Abstract
The present study examined whether preoperatively reduced cerebrovascular contractile reactivity to hypocapnia by hyperventilation is associated with development of cerebral hyperperfusion syndrome after arterial bypass surgery for adult patients with cerebral misery perfusion due to ischemic moyamoya disease. Among 65 adult patients with ischemic moyamoya disease, 19 had misery perfusion in the precentral region on preoperative 15O positron emission tomography and underwent arterial bypass surgery for that region. Brain technetium-99 m-labeled ethyl cysteinate dimer single-photon emission computed tomography (SPECT) was preoperatively performed with and without hyperventilation challenge and relative cerebrovascular contractile reactivity to hypocapnia (RCVCRhypocap) (%/mmHg) was calculated in the precentral region. Development of cerebral hyperperfusion syndrome was determined using perioperative changes of symptoms and brain N-isopropyl-p-[123I]-iodoamphetamine SPECT performed after surgery. RCVCRhypocap was significantly lower in the 6 patients with cerebral hyperperfusion syndrome (−2.85 ± 1.10%/mmHg) than in the 13 patients without cerebral hyperperfusion syndrome (0.18 ± 1.97%/mmHg; p = 0.0050). Multivariate analysis demonstrated low RCVCRhypocap as an independent predictor of cerebral hyperperfusion syndrome (95% confidence interval, 0.04–0.96; p = 0.0433). Preoperatively reduced cerebrovascular contractile reactivity to hypocapnia by hyperventilation is associated with development of cerebral hyperperfusion syndrome after arterial bypass surgery for adult patients with cerebral misery perfusion due to ischemic moyamoya disease.
Keywords: Moyamoya disease, hyperperfusion syndrome, hypocapnia, misery perfusion, single-photon emission computed tomography
Introduction
Moyamoya disease is a chronic, occlusive cerebrovascular disease of unknown etiology characterized by bilateral steno-occlusive changes in the terminal portion of the internal carotid artery and an abnormal vascular network at the base of the brain.1,2 Arterial bypass surgery, such as superficial temporal artery (STA)-middle cerebral artery (MCA) anastomosis, is generally employed as the standard surgical treatment for moyamoya disease with the onset of ischemic symptoms as well as intracerebral hemorrhages.3 This procedure prevents further cerebral ischemic attacks in the former4,5 and reduces the risk of rebleeding in the latter.6
Cerebral hyperperfusion following arterial bypass surgery is known as a surgical complication of moyamoya disease and is defined as an acute substantial increase in ipsilateral cerebral blood flow (CBF) well above the metabolic demands of the brain tissue.7–9 Cerebral hyperperfusion syndrome after arterial bypass surgery for moyamoya disease is a complication of cerebral hyperperfusion, with an incidence of approximately 30% and with characteristic features including transient aphasia, hemiparesis, and dysarthria.7,10,11 Cerebral hyperperfusion also leads to intracerebral hemorrhage and/or subarachnoid hemorrhage.11,12 The incidence of such hemorrhage ranges from 3 to 5%, which could potentially result in permanent neurological deficit and/or mortality.3 Furthermore, the incidence of cerebral hyperperfusion syndrome including hemorrhage is considerably greater in adult patients than in pediatric patients.10,13
Mechanisms to explain the development of cerebral hyperperfusion after arterial bypass surgery for moyamoya disease have been proposed.11,13 When steno-occlusive changes at the terminal portion of the internal carotid artery develop with deficient collateral circulation, perfusion pressure is decreased in the cerebral hemisphere distal to the affected artery. Reduction of perfusion pressure below the compensatory capacity of autoregulatory mechanisms leads to maximal dilation of resistance vessels and misery perfusion. When the perfusion pressure is restored following arterial reconstructive surgery, several days may be required for chronically impaired autoregulatory mechanisms to adjust to the new steady state, resulting in temporary ongoing hyperperfusion. Thus, absent or insufficient contraction of maximally dilated resistance vessels in response to restored perfusion pressure may cause cerebral hyperperfusion after arterial bypass surgery for moyamoya disease. However, whether this hypothesis is correct remains unclear.
In the healthy human brain, hypocapnia induced by hyperventilation contracts the arterioles, resulting in reduced CBF.14 This phenomenon can be displayed using positron emission tomography (PET).15 Technetium-99 m-labeled (99mTc) ethyl cysteinate dimer (ECD), an accumulative radioligand, is used as a brain perfusion tracer for single-photon emission computed tomography (SPECT) to image reduced blood flow in the cerebral hemisphere with internal carotid artery or MCA occlusive disease.16 Use of 99mTc-ECD SPECT can also image CBF changes by hypocapnia induced by hyperventilation in patients with moyamoya disease.17
The purpose of the present study using PET and SPECT was to determine whether preoperatively reduced cerebrovascular contractile reactivity to hypocapnia by hyperventilation is associated with development of cerebral hyperperfusion syndrome after arterial bypass surgery for adult patients with cerebral misery perfusion due to ischemic moyamoya disease.
Materials and methods
Healthy subjects
Twenty healthy male adults meeting the following criteria were prospectively enrolled to obtain normal control values of brain 15O gas PET and 99mTc-ECD SPECT: age >30 years but <60 years; absence of any history of hypertension, diabetes mellitus or dyslipidemia; absence of leukoaraiosis or asymptomatic lacunar infarction on conventional brain magnetic resonance (MR) imaging. These 20 healthy subjects were assigned to one of two groups (n = 10 each) who underwent 15O-gas PET or 99mTc-ECD SPECT, respectively.
Patients inclusion criteria
Patients who were diagnosed with moyamoya disease according to the diagnostic criteria of the Research Committee on Spontaneous Occlusion of the Circle of Willis of the Ministry of Health, Labor, and Welfare, Japan were prospectively selected for the present study if they satisfied the following clinical inclusion criteria: age >30 years but <60 years; useful residual function (modified Rankin disability scale 0 or 1); presence of episodes of ipsilateral carotid territory ischemic symptoms that had occurred ≤3 months before presentation to our department; and absence of major cerebral infarction on MR imaging. These selected patients first underwent brain 15O-gas PET according to the methods described below (see “Preoperative brain 15O-gas PET study” section). When a patient showed symptomatic misery perfusion on the 15O PET study (see “Data analysis and definition of preoperative misery perfusion and postoperative hyperperfusion” section), the patient was considered a candidate for arterial bypass surgery and was finally included into the present study.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee Iwate Medical University School of Medicine (H22-3) and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards, and written, informed consent was obtained from all subjects or their next of kin prior to the patient participation.
Preoperative brain 15O-gas PET study
PET studies were performed using an SET-3000GCT/M scanner (PET/CT; Shimadzu, Kyoto, Japan) for healthy subjects and patients. In the latter group, these studies were performed more than three weeks after the last ischemic event. Before emission scans, a transmission scan (3 min) with a 137Cs point source was performed using a bismuth germanate transmission detector ring coaxially attached to the gadolinium silica oxide emission detector ring. CBF was determined while the subject continuously inhaled C15O2 through a mask. Measurements of the cerebral metabolic rate of oxygen (CMRO2) and oxygen extraction fraction (OEF) were obtained during continuous inhalation of 15O2. Data were collected for 5 min. A single breath of C15O was used to measure cerebral blood volume (CBV). CBF, CMRO2 and OEF were calculated using the steady-state method,18 and CMRO2 and OEF were corrected by CBV.19
Preoperative brain 99mTc-ECD SPECT study with and without hyperventilation
Within five days after PET, 99mTc-ECD SPECT studies were performed for patients using a triple-head gamma camera (GCA-9300R; Toshiba Medical Systems, Tochigi, Japan). The spatial resolution of the gamma camera with a high-resolution fan-beam collimator was 8.5 mm full width at half maximum. The SPECT acquisition protocol consisted of a matrix size of 128 × 128 and 30-min continuous acquisition (5 min/rotation) over 360° in 4° steps. Post-acquisition, data were corrected for scatter with the triple energy window method and then reconstructed by filtered back-projection. A Butterworth preprocessing filter was applied, with a cutoff frequency of 0.08 cycles per pixel (1 pixel = 1.72 mm). For attenuation correction, the iterative Chang method was used, and the attenuation map was generated by extracting the skin contour and assuming the inner region side as a uniform attenuation body. The attenuation coefficient was 0.146 cm−1.
First, each patient was given an intravenous bolus injection of 740 MBq of 99mTc-ECD from a commercially supplied kit and scanning with the above-mentioned collimator was started 5 min after tracer injection with a scanning duration of 10 min. Two days later, the same patient underwent 99mTc-ECD SPECT with hyperventilation challenge. Under transcutaneous CO2 partial pressure (PCO2) monitoring using a single earlobe sensor (SENTEC Digital Monitor System; SENTEC, Ringstrasse, Switzerland), hyperventilation was performed for 90 s and 99mTc-ECD was administered immediately after the end of hyperventilation. Five minutes later, SPECT was performed in the same manner as at resting state. For each patient, PCO2 at the end of hyperventilation and the lowest PCO2 within 1 min after stopping were averaged, and the averaged value was subtracted from a pre-hyperventilation value. This difference was defined as ΔPCO2 (mmHg).
Postoperative brain N-isopropyl-p-[123I]-iodoamphetamine (IMP) SPECT study
To detect postoperative cerebral hyperperfusion, 123I-IMP SPECT studies were performed using the same scanner as 99mTc-ECD SPECT studies at one day and at one month after surgery. After intravenous infusion of 222 MBq of 123I-IMP, data acquisition was performed at a mid-scan time of 30 min for a scan duration of 20 min.
Data analysis and definitions of preoperative misery perfusion and postoperative hyperperfusion
All PET and SPECT images were transformed into the standard brain size and shape by linear and nonlinear transformation using SPM2 for anatomic standardization.20 Brain images from all subjects thus had the same anatomic format. A total of 318 constant regions of interest (ROIs) were automatically placed in both the cerebral and cerebellar hemispheres using a three-dimensional stereotaxic ROI template (3DSRT) with SPM2 (FUJIFILM RI Pharma, Tokyo, Japan).21 ROIs were grouped into 10 segments (callosomarginal, pericallosal, precentral, central, parietal, angular, temporal, posterior, hippocampal, and cerebellar) in each hemisphere according to the arterial supply. Of these 10 segments, five (precentral, central, parietal, angular, and temporal) perfused by the MCA and cerebellar ROI were used for the following analyses (Figure 1).
Figure 1.
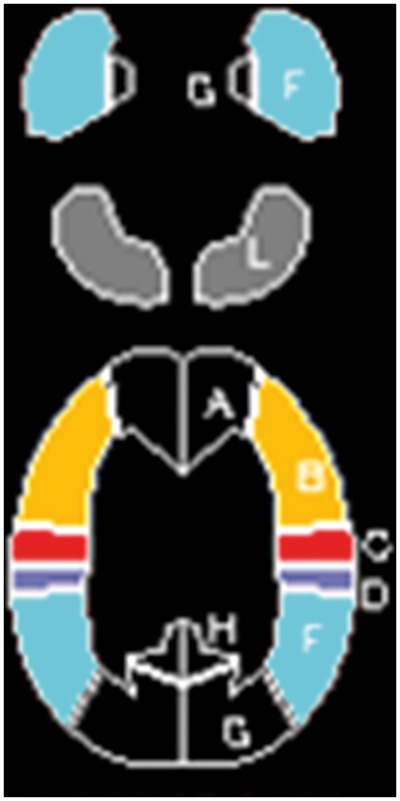
Diagrams showing the regions of interest (ROIs) of a three-dimensional stereotaxic ROI template. Orange, red, blue, cyan, and grey ROIs indicate precentral, central, parietal, temporal, and cerebellar regions, respectively.
Mean values of CBF, CBV, CMRO2 and OEF on PET images were measured in the five MCA ROIs in bilateral cerebral hemispheres. In each MCA ROI of each patient, when CBF or CMRO2 was lower than the lower limit of the 95% confidence interval (CI) of control values obtained from 10 healthy subjects, this ROI was defined as displaying abnormally reduced CBF or CMRO2, respectively; when CBV or OEF was greater than the upper limit of the 95%CI of the control values, this ROI was defined as having abnormally elevated CBV or OEF, respectively. Furthermore, when one or more MCA ROIs in the symptomatic hemisphere exhibited abnormally elevated OEF, the patient was defined as having symptomatic misery perfusion.
The mean value of radioactive counts on preoperative 99mTc-ECD SPECT images was measured in the five MCA ROIs in bilateral cerebral hemispheres and bilateral cerebellar ROIs. For each patient, the ratio of the mean radioactive count in each MCA ROI with misery perfusion in the symptomatic cerebral hemisphere to that in the ipsilateral cerebellar ROI was calculated. Relative cerebrovascular contractile reactivity to hypocapnia (RCVCRhypocap) (%/mmHg) was defined as follows: 100*(Ratiorest – RatioHV)/ Ratiorest *ΔPCO2), where, Ratiorest and RatioHV are the ECD SPECT ratios at resting state and with hyperventilation challenge, respectively. In each MCA ROI of each patient, when RCVCRhypcap was lower than the lower limit of the 95%CI of control values obtained from 10 healthy subjects, this ROI was defined as having abnormally reduced cerebrovascular contractile reactivity to hypocapnia; when RCVCRhypocap was greater than the upper limit of the 95%CI of control values, this ROI was defined as having abnormally increased cerebrovascular contractile reactivity to hypocapnia; and ROIs with other values of RCVCRhypocap were defined as having normal cerebrovascular contractile reactivity to hypocapnia.
When a focal increase in CBF was visually observed in one or more MCA ROIs ipsilateral to surgery and a ratio (defined as RCBFpostope) of the mean radioactive count in those MCA ROIs to that in the cerebellar ROI ipsilateral to surgery was >1.5 on 123I-IMP SPECT images one day after surgery, the patient was suspected as having cerebral hyperperfusion.13 Such patients were finally defined as having cerebral hyperperfusion when the focal increase in CBF at one day after surgery visually resolved on 123I-IMP SPECT images one month after surgery. Cerebral hyperperfusion syndrome was determined using the following criteria: (1) headache, seizure, alteration in level of consciousness, and/or focal neurologic signs such as aphasia, hemiparesis, and dysarthria that newly developed or worsened between 12 h and 30 days after surgery; and (2) presence of cerebral hyperperfusion on 123I-IMP SPECT.
Intra- and postoperative management
Patients underwent a single STA-MCA anastomosis without indirect revascularization between one month and four months after the last ischemic event. The M4 perfusing an MCA ROI with the misery perfusion in the symptomatic cerebral hemisphere was selected as a recipient artery. For patients with misery perfusion in bilateral cerebral hemispheres and bilateral carotid territory ischemic symptoms that had occurred ≤3 months before presentation to our department, the hemisphere with the MCA ROI with the higher OEF underwent surgery first; and three months later, the contralateral hemisphere underwent the surgery.
Postoperatively, all patients were strictly managed to avoid hypovolemia and anemia. Attempts were made to keep the systolic blood pressure between 110 and 130 mmHg. When a patient was suspected as having cerebral hyperperfusion on 123I-IMP SPECT images one day after surgery and headache, seizure, alteration in level of consciousness, and/or focal neurologic signs newly developed or worsened after surgery, a propofol coma was induced.
One month after surgery, MR angiography was performed to confirm patency of the arterial bypass.
Statistical analysis
Data are expressed as the mean ± standard deviation (SD). The results of 15O-gas PET and 99mTc-ECD SPECT were compared between controls and patients using the Mann–Whitney U test. The relationship between each variable and development of cerebral hyperperfusion on postoperative 123I-IMP SPECT imaging or cerebral hyperperfusion syndrome was evaluated with univariate analysis using the Mann–Whitney U test or χ2 test. A multivariate statistical analysis of factors related to development of cerebral hyperperfusion on postoperative 123I-IMP SPECT imaging or cerebral hyperperfusion syndrome was also performed using logistic regression modeling. Variables showing values of p < 0.2 in univariate analyses were selected for analysis in the final model. Statistical significance for all analyses was set at the p < 0.05 level.
Results
From May 2010 to December 2015, a total of 65 patients satisfied the clinical inclusion criteria and underwent brain 15O-gas PET. Of these 65 patients, 20 were determined as having misery perfusion in the symptomatic hemispheres, and 19 subsequently underwent preoperative 99mTc-ECD SPECT, arterial bypass surgery and postoperative 123I-IMP SPECT. The remaining patient refused these further examinations and arterial bypass surgery, and was excluded from the present analyses. Of the 19 patients included, one satisfied all the inclusion criteria in bilateral cerebral hemispheres. Although this patient eventually underwent arterial bypass surgery in bilateral hemispheres, the second surgery was consequently performed more than six months after the last ischemic event. Thus, only data from the first surgery were analyzed in this patient.
Mean age of the 19 patients (4 men, 15 women) was 44 ± 8 years (range, 32–57 years). Concomitant disease states and symptoms were recorded, including four patients with hypertension, two patients with diabetes mellitus, and four patients with dyslipidemia; 14 patients had TIA alone and 5 had minor stroke with or without preceding TIA. The STA was anastomosed to the M4 in the precentral region in all 19 patients. The M4 of the other regions was not selected as a recipient artery. Duration of intraoperative temporary occlusion for the recipient artery ranged from 21 to 40 min (mean, 30 ± 5 min)
Mean age of the 10 healthy male adults who were enrolled to obtain normal control values of brain 15O-gas PET was 42 ± 7 years (range, 30–55 years). Mean, SD and 95% CI of PET-CBF, CBV, CMRO2 and OEF in the MCA ROI of the precentral region in controls and patients are shown in Table 1. CBF was significantly lower in patients than in controls. CBV and OEF were significantly greater in patients than in controls. CMRO2 did not differ between controls and patients. The MCA ROI of the precentral region in all 19 patients exhibited abnormally reduced CBF and abnormally elevated CBV, and none of the MCA ROIs of the precentral region exhibited abnormally reduced CMRO2.
Table 1.
Comparison of 15O-gas PET and 99mTc-ECD SPECT values in MCA ROI of the precentral region in 20 cerebral hemispheres of 10 healthy subjects as controls and in 19 affected cerebral hemispheres of 19 patients.
| Controls | Patients | p | |
|---|---|---|---|
| (n = 20) | (n = 19) | ||
| CBF (ml/100 g/min) | <0.0001 | ||
| Mean | 46.7 | 34.0 | |
| SD | 4.6 | 2.8 | |
| 95% CI | 37.5 to 55.9 | 27.9 to 40.1 | |
| CBV (ml/100 g) | <0.0001 | ||
| Mean | 3.74 | 5.50 | |
| SD | 0.59 | 0.30 | |
| 95% CI | 2.47 to 5.01 | 4.85 to 6.15 | |
| CMRO2 (ml/100 g/min) | 0.3185 | ||
| Mean | 3.50 | 3.31 | |
| SD | 0.55 | 0.27 | |
| 95%CI | 2.32 to 4.68 | 2.73 to 3.89 | |
| OEF (%) | <0.0001 | ||
| Mean | 42.6 | 54.7 | |
| SD | 4.3 | 2.6 | |
| 95%CI | 33.5 to 51.8 | 49.2 to 60.2 | |
| RCVCRhypocap (%/mmHg) | 0.2021 | ||
| Mean | −0.14 | −0.77 | |
| SD | 0.47 | 2.24 | |
| 95%CI | −1.12 to 0.86 | −5.26 to 3.70 | |
PET: positron emission tomography; 99mTc-ECD: technetium-99 m-labeled ethyl cysteinate dimer; SPECT: single-photon emission computed tomography; MCA: middle cerebral artery; ROI: region of interest; CBF: cerebral blood flow; SD: standard deviation; CI: confidence interval; CBV: cerebral blood volume; CMRO2: cerebral metabolic rate of oxygen; OEF: oxygen extraction fraction; RCVCRhypocap: relative cerebrovascular contractile reactivity to hypocapnia.
The mean age of the 10 healthy male adults who were enrolled to obtain normal control values of RCVCRhypocap on 99mTc-ECD SPECT was 43 ± 8 years (range, 30–55 years). None of the healthy subjects or patients experienced the new development or deterioration of neurological symptoms during the hyperventilation challenge. In patients, ΔPCO2 was 8 ± 3 mmHg (range, 5–15 mmHg). Mean, SD and 95%CI of RCVCRhypocap in the MCA ROI of the precentral region in controls and patients are shown in Table 1. RCVCRhypocap did not differ between controls and patients. The MCA ROI of the precentral region in nine and five patients exhibited abnormally reduced and increased cerebrovascular contractile reactivity to hypocapnia, respectively.
Of the 19 patients analyzed, 10 (53%) were suspected as having cerebral hyperperfusion on 123I-IMP SPECT imaging one day after surgery. All these patients were finally determined as having cerebral hyperperfusion on 123I-IMP SPECT images one month after surgery. In these 10 patients, the MCA ROI perfused by an anastomosed M4 of the MCA was identical to the MCA ROI with cerebral hyperperfusion. Six (60%) of these 10 patients had cerebral hyperperfusion syndrome (Table 2). Three, two and one patients developed aphasia, seizure and hemiparesis, respectively. These symptoms developed two to nine days after surgery. Duration of propofol coma ranged from three to five days. Five patients recovered from this coma without new neurological deficits. The systolic blood pressure during propofol coma in the remaining patient (Patient 1) was kept between 110 and 130 mmHg of the systolic blood pressure, but that patient developed right hemiparesis after recovering from the coma (Figure 2). This hemiparesis remained one month after surgery, when ischemic lesions developed in the left central region on MR imaging. An arterial bypass was patent on postoperative MR angiography in all 19 patients.
Table 2.
Clinical data in patients with cerebral hyperperfusion syndrome after surgery.
| Patient no. | Age (years) | Sex | Symptoms at onset | Hyperperfusion syndrome |
||
|---|---|---|---|---|---|---|
| Symptoms | Onset (POD) | Duration of propofol coma (days) | ||||
| 1 | 52 | W | TIA | Aphasia | 3 | 5 |
| 2 | 55 | M | Stroke | Seizure | 5 | 3 |
| 3 | 32 | W | TIA | Hemiparesis | 2 | 4 |
| 4 | 47 | M | TIA | Aphasia | 9 | 4 |
| 5 | 57 | W | Stroke with TIA | Seizure | 6 | 3 |
POD: postoperative day; W: woman; M: man; TIA: transient ischemic attack.
Figure 2.
A 52-year-old woman with transient ischemic attacks of right hemiparesis and motor aphasia due to moyamoya disease. (a) Preoperative 15O gas positron emission tomography images show a decrease in cerebral blood flow (CBF) and an increase in cerebral blood volume (CBV) in the left precentral and central regions where cerebral metabolic rate of oxygen (CMRO2) and oxygen extraction fraction (OEF) are slightly decreased and markedly increased, respectively. (b) Preoperative Technetium-99 m-labeled (99mTc) ethyl cysteinate dimer (ECD) single-photon emission computed tomography (SPECT) images at rest (left) show a decrease in tracer uptake in the left precentral and central regions relative to that in the left cerebellar region. This difference in tracer uptake is decreased on 99mTc-ECD SPECT images with hyperventilation challenge (right). The left-to-right precentral and central differences in tracer uptake are also decreased on 99mTc-ECD SPECT images with hyperventilation challenge when compared with those at rest. (c) N-isopropyl-p-[123I]-iodoamphetamine (IMP) SPECT images one day after arterial bypass surgery reveal a focal increase in CBF in the left precentral region perfused by an anastomosed M4 of the left middle cerebral artery (left). Aphasia developed three days after surgery and propofol coma continued for five days. Right hemiparesis developed after recovery from this coma. This hemiparesis remained at one month after surgery when 123I-IMP SPECT images demonstrate resolution of a focal increase in CBF in the left precentral region and development of a focal decrease in CBF in the left central region (right).
Figure 3 shows the relationship between the development of cerebral hyperperfusion on postoperative 123I-IMP SPECT imaging or cerebral hyperperfusion syndrome and the preoperative RCVCRhypocap in the MCA ROI perfused by an anastomosed M4 of the MCA in patients. The RCVCRhypocap was significantly lower in patients with postoperative cerebral hyperperfusion than in those without postoperative cerebral hyperperfusion (Table 3) and in patients with cerebral hyperperfusion syndrome than in those without cerebral hyperperfusion syndrome (Table 4). Cerebral hyperperfusion showed abnormally reduced and normal cerebrovascular contractile reactivities to hypocapnia in 9 and 1 of the 10 patients, respectively; no patients with abnormally increased cerebrovascular contractile reactivity to hypocapnia experienced cerebral hyperperfusion. All six patients with cerebral hyperperfusion syndrome displayed abnormally reduced cerebrovascular contractile reactivity to hypocapnia.
Figure 3.
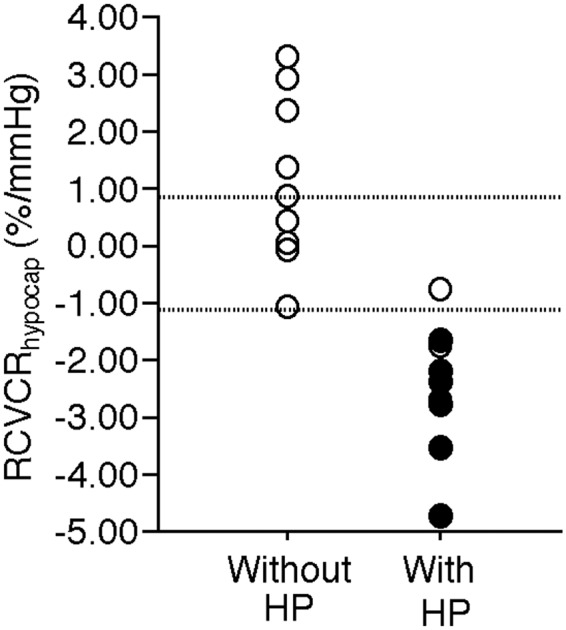
Relationship between cerebral hyperperfusion (HP) on postoperative 123I-IMP SPECT imaging or cerebral hyperperfusion syndrome and preoperative RCVCRhypocap in the MCA ROI of the precentral region perfused by an anastomosed MCA. Closed and open circles denote patients with and without cerebral hyperperfusion syndrome, respectively. Upper and lower dotted horizontal lines denote upper and lower limits of the 95% confidence interval of control values obtained from healthy subjects, respectively.
Table 3.
Factors related to development of cerebral hyperperfusion on postoperative SPECT in patients.
| Risk factor | Postoperative hyperperfusion |
Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|---|
| Yes (n = 10) | No (n = 9) | p | p | 95% CI | |
| Age (years) | 46.1 ± 8.8 | 41.9 ± 7.8 | 0.1902 | 0.7302 | 0.62 to 1.80 |
| Male sex | 2 (20%) | 2 (22%) | >0.9999 | ||
| Hypertension | 2 (20%) | 2 (22%) | >0.9999 | ||
| Diabetes mellitus | 1 (10%) | 1 (11%) | >0.9999 | ||
| Dyslipidemia | 2 (20%) | 2 (22%) | >0.9999 | ||
| TIA alone before surgery | 7 (70%) | 7 (78%) | >0.9999 | ||
| Preoperative PET-CBF (ml/100 g/min) | 33.1 ± 3.2 | 35.0 ± 2.0 | 0.2358 | ||
| Preoperative PET-CBV (ml/100 g) | 5.46 ± 0.31 | 5.55 ± 0.29 | 0.4618 | ||
| Preoperative PET-CMRO2 (ml/100 g/min) | 3.27 ± 0.35 | 3.36 ± 0.17 | 0.2701 | ||
| Preoperative PET-OEF (%) | 55.5 ± 3.2 | 53.8 ± 1.3 | 0.4140 | ||
| Preoperative RCVRhypocap (%/mmHg) | −2.50 ± 1.08 | 1.13 ± 1.47 | 0.0003 | 0.1276 | 0.07 to 4.12 |
| Duration of intraoperative temporary occlusion | 30 ± 7 | 29 ± 5 | 0.7126 | ||
Values represent number and percentage, or mean ± SD.
Table 4.
Factors related to development of postoperative cerebral hyperperfusion syndrome in patients.
| Risk factor | Hyperperfusion syndrome |
Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|---|
| Yes (n = 6) | No (n = 13) | p | p | 95% CI | |
| Age (years) | 46.0 ± 11.0 | 43.2 ± 7.3 | 0.4817 | ||
| Male sex | 2 (33%) | 2 (15%) | 0.5573 | ||
| Hypertension | 2 (33%) | 2 (15%) | 0.5573 | ||
| Diabetes mellitus | 1 (17%) | 1 (8%) | >0.9999 | ||
| Dyslipidemia | 2 (33%) | 2 (15%) | 0.5573 | ||
| Only TIA before surgery | 4 (67%) | 10 (77%) | >0.9999 | ||
| Preoperative PET-CBF (ml/100 g/min) | 33.6 ± 3.2 | 34.2 ± 2.8 | 0.7922 | ||
| Preoperative PET-CBV (ml/100 g) | 5.60 ± 0.34 | 5.46 ± 0.28 | 0.3340 | 0.1740 | 0.24 to 99.12 |
| Preoperative PET-CMRO2 (ml/100 g/min) | 3.34 ± 0.35 | 3.30 ± 0.24 | 0.5986 | ||
| Preoperative PET-OEF (%) | 55.9 ± 3.7 | 54.2 ± 1.8 | 0.4827 | ||
| Preoperative RCVRhypocap (%/mmHg) | −2.85 ± 1.10 | 0.18 ± 1.97 | 0.0050 | 0.0433 | 0.04 to 0.96 |
| Duration of intraoperative temporary occlusion | 29 ± 7 | 30 ± 6 | 0.7250 | ||
Note: Values represent number and percentage or mean ± SD.
Table 3 shows the results of univariate analyses of factors related to the development of cerebral hyperperfusion on postoperative 123I-IMP SPECT. No variables other than RCVCRhypocap in the MCA ROI perfused by an anastomosed M4 of the MCA were associated with development of postoperative cerebral hyperperfusion. After closely related variables were eliminated in univariate analyses, the following confounders (p < 0.2), age and RCVCRhypocap in the MCA ROI perfused by an anastomosed M4 of the MCA were included in the logistic regression model for multivariate analysis. No factors included in multivariate analysis were identified as independent predictors of the development of postoperative cerebral hyperperfusion.
Table 4 shows the results of univariate analyses of factors related to the development of cerebral hyperperfusion syndrome. No variables other than RCVCRhypocap in the MCA ROI perfused by an anastomosed M4 of the MCA were associated with development of cerebral hyperperfusion syndrome. Likewise, no closely related variables showed values of p < 0.2 in univariate analysis except RCVCRhypocap. To eliminate closely related variables in univariate analyses, CBV with p = 0.3340 in addition to RCVCRhypcap was included in the logistic regression model for multivariate analysis. On multivariate analysis, a low RCVCRhypocap was an independent predictor of the development of cerebral hyperperfusion syndrome.
Discussion
The present study demonstrated that preoperatively reduced cerebrovascular contractile reactivity to hypocapnia by hyperventilation is associated with development of cerebral hyperperfusion syndrome after arterial bypass surgery for adult patients with cerebral misery perfusion due to ischemic moyamoya disease.
In the brain, 99mTc-ECD reaches a maximum within 1 min after tracer injection and remains that level for the next 15 min, even when the distribution of CBF is changed several minutes after tracer injection.22 Thus, 99mTc-ECD SPECT with hyperventilation challenge in the present study displayed brain perfusion images within 1 min after the end of hyperventilation. Conversely, 99mTc-ECD SPECT enables imaging of a “snapshot” of regional CBF in this way,23 but underestimates high CBF due to the limited first-pass extraction fraction.24 We measured relative CBF reduction by hypocapnia on 99mTc-ECD SPECT and this CBF might be reduced below that at rest. Underestimation of 99mTc-ECD SPECT was therefore considered to have minimally influenced the present results. In contrast, because 123I-IMP is a good first-pass extraction fraction near 15O in the brain,24 SPECT using this tracer was performed to detect postoperative cerebral hyperperfusion.
In patients with symptomatic chronic internal carotid artery or MCA occlusive disease due to atherosclerosis, presence of misery perfusion in the affected hemisphere reportedly increases the risk of further ischemic events.25,26 Based on this finding, only patients with misery perfusion were considered as candidates for arterial bypass surgery for the present study period in our institution. As a result, all patients included into the present study had MCA ROIs with abnormally reduced CBF and abnormally elevated CBV.
The present study enrolling patients with such critical conditions of cerebral hemodynamics showed that preoperatively reduced cerebrovascular contractile reactivity to hypocapnia by hyperventilation was significantly associated with the development of cerebral hyperperfusion syndrome, supporting the hypothesis that contraction of maximally dilated resistance vessels insufficient to restore perfusion pressure may cause cerebral hyperperfusion syndrome after arterial bypass surgery. In contrast, preoperative CBV was not an independent predictor of cerebral hyperperfusion syndrome, which differed from results of a previous study.13 Whereas only half of the patients included in the previous study exhibited an abnormal increase in CBV, all our patients were determined as having an abnormal increase in CBV. Furthermore, a quarter of patients with abnormal increases in CBV showed increased cerebrovascular contractile reactivity to hypocapnia, while half showed reduced cerebrovascular contractile reactivity to hypocapnia. Only the latter may have had resistant vessels that either do not contract or contract insufficiently to restore perfusion pressure, resulting in cerebral hyperperfusion syndrome. An abnormal increase in CBV may thus be a necessary but not sufficient condition for the development of cerebral hyperperfusion syndrome. This may be one reason for the discrepancies between the previous and present results.
In children with ischemic moyamoya diseases, marked reduction of CBF was reportedly observed on the ischemic region during re-build-up phenomenon on electroencephalography after hyperventilation,17,27 suggesting relatively increased cerebrovascular contractile reactivity to hypocapnia in that region. These findings differed from our data of relatively decreased cerebrovascular contractile reactivity to hypocapnia in the ischemic region in adult patients with ischemic moyamoya diseases. This difference may explain why the incidence of cerebral hyperperfusion syndrome is considerably greater in adult patients than in pediatric patients.10,13
Several investigators have demonstrated that postoperative prophylactic blood pressure control (systolic blood pressure, 110–130 mmHg) combined with intra- and postoperative administration of minocycline hydrochloride, a neuro-protective antibiotic, blocking the deleterious inflammatory cascade by the activation of matrix metalloproteinase-9, significantly reduce the incidence of cerebral hyperperfusion syndrome.3,28 On the other hand, routine use of blood pressure control for moyamoya disease may induce development of ischemia in the regions that are not perfused via an anastomosed STA like our one patient. Thus, such perioperative preventative treatments should be applied for only patients who are determined as having reduced cerebrovascular contractile reactivity to hypocapnia by hyperventilation.
The present study has several limitations that require discussion. First, we used cerebrovascular contractile reactivity to hypocapnia by hyperventilation as an indicator of the vasoconstrictive capacity of dilated resistance vessels to restore perfusion pressure. Dilatation and constriction of the small arterioles or intraparenchymal vessels contribute to CBF change with PCO2 change,14 and these vessels differ from the resistance vessels that play a main role in cerebrovascular autoregulation: the former is peripheral to the latter. Cerebrovascular contractile reactivity to hypocapnia by hyperventilation is thus not strictly an indicator of the vasoconstrictive capacity of resistance vessels. However, Tajima et al. recently reported a significant negative correlation between autoregulatory vasodilation and diameter response to hypercapnia in mouse arterioles, indicating that arterioles play key roles in both autoregulatory and hypercapnic vasodilation.29 These findings support our data, suggesting that the reactivity to hypocapnia in the small arterioles or intraparenchymal vessels may correlate with the vasoconstrictive capacity of resistance vessels to increases in perfusion pressure under special hemodynamic conditions as in the patients in the present study. Second, among adult patients with histories of ischemic symptoms due to moyamoya disease, only patients with misery perfusion were considered as candidates for arterial bypass surgery for the present study period in our institution. This surgical indication is based on the finding regarding ICA or MCA occlusive diseases due to atherosclerosis.25,26 Whether the finding is correctly applied for adult patients with a history of ischemic symptoms due to moyamoya disease remained unclear and the validity of this determination should be validated. We will analyze long-term outcomes of adult patients without misery perfusion despite the presence of histories of ischemic symptoms due to moyamoya disease who did not undergo arterial bypass surgery. Lastly, as described above, because only patients with misery perfusion were included into the present study, the sample size was small.
In conclusion, the present study demonstrated that preoperatively reduced cerebrovascular contractile reactivity to hypocapnia by hyperventilation is associated with development of cerebral hyperperfusion syndrome after arterial bypass surgery for adult patients with cerebral misery perfusion due to ischemic moyamoya disease.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was partly funded by a Grant-in-Aid for Strategic Medical Science Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (S1491001) and Scientific Research from the Japan Society for the Promotion of Science (JP15K10313).
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Kuniaki Ogasawara has received research grants from consigned research funds from Nihon Medi-Physics Co., Ltd and Bristol-Myers Squibb. All other authors declare that they have no conflict of interest.
Authors’ contributions
All authors approved the version of the paper to be published. Shinpei Sato has contributed to the conception, design and implementation of the study. He analyzed and interpreted the data and drafted the article. Daigo Kojima has contributed to the analysis of the data and revised the manuscript critically for important intellectual content. Yasuyoshi Shimada has contributed to the analysis of the data and revised the manuscript critically for important intellectual content. Jun Yoshida has contributed to the analysis of the data and revised the manuscript critically for important intellectual content. Kentaro Fujimato has contributed to the analysis of the data and revised the manuscript critically for important intellectual content. Shunrou Fujiwara has contributed to the design, implementation of the study and revised the manuscript critically for important intellectual content. Masakazu Kobayashi has contributed to the design, implementation of the study and revised the manuscript critically for important intellectual content. Yoshitaka Kubo has contributed to the design, implementation of the study and revised the manuscript critically for important intellectual content. Kenji Yoshida has contributed to the design, implementation of the study and revised the manuscript critically for important intellectual content. Kazunori Terasaki has contributed to the design, implementation of the study and revised the manuscript critically for important intellectual content. Shouta Tsutsui has contributed to the analysis of the data and revised the manuscript critically for important intellectual content. Kenya Miyoshi has contributed to the analysis of the data and revised the manuscript critically for important intellectual content. Kuniaki Ogasawara has contributed to the conception, design and implementation of the study, and interpretation of the data. He has also revised the manuscript critically for important intellectual content.
References
- 1.Suzuki J, Takaku A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol 1969; 20: 288–299. [DOI] [PubMed] [Google Scholar]
- 2.Research Committee on the Pathology and treatment of Spontaneous Occlusion of the Circle of Willis; Health Labour Sciences Research Grant for Research on Measures for Intractable Diseases. Guidelines for diagnosis and treatment of moyamoya disease (Spontaneous occlusion of the circle of Willis). Neurol Med Chir 2012; 52: 245–266. [DOI] [PubMed] [Google Scholar]
- 3.Fujimura M, Tominaga T. Current status of revascularization surgery for moyamoya disease: special consideration for its ‘internal carotid-external carotid (IC-EC) conversion’ as the physiological reorganization system. Tohoku J Exp Med 2015; 236: 45–53. [DOI] [PubMed] [Google Scholar]
- 4.Fukui M. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis (‘moyamoya’ disease). Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) of the Ministry of Health and Welfare, Japan. Clin Neurol Neurosurg 1997; 99: S238–S240. [PubMed] [Google Scholar]
- 5.Houkin K, Ishikawa T, Yoshimoto T, et al. Direct and indirect revascularization for moyamoya disease surgical techniques and peri-operative complications. Clin Neurol Neurosurg 1997; 99: S142–S145. [DOI] [PubMed] [Google Scholar]
- 6.Miyamoto S, Yoshimoto T, Hashimoto N, et al. Effects of extracranial-intracranial bypass for patients with hemorrhagic moyamoya disease: results of the Japan Adult Moyamoya Trial. Stroke 2014; 45: 1415–1421. [DOI] [PubMed] [Google Scholar]
- 7.Fujimura M, Kaneta T, Mugikura S, et al. Temporary neurologic deterioration due to cerebral hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in patients with adult-onset moyamoya disease. Surg Neurol 2007; 67: 273–282. [DOI] [PubMed] [Google Scholar]
- 8.Kim JE, Oh CW, Kwon OK, et al. Transient hyperperfusion after superficialtemporal artery/middle cerebral artery bypass surgery as a possible cause of postoperative transient neurological deterioration. Cerebrovasc Dis 2008; 25: 580–586. [DOI] [PubMed] [Google Scholar]
- 9.Ohue S, Kumon Y, Kohno K, et al. Postoperative temporary neurological deficits in adults with moyamoya disease. Surg Neurol 2008; 69: 281–286. [DOI] [PubMed] [Google Scholar]
- 10.Fujimura M, Mugikura S, Kaneta T, et al. Incidence and risk factors for symptomatic cerebral hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in patients with moyamoya disease. Surg Neurol 2009; 71: 442–447. [DOI] [PubMed] [Google Scholar]
- 11.Fujimura M, Shimizu H, Inoue T, et al. Significance of focal cerebral hyperperfusion as a cause of transient neurologic deterioration after EC-IC bypass for moyamoya disease: comparative study with non-moyamoya patients using 123I-IMP SPECT. Neurosurgery 2011; 68: 957–965. [DOI] [PubMed] [Google Scholar]
- 12.Fujimura M, Shimizu H, Mugikura S, et al. Delayed intracerebral hemorrhage after superficial temporal artery-middle cerebral artery anastomosis in a patient with moyamoya disease: possible involvement of cerebral hyperperfusion and increased vascular permeability. Surg Neurol 2009; 71: 223–227. [DOI] [PubMed] [Google Scholar]
- 13.Uchino H, Kuroda S, Hirata K, et al. Predictors and clinical features of postoperative hyperperfusion after surgical revascularization for moyamoya disease: a serial single photon emission CT/positron emission tomography study. Stroke 2012; 43: 2610–2616. [DOI] [PubMed] [Google Scholar]
- 14.Kobari M, Gotoh F, Fukuuchi Y, et al. Quantitative measurement of blood flow velocity in feline pial arteries during hemorrhagic hypotension and hypercapnia. Stroke 1987; 18: 457–463. [DOI] [PubMed] [Google Scholar]
- 15.Nariai T, Senda M, Ishii K, et al. Posthyperventilatory steal response in chronic cerebral hemodynamic stress: a positron emission tomography study. Stroke 1998; 29: 1281–1292. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto Y, Oikawa K, Nomura J, et al. Optimal brain 99mTc-ECD SPECT imaging and analysis to detect misery perfusion on 15O PET imaging in patients with chronic occlusive disease of unilateral major cerebral artery. Clin Nucl Med 2017; 42: 499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matheja P, Weckesser M, Debus O, et al. Moyamoya syndrome: impaired hemodynamics on ECD SPECT after EEG controlled hyperventilation. Nuklearmedizin 2002; 41: 42–46. [PubMed] [Google Scholar]
- 18.Frackowiak RS, Lenzi GL, Jones T, et al. Quantitative measurement of regional cerebral blood flow and oxygen metabolism in man using 15O and positron emission tomography: theory, procedure, and normal values. J Comput Assist Tomogr 1980; 4: 727–736. [DOI] [PubMed] [Google Scholar]
- 19.Lammertsma AA, Jones T. Correction for the presence of intravascular oxygen-15 in the steady-state technique for measuring regional oxygen extraction ratio in the brain: 1. Description of the method. J Cereb Blood Flow Metab 1983; 3: 416–424. [DOI] [PubMed] [Google Scholar]
- 20.Nishimiya M, Matsuda H, Imabayashi E, et al. Comparison of SPM and NEUROSTAT in voxelwise statistical analysis of brain SPECT and MRI at the early stage of Alzheimer’s disease. Ann Nucl Med 2008; 22: 921–927. [DOI] [PubMed] [Google Scholar]
- 21.Takeuchi R, Matsuda H, Yoshioka K, et al. Cerebral blood flow SPET in transient global amnesia with automated ROI analysis by 3DSRT. Eur J Nucl Med Mol Imaging 2004; 31: 578–589. [DOI] [PubMed] [Google Scholar]
- 22.Vallabhajosula S, Zimmerman RE, Picard M, et al. Technetium-99m ECD: a new brain imaging agent: in vivo kinetics and biodistribution studies in normal human subjects. J Nucl Med 1989; 30: 599–604. [PubMed] [Google Scholar]
- 23.Lewis DH. Functional brain imaging with cerebral perfusion SPECT in cerebrovascular disease, epilepsy, and trauma. Neurosurg Clin N Am 1997; 8: 337–344. [PubMed] [Google Scholar]
- 24.Iida H, Akutsu T, Endo K, et al. A multicenter validation of regional cerebral blood flow quantitation using [123I] iodoamphetamine and single photon emission computed tomography. J Cereb Blood Flow Metab 1996; 16: 781–793. [DOI] [PubMed] [Google Scholar]
- 25.Yamauchi H, Higashi T, Kagawa S, et al. Is misery perfusion still a predictor of stroke in symptomatic major cerebral artery disease? Brain 2012; 135: 2515–2526. [DOI] [PubMed] [Google Scholar]
- 26.Grubb RL, Jr, Derdeyn CP, Fritsch SM, et al. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA 1998; 280: 1055–1060. [DOI] [PubMed] [Google Scholar]
- 27.Kazumata K, Kuroda S, Houkin K, et al. Regional cerebral hemodynamics during re-build-up phenomenon in childhood moyamoya disease. An analysis using 99mTc-HMPAO SPECT. Childs Nerv Syst 1996; 12: 161–165. [DOI] [PubMed] [Google Scholar]
- 28.Fujimura M, Niizuma K, Inoue T, et al. Minocycline prevents focal neurological deterioration due to cerebral hyperperfusion after extracranial-intracranial bypass for moyamoya disease. Neurosurgery 2014; 74: 163–170. [DOI] [PubMed] [Google Scholar]
- 29.Tajima Y, Takuwa H, Kokuryo D, et al. Changes in cortical microvasculature during misery perfusion measured by two-photon laser scanning microscopy. J Cereb Blood Flow Metab 2014; 34: 1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]