Abstract
Age-related white matter hyperintensities (WMH) are a manifestation of white matter damage seen on magnetic resonance imaging (MRI). They are related to vascular risk factors and cognitive impairment. This study investigated the cognitive profile at different stages of WMH in a large community-dwelling sample; 849 subjects aged 21 to 79 years were classified on the 4-stage Fazekas scale according to hyperintense lesions seen on individual T2-weighted fluid-attenuated inversion recovery MRI scans. The evaluation of cognitive functioning included seven domains of cognitive performance and five domains of subjective impairment, as proposed by the DSM-5. For the first time, the impact of age-related WMH on Theory of Mind was investigated. Differences between Fazekas groups were analyzed non-parametrically and effect sizes were computed. Effect sizes revealed a slight overall cognitive decline in Fazekas groups 1 and 2 relative to healthy subjects. Fazekas group 3 presented substantial decline in social cognition, attention and memory, although characterized by a high inter-individual variability. WMH groups reported subjective cognitive decline. We demonstrate that extensive WMH are associated with specific impairment in attention, memory, social cognition, and subjective cognitive performance. The detailed neuropsychological characterization of WMH offers new therapeutic possibilities for those affected by vascular cognitive decline.
Keywords: Cognition, magnetic resonance imaging, small vessel disease, white matter disease, vascular cognitive impairment
Introduction
Aging-related structural and functional brain changes are crucial determinants of individual health, behavior, and cognition in later life.1–3 Besides grey matter atrophy, white matter changes are a frequent finding among the elderly that can affect cognitive functioning. White matter hyperintensities (WMH) are the main manifestation of white matter changes. They are investigated with T2-weighted fluid-attenuated inversion recovery (FLAIR) magnetic resonance imaging (MRI). Although the etiology is not entirely known, small vessel disease is considered the main mechanism behind age-related WMH.4 It reduces myelination and leads to gliosis and axonal damage due to thickened vessel walls and narrowing of the lumen.5 Vascular risk factors such as hypertension, diabetes mellitus, cholesterol, smoking, and increased homocysteine levels are crucial for WMH development.4,6–8 The prevalence of WMH increases exponentially with age.9 In the general population, 11–21% of adults in their mid-60s,10 and more than 90% of the healthy elderly older than 80 years are affected.10–13 WMH vary highly in their degree and location across individuals, ranging from small punctate lesions to extensive damage in the deep or periventricular white matter.5
Although WMH are associated with an increased risk of stroke, cognitive dysfunction, incident dementia, and death,10 ‘an up-to-date, domain-specific quantification of the effect of WMHs on cognition in the normal population is unavailable’ (see Kloppenborg et al.,12 p. 2127). This may be due to a variety of WMH classification scales and the diversity of applied neuropsychological measures. Yet, the cognitive profile of age-related WMH may be characterized by global functional decline on the one hand10 or domain-specific impairment on the other. The latter has previously been described as frontal lobe syndrome with a predominant impairment in processing speed and executive functioning,1,5,6,10,12,14–17 while memory was not consistently affected.5,10,12,15,18,19 In their meta-analysis, Kloppenborg et al.12 showed that the WMH-associated memory decline was small but robust. Although processing speed and executive functions are predominantly affected as disease progresses,12 WMH seem to cause a more complex deficit-pattern.
Recently, the Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM-520) re-defined the dementia concept by introducing neurocognitive disorder (NCD) as new term for acquired cognitive dysfunction. The NCD diagnostic procedure requires the domain-specific evaluation of cognitive performance based on the integration of multiple neuropsychological measures. Thus, the DSM-5 shifts the focus from the (Alzheimer-centristic) assessment of memory (dys-)function towards an integrative approach additionally covering complex attention, executive functions, language, visuospatial skills, and social cognition. Following these guidelines, language and visuospatial skills have been omitted in the context of WMH. Moreover, social cognition has been rarely investigated in this context, although it is essential for social interactions,21 and several mental conditions and neurodegenerative diseases present social cognitive deficits.22–25 One major component of social cognition is ‘Theory of Mind’ (ToM). ToM is described as the ability to identify mental states of oneself and others.26 This information can be used to predict another person's future behavior, and to react and interact adequately. The consequences of white matter changes have been explored specifically in individuals with ToM deficits, e.g. autism27 or schizophrenia,28–30 but also in normal adults31,32 using different brain connectivity measures derived with MRI. Studies analyzing white matter lesion volume in the context of ToM are scarce and no relation was found in an elderly cohort,32 or in a cohort of multiple sclerosis patients.33 In the current study, ToM was assessed with the ‘Reading the Mind in the Eyes’ Test (RMET).34,35 This test is frequently applied in research and clinical practice and it has been shown to reliably detect ToM deficits (e.g. in autism34). The RMET captures the ability to attribute mental states from eye gaze. Previous studies consistently reported a decline in RMET performance with aging,36–38 but it has never been related to white matter damage before. Thus, this is the first study investigating RMET performance in the context of age-related WMH.
The DSM-5 diagnostic criteria further include the assessment of subjective cognitive complaints, as they mark subsequent cognitive deficits and conversion to dementia.39 Subjective cognitive failures are frequent among individuals with WMH.40 They are predictive of global cognitive deficits within 12 months41 and increase the risk of conversion to dementia.42 However, most studies assessed only global markers of cognition,41–43 or focused on single domains (e.g. memory complaints) for subjective impairment rating.40,44
In this study, we apply the DSM-5 criteria for the diagnosis of NCD to investigate domain-specific cognitive performance and subjective complaints associated with different stages of WMH. Besides attention, executive function and memory, the previously neglected domains language and visuoconstruction are investigated. Moreover, a special focus lies on social cognitive abilities that have been rarely explored in the context of WMH.
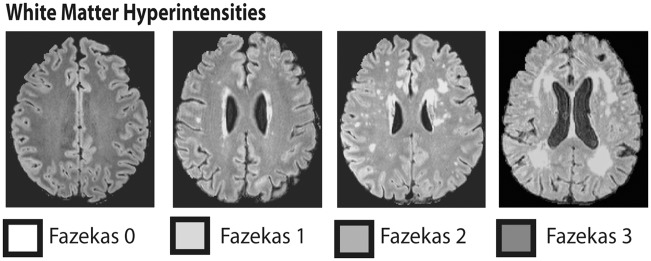
WMHs were assessed on the well-known Fazekas scale in FLAIR images (Figure 1) as this reliable scale is broadly applied in clinical routine in contrast to new quantitative algorithms. A very large population-based cohort was investigated, including 849 persons with a broad age range from 21 to 79 years. Based on previous findings, we expected attention, executive and memory performance to be negatively associated with WMH burden. Also, subjective complaints were assumed to be more frequent in individuals with extensive WMH.
Figure 1.
Axial FLAIR images showing the classification of white matter hyperintensities on the four stage Fazekas scale: the healthy brain (Fazekas 0) contrasted with punctiform (Fazekas 1), early confluent (Fazekas 2) and diffuse confluent (Fazekas 3) white matter hyperintensities.
Materials and methods
Participants
The current study is part of the population-based LIFE-adult study at the University of Leipzig, Germany.45 The total sample comprised 10,000 randomly selected residents of the city of Leipzig, of whom 2600 completed structural and functional brain MRI. Adults aged 40 to 79 years completed in-depth-testing, including neuropsychological assessment, medical examinations, and interviews on individual lifestyle conditions. Persons younger than 60 years completed a less extensive test battery. For the current study, individuals with the following characteristics were excluded: (a) incidental findings on brain MRI (vascular malformations, aneurysm, tumor, congenital lesions), (b) history of neurological diseases (e.g. epilepsy, Parkinson's disease, multiple sclerosis), (c) history of non-silent strokes, (d) cardio-vascular events (cardiac infarction, coronary heart disease, cardiac insufficiency), (e) intake of medication active on the central nervous system (opioids, hypnotics and sedatives, anti-parkinsonian drugs, anxiolytics, antipsychotics, anti-epileptic drugs), or (f) actual cancer therapy. In total, 849 persons (age: M = 60, SD = 13.1, range 21–79; 54% male) with full WMH rating on the Fazekas scale46 were analyzed. Written, informed consent was obtained from each subject. The research protocol was approved by the ethics committee of the medical faculty of the University of Leipzig (approval number 263-2009-14122009), and was in accordance with the latest version of the Declaration of Helsinki.
Quantification of white matter hyperintensities
T2-weighted FLAIR MRI scans (3 Tesla, repetition time 5000 ms, inversion time 1800 ms, echo time 395 ms, 1 mm isotropic resolution, acquisition time 7.02 min) were used for the classification of cerebral white matter lesions. The amount of WMH was quantified on the four-stage Fazekas scale.46 Three experienced neuroradiologists, blinded to the individual diagnosis, rated the T2 FLAIR MRI scans of all individuals. Raters were trained for rating WMH with this scale in clinical routine. MRI scans were randomly assigned to the three raters. The Fazekas scale ranges from zero to three and the value reflects the overall burden of cerebral WMH. Figure 1 illustrates the stages on this scale with MRI examples. Here, we concentrate on the conservative visual rating of WMH using the Fazekas scale, as this is the most frequently applied method in everyday clinical practice. The application is quick and easy, and the amount of WMH can be reliably quantified.5,12,47–49
Neuropsychological assessment
Cognitive performance was assessed with a set of standard neuropsychological tests. The detailed assessment procedure is described elsewhere.45 In the current study, the results of the Stroop Test, the Reading the Mind in the Eyes Test (RMET) and the extended version of the Consortium to Establish a Registry of Alzheimer's Disease (CERADplus) test battery were analyzed. Data were carefully checked for missing values and plausibility.
We applied the cognitive domains and neuropsychological measures proposed by the DSM-520 for the investigation of cognitive performance and subjective cognitive complaints. In particular, neuropsychological measures representing the DSM-5 domains attention (trail making test [TMT] part A: time to complete, Stroop neutral: reaction time [RT]), executive function (TMT time to complete part B/part A, Stroop RT incongruent/RT neutral), memory (CERADplus wordlist sum trial 1-3, wordlist delayed recall, wordlist recognition, figure delayed recall), language (Boston Naming Test), visuoconstructive abilities (CERADplus figure copy), and social cognition (RMET) were combined. Recent publications support the robust assignment of neuropsychological measures to cognitive domains.50,51 We expanded the DSM-5 dimensional approach by verbal fluency (phonematic fluency, semantic fluency) because verbal fluency tasks require more abilities than solely language.52,53 Furthermore, it is supposed to be an independent marker of cognitive decline.54
Subtest results were translated into scores informative of cognitive performance. Thus, individual test scores were z-standardized (M = 0, SD = 1) to the mean of the corresponding age group (<65, 65–69, 70–74, 75 + years; Table S2). Cognitive tests were age-standardized within the study sample, as standard scores are not published for all measures, i.e. the RMET and the ‘Stroop Test’. Furthermore, the CERADplus neuropsychological battery is not standardized for individuals younger than 49 years (‘basic tests’) and 55 years (‘plus tests’), and, importantly, our study sample is larger than the official CERAD standardization sample for the ‘plus tests’. Correlation analyses revealed no relation between age and performance in cognitive subtests within the age group younger than 65 years for most tests, justifying this broadly defined age group. Only the time to complete TMT A (r = .394, p < 0.001) and Stroop test neutral (r = .441, p < 0.001) were related to age. Overall, standardization within our sample not only grants the homogeneity of the measures’ analyses, but also relies on large sample sizes appropriate for standardization.
Standard scores pertaining to identical cognitive domains were then averaged to seven composite scores reflecting domain-specific cognitive performance (see van Boxtel et al.53). Composite scores were only computed if all measures of the respective domain were available. Scores of attention, executive function and verbal fluency were computed for 99% of the sample. Due to the design of the LIFE-adult study, focusing on in-depth phenotyping of the cohort aged 60 years and older, performance scores for memory, social cognition, language, and visuoconstruction were available for fewer participants with Fazekas 0. Yet, the number of Fazekas 0 individuals exceeds 100 subjects per domain. Table S1 provides detailed information about the number of individuals per Fazekas group that have been included in the analyses.
The inter-dependency between cognitive tasks has been widely discussed. The contribution of executive functions on ToM was of special interest.31,55–57 We investigated the inter-dependencies between cognitive scores with partial correlations in the whole sample (Table S3), corrected for the effects of age, sex, education, and Fazekas score. No significant relation between social cognition and executive function was found, being in line with other studies.31 However, the used ToM task depends highly on verbal skills, as reflected in the significant association with language, verbal fluency and verbal memory in our study. Also, attention as a basic cognitive ability is related to ToM performance. Although this inter-relatedness of cognitive domain scores might generally be considered by including them as covariates in the statistical analyses, the application of non-parametric statistics makes the inclusion of covariates difficult and we abstained from a statistical adjustment of other cognitive abilities.
Subjective cognitive complaints were assessed with the 20-item Dysexecutive questionnaire (DEX) of the behavioral assessment of the dysexecutive syndrome58 battery. Each item captures the frequency (range: 1 = never; 5 = very often) of a certain event. As the factorial structure of the DEX has been controversially discussed, and there is no straightforward evidence for a universal factorial structure,59 items were categorized to five domains based on their content: attention (items 10, 18), executive function (items 2, 4, 5, 7, 8, 12, 14, 15, 16, 19), memory (items 3, 6), language (item 1), and social cognition (items 9, 11, 13, 17, 20) to capture domain-specific subjective impairment. The values of all items assigned to a domain were summed and subsequently age-standardized (M = 0, SD = 1) to the mean of the corresponding age group (<65, 65–69, 70–74, 75 + years; Table S2). Individual domain scores were calculated for all individuals with full DEX information. Table S1 provides information about the number of individuals per Fazekas group.
Statistical analyses
Firstly, we tested potential differences between the four WMH groups on age, education and sex. Age-differences between groups were tested with a univariate ANOVA and differences between each pair of Fazekas groups were further tested with separate independent sample Student's t-tests. To control for multiple comparisons, the alpha level was Bonferroni-adjusted to p < 0.008 (six tests conducted). In case of non-equal variances (as tested with Levene's test), adjusted degrees of freedom are reported. Separate Chi-square statistics tested differences in ratios of sex, education, diabetes and hypercholesterolemia according to medical history, and arterial hypertension (as defined by the diagnostic guidelines of the European Society of Hypertension and of the European Society of Cardiology60) between Fazekas groups.
Differences between the four Fazekas groups in domain-specific measures of (1) objective cognitive performance (seven scores) and (2) subjective cognitive complaints (five scores) were investigated with the nonparametric rank-sum test with data-alignment (p < 0.05; see Bortz et al.,61 p. 239). Nonparametric statistics were applied due to (a) differing numbers of individuals between Fazekas groups, and (b) distribution characteristics of the outcome variables did not fulfill the requirements for parametric testing. Since the individual subtest scores and cognitive domain scores have been adjusted for the effects of age in a previous step, data were aligned for the effects of sex and education and their interaction with the Fazekas score, and corrected for identical ranks (see Bortz et al.,61 p. 231 and Schaich and Hamerle63). Significant differences were further examined using the single comparison algorithm proposed by Schaich and Hamerle (see Bortz et al.,61 p. 233 and Durlak64). For each of the seven cognitive performance scores, and each of the five subjective complaints scores, six post hoc comparisons were conducted to test differences between each pair of Fazekas groups. To control for multiple comparisons, the Bonferroni corrected significance level was, accordingly, set to p < 0.008 (p = 0.005/6).
Effect size measures inform about the magnitude and direction of the difference between two groups or variables, and thus provide valuable information about the relevance of an effect beyond the p-value of statistical tests.64 Here, we computed Hedges g* and respective 95%-confidence intervals for each cognitive score as a standardized mean difference between healthy individuals (Fazekas 0) and individuals at different Fazekas stages (see Durlak,64 p. 927 (1)). Fazekas group 0 was defined as reference group, while any other Fazekas group served as ‘experimental’ group. A negative effect size indicates worse performance for these groups compared to Fazekas 0. Hedges g* is the measure of choice for effect size analysis of samples with different numbers of individuals. As standardized measure, effect sizes can be directly compared in magnitude and direction. Statistical analyses were performed using IBM SPSS Statistics for Windows.65 All graphs were created using Microsoft Excel66 and Adobe Illustrator CS5.67
Results
Epidemiological and clinical characteristics
Table 1 illustrates the epidemiological and clinical characteristics of the cohort; 43.3% of the sample were free of age-related WMH as rated in FLAIR MRI scans (Fazekas 0), whereas WMH were detected in the larger proportion of the sample. In 43.8% only focal lesions (Fazekas 1) were found, while 11% displayed early confluent WMH (Fazkeas 2); 1.9% of the sample was diagnosed with severe and widespread changes in white matter (Fazekas 3).
Table 1.
Demographic and clinical characteristics of the four groups as defined by amount of white matter hyperintensities.
| Fazekas score |
|||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | Group comparisons | |
| N (%) | 368 (43.3) | 372 (43.8) | 93 (11) | 16 (1.9) | |
| Mean age (SD) | 53.1 (13.2) | 64.2 (10.5) | 69.4 (7.9) | 71 (7.7) | F(3845) = 87.326*** |
| Sex (% male) | 59.8 | 50 | 51.6 | 31.3 | χ2(3849) = 10.898* |
| Education (% ≥ 10 years) | 87.5 | 69.8 | 58.1 | 43.8 | χ2(3848) = 60.652*** |
| Diabetes# (%) | 6.9 | 13.3 | 15.7 | 20 | χ2(3833) = 11.16** |
| Arterial hypertension## (%) | 47.3 | 59.8 | 84.4 | 86.6 | χ2(3827) = 47.87*** |
| Hypercholesterolemia# (%) | 30.7 | 35.2 | 39.7 | 37.5 | χ2(3807) = 3.265 |
***p < 0.001, **p < 0.01, *p < 0.05 as tested with univariate ANOVA or Chi-square statistics.
#According to medical history, ## defined by the diagnosis guidelines of European Society of Hypertension and of the European Society of Cardiology.61
As expected, age was strongly associated with WMH burden. Healthy individuals (Fazekas 0) were significantly younger than persons with WMH (Fazekas 1: t(699.697) = −12.738, p < 0.001; Fazekas 2: t(236.591) = 15.255, p < 0.001; Fazekas 3: t(19.016) = −8.746, p < 0.001). Fazekas group 1 was significantly younger than Fazekas group 2 (t(181.803) = −5.249, p < 0.008; Student's t-tests, respectively). Furthermore, sex, education, arterial hypertension and diabetes were significantly associated with Fazekas scores (see Table 1), which was not the case for hypercholesterolemia (Chi-square tests). Note that data were adjusted for the effects of age, sex and education in the following analyses to control for a potential bias.
Objective cognitive performance
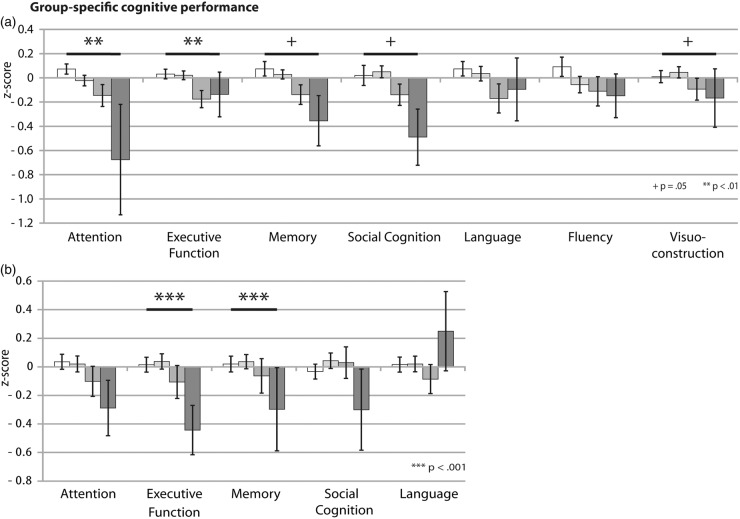
Performance in cognitive tests and results of the statistical analyses and post hoc comparisons are illustrated in Figure 2 and Table 2. Fazekas groups significantly differed in attention (χ2(3846) = 13.773, p < 0.01) and executive functions (χ2(3846) = 12.838, p < 0.01), independent of age, sex and education. Analyses yielded marginally significant group differences for memory performance (χ2(3438) = 7.74, p = 0.05), social cognition (χ2(3679) = 7.78, p = 0.05) and visuoconstructive abilities (χ2(3434) = 7.739, p = 0.05). On these cognitive domains, performance significantly declines with larger WMH burden (Fazkas stage 2 and 3) as shown with post hoc tests, while small, punctate lesions (Fazekas 1) were generally not associated with a deterioration of cognitive performance (Figure 2(b)). For attention, post hoc analyses yielded significant differences between Fazekas 0 and 2 (χ2(1460) = 93.61, p = 0.001) and Fazekas 2 and 3 (χ2(1109) = 54.66, p = 0.001). Persons without WMH and individuals classified as Fazekas 1 outperformed persons with moderate WMH (Fazekas 2) in measures of executive function (χ2(1460) = 62.73, p = 0.001). Memory performance was significantly worse for individuals with Fazekas grade 2 (χ2(1184) = 45.8, p = 0.001) and 3 (χ2(1118) = 46.92, p = 0.001) compared to the healthy subgroup. Persons with progressed WMH had significantly worse visuoconstructive abilities compared to lesion-free (Fazekas 2: χ2(1183) = 39.76, p = 0.001; Fazekas 3: χ2(1118) = 128.06, p = 0.001) or individuals with Fazekas grade 1 (Fazekas 3: χ2(1251) = 115.92, p = 0.001). Social cognitive abilities were significantly worse for Fazekas group 3 compared to Fazekas groups 1 (χ2(1317) = 102, p = 0.001) and 2 (χ2(1,83) = 68.17, p = 0.001).
Figure 2.
Effects of WMH on (a) seven domains of objective cognitive performance and (b) five domains of subjective cognitive complaints covering the whole cognitive spectrum. The graphs depict domain-specific means and standard mean errors of the age-standardized cognitive scores for each of the four Fazekas groups. P-values indicate the main effect of Fazekas score on the respective cognitive domain. The significant main effect of the factor group is marked as follows: ***p < 0.001, **p < 0.01, + p = 0.05. The color code for each Fazekas group is displayed in Figure 1.
Table 2.
Results of the global tests for the different Fazekas groups for all measures of objective performance and subjective complaints.
| Fazekas group [M (SD)] |
Significant post hoc comparisons## | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||
| Objective performance# | |||||
| Attention** | 0.753 (0.804) | −0.023 (0.832) | −0.146 (0.873) | −0.675 (1.82) | 0 > 2, 0 > 3 |
| Executive function** | 0.316 (0.755) | 0.021 (0.692) | −0.175 (0.683) | −0.137 (0.715) | 0 > 2, 1 > 2 |
| Memory+ | 0.074 (0.619) | 0.028 (0.586) | −0.138 (0.72) | −0.355 (0.748) | 0 > 2, 0 > 3 |
| Language | 0.074 (0.803) | 0.035 (1.047) | −0.169 (1.045) | −0.095 (0.651) | |
| Verbal fluency | 0.097 (0.619) | −0.056 (0.896) | −0.111 (0.873) | −0.147 (0.964) | |
| Visuoconstruction+ | 0.009 (0.993) | 0.045 (0.952) | −0.094 (1.14) | 0.167 (0.864) | 0 > 2, 0 > 3 1 > 2, 1 > 3 |
| Social cognition+ | 0 (1.01) | 0.05 (0.99) | −0.14 (0.96) | −0.49 (1) | |
| Subjective complaints# | |||||
| Attention | 0.035 (0.977) | −0.005 (1.03) | −0.101 (0.972) | −0.289 (0.726) | |
| Executive function*** | 0.015 (0.985) | 0.037 (1) | −0.106 (1.063) | −0.443 (0.646) | 0 > 3, 1 > 3, 2 > 3 |
| Memory*** | −0.005 (1.037) | 0.036 (0.927) | −0.063 (1.112) | −0.296 (1.081) | 2 > 3 |
| Language | 0.015 (0.992) | −0.005 (1.022) | −0.085 (0.927) | 0.249 (1.038) | |
| Social cognition | −0.033 (0.976) | 0.043 (1.01) | 0.029 (1.01) | −0.299 (1.061) | |
M mean; SD standard deviation.
***p < 0.001, **p < 0.01, + p = 0.05, Statistical analysis with # nonparametric rank-sum test with alignment for the effects of sex and education and ##post hoc algorithm by Schaich and Hamerle.62 Post hoc comparisons were carried out if analyses indicated a significant main effect of Fazekas group. All depicted post hoc comparisons are significant at p < 0.001.
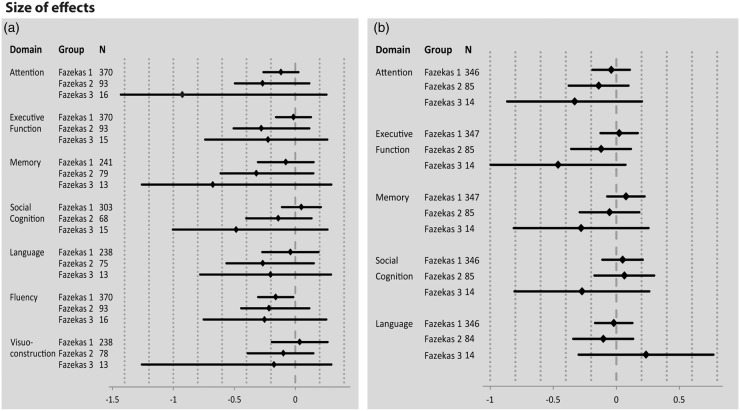
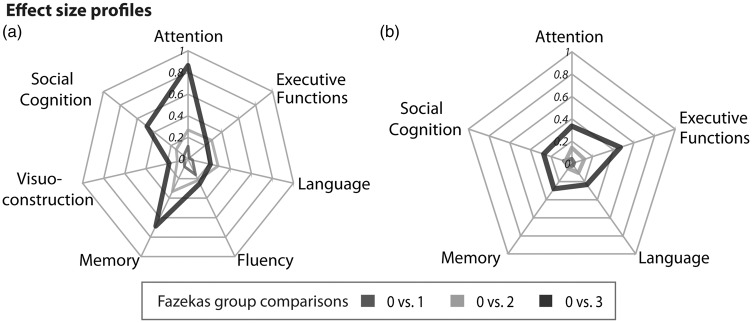
The results of the effect size analyses are displayed in Figures 3 and 4. While Figure 3 gives an extensive formal overview about the domain-specific effect sizes and respective 95% confidence intervals for each group, Figure 4 illustrates the cognitive profile at distinct stages of white matter damage. Effect sizes can be classified as small (≤|0.4|), medium (|0.4|–|0.8|), and large (≥|0.8|). Figures 3 and 4 illustrate that differences in cognitive outcomes become more substantial with larger WMH burden. The cognitive profile of persons with low lesion WMH burden (Fazekas 1) differs only slightly from healthy individuals (Figure 4(a)). Negative Hedges g* values indicate a performance decrease. The effect can be interpreted as small, ranging from −0.15 (fluency) to 0.03 (social cognition, visuoconstruction). At the stage of moderate WMH (Fazekas 2), performance on all cognitive domains slightly declines compared to healthy individuals. Effect sizes are more negative compared to Fazekas 1, but still in a small to medium range (range −0.32 to −0.1). For Fazkeas group 3, negative values for attention (g* = −0.88), social cognition (g* = −0.46), and memory (g* = −0.65) illustrate a substantial impairment in these domains compared to healthy individuals. Interestingly, effect sizes of visuoconstruction (g* = −0.17), language (g* = −0.2), fluency (g* = −0.24) and executive functions (g* = −0.21) are similar to individuals with moderate WMH (Fazekas 2).
Figure 3.
Effect sizes (Hedges g*) and the respective 95% confidence intervals for domain-specific (a) objective cognitive impairment and (b) subjective cognitive complaints for Fazekas groups 1, 2, and 3, relative to Fazekas group 0. Negative values indicate cognitive decline or more frequent cognitive complaints compared to healthy individuals (Fazekas 0). Based on the established conventions, effect size values are interpreted as follows: g* < 0.2 small effect, g* = 0.5 medium effect, g* > 0.8 large effect.
Figure 4.
Hedges g* was calculated for all cognitive domain scores of (a) objective cognitive performance and (b) subjective cognitive complaints. Mean scores of the Fazekas groups 1, 2 and 3 were referenced against the means of Fazekas group 0. According to the established conventions, effect sizes are interpreted as follows: g*<0.2 small effect, g* = 0.5 medium effect, g* > 0.8 large effect.
Individual cognitive profile of the Fazekas 3 group
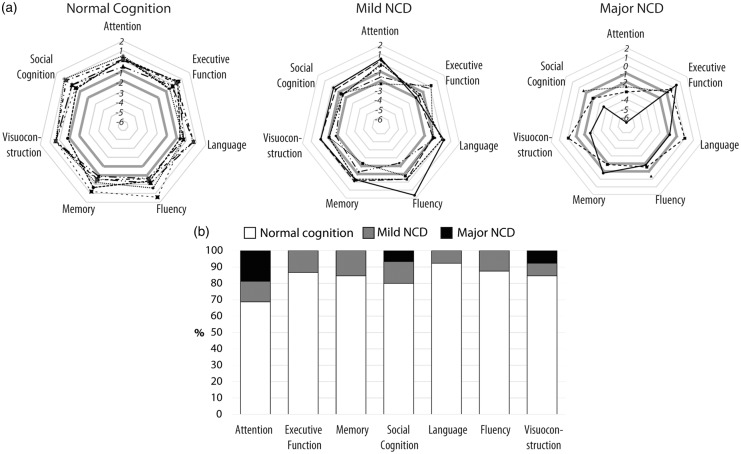
To evaluate the relevance of cognitive deficits associated with Fazekas score 3 on an individual level, we conducted a detailed analysis here. Results are illustrated in Figure 5 and Table S4. While the Fazekas 3 group showed severe cognitive impairment on specific domains compared to persons without WMH on a group level, the examination of the individual cognitive profiles revealed a broad variability in cognitive (dys-)functioning (Figure 5(a)). The clinical categorization of the Fazekas 3 group according to the diagnostic criteria for mild and major NCD20 showed that 7 out of 16 individuals (44%) presented normal cognition, as no domain had a score below −1 SD. Six individuals were classified with mild NCD (one or more domain scores <−1 SD and >−2 SD), and three had major NCD (one or more domain scores <−2 SD). Interestingly, no person presented executive dysfunctions, deficits in memory, language, or fluency as severe as major NCD (Figure 5(b)). Attention was the cognitive domain most consistently affected in our Fazekas 3 sample: only 11 out of 16 persons performed normal. Social cognition was the second most affected domain. Interestingly, only two persons had mild deficits in memory that were, however, non-exclusive, as they came along with an impairment in attention.
Figure 5.
(a) Individual cognitive domain score profiles of the Fazekas 3 group (N = 16). The diagnostic criteria for Neurocognitive Disorder (NCD) as defined by the Diagnostic and Statistical Manual of Mental Disorders 5th edition20 were applied to categorize Fazekas 3 individuals with normal cognition (all domain scores > −1 standard deviation [SD]), mild NCD (one or more domains <−1 SD and >−2 SD), and major NCD (one or more domains <−2 SD). The critical SDs for classification (−1 and −2) are marked bold grey in each diagram. (b) Domain-specific percentage of Fazekas 3 individuals classified as cognitively normal (white), mild NCD (grey) and major NCD (black).
Subjective cognitive complaints
Results for cognitive complaints are illustrated in Figure 2(b) and Table 2. Fazekas groups significantly differed in memory complaints (χ2(3796) = 18.805, p = 0.001). Fazekas group 3 reported higher impairment than Fazekas group 2 (χ2(1,99) = 34.86, p = 0.001). Further, significant differences on self-reported executive dysfunction were obtained (χ2(3796) = 93.889, p = 0.001). Fazekas group 3 worried significantly more than the other groups (Fazekas 0: χ2(1364) = 155.59, p = 0.001; Fazekas 1: χ2(1361) = 145.48, p = 0.001; Fazekas 2: χ2(1,99) = 104.75, p = 0.001).
Figure 4(b) shows that the effect size profiles of persons with small (Fazekas 1; range −0.04 to 0.07) and moderate WMH burden (Fazekas 2; range −0.13 to 0.06) largely overlap. In both cases, small effect sizes obtained for all domains indicate low levels of subjective deterioration. The effect size profile of Fazekas 3 shows a general increase of subjective complaints compared to the other groups (range −0.44 to −0.26). The only exception is language. Relative to healthy individuals, persons with Fazekas grade 1 and 2 reported language impairment more often than persons at Fazekas stage 3. Effect sizes for subjective decline (Figures 3(b) and 4(b)) are generally smaller compared to objective impairment (Figures 3(a) and 4(a)).
Discussion
Here, we investigated the complete cognitive profile of WMH applying the most up-to-date criteria published by the DSM-520 in 849 participants aged 21 to 79 years. This not only includes domain-specific objective cognitive performance, but also subjective cognitive complaints. The investigation of the effects of age-related WMH on social cognitive abilities in this large cohort study is of special interest. WMHs were assessed in FLAIR images with the Fazekas scale. As this well-validated assessment tool is broadly applied in clinical routine in contrast to new quantitative algorithms, we enable a translation of our results into clinical practice.
The overall neuropsychological profile suggests a decline in cognitive performance as a function of WMH burden, which becomes evident at progressed stages of white matter damage (starting from Fazekas score 2). Thus, our results are well in line with previous findings.12 Effect sizes are especially important for inferring clinical relevance as they provide valuable information about actual performance differences. When comparing the performance of Fazekas groups 1 and 2 relative to healthy individuals, small and predominantly negative effect sizes for all cognitive domains indicate only a slight performance deficit which barely differs from normal cognition. There is a general tendency towards a decrease in global cognitive functioning that goes along with moderate white matter changes (Fazekas 2), but without specific impairment of single cognitive functions. Compared to healthy individuals, the pattern of deterioration is more specific at severe stages of white matter damage (Fazekas 3), in particular for attention, memory and social cognition. These results speak in favor for a late impact of white matter lesions on specific cognitive functions, while small and moderate white matter changes are functionally less relevant.18 Our results also correspond to pathological mechanisms of small vessel disease. In the initial stages, lesions affect only small proportions of white matter and most neural structures remain intact. Intra- and inter-neural communication is less efficient, but functions are still preserved.5 Additionally, neural plasticity may be a crucial factor for the delay of functional consequences. As the neural tissue adjacent to the lesion is highly vulnerable to subsequent spread of injury,19 it may be primarily affected by morphological and structural changes. Later, alterations in neural signal transmission and metabolic functions impact subcortical networks and cortical connections critical to higher-order cognition, causing functional consequences.9
Functional networks engaged in attention, memory and social cognition may be mainly affected by WMH, as Fazekas 3 individuals presented major cognitive decline. Remarkably, the expected decline in executive functioning in our study was only of minor importance in contrast to deficits in attention and memory.12 This may be due to the nature of executive functions to be a broad construct assessed with a wide range of tests. Here, we focused on executive measures per se by assessing task-switching, flexibility and inhibition of automatic responses. Overall, results indicate a trend towards a functional decline in these subcomponents at progressed stages of WMH, but their association may be less strong than previously assumed.12
Most interestingly, our study adds information on the consequences of WMH on domains that have been underrepresented or even never investigated in recent publications: language, visuoconstruction, and social cognition. Here, the association between extensive WMH (Fazekas 3) and a functional loss in social cognitive abilities is of decisive interest. In this study, we assessed ToM, a specific aspect of social cognition crucial for the identification of mental states, knowledge and beliefs of another person. In a previous study, a lower RMET accuracy was related to an age-related decline in the structural integrity of white matter bundles inter-connecting cortical brain areas.31 As studies analyzing white matter lesion volume in the context of ToM are rare,32,33 this is the first study showing that extensive, age-associated white matter damage as measured with the Fazekas scale is functionally related to ToM deficits. We assume that progression of small vessel pathology, as discussed above, finally also disrupts connections between cortical areas commonly involved in ToM tasks.21
A closer inspection of the individual neuropsychological profiles of the Fazekas 3 group adds a new perspective on white matter-associated cognitive deficits. Although this group generally presents large WMH burden, 7 out of 16 individuals can be classified as cognitively normal according to the DSM-5 classification criteria.20 In total, only three persons presented cognitive deficits as severe as major NCD, comparable to vascular dementia. These results demonstrate the high interindividual variability of cognitive consequences of WMH, as most Fazekas 3 individuals were either un- or only minimally affected by these structural brain changes, supporting the concept of an effective coping with cognitive deficits. Not only individual lifestyle factors, such as cognitive and social resources, sports and nutrition may contribute to the cognitive outcome but also lesion location and a genetic predisposition may be crucial factors in this relation. Consequently, these results highlight the necessity of tailored clinical diagnostics and therapy in the framework of personalized medicine. Of note, results of the individualized analysis support the assumption that cognitive profiles differ between small vessel and Alzheimer's disease (see further discussion in the limitations section).
Not surprisingly, the profile of subjective cognitive impairment points in the same direction like objective cognitive impairment, as larger WMH burden is associated with more cognitive complaint. Individuals with mild and moderate WMH rarely experience cognitive impairment and don't report cognitive complaint, while persons with severe lesions suffer from substantial cognitive decline. They are frequently confronted with cognitive deficits in everyday life, and report more cognitive incidents. The effect sizes of subjective impairment are generally lower compared to objective performance and can be classified as medium-sized. The discrepancy between objective and subjective cognitive performance may come from the ability to cope with cognitive deficits in daily life. Coping enables individuals to manage their everyday activities and thus, they feel less affected by cognitive deficits. Alternatively, this finding may indicate problems to integrate cognitive deficits in the individual self-concept, leading to denial or unawareness of functional loss, or desirable response behavior in persons with high WMH burden. This may then be a behavioral consequence of WMH-associated cognitive decline.
Study limitations
Although this study was carefully conducted, some critical remarks must be mentioned. The effects of WMH on cognition were investigated with a cross-sectional design, limiting inference on cause-effect associations. Furthermore, quantification of WMH may be interpreted as conservative approach, as WMH can potentially be extracted with the help of (semi-) automated computer algorithms, and no spatial information about the lesion was considered. Although location information may be relevant in the context of WMH-associated cognitive deficits,4,6,12,15,18 our method was guided by common practice in clinical settings, where the usage of WMH rating scales is the method of choice rather the application of extraction algorithms. Thus, we followed this clinical approach to investigate the relation between global WMH burden and the cognitive profile as defined in the DSM-5. We used the Fazekas scale, as WMH classification is simple, reliable and highly correlated with volumetric measures of WMH.5,12,47–49 Compared to WMH volume, ceiling effects of the Fazekas scale may limit definite conclusions about the association of WMH and cognition.47 The results of the actual study shall be verified with volumetric measures. In future, extraction algorithms may be more often applied in clinical practice, as few algorithms (e.g. Lesion TOADS68) have been validated for patients with multiple sclerosis. Due to differing pathological mechanisms underlying multiple sclerosis and age-related WMH, a specification of the algorithms is necessary for its valid extraction in future.
Another limitation refers to the small number of individuals classified as Fazekas 3. As participation was voluntary, a selection bias cannot be completely ruled out. Handicapped persons suffering from severe mental or physical impairment may not have participated in the study, but also limited time capacity or the nursing of a spouse may have prevented successfully aged elderly from study participation. Accordingly, the distribution of individuals to Fazekas groups may thus be representative for the general population, cf.11 Given this statistically unideal differences sample size, combined with distribution characteristics of some cognitive outcomes that do not match requirements for parametric testing, non-parametric statistics can be regarded as best-practice. Additionally, we included Hedges g* as an effect size measure as it adjusts for differences in sample size and adds valuable information about the relevance of group differences in cognition beyond p-values.
The interplay between Alzheimer's disease and small vessel disease pathological processes is discussed in the literature.69,70 Based on the medical history of all individuals, no participant was previously diagnosed with Alzheimer's disease or other types of dementia. The detailed examination of the cognitive profiles of the Fazekas 3 individuals (Figure 5, Table S4) revealed that no person showed cognitive deficits indicative for (prodromal) Alzheimer's disease, or had a selective amnestic cognitive impairment prototypical to prodromal Alzheimer's disease. Additionally, we compared the hippocampal volumes of the Fazekas 3 group to randomly selected age, sex and education matched controls from Fazekas groups 0, 1 and 2. Mean hippocampal volumes did not differ significantly between Fazekas groups (F(3,60) = 0.38, p > 0.05), indicating that the Fazekas 3 group does not show larger hippocampal atrophy compared to the other groups. Thus, prodromal Alzheimer's disease may not be a confounding factor in this sample.
Regarding the validity of neuropsychological measures, ceiling effects are an issue to be discussed. Most participants maximally scored on Boston Naming Test and the CERADplus visuoconstruction task. Because of the low response variability, slight differences in absolute test scores result in major differences in the z-transformed scores. Thus, slight differences in verbal and visuoconstructive abilities may be overemphasized under these conditions.
Finally, the DEX was used to evaluate domain-specific subjective cognitive impairment. The underlying factor structure is strongly debated, and it may have different subscales. As instruments systematically assessing domain-specific subjective impairment are still underdeveloped, items of the DEX were grouped to the DSM-5 cognitive domains, based on their content. Considering, that DEX is assessing dysexecutive symptoms, executive functions are over-represented, while other domains (e.g. language) are only poorly represented. More research is needed to improve available tools and construct new instruments for the reliable assessment of subjective complaints on the domain-level.
Conclusions
In conclusion, the study shows that cognitive impairment and subjective impairment are associated with severe WMH, while small und moderate WMH hardly affect cognition. This study is the first to fully investigate the cognitive profile and to include social cognition changes associated with different stages of WMH in a population-based study. Processing speed, memory and social cognition were predominantly impaired at progressed stages of white matter damage. However, at an individual level, the cognitive spectrum associated with large WMH burden is widespread as it ranges from cognitive functioning on a normal level to impairment as severe as major NCD. The role of lesion volume and lesion location on the cognitive profile in WMH will be subject of future investigation. By disentangling WMHs' whole neuropsychological profile, our study suggests that new diagnostic and therapeutic approaches for this disease in the future.
Supplementary Material
Acknowledgments
We thank all members of the LIFE study center for conducting the LIFE-ADULT Study as well as all participants for their good collaboration.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study has been supported by LIFE – Leipzig Research Center for Civilization Diseases at the University of Leipzig – funded by the European Union, European Regional Development Fund and by the Free State of Saxony within the framework of the excellence initiative (TL, KA, SRH, AV & MLS). Furthermore, MLS has been supported by the German Consortium for Frontotemporal Lobar Degeneration, funded by the German Federal Ministry of Education and Research, by the Parkinson's Disease Foundation (grant number PDF-IRG-1307), by the Michael J Fox Foundation (grant number MJFF-11362), and JK by the Max-Planck International Research Network on Aging.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors' contributions
JK planned and conducted statistical analyses, wrote the first draft of the manuscript including creation of the figures, and modified all subsequent drafts. LL processed all MRI data and contributed to all drafts of the manuscript. TL, SF, KA, KTH, ML, SGRH designed the study and reviewed the final draft of the manuscript. AV designed the study and contributed to interpretation of the data. MLS designed the study, supervised data analyses, substantially contributed to interpretation of the results and made substantial modifications to all drafts of the manuscript.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Schaefer A, Quinque EM, Kipping JA, et al. Early small vessel disease affects frontoparietal and cerebellar hubs in close correlation with clinical symptoms – a resting-state fMRI study. J Cereb Blood Flow Metab 2014; 34: 1091–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith GE, Bondi MW. Mild cognitive impairment and dementia, Oxford: American Academy of Clinical Neuropsychology, 2013. [Google Scholar]
- 3.Stevens RD, Hannawi Y, Sair H. Small vessel disease and the resting functional architecture of the brain. J Cereb Blood Flow Metab 2014; 34: 1089–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pantoni L, Poggesi A, Inzitari D. The relation between white-matter lesions and cognition. Curr Opin Neurobiol 2007; 20: 390–397. [DOI] [PubMed] [Google Scholar]
- 5.Frisoni GB, Galluzzi S, Pantoni L, et al. The effect of white matter lesions on cognition in the elderly – small but detectable. Nature Clin Pract Neurol 2007; 3: 620–627. [DOI] [PubMed] [Google Scholar]
- 6.Breteler MM. Vascular involvement in cognitive decline and dementia – epidemiologic evidence from the Rotterdam study and the Rotterdam scan study. Ann N Y Acad Sci 2006; 903: 457–465. [DOI] [PubMed] [Google Scholar]
- 7.Habes M, Erus G, Toledo JB, et al. White matter hyperintensities and imaging patterns of brain ageing in the general population. Brain 2016; 139: 1164–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiong YY, Mok V. Age-related white matter changes. J Aging Res 2011; 2011: 617927, . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galluzzi S, Lanni C, Pantoni L, et al. White matter lesions in the elderly: pathophysiological hypothesis on the effect on brain plasticity and reserve. J Neurol Sci 2008; 273: 3–9. [DOI] [PubMed] [Google Scholar]
- 10.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 2010; 341: c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Leeuw FE, de Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatr 2001; 70: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kloppenborg RP, Nederkoorn PJ, Geerlings MI, et al. Presence and progression of white matter hyperintensities and cognition – a meta-analysis. Neurology 2014; 82: 2127–2138. [DOI] [PubMed] [Google Scholar]
- 13.Nichtweiss M, Weidauer S, Treusch N, et al. White matter lesions and vascular cognitive impairment: part 1: typical and unusual causes. Clin Neuroradiol 2012; 22: 193–210. [DOI] [PubMed] [Google Scholar]
- 14.Quinque EM, Arelin K, Dukart J, et al. Identifying the neural correlates of executive functions in early cerebral microangiopathy: a combined VBM and DTI study. J Cereb Blood Flow Metab 2012; 32: 1869–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt R, Grazer A, Enzinger C, et al. MRI-detected white matter lesions: do they really matter? J Neural Transm 2011; 118: 673–681. [DOI] [PubMed] [Google Scholar]
- 16.Schroeter ML, Bucheler MM, Preul C, et al. Spontaneous slow hemodynamic oscillations are impaired in cerebral microangiopathy. J Cereb Blood Flow Metab 2007; 27: 1094–1094. [DOI] [PubMed] [Google Scholar]
- 17.Schroeter ML, Cutini S, Wahl MM, et al. Neurovascular coupling is impaired in cerebral micrangiopathy - An event-related Stroop study. NeuroImage 2007; 34(1): 26–34. [DOI] [PubMed] [Google Scholar]
- 18.de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol 2000; 47: 145–151. [DOI] [PubMed] [Google Scholar]
- 19.Maillard P, Carmichael O, Fletcher E, et al. Coevolution of white matter hyperintensities and cognition in the elderly. Neurology 2012; 79: 442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 5th ed Arlington, VA: American Psychiatric Publishing, 2013. [Google Scholar]
- 21.Fiske ST, Taylor SE. Social cognition – from brains to culture, London: Sage, 2013. [Google Scholar]
- 22.Baron-Cohen S, Bowen DC, Holt RJ, et al. The “Reading the Mind in the Eyes” test: complete absence of typical sex difference in ∼400 men and women with Autism. PLoS One 2015; 10: e0136521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brune M, Brune-Cohrs U. Theory of mind – evolution, ontogeny, brain mechanisms and psychopathology. Neurosci Biobehav Rev 2006; 30: 437–455. [DOI] [PubMed] [Google Scholar]
- 24.Schroeter M. Considering the frontomedian cortex in revised criteria for behavioural variant frontotemporal dementia. Brain 2012, pp. 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schroeter ML, Laird AR, Chwiesko C, et al. Conceptualizing neuropsychiatric diseases with multimodal data-driven meta-analyses – the case of behavioral variant frontotemporal dementia. Cortex 2014; 57: 22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Premack D, Woodruff G. Chimpanzee problem-solving: a test for comprehension. Science 1978; 202: 532–535. [DOI] [PubMed] [Google Scholar]
- 27.Ellis HD, Gunter HL. Asperger syndrome: a simple matter of white matter? TICS 1999; 3: 192–200. [DOI] [PubMed] [Google Scholar]
- 28.Chisholm K, Lin A, Abu-Akel A, et al. The association between autism and schizophrenia spectrum disorders: a review of eight alternate models of co-occurrence. Neurosci Biobehav Rev 2015; 55: 173–183. [DOI] [PubMed] [Google Scholar]
- 29.Kana RK, Libero LE, Moore MS. Disrupted cortical connectivity theory as an explanatory model for autism spectrum disorders. Phys Life Rev 2011; 8: 410–437. [DOI] [PubMed] [Google Scholar]
- 30.Kana RK, Libero LE, Hu CP, et al. Functional brain networks and white matter underlying theory-of-mind in autism. SCAN 2014; 9: 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cabinio M, Rossetto F, Blasi V, et al. Mind-Reading ability and structural connectivity changes in aging. Front Psychol 2015; 6: 1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charlton RA, Barrick TR, Markus HS, et al. Theory of mind associations with other cognitive functions and brain imaging in normal aging. Psychol Aging 2009; 24: 338–348. [DOI] [PubMed] [Google Scholar]
- 33.Mike A, Strammer E, Aradi M, et al. Disconnection mechanism and regional cortical atrophy contribute to impaired processing of facial expressions and theory of mind in multiple sclerosis: a structural MRI study. PLoS One 2013; 8: e82422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baron-Cohen S, Wheelwright S, Hill J, et al. The “Reading the Mind in the Eyes” Test revised version: a study with normal adults and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry 2001; 42: 241–251. [PubMed] [Google Scholar]
- 35.Bölte S. Reading the mind in the eyes test für Erwachsene (dt. Fassung) von S. Baron-Cohen. Frankfurt am Main: J.W. Goethe-Universität, 2005.
- 36.Pardini M, Nichelli PF. Age-related decline in mentalizing skills across adult life span. Exp Aging Res 2009; 35: 98–106. [DOI] [PubMed] [Google Scholar]
- 37.Baglio F, Castelli I, Alberoni M, et al. Theory of mind in amnestic mild cognitive impairment: an fMRI study. J Alzheimers Dis 2012; 29: 25–37. [DOI] [PubMed] [Google Scholar]
- 38.Kirkland RA, Peterson E, Baker CA, et al. Metaanalysis reveals adult female superiority in “Reading the mind in the eyes” test. N Am J Psychol 2013; 15: 449–458. [Google Scholar]
- 39.Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry 2000; 15: 983–991. [DOI] [PubMed] [Google Scholar]
- 40.de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and subjective cognitive dysfunction. The Rotterdam Scan Study. Neurology 2001; 56: 1539–1545. [DOI] [PubMed] [Google Scholar]
- 41.Haley AP, Hoth KF, Gunstad J, et al. Subjective cognitive complaints relate to white matter hyperintensities and future cognitive decline in patients with cardiovascular disease. Am J Geriatr Psychiat 2009; 17: 976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benedictus MR, van Harten AC, Leeuwis AE, et al. White matter hyperintensities relate to clinical progression in subjective cognitive decline. Stroke 2015; 46: 2661–2664. [DOI] [PubMed] [Google Scholar]
- 43.Uiterwijk R, Huijts M, Staals J, et al. Subjective cognitive failures in patients with hypertension are related to cognitive performance and cerebral microbleeds. Hypertension 2014; 64: 653–657. [DOI] [PubMed] [Google Scholar]
- 44.Jorm AF, Butterworth P, Anstey KJ, et al. Memory complaints in a community sample aged 60–64 years: associations with cognitive functioning, psychiatric symptoms, medical conditions, APOE genotype, hippocampus and amygdala volumes, and white-matter hyperintensities. Psychol Med 2004; 34: 1495. [DOI] [PubMed] [Google Scholar]
- 45.Loeffler M, Engel C, Ahnert P, et al. The LIFE-Adult-Study: objectives and design of a population-based cohort study with 10,000 deeply phenotyped adults in Germany. BMC Public Health 2015; 15: 691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fazekas F, Chawluk JB, Alavi A, et al. MR signal abnormalities at 1.5-T in Alzheimers dementia and normal aging. Am J Neuroradiol 1987; 8: 421–426. [DOI] [PubMed] [Google Scholar]
- 47.Gao FQ, Swartz RH, Scheltens P, et al. Complexity of MRI white matter hyperintensity assessments in relation to cognition in aging and dementia from the Sunnybrook Dementia Study. J Alzheimers Dis 201126 Suppl 3, 379–388. [DOI] [PubMed] [Google Scholar]
- 48.Scheltens P, Erkinjunti T, Leys D, et al. White matter changes on CT and MRI: an overview of visual rating scales. Eur Neurol 1998; 39: 80–89. [DOI] [PubMed] [Google Scholar]
- 49.Valdes Hernandez MC, Morris Z, Dickie DA, et al. Close correlation between quantitative and qualitative assessments of white matter lesions. Neuroepidemiology 2013; 40: 13–22. [DOI] [PubMed] [Google Scholar]
- 50.Beck IR, Schmid NS, Berres M, et al. Establishing robust cognitive dimensions for characterization and differentiation of patients with Alzheimer's disease, mild cognitive impairment, frontotemporal dementia and depression. Int J Geriatr Psychiatry 2014; 29: 624–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Madureira S, Verdelho A, Ferro J, et al. Development of a neuropsychological battery for the Leukoaraiosis and Disability in the Elderly Study (LADIS): experience and baseline data. Neuroepidemiology 2006; 27: 101–116. [DOI] [PubMed] [Google Scholar]
- 52.Henry LA, Messer DJ, Nash G. Executive functioning and verbal fluency in children with language difficulties. Learn Inst 2015; 39: 137–147. [Google Scholar]
- 53.van Boxtel MP, Buntinx F, Houx PJ, et al. The relation between morbidity and cognitive performance in a normal aging population. J Gerontol Biol Sci Med Sci 1998; 53: M147–M154. [DOI] [PubMed] [Google Scholar]
- 54.Mueller KD, Koscik RL, LaRue A, et al. Verbal fluency and early memory decline: results from the Wisconsin Registry for Alzheimer's Prevention. Arch Clin Neuropsychol 2015; 30: 448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ahmed FS, Miller LS. Executive function mechanisms of theory of mind. J Autism Dev Disord 2011; 41: 667–678. [DOI] [PubMed] [Google Scholar]
- 56.Peterson E, Miller S. The eyes test as a measure of individual differences: how much of the variance reflects verbal IQ? Front Psychol 2012; 3: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pinkham AE, Penn DL, Green MF, et al. Social cognition psychometric evaluation: results of the initial psychometric study. Schizophr Bull 2014; 40: 813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilson BA, Alderman N, Burgess PW, et al. Behavioural assessment of the dysexecutive syndrome (BADS). Bury St Edmunds, UK: Thames Valley Test Company Harcourt Assessment, 1996.
- 59.Pedrero-Perez EJ, Ruiz-Sanchez-de-Leon JM, Winpenny-Tejedor C. Dysexecutive Questionnaire (DEX): unrestricted structural analysis in large clinical and non-clinical samples. Neuropsychol Rehabil 2015; 25: 879–894. [DOI] [PubMed] [Google Scholar]
- 60.The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). ESH/ESC guidelines for the management of arterial hypertension. Eur Heart J 2013; 34: 2159–219. [DOI] [PubMed] [Google Scholar]
- 61.Bortz J, Lienert GA, Boehnke K. Verteilungsfreie methoden in der biostatistik, 3rd ed Berlin, Heidelberg: Springer-Verlag, 2008. [Google Scholar]
- 62.Conover WJ. Practical nonparametric statistics, New York: Wiley, 1971. [Google Scholar]
- 63.Schaich E, Hamerle A. Verteilungsfreie statistische prüfverfahren, Berlin: Springer, 1984. [Google Scholar]
- 64.Durlak JA. How to select, calculate, and interpret effect sizes. J Pediatr Psychol 2009; 34: 917–928. [DOI] [PubMed] [Google Scholar]
- 65.IBM Corp. IBM SPSS statistics for windows, version 22.0, Armonk, NY: IBM Corp, 2013. [Google Scholar]
- 66.Microsoft. Microsoft excel 2010, Redmond, WA: Microsoft, 2010. [Google Scholar]
- 67.Adobe Systems Incorporated. Adobe illustrator CS5, version 15.1.1, San Jose, CA: Adobe Systems, 2010. [Google Scholar]
- 68.Shiee N, Bazin P-L, Ozturk A. A topology-preserving approach to the segmentation of brain images with multiple sclerosis lesions. Neuroimage 2010; 49: 1524–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kester MI, Goos JDC, Teunissen CE. Associations between cerebral small-vessel disease and Alzheimer disease pathology as measured by cerebrospinal fluid biomarkers. JAMA Neurol 2014; 71: 855–862. [DOI] [PubMed] [Google Scholar]
- 70.Mortamais M, Artero S, Ritchie K. White matter hyperintensities as early and independent predictors of Alzheimer's disease risk. J Alzheimers Dis 2014; 42: 393–400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.