Abstract
Severe anemia and iron deficiency are common complications in chronic kidney disease. The cause of renal anemia is multifactorial and includes decreased erythropoietin (Epo) production, iron deficiency, and inflammation, and it is currently treated with injections of synthetic Epo. However, the use of recombinant Epo has several adverse effects. We previously reported that high fibroblast growth factor 23 (FGF23) levels in mice are associated with decreased red blood cell production, whereas genetic inactivation of Fgf23 results in expansion of the erythroid lineage. The present study is the first to show that high FGF23 levels in a mouse model of renal failure contribute to renal anemia, and inhibiting FGF23 signaling stimulates erythropoiesis and abolishes anemia and iron deficiency. Moreover, we show that inhibition of FGF23 signaling significantly decreases erythroid cell apoptosis and influences the commitment of hematopoietic stem cells toward the erythroid linage. Furthermore, we show that blocking FGF23 signaling attenuates inflammation, resulting in increased serum iron and ferritin levels. Our data clearly demonstrate that elevated FGF23 is a causative factor in the development of renal anemia and iron deficiency, and importantly, blocking FGF23 signaling represents a novel approach to stimulate erythropoiesis and possibly improve survival for millions of chronic kidney disease patients worldwide.—Agoro, R., Montagna, A., Goetz, R., Aligbe, O., Singh, G., Coe, L. M., Mohammadi, M., Rivella, S., Sitara, D. Inhibition of fibroblast growth factor 23 (FGF23) signaling rescues renal anemia.
Keywords: chronic kidney disease, erythropoietin, FGF23, iron
Chronic kidney disease (CKD) is a global growing public health problem currently affecting millions of Americans and many other people worldwide (1, 2). As renal function declines, CKD patients experience several secondary complications, including cardiovascular disease, aberrant mineral metabolism, and anemia. Renal anemia is a risk factor for cardiovascular disease in CKD patients, affecting more than 80% of these individuals, and it has been associated with increased mortality and adverse long-term outcomes (3, 4). This triad of CKD, cardiovascular disease, and anemia is known as the cardio–renal–anemia syndrome; each of the 3 conditions is capable of causing or being caused by each other (5). The cause of anemia in the cardio–renal syndrome is multifactorial and includes decreased erythropoietin (Epo) production due to renal dysfunction, iron deficiency, inflammation, and inhibition of the renin–angiotensin system by the use of angiotensin-converting enzyme inhibitors (6, 7). Epo and iron are both essential for erythropoiesis and are involved at different stages of the process of differentiation and maturation, from pluripotent stem cell to erythrocyte.
Fibroblast growth factor (FGF) 23 is an osteocyte-secreted protein that acts on the kidneys to regulate phosphate and vitamin D homeostasis and bone mineralization (8, 9). FGF23 requires Klotho as a coreceptor for binding with high affinity to the fibroblast growth factor receptors (FGFR) (10, 11). Fgf23 deficiency results in hyperphosphatemia, elevated vitamin D levels, aberrant bone mineralization, and tissue and vascular calcifications (12, 13). In contrast, circulating FGF23 levels are increased in CKD patients as well as in several bone disorders, including autosomal-dominant hypophosphatemic rickets and X-linked hypophosphatemia (14, 15). In CKD, serum FGF23 levels progressively increase as renal function declines, reaching up to 10,000-fold above the normal range in advanced renal failure. While this massive increase in FGF23 levels is a compensatory mechanism to maintain normal serum phosphate levels in response to reduced renal phosphate excretory capacity (16, 17), high FGF23 levels are associated with greater risk for cardiovascular disease, cardiac hypertrophy, and mortality in dialysis patients (18, 19).
In our previous studies, we reported that FGF23 negatively regulates erythropoiesis, thus providing the first link between high FGF23 levels and decreased red blood cell production (20). Moreover, we showed that inactivation of Fgf23 results in increased erythroid cell populations both prenatally (in fetal livers) and postnatally [in bone marrow (BM) and peripheral blood]. This novel function of FGF23 is both direct by influencing the commitment of hematopoietic stem cells (HSCs) toward the erythroid linage, and indirect by regulating Epo (20). These findings set the stage for the study described here.
Given its strong link to CKD progression and its role on erythropoiesis, in the present study, we sought to investigate if elevated FGF23 is a causal factor in the development of renal anemia, and whether blocking FGF23 activity can improve erythropoiesis and ameliorate anemia. For this, we used the 72 aa long C-terminal tail peptide of FGF23 as a tool to specifically block FGF23 signaling (21), then compared its effect to those of Epo, which is the current mainstay of therapy for CKD-associated anemia. However, although recombinant Epo is the primary therapeutic option for the management of renal anemia, it is rather a symptomatic treatment and does not target the ultimate cause of CKD-associated anemia.
In the present study, we provide evidence for a causative role of FGF23 in the development of CKD-associated anemia and the therapeutic potential of blocking FGF23 signaling.
MATERIALS AND METHODS
Animals
Male C57BL/6J mice (45 d old) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA), where renal insufficiency was induced by a 2-step polectomy surgery. This procedure reduces the original functional renal mass by five sixths [5/6 nephrectomy (Nx)]. The second surgery occurred 1 wk after the first surgery. Control mice underwent sham surgery at the same time. After the second surgery, the mice were transported to New York University College of Dentistry animal facility, where they were housed and kept on a 12-h light/dark cycle at 23°C, and received food (standard lab chow) and water ad libitum. All animal studies were approved by the Institutional Animal Care and Use Committee at New York University. Treatment started 8 wk after surgery to allow establishment of renal failure. Mice were randomized into 4 groups: sham surgery or 5/6 Nx with or without intraperitoneal injections of vehicle or compound. Homozygous Fgf23−/− mice were obtained from Fgf23+/− breeding.
Treatments
Mice that underwent 5/6 Nx or sham surgery were treated with one of the following compounds. A single injection (10 mg/kg, i.p.) of the FGF23 blocking peptide (provided by M. Mohammadi, New York University School of Medicine) or vehicle (HEPES-buffered saline; 25 mM HEPES-NaOH pH 7.5, 150 mM NaCl). The FGF23 blocking peptide is the 72 aa long C-terminal tail of human FGF23 (residues Ser180-Ile251), which corresponds to the C-terminal fragment of FGF23 generated by proteolytic cleavage at the RXXR motif (21). The injected mice were humanely killed 12 h after injection. Human recombinant Epo (Amgen, Thousand Oaks, CA, USA) or vehicle (PBS) was injected (200 IU, i.p.) every day for 3 successive days (22). The injected mice were humanely killed 24 h after the last injection.
Serum measurements
Serum FGF23 levels were measured using the mouse FGF23 C-terminal ELISA kit (Immutopics International, San Clemente, CA, USA), which measures both the intact and C-terminal fragments of FGF23. Serum Epo and ferritin concentrations were measured using the Quantikine Rat/Mouse EPO Immunoassay kit (R&D Systems, Minneapolis, MN, USA) and the Mouse Ferritin ELISA Kit (Abcam, Cambridge, United Kingdom), respectively. Serum parathyroid hormone (PTH) levels were measured using the mouse PTH 1-84 ELISA Kit (Immutopics International). Serum iron and transferrin saturation were measured using the Iron-TIBC Kit from Pointe Scientific (Canton, MI, USA). Serum hepcidin was measured using the Hepcidin Murine-Compete ELISA kit (Intrinsic LifeSciences, La Jolla, CA, USA). Serum creatinine and urea nitrogen were measured using the Stanbio Direct Creatinine LiquiColor Kit and Stanbio Urea Nitrogen (blood urea nitrogen, BUN) kit, respectively, whereas serum and urinary phosphate were measured using the Stanbio Phosphorus Liqui-UV Kit (Stanbio Laboratory, Boerne, TX, USA).
Isolation and assessment of BM cells by flow cytometry analysis
BM cell suspensions were prepared from dissected tibiae and femora from mice with 5/6 Nx or sham surgery in Iscove modified Dulbecco medium (IMDM) (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 20% fetal bovine serum (20% IMDM) through a 26-gauge Becton Dickinson (BD Biosciences, San Jose, CA, USA) needle, as described elsewhere (20). BM cells were dispersed by manual agitation then filtered to remove foreign particles. Flow cytometry analysis for BM cells was carried out in a BD FACSort flow cytometer equipped with 488 argon lasers (BD Biosciences). For immunostaining, cells were washed and resuspended in 1× PBS containing 0.1% bovine serum albumin. Mouse FcR was blocked before staining using CD16/32 antibody to reduce nonspecific binding. After the addition of antibodies, cells were incubated for 40 min on ice. Labeled cells were then washed with 1× PBS and analyzed by flow cytometry. Appropriate isotype controls were kept for each set. Forward- and side-scatter patterns were gated excluding the debris. A total of 20,000 events were collected and analyzed by FlowJo 7.6.5 software (FlowJo, Ashland, OR, USA). Erythroid lineage was assessed using Ter119-APC/CD71-PE/CD44-FITC markers (BD Pharmingen, San Jose, CA, USA) combined with the forward-scatter properties (23, 24). HSCs/progenitor cells were differentiated using CD150-PE/CD48-APC (SLAM) markers (eBioscience, San Diego, CA, USA).
Colony-forming unit assay
Cell suspensions were prepared from BM in 20% IMDM from mice with 5/6 Nx or sham surgery. Aliquots were then plated in a semisolid methylcellulose medium supplemented with recombinant cytokines for colony assays of murine cells (Methocult M3434; STEMCELL Technologies, Vancouver, BC, Canada). Cytokines included: IL-3 (4 ng/10 μl), stem cell factor (20 ng/10 μl), granulocyte macrophage-colony stimulating factor (2 ng/10 μl), and Epo (2 U/10 μl). BM cells were plated at a concentration of 1 × 104 and incubated in a humidified chamber at 37°C with 5% CO2. Burst-forming unit–erythroid (BFU-E) colonies were scored between 8 and 12 d after culture.
RNA isolation
Total RNA was extracted from kidneys, liver, bone, and BM isolated from femora and tibiae of mice that underwent 5/6 Nx or sham surgery using Trizol (Ambion; Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s protocol (Molecular Research Center, Cincinnati, OH, USA). Synthesis of cDNA was performed using the High Capacity cDNA Reverse Transcription Kit as described by the manufacturer (Applied Biosystems; Thermo Fisher Scientific, Waltham, MA, USA). cDNA was amplified by quantitative PCR (qPCR) using the PerfecCTa SYBR Green SuperMix (Quanta Biosciences, Gaithersburg, MD, USA). The following primers were used: Epo, 5′-TCTACGTAGCCTCACTTCACT-3′ and 5′-ACCCGGAAGAGCTTGCAGAAA-3′; hypoxia-induced factor 1-α (Hif1α), 5′-TCTCGGCGAAGCAAAGAGTCT-3′ and 5′-TAGACCACCGGCATCCAGAAG-3′; hypoxia-induced factor 2-α (Hif2α), 5′-GGGAACACTACACCCAGTGC-3′ and 5′-TCTTCAAGGGATTCTCCAAGG-3′; bone morphogenetic protein 6 (Bmp6), 5′-GTGTGGGCCTCCGAAGAA-3′ and 5′-ACACTCAGCTGGAGTCCCATGT-3′; hepcidin (Hamp), 5′-CACCACCTATCTCCATCAACAG-3′ and 5′-GTTGGTGTCTCTCTTCCTTCTC-3′; Ifn-γ, 5′-GGCTGTCCCTGAAAGAAAGC-3′ and 5′-GAGCGAGTTATTTGTCATTCGG-3′; Il-6, 5′-ATCCAGTTGCCTTCTTGGGACTGA-3′ and 5′-TAAGCCTCCGACTTGTGAAGTGGT-3′; Tnf-α, 5′-AAGGGAGAGTGGTCAGGTTGCC-3′ and 5′-CCTCAGGGAAGAGTCTGGAAAGG-3′; Fgf23, 5′-ACTTGGCCTTTATTAGCCGGGTCT-3′ and 5′-AGATGGCCTCTTCCCTGTGTTCAA-3′; Klotho, 5′-AAATGGCTGGTTTGTCTCGGGAAC-3′ and 5′-TATGCCACTCGAAACCGTCCATGA-3′; and hypoxanthine–guanine phosphoribosyl transferase (Hprt housekeeping gene), 5′-AAGCCTAAGATGAGCGCAAG-3′ and 5′-TTACTAGGCAGATGGCCACA-3′. Forty cycles (95°C, 15 s; 60°C, 30 s; 72°C, 30 s) were run on a MasterCycler Realplex2 (Eppendorf, Hamburg, Germany), and reactions were analyzed. Melting curve and gel analyses (sizing and sequencing) verified single products of the appropriate base pair size.
Cell cycle and apoptosis
C57BL/6J mice were treated intraperitoneally with the following: recombinant FGF23 protein (5 μg; R&D Systems) for 24 h; FGF23 blocking peptide (10 mg/kg) for 12 h; or FGF23 protein for 24 h (to allow FGF23 levels to increase), followed by FGF23 blocking peptide for 12 h. Treated and untreated C57BL/6J mice, and Fgf23−/− mice were then further injected with bromodeoxyuridine (BrdU; 1 mg, i.p.), according to the FITC BrdU Flow kit instructions (BD Biosciences). After 1 h, BM cells were isolated from dissected tibiae and femora and labeled with BD IMag anti-mouse Ter-119 Particles–DM (BD Biosciences). The labeled Ter119+ cell suspensions were separated from erythroid lineage–negative cells (Ter119−) using the BD IMagnet (BD Biosciences) and stained with Ter119-APC and CD44-PE antibodies (BD Biosciences). They were then fixed and stained with anti–BrdU-FITC and the DNA marker 7-aminoactinomycin (7-AAD), as described in the FITC BrdU Flow Kit manual. Cell cycle profiles were assessed by flow cytometry.
For apoptosis, labeled Ter119+ BM cells were stained with Ter119-APC and CD44-PE antibodies and counterstained with FITC-conjugated annexin V antibody (5 μl; BD Biosciences). Late stage apoptotic and necrotic cells were excluded from the analysis by counterstaining with propidium iodide (5 μl of 250 μg/ml stock; BD Biosciences). The percentage of annexin V+ and Ter119+ cells was gated and analyzed by flow cytometry. The annexin V+ fraction was detected as apoptotic.
Statistical analysis
Statistical significance was evaluated by Student’s t test for comparison between 2 groups or by 1-way ANOVA followed by Tukey’s test for multiple group comparisons. All analyses were performed by GraphPad Prism 5.0 software (GraphPad Software, La Jolla, CA, USA), and all values were expressed as mean ± se. Values of P < 0.05 were considered significant.
RESULTS
Evaluation of renal insufficiency after 5/6 Nx in mice
In the present study, we used an established and widely used mouse model of CKD and associated anemia in which renal failure is induced by 5/6 Nx. Mice that underwent 5/6 Nx were first examined for successful induction of renal insufficiency 8 wk after surgery. Body weight and total kidney weight were significantly reduced in nephrectomized mice compared to sham-treated mice (Table 1). Kidney function was appropriately low after 5/6 Nx, as determined by elevated serum creatinine and BUN concentrations (Table 1). Mineral disturbances (e.g., hyperphosphatemia and hypocalcemia) and hormone imbalances (e.g., calcitriol deficiency and secondary hyperparathyroidism) are common complications of CKD, along with elevated circulating FGF23 levels, increased fractional excretion of phosphate (FEPi), and decreased renal Klotho expression (25, 26). As expected, serum FGF23 levels were highly elevated in 5/6 Nx mice, which also developed hyperphosphatemia, and experienced increased FEPi and secondary hyperparathyroidism (Table 1). In addition, bone Fgf23 expression was significantly up-regulated, whereas renal Klotho expression was significantly decreased after 5/6 Nx (Supplemental Fig. 1A, B). Therefore, these findings clearly demonstrate the development of kidney disease after 5/6 Nx in our mouse model.
TABLE 1.
Blood and urinary parameters in mice with 5/6 Nx and sham surgery
| Parameter | Sham surgery (n = 9) | 5/6 Nx (n = 6) | P |
|---|---|---|---|
| Body weight (g) | 25.5 ± 0.4 | 21.6 ± 0.6 | <0.001 (0.0007) |
| Kidney weight (g) | 0.33 ± 0.007 | 0.12 ± 0.002 | <0.0001 |
| Serum creatinine (mg/dl) | 0.77 ± 0.17 | 2.2 ± 0.3 | <0.001 (0.0007) |
| Serum BUN (mg/dl) | 25.4 ± 0.67 | 60.3 ± 3.8 | <0.0001 |
| Serum FGF23 (pg/ml) | 306.3 ± 6.0 | 801.2 ± 38.0 | <0.0001 |
| Serum phosphate (mg/dl) | 7.6 ± 0.2 | 12.1 ± 0.7 | <0.0001 |
| FEPi (%) | 26.2 ± 3.2 | 71.7 ± 2.25 | <0.0001 |
| Intact PTH (pg/ml) | 69.5 ± 2.02 | 229.8 ± 52.49 | <0.01 (0.0086) |
| Hemoglobin (g/dl) | 15.6 ± 0.05 | 11.2 ± 0.2 | <0.01 (0.0021) |
Development of anemia and iron deficiency in 5/6 Nx model
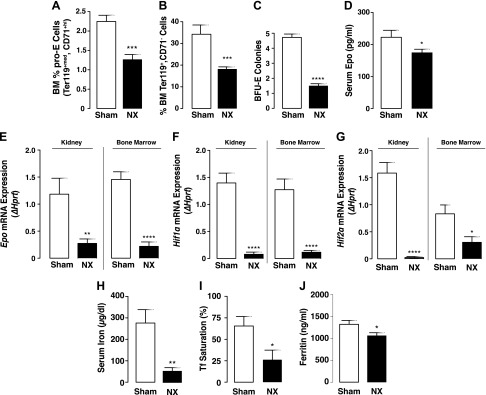
Red blood cell production is driven by renal Epo secretion in response to hypoxia. Anemia is a common complication of CKD that develops primarily as a result of the progressive decline in renal Epo secretion associated with the loss of kidney function (27, 28). Consistent with the presence of anemia in CKD patients, low levels of hemoglobin and hematocrit have also been reported in mice after 5/6 Nx (29, 30). However, the degree of anemia in these mice remains unknown. In our studies, we confirmed low hemoglobin levels in the 5/6 Nx mice (Table 1). To assess the extent of anemia after Nx, we carried out analysis of the erythroid cell populations and examined the maturation stages of erythroblasts (Ter119+) by the loss of CD71 expression, as reported by Asari et al. (23). Our results show a significant decrease in the percentage of early (proerythroblast, pro-E; Ter119+med CD71+hi), as well as terminally differentiated erythroid cells (Ter119+CD71−) in the BM (Fig. 1A, B) of 5/6 Nx mice compared to the sham-treated group.
Figure 1.
Establishment of anemia in mice with 5/6 Nx. A, B) Flow cytometry analysis of BM cell populations from mice with sham surgery or 5/6 Nx. Percentage of pro-E stained positive for Ter119med and CD71high (B), and terminally differentiated erythroid cells stained positive for Ter119high and negative for CD71 (B) (sham, n = 9; Nx, n = 7). C) Colony-forming assay for erythroid (BFU-E) progenitors (sham, n = 15; Nx, n = 14). Cells from each mouse were plated in duplicate, and number of colonies in each plate was counted. D) Serum Epo concentrations measured by ELISA in sham-treated (n = 11) and 5/6 Nx mice (n = 18). Samples were measured in duplicate. E–G) qPCR Epo (E), Hif1α (F), and Hif2α (G) mRNA expression in kidney (sham, n = 18; Nx, n = 14) and BM (sham, n = 14; Nx, n = 13). Data are represented as fold change (Δ) relative to housekeeping gene Hprt. H–J) Serum iron parameters. Iron (H), transferrin saturation (I), and ferritin (J) in sham-treated and 5/6 Nx mice (n = 7). Samples were measured in duplicate. Data are represented as means ± sem. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Moreover, we assessed the presence of erythroid progenitors in the BM of sham-treated and 5/6 Nx mice by colony-forming unit assay, an in vitro functional assay for enumerating multipotential and lineage-committed hematopoietic progenitor cells. BFU-E, which are committed erythroid progenitor cells, are detected by the formation of discrete erythroid colonies after in vitro culture in methylcellulose in the presence of growth factors. The number of colonies formed provides a measure of the number of viable and functional progenitors present. As shown in Fig. 1C, BM cells from the 5/6 Nx mice generated significantly less BFU-E colonies in vitro compared to BM cells from the sham-treated group, suggesting that BM cells from the 5/6 Nx mice contain less functional erythroid progenitors.
Furthermore, we examined whether the reduction in erythroid cell numbers is associated with decreased renal Epo secretion. As expected, our data show that circulating Epo levels were lower in the 5/6 Nx mice compared to the sham-treated group (Fig. 1D). In addition, we found that Epo mRNA expression was significantly suppressed in the kidney and BM of 5/6 Nx mice (Fig. 1E) as a result of diminished Hif levels, as determined by significant down-regulation of the hypoxia-inducible transcription factors (HIF) Hif1α (Fig. 1F) and Hif2α mRNA expression (Fig. 1G). Because renal anemia is associated with iron deficiency in CKD patients (31), we examined iron levels in 5/6 Nx and sham-treated mice. In agreement with the patient data, 5/6 Nx mice have significantly lower serum iron levels, transferrin saturation, and serum ferritin levels compared to sham-treated mice (Fig. 1H–J). These data confirm that the 5/6 Nx mouse model shows consistent characteristics of renal anemia and iron deficiency.
Inhibition of FGF23 signaling stimulates erythropoiesis and induces Epo secretion in 5/6 Nx mice
We recently reported that mice deficient in Fgf23 (Fgf23−/−) have significantly increased erythropoiesis (20). Therefore, we investigated whether inhibition of FGF23 signaling can restore erythropoiesis and correct anemia in the setting of CKD. After developing renal failure and anemia, 5/6 Nx mice and the sham-treated controls were randomized to receive the FGF23 blocking peptide, Epo or vehicle (untreated). The FGF23 blocking peptide competes with the full-length FGF23 for binding to the FGFR-Klotho complex, to antagonize the activity of full-length FGF23 (21). To verify the inhibitory efficacy of the FGF23 C-tail blocking peptide, we measured serum phosphate and FEPi in sham-treated and 5/6 Nx mice after injection. Consistent with previous findings (21), the FGF23 blocking peptide inhibited renal phosphate excretion and induced hyperphosphatemia in sham-treated mice compared to vehicle-treated animals (Supplemental Table 1). Moreover, FEPi was decreased in 5/6 Nx mice after injection of the FGF23 blocking peptide (Supplemental Table 1), as described in other studies using FGF23 neutralizing antibodies (32, 33). Interestingly, serum phosphate levels also dropped in 5/6 Nx mice, although they still remained hyperphosphatemic (Supplemental Table 1). Serum PTH levels were induced in sham-treated mice and remained elevated in 5/6 Nx mice after treatment with the FGF23 blocking peptide (Supplemental Table 1), in agreement with reports showing that FGF23 signaling directly inhibits PTH gene expression and secretion in the parathyroid gland (34).
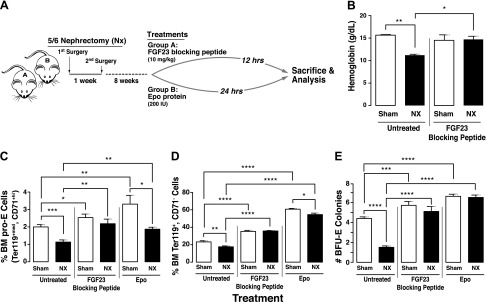
We evaluated the effect of the FGF23 blocking peptide on erythropoiesis and compared it to the traditional Epo treatment for anemia in CKD. A schematic of the experimental design is shown in Fig. 2A. A single injection of the FGF23 blocking peptide normalized hemoglobin levels (Fig. 2B) and significantly increased both early (pro-E) and terminally differentiated BM erythroid cell populations in 5/6 Nx mice compared to untreated 5/6 Nx mice to levels that were at least comparable to sham-treated mice, showing complete rescue of Nx-induced anemia (Fig. 2C, D). Furthermore, treatment with the FGF23 blocking peptide markedly increased the number of BFU-E colonies formed in vitro (Fig. 2E). Taken together, our data suggest that inhibiting FGF23 signaling stimulates erythropoiesis and rescues renal anemia. As expected, Epo treatment similarly increased early and terminally differentiated BM erythroid cell populations in 5/6 Nx mice (Fig. 2C, D) and the number of BFU-E colonies formed in vitro (Fig. 2E).
Figure 2.
Inhibition of FGF23 signaling rescues erythroid cell populations in mouse model of Nx-induced anemia. A) Experimental design. C57BL/6J mice underwent 2-step 5/6 Nx or sham surgery. Treatment started 8 wk after surgery. Mice were humanely killed 12 h after treatment with FGF23 blocking peptide or 24 h after treatment with Epo. B) Blood hemoglobin levels in sham-treated and 5/6 Nx mice untreated (vehicle-treated) or treated with FGF23 blocking peptide (sham, n = 4; Nx, n = 4). C, D) Flow cytometry analysis of BM cell populations from sham-treated or 5/6 Nx mice that were untreated or treated (with FGF23 blocking peptide or Epo). Percentage of pro-E (C) stained positive for Ter119med and CD71high (sham, n = 9; Nx, n = 7), and terminally differentiated erythroid cells (D) stained positive for Ter119high and negative for CD71 (sham, n = 9; Nx, n = 7). E) Colony-forming assay for erythroid (BFU-E) progenitors (n = 15 for all groups). Cells from each mouse were plated in duplicate, and number of colonies in each plate was counted. Data are represented as means ± sem. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
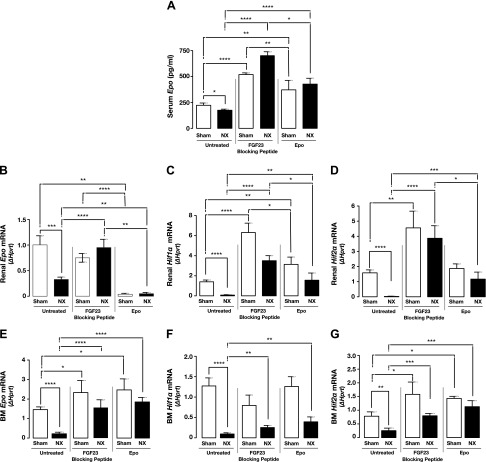
Moreover, we investigated whether blocking FGF23 signaling has an effect on Epo secretion. Inhibition of FGF23 signaling led to a significant increase in serum Epo levels (Fig. 3A) as well as renal and BM Epo mRNA expression in both sham-treated and 5/6 Nx mice (Fig. 3B, E). This increase in Epo was due to a robust induction of Hif mRNA expression in the kidneys (Fig. 3C, D) and BM (Fig. 3F, G). Circulating Epo levels were also increased in sham-treated and 5/6 Nx mice after treatment with recombinant Epo (Fig. 3A), albeit to a lesser extent than after injection with the FGF23 blocking peptide. Despite the increase in serum Epo, renal Epo transcription was markedly suppressed after Epo treatment (Fig. 3B). This is most likely due to a feedback mechanism that signals the kidneys to turn off Epo secretion when they sense elevation in serum Epo levels.
Figure 3.
Inhibition of FGF23 signaling induces Epo secretion in Nx mice. A) Serum Epo concentrations measured by ELISA in sham-treated and 5/6 Nx mice untreated (vehicle-treated) or treated with FGF23 blocking peptide or Epo (sham, n = 11; Nx, n = 10). Samples were measured in duplicate. B–G) qPCR in sham-treated and 5/6 Nx mice untreated or treated with FGF23 blocking peptide or Epo. Data are represented as fold change (Δ) relative to Hprt. Renal mRNA expression of Epo (B), Hif1α (C), and Hif2α (D) (sham, n = 15; Nx, n = 13); BM mRNA expression of Epo (E), Hif1α (F), and Hif2α (G) (sham, n = 14; Nx, n = 13). Data are represented as means ± sem. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Inhibition of FGF23 signaling abolishes iron deficiency and decreases inflammation in 5/6 Nx mice
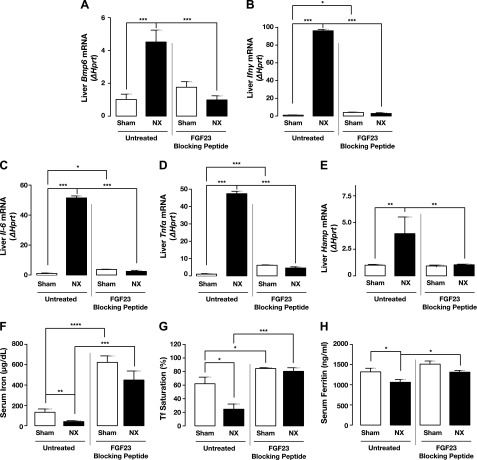
Proper production of red blood cells requires not only Epo but also iron, which is an essential element for hemoglobin synthesis and normal differentiation and proliferation of erythroid progenitor cells. Systemic iron homeostasis is regulated by the liver hormone hepcidin, a master regulator of iron metabolism, which controls iron release from iron stores into the circulation. Chronic inflammation can lead to anemia (anemia of chronic disease, also known as anemia of inflammation), mediated principally by up-regulation of hepcidin (35, 36). Patients with CKD are in a state of chronic inflammation, as evidenced by elevated levels of proinflammatory cytokines (e.g., IL-6, IL-1β, TNF-α), leading to up-regulation of hepcidin. Hepcidin in turn inhibits intestinal iron absorption, thereby limiting available iron to be transported to the circulation and resulting in iron deficiency (37, 38). Consistent with patient data, inflammatory markers (Ifn-γ, Il-6, and Tnf-α) and the hepcidin inducer Bmp6 were significantly increased in the liver of the 5/6 Nx mice (Fig. 4A–D) compared to sham-treated mice, resulting in up-regulation of hepcidin (Hamp) mRNA expression (Fig. 4E). Importantly, inhibiting FGF23 signaling by the FGF23 blocking peptide significantly reduced these inflammatory markers (Fig. 4A–D) and normalized hepcidin expression in the 5/6 Nx mice (Fig. 4E). Similarly, serum hepcidin levels were significantly reduced in both sham-treated and 5/6 Nx mice after treatment with the FGF23 blocking peptide (data not shown).
Figure 4.
Inhibition of FGF23 signaling corrects iron deficiency related to inflammation. A–E) qPCR. Bmp6 (A), Ifn-γ (B), Il-6 (C), Tnf-α (D), and hepcidin (E) (Hamp) mRNA expression in liver of sham-treated and 5/6 Nx mice untreated (vehicle-treated) or treated with FGF23 blocking peptide (sham, n = 12; Nx, n = 12). Data are represented as fold change (Δ) relative to Hprt. F–H) Serum iron parameters in sham-treated and 5/6 Nx mice untreated or treated with FGF23 blocking peptide. Iron (F), transferrin saturation (G), and ferritin (H) (sham, n = 7; Nx, n = 7). Samples were measured in duplicate. Data are represented as means ± sem. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Hepcidin production is inversely correlated with plasma iron levels. Iron deficiency is common among CKD patients with anemia. In addition, iron deficiency can stimulate FGF23 transcription in osteocytes (39, 40), whereas rapid intravenous iron loading can decrease C-terminal FGF23 levels (41). In our studies, we confirmed iron deficiency in the 5/6 Nx mice (Fig. 4F–H) and found that treatment with the FGF23 blocking peptide completely ameliorated iron deficiency in these mice. Serum iron levels, transferrin saturation, and serum ferritin were significantly increased by inhibition of FGF23 signaling in 5/6 Nx mice (Fig. 4F–H).
Blocking FGF23 signaling decreases apoptosis and affects cell cycle progression of erythroid cells
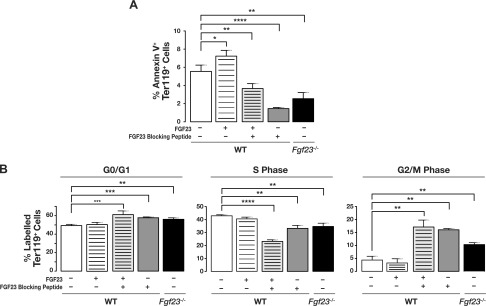
To determine whether the increase in erythropoiesis is due to reduced cell death and/or increased cell proliferation, we examined the effect of FGF23 signaling inhibition on erythroid cell cycling and apoptosis. We treated C57BL/6J mice with recombinant FGF23 protein, FGF23 blocking peptide, or a combination of the two, and compared them to untreated C57BL/6J and Fgf23−/− mice. Our data clearly show that inhibiting FGF23, by the use of an FGF23 blocking peptide or genetic inactivation (Fgf23−/−), significantly decreased apoptosis of erythroid cells, as demonstrated by significantly decreased annexin V staining on the surface of Ter119+ BM cells. Conversely, injecting recombinant FGF23 protein resulted in a modest increase in erythroid cell apoptosis (Fig. 5A). Moreover, treatment of C57BL/6J with recombinant FGF23 protein and FGF23 blocking peptide together significantly reduced the fraction of apoptotic erythroid cells (Fig. 5A), demonstrating the inhibitory efficacy of the FGF23 blocking peptide in the presence of high FGF23 levels.
Figure 5.
Blocking FGF23 signaling decreases apoptosis and inhibits cell cycle progression of erythroid cells. Flow cytometry analysis of (A) apoptosis and (B) cell cycle. BM erythroid cell populations isolated from untreated C57BL/6J mice; C57BL/6J mice treated with either recombinant FGF23, FGF23 blocking peptide, or combination of FGF23 protein and FGF23 blocking peptide; and Fgf23−/− mice, were stained with Ter119 and CD44 antibodies and further stained with either annexin V antibody (for apoptosis) or BrdU antibody and 7-aminoactinomycin (7-AAD) (for cell cycle) (untreated, n = 13; +FGF23, n = 10; +FGF23 blocking peptide, n = 4; +FGF23 + FGF23 blocking peptide, n = 9; Fgf23−/−, n = 10). Data are represented as means ± sem. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
To assess whether inhibition of FGF23 signaling affected the cell cycle distribution of erythroid progenitors, BM erythroid cells were collected 1 h after BrdU injection of treated and untreated C57BL/6J and Fgf23−/− mice. Cell cycle status was assessed by flow cytometry. Our data show that, relative to untreated C57BL/6J, blocking FGF23 signaling reduced the fraction of erythroid cells in the S phase of the cell cycle (Fig. 5B). In addition to a slight increase in the fraction of erythroid cells in G0/G1 phase, inhibition of FGF23 increased the fraction of erythroid cells in the G2/M phase compared to cells from untreated C57BL/6J (Fig. 5B).
DISCUSSION
High FGF23 levels are associated with higher mortality, exacerbated CKD progression, and higher risk of cardiovascular events in CKD patients. Severe anemia is a common complication in CKD and is a risk factor for cardiovascular disease and heart failure in CKD patients. The cause of renal anemia is multifactorial and includes decreased Epo production, iron deficiency, and inflammation. At present, no association has been reported between high FGF23 levels and renal anemia according to animal or clinical studies. However, an observational study of 53 CKD patients showed that high FGF23 levels are negatively correlated with hemoglobin levels (42).
Here we investigated the association between high FGF23 levels and development of anemia in a mouse model of CKD, and evaluated the effect of pharmacologically blocking FGF23 signaling in the anemia of renal failure. We used the FGF23 C-terminal tail peptide as a tool to specifically inhibit FGF23 signaling (21). This peptide comprises the 72-aa long FGF23 C-terminal domain that is generated by inactivating proteolytic cleavage of FGF23 (43, 44). It has been shown in binding and cell signaling studies that the peptide can compete with the intact FGF23 for binding to the FGFR-Klotho complex (21). Therefore, the peptide can inhibit FGF23 signaling and antagonize the phosphaturic activity of FGF23 in vivo (21).
FGF23 acts directly on the renal proximal tubular cells to suppress phosphate reuptake and expression of 1α-hydroxylase, the rate-limiting enzyme for vitamin D production, by binding to FGFR1 and to FGFR3 and FGFR4, respectively (9, 45). Moreover, FGF23 acts directly on the distal tubular cells to stimulate calcium and sodium reabsorption (46, 47). In both proximal and distal tubular epithelium, FGF23 binds to the binary FGFR-Klotho complex and induces signaling mainly through activation of ERK1/2 (Ras/MAPK), but also PI3K/Akt, and PLCγ/PKC pathways (48). Studies have shown that FGF23 also acts directly on cardiomyocytes by binding to FGFR4 independent of Klotho and signals primarily through the PLCγ/PKC pathway to induce left ventricular hypertrophy (49, 50). We have reported that Fgf23, Klotho, and FGFRs 1 and 4 are all expressed in wild-type BM erythroid cells (20), thus identifying erythroid cells as target cells of FGF23, and suggesting that they are capable of responding to FGF23 signaling, as well as to its inhibition. However, further studies are needed to identify which FGFR and signaling pathway FGF23 uses to regulate erythropoiesis, and to address the mechanism by which FGF23 acts on Epo-producing cells. Furthermore, receptor studies would be important to assess expression of FGFRs and Klotho in Epo-producing cells. Epo is primarily synthesized by peritubular interstitial cells in the renal cortex, and to a lesser extent by hepatocytes and osteoblasts. Currently there are no studies describing expression of FGFRs and Klotho in renal peritubular interstitial cells. Moreover, to our knowledge, there is no available renal cell line producing Epo to study expression of FGFRs and Klotho and the downstream signaling pathways involved in the regulation of Epo expression by FGF23. Although genetic approaches have been used to isolate a pure population of cells essential for renal Epo production (51, 52), and although attempts have been made to establish a renal cell line producing Epo (53), it still remains challenging to isolate, purify, culture, and handle this rare cell population.
Many of the mineral disturbances and comorbidities observed in CKD patients are present in the 5/6 Nx model, including hyperphosphatemia, increased serum creatinine, BUN, and FGF23 levels, and reduced renal Klotho expression (Supplemental Fig. 1 and Table 1). Consistent with previous findings in healthy rats (21), treatment with the FGF23 blocking peptide reduced renal phosphate excretion and induced hyperphosphatemia in sham-treated mice (Supplemental Table 2), confirming the effectiveness of the FGF23 blocking peptide. Moreover, FEPi was decreased in 5/6 Nx mice after injection of the FGF23 blocking peptide, as described in other studies using FGF23-neutralizing antibodies (32, 33). It would be expected that the decrease in renal phosphate excretion in 5/6 Nx mice would result in a further rise in serum phosphate levels in these mice. Interestingly, we observed that serum phosphate levels also dropped in 5/6 Nx mice after treatment with the FGF23 blocking peptide, although they still remained hyperphosphatemic (Supplemental Table 1). This paradoxical finding could possibly be caused by increased phosphate uptake in bone after treatment with the FGF23 blocking peptide, or end-organ resistance to FGF23 that the 5/6 Nx mice develop as a result of Klotho deficiency. Studies of both human and mouse genetic disorders have shown that loss of αKlotho results in end-organ resistance to FGF23 (54, 55).
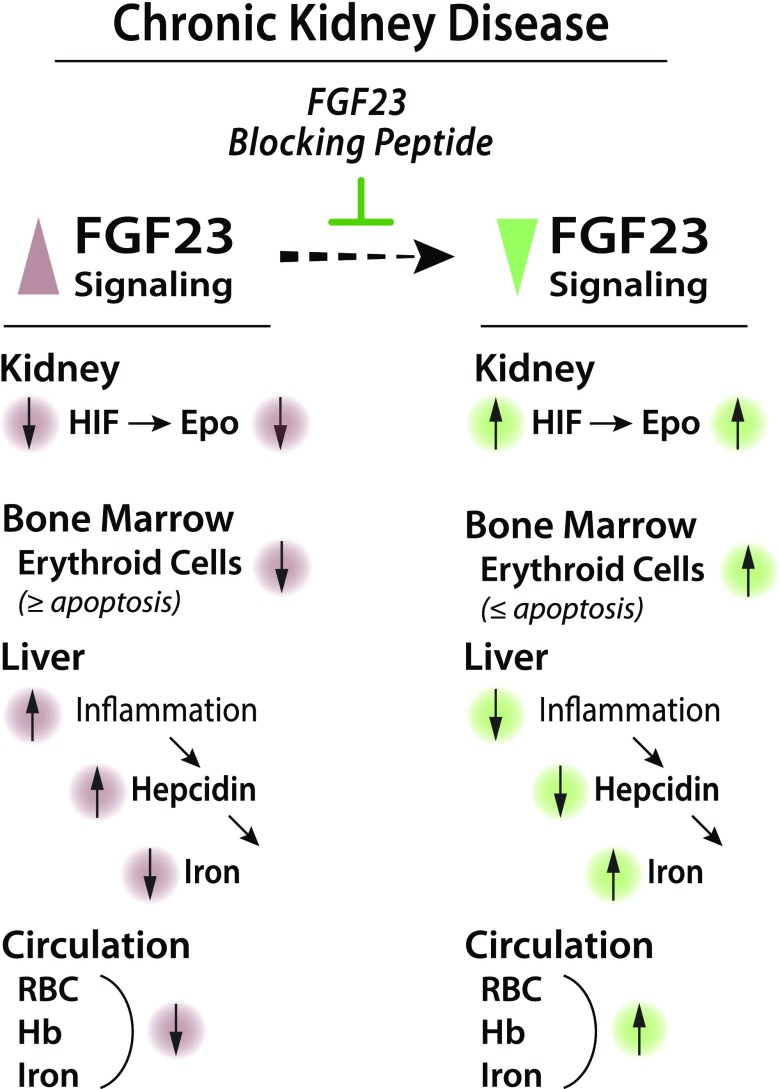
Importantly, our study provides compelling evidence that blocking FGF23 activity stimulates erythropoiesis and rescues anemia and iron deficiency in a CKD mouse model. Specifically, we show that inhibition of FGF23 signaling normalized hemoglobin levels; increased early and late stage erythroid populations by decreasing erythroid cell apoptosis; induced renal and BM Epo expression, resulting in elevated serum Epo levels by creating a hypoxic environment that activates Epo-induced erythropoiesis; and ameliorated iron deficiency and increased serum iron and ferritin levels by attenuating inflammation (Fig. 6).
Figure 6.
Proposed model for role of FGF23 in renal anemia. High FGF23 levels in CKD are associated with decreased Epo secretion, reduced erythroid cells in BM, and inflammation, leading to anemia and iron deficiency. However, blocking FGF23 signaling results in stimulation of Epo secretion, increased BM erythroid cells, and reduced inflammation, leading to amelioration of anemia and iron deficiency.
Interestingly, we found that inhibition of FGF23 signaling diminished erythroid cell apoptosis, indicating that the increase in erythropoiesis after inhibition of FGF23 is caused by accumulation of erythroid cells due to reduced cell death, demonstrating that FGF23 regulates apoptosis during erythroid differentiation. We also showed that blocking FGF23 signaling affects erythroid cell cycling, suggesting that FGF23 is key to the control of cell cycle progression of erythroid cells.
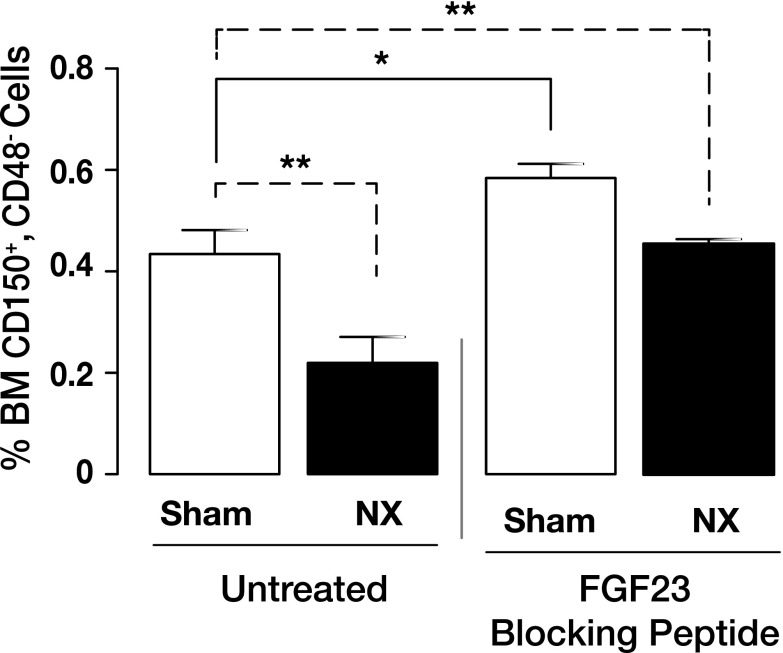
The finding that treatment with the FGF23 blocking peptide increased Epo secretion, even though 5/6 Nx reduced the available number of renal Epo-producing cells, is intriguing. To understand this better, we evaluated the effect of the blocking peptide on extrarenal Epo expression in BM and liver. BM Epo mRNA expression was significantly lower in 5/6 Nx mice compared to sham-treated mice, and it was robustly increased after treatment with the FGF23 blocking peptide (Fig. 3E). Conversely, 5/6 Nx mice exhibited a significant increase in hepatic Epo mRNA expression compared to sham-treated mice, to compensate for reduced renal Epo production after Nx. However, hepatic Epo expression remained at control levels in both sham-treated and 5/6 Nx mice after treatment with the FGF23 blocking peptide (data not shown). This led us to hypothesize that FGF23 has a strong effect on the local BM environment and possibly affects recruitment of HSCs to the erythroid lineage. In a previous study (20), we showed that loss of Fgf23 results in increased frequency of CD150+CD48− (SLAM) cells, enriched for HSCs. In agreement with our previous findings, here we confirm this hypothesis by showing that blocking FGF23 signaling results in significantly increased frequency of early hematopoietic progenitors (SLAM cells) in both sham-treated and 5/6 Nx mice (Fig. 7). As we reported previously (20), the increase in HSCs after inactivation of Fgf23 is due to reduced HSC apoptosis.
Figure 7.
Inhibition of FGF23 signaling increases HSC populations in mouse model of Nx-induced anemia. Flow cytometry analysis of BM HSC populations in untreated (vehicle-treated) and treated (with FGF23 blocking peptide) mice with sham surgery or 5/6 Nx. Graph represents percentage of HSC populations in BM stained for SLAM (sham, n = 8; Nx, n = 6). Data are represented as means ± sem. *P < 0.05, **P < 0.01.
In addition to reduced Epo secretion, iron deficiency is common among CKD patients and it is largely attributed to the presence of inflammation in these patients (7, 56). Inflammation has a potent effect on iron homeostasis by stimulating hepcidin, a key regulator of iron homeostasis produced in the liver. High hepcidin concentrations result in inhibition of intestinal iron absorption as well as sequestration of iron in macrophages and hepatocytes, thereby decreasing serum iron levels (38, 57). The relationship between iron and FGF23 has been described in several studies (39–41). However, there have been conflicting reports on the correlation between inflammation and FGF23 (58–61), although most tend to agree that high FGF23 levels are associated with inflammation in CKD. Two recent studies also show that FGF23 can directly stimulate hepatic secretion of inflammatory cytokines (61), and inflammation and iron deficiency stimulate FGF23 production (59). Here we show that inflammatory markers are significantly increased in the liver of 5/6 Nx mice, causing up-regulation of liver hepcidin (Hamp) mRNA expression and resulting in iron deficiency. Importantly, inhibition of FGF23 signaling by the FGF23 blocking peptide significantly reduced these inflammatory markers, normalized hepcidin (Hamp) mRNA expression, and completely ameliorated iron deficiency in the 5/6 Nx mice.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank M. N. T. Wada (University of Sao Paulo, Sao Paulo, Brazil and New York University School of Dentistry, New York, USA) and R. C. Bernstein (New York University College of Dentistry) for technical assistance. The authors are grateful to W. R. Abrams (New York University College of Dentistry) for invaluable assistance with the technical visualization of the data, and M. Gregory and P. Lopez (New York University School of Medicine) for assistance with flow cytometry and flow data analyses. This work was supported in part by funds from the American Heart Association (12SDG12080152) and the U.S. Department of Defense (W81XWH-16-1-0598; to D.S.), and the U.S. National Institutes of Health, National Institute of Dental and Craniofacial Research (DE 13686; to M.M.). The authors declare no conflicts of interest.
Glossary
- BFU-E
burst-forming unit–erythroid
- BM
bone marrow
- BrdU
bromodeoxyuridine
- BUN
blood urea nitrogen
- CKD
chronic kidney disease
- Epo
erythropoietin
- FEPi
fractional excretion of phosphate
- FGF
fibroblast growth factor
- FGFR
fibroblast growth factor receptor
- HIF
hypoxia-inducible factor
- HSC
hematopoietic stem cell
- IMDM
Iscove modified Dulbecco medium
- Nx
nephrectomy
- pro-E
proerythroblast
- PTH
parathyroid hormone
- qPCR
quantitative PCR
- SLAM
CD150+CD48−
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
D. Sitara designed research; R. Agoro, A. Montagna, O. Aligbe, G. Singh, L. M. Coe, and D. Sitara performed research; R. Goetz and M. Mohammadi contributed new reagents or analytic tools and provided scientific advice on the use of the FGF23 blocking peptide; R. Agoro, A. Montagna, L. M. Coe, and D. Sitara analyzed data; S. Rivella assisted with the data interpretation and manuscript preparation; and D. Sitara wrote the manuscript and oversaw the study.
REFERENCES
- 1.Jha V., Garcia-Garcia G., Iseki K., Li Z., Naicker S., Plattner B., Saran R., Wang A. Y., Yang C. W. (2013) Chronic kidney disease: global dimension and perspectives. Lancet 382, 260–272 https://doi.org/10.1016/S0140-6736(13)60687-X [DOI] [PubMed] [Google Scholar]
- 2.Levey A. S., Atkins R., Coresh J., Cohen E. P., Collins A. J., Eckardt K. U., Nahas M. E., Jaber B. L., Jadoul M., Levin A., Powe N. R., Rossert J., Wheeler D. C., Lameire N., Eknoyan G. (2007) Chronic kidney disease as a global public health problem: approaches and initiatives—a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 72, 247–259 https://doi.org/10.1038/sj.ki.5002343 [DOI] [PubMed] [Google Scholar]
- 3.Go A. S., Chertow G. M., Fan D., McCulloch C. E., Hsu C. Y. (2004) Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 351, 1296–1305 https://doi.org/10.1056/NEJMoa041031 [DOI] [PubMed] [Google Scholar]
- 4.Go A. S., Yang J., Ackerson L. M., Lepper K., Robbins S., Massie B. M., Shlipak M. G. (2006) Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) study. Circulation 113, 2713–2723 https://doi.org/10.1161/CIRCULATIONAHA.105.577577 [DOI] [PubMed] [Google Scholar]
- 5.Silverberg D., Wexler D., Blum M., Wollman Y., Iaina A. (2003) The cardio-renal anaemia syndrome: does it exist? Nephrol. Dial. Transplant. 18, viii7–viii12 [DOI] [PubMed] [Google Scholar]
- 6.Cole J., Ertoy D., Lin H., Sutliff R. L., Ezan E., Guyene T. T., Capecchi M., Corvol P., Bernstein K. E. (2000) Lack of angiotensin II–facilitated erythropoiesis causes anemia in angiotensin-converting enzyme–deficient mice. J. Clin. Invest. 106, 1391–1398 https://doi.org/10.1172/JCI10557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalantar-Zadeh K., Rodriguez R. A., Humphreys M. H. (2004) Association between serum ferritin and measures of inflammation, nutrition and iron in haemodialysis patients. Nephrol. Dial. Transplant. 19, 141–149 https://doi.org/10.1093/ndt/gfg493 [DOI] [PubMed] [Google Scholar]
- 8.Perwad F., Zhang M. Y., Tenenhouse H. S., Portale A. A. (2007) Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitro. Am. J. Physiol. Renal Physiol. 293, F1577–F1583 https://doi.org/10.1152/ajprenal.00463.2006 [DOI] [PubMed] [Google Scholar]
- 9.Shimada T., Hasegawa H., Yamazaki Y., Muto T., Hino R., Takeuchi Y., Fujita T., Nakahara K., Fukumoto S., Yamashita T. (2004) FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 19, 429–435 https://doi.org/10.1359/JBMR.0301264 [DOI] [PubMed] [Google Scholar]
- 10.Kurosu H., Ogawa Y., Miyoshi M., Yamamoto M., Nandi A., Rosenblatt K. P., Baum M. G., Schiavi S., Hu M. C., Moe O. W., Kuro-o M. (2006) Regulation of fibroblast growth factor-23 signaling by klotho. J. Biol. Chem. 281, 6120–6123 https://doi.org/10.1074/jbc.C500457200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urakawa I., Yamazaki Y., Shimada T., Iijima K., Hasegawa H., Okawa K., Fujita T., Fukumoto S., Yamashita T. (2006) Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444, 770–774 https://doi.org/10.1038/nature05315 [Google Scholar]
- 12.Shimada T., Kakitani M., Yamazaki Y., Hasegawa H., Takeuchi Y., Fujita T., Fukumoto S., Tomizuka K., Yamashita T. (2004) Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J. Clin. Invest. 113, 561–568 https://doi.org/10.1172/JCI200419081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sitara D., Razzaque M. S., Hesse M., Yoganathan S., Taguchi T., Erben R. G., Jüppner H., Lanske B. (2004) Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol. 23, 421–432 https://doi.org/10.1016/j.matbio.2004.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ADHR Consortium . (2000) Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat. Genet. 26, 345–348 https://doi.org/10.1038/81664 [DOI] [PubMed] [Google Scholar]
- 15.Jonsson K. B., Zahradnik R., Larsson T., White K. E., Sugimoto T., Imanishi Y., Yamamoto T., Hampson G., Koshiyama H., Ljunggren O., Oba K., Yang I. M., Miyauchi A., Econs M. J., Lavigne J., Jüppner H. (2003) Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N. Engl. J. Med. 348, 1656–1663 https://doi.org/10.1056/NEJMoa020881 [DOI] [PubMed] [Google Scholar]
- 16.Fliser D., Kollerits B., Neyer U., Ankerst D. P., Lhotta K., Lingenhel A., Ritz E., Kronenberg F., Kuen E., König P., Kraatz G., Mann J. F., Müller G. A., Köhler H., Riegler P.; MMKD Study Group . (2007) Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) study. J. Am. Soc. Nephrol. 18, 2600–2608 https://doi.org/10.1681/ASN.2006080936 [DOI] [PubMed] [Google Scholar]
- 17.Isakova T., Wahl P., Vargas G. S., Gutiérrez O. M., Scialla J., Xie H., Appleby D., Nessel L., Bellovich K., Chen J., Hamm L., Gadegbeku C., Horwitz E., Townsend R. R., Anderson C. A., Lash J. P., Hsu C. Y., Leonard M. B., Wolf M. (2011) Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 79, 1370–1378 https://doi.org/10.1038/ki.2011.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gutiérrez O. M., Mannstadt M., Isakova T., Rauh-Hain J. A., Tamez H., Shah A., Smith K., Lee H., Thadhani R., Jüppner H., Wolf M. (2008) Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N. Engl. J. Med. 359, 584–592 https://doi.org/10.1056/NEJMoa0706130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scialla J. J., Xie H., Rahman M., Anderson A. H., Isakova T., Ojo A., Zhang X., Nessel L., Hamano T., Grunwald J. E., Raj D. S., Yang W., He J., Lash J. P., Go A. S., Kusek J. W., Feldman H., Wolf M.; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators . (2014) Fibroblast growth factor-23 and cardiovascular events in CKD. J. Am. Soc. Nephrol. 25, 349–360 https://doi.org/10.1681/ASN.2013050465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coe L. M., Madathil S. V., Casu C., Lanske B., Rivella S., Sitara D. (2014) FGF-23 is a negative regulator of prenatal and postnatal erythropoiesis. J. Biol. Chem. 289, 9795–9810 https://doi.org/10.1074/jbc.M113.527150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goetz R., Nakada Y., Hu M. C., Kurosu H., Wang L., Nakatani T., Shi M., Eliseenkova A. V., Razzaque M. S., Moe O. W., Kuro-o M., Mohammadi M. (2010) Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23-FGFR-Klotho complex formation. Proc. Natl. Acad. Sci. USA 107, 407–412 https://doi.org/10.1073/pnas.0902006107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Q., Davidoff O., Niss K., Haase V. H. (2012) Hypoxia-inducible factor regulates hepcidin via erythropoietin-induced erythropoiesis. J. Clin. Invest. 122, 4635–4644 https://doi.org/10.1172/JCI63924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asari S., Sakamoto A., Okada S., Ohkubo Y., Arima M., Hatano M., Kuroda Y., Tokuhisa T. (2005) Abnormal erythroid differentiation in neonatal bcl-6-deficient mice. Exp. Hematol. 33, 26–34 https://doi.org/10.1016/j.exphem.2004.10.001 [DOI] [PubMed] [Google Scholar]
- 24.Koulnis M., Pop R., Porpiglia E., Shearstone J. R., Hidalgo D., Socolovsky M. (2011) Identification and analysis of mouse erythroid progenitors using the CD71/TER119 flow-cytometric assay. J. Vis. Exp. 5(54), 2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gutierrez O., Isakova T., Rhee E., Shah A., Holmes J., Collerone G., Jüppner H., Wolf M. (2005) Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J. Am. Soc. Nephrol. 16, 2205–2215 https://doi.org/10.1681/ASN.2005010052 [DOI] [PubMed] [Google Scholar]
- 26.Komaba H., Goto S., Fujii H., Hamada Y., Kobayashi A., Shibuya K., Tominaga Y., Otsuki N., Nibu K., Nakagawa K., Tsugawa N., Okano T., Kitazawa R., Fukagawa M., Kita T. (2010) Depressed expression of Klotho and FGF receptor 1 in hyperplastic parathyroid glands from uremic patients. Kidney Int. 77, 232–238 https://doi.org/10.1038/ki.2009.414 [DOI] [PubMed] [Google Scholar]
- 27.Lipkin G. W., Kendall R. G., Russon L. J., Turney J. H., Norfolk D. R., Brownjohn A. M. (1990) Erythropoietin deficiency in acute renal failure. Nephrol. Dial. Transplant. 5, 920–922 https://doi.org/10.1093/ndt/5.11.920 [DOI] [PubMed] [Google Scholar]
- 28.Zhang F., Laneuville P., Gagnon R. F., Morin B., Brox A. G. (1996) Effect of chronic renal failure on the expression of erythropoietin message in a murine model. Exp. Hematol. 24, 1469–1474 [PubMed] [Google Scholar]
- 29.Leelahavanichkul A., Yan Q., Hu X., Eisner C., Huang Y., Chen R., Mizel D., Zhou H., Wright E. C., Kopp J. B., Schnermann J., Yuen P. S., Star R. A. (2010) Angiotensin II overcomes strain-dependent resistance of rapid CKD progression in a new remnant kidney mouse model. Kidney Int. 78, 1136–1153 https://doi.org/10.1038/ki.2010.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Querbes W., Bogorad R. L., Moslehi J., Wong J., Chan A. Y., Bulgakova E., Kuchimanchi S., Akinc A., Fitzgerald K., Koteliansky V., Kaelin W. G., Jr. (2012) Treatment of erythropoietin deficiency in mice with systemically administered siRNA. Blood 120, 1916–1922 https://doi.org/10.1182/blood-2012-04-423715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fishbane S., Pollack S., Feldman H. I., Joffe M. M. (2009) Iron indices in chronic kidney disease in the National Health and Nutritional Examination Survey, 1988–2004. Clin. J. Am. Soc. Nephrol. 4, 57–61 https://doi.org/10.2215/CJN.01670408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasegawa H., Nagano N., Urakawa I., Yamazaki Y., Iijima K., Fujita T., Yamashita T., Fukumoto S., Shimada T. (2010) Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 78, 975–980 https://doi.org/10.1038/ki.2010.313 [DOI] [PubMed] [Google Scholar]
- 33.Shalhoub V., Shatzen E. M., Ward S. C., Davis J., Stevens J., Bi V., Renshaw L., Hawkins N., Wang W., Chen C., Tsai M. M., Cattley R. C., Wronski T. J., Xia X., Li X., Henley C., Eschenberg M., Richards W. G. (2012) FGF23 neutralization improves chronic kidney disease–associated hyperparathyroidism yet increases mortality. J. Clin. Invest. 122, 2543–2553 https://doi.org/10.1172/JCI61405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ben-Dov I. Z., Galitzer H., Lavi-Moshayoff V., Goetz R., Kuro-o M., Mohammadi M., Sirkis R., Naveh-Many T., Silver J. (2007) The parathyroid is a target organ for FGF23 in rats. J. Clin. Invest. 117, 4003–4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ganz T. (2003) Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 102, 783–788 https://doi.org/10.1182/blood-2003-03-0672 [DOI] [PubMed] [Google Scholar]
- 36.Nemeth E., Rivera S., Gabayan V., Keller C., Taudorf S., Pedersen B. K., Ganz T. (2004) IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Invest. 113, 1271–1276 https://doi.org/10.1172/JCI200420945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barreto D. V., Barreto F. C., Liabeuf S., Temmar M., Lemke H. D., Tribouilloy C., Choukroun G., Vanholder R., Massy Z. A.; European Uremic Toxin Work Group (EUTox) . (2010) Plasma interleukin-6 is independently associated with mortality in both hemodialysis and pre-dialysis patients with chronic kidney disease. Kidney Int. 77, 550–556 https://doi.org/10.1038/ki.2009.503 [DOI] [PubMed] [Google Scholar]
- 38.Nemeth E., Tuttle M. S., Powelson J., Vaughn M. B., Donovan A., Ward D. M., Ganz T., Kaplan J. (2004) Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306, 2090–2093 https://doi.org/10.1126/science.1104742 [DOI] [PubMed] [Google Scholar]
- 39.Farrow E. G., Yu X., Summers L. J., Davis S. I., Fleet J. C., Allen M. R., Robling A. G., Stayrook K. R., Jideonwo V., Magers M. J., Garringer H. J., Vidal R., Chan R. J., Goodwin C. B., Hui S. L., Peacock M., White K. E. (2011) Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc. Natl. Acad. Sci. USA 108, E1146–E1155 https://doi.org/10.1073/pnas.1110905108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Imel E. A., Peacock M., Gray A. K., Padgett L. R., Hui S. L., Econs M. J. (2011) Iron modifies plasma FGF23 differently in autosomal dominant hypophosphatemic rickets and healthy humans. J. Clin. Endocrinol. Metab. 96, 3541–3549 https://doi.org/10.1210/jc.2011-1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolf M., Koch T. A., Bregman D. B. (2013) Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J. Bone Miner. Res. 28, 1793–1803 https://doi.org/10.1002/jbmr.1923 [DOI] [PubMed] [Google Scholar]
- 42.Tsai M. H., Leu J. G., Fang Y. W., Liou H. H. (2016) High fibroblast growth factor 23 levels associated with low hemoglobin levels in patients with chronic kidney disease stages 3 and 4. Medicine (Baltimore) 95, e3049 https://doi.org/10.1097/MD.0000000000003049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shimada T., Muto T., Urakawa I., Yoneya T., Yamazaki Y., Okawa K., Takeuchi Y., Fujita T., Fukumoto S., Yamashita T. (2002) Mutant FGF-23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endocrinology 143, 3179–3182 https://doi.org/10.1210/endo.143.8.8795 [DOI] [PubMed] [Google Scholar]
- 44.White K. E., Carn G., Lorenz-Depiereux B., Benet-Pages A., Strom T. M., Econs M. J. (2001) Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 60, 2079–2086 https://doi.org/10.1046/j.1523-1755.2001.00064.x [DOI] [PubMed] [Google Scholar]
- 45.Andrukhova O., Zeitz U., Goetz R., Mohammadi M., Lanske B., Erben R. G. (2012) FGF23 acts directly on renal proximal tubules to induce phosphaturia through activation of the ERK1/2-SGK1 signaling pathway. Bone 51, 621–628 https://doi.org/10.1016/j.bone.2012.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andrukhova O., Slavic S., Smorodchenko A., Zeitz U., Shalhoub V., Lanske B., Pohl E. E., Erben R. G. (2014) FGF23 regulates renal sodium handling and blood pressure. EMBO Mol. Med. 6, 744–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andrukhova O., Smorodchenko A., Egerbacher M., Streicher C., Zeitz U., Goetz R., Shalhoub V., Mohammadi M., Pohl E. E., Lanske B., Erben R. G. (2014) FGF23 promotes renal calcium reabsorption through the TRPV5 channel. EMBO J. 33, 229–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin A., David V., Quarles L. D. (2012) Regulation and function of the FGF23/klotho endocrine pathways. Physiol. Rev. 92, 131–155 https://doi.org/10.1152/physrev.00002.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Faul C., Amaral A. P., Oskouei B., Hu M. C., Sloan A., Isakova T., Gutiérrez O. M., Aguillon-Prada R., Lincoln J., Hare J. M., Mundel P., Morales A., Scialla J., Fischer M., Soliman E. Z., Chen J., Go A. S., Rosas S. E., Nessel L., Townsend R. R., Feldman H. I., St John Sutton M., Ojo A., Gadegbeku C., Di Marco G. S., Reuter S., Kentrup D., Tiemann K., Brand M., Hill J. A., Moe O. W., Kuro-O M., Kusek J. W., Keane M. G., Wolf M. (2011) FGF23 induces left ventricular hypertrophy. J. Clin. Invest. 121, 4393–4408 https://doi.org/10.1172/JCI46122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grabner A., Amaral A. P., Schramm K., Singh S., Sloan A., Yanucil C., Li J., Shehadeh L. A., Hare J. M., David V., Martin A., Fornoni A., Di Marco G. S., Kentrup D., Reuter S., Mayer A. B., Pavenstädt H., Stypmann J., Kuhn C., Hille S., Frey N., Leifheit-Nestler M., Richter B., Haffner D., Abraham R., Bange J., Sperl B., Ullrich A., Brand M., Wolf M., Faul C. (2015) Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell Metab. 22, 1020–1032 https://doi.org/10.1016/j.cmet.2015.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pan X., Suzuki N., Hirano I., Yamazaki S., Minegishi N., Yamamoto M. (2011) Isolation and characterization of renal erythropoietin-producing cells from genetically produced anemia mice. PLoS One 6, e25839 https://doi.org/10.1371/journal.pone.0025839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Souma T., Suzuki N., Yamamoto M. (2015) Renal erythropoietin-producing cells in health and disease. Front. Physiol. 6, 167 https://doi.org/10.3389/fphys.2015.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frede S., Freitag P., Geuting L., Konietzny R., Fandrey J. (2011) Oxygen-regulated expression of the erythropoietin gene in the human renal cell line REPC. Blood 117, 4905–4914 https://doi.org/10.1182/blood-2010-07-298083 [DOI] [PubMed] [Google Scholar]
- 54.Ichikawa S., Imel E. A., Kreiter M. L., Yu X., Mackenzie D. S., Sorenson A. H., Goetz R., Mohammadi M., White K. E., Econs M. J. (2007) A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J. Clin. Invest. 117, 2684–2691 https://doi.org/10.1172/JCI31330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Segawa H., Yamanaka S., Ohno Y., Onitsuka A., Shiozawa K., Aranami F., Furutani J., Tomoe Y., Ito M., Kuwahata M., Imura A., Nabeshima Y., Miyamoto K. (2007) Correlation between hyperphosphatemia and type II Na-Pi cotransporter activity in klotho mice. Am. J. Physiol. Renal Physiol. 292, F769–F779 https://doi.org/10.1152/ajprenal.00248.2006 [DOI] [PubMed] [Google Scholar]
- 56.Sun C. C., Vaja V., Babitt J. L., Lin H. Y. (2012) Targeting the hepcidin–ferroportin axis to develop new treatment strategies for anemia of chronic disease and anemia of inflammation. Am. J. Hematol. 87, 392–400 https://doi.org/10.1002/ajh.23110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ganz T. (2007) Molecular control of iron transport. J. Am. Soc. Nephrol. 18, 394–400 https://doi.org/10.1681/ASN.2006070802 [DOI] [PubMed] [Google Scholar]
- 58.Braithwaite V., Prentice A. M., Doherty C., Prentice A. (2012) FGF23 is correlated with iron status but not with inflammation and decreases after iron supplementation: a supplementation study. Int. J. Pediatr. Endocrinol. 2012, 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.David V., Martin A., Isakova T., Spaulding C., Qi L., Ramirez V., Zumbrennen-Bullough K. B., Sun C. C., Lin H. Y., Babitt J. L., Wolf M. (2016) Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int. 89, 135–146 https://doi.org/10.1038/ki.2015.290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Munoz Mendoza J., Isakova T., Cai X., Bayes L. Y., Faul C., Scialla J. J., Lash J. P., Chen J., He J., Navaneethan S., Negrea L., Rosas S. E., Kretzler M., Nessel L., Xie D., Anderson A. H., Raj D. S., Wolf M.; CRIC Study Investigators . (2017) Inflammation and elevated levels of fibroblast growth factor 23 are independent risk factors for death in chronic kidney disease. Kidney Int. 91, 711–719 https://doi.org/10.1016/j.kint.2016.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh S., Grabner A., Yanucil C., Schramm K., Czaya B., Krick S., Czaja M. J., Bartz R., Abraham R., Di Marco G. S., Brand M., Wolf M., Faul C. (2016) Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int. 90, 985–996 https://doi.org/10.1016/j.kint.2016.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.