Abstract
The mitochondrial protein prohibitin (PHB) has emerged as an important modulator of neuronal survival in different injury modalities. We previously showed that viral gene transfer of PHB protects CA1 neurons from delayed neurodegeneration following transient forebrain ischemia through mitochondrial mechanisms. However, since PHB is present in all cell types, it is not known if its selective expression in neurons is protective, and if the protection occurs also in acute focal ischemic brain injury, the most common stroke type in humans. Therefore, we generated transgenic mice overexpressing human PHB1 specifically in neurons (PHB1 Tg). PHB1 Tg mice and littermate controls were subjected to transient middle cerebral artery occlusion (MCAo). Infarct volume and sensory-motor impairment were assessed three days later. Under the control of a neuronal promoter (CaMKIIα), PHB1 expression was increased by 50% in the forebrain and hippocampus in PHB1 Tg mice. The brain injury produced by MCAo was reduced by 63 ± 11% in PHB1 Tg mice compared to littermate controls. This reduction was associated with improved sensory-motor performance, suggesting that the salvaged brain remains functional. Approaches to enhance PHB expression may be useful to ameliorate the devastating impact of cerebral ischemia on the brain.
Keywords: Cerebral ischemia, cerebral blood flow, neuroprotection, prohibitin, transgenic mice
Introduction
Stroke remains a leading cause of death and disability worldwide.1 Despite decades of research, reestablishing cerebral perfusion with tissue plasminogen activator (tPA) and endovascular devices are the only available treatment options.2,3 However, due to a narrow therapeutic time window, strict diagnostic requirements, and potential contraindications, currently only 3–5% of stroke patients are able to benefit from these interventions.4–6 Consequently, the majority of stroke patients receive only supportive care, highlighting the need for new effective treatments.
Mitochondria have long been implicated in the tissue damage caused by ischemia-reperfusion, both through impairment of energy production and as mediators of cell death.7,8 The lack of oxygen leads to impaired mitochondrial ATP production with bioenergetics failure, resulting in calcium overload in the organelle.9 In turn, the elevated calcium level is thought to induce the mitochondrial permeability transition (MPT), leading to the collapse of the mitochondrial membrane potential and activation of intrinsic cell death pathways.10 In addition, impaired mitochondria are a major source of post-ischemic reactive oxygen species (ROS)11 further compromising cells by oxidizing proteins, lipids and nucleic acids. Therefore, alleviating mitochondrial bioenergetic failure may represent an effective strategy to preserve neuronal integrity and maintain function in the ischemic territory.7,12
The mitochondrial protein prohibitin (PHB) has emerged as a key regulator of mitochondrial stability and function.13–16 PHB comprises a highly conserved and ubiquitously expressed family of proteins that are predominantly localized to the inner mitochondrial membrane.14,15 Multiple heterodimers composed of homologous PHB1 and PHB2 subunits form large ring complexes (>1 MDa) thought to function as protein and lipid scaffolds.17,18 PHB is indispensable for the maintenance of mitochondrial structure, function, and genome stability,19 and deletion of PHB in C. elegans and mice is embryonically lethal.20,21
PHB overexpression has been shown to mediate cell survival by stabilizing mitochondria and suppressing oxidative stress.17,22 Consistent with this role, we have previously shown that PHB1 expression protects neurons from different injury modalities through stabilization of respiratory chain complex I, and reduces generation of ROS.23 Furthermore, PHB viral-gene transfer in the hippocampus, leading to PHB overexpression in all cell types, protected CA1 neurons from the delayed neurodegeneration associated with forebrain ischemia.24 However, it remains to be established whether PHB is effective also in acute focal ischemic injury, which underlies most ischemic strokes in humans, and whether PHB expression in neurons is sufficient to confer brain protection and improve neurological outcome.
In this study, we used a novel transgenic mouse model that overexpresses PHB1 in forebrain neurons to determine if neuronal PHB1 upregulation is sufficient to afford neuroprotection in a model of acute focal cerebral ischemia. We found that selective neuronal expression of PHB1 is sufficient to reduce brain damage and improve sensory-motor deficits following middle cerebral artery occlusion (MCAo). The data highlight the strong protective effect of PHB in focal ischemic injury and establish that PHB is able to provide cell autonomous protection in neurons.
Material and methods
Animals
The animal protocol was approved by the Institutional Animal Care and Use Committee of Weill Cornell Medicine. All animal experiments were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health as well as the ARRIVE guidelines.25 All experiments were performed in a blinded fashion and mice were selected randomly. Experiments were performed in 8–10-week-old male PHB1 Tg mice on a C57BL/6 genetic background with age-matched non-transgenic littermates served as controls. Mice were housed in a temperature and humidity controlled facility on a 12-h light/dark cycle and food and water were provided ad libitum.
Generation of a neuronal PHB1 expressing mouse line
The primer sequences used for the amplification of the human PHB1 gene were the following:
Forward primer: 5-GCGGATCCGCCACCatgGCTGCCAAAGTGTTTGAGTCC; reverse primer: 5-GCGAATTCACTGGGGCAGCTGGAGGAGC. DNA fragments including human PHB1 cDNA coding region, CamKIIα promoter, IRES and eGFP were cloned into pcDNA3.1 plasmid and propagated (Figure S1). The transgene construct was digested from the plasmid with MluI and EagI restriction enzymes. After purification, this DNA fragment was used for pronuclear transfer into zygotes from C57Bl/6 J mice following standard protocols (Transgenic Mouse Core of Weill Cornell Medicine). Two founder animals were identified by southern blot and experiments were performed using the higher copy line (10 copies. Figure S1).
Y-maze spontaneous alteration test
Working memory in mice was evaluated by the Y-maze test, as previously described.24,26 This test was selected because it takes advantage of the natural tendency of rodents to explore new environments, making it less stressful and more consistent with the natural behavior of mice than other spatial memory tests. Experiments were performed using adult (five to six month old) PHB1 Tg mice and age-matched WT controls with an operator blinded to mouse genotype. Sessions were video recorded and replayed for determination of the parameters of interest. Briefly, each mouse was placed at the end of one arm and allowed to freely explore the apparatus for 8 min. The sequence and number of all arm entries were recorded for each animal throughout the period. Alternation rate was defined as entries into all three arms on consecutive occasions using the following formula: alternation rate (%) = number of alternations/(number of total arm entries–2) × 100. Trials in which the number of total arm entries was <10 were not included in the analysis.
Novel object recognition test
The novel object recognition test (NOR) was conducted following a procedure published previously.27 Prior to the procedure, mice were placed in the test chamber to get familiar with the testing environment. Twenty-four hours later, mice were returned to the same chamber pre-arranged with two identical sample objects and allowed to explore for 5 min. Two hours later, mice were placed in the same box but one of the two objects was replaced by a novel object. Mice were allowed to explore freely for 5 min. The entire exploration session was video recorded and the time spent on familiar and novel object was measured by a technician blinded to the mouse genotype. Exploration of an object was defined as the mouse actively sniffing or touching the object. The amount of time spent on exploring the novel object was expressed as percentage of the total exploration time and is used as an index of recognition memory.
Immunofluorescence staining of PHB1-Tg brain tissue
As described previously,28 mice were deeply anesthetized and perfused transcardiacally with heparin and 4% paraformaldehyde (PFA). Brains were removed, post-fixed, and cut with a Vibratome. Coronal sections (40 µm) were first boiled in 10 mM sodium citrate for 10 min and incubated with bovine albumin serum (5% in PBS) for 1 h followed by primary antibody incubation overnight at 4℃ (GFP 1:100, NeuN 1:200, GFAP 1:200 dilutions, Thermo Scientific). The brain sections were washed twice in ice cold PBS followed by incubation with FITC or Cy5-conjugated secondary antibody against either mouse or rabbit (1:200, Jackson Immuno Inc.). The sections were washed in PBS, mounted on slides, and covered with fluorescence mounting medium (Vector Laboratories). Fluorescence images were acquired using a confocal microscope (Leica).
MCAo model
Transient focal cerebral ischemia was induced in 8–10-week-old male mice using an intraluminal filament model of middle cerebral artery (MCA) occlusion.29–32 Briefly, mice were anesthetized with 1.5–2% isoflurane and rectal temperature was maintained at 37.3 ± 0.3℃ using a heating pad (TC-1000, CWE Inc., Fort Wayne, IN) during the surgical procedure and in the recovery period until the animals regained full consciousness.
A heat-blunted suture (6–0 suture) was inserted via the right external carotid artery until it obstructed the proximal part of the MCA and the common carotid artery simultaneously ligated for the duration of the ischemic period (35 min). Relative cerebral blood flow (CBF) was measured with a transcranial Laser Doppler flowmetry (Periflux System 5010, Perimed, King Park, NY) in the center (coordinates: 2 mm posterior, 5 mm lateral to bregma) of the ischemic territory. After 35 min, the filament was retracted and the CBF reestablished. Only animals that exhibited a reduction in CBF of >85% during MCA occlusion and in which CBF recovered by >80% after 10 min of reperfusion, were included in the study. There was a single mortality in the MCAo group (PHB1 Tg: n = 1; WT littermates: n = 0). Four animals were excluded due to subarachnoid hemorrhage during surgery indicated by insufficient restoration of relative cerebral blood flow on Laser Doppler, following removal of the filament (PHB1 Tg: n = 1; WT littermates: n = 3).
Measurement of infarct volume
As described previously,31,33,34 infarct volume was measured in coronal Nissl stained brain sections (thickness: 30 µm; interval: 600 µm) throughout the infarcted territory (MCID, Imaging Research, UK). Post-ischemic edema was corrected by quantifying the difference in brain volume between the ischemic hemisphere and the contralateral side according to the method described previously.34,35
Sensory-motor testing
To detect a functional impairment three days post-MCAo, we used well established behavioral tests.36,37 The hanging wire test was selected to measure differences in grip strength, balance and endurance, whereas the corner test was used to determine sensory and motor asymmetries. To exclude differences in motor-sensory function at baseline, PHB1 Tg and non-transgenic littermates were first tested two days before surgery. Mice that did not exhibit spontaneous movement into the corner of the testing apparatus during the 10-min testing period were excluded from the test. Consequently, the performance of 2 WT littermates and 1 PHB1 Tg mouse could not be evaluated three days after MCAo and had to be excluded. However, these animals exhibited intact gross motor function otherwise.
Body weight and arterial blood pressure
Body weight and systolic arterial pressure (SAP) were determined once a week in four- to nine-week-old PHB1 Tg and wild type littermates. SAP was measured by a noninvasive tail cuff system (Model MC4000; Hatteras Instruments). Ten systolic blood pressure measurements were taken in each mouse over a 20-min session. The averaged values for each animal were combined to determine the mean group SAP.38
Resting CBF
The resting CBF was quantitatively assessed by ASL-MRI on a 7.0 Tesla 70/30 Bruker Biospec small-animal MRI system with 450 mT/m gradient amplitude and a 4500 T/m/s slew rate as previously described.39 Briefly, a volume coil was used for transmission and a surface coil for reception. Anatomical localizer images were acquired to find the transversal slice at the level of bregma. One axial slice was acquired with a field of view of 15 × 15 mm, spatial resolution of 0.234 × 0.234 × 2 mm, TE of 5.368 ms, effective TE of 48.32 ms, recovery time of 10 s, and a RARE factor of 72; 22 TIR values ranging from 30 to 2300 ms were used, and the inversion slab thickness was 4 mm. For computation of CBF, the Bruker ASL perfusion processing macro was used. The masked CBF images were exported and further processed using customized software. The processed data were subsequently analyzed using NIH Image J software and reported as CBF (milliliters per 100 g brain tissue/min). Heating maps were generated using MRIcroX viewer software (http://www.mccauslandcenter.sc.edu/CRNL/tools/mricro-viewer).
Visualization of cerebral vessels
To evaluate large cerebral arteries in PHB1 Tg compared to WT littermates, we used India ink perfusions and post fixation with 4% paraformaldehyde to visualize the circle of Willis and major cerebral vessels as described previously.40 Deeply anesthetized mice were transcardially perfused with 15 ml of heparinized phosphate-buffered saline to remove blood cells. Subsequently, mice were perfused with 7 ml of 30% India ink, and their brains removed and post-fixed in 4% paraformaldehyde. Images were acquired with a digital microscope using MicroCapture Pro software (DNT, Germany) at 10 × magnification.
Immunoblotting
Whole tissue lysates from indicated brain regions (cortex, cerebellum, hippocampus, cerebellum) were prepared in RIPA lysis buffer with protease inhibitor cocktail (Roche, Mannheim, Germany) using 2 ml Teflon tissue homogenizer. For immunoblot analysis, proteins were separated with a 10% SDS gel, transferred to a PVDF membrane. PHB1 was detected using a monoclonal mouse anti-PHB1 antibody (Clone II-14-10) in a 1:500 dilution (Thermo Fisher Scientific, Warm Springs, CA). Immunoreactive bands were visualized using fluorescent secondary antibodies and imaged with LI-COR scanner system (LI-COR, Lincoln, NE). Beta-actin staining (1:1000, Cell Signaling Technologies, Danvers, MA) was used to monitor equal gel loading.
Subcellular fractionation
Subcellular fractionation of mouse brain was performed using ProteoExtract Subcellular Proteome Extraction kit (Calbiochem, San Diego, CA) following the manufacturer’s instructions. Proteins for the mitochondrial, nuclear, membrane, and cytosolic fractions were loaded on 10% SDS gels in equal amounts and the subcellular localization of PHB1 was determined by Western blotting.
Data analysis
GraphPad Prism software (version 6.0, GraphPad Software, San Diego, CA) was used for all statistical analysis. Data are expressed as mean ± SE. Intergroup differences were analyzed by unpaired Student’s t test or non-parametric Mann–Whitney U rank-sum test, as appropriate. Differences were considered statistically significant for *p < 0.05, **p < 0.001, ***p < 0.0001). The number of experimental units required to detect a standardized effect size > 0.25 was calculated by a priori power analysis using PS sample size and power calculation software version 19 with the following assumptions: power = 0.8 and α = 0.05, SD 20% of the mean for in vivo MCAo experiments.
Results
Neuron-specific PHB1 overexpressing mice
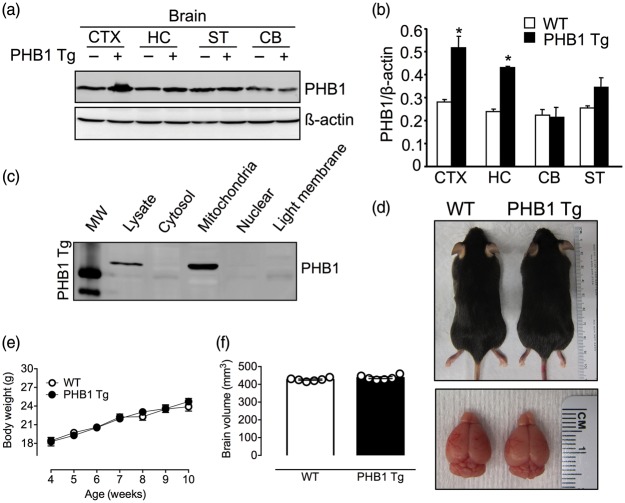
To specifically study the protective potential of PHB1 expression in neurons, we generated transgenic mice with PHB1 overexpression in neurons (PHB1 Tg). The human PHB1 gene was expressed under the control of the CaMKIIα promoter41 (Figure S1(a)), resulting in a postnatal PHB1 increase in forebrain neurons. Germline transmission was confirmed and copy number of the PHB1 transgene in heterozygous PHB1 Tg was determined using southern blotting (Figure S1(b)). The line utilized in this study contained 10 copies of the transgene per genome. Consistent with the spatial expression pattern predicted for the CaMKIIα promoter,42,43 immunoblotting analyses of brain lysates of PHB1 Tg mice showed a significant (p < 0.05) increase in PHB protein levels in forebrain regions, including the neocortex (51 ± 7%; n = 4) and hippocampus (48 ± 8%) (Figure 1(a) and (b)). PHB1 expression was increased in the striatum, but the change did not reach statistical significance (p > 0.05) (Figure 1(b)). PHB1 expression was not increased in cerebellum (Figure 1(a) and (b)) or peripheral organs (Figure S1(c)). As anticipated, PHB was present in the brain mitochondrial fraction (Figure 1(c)).
Figure 1.
CaMKIIa mediated overexpression of the human PHB1 gene in forebrain neurons. (a, b) Representative immunoblot and immunoblot analysis of tissue lysates from the indicated brain regions of PHB1 Tg and littermate controls (WT) at nine weeks of age using PHB1 and beta-actin specific antibodies. Cortex (CTX), hippocampus (HC), striatum (ST), cerebellum (CB). Beta-actin was used to monitor equal gel loading. (c) Brain tissue fractionation analysis confirming the mitochondrial localization of the PHB1 transgene protein. (d) Representative images of a nine-week-old PHB1 Tg and littermate control indicating an undistinguishable phenotype. (e) Body weight of PHB1 Tg and age-matched WT controls (week 4–5: n = 4–5 mice/group, week: 6–7: n = 14–18 mice/group, week 8: n = 17–24 mice/group, week 9: n = 12–18 mice/group, week 10: n = 5 animals/group); p > 0.05; multiple t-tests. (f) Brain volume assessed using T2-weighted MRI showing no difference between nine-week-old PHB1 Tg and WT mice; n = 6 mice/group; p > 0.05; Mann–Whitney U rank-sum test.
To demonstrate that CamkIIα promoter directs a neuronal specific expression in PHB1 Tg mouse, we performed a double immunofluorescence staining of GFP, expressed from the IRES cassette in the transgene under CamKIIα promoter, and NeuN or GFAP as neuron and astrocyte marker, respectively. Because of high degree of protein sequence similarity in mouse and human PHB1, PHB1 antibodies cannot differentiate endogenous mouse PHB1 and human PHB1 from transgene. We therefore used GFP expressed from the bicistronic cassette as a marker for PHB1 transgene expression. Immunofluorescence double staining of GFP, and neuronal marker NeuN or astrocyte marker GFAP, revealed that CaMKIIα promoter directed a neuronal transgene expression. GFP positive cells in the cortex co-localized with NeuN positive cells (Figure 2(a)), but not astrocytes (Figure 2(b)), demonstrating PHB1 transgene expression in cortical neurons.
Figure 2.

Immunofluorescence double staining of PHB1 Tg brain. (a) Confocal images of coronal sections of a PHB1 Tg mouse (10 weeks old) doubly stained with GFP (green, as PHB1 transgene expression marker) and NeuN (red, as neuronal marker). Merged images show that GFP positive cells are also NeuN positive, suggesting neuronal specific expression of the transgene. (b) Confocal images of double staining of GFP and GFAP (red, astrocyte marker). Merged panels show no co-localization between GFP and GFAP positive cells. Top panels in both (a) and (b) are images acquired with a low magnification lens (5×); lower panels were acquired with a higher magnification lens (63×) from the regions indicated by the square in the top panels. Size bars are 500 um and 25 um as indicated.
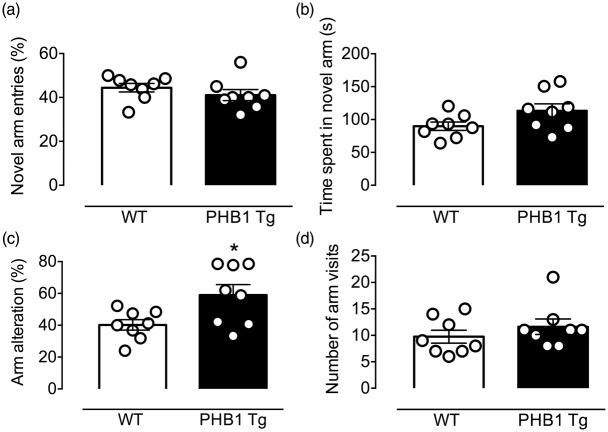
Heterozygous PHB1 Tg mice were born at expected mendelian ratios, were viable and showed normal fertility. PHB1 Tg mice were phenotypically indistinguishable from wild type (WT) littermates (Figure 1(d)) and gained weight at the same rate as littermates (Figure 1(e)). Brain volume and gross cerebral anatomy did not differ from age matched WT littermate controls (Figure 1(d) and (f)). To determine whether PHB1 expression alters cognitive function, we examined the performance of the mice at the Y-maze (Figure 3). We found a small but significant increase in arm alternation in PHB1 Tg mice at baseline, suggesting a possible improvement in spatial working memory (Figure 3(c); *p = 0.033; n = 8/group). However, the NOR test27 revealed no difference in recognition memory between PHB1 Tg and WT controls (Figure 3S).
Figure 3.
PHB1 expression marginally improves cognitive function assessed using the Y maze. (a) No difference in novel arm entry, time spent in novel arm (b) and number of arm visits (d) was observed between five-and six-month-old, naïve PHB1 Tg and WT littermates; n = 8 mice/group; p > 0.05; unpaired t-test. (c) The percentage of arm alterations is increased in PHB1 Tg mice, indicating a small but significant improvement in spatial-working memory; n = 8 mice/group; *p < 0.05; unpaired t-test.
PHB1 Tg mice are protected from focal ischemic brain injury
To assess the effect of neuron-specific overexpression of PHB1 on focal cerebral ischemia, we subjected two-month-old male PHB1 Tg mice to a transient occlusion of the right middle cerebral artery (MCAo) and measured the consequent tissue damage three days later in Nissl stained sections. Infarct volumes in PHB1 Tg mice were reduced by 63 ± 11% (p = 0.0002; n = 10/group; Figure 4(a) and (b)) compared to WT mice. The reduction in injury volume was observed both in neocortex (PHB1 Tg: 7.8 ± 3.7 mm3; WT: 27.1 ± 6.0 mm3; p = 0.0051) and striatum (PHB1 Tg: 10.1 ± 1.8 mm3; WT: 24.0 ± 3.0 mm3; p = 0.0011). In parallel, the PHB1 Tg mice lost less post-injury body weight (PHB1 Tg: 3.1 ± 0.6 g; WT: 5.4 ± 0.9 g; p = 0.0378; Figure 5(d)).
Figure 4.
Ischemic injury is attenuated in PHB1-expressing mice. (a) Representative images of corresponding coronal Nissl-stained brain section of a PHB1 Tg and littermate control (WT) mouse 72 h after MCAo with the red dashed line indicating the infarct area. (b) PHB1 Tg mice show markedly reduced infarcts compared to WT controls; n = 9–10 mice/group; ***p = 0.0002; Mann–Whitney U rank-sum test. (c) The degree of focal cerebral ischemia and reperfusion are similar in the ischemic territory in PHB1 Tg and WT; n = 9–10 mice/group; p > 0.05; multiple t-tests.
Figure 5.
Effect of neuronal PHB1 expression on neurological impairment and functional outcome 72 h after MCAo. (a) PHB1 Tg mice show a significant improvement in sensory-motor performance (Corner test; n = 6–7; *p < 0.01; t-test) and markedly reduced motor impairment indicated by hanging wire test (n = 8 mice/group; *p < 0.048, Mann–Whitney U rank-sum test) and modified Bederson score; n = 10 mice/group; *p < 0.05, Mann–Whitney test) (b, c) compared to age-matched WT controls. (d) Body weight loss analysis of PHB1 Tg and WT controls; n = 10 mice/group; *p < 0.05; unpaired t-test.
The reduction in tissue damage in PHB1 Tg mice was also associated with an improved neurological function 72 h after the ischemic insult. Compared to WT littermates, PHB1 Tg mice exhibited an increased latency to fall at the hanging wire test (p = 0.044), attenuated functional impairment in the modified Bederson score (p = 0.022) and improved sensory-motor performance at the corner test (p = 0.019; Figure 5).
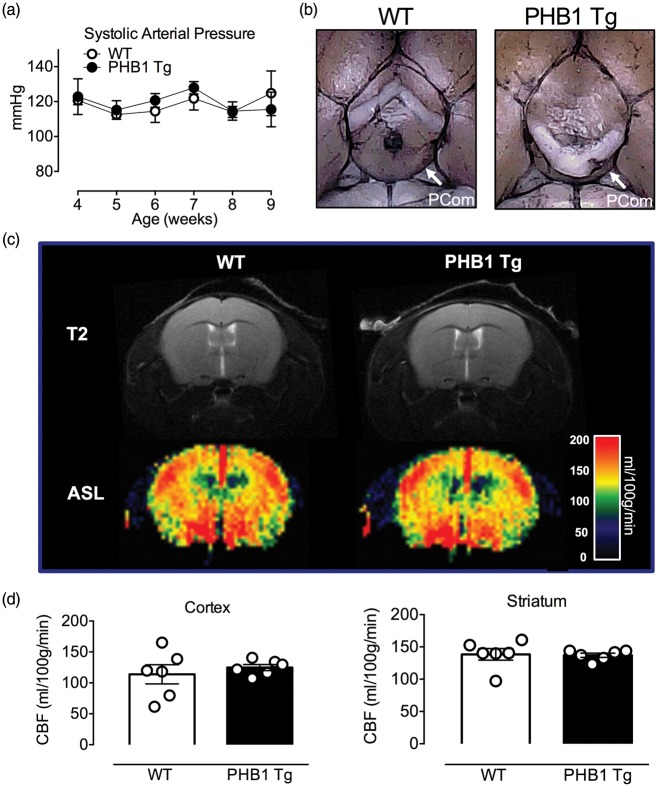
Cerebrovascular anatomy, resting and intra-ischemic CBF did not differ between PHB1 Tg and WT littermates
The anatomy of the circle of Willis and its major branches, including the posterior communicating artery,44 was comparable between WT and PHB1 Tg (Figure 6(a) and (b)). Furthermore, no differences in resting CBF (ml/100 g/min), assessed quantitatively by MRI-ASL (Figure 6(c) and (d)), or intra-ischemic CBF after MCAo, assed by laser-Doppler flowmetry, (Figure 6(c)) were observed. Similarly, no differences in arterial pressure, body temperature blood glucose, hematocrit or pO2, pCO2, and pH were observed between PHB1 Tg and WT littermates (Figure S2).
Figure 6.
No difference in stroke relevant parameters in eight-week-old naïve PHB1 Tg and WT mice. (a) Neuronal overexpression of PHB1 did not affect arterial blood pressure; n = 12 mice/group; p > 0.05, unpaired t-test. (b) Representative images of India ink perfused brains from PHB1 Tg and WT mice illustrating the circle of Willis and major cerebral arteries. White arrows indicate the posterior communicating artery (PCom). (c, d) Resting CBF, measured quantitatively using ASL-MRI, is similar in naïve PHB1 Tg and WT; n = 6 mice/group; p > 0.05, unpaired t-test.
Discussion
Major findings of the study
We developed a transgenic mouse model overexpressing human PHB1 in postnatal glutamatergic forebrain neurons and used it to assess the effect of PHB1 overexpression on acute brain injury produced by transient MCAo. We demonstrate that PHB1 transgene expression under the CaMKIIα promoter is restricted to neurons and found that enhancing PHB1 expression by 50% in forebrain was sufficient to confer a sizable injury reduction after focal cerebral ischemia. Importantly, the smaller infarct size was accompanied by an improvement in sensory-motor performance, suggesting that the salvaged brain remained functional. Additionally, PHB1 expression resulted in less body weight loss after ischemic injury supporting its beneficial effect on their overall health. This robust protection was not attributable to differences in pre- and post- ischemic CBF or to differences in other critical systemic physiological variables relevant to stroke pathophysiology. The data indicate that PHB upregulation in neurons is able to protect the brain tissue from acute ischemic injury and to preserve its function, and that neuronal PHB is able to confer cell-autonomous protection with beneficial effects for the whole tissue. In the transient MCAo model, substantial neuronal loss occurs after 24–72 h reperfusion, resulting in significant infarct growth during this period.45,46 This is when the lesion becomes stable and we and others have used the time point in this period extensively over the years for infarct volume measurement.47–53 However, it would be of interest to determine whether the protection is sustained in time in future studies.
Potential underlying mechanisms of PHB mediated neuroprotection
Despite accumulating evidence supporting neuroprotective potential from PHB expression, its underlying mechanism remains to be fully elucidated. In view of the central role of PHB in maintaining mitochondrial structure and function, it is highly likely that PHB overexpression ameliorates the deleterious effect of ischemia-reperfusion injury through mitochondrial mechanisms.
Functionally, PHB knockdown in neuronal cultures as well as in endothelial cells was shown to increase mitochondrial ROS production via decreased activity of Complex I and partial blockade of the respiratory electron transport chain.23,54 PHB may also affect mitochondrial function by promoting and stabilizing super-complex formation. Recently, the classical paradigm of mitochondrial respiratory chain complexes acting as individual functional entities in the inner mitochondrial membrane has been complemented by a dynamic supramolecular interaction model.55,56 In this solid structural organization model, mitochondrial respiratory complexes associate with each other in varying ratios to form supermolecular complexes, leading to increased energy conversion efficiency and decreased ROS emission. This model in combination with our recent data on the robust protection of neuronal PHB1 expression indicates a potential beneficial link between elevated PHB1 levels and mitochondrial supercomplex formation. The level of PHB1 transgene expression driven by the CaMKIIα promoter is moderate, as detected by Western blotting. One plausible explanation is the dilution effect from non-neuronal cells in the brain tissues. Nevertheless, such increased PHB1 expression selectively in neurons, albeit small, is able to afford protection against ischemia. This is in agreement with our early cell based studies in which PHB expression at moderate level was able to protect neuronal culture against hypoxic and oxidative stresses,23 attesting to the powerful neuroprotective effect of PHB1.
PHB expression and its implications for cognitive function
Recent data implicate PHBs in the maintenance of brain health. Postnatal deletion of PHB2 in the mouse forebrain leads to extensive neurodegeneration characterized by impaired mitochondrial architecture, early onset tau hyperphosphorylation and neurofibrillary tangle formation. This phenotype was accompanied by severe behavioral and cognitive dysfunction,57 suggesting that PHB, possibly through its effect on mitochondria, may be required for normal cognitive function. Although, our data are in line with this hypothesis by demonstrating that increased PHB1 expression in neurons modulates spatial memory, testing at the NOR task showed no difference in recognition memory between PHB1 Tg and WT littermates (Figure S3). Therefore, the effect of PHB expression on baseline cognition seems modest. However, it will be interesting to determine if PHB1 overepxression is able to rescue cognition in models of aging or neurodegeneration.
Conclusion
The present study used a novel transgenic mouse model to explore the in vivo effects of neuronal-specific PHB1 expression in a disease-relevant paradigm. Our findings provide the first evidence that specific expression of PHB1 in neurons is sufficient to reduce focal cerebral ischemic injury and preserve neurological function. The mechanisms of the protection remain to be fully elucidated, but they may involve maintenance of mitochondrial integrity and suppression of ROS production, early pathophysiological events in ischemic stroke. Therefore, a PHB-targeted approach counteracting these detrimental effects early in the ischemic cascade may represent a novel strategy to ameliorate the devastating impact of cerebral ischemia.
Supplementary Material
Acknowledgements
We thank Ms. Huihong Li for her excellent technical assistance.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH grants NS067078, NS034179, NS095692. AK was recipient of a post-doctoral research grant from the Deutsche Forschungsgemeinschaft (KA 3810/1-1).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
AK, CA, LQ and HV performed research, collected and analyzed data; CI, PZ, AK and GM designed research; AK, CI, PZ, and GM wrote the paper.
Supplementary material
Supplementary material for this paper can be found at the journal website http://journals.sagepub.com/home/jcb.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics – 2016 update: a report from the American Heart Association. Circulation 2016; 133: e38–e360. [DOI] [PubMed] [Google Scholar]
- 2.Campbell BC, Meretoja A, Donnan GA, et al. Twenty-year history of the evolution of stroke thrombolysis with intravenous alteplase to reduce long-term disability. Stroke 2015; 46: 2341–2346. [DOI] [PubMed] [Google Scholar]
- 3.Campbell BC, Donnan GA, Lees KR, et al. Endovascular stent thrombectomy: the new standard of care for large vessel ischaemic stroke. Lancet Neurol 2015; 14: 846–854. [DOI] [PubMed] [Google Scholar]
- 4.Demaerschalk BM, Kleindorfer DO, Adeoye OM, et al. Scientific rationale for the inclusion and exclusion criteria for intravenous alteplase in acute ischemic stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2016; 47: 581–641. [DOI] [PubMed] [Google Scholar]
- 5.Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014; 384: 1929–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359: 1317–1329. [DOI] [PubMed] [Google Scholar]
- 7.Sims NR, Muyderman H. Mitochondria, oxidative metabolism and cell death in stroke. Biochim Biophys Acta 2010; 1802: 80–91. [DOI] [PubMed] [Google Scholar]
- 8.Sims NR, Anderson MF. Mitochondrial contributions to tissue damage in stroke. Neurochem Int 2002; 40: 511–526. [DOI] [PubMed] [Google Scholar]
- 9.Kristian T. Metabolic stages, mitochondria and calcium in hypoxic/ischemic brain damage. Cell Calcium 2004; 36: 221–233. [DOI] [PubMed] [Google Scholar]
- 10.Kalogeris T, Baines CP, Krenz M, et al. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol 2012; 298: 229–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pundik S, Xu K, Sundararajan S. Reperfusion brain injury: focus on cellular bioenergetics. Neurology 2012; 79: S44–S51. [DOI] [PubMed] [Google Scholar]
- 12.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron 2010; 67: 181–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Artal-Sanz M, Tsang WY, Willems EM, et al. The mitochondrial prohibitin complex is essential for embryonic viability and germline function in Caenorhabditis elegans. J Biol Chem 2003; 278: 32091–32099. [DOI] [PubMed] [Google Scholar]
- 14.Artal-Sanz M, Tavernarakis N. Prohibitin and mitochondrial biology. Trends Endocrinol Metab 2009; 20: 394–401. [DOI] [PubMed] [Google Scholar]
- 15.Osman C, Merkwirth C, Langer T. Prohibitins and the functional compartmentalization of mitochondrial membranes. J Cell Sci 2009; 122: 3823–3830. [DOI] [PubMed] [Google Scholar]
- 16.Mishra S, Murphy LC, Murphy LJ. The Prohibitins: emerging roles in diverse functions. J Cell Mol Med 2006; 10: 353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merkwirth C, Langer T. Prohibitin function within mitochondria: essential roles for cell proliferation and cristae morphogenesis. Biochim Biophys Acta 2009; 1793: 27–32. [DOI] [PubMed] [Google Scholar]
- 18.Tatsuta T, Model K, Langer T. Formation of membrane-bound ring complexes by prohibitins in mitochondria. Mol Biol Cell 2005; 16: 248–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merkwirth C, Dargazanli S, Tatsuta T, et al. Prohibitins control cell proliferation and apoptosis by regulating OPA1-dependent cristae morphogenesis in mitochondria. Genes Dev 2008; 22: 476–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He B, Feng Q, Mukherjee A, et al. A repressive role for prohibitin in estrogen signaling. Mol Endocrinol 2008; 22: 344–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park SE, Xu J, Frolova A, et al. Genetic deletion of the repressor of estrogen receptor activity (REA) enhances the response to estrogen in target tissues in vivo. Mol Cell Biol 2005; 25: 1989–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Theiss AL, Sitaraman SV. The role and therapeutic potential of prohibitin in disease. Biochim Biophys Acta 2011; 1813: 1137–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou P, Qian L, D'Aurelio M, et al. Prohibitin reduces mitochondrial free radical production and protects brain cells from different injury modalities. J Neurosci 2012; 32: 583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurinami H, Shimamura M, Ma T, et al. Prohibitin viral gene transfer protects hippocampal CA1 neurons from ischemia and ameliorates postischemic hippocampal dysfunction. Stroke 2014; 45: 1131–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kilkenny C, Altman DG. Improving bioscience research reporting: ARRIVE-ing at a solution. Lab Animals 2010; 44: 377–378. [DOI] [PubMed] [Google Scholar]
- 26.Park L, Zhou J, Zhou P, et al. Innate immunity receptor CD36 promotes cerebral amyloid angiopathy. Proc Natl Acad Sci U S A 2013; 110: 3089–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leger M, Quiedeville A, Bouet V, et al. Object recognition test in mice. Nat Protoc 2013; 8: 2531–2537. [DOI] [PubMed] [Google Scholar]
- 28.Park L, Wang G, Moore J, et al. The key role of transient receptor potential melastatin-2 channels in amyloid-beta-induced neurovascular dysfunction. Nat Commun 2014; 5: 5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho S, Park EM, Febbraio M, et al. The class B scavenger receptor CD36 mediates free radical production and tissue injury in cerebral ischemia. J Neurosci 2005; 25: 2504–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hata R, Mies G, Wiessner C, et al. A reproducible model of middle cerebral artery occlusion in mice: hemodynamic, biochemical, and magnetic resonance imaging. J Cereb Blood FlowMetab 1998; 18: 367–375. [DOI] [PubMed] [Google Scholar]
- 31.Jackman K, Kunz A, Iadecola C. Modeling focal cerebral ischemia in vivo. Meth Mol Biol 2011; 793: 195–209. [DOI] [PubMed] [Google Scholar]
- 32.Clark WM, Lessov NS, Dixon MP, et al. Monofilament intraluminal middle cerebral artery occlusion in the mouse. Neurol Res 1997; 19: 641–648. [DOI] [PubMed] [Google Scholar]
- 33.Tureyen K, Vemuganti R, Sailor KA, et al. Infarct volume quantification in mouse focal cerebral ischemia: a comparison of triphenyltetrazolium chloride and cresyl violet staining techniques. J Neurosci Meth 2004; 139: 203–207. [DOI] [PubMed] [Google Scholar]
- 34.Iadecola C, Zhang F, Casey R, et al. Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. J Neurosci 1997; 17: 9157–9164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin TN, He YY, Wu G, et al. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke 1993; 24: 117–121. [DOI] [PubMed] [Google Scholar]
- 36.Balkaya M, Krober JM, Rex A, et al. Assessing post-stroke behavior in mouse models of focal ischemia. J Cereb Blood FlowMetab 2013; 33: 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abe T, Shimamura M, Jackman K, et al. Key role of CD36 in Toll-like receptor 2 signaling in cerebral ischemia. Stroke 2010; 41: 898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coleman A, Steel S, deGreeff A, et al. Validation of the Lloyds pharmacy BP11 oscillometric blood pressure monitor according to the international protocol of the European society of hypertension. Blood Pressure Monit 2010; 15: 163–166. [DOI] [PubMed] [Google Scholar]
- 39.Jackman KA, Zhou P, Faraco G, et al. Dichotomous effects of chronic intermittent hypoxia on focal cerebral ischemic injury. Stroke 2014; 45: 1460–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xue S, Gong H, Jiang T, et al. Indian-ink perfusion based method for reconstructing continuous vascular networks in whole mouse brain. PloS One 2014; 9: e88067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X, Zhang C, Szabo G, et al. Distribution of CaMKIIalpha expression in the brain in vivo, studied by CaMKIIalpha-GFP mice. Brain Res 2013; 1518: 9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mayford M, Bach ME, Huang YY, et al. Control of memory formation through regulated expression of a CaMKII transgene. Science 1996; 274: 1678–1683. [DOI] [PubMed] [Google Scholar]
- 43.Burgin KE, Waxham MN, Rickling S, et al. In situ hybridization histochemistry of Ca2+/calmodulin-dependent protein kinase in developing rat brain. The J Neurosci 1990; 10: 1788–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujii M, Hara H, Meng W, et al. Strain-related differences in susceptibility to transient forebrain ischemia in SV-129 and C57black/6 mice. Stroke 1997; 28: 1805–1810. discussion 11. [DOI] [PubMed] [Google Scholar]
- 45.Zhang RL, Chopp M, Chen H, et al. Temporal profile of ischemic tissue damage, neutrophil response, and vascular plugging following permanent and transient (2H) middle cerebral artery occlusion in the rat. J Neurol Sci 1994; 125: 3–10. [DOI] [PubMed] [Google Scholar]
- 46.Chen H, Kim GS, Okami N, et al. NADPH oxidase is involved in post-ischemic brain inflammation. Neurobiol Dis 2011; 42: 341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benakis C, Brea D, Caballero S, et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nat Med 2016; 22: 516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kawano T, Anrather J, Zhou P, et al. Prostaglandin E2 EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nat Med 2006; 12: 225–229. [DOI] [PubMed] [Google Scholar]
- 49.Garcia-Bonilla L, Racchumi G, Murphy M, et al. Endothelial CD36 contributes to postischemic brain injury by promoting neutrophil activation via CSF3. J Neurosci 2015; 35: 14783–14793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shindo A, Maki T, Mandeville ET, et al. Astrocyte-derived pentraxin 3 supports blood-brain barrier integrity under acute phase of stroke. Stroke 2016; 47: 1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qiu J, Xu J, Zheng Y, et al. High-mobility group box 1 promotes metalloproteinase-9 upregulation through Toll-like receptor 4 after cerebral ischemia. Stroke 2010; 41: 2077–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim GS, Jung JE, Niizuma K, et al. CK2 is a novel negative regulator of NADPH oxidase and a neuroprotectant in mice after cerebral ischemia. J Neurosci 2009; 29: 14779–14789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Donath S, An J, Lee SL, et al. Interaction of ARC and Daxx: a novel endogenous target to preserve motor function and cell loss after focal brain ischemia in mice. J Neurosci 2016; 36: 8132–8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schleicher M, Shepherd BR, Suarez Y, et al. Prohibitin-1 maintains the angiogenic capacity of endothelial cells by regulating mitochondrial function and senescence. J Cell Biol 2008; 180: 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Genova ML, Lenaz G. Functional role of mitochondrial respiratory supercomplexes. Biochim Biophys Acta 2014; 1837: 427–443. [DOI] [PubMed] [Google Scholar]
- 56.Acin-Perez R, Fernandez-Silva P, Peleato ML, et al. Respiratory active mitochondrial supercomplexes. Mol Cell 2008; 32: 529–539. [DOI] [PubMed] [Google Scholar]
- 57.Merkwirth C, Martinelli P, Korwitz A, et al. Loss of prohibitin membrane scaffolds impairs mitochondrial architecture and leads to tau hyperphosphorylation and neurodegeneration. PLoS Genet 2012; 8: e1003021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.