Abstract
Background
Impairment of gastric digestion due to pH elevation increases the risk for food allergy induction. As patients after Roux-en-Y gastric bypass (RYGB) surgery have lower gastric acidity and less gastric gland secretion we aimed to analyse in a prospective study the effect of limited gastric digestion capacity by surgical intervention on the immune response towards allergens.
Methods
Nine patients undergoing RYGB surgery for morbid obesity and one control patient having undergone surgery for treatment of an incisional hernia were enrolled in the study. Before and 1, 3, 6, 9 and 12 months after surgery blood was collected for analysis of specific IgE antibodies and patients were subjected to skin prick testing with 16 food and 18 aero-allergens.
Results
Skin prick test results revealed an increase of positive reactions indicating sensitizations towards the tested food and aero-allergens in 77.8% and 88.9% of the patients, respectively, after surgical elimination of gastric digestion. These results were in line with elevated titers of food- and aeroallergen-specific IgE antibodies in 7 out of 9 (7/9) and 5/9 patients, respectively, after RYGB surgery. Serum cytokine levels revealed a mixed response for IFN-γ and were mostly beneath detection limit for IL-4.
Conclusion
A change of IgE reactivity pattern occurred after impairment of gastric digestion due to surgical elimination underlining the important gastric gate keeping function during oral sensitization. Even though this study indicates an increased allergy risk for gastric bypass patients, further studies are needed to in depth investigate the immunological changes associated with RYGB surgery.
Keywords: Bariatric surgery, Laparoscopic gastric bypass, Food specific IgE, Sensitization, Skin prick test
Introduction
Globally, there are more than 1 billion overweight adults, at least 500 million of them obese [1]. Once considered a problem only in high income countries, overweight and obesity are now dramatically on the rise in low- and middle-income countries, particularly in urban settings [2]. Overweight and obesity are major risk factors for a number of chronic diseases, including diabetes, sleep apnoea, cardiovascular diseases, hypertension stroke and certain forms of cancer. Obesity is defined as a body mass index (BMI; weight in kg/(height in meters)2) ≥30 kg/m2, in an overall classification in which the healthy range of weight is a BMI between 18.5 and 24.9 kg/m2.
Roux-en-Y gastric bypass (RYGB) is a type of procedure performed on people who are dangerously obese, for the purpose of losing weight. Following the general criteria for eligibility for bariatric surgery only patients with morbid obesity, which is defined with a BMI ≥40 kg/m2, might be considered surgical candidates. Patients with less severe obesity (BMI ≥35 kg/m2) might be included in surgery if they have comorbid conditions such as cardiopulmonary problems, e.g. severe sleep apnoea or obesity-related cardiomyopathy, or type 2 diabetes mellitus. The weight loss by bariatric surgery is usually achieved by reducing the size of the stomach with an implanted medical device (gastric banding) or through removal of a portion of the stomach (sleeve gastrectomy or biliopancreatic diversion with duodenal switch) or by resecting and re-routing the small intestines to a small stomach pouch, which is also termed gastric bypass surgery. In the late 1970s, the gastric bypass was developed on the basis of information gathered from gastrectomy procedures and then modified to a RYGB anastomosis. In RYGB, the upper part of the stomach is transected; thus, a very small proximal gastric pouch, measuring 15–25 ml, is created. The gastric pouch is anastomosed to a Roux-en-Y proximal jejunal segment, bypassing the remaining stomach, duodenum, and a small portion of jejunum. The standard Roux (alimentary) limb length is ~50–150 cm, and the biliopancreatic limb is 15–50 cm. As a result, the RYGB limits food intake and induces some nutrient malabsorption [3]. Currently, most bariatric procedures are performed laparoscopically [4]. This surgical approach has the advantages of fewer wound complications, less postoperative pain, a briefer hospital stay, and faster postoperative recovery with comparable efficacy [5–7].
With regards to its physiological function, a massive impairment of the gastrointestinal tract’s digestive capacity can be expected after RYGB operations. Taking protein digestion as an example, the activation of the major gastric protease pepsin as well as the secretion of the pancratic proteases depends on the gastric acidity. Hydrochlorid acid, however, is produced by the parietal cells located in the gastric glands of the fundic area [8]. After RYGB operation only parts of the gastric cardia region remain present with major impact on the entire physiologic function of the stomach. It is well recognized that gastric bypass surgery is associated with inadequate gastric gland secretions as indicated by significantly reduced luminal intrinsic factor, which is produced by the hydrochlorid acid secreting parietal cells [9]. Furthermore, lower levels of postprandial acidity was reported for RYGB patients compared to normal individuals or GERD patients [10]. Interestingly, intake of acid suppression medication was found to decrease after bariatric surgery. RYGB was recently even described as the most efficient procedure to reduce the usage of these pharmaceuticals as compared to before surgery [11]. Thus, gastric hypoacidity might be the result with effect on gastric as well as pancreatic protease activation [12]. It is known that metabolic complications involving macro- and micronutrient deficiencies are common among patients who undergo restrictive-malabsorptive surgical procedures like RYGB [13–16]. Therefore early identification, routine prophylactic macro- and micronutrient supplementation and appropriate treatment are critically important in the successful management of the bariatric patient. Nevertheless, interference with the digestive function of the gastrointestinal tract might also have an additional influence on the protective role of gastrointestinal digestion on sensitization via the oral route. We have previously reported that, interference with the gastric digestion represents a major risk not only for the development of food allergies but also in situations when IgE is already present due to a reduced amount of tolerated food allergens [17].
Therefore, in the present study we aimed to analyze the impact of surgical elimination of the gastric digestion on sensitization via the oral route.
Materials and Methods
Study population and patient selection criteria
All patients between 18-70 years, who underwent RYGB for morbid obesity (BMI>40) as well as sex- and age-matched surgical control patients, who underwent incisional hernia repair at the Department of Surgery of the Medical University of Vienna were investigated for eligibility to be included in this study. Patients could not be included in the study if they were treated with anti-ulcer drugs (sucralfate, H2 receptor blockers or proton pump inhibitors) prior to the study or control patients who received these drugs for longer than the perioperative period, i.e. 2 weeks after surgery. Furthermore, patients with a clinical history of IgE-mediated allergic diseases were not included in the study. The study protocol was approved by the ethics committee of the Medical University Vienna (number: 261/2006) and written informed consent was obtained from all individual participants included in the study.
Based on these stringent criteria 9 RYGB patients and 1 control patient were included in the allergological evaluation and the clinical follow-up for 12 months during the patient recruitment period February to October 2007 (Table 1). During this period nearly 90 patients underwent RYGB for morbid obesity at the Department of Surgery of the Medical University of Vienna. The first patient was included on February 19, 2007. The last allergological screening of a RYGB patient was performed on September 29, 2008. To enable further recruitment of control patients, we extended the patient recruitment period until end of 2009, however, no further patients were included either because of the above mentioned stringent inclusion criteria or due to patients’ refusal to participate.
Table 1.
Patients’ Characteristics
| Pat. | Sex | Age at operation | Month of operation | Follow-up Time-period | Preoperative BMI | BMI at 12 Mo postoperative |
|---|---|---|---|---|---|---|
| 1 | f | 23 years | February | 6 months | 48.5 | 36.2; pregnant |
| 2 | f | 34 years | February | 12 months | 45.9 | 23.5 |
| 3 | f | 21 years | February | 6 months | 49.2 | 34.3; pregnant |
| 4 | f | 45 years | March | 12 months | 61.7 | 32.3 |
| 5 | m | 64 years | April | 12 months | 38.6 | 26.6 |
| 6 | f | 33 years | May | 12 months | 49.2 | 27.3 |
| 7 | f | 18 years | May | 12 months | 41.9 | 23.6 |
| 8 | f | 33 years | June | 12 months | 48.5 | 25.0 |
| 9 | f | 51 years | October | 12 months | 39.4 | 24.5 |
| Co | f | 36 years | August | 12 months | 21.6 | 21.9 |
Surgical procedures and postoperative care
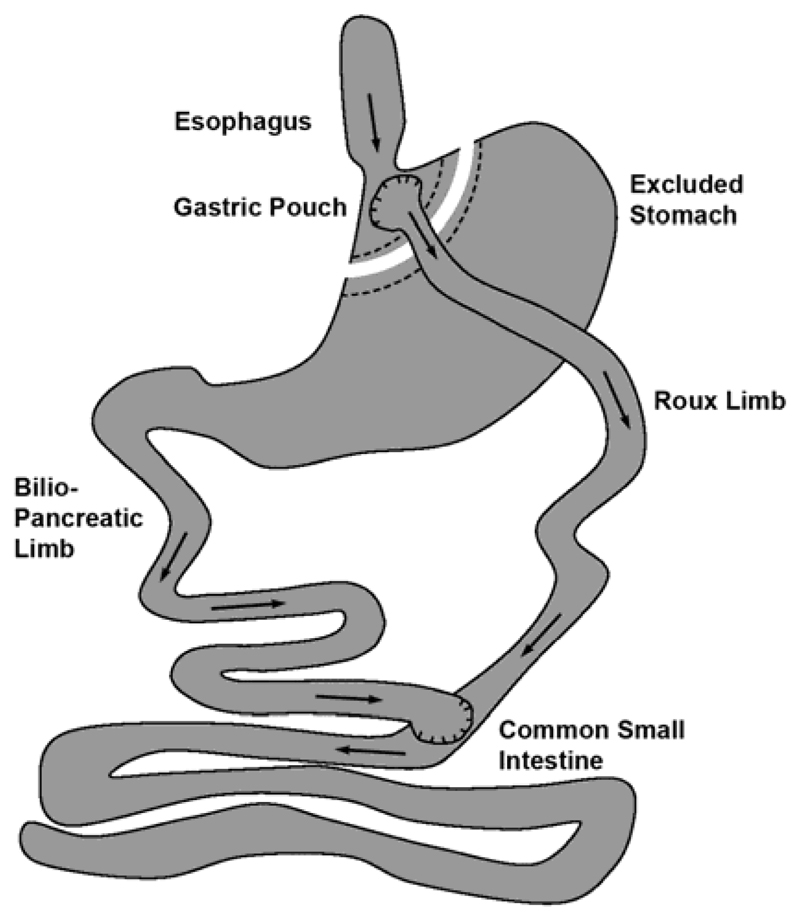
In all 9 RYGB patients the surgical procedure was performed according to the standard procedure of RYGB operations (Fig. 1). This method works by combining both restrictive and malabsorptive elements. The restrictive part can be achieved by stapling the stomach into two sections. The top section becomes a small pouch (15–25 ml) limiting the amount of food intake. The malabsorptive part is achieved by surgically dividing the small intestine. The lower part of the intestine is pulled up to directly connect to the small pouch (gastro-jejunostomy). The other end of this divided intestine is surgically sewn back at a specific point (100-150cm) further down the small intestine (jejuno-jejunostomy). Oral nutrition was started on the 2nd postoperative day after an uneventful postoperative course, as was the case for all included patients. All patients were discharged from hospital after 5 days of postoperative care.
Fig. 1. Schematic diagram of the gastrointestinal digestive tract after RYGB surgery.
Arrows indicate the passage of chyme and bile components through the digestive system with both substances being mixed in the lower small intestinal tract.
The control patient underwent open hernia repair with the implantation of a mesh prothesis in a sublay technique, which is the standard treatment for incisional hernias larger than 5cm in diameter at our institution. After dissection and resection of the hernial sac, the synthetic prothesis was placed extraperitoneally over the dorsal sheath of the rectus fascia and below the body of the rectus muscle. The bodies of the rectus muscles were adapted loosely and the ventral sheath of the rectus fascia was closed thereafter. Postoperatively, the patient resumed oral feeding on the first postoperative day and was discharged from hospital after removal of the wound drainage tubes 2-3 days after surgery.
Allergological evaluation
Allergological evaluations of study and control patients were scheduled preoperatively and 1, 3, 6, 9, and 12 months after surgery. At each of the time-points the patients were available, blood was withdrawn for evaluation of allergen-specific IgE and serum cytokine levels and skin testing was performed.
Allergen-specific IgE antibodies in patient’s sera were measured in enzyme linked immunosorbent assay (ELISA). Standard series of 18 inhalant (birch, ash tree, grass, rye, plantain, ragweed, mugwort, Candida albicans, Alternaria, Cladosporium, house dust mite, storage mite, dog, horse, guinea pig, cat and two latex extracts) and 16 food allergens (egg white, egg yolk, codfish, soy, milk, pea, peanut, hazelnut, orange, walnut, tomato, celery, wheat, rye, carrot, potato) were tested. Microtiter plates were coated with 100µl skin prick test substances diluted 1:20 in coating buffer (50mM NaHCO3, pH 9.6) over night at 4°C. For determination of IgE serum concentration also a 2-fold serial dilution of a human IgE standard (Alpha Diagnostic Intl. Inc., San Antonio, USA) was included. After repeated washing cycles, unspecific binding was blocked by 2 hours incubation with Tris-buffered saline containing 0.05% TWEEN 20 (TBST) and 1% bovine serum albumin (BSA). Sera were diluted 1: 5 in TBST containing 0.1% BSA and incubated over night at 4°C. Plates were washed and incubated for 2 hours using HRP-labelled goat anti human-IgE (KPL Gaithersburg, MD, USA) diluted 1:1000 in TBST containing 0.1% BSA. Detection was performed using 100µl/well optEIA TMB Substrate set (BD Bioscience, San Jose, USA) according to the manufacturer’s instruction. The reaction was stopped after 2 minutes using 1.8M H2SO4 and absorption measured at 405 and 650 nm. Serum IgE concentrations were calculated by comparison to the standard curve.
Skin prick tests were performed with the 16 food and 18 inhalant allergen extracts (ALK-Abello, Horsholm, Denmark) according to the position paper of the European Academy of Allergology and Clinical Immunology, where a diameter above 3 mm was defined as positive (+) [18]. Each patient was accurately interviewed for any clinical symptoms due to food hypersensitivity (e.g. skin rash, urticaria, angioedema, gastrointestinal symptoms, hypotension, rhinitis, itching).
Circulating IL-4 and IFN-γ in the patients’ sera was determined by commercial ELISA using monoclonal antibodies against human IL-4 and IFN-γ (Bioscience, San Diego, CA). The assays were performed following the manufacturer’s instructions. In short, microtiter plates were coated with the respective capture antibody and incubated overnight at 4°C. After washing, the plates were blocked with assay diluent for 1 hour. Serum samples and standard dilutions were incubated overnight at 4°C. Next the detection antibody was applied for 1 hour followed by Avidin-HRP for 30 minutes. The color reaction was developed with TMB and stopped after approximately 15 minutes. Plates were measured at 450 – 570 nm. Cytokine concentrations were calculated according to the standard curve. Detection sensitivity for IFN-γ was 4 pg/mL and 2 pg/mL for IL-4.
Results
Surgical characteristic and postoperative clinical follow up
The 9 RYGB patients had all an uncomplicated laparoscopic gastric bypass surgery with a mean preoperative BMI of 46.4kg/m2. Mean BMI and excess weight loss at 12 months follow-up were 28.3kg/m2 and 81.63%, respectively, which is equivalent to the general results after laparoscopic gastric bypass (Table 1). Patients 5 and 9 had glucose intolerance/diabetes before surgery with the need for oral antidiabetic medication in patient 5. Both lost their glucose intolerance after RYGB surgery with normal HbA1c levels at the 12 month time-point.
At every medical consultation during the follow-up period all patients were carefully interviewed regarding intake of medication interfering with gastric digestive capacity, signs of allergic disorders and regarding the presence of digestive complaints. Patients 1, 2, 5 and 7 were under gastric acid suppression immediately following RYGB surgery for a period of 43, 42, 16 and 22 days, respectively. None of the patients reported any clinical symptoms that could be seen in association with either inhalant or food adverse type 1 reaction in the detailed interviews. Only patient 8 reported symptoms of food adverse reactions developed after gastric bypass surgery, without further specification which food compound might be the causative trigger.
Gastric bypass surgery increases number of positive SPTs to food and aeroallergens
The evaluation of skin prick test results in all patients participating in the study revealed increasing numbers of positive skin reactions indicating sensitizations towards the tested food compounds in 7 out of 9 patients (7/9) and towards aeroallergens in 8/9 patients, but not in the control patient (Tables 2 and 3). As sensitization especially towards aeroallergens might be observed in connection with the pollen season we also paid special attention to the time-point of operation with regards to the pollen season. In patients 1-4 surgery was performed during the blooming season of trees, whereas patients 6-8 and the control patient were included in our study during grass and weed pollen season. Only in patient 9 gastric bypass surgery was done after the pollen season (Table 1). However, when taking into consideration a possible interaction between the observed skin test reactivity and the time-point of bariatric surgery, we could not determine any significant association for the 2 groups of patients. Despite the fact that there was a pronounced increase in positive skin prick test numbers, the results of the positive wheal-and-flare reactions was not fully consistent regarding the tested allergens within the single patients throughout the entire follow-up period of 12 months. Skin prick test results in the control patient did not reveal any increasing numbers of positive skin reactions towards food proteins or aeroallergens.
Table 2.
Number of positive skin prick tests with food allergens
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Control patient | |
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | 0 | 2 | 1 | 3 | 0 | 0 | 0 | 3 | 0 | 2 |
| 1 month | 1 | 1 | 0 | 0 | 0 | 3 | 2 | 2 | 0 | |
| 3 months | 1 | 2 | 1 | 8 | 0 | 3 | 6 | 0 | ||
| 6 months | 3 | 2 | 0 | 10 | 0 | 2 | 1 | |||
| 9 months | 8 | 3 | 2 | |||||||
| 12 months | 4 | 3 | 2 | 0 | 7 | 1 | 2 | 0 |
Table 3.
Number of positive skin prick tests with aeroallergens
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Control patient | |
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | 0 | 2 | 4 | 3 | 1 | 0 | 0 | 1 | 2 | 2 |
| 1 month | 2 | 3 | 2 | 0 | 1 | 2 | 3 | 0 | 0 | |
| 3 months | 4 | 2 | 5 | 3 | 3 | 7 | 6 | 1 | ||
| 6 months | 8 | 2 | 3 | 9 | 0 | 0 | 1 | |||
| 9 months | 5 | 3 | 3 | |||||||
| 12 months | 3 | 6 | 5 | 3 | 5 | 0 | 2 | 0 |
Increasing titers of specific IgE antibodies are detected in the patient’s sera after surgical elimination of gastric digestion
To determine changes occurring after surgical intervention, increases of allergen-specific IgE antibody titers from before to after elimination of gastric digestion were calculated. After surgical elimination of gastric digestion we determined increasing amounts of specific IgE antibodies in 7/9 patients towards food compounds and in 5/9 patients towards aeroallergens (Table 4) confirming the results from SPTs.
Table 4.
Allergens recognized by patients’ IgE with increasing titers during follow-up period. Marginal changes of IgE titers of 0.2 μg/ml (0.08 kU/L) and below were considered to be of minor clinical relevance and are therefore not included in the table.
| Patient (time-point of measurement) | Food allergens (CAP class) | Aeroallergens (CAP class) |
|---|---|---|
| patient 1 (6 month) | Codfish (1) | -- |
| patient 2 (9 month) | Codfish (2) | Candida albicans (2) |
| patient 3 (6 month) | Egg white (0), carrot (0) | -- |
| patient 4 (9 month) | -- | Birch pollen (1), ribwort (1) |
| patient 5 (3 month) | Tomato (2), carrot (0) | Cladosporium sp. (1), storage mite (1) |
| patient 6 (12 month) | Codfish (2), cow milk (0) | Candida albicans (0), Alternaria alternata (1) |
| patient 7 (3 month) | Soy (1), walnut (0) | -- |
| patient 8 (9 month) | Egg yolk (1), soy (2), cow milk (2), wheat (1), rye (0) | Horse (2), ribwort (2) |
| patient 9 (12 month) | -- | -- |
| Co patient (6 month) | -- | -- |
Even though, for some allergens a steady increase of allergen-specific IgE levels was observed, we also detected decreases of IgE levels during the follow-up period. Neither the increase nor the drop of IgE levels could be explained by pollen contact during the respective season, which in general could impact on food allergen-specific IgE levels due to cross-reactive allergens.
No increased allergen-specific IgE levels were detected in serum samples of the control patient from before to after surgery (Table 4).
Gastric bypass surgery affects Th1 and Th2 cytokines
With regards to cytokine levels we measured very low amounts of the Th1 cytokine IFN-γ in serum samples taken during the follow-up visit. Additionally, titers were mostly below detection limits for the Th2 cytokine IL-4 (Table 5). In 5/9 patients we observed a slight increase of IFN-γ, mostly at the 1- or 3-month time-point of follow-up. The IFN-γ increase was most prominent in patient 8 one month after RYGB surgery. For patients 7 and 9 a marginal decrease of IFN-γ was measured. Regarding IL-4 we observed a very slight increase in patient 8 with highest levels at the 1-month time-point; all other values remained below detection limit. Both IFN-γ and IL-4 levels remained below detection limits in serum samples of patients 3 and 5 as well as the control patient during the entire follow-up period (Table 5).
Table 5.
Evaluations of serum cytokine levels of IFNγ and IL-4 (concentrations given as pg/mL)
| IFNγ IL-4 |
Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Control patient |
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | - - |
4 - |
- - |
- - |
- - |
- - |
8 - |
105 - |
6 - |
- - |
| 1 month | 4 - |
5 - |
- - |
7 - |
- - |
4 - |
4 - |
293 3.54 |
- - |
|
| 3 months | 24 - |
5 - |
- - |
4 - |
- - |
7 - |
4 - |
4 - |
||
| 6 months | 4 - |
8 - |
- - |
4 - |
4 - |
270 2.05 |
- - |
|||
| 9 months | - - |
|||||||||
| 12 months | 4 - |
- - |
- - |
- - |
66 - |
5 - |
- - |
Discussion
In 2008 and 2011 extensive investigations of bariatric surgery procedures and numbers were performed worldwide including all 50 nations or national subgroups affiliated to the International Federation for the Surgery of Obesity and Metabolic Disorders [19,20]. These surveys revealed that the total number of bariatric surgery operations has marginally decreased between 2008 (344,221) and 2011 (340,768). In 2011 the most commonly performed operation was the RYGB (46.6%), followed by sleeve gastrectomy accounting for a total number of 94,734 operations worldwide. Thus, without any doubt any adverse effects associated with this surgical procedure affects an extensive number of patients worldwide.
Out of the nearly 90 patients being subjected to RYGB operations during the period of patients’ recruitment at our centre we included 9 RYGB patients (approximately 10%) in the present study. These patients were subjected to allergological evaluations during the year succeeding surgical intervention to analyse the association between surgical elimination of gastric digestion and sensitization to allergenic proteins.
In the past the impact of bariatric operations on weight loss and nutritional status of surgery patients has been repeatedly evaluated and has led to the recommendation of lifelong vitamin and mineral supplementation after all bariatric procedures [21–23]. Despite universal supplementation, the prevalence of nutritional deficiencies was found to be comparable between different types of bariatric procedures limiting the gastric and intestinal nutrient digestive and absorptive capacity, with 25-hydroxy vitamin D deficiency as the most common [24]. Our study population with a mean BMI reduction to 28.3kg/m2 and an excess weight loss of 81.63% at the 12 months follow-up time-point was within the range of general results after laparoscopic gastric bypass.
As nutrient deficiencies might be associated with impaired immune responses [25], the observed macronutrient and micronutrient deficiencies of postbariatric patients were suggested to influence wound healing and immune function [26]. However, in context of bariatric surgery and chronically obese patients not only nutritional deficiencies might influence the adequate immune response but also the adipose tissue itself. Obesity is considered as a chronic low-grade inflammation, evidenced by increased levels of systemic acute phase proteins and inflammatory markers such as IL-6, IL-1, IL-9, IL-10, IL-8 and RANTES [27,28]. Morbid obesity was found to be associated with a continuous activation of the innate immune response, especially neutrophils [29]. Other studies revealed elevated counts of eosinophils and monocytes, but reduced numbers of monocyte and neutrophil CD62L in severely obese patients [30]. These deviated levels reversed rapidly by surgically-induced weight loss, thus, gastric bypass surgery was termed an “immune restorative operation” [30]. In line with this study, bariatric surgery was described to modify many immunological parameters and reverse obesity induced impairment of immune function [31,32]. Inflammatory factors in patients’ sera were found to follow a biphasic pattern in the postoperative phase, decreasing sharply 3 months after bariatric surgery. At 6 months they were found to again raise to pre-surgical levels and then attenuate in an overall reduction at 12 months after surgical intervention [27]. In contrast to these data, in our cytokine determination we observed a slight increase of IFN-γ at the 1 or 3 month time-point of follow-up. Regarding IL-4 a very slight increase was observed in 1 patient with highest levels 1 month after surgery.
In our study population we observed a marked increase of IgE antibodies specific for food as well as inhalant allergens after surgically-induced weight loss. Of interest, animal experiments demonstrated an impairment of humoral immune response associated with decreased total body fat by partial lipectomy which was suggested to be due to the reduced energy available [33]. Additional to the elevated IgE levels, patients developed increasing numbers of positive SPT reactivities indicating sensitization throughout the post-surgical follow-up period. Even though persistent vomiting is observed in post-bariatric patients [34], the only connection between this type of surgical intervention and atopic disorders described in literature has been reported in regard to asthma. However, in asthma patients bariatric surgery was reported to ameliorate the clinical situation [35]. In our study population, the observed reactivity pattern towards food proteins might be seen in association with the gastric gate keeping function against food allergens [17]. Impairment of gastric digestion was found to be associated with elevated risk for de novo sensitization towards regular constituents of the daily diet [36,37]. Nevertheless, these previous data do not provide an explanation for the observed increase of IgE and SPT reactivity towards inhalative allergens, as for these allergens the gastrointestinal route should not be decisive for sensitization.
Conclusions
This study is the logical consequence of our previous investigations indicating that an impairment of the gastric digestive function either by medication or by surgical gastric elimination supports allergic sensitizations. We investigated the causative association between surgical elimination of gastric digestion and type 1 hypersensitivity reactions. We observed an increasing number of positive skin tests associated with elevated IgE levels towards both, food as well as aeroallergens. Even though the patients did not report clinical symptoms during the follow-up period, we observed sensitisation in over 70% of the patients which represents a risk to experience adverse gastrointestinal or respiratory reactions upon encounter with the respective allergen.
This pioneer study with a low number of surgery patients and limited comparability to the single control patient, may found the basis of more detailed studies, which are urgently needed. Based on our data we conclude that bypassing the stomach by surgical intervention likely has impact of on the immune function of the gut.
Funding
This study was funded by OeNB Jubiläumsfond 11375 and grants P21577-B11, P21884-B11, KLI284 and SFB F4606-B19 of the Austrian Science Funds FWF.
Footnotes
Conflict of Interest Statement: Eva Untersmayr has received research grants from the Austrian Science Fund FWF and from the Austrian National Bank Jubiläumsfond during the conduct of the study. Soheila Shakeri-Leidenmühler, Anna Lukschal, Cornelia Schultz, Arthur Bohdjalian, Felix Langer, Tudor Birsan, Susanne C. Diesner, Elli K. Greisenegger, Otto Scheiner, Tamara Kopp, Erika Jensen-Jarolim and Gerhard Prager declare that they have no conflict of interest.
Statement of Informed Consent: Informed consent was obtained from all individual participants included in the study.
Statement of Human Rights: The study protocol has been approved by the institutional ethics committee of the Medical University of Vienna (number: 261/2006). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Sise A, Friedenberg FK. A comprehensive review of gastroesophageal reflux disease and obesity. Obes Rev. 2008;9:194–203. doi: 10.1111/j.1467-789X.2007.00459.x. [DOI] [PubMed] [Google Scholar]
- 2.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elder KA, Wolfe BM. Bariatric surgery: a review of procedures and outcomes. Gastroenterology. 2007;132:2253–2271. doi: 10.1053/j.gastro.2007.03.057. [DOI] [PubMed] [Google Scholar]
- 4.Buchwald H, Williams SE. Bariatric surgery worldwide 2003. Obes Surg. 2004;14:1157–1164. doi: 10.1381/0960892042387057. [DOI] [PubMed] [Google Scholar]
- 5.Schauer PR, Ikramuddin S. Laparoscopic surgery for morbid obesity. Surg Clin North Am. 2001;81:1145–1179. doi: 10.1016/s0039-6109(05)70189-4. [DOI] [PubMed] [Google Scholar]
- 6.Davila-Cervantes A, Borunda D, Dominguez-Cherit G, Gamino R, Vargas-Vorackova F, et al. Open versus laparoscopic vertical banded gastroplasty: a randomized controlled double blind trial. Obes Surg. 2002;12:812–818. doi: 10.1381/096089202320995619. [DOI] [PubMed] [Google Scholar]
- 7.Schauer PR, Ikramuddin S, Gourash W, Ramanathan R, Luketich J. Outcomes after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Ann Surg. 2000;232:515–529. doi: 10.1097/00000658-200010000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger H. The distribution of parietal cells in the stomach: A histotopographic study. American Journal of Anatomy. 1934;54:87–114. [Google Scholar]
- 9.Marcuard SP, Sinar DR, Swanson MS, Silverman JF, Levine JS. Absence of luminal intrinsic factor after gastric bypass surgery for morbid obesity. Dig Dis Sci. 1989;34:1238–1242. doi: 10.1007/BF01537272. [DOI] [PubMed] [Google Scholar]
- 10.Herbella FA, Vicentine FP, Del Grande JC, Patti MG, Arasaki CH. Postprandial proximal gastric acid pocket in patients after Roux-en-Y gastric bypass. J Gastrointest Surg. 2010;14:1742–1745. doi: 10.1007/s11605-010-1309-5. [DOI] [PubMed] [Google Scholar]
- 11.Varban OA, Hawasli AA, Carlin AM, Genaw JA, English W, et al. Variation in utilization of acid-reducing medication at 1 year following bariatric surgery: results from the Michigan Bariatric Surgery Collaborative. Surg Obes Relat Dis. 2015;11:222–228. doi: 10.1016/j.soard.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 12.Pali-Schöll I, Jensen-Jarolim E. Anti-acid medication as a risk factor for food allergy. Allergy. 2011;66:469–477. doi: 10.1111/j.1398-9995.2010.02511.x. [DOI] [PubMed] [Google Scholar]
- 13.Bal BS, Finelli FC, Shope TR, Koch TR. Nutritional deficiencies after bariatric surgery. Nat Rev Endocrinol. 2012;8:544–556. doi: 10.1038/nrendo.2012.48. [DOI] [PubMed] [Google Scholar]
- 14.Harbottle L. Audit of nutritional and dietary outcomes of bariatric surgery patients. Obes Rev. 2011;12:198–204. doi: 10.1111/j.1467-789X.2010.00737.x. [DOI] [PubMed] [Google Scholar]
- 15.Jeffreys RM, Hrovat K, Woo JG, Schmidt M, Inge TH, et al. Dietary assessment of adolescents undergoing laparoscopic Roux-en-Y gastric bypass surgery: macro- and micronutrient, fiber, and supplement intake. Surg Obes Relat Dis. 2012;8:331–336. doi: 10.1016/j.soard.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stocker DJ. Management of the bariatric surgery patient. Endocrinol Metab Clin North Am. 2003;32:437–457. doi: 10.1016/s0889-8529(03)00002-1. [DOI] [PubMed] [Google Scholar]
- 17.Untersmayr E, Jensen-Jarolim E. The role of protein digestibility and antacids on food allergy outcomes. J Allergy Clin Immunol. 2008;121:1301–1308. doi: 10.1016/j.jaci.2008.04.025. quiz 1309-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malling HJ. Methods of skin testing. Allergy. 1993;48:55–56. [Google Scholar]
- 19.Buchwald H, Oien DM. Metabolic/bariatric surgery Worldwide 2008. Obes Surg. 2009;19:1605–1611. doi: 10.1007/s11695-009-0014-5. [DOI] [PubMed] [Google Scholar]
- 20.Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obes Surg. 2013;23:427–436. doi: 10.1007/s11695-012-0864-0. [DOI] [PubMed] [Google Scholar]
- 21.Mechanick JI, Kushner RF, Sugerman HJ, Gonzalez-Campoy JM, Collazo-Clavell ML, et al. American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery Medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Endocr Pract. 2008;14(Suppl 1):1–83. doi: 10.4158/EP.14.S1.1. [DOI] [PubMed] [Google Scholar]
- 22.Mechanick JI, Youdim A, Jones DB, Garvey WT, Hurley DL, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient--2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity (Silver Spring) 2013;21(Suppl 1):S1–27. doi: 10.1002/oby.20461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snyder-Marlow G, Taylor D, Lenhard MJ. Nutrition care for patients undergoing laparoscopic sleeve gastrectomy for weight loss. J Am Diet Assoc. 2010;110:600–607. doi: 10.1016/j.jada.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 24.Moize V, Andreu A, Flores L, Torres F, Ibarzabal A, et al. Long-term dietary intake and nutritional deficiencies following sleeve gastrectomy or Roux-En-Y gastric bypass in a mediterranean population. J Acad Nutr Diet. 2013;113:400–410. doi: 10.1016/j.jand.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Calder PC. Feeding the immune system. Proc Nutr Soc. 2013;72:299–309. doi: 10.1017/S0029665113001286. [DOI] [PubMed] [Google Scholar]
- 26.Agha-Mohammadi S, Hurwitz DJ. Potential impacts of nutritional deficiency of postbariatric patients on body contouring surgery. Plast Reconstr Surg. 2008;122:1901–1914. doi: 10.1097/PRS.0b013e31818d20d6. [DOI] [PubMed] [Google Scholar]
- 27.Dalmas E, Rouault C, Abdennour M, Rovere C, Rizkalla S, et al. Variations in circulating inflammatory factors are related to changes in calorie and carbohydrate intakes early in the course of surgery-induced weight reduction. Am J Clin Nutr. 2011;94:450–458. doi: 10.3945/ajcn.111.013771. [DOI] [PubMed] [Google Scholar]
- 28.de Heredia FP, Gomez-Martinez S, Marcos A. Obesity, inflammation and the immune system. Proc Nutr Soc. 2012;71:332–338. doi: 10.1017/S0029665112000092. [DOI] [PubMed] [Google Scholar]
- 29.Nijhuis J, Rensen SS, Slaats Y, van Dielen FM, Buurman WA, et al. Neutrophil activation in morbid obesity, chronic activation of acute inflammation. Obesity (Silver Spring) 2009;17:2014–2018. doi: 10.1038/oby.2009.113. [DOI] [PubMed] [Google Scholar]
- 30.Cottam DR, Schaefer PA, Shaftan GW, Velcu L, Angus LD. Effect of surgically-induced weight loss on leukocyte indicators of chronic inflammation in morbid obesity. Obes Surg. 2002;12:335–342. doi: 10.1381/096089202321088101. [DOI] [PubMed] [Google Scholar]
- 31.Moulin CM, Marguti I, Peron JP, Halpern A, Rizzo LV. Bariatric surgery reverses natural killer (NK) cell activity and NK-related cytokine synthesis impairment induced by morbid obesity. Obes Surg. 2011;21:112–118. doi: 10.1007/s11695-010-0250-8. [DOI] [PubMed] [Google Scholar]
- 32.Valezi AC, Cabrera EJ, Delfino VD, Barbosa DS, Mali Junior J, et al. Roux-en-Y gastric bypass and inflammatory activity of the adipose tissue. Rev Col Bras Cir. 2011;38:161–166. doi: 10.1590/s0100-69912011000300004. [DOI] [PubMed] [Google Scholar]
- 33.Demas GE, Drazen DL, Nelson RJ. Reductions in total body fat decrease humoral immunity. Proc Biol Sci. 2003;270:905–911. doi: 10.1098/rspb.2003.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Netto BD, Moreira EA, Patino JS, Beninca JP, Jordao AA, et al. Influence of Roux-en-Y gastric bypass surgery on vitamin C, myeloperoxidase, and oral clinical manifestations: a 2-year follow-up study. Nutr Clin Pract. 2012;27:114–121. doi: 10.1177/0884533611431462. [DOI] [PubMed] [Google Scholar]
- 35.Evans S, Kurukulaaratchy RJ. The effect of bariatric surgery in the difficult asthma-obesity phenotype: a case report. J Asthma. 2013;50:52–55. doi: 10.3109/02770903.2012.741639. [DOI] [PubMed] [Google Scholar]
- 36.Schöll I, Untersmayr E, Bakos N, Roth-Walter F, Gleiss A, et al. Antiulcer drugs promote oral sensitization and hypersensitivity to hazelnut allergens in BALB/c mice and humans. Am J Clin Nutr. 2005;81:154–160. doi: 10.1093/ajcn/81.1.154. [DOI] [PubMed] [Google Scholar]
- 37.Untersmayr E, Bakos N, Scholl I, Kundi M, Roth-Walter F, et al. Anti-ulcer drugs promote IgE formation toward dietary antigens in adult patients. FASEB J. 2005;19:656–658. doi: 10.1096/fj.04-3170fje. [DOI] [PubMed] [Google Scholar]