–MS/MS to obtain retention times and diagnostic MS/MS spectra just before all experiments using acquisition parameters as previously described (6).
Human leukocyte isolation and MΦ differentiation
Peripheral blood monocytes were prepared in accordance with the approved Partners Human Research Committee Protocol (1999P001297). Human MΦs were differentiated by culturing freshly isolated peripheral blood monocytes in Roswell Park Memorial Institute (RPMI) 1640 medium that was supplemented with 10% fetal bovine serum and recombinant human GM-CSF (10 ng/ml; R&D Systems, Minneapolis, MN, USA) for 7 d.
Human MΦ phagocytosis
MΦs were plated onto 96-well plates (50 × 103 cells/well in PBS++), and phagocytosis was carried out 24 h later. MΦs were incubated with MK571 (1 µM; 15 min at 37°C) and synthetic MCTRs (0.1, 1, or 10 nM; 15 min at 37°C) (5), followed by incubation with fluorescent-labeled Escherichia coli (50:1; BacLight Green; Molecular Probes, Eugene, OR, USA) for 60 min at 37°C. Plates were gently washed, extracellular fluorescence was quenched by trypan blue, and phagocytosis was determined by measuring total fluorescence (Ex 493 nm/Em 535 nm) using the SpectraMax M3 Plate Reader (Molecular Devices, Sunnyvale, CA, USA). For real-time imaging, MΦs were plated onto 8-well glass chamber slides (100 × 103 cells/well) and kept in a Stage Top Incubation system for imaging with a microscope that was equipped with a built-in digital gas mixer and temperature regulator (INUF-K14; Tokai Hit, Fujinomiya, Japan). MK571 (1 µM; 15 min at 37°C) was added, followed by MCTR3 (1 nM; 15 min at 37°C), then BacLight Green–labeled E. coli (5 × 106 colony-forming units). Images were acquired every 10 min for 2 h (37°C) with a Keyence BZ-9000 (Biorevo, Osaka, Japan) inverted fluorescence phase-contrast microscope (×40 objective) that was equipped with a monochrome/color switching camera using BZ-II Viewer Software (Keyence, Itasca, IL, USA). Green fluorescence intensity was quantified by using the BZ-II Analyzer.
Vascular leakage
Mice were anesthetized by injection (200 µl, i.p.) of saline containing 3 mg ketamine HCl and 0.6 mg xylazine. Mice were administered LTD4 (5 µM, 300 µl/mouse; i.e., 1.5 nmol/mouse), plus MCTR1 and MCTR2 (0.5 µM each, 300 µl/mouse; i.e., 0.15 nmol/mouse) or vehicle (saline containing 0.01% ethanol) via intrascrotal injection immediately prior to administration of Alexa 594-labeled Albumin (25 mg/kg of body weight) via intravenous injection. After 10 min, the cremasters were harvested and vessels stained with anti-CD31 antibody. Tissues were then mounted and imaged using a Zeiss LSM501META confocal microscope.
Electrical cell-substrate impedance-sensing system
Ligand–receptor interactions were determined by measuring impedance across CHO cell monolayers, carried out essentially as in Krishnamoorthy et al. (13), by using an electrical cell-substrate impedance-sensing system (ECIS; Applied Biophysics, Troy, NY, USA). In brief, human CysLT1 or wild-type CHO cells were plated at 0.1 × 106 cells/well in an 8-well ECIS array (8W10E+). Test compounds were added to the chambers in serum-free medium, and real-time impedance changes were monitored—one measurement per 4 s for 10 min at 37°C—followed by the addition of LTD4 (1 nM) for an additional 10 min.
Measurement of heart beats of Ciona intestinalis (sea squirt)
Fresh live sea squirts were purchased from Marine Biologic Laboratory Woods Hole Oceanographic Institution (Woods Hole, MA, USA) and kept in seawater at room temperature. Hearts were gently isolated from surrounding tissues and dissected into halves. Each half was placed in a 24-well plate in seawater (0.5 ml/well). Basal heart rates were counted for 30 s. LTD4 (1, 10, or 100 nM in 0.5 ml seawater), MCTR (100 nM in 0.5 ml seawater), or vehicle (seawater with 0.01% ethanol) were added to seawater that contained isolated hearts, and heart rates were determined for 30-s intervals at 2, 5, 10, 15, 25, and 30 min. In separate sets of experiments, MCTR (1, 10 or 100 nM) or vehicle were added to hearts in seawater for 30 min, followed by LTD4 (10 nM), and heart rates were counted for 30 s at 30-min time intervals. Excised tunicate hearts were imaged for video capture with a Qicam Fast 1394 Digital CCD Camera (QImaging, Surrey, BC, Canada) that was paired with a Zeiss Axiovert 40 CFL microscope (×40 magnification). Captured TIFF stack files were converted to video files via ImageJ (National Institutes of Health, Bethesda, MD, USA; Supplemental Video 1).
[3H]-LTD4 radioligand binding and CysLT1 surface expression
[3H]-LTD4 (specific activity, ∼130 Ci/mmol; PerkinElmer, Waltham, MA, USA) binding was carried out with CHO cells that were transfected with CysLT1 or human MΦs in Dulbecco’s PBS with CaCl2 and MgCl2 (14). For competition binding, cells (2 × 106 cells/0.1 ml Dulbecco’s PBS with Ca2+ and Mg2+) were incubated with ∼2 nM of [3H]-LTD4 in the absence or presence of unlabeled LTD4, MCTR1, MCTR2, or MCTR3 for 60 min at 4°C. Bound and unbound radioligands were separated by filtration through Whatman GF/C filters (Thermo Fisher Scientific, Waltham, MA, USA). Filters were washed 2 times with 5 ml of ice-cold Dulbecco’s PBS. Radioactivity retained on the filter was determined by using a scintillation counter (Beckman, Fullerton, CA, USA). Nonspecific binding was determined in the presence of 1 μM of unlabeled homoligand (LTD4).
CHO-CysLT1 cells or human MΦs (0.5 × 106 cells/0.1 ml) were incubated with or without rabbit anti-human CysLT1 Ab (1:50 dilution; GTX108112; GeneTex, Irvine, CA, USA) for 30 min at 4°C, followed by PE-conjugated anti-rabbit IgG (1:200 dilution; eBiosciences, Waltham, MA, USA) for 30 min at 4°C. Flow cytometry was carried out by using FACSCantoII (BD Biosciences, San Jose, CA, USA).
Statistical analysis
Statistical analysis was performed by using Student’s t test for 2-group comparisons and 1-way ANOVA for multiple group comparisons with post hoc analysis using Tukey’s test (Prism; GraphPad Software, La Jolla, CA, USA). Values of P < 0.05 were considered statistically significant.
RESULTS
MCTRs regulate LTD4-mediated vascular leakage
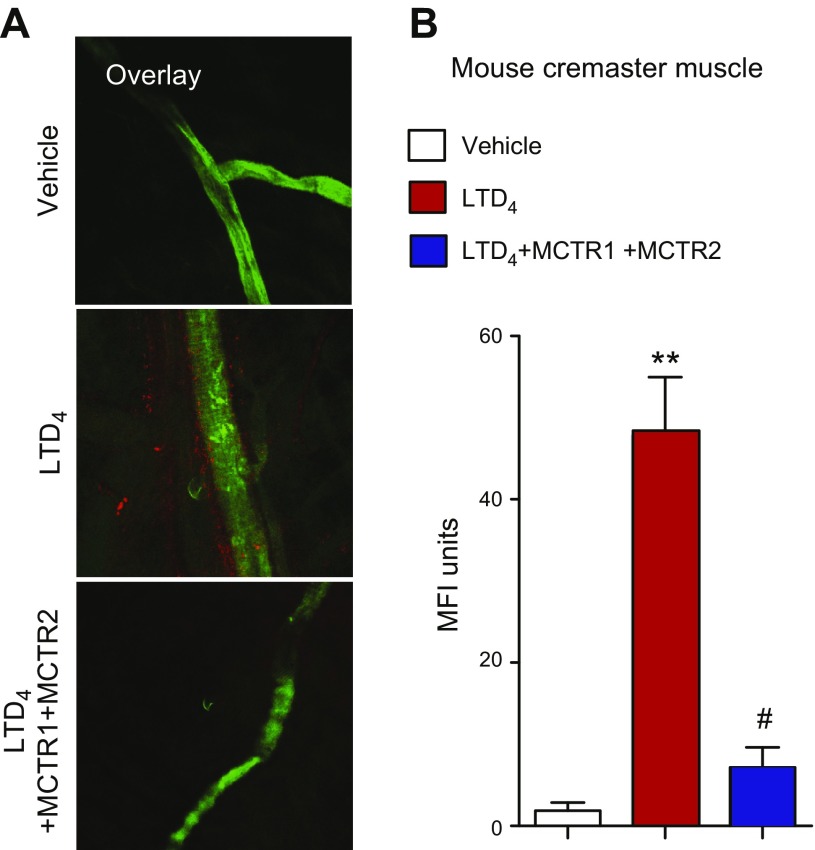
Given that LTD4 potently promotes vascular leakage (7, 8), we investigated whether MCTRs stimulate vascular leak or regulate LTD4-initiated vascular leakage in mouse cremaster vessels. Mice were administered LTD4 (1.5 nmol per mouse) via intrascrotal injection, followed by intravenous injection of Alexa Fluor 594–labeled albumin administered systemically. After 10 min, cremaster muscles were harvested and vessels were stained with an anti-CD31 Ab (green fluorescence; Fig. 1A). Tissues were then mounted and imaged using a confocal microscope. LTD4 induced marked vascular leakage as monitored by fluorescence intensity (red fluorescence; Fig. 1A, B). MCTR1 and MCTR2 (0.15 nmol each) significantly blocked (>75%) LTD4 (1.5 nmol/mouse) -stimulated vascular leakage in cremaster vessels (Fig. 1B). These results demonstrate that MCTR functionally antagonized LTD4-initiated vascular leakage.
Figure 1.
MCTRs regulate LTD4-mediated vascular leakage. Mice were administered MCTR1 and MCTR2 (each at 0.15 nmol/mouse) or vehicle (saline that contained 0.01% EtOH) and LTD4 (1.5 nmol/mouse) via intrascrotal injection immediately before Alexa Fluor 594–labeled albumin (25 mg/kg) via intravenous injection. After 10 min, cremasters were harvested and vessels were stained with anti-CD31 Ab. Tissues were then mounted and imaged by using a Zeiss LSM501META confocal microscope. A) Representative images of 3 mice per group. B) Vascular leakage was quantified by using ImageJ software as mean fluorescence intensity (MFI) per field. Results are presented as means ± sem (n = 3 mice/group). **P < 0.01 vs. vehicle alone, #P < 0.05 vs. LTD4 alone.
MCTRs reverse inotropic actions on primordial hearts
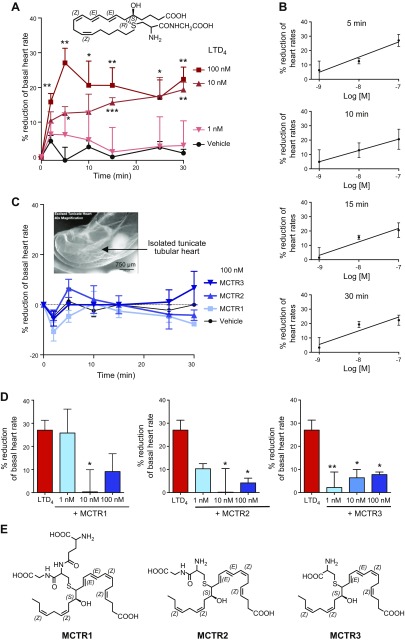
CysLTs reduce contraction of isolated guinea pig hearts, a negative inotropic effect (10). This seems to result from the activation of the pertussis toxin–sensitive G protein–gated muscarinic K+ channel (IK.Ach) in cardiac myocytes (11). As MCTR prevented LTD4-stimulated vascular leak (Fig. 1), we investigated whether LTD4 and MCTR regulate heart rates by using isolated primitive hearts from C. intestinalis, known as the sea squirt. The heart of this primordial chordate (C. intestinalis) consists of a valveless myocardial tube that fits in a pericardial sac (15). Freshly isolated sea squirt hearts were kept in seawater and displayed basal rates of ∼15 beats per 30 s (Supplemental Video 1). The addition of LTD4 to seawater (10 or 100 nM in 0.5 ml) significantly lowered heart rates in a dose-dependent manner, which resulted in ∼20–30% reductions (Fig. 2A). This negative inotropic action of LTD4 (1–100 nM) was concentration dependent between 5 and 30 min, with an estimated EC50 of ∼10 nM (Fig. 2B). In comparison, each MCTR alone (100 nM) did not elicit significant changes in heart rate (Fig. 2C).
Figure 2.
MCTRs block LTD4-stimulated negative inotropic action on tunicate hearts. Tunicate hearts were isolated and placed in seawater, and the basal heart rate was recorded for 30 s. A, B) LTD4 (1, 10, or 100 nM; structure shown in inset) was added to hearts kept in seawater, and heart rates were counted for 30 s at 2-, 10-, 15-, 25-, and 30-min intervals. Results are expressed as the percent reduction of heart rates; time course (2–30 min; A) and dose response (1–100 nM LTD4; B) at 5, 10, 15, and 30 min. C) MCTR1, MCTR2, and MCTR3 (100 nM) were added to hearts kept in seawater, and heart rates were counted for 30 s at 2-, 10-, 15-, 25-, and 30-min intervals. A representative isolated tunicate tubular heart (inset). D) MCTRs (1, 10, or 100 nM) or vehicle were added to hearts in seawater for 30 min, followed by LTD4 (10 nM), and heart rates were counted for 30 s at 30-min time intervals. E) Chemical structures of MCTR1, MCTR2, and MCTR3 with stereochemical assignments. Results are presented as means ± sem (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001 compared with hearts incubated with vehicle alone (A); *P < 0.05; **P < 0.01 compared with hearts incubated with LTD4 alone (D).
We next assessed whether MCTRs regulate LTD4-stimulated negative inotropic action. MCTR1, MCTR2, or MCTR3 (1–100 nM) were each added to hearts in seawater suspension for 30 min at room temperature before the addition of LTD4 (10 nM). Each MCTR dose-dependently prevented LTD4-reduced heart rates (Fig. 2D), and MCTR3 resulted in a ∼90% reversal at 1 nM. The complete stereochemistries of all 3 MCTRs have been established (3, 5, 6), and their chemical structures with stereochemical assignments are shown in Fig. 2E. These results demonstrate that MCTRs blocked LTD4-induced negative inotropic actions in isolated primordial hearts.
Interactions of MCTRs with human recombinant CysLT1: ligand–receptor-dependent changes in impedance
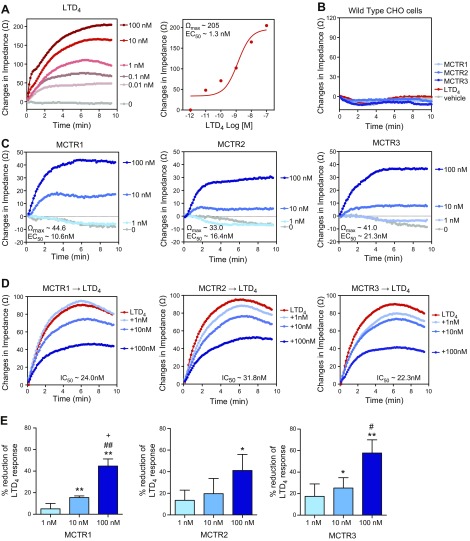
As MCTRs countered the vascular actions of LTD4, we next questioned whether MCTRs directly interact with CysLT1, a LTD4 receptor. To this end, we carried out ECIS to monitor rapid changes in impedance upon ligand binding to receptors (16). In this system, LTD4 (0.01–100 nM) dose-dependently elicited rapid changes in impedance with CHO cells that overexpressed recombinant human CysLT1 (CHO-CysLT1), which resulted in a maximal impedance change (Ωmax) of ∼205 Ω at 100 nM (37°C, pH 7.45) and an estimated EC50 of 1.0 nM (Fig. 3A). LTD4 did not alter impedance in CHO wild-type cells (Fig. 3B). In comparison, MCTR1, MCTR2, and MCTR3 each dose-dependently elicited impedance changes, with lower maximal responses (Ωmax, ∼44.6, 33.0, and 41.0 Ω for MCTR1, MCTR2, and MCTR3, respectively, at 100 nM; Fig. 3C) and higher EC50 values (∼10.6, 16.4, and 21.3 nM for MCTR1, MCTR2, and MCTR3, respectively; Fig. 3C) compared with LTD4. In addition, incubation of MCTRs (1–100 nM; 10 min) with CHO-CysLT1 cells before the addition of LTD4 (1 nM) significantly reduced the LTD4-elicited impedance change, with estimated half-maximal inhibitory concentrations (IC50) of ∼24.0, 31.8, and 22.3 nM for MCTR1, MCTR2, and MCTR3, respectively (Fig. 3D). Among the 3 MCTRs, MCTR3 displayed the highest potency, with ∼25 and 60% reductions of LTD4-initiated impedance change at 10 and 100 nM, respectively (Fig. 3E). Together, these results indicate that MCTRs can directly interact with human recombinant CysLT1 expressed in CHO cells to impact LTD4-mediated responses in vitro.
Figure 3.
MCTRs interact with human recombinant CysLT1 expressed in CHO cells: ligand-receptor–dependent changes in impedance. CHO cells were transfected with recombinant CysLT1. Impedance was recorded using real-time monitoring across cell monolayers with an ECIS system for 10 min (one measurement per 4 s). A–C) CHO-CysLT1 or CHO wild-type cells were incubated with LTD4 (0.01–100 nM; A); LTD4, MCTR1, MCTR2, and MCTR3 (100 nM; B); and MCTRs (1–100 nM) or medium (C), and impedance was recorded for 10 min. D, E) CHO-CysLT1 cells were incubated with MCTRs (1–100 nM) or vehicle for 15 min, followed by the addition of LTD4 (1 nM), and impedance was recorded for 10 min. Results are representative of n = 3 (A, B); mean of n = 3 (C, D); and mean ± sem (n = 3; E). *P < 0.05, **P < 0.01 vs. LTD4 alone, #P < 0.05, ##P < 0.01 vs. 1 nM, +P < 0.05 vs. 10 nM.
Stimulation of MΦ phagocytosis of E. coli: proresolving immune-modulatory actions
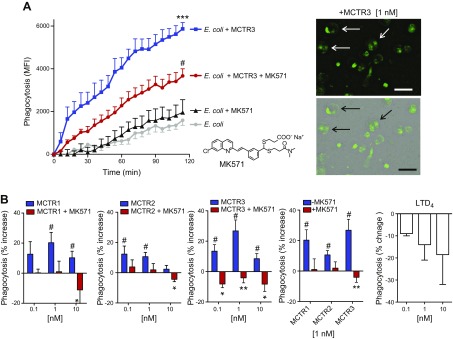
To investigate whether blockade of the CysLT1 receptor can regulate MCTR actions in stimulating MΦ phagocytosis, we monitored human MΦ phagocytosis of fluorescently labeled E. coli by using real-time fluorescence imaging (Fig. 4A). Human MΦs that were incubated with MCTR3 (1 nM) demonstrated enhanced phagocytosis of E. coli compared with MΦs that were incubated with E. coli alone. Incubation of MΦs with MK571, a CysLT1 antagonist (1 µM, structure shown in Fig. 4A inset), significantly reduced MCTR3-enhanced phagocytosis; MK571 alone did not significantly alter E. coli phagocytosis. We next assessed dose responses of MCTR1, MCTR2, and MCTR3 in the regulation of MΦ phagocytosis of fluorescently labeled E. coli (Materials and Methods). MCTR1, MCTR2, and MCTR3 (0.1, 1, or 10 nM) each increased phagocytosis of fluorescently labeled E. coli that was significantly reduced in the presence of MK571 (1 µM; Fig. 4B). For direct comparison, LTD4 alone (0.1–10 nM) did not significantly increase phagocytosis (Fig. 4B). These results indicate that MK571 also functionally blocked MCTR-stimulated phagocytosis with human MΦs.
Figure 4.
MCTRs stimulate MΦ phagocytosis of E. coli: blockage by the CysLT1 antagonist. A) Human MΦs were plated onto slide chambers (1 × 105 cells/well), incubated with MK571 (1 μM, 15 min, 37°C; structure shown in inset in left panel), or vehicle, followed by MCTR3 (1 nM, 15 min, 37°C) or vehicle. Phagocytosis was then initiated by adding BacLight Green–labeled E. coli (E. coli:MΦ 50:1). Fluorescent images were recorded every 10 min for 120 min. Results are mean fluorescence intensity (MFI); means ± sem (n = 4); ***P < 0.001 vs. E. coli alone, #P < 0.05 vs. E. coli + MCTR3 (left). Representative images: fluorescence (top right) and overlay of fluorescence and bright field images (bottom right). Arrows indicate MΦs with ingested fluorescent E. coli. Scale bar, 50 μm. B) Human MΦs were plated onto 96-well plates (0.5 × 105 cells/well) and incubated with MK571 (1 µM, 15 min, 37°C) or vehicle, followed by MCTRs (0.1–10 nM, 15 min, 37°C). Phagocytosis was initiated by adding BacLight Green–labeled E. coli (E. coli:MΦ 50:1) for 1 h. Fluorescence was determined on a microplate reader. Results are expressed as percent increases of phagocytosis above vehicle. Means ± sem (n = 3). #P < 0.05 MCTRs vs. vehicle, *P < 0.05, **P < 0.01 MCTRs vs. MCTRs + MK571.
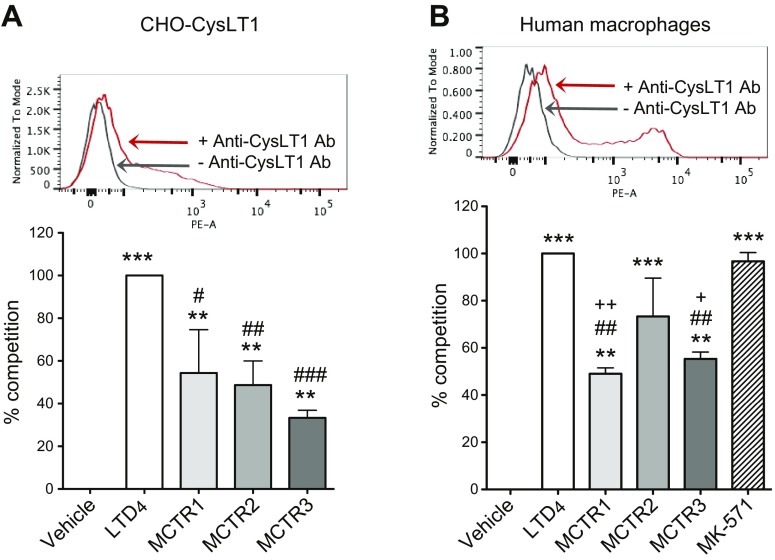
MCTRs compete for [3H]-LTD4 binding with human recombinant CysLT1 and macrophages
Given that MCTRs interact with human recombinant CysLT1 expressed in CHO cells and counter LTD4 responses (Fig. 3), we examined whether MCTRs directly compete for [3H]-LTD4 binding with human recombinant CysLT1. CHO-CysLT1 cells were incubated with 2 nM [3H]-LTD4 in the absence or presence of unlabeled homoligand LTD4 or individual MCTRs. MCTR1, MCTR2, and MCTR3 each significantly competed for specific [3H]-LTD4 binding (∼63, 49, and 33%, respectively), albeit significantly less potent (P < 0.05, < 0.01, and < 0.001 for MCTR1, MCTR2, and MCTR3, respectively) than LTD4 at equal molar concentrations (Fig. 5A). These results indicated that each MCTR directly competed for [3H]-LTD4 binding to recombinant human CysLT1, demonstrating lower affinities than native homoligand LTD4.
Figure 5.
[3H]-LTD4 binding with human MΦs and recombinant CysLT1: competition by MCTRs. A) CHO-CysLT1 cells. Expression of CysLT1; representative flow cytometry (n = 3; top). CHO-CysLT1 cells (2 × 106) were incubated with 2 nM [3H]-LTD4, together with vehicle, unlabeled LTD4, MCTR1, MCTR2, or MCTR3 (1 μM each) for 60 min at 4°C. B) Human MΦs. Expression of CysLT1; representative flow cytometry (n = 3; top). MΦs (1 × 106 cells) were incubated with 2 nM [3H]-LTD4, together with vehicle, unlabeled LTD4, MCTR1, MCTR2, MCTR3, or MK-571 (1 μM each) for 60 min at 4°C. Results are expressed as percent competition of total [3H]-LTD4 binding. Competition of [3H]-LTD4 total binding by unlabeled LTD4 (1 μM) is taken as 100%. Results are presented as means ± sem from 3 independent experiments and 2 replicates in each experiment. **P < 0.01, ***P < 0.001 compared with [3H]-LTD4 binding in the absence of unlabeled LTD4 (veh), #P < 0.05, ##P < 0.01, ###P < 0.001 compared with [3H]-LTD4 binding in the presence of unlabeled LTD4 (LTD4), +P < 0.05, ++P < 0.01 compared with 3H-LTD4 binding in the presence of unlabeled MK571.
As MCTRs also stimulated human MΦ phagocytosis of E. coli—an action blocked by CysLT1 antagonist MK571—we next examined whether MCTRs compete with [3H]-LTD4 binding with human MΦs (Fig. 5B). Human MΦs were incubated with 2 nM [3H]-LTD4 in the absence or presence of unlabeled homoligand LTD4 or individual MCTRs. MCTR1, MCTR2, and MCTR3 each significantly competed for specific [3H]-LTD4 binding, which resulted in ∼49, 73, and 55% competition, respectively. In comparison, MK571 at equal molar concentrations competed for 97% of specific [3H]-LTD4 binding. These results suggest that it is likely that MCTRs functionally interact with endogenous CysLT1 on human MΦs, with lower affinities than LTD4 and MK-571.
DISCUSSION
Results in the current study demonstrate that MCTRs functionally antagonize LTD4-stimulated vascular responses, including vascular permeability in mouse cremaster vessels and negative inotropic (cardiac depressant) actions on primordial tunicate hearts. We also observed that MCTRs directly interact with the recombinant human CysLT1 receptor expressed in vitro in CHO cells by using ECIS-monitored intracellular signaling. With human MΦs, each of the MCTRs stimulated phagocytosis of E. coli, an action not shared by LTD4. CysLTs are well-known sulfido-conjugated leukotrienes—for example, LTC4, LTD4, and LTE4—from arachidonic acid. LTC4 and LTD4 are potent bronchoconstrictors and increase vascular permeability (7, 8). LTE4 was previously considered to be inactive. In addition, LTD4 binds to human recombinant CysLT1 receptor with a dissociation constant (Kd) of 0.3 nM, whereas LTE4 is at least 100 times less potent than LTD4 (8). In comparison, MCTRs, a new family of sulfido-conjugated lipid mediators, all carry potent proresolving actions that stimulate MΦs and tissue regeneration at picomolar to nanomolar concentrations (3, 4).
We used ECIS to monitor rapid impedance changes upon ligand activation of GPCR. This cell-based and radiolabel-free system can quantitatively determine direct GPCR activation and distinguish Gαs, Gαi, and Gαq coupling. Gαi coupling results in an increase of impedance, Gαs coupling induces a decrease of impedance, and Gαq coupling demonstrates a transient dip, followed by an increase of impedance (16). Here, in CHO cells that express recombinant human CysLT1, LTD4 dose-dependently increased impedances, which suggests that CysLT1 couples with Gαi in CHO cells (Fig. 3A). Along these lines, in human monocyte-like U937 cells, LTD4-stimulated intracellular Ca2+ release via CysLT1 is partially sensitive to pertussis toxin, which suggests that CysLT1 couples with Gαi and other G proteins, such as Gαq (17). Thus, there could be some CysLT1 signaling components that are relevant to MΦ functions, such as phagocytosis, that are not fully present in CHO cells. Yet the intracellular downstream signals of CysLT1 in monocytes and MΦs and in CHO-CysLT1 cells might have overlapping components that are initiated by Gαi coupling.
In the present report, we found that each MCTR stimulated phagocytosis and regulated heart rates in the nanomolar concentration range (Figs. 2 and 4). In addition, each of the MCTRs directly activated recombinant human CysLT1 at 1–100 nM, albeit to a lesser extent than did LTD4, and reduced LTD4-initiated impedance changes with CHO-CysLT1 cells (Fig. 3). Thus, sulfido-conjugated CysLTs—biosynthesized from arachidonic acid via a 5,6-epoxide intermediate (i.e., LTA4) —and the sulfido-conjugated MCTRs—biosynthesized from docosahexaenoic acid via a 13S,14S-epoxide intermediate [i.e., eMaR] —both functionally interact with human recombinant CysLT1, yet display distinct and apparently opposing bioactions in the regulation of MΦ phagocytosis and vascular responses.
Of note, MCTRs (0.15 nmol) in vivo at a dose 10 times lower than that of LTD4 (1.5 nmol) reduced LTD4 responses >75% (Fig. 1). In addition, MCTRs were active at doses as low as 0.1 nM on human MΦs in stimulating phagocytosis (Fig. 4). In comparison, with human recombinant CysLT1 at an equal molar concentration at 100 nM, MCTRs alone (Ωmax, 33–45) were much less potent than LTD4 (Ωmax, 205) in eliciting impedance changes (Fig. 3A, C). In addition, MCTRs (10–100 nM) at concentrations that were much higher than that of LTD4 (1 nM) were required to block LTD4 responses (Fig. 3D, E). Results obtained with ECIS are consistent with those obtained with [3H]-LTD4–specific binding in which MCTRs were apparently less potent than LTD4 in competing for [3H]-LTD4 binding with CHO-CysLT1 cells and with human MΦs (Fig. 5). Together, these findings suggest that, in addition to pharmacologic interaction with CysLT1, MCTRs may each interact with additional receptors that are present on human MΦs and mouse endothelium with higher affinity. These putative MCTR receptors could contribute to the potent actions of MCTRs at low nanomolar range in vitro, as well as physiologic proresolving and organ-protective actions of MCTRs in vivo (3).
MCTRs are potent proresolving mediators. By definition, they stimulate the resolution of E. coli infection, clear apoptotic polymorphonuclear neutrophils (PMN) (efferocytosis), and enhance killing of E. coli (Fig. 4) (3, 4). They thus qualify as immunoresolvents given that they stimulate host innate immune functions without immunosuppression. Leukotriene-based therapeutics include CysLT receptor antagonists and 5-lipoxygenase inhibitors that were developed for the treatment of asthma and allergic rhinitis. CysLT receptor antagonists, such as montelukast (Singular; Merck, Kenilworth, NJ, USA) and zafirlukast (Accolate; AstraZeneca Pharmaceutical, Cambridge, United Kingdom), are currently widely used for the treatment of asthma and allergy, because CysLTs are potent stimulators of vascular permeability and bronchoconstriction (7–9); however, these drugs are associated with respiratory tract infections, one of the most frequent adverse drug reactions (18). In the present experiments, we found that the CysLT1 receptor antagonist, MK571, also blocked MCTR-stimulated MΦ phagocytosis of E. coli (Fig. 4). In addition, each MCTR competed for specific [3H]-LTD4 binding with MΦs, with lower affinity than either LTD4 or MK571 (Fig. 5B), which suggests that MCTRs could interact with additional receptors that may be present on human MΦs other than with CysLT1. Hence, in addition to blocking the actions of CysLT1, CysLT1 antagonists may also compromise the proresolving actions of MCTRs via CysLT1 and/or additional receptors. In this regard, the MK571 structure (19) may be useful as a scaffold (Fig. 4A, inset) to obtain more selective agonists and antagonists of MCTR receptors yet to be identified.
CysLTs display a negative inotropic effect on the contractile response of guinea pig and human myocardium in vitro (10). As CysLTs are released during a variety of inflammatory, allergic, and infectious conditions (7, 8), their potent myocardial depressant effect might contribute to the myocardial dysfunction that is associated with systemic anaphylactic and septic shock. In the present experiments, we used the sea squirt, C. intestinalis, which is known to produce lipid mediators, including lipoxin-like compounds and eicosapentaenoic acid–derived lipoxygenase (LOX) products (20, 21). Sea squirts also produce the cyclo-oxygenase products, PGE2, PGF2α, and thromboxanes, from endogenous arachidonic acid (20, 21). Of interest, the C. intestinalis genome (http://www.ncbi.nlm.nih.gov/nuccore/330855459) contains predicted sequences for 5-LOX (LOC100178342; XM_009862295.1) and CysLT1 (LOC100186833; XM_002122952.2). We found that, with the isolated hearts of C. intestinalis, LTD4 resulted in a significant depression of heart rates—from ∼30 beats/min to ∼20 beats/min—in a time- and dose-dependent manner, an action that was reversed by MCTRs (Fig. 2). Thus, we identified the cardioprotective action of MCTRs by using a direct assay of isolated primordial hearts.
MCTRs are potent stimulators for planarian tissue regeneration (3). These known actions of MCTRs, together with our present results, suggest that the organ protective actions of MCTRs may be conserved among species from primordial lower-phylum species, such as planaria (3) and sea squirts (Fig. 2), to mammals [e.g., reducing vascular leakage (Fig. 1) and ischemia-reperfusion–initiated second organ injury in mice (3)]. Along these lines, other SPMs, including D-series Rvs and MaR1, also demonstrated potent cardioprotective actions. For example, both RvD1 and MaR1 attenuate neointimal hyperplasia, which reduces vascular injury after carotid artery ligation (22). RvD1 and RvD2 attenuate abdominal aortic aneurysm (23). In addition, after myocardial infarction, RvD1 reduces fibrosis, thereby delaying the onset of heart failure (24). In advanced atherosclerosis, RvD1 promotes plaque stability, reduces lesional necrosis, and increases fibrous cap thickness (25). In addition to cardioprotective actions, MaR1 induces potent neuroprotection after spinal cord injury in mice, which accelerates the clearance of neutrophils, reduces proinflammatory cytokines at the lesion site, and improves functional neurologic recovery (26). Taken together, resolvins, MaR1, and MCTR demonstrate organ protection and promote the resolution of inflammation-associated tissue injuries in experimental models in vivo.
In summary, the present results demonstrate that, in addition to regulating MΦ phagocytosis, which is critical in the host innate immune response, newly discovered MCTRs can pharmacologically counter both vascular and negative inotropic actions that are stimulated by the leukotriene, LTD4. Cardioprotective actions of MCTR that are demonstrable in primordial hearts of the sea squirt may be relevant to human tissues, warranting additional studies. Our results clearly demonstrate the ability of MCTRs to interact with human CysLT1 and counter-regulate the functions of LTD4 in mice and primordial hearts. From these results, it is also likely that additional receptor networks contribute to the physiologic actions of MCTRs in vivo in humans. Whereas the present results indicate that MCTRs can block LTD4 vascular responses in model organisms, MCTRs do not seem to stimulate intracellular signal transduction via CysLT1 relevant to the physiologic actions of MCTRs, which suggests additional receptors yet to be identified. Taken together, these findings suggest new opportunities with which to address the counter-regulation of CysLTs and their roles in the pathobiology of diseases (8). They demonstrate the opposing bioactions of CysLTD4 and MCTRs in vivo and in vitro that are relevant to the pharmacologic interactions of these mediators and, potentially, to the physiologic roles of MCTRs.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Dr. Enis Hrustić (Center for Marine Research, Ruđer Bošković Institute, Rovinj, Croatia) for helpful discussion, and Mary Small (Brigham and Women’s Hospital, Boston, MA, USA) for assistance with manuscript preparation. This work was supported, in part, by U.S. National Institutes of Health, National Institute of General Medical Sciences Grants P01-GM095467 and R01-GM38765 (to C.N.S.). The authors declare no conflicts of interest.
Glossary
- CysLT
cysteinyl leukotriene
- CysLT1
cysteinyl leukotriene receptor 1
- ECIS
electrical cell-substrate impedance sensing
- LTC4
leukotriene C4
- LTD4
leukotriene D4
- MaR
maresin
- MCTR
maresin conjugates in tissue regeneration
- MΦ
macrophage
- PG
prostaglandin
- Rv
resolvin
- SPM
specialized proresolving mediator
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
C. N. Serhan designed research; N. Chiang and C. N. Serhan wrote the manuscript; N. Chiang, I. R. Riley, and J. Dalli designed and carried out experiments, analyzed data, and contributed to manuscript preparation; and A. R. Rodriguez and B. W. Spur carried out total organic synthesis of MCTRs.
REFERENCES
- 1.Serhan C. N., Chiang N., Dalli J. (2017) New pro-resolving n-3 mediators bridge resolution of infectious inflammation to tissue regeneration [E-pub ahead of print]. Mol. Aspects Med. 10.1016/j.mam.2017.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perretti M., Leroy X., Bland E. J., Montero-Melendez T. (2015) Resolution pharmacology: opportunities for therapeutic innovation in inflammation. Trends Pharmacol. Sci. 36, 737–755 [DOI] [PubMed] [Google Scholar]
- 3.Dalli J., Chiang N., Serhan C. N. (2014) Identification of 14-series sulfido-conjugated mediators that promote resolution of infection and organ protection. Proc. Natl. Acad. Sci. USA 111, E4753–E4761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalli J., Sanger J. M., Rodriguez A. R., Chiang N., Spur B. W., Serhan C. N. (2016) Identification and actions of a novel third maresin conjugate in tissue regeneration: MCTR3. PLoS One 11, e0149319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez A. R., Spur B. W. (2015) First total synthesis of pro-resolving and tissue regenerative maresin sulfido-conjugates. Tetrahedron Lett. 56, 3936–3940 [Google Scholar]
- 6.Dalli J., Vlasakov I., Riley I. R., Rodriguez A. R., Spur B. W., Petasis N. A., Chiang N., Serhan C. N. (2016) Maresin conjugates in tissue regeneration biosynthesis enzymes in human macrophages. Proc. Natl. Acad. Sci. USA 113, 12232–12237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samuelsson B. (2012) Role of basic science in the development of new medicines: examples from the eicosanoid field. J. Biol. Chem. 287, 10070–10080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimizu T. (2009) Lipid mediators in health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu. Rev. Pharmacol. Toxicol. 49, 123–150 [DOI] [PubMed] [Google Scholar]
- 9.Levi R., Krell R. D., eds. (1988) Biology of the Leukotrienes, New York Academy of Sciences, New York [Google Scholar]
- 10.Hattori Y., Levi R. (1984) Negative inotropic effect of leukotrienes: leukotrienes C4 and D4 inhibit calcium-dependent contractile responses in potassium-depolarized guinea-pig myocardium. J. Pharmacol. Exp. Ther. 230, 646–651 [PubMed] [Google Scholar]
- 11.Kurachi Y., Ito H., Sugimoto T., Shimizu T., Miki I., Ui M. (1989) Arachidonic acid metabolites as intracellular modulators of the G protein-gated cardiac K+ channel. Nature 337, 555–557 [DOI] [PubMed] [Google Scholar]
- 12.Wan M., Godson C., Guiry P. J., Agerberth B., Haeggström J. Z. (2011) Leukotriene B4/antimicrobial peptide LL-37 proinflammatory circuits are mediated by BLT1 and FPR2/ALX and are counterregulated by lipoxin A4 and resolvin E1. FASEB J. 25, 1697–1705 [DOI] [PubMed] [Google Scholar]
- 13.Krishnamoorthy S., Recchiuti A., Chiang N., Fredman G., Serhan C. N. (2012) Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. Am. J. Pathol. 180, 2018–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gronert K., Martinsson-Niskanen T., Ravasi S., Chiang N., Serhan C. N. (2001) Selectivity of recombinant human leukotriene D4, leukotriene B4, and lipoxin A4 receptors with aspirin-triggered 15-epi-LXA4 and regulation of vascular and inflammatory responses. Am. J. Pathol. 158, 3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson B. (2007) Ciona intestinalis as a model for cardiac development. Semin. Cell Dev. Biol. 18, 16–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peters M. F., Scott C. W. (2009) Evaluating cellular impedance assays for detection of GPCR pleiotropic signaling and functional selectivity. J. Biomol. Screen. 14, 246–255 [DOI] [PubMed] [Google Scholar]
- 17.Capra V., Ravasi S., Accomazzo M. R., Parenti M., Rovati G. E. (2004) CysLT1 signal transduction in differentiated U937 cells involves the activation of the small GTP-binding protein Ras. Biochem. Pharmacol. 67, 1569–1577 [DOI] [PubMed] [Google Scholar]
- 18.Aagaard L., Hansen E. H. (2014) Adverse drug reactions associated with asthma medications in children: systematic review of clinical trials. Int. J. Clin. Pharm. 36, 243–252 https://doi.org/10.1007/s11096-014-9924-y [DOI] [PubMed] [Google Scholar]
- 19.Piper P. J., ed. (1981) SRS-A and Leukotrienes, Research Studies Press, Chichester, United Kingdom [Google Scholar]
- 20.Knight J., Taylor G. W., Wright P., Clare A. S., Rowley A. F. (1999) Eicosanoid biosynthesis in an advanced deuterostomate invertebrate, the sea squirt (Ciona intestinalis). Biochim. Biophys. Acta 1436, 467–478 [DOI] [PubMed] [Google Scholar]
- 21.Pope E. C., Rowley A. F. (2002) The heart of Ciona intestinalis: eicosanoid-generating capacity and the effects of precursor fatty acids and eicosanoids on heart rate. J. Exp. Biol. 205, 1577–1583 [DOI] [PubMed] [Google Scholar]
- 22.Akagi D., Chen M., Toy R., Chatterjee A., Conte M. S. (2015) Systemic delivery of proresolving lipid mediators resolvin D2 and maresin 1 attenuates intimal hyperplasia in mice. FASEB J. 29, 2504–2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pope N. H., Salmon M., Davis J. P., Chatterjee A., Su G., Conte M. S., Ailawadi G., Upchurch G. R., Jr (2016) D-series resolvins inhibit murine abdominal aortic aneurysm formation and increase M2 macrophage polarization. FASEB J. 30, 4192–4201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kain V., Ingle K. A., Colas R. A., Dalli J., Prabhu S. D., Serhan C. N., Joshi M., Halade G. V. (2015) Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function. J. Mol. Cell. Cardiol. 84, 24–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fredman G., Hellmann J., Proto J. D., Kuriakose G., Colas R. A., Dorweiler B., Connolly E. S., Solomon R., Jones D. M., Heyer E. J., Spite M., Tabas I. (2016) An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat. Commun. 7, 12859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francos-Quijorna I., Santos-Nogueira E., Gronert K., Sllivan A. B., Kopp M. A., Brommer B., David S., Schwab J. M., López-Vales R. (2017) Maresin 1 promotes inflammatory resolution, neuroprotection, and functional neurological recovery after spinal cord injury. J. Neurosci. 37, 11731–11743 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.