Abstract
Stroke is a devastating disorder that significantly contributes to death, disability and healthcare costs. In ischemic stroke, the only current acute therapy is recanalization, but the narrow therapeutic window less than 6 h limits its application. The current challenge is to prevent late cell death, with concomitant therapy targeting the ischemic cascade to widen the therapeutic window. Among potential neuroprotective drugs, cyclin-dependent kinase inhibitors such as (S)-roscovitine are of particular relevance. We previously showed that (S)-roscovitine crossed the blood–brain barrier and was neuroprotective in a dose-dependent manner in two models of middle cerebral artery occlusion (MCAo). According to the Stroke Therapy Academic Industry Roundtable guidelines, the pharmacokinetics of (S)-roscovitine and the optimal mode of delivery and therapeutic dose in rats were investigated. Combination of intravenous (IV) and continuous sub-cutaneous (SC) infusion led to early and sustained delivery of (S)-roscovitine. Furthermore, in a randomized blind study on a transient MCAo rat model, we showed that this mode of delivery reduced both infarct and edema volume and was beneficial to neurological outcome. Within the framework of preclinical studies for stroke therapy development, we here provide data to improve translation of pre-clinical studies into successful clinical human trials.
Keywords: Brain ischemia, brain recovery, cell cycle, neuroprotection, pharmacokinetics
Introduction
Stroke is the third most frequent cause of death and the first cause of acquired disability in adults.1 Stroke is classified as hemorrhagic or ischemic strokes, with ischemic strokes accounting for about 80% of cases. To date, the only emergency therapeutic solution is recanalization through thrombolysis and/or thrombectomy. However, notably due to the short intervention window (< 6 hours) and hemorrhagic transformation risk, only a small percentage of acute ischemic stroke patients are eligible for this treatment.2 Therefore, developing a new therapeutic strategy is a major public health issue.
Although numerous clinical trials failed before the advent of recanalization, neuroprotection, associated to recanalization, remains a potentially promising strategy to widen the therapeutic window. The current challenge is to develop pleiotropic molecules targeting several events in the ischemic cascade.1,3
Among potentially promising drugs to treat ischemia, the cyclin-dependent kinase (CDK) inhibitors 2, 6, 9-trisubstituted purine analogs, such as olomoucine, roscovitine and flavopiridol, are of particular relevance.4 There is increasing evidence for a neuroprotective effect of roscovitine, a potent inhibitor of CDK 1, 2, 5, 7, and 95,6 in neurological diseases including stroke,7–9 traumatic brain injury,10 Niemann-Pick disease,11 amyotrophic lateral sclerosis12 and Alzheimer’s disease.13
Roscovitine exists in two stereoisomers, (R)- and (S)-roscovitine, each of which displayed neuroprotective effectiveness in in vitro and in vivo neuronal death models of global and focal cerebral ischemia.8,9 This neuroprotective effect is mediated by a wide range of biological pathways, such as excitotoxicity, apoptosis and inflammation.4,8,14,15 Although the dextrogyre (R) isomer of roscovitine displays slightly better in vitro inhibitory activity on CDKs than its levogyre (S) counterpart,5,6 we previously showed that (S)-roscovitine had a significantly stronger neuroprotective effect in primary neuronal culture following kainic acid treatment.9 In addition, we also previously showed that (S)-roscovitine dose-dependently reduced brain infarct volume in rodent models of both permanent and transient focal ischemia.9
Because of numerous unsuccessful attempts to translate promising pre-clinical trials into positive outcomes in humans,1 the Stroke Academic Industry Roundtable (STAIR) made recommendations to improve the quality of pre-clinical trials, such as defining the pharmacokinetics and minimum effective and maximum tolerated doses with respect to functional endpoints that are more relevant to human clinical trials.16,17 In accordance with the STAIR guidelines, we investigated the pharmacokinetic properties of (S)-roscovitine, with a view to optimizing its mode of delivery. A randomized blind study subsequently established dose-response relationships between different doses of (S)-roscovitine and several functional and histological outcomes. The data showed that the combination of two (S)-roscovitine delivery modes induced a significant neuroprotective effect, associated with improved functional outcome and reduced brain edema.
Methods
Animals
Adult male Sprague-Dawley rats (275–320 g body weight) were purchased from Janvier Labs (Le Genest-Saint-Isle, France) and housed in pairs. All rats were kept in a temperature-controlled room (21 ± 0.5℃) under a 12-h light/dark cycle. All experiments were conducted in accordance with directives from the European Community Council (2010/63/EU) and French legislation (Act no. 87-848, Ministère de l'Agriculture et de la Forêt) on animal experimentation. Procedures were validated by the institutional review board of Normandy, France (CENOMEXA) under the approval number A14118015, and are reported in compliance with the ARRIVE (Animal Research: Reporting in Vivo Experiments) guidelines. All rats had ad libitum access to food and water.
Drug synthesis
(S)-roscovitine was synthesized and provided by Kaïronkem (Marseille, France). (S)-Roscovitine (batch N° K401.6) was 98% pure (HPLC).
(S)-roscovitine solution for intravenous (IV) bolus injection was prepared with 4 mg/ml (S)-roscovitine dissolved in a vehicle solution containing 30% hydroxypropyl ß-cyclodextrin (HPbCD) in 0.1 M phosphate buffer (PB) (pH = 7.4). The solution for subcutaneous (SC) infusion was prepared with 0.14 mg/ml (0.1 mg/kg body weight) or 1.43 mg/ml (1 mg/kg body weight) dissolved in saline containing 50 mM HCl vehicle solution (pH = 1.5).
Experimental design
Two independent experiments were carried out.
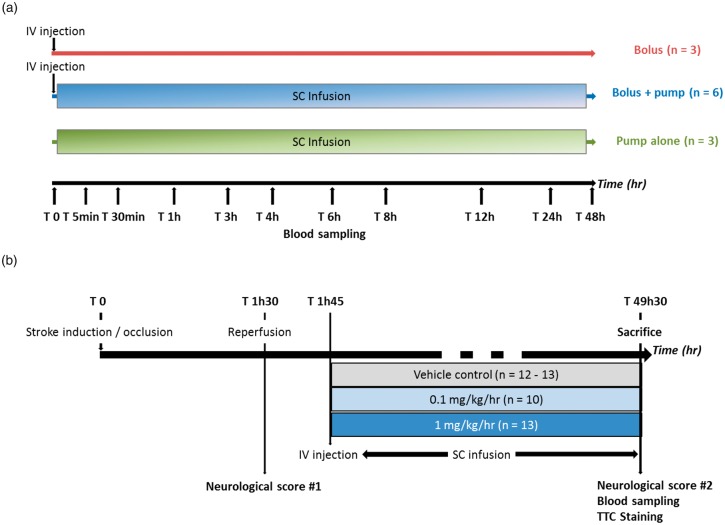
Experiment 1 was carried out to assess the effect of different (S)-roscovitine delivery modes on pharmacokinetic parameters in healthy rats (Figure 1(a)).
Figure 1.
Experimental design. (a) Experiment 1: Effect of delivery mode of (S)-roscovitine on pharmacokinetic parameters in healthy rats: Healthy rats received (S)-roscovitine at T0 either by IV bolus (25 mg/kg, n = 3), SC infusion (1 mg/kg/h for 48 h, n = 3) or both (25 mg/kg + 1 mg/kg/h for 48 h, n = 6). In order to measure (S)-roscovitine concentration in plasma, blood was collected at T0, T 5 min, T 30 min, T 1 h, T 3 h, T 6 h, T 8 h, T 24 h and T 48 h for bolus and bolus + pump groups and at T 0, T 1 h, T 4 h, T 12 h, T 24 h and T 48 h. (b) Experiment 2: Dose-response effect of (S)-roscovitine administration after transient middle cerebral artery occlusion on histopathological and neurological score in rats: Transient focal cerebral ischemia was induced in adult rats for 90 min. (S)-roscovitine or its vehicle control was administered 15 min post-reperfusion by an IV bolus (25 mg/kg body weight) followed by SC infusion for 48 hours: Vehicle (n = 12–13), 0.1 mg/kg/h (n = 10) or 1 mg/kg/h (n = 13). Neurological scoring was performed just prior to reperfusion and 48 h post-tMCAo. Blood was collected 48 h post-tMCAo to measure (S)-roscovitine concentration in plasma, then animals were euthanized and brain infarction volumes were measured by TTC staining.
(S)-roscovitine was administered at T0, either by IV bolus of 1.9 ml at 25 mg/kg body weight (“bolus group”, n = 3) or by SC infusion (“pump group”, n = 3). For one group of animals, bolus injection was immediately followed by SC infusion of 1 mg/kg/h for 48 h (“bolus + pump” group, n = 6). Blood was sampled at T0, T 5 min, T 30 min, T 1 h, T 3 h, T 6 h, T 8 h, T 24 h and T 48 h for the bolus and bolus + pump groups and at T 0, T 1 h, T 4 h, T 12 h, T 24 h and T 48 h for the pump group, to assess (S)-roscovitine concentration in plasma.
Experiment 2 was carried out to assess the dose-response effects of (S)-roscovitine on neurological score, infarct volume and brain edema in tMCAo rats (Figure 1(b)).
(S)-roscovitine solution (1.9 ml) was administered 15 min post-reperfusion by IV bolus (25 mg/kg body weight) into the external jugular vein at a constant rate (0.5 ml/min) using a syringe pump. At 20-min post-reperfusion, the InfuDisk pump (Med-E-Cell) was fitted for SC infusion of (S)-roscovitine, and animals received a 0.1 mg/kg/h (“0.1 mg/kg group”, n = 10) or 1 mg/kg/h dose (“1 mg/kg group”, n = 13). The pumps were secured to the animal with a back-pack apparatus and left in place for 48 h. Infusion pumps delivered 10 ml of solution at a rate of 0.21 ml/h and were weighed before and after treatment to determine the effective (S)-roscovitine dose delivered. Control animals received an IV bolus of vehicle solution + SC vehicle solution (“vehicle group”, n = 13). The neurological score of each rat was measured just prior reperfusion after anesthesia recovery (T 1h30) and 48 h post-occlusion (T 49h30). Blood was then sampled, and the rats were euthanized for histological analyses.
Pharmacokinetics
Blood sampling
For pharmacokinetic analysis, in healthy animals, blood samples (200 µL) were collected from the saphenous vein with Microvette capillary blood collection tubes treated with Lithium Heparin (Sarstedt, France). Blood was sampled at T 0, T 5 min, T 30 min, T 1 h, T 3 h, T 4 h T 6 h, T 8 h, 12 h, T 24 h and T 48 h. A saline solution (200 µL) was administered subcutaneously to compensate for the lost blood volume. Samples were centrifuged at 2000g for 5 min at 4℃ to separate the plasma.
(S)-roscovitine plasma assay by HPLC-MS/MS
(S)-roscovitine was administered by IV bolus or/and SC infusion (0.1 or 1 mg/kg/h) under isoflurane gas anesthesia (3 to 10 rats per time point). An internal standard solution of olomoucine (0.500 ng/µL in ethanol) was added to plasma samples (25 µL). They were then prepared by liquid–liquid extraction and injected for HPLC-MS/MS analysis.
Pharmacokinetic parameters
Pharmacokinetic analysis of plasma (S)-roscovitine was performed using the Phoenix®, WinNonlin® pharmacokinetic software package (version 4.1) (Pharsight, USA). The pharmacokinetic parameters evaluated comprised: area under concentration-time curve extrapolated to infinity (AUC inf), area under the concentration-time curve between 8 and 48 h (AUC 8-48 h), extrapolated concentration at time 0 h (C0), distribution half-life (T1/2 -α), elimination half-life (T1/2-β), clearance (Cl), and volume of distribution (Vd).
Transient middle cerebral artery occlusion (tMCAo) model
tMCAo and reperfusion procedures
tMCAo was achieved as previously described.18 Briefly, rats were deeply anesthetized and maintained with 2% isoflurane in a 70%/30% gas mixture (N2O/O2). Rectal temperature was maintained at 37 ± 0.5℃ with a heating pad. Cerebral blood flow (CBF) was continuously recorded by laser Doppler flowmetry with a miniature laser-Doppler probe directly fixed over a region of the cortex supplied by the middle cerebral artery (MCA) throughout all ischemia and reperfusion procedures. After temporary suture of the common (CCA) and external carotid arteries (ECA) and coagulation of the ECA and of pterygopalatine artery, a 4–0 monofilament suture (4037PK5Re, Doccol Corporation) was introduced into the right internal carotid artery lumen and advanced until resistance was felt (∼20 mm) and until CBF fell by at least 60% compared to baseline. After anesthesia recovery, neurological score was performed just before 90 min of ischemia onset. Then animals were anesthetized again for withdrawal of monofilament to allow MCA reperfusion. The temporary suture on the ECA was permanently tied off to prevent blood loss, and the caudal temporary suture placed around the CCA was removed to allow CBF release. Post-operative care and observation were carried out until the animal recovered consciousness, and included postoperative subcutaneous injection of saline solution and provision of softened food.
Quality control for data collection and data processing
Experiments were performed in accordance with the STAIR committee recommendations for good scientific enquiry.
Rats were randomly allocated to treatment groups by an independent experimenter using a randomization table. All outcomes were assessed under blind conditions, and infarct volume and edema were measured by two independent researchers (ER, BM).
Inclusion and exclusion criteria were applied as follows:
Inclusion criteria:
– CBF < 60% baseline;
– minimum combined neurological score of 3 (minimum score of 1 on rotation and 2 on tail suspension) just prior reperfusion (T 1h15).
Exclusion criteria:
– rats presenting CBF ≥ 60% baseline or blood-flow restoration following occlusion were immediately excluded and euthanized;
– rats with combined neurological score < 3 just before reperfusion (T 1h15) were immediately excluded and euthanized;
– rats that died within 48 h were excluded;
– as were rats with hemorrhage.
For the final behavioral analysis, 13 animals were included in the vehicle group, 10 in the 0.1 mg/kg/h group and 13 in the 1 mg/kg/h group. To avoid bias of infarct volume measures, one animal was removed in the vehicle group because of lack of all brain slices (n = 12) (Supplementary Figure 1).
Outcomes
Neurological scoring procedure
The primary end-point was behavioral assessment, indicating potential functional recovery. Just prior to reperfusion and 48 h post-surgery, behavioral deficit was assessed on a gross neurological score, adapted from the method of Jiang et al.,19 consisting in summing of the results of two behavioral tests scored on a 0-to-4 scale each (Supplementary Table 1).
To assess a potential beneficial effect of treatment on behavioral outcome, recovery level was calculated as follows
Blood collection and (S)-roscovitine plasma assay by HPLC-MS/MS
Blood collection and (S)-roscovitine plasma assay by HPLC-MS/MS were performed in vehicle (n = 2), 0.1 mg/kg (n = 10) and 1 mg/kg (n = 4) groups 48 h after occlusion, as described in subsections Blood sampling and (S)-roscovitine plasma assay above.
Evaluation of infarct volume and edema
Immediately after blood collection, rats were decapitated and brains immediately removed; 2-mm-thick coronal sections were cut into brain slicer matrix (Zivic Instruments, Pittsburgh, USA). The brain sections were immersed into 1% 2,3,5-triphenyltetrazolium chloride (TTC) for 5 min at 37℃, fixed in 4% paraformaldehyde overnight and conserved at −20℃ in cryopreservation solution.
Images from the stained coronal sections were measured with ImageJ (NIH) image processing software. Cortical and subcortical areas were delineated for a total of seven 2-mm-thick sections. Total area was determined as the sum of both cortical and subcortical areas.
Actual volumes of the various areas were calculated as follows
with factor 2 corresponding to brain slice thickness.
Edema formation was measured as percentage size of the contralateral hemisphere20
The actual infarct volumes were measured and corrected to compensate for brain swelling in the infarcted hemisphere, as previously described,21 according to the STAIR recommendations22
Lesion volume was also measured, under blind conditions by two independent operators. The final lesion volume for each animal corresponded to the arithmetic mean of the two measured volumes.
Measurement of effective delivered dose and correlation with infarct volume
For each animal, (S)-roscovitine was assayed by HPLC-MS/MS in the stock solutions used for IV injection and SC infusion. Infusion pumps were weighed before and after treatment to determine the effective delivered volume. The effective delivered dose of (S)-roscovitine corresponded to the sum of the concentration multiplied by the volume of each (S)-roscovitine solution.
Statistical analyses
Sample size was determined based on previous roscovitine pharmacokinetics studies which showed that the plasma (R)-roscovitine concentration fitted a bi-compartmental model, with low variability, after a single i.p. administration of 25 mg/kg in adult rats.23,24 In this experiment, three animals were used per condition as it is the case in our experiment. Regarding the efficacy study, the sample size was determined based on preliminary data with the same inclusion criteria and previous ischemia study performed in our laboratory, which demonstrated a beneficial effect of (S)-roscovitine with a minimal number of animals.9 We calculated that 12 animals would be necessary in each group for the present study for a power of 80% and a significance of p = 0.05.
Continuous data were presented as mean ± standard deviation (mean ± SD) and categorical data as median and interquartile range. Statistical analyses were performed with GraphPad Prism 5 software (San Diego, USA). Gaussian distributed continuous variables were analyzed by two-tailed unpaired Student’s t-test and non-Gaussian continuous variables by non-parametric Mann–Whitney U test. Neurological scores were analyzed by Kruskal–Wallis test to compare behavioral deficit between the three groups (vehicle vs. 0.1 mg/kg vs 1 mg/kg) at each time T 1h30 and T 49h30 with post-hoc Dunn’s multiple comparison test. The potential recovery between T 1h30 and T 49h30 was analyzed in each group by Wilcoxon matched-pairs test. Fisher’s exact test was used to analyze mortality data. The alpha level was set at 0.05.
Results
Pharmacokinetics
SC infusion is necessary to maintain long-term minimal plasma (S)-roscovitine concentration after bolus injection (Figure 2)
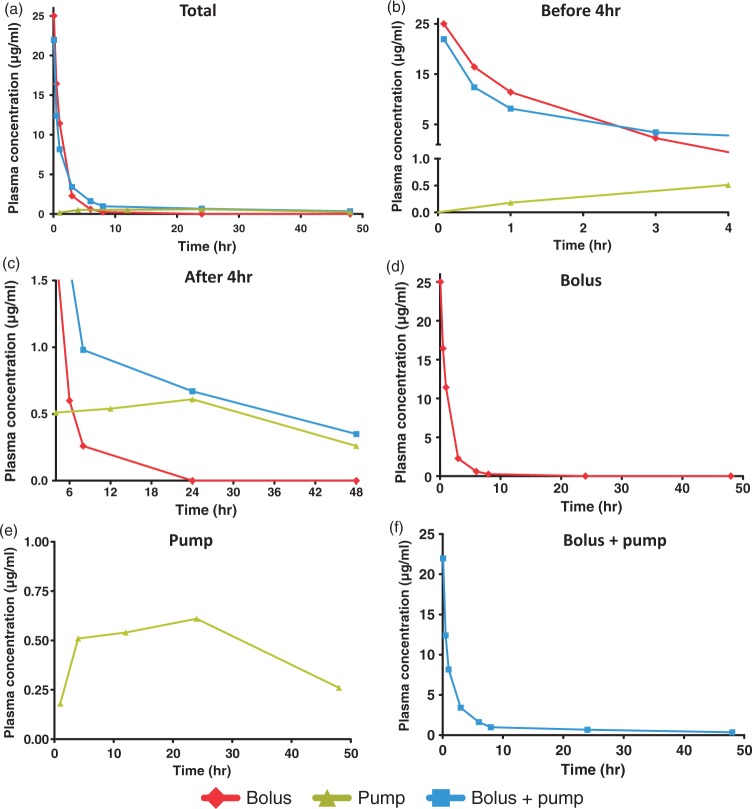
Figure 2.
Plasma-time concentration curves. Rats received either IV bolus (25 mg/kg), SC pump infusion (1 mg/kg/h for 48 h) or both. Plasma concentrations were measured by HPLC-MS/MS between T0 and T 48 h. Plasma-time concentrations curves of (S)-roscovitine are represented for Bolus (n = 3), Pump (n = 3) and Bolus + pump (n = 6) between T0 and T 48 h (a). Curves are zoomed before T 4 h (b) and after T 4 h (c). The plasma-time concentration curves are also independently represented for (d) Bolus, (e) Pump and (f) Bolus + pump. Data are expressed as means of three to six independent values.
To dissect out the contribution of each (S)-roscovitine delivery mode to plasma concentration, pharmacokinetic profiles were analyzed for the bolus (n = 3), pump (n = 3) and bolus + pump (n = 6) groups. Plasma-time concentration curves are reported in Figure 2.
Injection of bolus alone induced a sharp increase in (S)-roscovitine plasma concentration at 25 µg/mL 5 min from onset, followed by a rapid decline 3 h after onset (2.3 µg/mL) and disappearance about 24 h after. Pump infusion induced a slight increase up to 4 h, then seemed to plateau around 0.5 µg/mL until 24 h, followed by a descending phase with a concentration of 0.3 µg/mL at 48 h. The combination of bolus injection and pump infusion provided the same sharp initial increase at 5 min (21.9 µg/mL) as in the bolus-alone group, followed by a rapid decline (3.4 µg/mL at 3 h, 1.6 µg/mL at 6 h), and maintained (S)-roscovitine plasma concentration at 0.7 µg at 24 h and at 0.4 µg at 48 h.
Combined IV bolus injection and SC infusion enables sustained plasma (S)-roscovitine concentration for 48 h due to increased elimination half-life (Figure 3, Table 1)
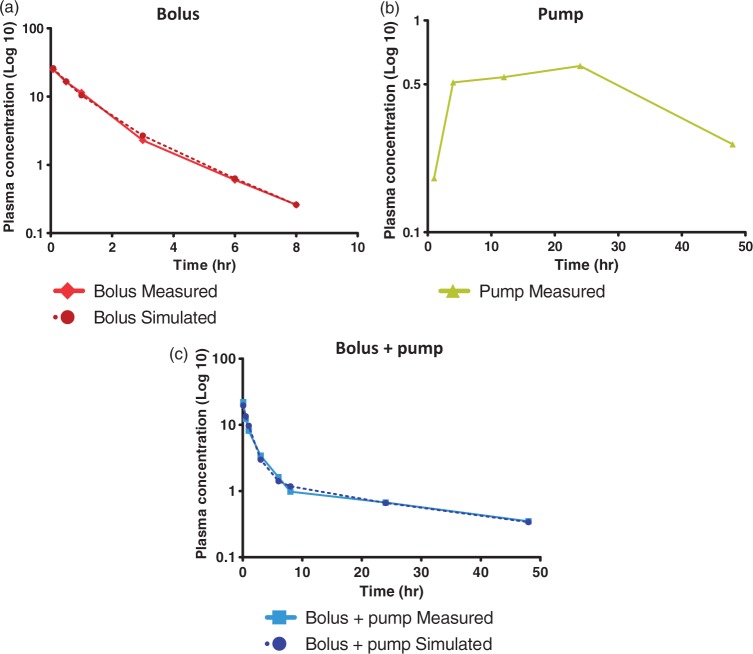
Figure 3.
Distribution and elimination model of (S)-roscovitine. Plasma-time concentration curves of (S)-roscovitine are represented according a semi-logarithmic scale for (a) Bolus (n = 3), (b) Pump (n = 3) and (c) Bolus + pump (n = 6). Comparative values calculated using a two-compartmental open model are also represented for (a) Bolus and (c) Bolus + pump. Data are expressed as means of three to six independent values.
Table 1.
Pharmacokinetic parameters of (S)-roscovitine in plasma of adult rats.
| Bolus (25 mg/kg) | Bolus (25 mg/kg) Pump (1 mg/kg/h) | |
|---|---|---|
| AUCinf (h. µg/ml) | 34.1 ± 4.24 | 75.71 ± 37.56 |
| AUC 8–48 h (h.µg/ml) | 5.29 ± 2.18 | 25.26 ± 13.09* |
| C0 (µg/ml) | 28.58 ± 2.56 | 21.03 ± 4.74* |
| T1/2 -α (h) | 0.52 ± 0.17 | 0.82 ± 0.29 |
| T1/2-β (h) | 1.58 ± 0.16 | 24.63 ± 18.90* |
| Cl (ml/h) | 222.0 ± 28.84 | 149.5 ± 81.95 |
| Vd (ml) | 503.3 ± 15.04 | 4093 ± 1457* |
Note: Rats received either IV bolus (25 mg/kg, n = 3), or pump SC infusion (1 mg/kg/h for 48 h, n = 3) or both (n = 6). Plasma (S)-roscovitine concentrations were measured by HPLC-MS/MS. Data were expressed as means ± SD. Sign indicate values different from Bolus: * p < 0.05.
AUC inf: the area under the concentration-time curve extrapolated to the infinity; AUC 8–48 h: area under the concentration-time curve between 8 h and 48 h; C0: extrapolated concentration at time 0 h; T1/2 -α: distribution half-life; T1/2-β: elimination half-life; Cl: clearance; Vd: volume of distribution.
The IV bolus-alone plasma-time concentration curve of (S)-roscovitine fitted a bi-compartmental open model, which appeared as two straight lines when plotted on a semi-logarithmic scale (Figure 3(a)).
Although the SC infusion-alone plasma-time concentration curve seemed to fit a tri-compartmental model, mainly due to the absorption phase related to extravascular administration (Figure 3(b)), the combination of IV bolus and SC infusion curves highlighted a two-compartmental model of distribution and elimination of (S)-roscovitine, as with bolus injection alone (Figure 3(c)).
Simulated values for bolus-alone and bolus + pump delivery were calculated as follows, according to a two-compartmental model
where
A, B: extrapolated distribution and elimination at time T0, respectively
α, β: distribution and elimination constants, respectively
Simulated values for the bolus-alone and bolus + pump delivery modes are reported in Figure 3(a) and (c), respectively. Overlaying the simulated and measured curves confirmed the validity of a bi-compartmental model for (S)-roscovitine delivery by bolus alone or bolus + pump.
Pharmacokinetic parameters related to these two modes of (S)-roscovitine delivery are reported in Table 1 and showed that the AUC between 8 and 48 h in the bolus + pump group was about 5-fold higher (25.26 ± 13.09 h.µg/ml) than in the bolus-alone group (5.29 ± 2.18 h. µg/ml; U = 0.0, p = 0.02). No difference was observed for distribution half-life (U = 3.0, p = 0.17) but volume of distribution was about 8-fold higher in the bolus + pump group than in the bolus alone group (4,093 ± 1,457 vs. 503.3 ± 15.04 ml; U = 0.0, p = 0.02). Although no significant difference was observed in clearance (U = 5.0, p = 0.38), elimination half-life was about 16-fold longer in the bolus + pump group than in the bolus-alone group (24.63 ± 18.90 vs. 1.58 ± 0.16 h; U = 0.0, p = 0.02), resulting in longer sustained (S)-roscovitine plasma concentration in rats that received SC bolus + pump infusion SC than those that received bolus injection alone. Furthermore, it should be noted that the initial concentration was significantly lower in the bolus + pump group than in the bolus-alone group (21.03 ± 4.74 vs. 28.58 ± 2.56 µg/ml; U = 1.0, p = 0.047).
Dose-response effect in tMCAo rats
The second study assessed the dose-response effect of (S)-roscovitine administration on infarct volume and neurological score in tMCAo rats.
Baseline characteristics:
Mortality and physiological parameters were recorded throughout the tMCAo procedure and until the day of sacrifice (Supplementary Table 2, Supplementary Figure 1).
Two out of 21 randomized treated animals died in the vehicle group, 3 out of 22 in the 0.1 mg/kg/h and 4 out of 20 in the 1 mg/kg/h in the (S)-roscovitine treated groups, i.e. 9.5%, 13.6% and 20%, respectively. No significant difference has been revealed between the groups (Fisher exact test: p = 0.83).
The percentage drop in CBF following occlusion was similar between the three groups. Body weight was similar between the three groups, both before surgery and before sacrifice. Body temperature was maintained around 37℃ throughout the anesthesia procedure and did not show significant difference at post-reperfusion. On the day of sacrifice, no difference was observed between the vehicle group and the 1 mg/kg/h group, but temperature was lower in the 0.1 mg/kg group than in vehicle group (37.15 ± 0.30 vs. 37.63 ± 0.49; t = 2.74, p = 0.01). Blood glucose concentration was also measured before, during and after occlusion, without showing any significant difference between the three groups.
To calculate the effective delivered dose for each animal, the effective injected sample volume was measured, and showed no significant difference between the three groups.
Neurological score
(S)-roscovitine improves neurological score in tMCAo rats, and its effect is dose-dependent.
Neurological score was measured both before treatment onset just prior reperfusion (neurological score #1, T 1h30) and after treatment just before sacrifice (neurological score #2, T 49h30). Results are reported in Figure 4 and Table 2.
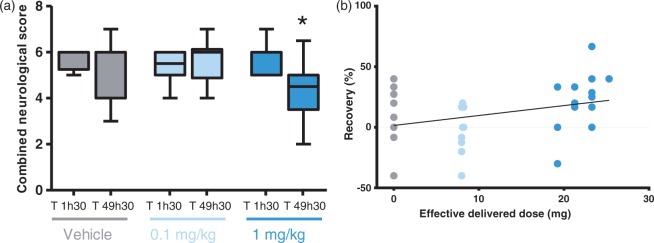
Figure 4.
Dose-dependent neuroprotective effect on neurological score. Rats underwent neurological scoring just prior to reperfusion (T 1h30) and 48 h post-MCAo (T 49h30). Figures show (a) the combined neurological score in the vehicle control group (n = 13) and (S)-roscovitine treatment groups: 0.1 mg/kg/h (n = 10) and 1 mg/kg/h (n = 13). Data are expressed as median and interquartile ranges. Data were analyzed by Wilcoxon matched-pairs test. Sign indicate value different from T 1h30: * p < 0.05. Figure (b) shows a significant positive correlation between recovery and the effective delivered dose. Pearson’s correlation coefficient: r = 0.332, r2 = 0.110, p = 0.048.
Table 2.
Dose-dependent neuroprotective effect on neurological score.
| T 1h30 |
T 49h30 |
Difference |
||||
|---|---|---|---|---|---|---|
| Median (95% CI) | Range | Median (95% CI) | Range | Median (95% CI) | Range | |
| Combined | ||||||
| Vehicle (n = 13) | 6.0 (5.5 – 6.0) | (5.3 – 6.0) | 6.0 (4.5 – 6.0) | (4.0 – 6.0) | 0.0 (−1.2 – 0.2) | (−1.8 – 0.0) |
| 0.1 mg/kg (n = 10) | 5.5 (4.9 – 5.9) | (5.0 – 6.0) | 6.0 (4.9 – 6.3) | (4.9 – 6.1) | 0.3 (−0.5 – 0.9) | (−1.0 – 1) |
| 1 mg/kg (n = 13) | 6.0 (5.3 – 6.1) | (5.0 – 6.0) | 4.5 (3.6 – 5.1)* | (3.5 – 5.0) | −1.5 (−2.1 – −0.5) # | (−2.0 – −0.5) |
| Tail suspension | ||||||
| Vehicle (n = 13) | 3.0 (3.0–3.0) | (3.0–3.0) | 3.0 (1.8–2.9) | (1.0–3.0) | 0.0 (−1.3 – −0.6) | (−2.0 – 0.0) |
| 0.1 mg/kg (n = 10) | 3.0 (3.0–3.0) | (3.0–3.0) | 3.0 (2.0–3.0) | (2.1–3.1) | 0.0 (−0.9 – 0.1) | (−1.0 – 0.0) |
| 1 mg/kg (n = 13) | 3.0 (2.8–3.1) | (3.0–3.0) | 2.0 (1.2–2.1)**# | (1.0–2.0) | −1.0 (−1.7 – −0.8) | (−2.0 – −1.0) |
| Spontaneous rotation | ||||||
| Vehicle (n = 13) | 3.0 (2.5–3.0) | (2.3–3.0) | 3.0 (2.5–3.3) | (3.0–3.0) | 0.0 (−0.4 – 0.6) | (0.0–0.5) |
| 0.1 mg/kg (n = 10) | 2.5 (1.9–2.9) | (2.0–3.0) | 3.0 (2.4–3.6) | (3.0–3.5) | 0.3 (−0.2 – 1.4) | (−1.0–1.3) |
| 1 mg/kg (n = 13) | 3.0 (2.4–3.1) | (2.0–3.0) | 3.0 (1.9–3.4) | (2.0–3.8) | 0.0 (−0.8 – 0.7) | (0.5–1.0) |
Note: Rats underwent neurological scoring (vehicle: n = 13, 0.1 mg/kg/h: n = 10 and 1 mg/kg/h: n = 13) just prior reperfusion (T 1h30) and 48 h post-MCAo (T 49h30). Data are presented as median and interquartile range at each time. The potential recovery was presented as difference T 49h30 – T 1h30. Data were analyzed by Kruskal–Wallis test to compare behavioral deficit between the three groups (vehicle vs. 0.1 mg/kg/h vs. 1 mg/kg/h) at each time T 1h30 and T 49h30 with post-hoc Dunn’s multiple comparison test. The potential recovery between T 1h30 and T 49h30 was analyzed in each group by Wilcoxon matched-pairs test. Signs indicate values different from T 1h30: *p < 0.05 and **p < 0.01 and different from 0.1 mg/kg/h: # p < 0.05.
Wilcoxon matched-pairs test revealed that only animals treated with the 1 mg/kg/h dose presented a lower combined neurological score at T 49h30 (4.5 [3.5–5.0]) than at T 1h30 (6.0 [5.0–6.0], W = 57.00, p = 0,0117). No improvement of combined neurological score was found in animals treated with the vehicle solution or the 0.1 mg/kg/h dose.
Regarding dose effect between groups at each times, no dose effect was found at T 1h30 (H = 1.44, n.s.) and T 49h30 (H = 5.31, n.s.), but analysis of difference between combined neurological scores measured at T 1h30 and T 49h30 revealed a significant improvement across the time in the behavioral impairment induced by occlusion in rats treated with the 1 mg/kg/h dose (1.5 [−2 – −0.5]) compared to those treated with the 0.1 mg/kg/hr dose (0.3 [−1.0 – 1], H = 8.39, p = 0.015) (Table 2).
More specifically, Wilcoxon matched-pairs test revealed that the suspension subscore measured at T 49h30 (2.0 [1.0–2.0]) was lower than at T 1h30 (3.0 [3.0–3.0], W = 66.00, p = 0.003) in rats treated with the 1 mg/kg/h dose (Table 2).
In addition, comparison between groups at T 49h30 showed that rats treated with the 1 mg/kg/h dose presented lower suspension subscore (2.0 [1.0–2.0]) than those receiving the 0.1 mg/kg/h (3.0 [2.1–3.1], H = 6.76, p = 0.03). No time or dose effect was found on spontaneous rotation test (Table 2).
Moreover, analysis of neurological score progression over time showed a significant positive correlation between recovery level and effective delivered dose (Figure 4(b), Pearson’s correlation: r = 0.332, r2 = 0.110, p = 0.048), suggesting a continuing beneficial effect of (S)-roscovitine dose on neurological score.
Infarct volume and edema
(S)-roscovitine reduces infarct volume and brain swelling in a dose-dependent manner in tMCAo rats
Total, cortical and sub-cortical infarcted regions volumes were measured at T 49h30 and corrected to compensate for brain swelling in the infarcted hemisphere (Figure 5(a)).
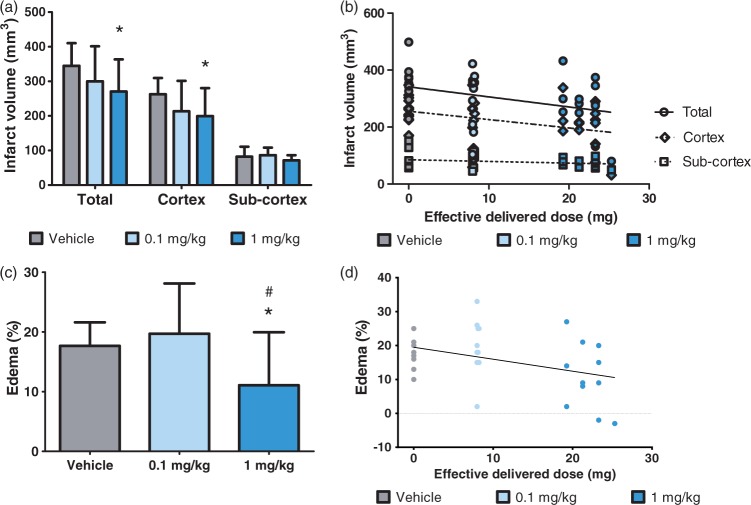
Figure 5.
Dose-dependent neuroprotective effect on infarct volume and brain edema. Brain lesions were measured by TTC staining 48 h post-MCAo. Figures show (a) the mean corrected infarction volumes and (c) edema measured as the percentage size of the contralateral hemisphere in the vehicle control group (n = 12) and (S)-roscovitine treatment groups: 0.1 mg/kg (n = 10) and 1 mg/kg (n = 13). Data are expressed as means ± SD. Data were analyzed by Student’s unpaired t-test. Signs indicate values different from vehicle: *p < 0.05 and different from 0.1 mg/kg: #p < 0.05. Figure (b) shows a negative correlation between infarct volume and the effective delivered dose in the total brain, cortex or subcortex. Pearson’s correlation coefficients: total brain: r = −0.376, r2 = 0.142, p = 0.03; cortex: r = −0.369, r2 = 0.137, p = 0.03; subcortex: r = 0.251, r2 = 0.063, p = 0.15 (N.S.). Figure (d) shows a negative correlation between edema and the effective delivered dose. Pearson’s correlation coefficient: r = −0.420, r2 = 0.177, p = 0.01.
Rat treated with the 1 mg/kg/h (S)-roscovitine dose exhibited a lower total infarction volume than those receiving the vehicle solution (−21.5%; t = 2.29, p = 0.03). No significant reduction of total infarction volume was observed in rats treated with the 0.1 mg/kg/h dose (−12.9%; n.s.).
More specifically, cortical infarction volume was lower in the 1 mg/kg/h group than in the control group (−24%. t = 2.36, p = 0.03). No significant difference was observed for cortical infarction volume between 0.1 mg/kg/h versus the vehicle control group (−18.7%; n.s.). No significant difference in sub-cortical infarction volume was found between the groups. Analysis of infarct volume according to effective delivered dose (Figure 5(b)) revealed a significant negative correlation for the total brain (Pearson’s correlation: r = −0.38, r2 = 0.14, p = 0.03) and cortex (Pearson’s correlation: r = −0.37, r2 = 0.14, p = 0.03). Analysis of edema (Figure 5(c)) revealed significantly less brain swelling in rats treated with 1 mg/kg/h of (S)-roscovitine than in the vehicle group (−37.3%, t = 2.36, p = 0.03) and the 0.1 mg/kg/h group (−43.8%, t = 2.36, p = 0.03). No difference was found between the 0.1 mg/kg/h and the vehicle groups; however, a significant negative correlation was observed between brain edema and effective delivered dose (Figure 5(d), Pearson’s correlation: r = −0.42, r2 = 0.18, p = 0.01).
Plasma (S)-roscovitine concentration at T 49h30 after focal ischemia is consistent with the concentration found in experiment 1 without ischemia
To confirm the association between (S)-roscovitine administration and beneficial effects on behavior and infarction volume, blood was collected at T 49h30 for (S)-roscovitine plasma assay. While (S)-roscovitine was not detected in the plasma of animals injected with vehicle solution (n = 2), comparison between (S)-roscovitine-injected groups showed a 19.2% higher (S)-roscovitine plasma level in the 1 mg/kg/h group (0.50 ± 0.25 µg/ml; n = 4) than in the 0.1 mg/kg/h group (0.02 ± 0.02 µg/ml; n = 10; U = 0.0, p = 0.006). Otherwise, it is interesting to note that plasma (S)-roscovitine concentration 48 h after treatment onset was similar in experiments 1 and 2 (0.4 and 0.5 µg/ml, respectively) in rats that received 1 mg/kg/h (S)-roscovitine by subcutaneous injection, as expected.
Discussion
Menn et al.9 first reported a neuroprotective effect of (S)-roscovitine in rodent models of cerebral ischemia. The present study sought to further explore the interest of (S)-roscovitine as a possible stroke therapy, with two main objectives: (1) to provide additional data on in vivo (S)-roscovitine pharmacokinetics in rat; (2) and to follow quality standards for preclinical studies of stroke therapy.
The study showed that sustained delivery of (S)-roscovitine for 48 h could be obtained by combining bolus and pump administration. This combination of two delivery modes provided a neuroprotective effect of (S)-roscovitine 1 mg/kg in a model of focal MCA infarction, with improved neurological score and a decrease in both infarction and brain edema volumes.
Mode of delivery and related pharmacokinetics
Two systemic delivery modes suitable for human therapy were tested, alone and in combination. To our knowledge, the present study is the first to measure the pharmacokinetic parameters of (S)-roscovitine.
The pharmacokinetic profile of (S)-roscovitine administered by IV bolus injection fitted a bi-compartmental model. The plasma time concentration curve was characterized by an early distribution phase of (S)-roscovitine in the plasmatic compartment, followed by a tissue distribution phase concomitant with elimination of the compound from the body. Several studies have previously reported the pharmacokinetic parameters of seliciclib, the (R) stereo-isomer of roscovitine, in adult Sprague-Dawley rats after IV or IP single-dose injection (25 mg/kg). Pharmacokinetic profiles in plasma also fitted a bi-compartmental model, but presented shorter distribution half-lives: between 4 to 6 min,24–26 compared to 31 min with (S)-roscovitine. The second phase corresponded to a rapid decline in (S)-roscovitine concentration in plasma, associated with a short elimination half-life (1.58 ± 0.16 h; i.e. 94.8 ± 9.6 min). Although these results revealed that this mode of delivery is not suitable for long-acting treatment, the elimination half-life was about 3-fold longer than that previously measured for (R)-roscovitine in the central compartment and in the brain (both < 30 min).24–26 As a result, the area under the plasma time concentration curve (AUC) of (S)-roscovitine was about 11.3-fold higher and the clearance 3-fold higher than that of (R)-roscovitine.24–26 It has been shown that stereoisomers may exhibit different pharmacokinetics27 in terms of absorption, distribution, protein binding, systemic clearance, first-pass metabolism and renal clearance. The present data show that combined-mode administration (S)-roscovitine displays the kind of pharmacokinetics that allows long and sustained brain tissue exposure, optimizing the therapeutic effect.
The plasma time concentration curve induced by SC infusion did not fit a bi-compartmental model: rather, (S)-roscovitine concentration in the plasma compartment increased progressively, reaching a plateau between 4 and 24 h, followed by a slow decrease until 48 h. The initial increase in concentration corresponded to an absorption phase related to extravascular infusion. Then, despite the low plasma concentration 24 h after treatment onset (0.61 ± 0.65 µg/ml), the plateau may correspond to an equilibrium concentration, enabling sustained delayed action of (S)-roscovitine.
Regarding the combination of bolus and pump injections, our data revealed that the plasma time concentration of (S)-roscovitine also fitted a bi-compartmental model.
It should be noted that, while initial concentrations in both the bolus and the bolus + pump groups were as expected, within the same order of magnitude and drastically higher than in the pump-alone group, the initial concentration (Co) was higher in the bolus group than in the combined bolus + pump group. This was related, under the present experimental conditions, to variability in the initial slope (initial diffusion-elimination phase related to the T1/2 -α values), without any significant impact on the general conclusions regarding the pharmacokinetic profiles.
In the combined bolus + pump group, distribution half-life was not significantly different than in the bolus-alone group, but distribution volume was 8-fold higher, reflecting the additional dose delivered by the SC pump. Elimination half-life was 16-fold longer (24.63 ± 18.90 vs.1.58 ± 0.16 h), associated with the 5-fold greater AUC between 8 and 48 h (25.26 ± 13.09 vs. 5.29 ± 2.18 h.µg/ml), suggesting substantially greater brain exposure in rats treated with bolus + pump compared to those that received a bolus alone. Although the precise therapeutic time window still needs to be determined,17 it is now recognized that the area of ischemic penumbra persisting after the acute phase of stroke is potentially salvageable for about 48 h in humans.28,29 Combining bolus and pump systemic injections is thus a delivery mode that compensates for the low absorption phase in subcutaneous infusion, enabling sustained action over a 48-h period, optimizing the benefit of (S)-roscovitine after ischemic stroke.
Quality standards for preclinical studies of stroke therapy
The present study adhered to the quality standards for preclinical studies of stroke therapy published by the editorial board of the Stroke journal.30–32
The main methodological improvements compared to our previous study9 were:
Randomization: This is the first randomized study of (S)-roscovitine. Randomization is a key methodological approach and is recommended by the STAIRS criteria (STAIRS 6). Although the prior publication9 was a blind study, it was not randomized.
Inclusion and exclusion criteria: the present inclusion and exclusion criteria were objective and quantitative. Cerebral blood flow (CBF) was continuously monitored by Laser Doppler. A threshold of a minimum drop of 60% compared to baseline was implemented to optimize inclusion. The percentage consecutive CBF-drop following occlusion, body weight before surgery and before sacrifice and body temperature throughout the anesthesia procedure and on post-reperfusion was compared between the three test groups.
Reproducibility: Previous studies reported the benefit of (S)-roscovitine in models of permanent and transient cerebral ischemia, in different animal species (mice and rats) and in a study performed by two independent laboratories. The present study provides additional evidence of (S)-roscovitine's neuroprotective efficacy in a transient model, with stronger quality standards.
The choice of ischemia duration was based on prior experiments.9 In the present study, we used 90-min ischemia. Since a pre-MCAo experiment showed that (S)-roscovitine significantly reduced infarction volume, it was important to show that it was also effective as post-MCAo treatment. Our previous work in transient MCAo showed significant efficacy of post-treatment (S)-roscovitine (pooled data at 1, 5 and 10 mg/ml), but did not study a single individual dose as in the present study. It was therefore important to determine the most effective dose.
(S)-roscovitine has a beneficial effect on functional outcomes, infarct and edema volumes
The plasma (S)-roscovitine concentration measured at T 49h30 (i.e. 48 h after treatment onset in tMCAo rats) was similar to that measured in the pharmacokinetic experiment in healthy rats, showing that pharmacokinetic parameters were not modified in ischemic conditions and further supporting the interest of combined bolus and pump delivery. Weight, glucose levels and body temperature were monitored throughout the tMCAo procedure until the day of sacrifice; no difference was observed between treated and control groups, suggesting that the neuroprotective effect of (S)-roscovitine was not due to differences in physiological parameters.
Functional outcome
The present study is the first to highlight the fact that the neuroprotective effect of (S)-roscovitine is associated with better functional recovery after focal ischemia, and that it is effective after ischemia onset and recanalization. This effect was mainly due to the tail suspension subscore, which seemed to be more sensitive in detecting motor impairment.
Comparison could be only performed with (R)-roscovitine, its dextrogyre counterpart. However, it has been shown that enantiomers can exhibit pharmacodynamic differences.27 Only one study reported a neuroprotective effect of (R)-roscovitine on brain damage, with improvement in neurological deficit in a 1-h tMCAo rat model; however, (R)-roscovitine was administered pre-occlusion by 48 h intra-cerebrovascular (ICV) infusion,8 which is not suitable for humans. A beneficial preventive effect of (R)-roscovitine administered by ICV injection for functional recovery was also reported in a rat model of brain trauma.10
Infarct volume
The beneficial effect of (S)-roscovitine on functional recovery was associated with a 24% decrease in infarct volume in the cortex. These results are consistent with those of our previous study, which showed that the (S) isomer of roscovitine decreased brain infarct volume in both permanent and transient focal cerebral ischemia models in rodents, and that a single bolus injection (25 mg/kg) combined with 48 h infusion of the low test dose (1 mg/kg/h) provided greater reduction in infarct volume than the high dose in a tMCAo rat model.9
Brain edema
To our knowledge, the present study is the first to demonstrate a beneficial effect of a CDK inhibitor on edema formation after ischemic stroke. Brain edema was reduced by 37.3% in the treatment group compared to the vehicle group. Only a few studies reported a beneficial effect of pharmacological or non-pharmacological treatment on brain swelling.1
The beneficial effect of (S)-roscovitine is dose-dependent
Although there were clusters due to different group conditions, improved recovery and infarct and edema size were significantly dose-dependent, suggesting a possibility of enhancing the beneficial effect of (S)-roscovitine by using higher dose. A higher dose of (S)-roscovitine was also tested (5 mg/kg/h), but the solution precipitated under the skin after subcutaneous injection. It is known that high-concentration (S)-roscovitine solution has an acidic pH and precipitates at physiological pH as found in the subcutaneous environment. The presence of subcutaneous deposits therefore led us to exclude this group from the study. As lower doses were less effective and higher doses were unusable, bolus injection (25 mg/kg) followed by 1 mg/kg/h infusion may be the optimal (S)-roscovitine dose for stroke therapy in animals, although there may be room for improvement for future human treatment.
Action mechanisms
The present findings are in line with several studies that reported similar beneficial effects of CDK inhibitors after ischemia in rats, using flavopiridol a CDK4/cyclin D1 inhibitor.33,34 More specifically, we previously showed that the neuroprotective effect of (S)-roscovitine was mediated by CDK5.9 Given that all CDKs except CDK5 are silent in post-mitotic neurons,35 there is increasing evidence that aberrant CDK5 activity is a primary cause of neuronal death during stroke36 and that CDK5 inhibitors are neuroprotective in neurodegenerative disorders and neurodegeneration associated with stroke.37 Gutierrez-Vargas et al.38 showed that, through RNAi, intracerebral hippocampal administration of silencing CDK5 during ischemia prevented reversal learning impairment at four months post-ischemia in rats.38 Another study reported that a serine protease inhibitor, inducing CDK5 down-regulation, was associated with improved memory performance and axonal regeneration after 2 h MCAo in rat.39 Moreover, Miranda-Barrientos et al.40 recently showed that CDK5 inhibition induced increased LTP levels in corticostriatal synapses in adult mice.
Brain edema may be the consequence of various brain injuries, including stroke, and may be classified as cytotoxic, vasogenic or even hydrocephalic.41 There is no clear delineation between cytotoxic and vasogenic edema. While cytotoxic edema is associated with dysfunction of the cell membrane ion pump, leading to uptake of brain water from the blood into brain parenchyma, vasogenic edema results from a breakdown of the blood–brain barrier (BBB), leading to extravasation of serum protein and water. Ischemia and rapid reperfusion cause changes in BBB permeability that may persist from some hours to several weeks after reperfusion,42,43 highlighting the relevance of long-acting treatment. BBB breakdown is associated with increased risk of hemorrhagic transformation, particularly with tissue plasminogen activator treatment (tPA) or surgery.44,45 Our previous study showed that (S)-roscovitine was able to cross the BBB in healthy animals,9 and that the plasma concentration was sufficient to provide a beneficial effect on functional outcomes. The strong decrease in edema formation in this study suggests that (S)-roscovitine may help maintain BBB integrity and thus reduce the risk of hemorrhagic transformation. Other studies reported that BBB disruption after experimental stroke was accompanied by activation of inflammatory processes, including microglial activation.46,47 (R)-roscovitine attenuates cell-cycle activation of glial cells, reducing glial scar formation and microglial activation, and thus improving behavioral outcome after stroke or brain trauma.8,10 According to these findings, (S)-roscovitine may also prevent edema formation by reducing inflammatory response.
Conclusion
Taken together, our results strongly support a beneficial effect of (S)-roscovitine on brain damage and functional outcome after ischemic stroke. This study provides new insight in line with the STAIR recommendations for preclinical studies. Our data revealed that it was necessary to combine two modes of (S)-roscovitine delivery to achieve immediate and sustained action covering a 48-h time-window. The pharmacokinetic parameters revealed a longer elimination half-life for the (S) stereoisomer of roscovitine compared to the (R) stereoisomer, making it a better candidate for stroke therapy. The present study showed for the first time a beneficial effect of (S)-roscovitine on functional outcome, which is the most relevant endpoint for clinical trials. The pleiotroipic effect of (S)-roscovitine on brain ischemia, neuronal death, inflammatory cells and brain edema, associated with administration suitable for humans, makes it a perfect candidate for human trials in the era of recanalization, whether performed by thrombolysis, thrombectomy or both.
Supplementary Material
Acknowledgments
The authors thank Pr Denis Vivien (INSERM UMR-S U919, Caen) and Benoit Haelewyn (Centre Universitaire de Ressources Biologiques (CURB), Caen) for welcoming Estelle Rousselet in their laboratory to perform animal stroke experiments. We thank the UBO (Université de Bretagne Occidentale) facility for help with animal experimentation, and acknowledge the help given by Dr. Séverine Loisel, Marie-Françoise Scoazec and Manuel Feillant (UBO, Brest, France). The article was re-edited by a native English speaker: Mr. Iain McGill.
Funding
This study was supported by ANR (Research French National Agency) Strokinin project: ANR-10-BIOT-005 and a start-up company (Neurokin) which is no longer in activity.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors have some competing financial interests to declare. BM and ST are Neurokin S.A. shareholders. ST received consultancy fees from Neurokin S.A. until 2011. This does not alter our adherence to all the JCBFM policies on sharing data and material.
Authors’ contributions
BM and ST designed and supervised the study. ER, BM, MLQ and YC performed the experiments. ER, AL, BM, YC, MLQ and ST analyzed the data. ER, AL, BM and ST wrote the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Moretti A, Ferrari F, Villa RF. Neuroprotection for ischaemic stroke: current status and challenges. Pharmacol Ther 2015; 146: 23–34. [DOI] [PubMed] [Google Scholar]
- 2.Khaja AM, Grotta JC. Established treatments for acute ischaemic stroke. Lancet 2007; 369: 319–330. [DOI] [PubMed] [Google Scholar]
- 3.Bordet R, Ouk T, Onteniente B, et al. Cerebral ischaemia: tomorrow's therapeutic tracks. Med Sci 2009; 25: 847–854. [DOI] [PubMed] [Google Scholar]
- 4.Knockaert M, Greengard P, Meijer L. Pharmacological inhibitors of cyclin-dependent kinases. Trends Pharmacol Sci 2002; 23: 417–425. [DOI] [PubMed] [Google Scholar]
- 5.Bach S, Knockaert M, Reinhardt J, et al. Roscovitine targets, protein kinases and pyridoxal kinase. J Biol Chem 2005; 280: 31208–31219. [DOI] [PubMed] [Google Scholar]
- 6.Meijer L, Borgne A, Mulner O, et al. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem 1997; 243: 527–536. [DOI] [PubMed] [Google Scholar]
- 7.Wen Y, Yang SH, Liu R, et al. Cdk5 is involved in NFT-like tauopathy induced by transient cerebral ischemia in female rats. Biochim Biophys Acta 2007; 1772: 473–483. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Q, Chen C, Lu J, et al. Cell cycle inhibition attenuates microglial proliferation and production of IL-1beta, MIP-1alpha, and NO after focal cerebral ischemia in the rat. Glia 2009; 57: 908–920. [DOI] [PubMed] [Google Scholar]
- 9.Menn B, Bach S, Blevins TL, et al. Delayed treatment with systemic (S)-roscovitine provides neuroprotection and inhibits in vivo CDK5 activity increase in animal stroke models. PLoS One 2010; 5: e12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hilton GD, Stoica BA, Byrnes KR, et al. Roscovitine reduces neuronal loss, glial activation, and neurologic deficits after brain trauma. J Cereb Blood Flow Metab 2008; 28: 1845–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang M, Li J, Chakrabarty P, et al. Cyclin-dependent kinase inhibitors attenuate protein hyperphosphorylation, cytoskeletal lesion formation, and motor defects in Niemann-Pick Type C mice. Am J Pathol 2004; 165: 843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Appert-Collin A, Hugel B, Levy R, et al. Cyclin dependent kinase inhibitors prevent apoptosis of postmitotic mouse motoneurons. Life Sci 2006; 79: 484–490. [DOI] [PubMed] [Google Scholar]
- 13.Lopes JP, Oliveira CR, Agostinho P. Role of cyclin-dependent kinase 5 in the neurodegenerative process triggered by amyloid-Beta and prion peptides: implications for Alzheimer's disease and prion-related encephalopathies. Cell Mol Neurobiol 2007; 27: 943–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rashidian J, Iyirhiaro GO, Park DS. Cell cycle machinery and stroke. Biochim Biophys Acta 2007; 1772: 484–493. [DOI] [PubMed] [Google Scholar]
- 15.Timsit S, Menn B. Cerebral ischemia, cell cycle elements and Cdk5. Biotechnol J 2007; 2: 958–966. [DOI] [PubMed] [Google Scholar]
- 16.Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke 1999; 30: 2752–2758. [DOI] [PubMed]
- 17.Fisher M, Feuerstein G, Howells DW, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 2009; 40: 2244–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989; 20: 84–91. [DOI] [PubMed] [Google Scholar]
- 19.Jiang SX, Lertvorachon J, Hou ST, et al. Chlortetracycline and demeclocycline inhibit calpains and protect mouse neurons against glutamate toxicity and cerebral ischemia. J Biol Chem 2005; 280: 3381133818. [DOI] [PubMed] [Google Scholar]
- 20.Gartshore G, Patterson J, Macrae IM. Influence of ischemia and reperfusion on the course of brain tissue swelling and blood-brain barrier permeability in a rodent model of transient focal cerebral ischemia. Exp Neurol 1997; 147: 353–360. [DOI] [PubMed] [Google Scholar]
- 21.Leach MJ, Swan JH, Eisenthal D, et al. BW619C89, a glutamate release inhibitor, protects against focal cerebral ischemic damage. Stroke 1993; 24: 1063–1067. [DOI] [PubMed] [Google Scholar]
- 22.Liu S, Zhen G, Meloni BP, et al. rodent stroke model guidelines for preclinical stroke trials (1st Edition). J Exp Stroke Transl Med 2009; 2: 2–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hassan M, Sallam H, Hassan Z. The role of pharmacokinetics and pharmacodynamics in early drug development with reference to the cyclin-dependent kinase (Cdk) inhibitor – roscovitine. Sultan Qaboos Univ Med J 2011; 11: 165–178. [PMC free article] [PubMed] [Google Scholar]
- 24.Sallam H, Jimenez P, Song H, et al. Age-dependent pharmacokinetics and effect of roscovitine on Cdk5 and Erk1/2 in the rat brain. Pharmacol Res 2008; 58: 32–37. [DOI] [PubMed] [Google Scholar]
- 25.Vita M, Abdel-Rehim M, Olofsson S, et al. Tissue distribution, pharmacokinetics and identification of roscovitine metabolites in rat. Eur J Pharm Sci 2005; 25: 91–103. [DOI] [PubMed] [Google Scholar]
- 26.Vita M, Meurling L, Pettersson T, et al. Analysis of roscovitine using novel high performance liquid chromatography and UV-detection method: pharmacokinetics of roscovitine in rat. J Pharm Biomed Anal 2004; 34: 425–431. [DOI] [PubMed] [Google Scholar]
- 27.Tracy TS. Stereochemistry in pharmacotherapy: when mirror images are not identical. Ann Pharmacother 1995; 29: 161–165. [DOI] [PubMed] [Google Scholar]
- 28.Phan TG, Wright PM, Markus R, et al. Salvaging the ischaemic penumbra: more than just reperfusion? Clin Exp Pharmacol Physiol 2002; 29: 1–10. [DOI] [PubMed] [Google Scholar]
- 29.Chavez JC, Hurko O, Barone FC, et al. Pharmacologic interventions for stroke: looking beyond the thrombolysis time window into the penumbra with biomarkers, not a stopwatch. Stroke 2009; 40: e558–e563. [DOI] [PubMed] [Google Scholar]
- 30.Lapchak PA. Recommendations and practices to optimize stroke therapy: developing effective translational research programs. Stroke 2013; 44: 841–843. [DOI] [PubMed] [Google Scholar]
- 31.Macleod MR, Fisher M, O'Collins V, et al. Good laboratory practice: preventing introduction of bias at the bench. Stroke 2009; 40: e50–e52. [DOI] [PubMed] [Google Scholar]
- 32.Vahidy F, Schabitz WR, Fisher M, et al. Reporting standards for preclinical studies of stroke therapy. Stroke 2016; 47: 2435–2438. [DOI] [PubMed] [Google Scholar]
- 33.Osuga H, Osuga S, Wang F, et al. Cyclin-dependent kinases as a therapeutic target for stroke. Proc Natl Acad Sci U S A 2000; 97: 10254–10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang F, Corbett D, Osuga H, et al. Inhibition of cyclin-dependent kinases improves CA1 neuronal survival and behavioral performance after global ischemia in the rat. J Cereb Blood Flow Metab 2002; 22: 171–182. [DOI] [PubMed] [Google Scholar]
- 35.Whittaker SR, Walton MI, Garrett MD, et al. The Cyclin-dependent kinase inhibitor CYC202 (R-roscovitine) inhibits retinoblastoma protein phosphorylation, causes loss of Cyclin D1, and activates the mitogen-activated protein kinase pathway. Cancer Res 2004; 64: 262–272. [DOI] [PubMed] [Google Scholar]
- 36.Meyer DA, Torres-Altoro MI, Tan Z, et al. Ischemic stroke injury is mediated by aberrant Cdk5. J Neurosci 2014; 34: 8259–8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mushtaq G, Greig NH, Anwar F, et al. Neuroprotective mechanisms mediated by CDK5 inhibition. Curr Pharm Des 2016; 22: 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gutierrez-Vargas JA, Moreno H, Cardona-Gomez GP. Targeting CDK5 post-stroke provides long-term neuroprotection and rescues synaptic plasticity. J Cereb Blood Flow Metab 2016; 37: 2208–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y, Li C, Wang J, et al. Nafamostat mesilate improves neurological outcome and axonal regeneration after stroke in rats. Mol Neurobiol . Epub ahead of print 22 June 2016. DOI: 10.1007/s12035-016-9999-7. [DOI] [PubMed] [Google Scholar]
- 40.Miranda-Barrientos J, Nieto-Mendoza E, Hernandez-Echeagaray E. The Cdk5 inhibitor Roscovitine increases LTP induction in corticostriatal synapses. ASN Neuro 2014; 6: AN20140006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang G, Yang GY. Aquaporin-4: a potential therapeutic target for cerebral edema. Int J Mol Sci 2016; 17: 1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pillai DR, Dittmar MS, Baldaranov D, et al. Cerebral ischemia-reperfusion injury in rats–a 3 T MRI study on biphasic blood-brain barrier opening and the dynamics of edema formation. J Cereb Blood Flow Metab 2009; 29: 1846–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strbian D, Durukan A, Pitkonen M, et al. The blood-brain barrier is continuously open for several weeks following transient focal cerebral ischemia. Neuroscience 2008; 153: 175–181. [DOI] [PubMed] [Google Scholar]
- 44.Wang X, Lo EH. Triggers and mediators of hemorrhagic transformation in cerebral ischemia. Mol Neurobiol 2003; 28: 229–244. [DOI] [PubMed] [Google Scholar]
- 45.Sumii T, Lo EH. Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke 2002; 33: 831–836. [DOI] [PubMed] [Google Scholar]
- 46.Pradillo JM, Denes A, Greenhalgh AD, et al. Delayed administration of interleukin-1 receptor antagonist reduces ischemic brain damage and inflammation in comorbid rats. J Cereb Blood Flow Metab 2012; 32: 1810–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McColl BW, Rothwell NJ, Allan SM. Systemic inflammation alters the kinetics of cerebrovascular tight junction disruption after experimental stroke in mice. J Neurosci 2008; 28: 9451–9462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.